Abstract
Site-directed mutagenesis was employed to create lesions in fimI, a gene of uncertain function located in the chromosomal gene cluster (fim) involved in Escherichia coli type 1 pilus biosynthesis. Chromosomal fimI mutations produced a piliation-negative phenotype. Complementation analysis indicated that a fimI′-kan insertion mutation and a fimI frameshift mutation produced polarity-like effects not seen with an in-frame fimI deletion mutation. Minicell analysis associated fimI with a 16.4-kDa noncytoplasmic protein product (FimI). We conclude that FimI has a required role in normal pilus biosynthesis.
Type 1 pili are filamentous proteinaceous appendages produced by many members of the Enterobacteriaceae (28). In Escherichia coli, the pili are made principally of a repeating monomer, FimA, the product of the fimA gene (11, 21), that is arrayed helically to form a hollow-cored fiber (5). There are at least three minor pilus proteins that are organized into structures seen on the ends of pili (10) and may also be present in the pilus fiber (24). One of these minor components, FimH (the product of the fimH [pilE] gene [15]), is the molecule that binds to mannose-containing receptors on eucaryotic cells (13).
The molecular interactions needed to construct E. coli type 1 pili have been examined in some detail (28). However, fimI, one of the nine fim genes clustered at centisome 98 on the E. coli genetic map, has not been well characterized and may have a role in the biosynthetic process. The fimI gene is found in both E. coli (12) and Salmonella enterica serovar Typhimurium (25). In both cases fimI was identified as an open reading frame located adjacent and 3′ to fimA and predicted to encode FimA-like proteins with mature molecular masses of ca. 17 kDa in the case of E. coli and 19 kDa in the case of S. enterica. Only recently have E. coli mutants been isolated with transposon insertions in fimI (2, 4). Fimbriation is eliminated in these mutants, suggesting a required role for the putative fimI product in pilus biosynthesis. However, polar effects of the insertions on the expression of downstream genes (whose products are known to be required for pilus assembly [20]) could not be ruled out (2, 4).
In this report, we employed site-directed mutagenesis to show that insertion and frameshift mutations in fimI indeed appeared polar. However, even when polarity was rendered undetectable by our employment of an in-frame ΔfimI mutant, fimI was still required for pilus biosynthesis. We also associated a 16.4-kDa noncytoplasmic protein with the fimI coding region.
Bacterial strains, plasmids, and growth conditions.
Bacterial strains, all E. coli K-12 derivatives, along with the plasmids used are listed in Table 1. Media consisted of L broth and L agar (17) except where otherwise noted. Antibiotic concentrations were as described previously (21).
TABLE 1.
Bacterial strains, phage, and plasmids used in the study
| Strain, phage, or plasmid | Description | Source or reference |
|---|---|---|
| E. coli | ||
| ORN103 | thr-1 leu-6 thi-1 Δ(argF-lac)U169xyl-7 ara-13 mtl-2 gal-6 rpsL tonA2 minA minB recA13 Δ(fimEAICDFGH) | 23 |
| ORN178 | thr-1 leuB thi-1 Δ(argF-lac)U169xyl-7 ara-13 mtl-2 gal-6 rpsL tonA2 supE44 pilG1, tetR inserted ca. 200 bp 3′ to the end of fimH, Tetrλr Pil+ (does not exhibit phase variation of piliation) | 26 |
| ORN172 | thr-1 leuB thi-1 Δ(argF-lac)U169xyl-7 ara-13 mtl-2 gal-6 rpsL tonA2 supE44 pilG1 λr Δ(fimBEAICDFGH) | 29 |
| ORN220 | ORN178 except that the fimI′-kan allele from pORN311 is in the chromosome and the strain is nalidixic acid resistant | This studya |
| ORN221 | ORN220 except that fimI′-PmeI from pORN313 is in the chromosome | This study |
| ORN228 | ORN220 except ΔfimI from pORN316 is in the chromo- some | This study |
| Bacteriophage | ||
| P1 | vir | Laboratory collection |
| Plasmids | ||
| pACYC184 | P15A Cmr Tcr | 6 |
| pBK-CMV | Kanr | Stratagene |
| pKAS32 | oriR6K oriT rpsL Apr | 27 |
| pSH2 | pACYC184 fim(BEAICDFGH) Cmr | 8 |
| pORN113 | pSH2 fimC::Tn5 (pilB::Tn5) | 21 |
| pORN140 | pSH2 except that the PstI site in fimA is replaced by an in-frame XhoI site, Pil+ | This studyb |
| pORN308 | pBK-CMV fimI | This study |
| pORN315 | pBK-CMV ΔfimI (an in-frame deletion) | This studyc |
| pORN309 | pBK-CMV fimI with the AseI site eliminated and modified by a PmeI linker insertion | This study |
| pORN310 | pORN140 with the fimI′-PmeI lesion | This study |
| pORN311 | pORN310 with a fimI′-kan insertion replacing the fimI′-PmeI lesion | This studyd |
| pRN2010 | ColE1 Spr Tcr | 21 |
| pORN104 | pRN2010 fimBEAICDFGH Spr | 21 |
| pORN312 | pRN2010 fimBEACDFGH fimI′-PmeI Spr | This studye |
| pORN313 | pKAS32 with the fimI′-PmeI allele from pORN309 carried on a ca. 1.5-kb XhoI-EcoRI fragment | This study |
| pORN316 | pKAS32 with the ΔfimI allele from pORN315 carried on a ca. 0.8-kb XhoI-EcoRI fragment | This study |
Strain ORN220 was constructed by performing a linear transformation of EcoRI-digested pORN311 using the methods of Russell and Orndorff (26). P1 phage was grown on Kanr Tets recombinants, and the lysate was used to introduce the fimI′-kan insertion mutation into strain ORN178 by transduction (26). A spontaneous nalidixic acid-resistant variant of an isolated transductant was obtained by standard methods.
Plasmid (pORN140) contains a unique, in-frame, XhoI site replacing the normal PstI site in the fimA gene. The steps in the construction were the same as those described by Orndorff and Falkow (22). The mutation created by the insertion is silent in this in-frame context.
Oligonucleotide primers (P1, 5′GCGCTTTTAGATCTCAGGCCTGGTTCTCTTTAACC, and P2, 5′GCGCTTTTAGATCTGATACTGAACCTTGAAGGTCGC) were used to generate pORN315 from PCR amplification of pORN309 as described in the text.
The kanamycin cassette from pACYC177 (Invitrogen) was obtained following PstI digestion; the ca. 1-kb fragment was blunt ended with DNA polymerase I and ligated into PmeI-digested pORN310. The ligation mixture was introduced into E. coli DH5α by transformation with selection for kanamycin and chloramphenicol.
Plasmid pORN312 was constructed by cloning the 11.2-kb SalI fragment from pORN310 containing the fim genes with the fimI′-PmeI lesion into SalI-digested pRN2010.
Site-directed mutagenesis of fimI.
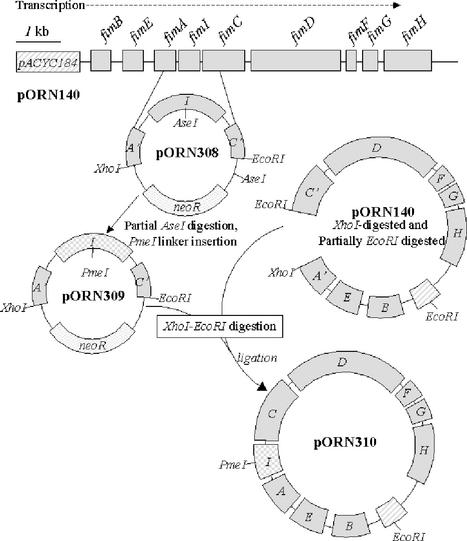
The steps necessary for the fimI′-kan insertion mutation and the PmeI linker insertion to generate a frameshift mutation are summarized in Fig. 1. Briefly, plasmid pORN308 containing the fimI gene flanked by EcoRI and XhoI restriction endonuclease sites was obtained from plasmid pORN140. The AseI restriction endonuclease site in fimI was modified by PmeI linker mutagenesis following the elimination of a vector-borne AseI site by end-filling and religation (22). The resulting pORN309 plasmid was then used in combination with the original pORN140 plasmid to create pORN310, a plasmid identical to the starting pORN140 plasmid except for the PmeI lesion in fimI. Subsequent addition of the kanamycin resistance cassette from Tn903 (7) into the site created by PmeI linker mutagenesis produced plasmid pORN311. The transcriptional orientation in the cassette was the same as that of the fim cluster. Plasmid pORN315 carrying the in-frame fimI deletion mutation (ΔfimI) was generated by means of PCR amplification of the PmeI-linearized plasmid pORN309. The amplicons, each having the beginning of fimI on one tip and the end of fimI on the other, were digested with BglII (a site present in the oligonucleotide primers) (Table 1) and ligated, and a representative clone was obtained by standard methods.
FIG. 1.
Construction of the fimI′-PmeI mutation. The parental pORN140 fimI allele on an XhoI-EcoRI restriction endonuclease fragment was ligated with XhoI-EcoRI-cleaved pBK-CMV (Stratagene) to create pORN308. This ligation mixture was introduced into E. coli DH5α (Invitrogen) by transformation (14). Partial AseI digestion and PmeI linker insertion followed, producing pORN309. The mutant fimI′-PmeI allele was then introduced into the original plasmid (pORN140) as diagrammed to create pORN310, and the neoR cassette was added at the unique PmeI site to produce pORN311. The neoR gene confers kanamycin resistance. The pACYC184 cloning vector confers chloramphenicol resistance. Additional details for the constructions are described in the text and in note b to Table 1.
Introduction of the mutant fimI alleles into the chromosome.
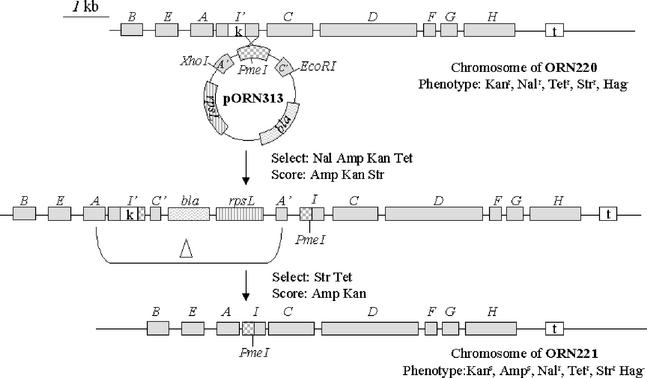
The fimI′-kan insertion mutation was introduced into the E. coli chromosome via linear recombination (26) to create strain ORN220. We subsequently replaced the insertion mutation with the unmarked fimI alleles by using the streptomycin counter-selection technique devised by Skorupski and Taylor (27) as illustrated for the fimI′-PmeI allele (Fig. 2). After chromosomal introduction, PCR amplicons of both unmarked mutant alleles were completely sequenced (University of North Carolina Automated DNA Sequencing Facility, Chapel Hill). The fimI′-PmeI allele was identical to the parental allele except that it contained an insertion in the central portion of the gene at the former AseI site of seven tandem PmeI sites [(GTTTAAAC)7], producing stop codons in all three reading frames and truncating the predicted product (normally, 160 amino acids long) after 118 amino acids. The ΔfimI mutation removed ca. 90% of the central region of fimI and had the substituted BglII restriction endonuclease site carried in frame.
FIG. 2.
Introduction of the fimI′-PmeI allele on pORN313 into the chromosome of ORN220. Mating and allelic exchange were performed according to the methods of Harris et al. (9). Capital letters indicate corresponding fim genes; rpsL and bla are genes for streptomycin resistance and ampicillin resistance, respectively. Phenotypic designations denote sensitivity (s) or resistance (r) to the following antibiotics: kanamycin (Kan), tetracycline (Tet), ampicillin (Amp), streptomycin (Str), and nalidixic acid (Nal). Hemagglutination is designated phenotypically as Hag. A delta (Δ) denotes a deletion. The neoR gene inserted into fimI and the tetR gene inserted adjacent to the fim cluster are denoted by boxes labeled with k and t, respectively. The neoR and tetR genes confer resistance to kanamycin and tetracycline, respectively.
Complementation.
All chromosomal fimI mutants were negative for piliation as indicated by the failure of the mutants to agglutinate guinea pig erythrocytes and to agglutinate in antiserum raised against purified pili (26). Further, no pili were visible upon electron microscopic examination (16) of negatively stained preparations (data not shown). Minicell analysis of the fimI′-kan insertion mutant strongly suggested that polarity could be a factor in reducing the normal expression levels of two downstream genes (fimC and fimD) (data not shown). To test whether polarity was a factor in the failure of the fimI mutants to produce pili, a recombinant plasmid containing all of the fim genes, except for fimC (a gene immediately downstream from fimI whose expression is required for piliation), was introduced into the strains containing the chromosomal mutant fimI alleles, and each strain was examined for its ability to agglutinate erythrocytes. The results (Table 2) indicated that the in-frame ΔfimI mutant was the only one that was successfully complemented to restore hemagglutination to levels that were statistically the same as for the parental strain bearing the same plasmid (Student's t test, P < 0.05). The reason for the somewhat suppressive effect of the complementing plasmid on parental piliation levels (Table 2) is unknown, but we attribute it to suboptimal ratios of various pilus components needed for the most efficient biogenesis. Levels of piliation in the parental and ΔfimI-complemented strains were similar also when the strains were viewed electron microscopically (data not shown).
TABLE 2.
Complementation of the chromosomal fimI mutant alleles in transa
| Strain | Relevant chromosomal property | Plasmid | Relevant plasmid propertyb | Erythrocyte agglutination as a % of value for parental strainc |
|---|---|---|---|---|
| ORN178 | Parental | pACYC184 | NA | 100 |
| ORN178 | Parental | pORN113 | fimBEAIDFGH fimC::Tn5 | 66 ± 5 |
| ORN220 | fimI′-kan | pACYC184 | NA | 0 ± 0 |
| ORN220 | fimI′-kan | pORN113 | fimBEAIDFGH fimC::Tn5 | 0 ± 0 |
| ORN221 | fimI′-PmeI | pACYC184 | NA | 0 ± 0 |
| ORN221 | fimI′-PmeI | pORN113 | fimBEAIDFGH fimC::Tn5 | 18 ± 8 |
| ORN228 | ΔfimI | pACYC184 | NA | 0 ± 0 |
| ORN228 | ΔfimI | pORN113 | fimBEAIDFGH fimC::Tn5 | 64 ± 2 |
Overnight cultures were concentrated ca. 10-fold and tested for their ability to agglutinate guinea pig erythrocytes in plate agglutination assays (9).
The fimC gene lies immediately 3′ to fimI. See the text for additional details. NA, not applicable.
Log2 values of the reciprocals of the agglutination titers were compared after normalization to the values for the parental strain (100%). Values are averages (± standard deviations) of the results of at least three separate experiments.
Association of a 16.4-kDa protein product with fimI.
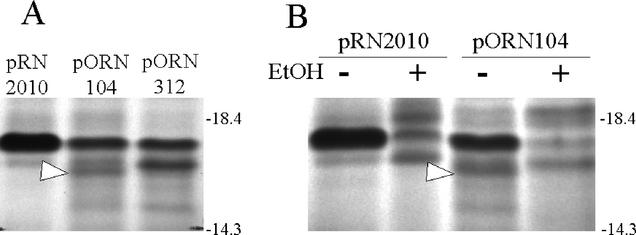
In order to identify the fimI product, the fim region containing the fimI-PmeI lesion from pORN310 was cloned into another vector (pRN2010), creating pORN312. This plasmid was used in conjunction with an earlier construct (pORN104 [21]) that employed the parental fim cluster in the same vector. The change in vector was necessary in part because we anticipated that vector-encoded bands from pACYC184 would obscure the region where the fimI product was expected to migrate. Transcription and translation of plasmid-encoded fimI in minicells revealed that a band migrating with an apparent molecular mass of 16.4 kDa was absent in minicells harboring a plasmid with a fimI′-PmeI lesion (pORN312) but not from minicells harboring an otherwise identical plasmid with the parental fimI allele (pORN104) (Fig. 3A). Experiments using 8% ethanol to inhibit signal peptide processing (21) revealed that the mature 16.4-kDa FimI band was absent when ethanol was present during radiolabeling (Fig. 3B). The predicted higher-molecular-weight form of FimI was not identified (possibly because of obscuring bands). Nevertheless, the results indicated a noncytoplasmic protein. Both the extracytoplasmic location and the apparent size of the FimI band were in good agreement with DNA sequence-based predictions (3). FimI production was also noted in a minicell analysis of pORN308, which contained just the fimI gene. However, noticeably lower levels of FimI were seen (data not shown). Low expression of fimI and/or FimI instability when the protein is synthesized separately from the rest of the fim cluster may explain why this clone did not complement any of the chromosomal fimI lesions.
FIG. 3.
(A) Transcription and translation of fimI in minicells. Minicells from strain ORN103 were isolated and labeled with [35S]cysteine. Radiolabeled products were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described by Orndorff and Falkow (21) except that the radiolabeled products were not hydrolyzed in acid prior to solubilization in the sample buffer and the ratio of acrylamide to bis-acrylamide was adjusted to 38:1. A portion of an autoradiogram of the gel is shown. Plasmids were pRN2010 (cloning vector), pORN104 (fimI), and pORN312 (fimI′-PmeI). An arrow denotes the putative fimI product. (B) Minicells were obtained and incubated as described above except that 8% ethanol (EtOH) was employed (where indicated) to inhibit signal sequence processing during radiolabeling. An arrow denotes the putative fimI product. The positions and sizes (in kilodaltons) of molecular markers (Invitrogen) are indicated.
Conclusions.
We compared the phenotypes of three chromosomal mutants, each bearing a site-directed mutation in fimI, a gene whose putative product is thought to have a role in type 1 pilus biogenesis. All lesions in fimI resulted in a piliation-negative phenotype. However, complementation analysis revealed that the insertion and frameshift lesions produced effects consistent with polarity on the transcriptionally downstream gene (fimC). Minicell analyses associated the fimI coding region with a noncytoplasmic protein with an apparent mature (processed) molecular mass of 16.4 kDa.
The proposed start site of fimI translation in E. coli K-12 is at nucleotide 4541188 (3). However, if both the predicted translation start site and the signal sequence cleavage site are assumed, then the predicted FimI precursor protein has an unusually long (55-amino-acid) signal sequence (a discrepancy often observed when the predicted translation initiation site is too far upstream [18]). Additionally, the proposed start site places the beginning of fimI translation within the preceding fimA coding region. In view of the foregoing, we feel that a more likely translation initiation site corresponds to the ATG codon beginning at nucleotide 4541296 (3). Such a start site eliminates translational overlap and predicts a more common 19-amino-acid signal sequence.
The role of the fimI product in pilus biosynthesis is unknown. Results of two recent studies in which fimI chromosomal insertion mutants were identified (2, 4) suggested that fimI is required for pilus biosynthesis. However, in both studies the authors acknowledged that insertion mutations might have polar effects and, thus, did not draw any conclusions as to the requirement for the putative fimI product in pilus biogenesis. Our results justify their concerns. In one of the studies (4), the authors concluded that FimH, the adhesive component of the pilus (20), could still be synthesized and become located on the cell surface in the absence of a fimI product and pilus. This conclusion was based upon the observation that one of their two fimI insertion mutants could still bind epithelial cells. Whereas our results support the idea that the fimI product is required for pilus biosynthesis, we found no FimH activity in any of our fimI mutants. However, we cannot rule out the possibility that under different assay conditions, fimI mutants might produce a more equivocal phenotype with regard to piliation and/or FimH expression.
Our observation that FimI did not have the characteristics of a cytoplasmic protein suggested that FimI has a direct role in pilus biosynthesis (as opposed to an indirect regulatory role). Regarding the possible presence of FimI in the pilus, biochemical evidence from purified type 1 pili (amino acid composition studies, N-terminal sequencing [19]) suggests that FimI cannot constitute a major portion of purified pili. (This assumes that FimI is not specifically lost during the pilus purification process.) However, FimI may be a minor component of the pilus or simply be required for some currently unappreciated phase of the assembly process (20).
One previous report (25) speculated that FimI could function analogously to PapH, a protein described by Baga et al. (1), whose loss through mutation produces mutants with long and cell-dissociated pyelonephritis-associated pili. This phenotype suggested to Baga et al. that PapH was involved in membrane anchoring and pilus length modulation. Our studies do not support such a role for FimI in that no long or dissociated pili were noted in any of the fimI mutants. Further work will be required to discern how FimI fits into the already-complicated picture of pilus biosynthesis (28).
Nucleotide sequence accession numbers
The parental fimI allele (from strain ORN178) was identical to the corresponding gene of E. coli K-12 (3) (accession number AE000502). The fimI′-PmeI and ΔfimI genes have accession numbers AF424784 and AY255626, respectively.
Acknowledgments
We thank Craig Altier for a critical reading of the manuscript.
This work was supported by a grant from the National Institutes of Health (AI22223) and the State of North Carolina.
REFERENCES
- 1.Baga, M., M. Norgren, and S. Normark. 1987. Biogenesis of E. coli Pap pili: PapH, a minor pilin subunit involved in cell anchoring and length modulation. Cell 49:241-251. [DOI] [PubMed] [Google Scholar]
- 2.Bahrani-Mougeot, F. K., E. L. Buckles, C. V. Lockatell, J. R. Hebel, D. E. Johnson, C. M. Tang, and M. S. Donnenberg. 2002. Type 1 fimbria and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45:1079-1093. [DOI] [PubMed] [Google Scholar]
- 3.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1462. [DOI] [PubMed] [Google Scholar]
- 4.Boudeau, J., N. Barnich, and A. Darfeuille-Michaud. 2001. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn's disease is involved in bacterial invasion of intestinal epithelial cells. Mol. Microbiol. 39:1272-1284. [DOI] [PubMed] [Google Scholar]
- 5.Brinton, C. C., Jr. 1965. The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans. N. Y. Acad. Sci. 27:1003-1054. [DOI] [PubMed] [Google Scholar]
- 6.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagberg, L., R. Hull, S. Hull, S. Falkow, R. Freter, and C. S. Edén. 1983. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect. Immun. 40:265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris, S. L., P. A. Spears, E. A. Havell, T. S. Hamrick, J. R. Horton, and P. E. Orndorff. 2001. Characterization of Escherichia coli type 1 pilus mutants with altered binding specificities. J. Bacteriol. 183:4099-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, C. H., J. S. Pinkner, R. Roth, J. Heuser, A. V. Nicholes, S. N. Abraham, and S. J. Hultgren. 1995. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc. Natl. Acad. Sci. USA 92:2081-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klemm, P. 1984. The fimA gene encoding the type-1 fimbrial subunit of Escherichia coli. Nucleotide sequence and primary structure of the protein. Eur. J. Biochem. 143:395-399. [DOI] [PubMed] [Google Scholar]
- 12.Klemm, P. 1992. FimC, a chaperone-like periplasmic protein of Escherichia coli involved in biogenesis of type 1 fimbriae. Res. Microbiol. 143:831-840. [DOI] [PubMed] [Google Scholar]
- 13.Krogfelt, K. A., H. Bergmans, and P. Klemm. 1990. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect. Immun. 58:1995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lederberg, E. M., and S. N. Cohen. 1974. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J. Bacteriol. 119:1072-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurer, L., and P. E. Orndorff. 1985. A new locus, pilE, required for the binding of type 1 piliated Escherichia coli to erythrocytes. FEMS Microbiol. Lett. 30:59-66. [Google Scholar]
- 16.Maurer, L., and P. E. Orndorff. 1987. Identification and characterization of genes determining receptor binding and pilus length of Escherichia coli type 1 pili. J. Bacteriol. 169:640-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 18.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 19.O'Hanley, P., D. Lark, S. Normark, S. Falkow, and G. K. Schoolnik. 1983. Mannose-sensitive and Gal-Gal binding Escherichia coli pili from recombinant strains. Chemical, functional, and serological properties. J. Exp. Med. 158:1713-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orndorff, P. E. 1994. Escherichia coli type 1 pili, p. 91-111. In V. L. Miller, J. B. Kaper, D. A. Portnoy, and R. R. Isberg (ed.), Molecular genetics of bacterial pathogenesis. American Society for Microbiology, Washington, D.C.
- 21.Orndorff, P. E., and S. Falkow. 1984. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J. Bacteriol. 159:736-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orndorff, P. E., and S. Falkow. 1985. Nucleotide sequence of pilA, the gene encoding the structural component of type 1 pili in Escherichia coli. J. Bacteriol. 162:454-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orndorff, P. E., P. A. Spears, D. Schauer, and S. Falkow. 1985. Two modes of control of pilA, the gene encoding type 1 pilin in Escherichia coli. J. Bacteriol. 164:321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponniah, S., R. O. Endres, D. L. Hasty, and S. N. Abraham. 1991. Fragmentation of Escherichia coli type 1 fimbriae exposes cryptic d-mannose-binding sites. J. Bacteriol. 173:4195-4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossolini, G. M., P. Musca, A Chiesurin, and G. Satta. 1993. Analysis of the salmonella fim gene cluster: identification of a new gene (fimI) encoding a fimbrin-like protein and located downstream of the fimA gene. FEMS Microbiol. Lett. 114:259-265. [DOI] [PubMed] [Google Scholar]
- 26.Russell, P. W., and P. E. Orndorff. 1992. Lesions in two Escherichia coli type 1 pilus genes alter pilus number and length without affecting receptor binding. J. Bacteriol. 174:5923-5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skorupski, K., and R. K. Taylor. 1996. Positive selection vectors for allelic exchange. Gene 169:47-52. [DOI] [PubMed] [Google Scholar]
- 28.Soto, G. E., and S. J. Hultgren. 1999. Bacterial adhesins: common themes and variations in architecture and assembly. J. Bacteriol. 181:1059-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodall, L. D., P. W. Russell, S. L. Harris, and P. E. Orndorff. 1993. Rapid, synchronous, and stable induction of type 1 piliation in Escherichia coli by using a chromosomal lacUV5 promoter. J. Bacteriol. 175:2770-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]