Abstract
Fetal exposure to excess glucocorticoids (GCs) programs the developing hypothalamo–pituitary–adrenal (HPA) axis, and may predispose offspring to adult-onset disease. During development, serotonin (5-HT) influences transcription of hippocampal GR mRNA via the 5-HT7 receptor. The effect of 5-HT on GR involves the transcription factor NGFI-A. Given the developmental changes which we have previously reported in hippocampal GR mRNA expression, we hypothesized that (1) there are progressive developmental changes in 5-HT7 receptor and NGFI-A mRNA expression in the fetal guinea-pig limbic system, and (2) repeated exposure to synthetic GC treatment will significantly modify developmental expression of these genes. 5-HT7 receptor mRNA was highly expressed in the hippocampus and thalamus at gestational day (gd) 40 (term ∼70 days), and significantly decreased (P < 0.05) with advancing gestation. Conversely, NGFI-A mRNA expression in the hippocampus and frontal cortex was almost undetectable at gd40, but was dramatically elevated (P < 0.05; 8-fold) near term. Changes in mRNA were refelected by NGFI-A protein levels. These changes were significantly correlated to hippocampal GR expression and fetal plasma cortisol concentrations. Synthetic GC treatment increased NGFI-A mRNA levels in CA1 and the cingulate cortex, but had no effect on 5-HT7 receptor expression. In conclusion our results suggest that (1) limbic 5-HT7 receptor expression is not directly linked to maturation of hippocampal GR in late gestation; (2) the up-regulation of NGFI-A expression near term is driven by glucocorticoid; and (3) premature exposure to synthetic glucocorticoid significantly increases NGFI-A-related transcriptional activity in the fetal limbic system.
Synthetic glucocorticoid is routinely administered to pregnant women at risk of preterm delivery (NIH Consensus Development Conference, 1995). Such treatment is highly effective in preventing pulmonary complications in newborn infants (Ballard & Ballard, 1996). Until recently, the prescribing of multiple course glucocorticoid therapy had become common practice in Australia, Europe and North America, if the risk of preterm delivery persisted (Brocklehurst et al. 1999; Smith et al. 2000). However, a recent NIH consensus update conference (2000) recommended that such practice be confined to ongoing clinical trials in which the efficacy of multiple course therapy is being investigated. Notwithstanding, practice in the late 1990s and early 2000s has led to an extremely large cohort of infants/children that received multiple exposure to synthetic glucocorticoids. Recent experimental data indicate that excess antenatal glucocorticoid exposure leads to alteration or programming of the fetal hypothalamo–pituitary–adrenal (HPA) system, resulting in altered regulation of the axis throughout life (Weinstock, 2001; Welberg & Seckl, 2001; Matthews, 2002). Studies in guinea-pigs, rats, and sheep indicate that repeated prenatal exposure to synthetic glucocorticoid leads to permanent changes in HPA function (Liu et al. 2001; Welberg et al. 2001; Sloboda et al. 2002). Long-term alterations in HPA function have been linked to the premature development of adult onset disease in the rat, sheep and human (Nyirenda et al. 2001; Reynolds et al. 2001a,b; Welberg et al. 2001; Phillips, 2002).
It is emerging from animal studies that perinatal programming of HPA function involves a permanent resetting of glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) levels in the hippocampus, hypothalamus and pituitary (Meaney et al. 2000; Liu et al. 2001; Welberg & Seckl, 2001; Matthews, 2002). In the guinea-pig, we have shown that prenatal exposure to synthetic glucocorticoid leads to adult offspring with permanently modified HPA function and altered central GR and MR expression, and that this effect is sex-specific (Liu et al. 2001). In recent studies, Meaney and colleagues have shown that hippocampal serotonin, via 5HT7 receptor activation, is central to the process of programming of GR (Meaney et al. 2000; Laplante et al. 2002). Activation of the 5HT7 receptor leads to increased levels of the transcription factor NGFI-A, which in turn interacts with the GR promoter, altering methylation profiles and permanently up-regulating hippocampal GR expression (Weaver et al. 2001). While NGFI-A and the 5-HT7 receptor are clearly linked to postnatal programming of HPA function in neonatal rats, it is not known whether such a relationship can occur during fetal life. Nothing is known about the prenatal expression of 5-HT7 receptors or NGFI-A in any species, or whether manipulation of the prenatal environment can modify their pattern of development.
Guinea-pigs, unlike rats and mice, give birth to neuroanatomically mature young, and this more closely resembles the situation in humans (Darlington et al. 1999). We have previously reported the developmental patterns of GR in the fetal guinea-pig brain and how GR expression is influenced by exposure to glucocorticoids (Matthews, 1998; McCabe et al. 2001; Owen & Matthews, 2003). The guinea-pig GR exhibits 4-fold lower binding affinity for synthetic glucocorticoid than that which is found in the human (Keightley et al. 1998). To accommodate this, we have chosen a dose (1mgkg−1) which is physiologically comparable to that received by humans in modern clinical practice (∼0.25mgkg−1 day−1) (NIH Consensus Development Conference, 1995). The relationship of fetal GR development with limbic 5-HT7 receptor or NGFI-A expression is not known. Therefore, the main objectives of this study were (1) to investigate the development of 5-HT7 receptor and NGFI-A expression over the second half of gestation in the limbic system of the fetal guinea-pig, and correlate these to developmental changes in GR mRNA expression in the same animals, and (2) to determine the effects of repeated course synthetic glucocorticoid treatment on the limbic 5-HT7 receptor and NGFI-A expression.
Methods
Animals and tissue processing
Female guinea-pigs were mated in our animal facility as previously described (Dean & Matthews, 1999). This method produces accurately time-dated pregnant guinea-pigs. These studies were performed using protocols approved by the Animal Care Committee at the University of Toronto and were in accordance with the Canadian Council for Animal Care. Fetal plasma cortisol concentrations were measured by radioimmunoassay, as previously described (Owen & Matthews, 2003).
Development studies
Maternal guinea-pigs were killed by rapid decapitation on gestational days (gd) 40, 50 or 60, and fetuses (n= 6 of each sex at each age, randomly selected from separate litters) quickly removed (term (70 days). Samples of trunk blood were obtained from each fetus. Brains were sagitally hemisected at an angle and slightly off midline, such that the left block contained the left hippocampus and the entire hypothalamus. The right hippocampus was dissected from the remaining block. Tissue was rapidly frozen and stored at −80°C until further processing.
Antenatal glucocorticoid treatment
Pregnant animals were injected in the intrascapulary region with 1mgkg−1 (n= 5) or 10mgkg−1 (n= 7) dexamethasone (DEX; dexamethasone-1-phosphate, Sigma-Aldrich Corp, Oakville, Canada), or vehicle (n= 8), on gd40, 41, 50, 51, 60 and 61, as previously described (McCabe et al. 2001). Pregnant guinea-pigs were killed on gd62, 24 h after the final injection. Fetal brains were removed and frozen with no further dissection.
In situ hybridization
The method for in situ hybridization has been described in detail elsewhere (Matthews, 1998). Coronal cryosections (10μm) were mounted onto poly-l-lysine-coated slides, dried and fixed in paraformaldehyde (4%). Antisense oligonucleotide probes for the 5-HT7 receptor, NGFI-A and GR mRNA were labelled using terminal deoxynucleotidyl transferase (Gibco, Burlington, ON, Canada) and [35S]dATP (1300 Ci mmol−1, Perkin Elmer, Woodbridge, ON, Canada) to a specific activity of 1.0 × 109 c.p.m.μg−1. Labelled probe in hybridization buffer (200μl) was applied to slides at a concentration of 1.0 × 105c.p.m.μl−1. Oligonucleotide probes were complementary to bases 1085–1129 of guinea-pig 5-HT7 receptor (Tsou et al. 1994) and bases 1062–1106 of rat NGFI-A mRNA (Milbrandt, 1987). The probe for guinea-pig GR mRNA has been previously described (Matthews, 1998). Slides were incubated overnight in a moist chamber at 42.5°C. After washing in 1×SSC (20min at 23°C, then 35min at 55°C), slides were rinsed, dehydrated in ethanol, dried and exposed to autoradiographic film (Biomax MR, Kodak, Perkin Elmer, Woodbridge, ON, Canada). Films were developed using an automatic processor (exposure: GR, NGFI-A, 14 days; 5-HT7, 42 days). Slides hybridized for NGFI-A mRNA expression were then dipped into photographic emulsion (LM, Amersham Pharmacia) and processed as previously described (Erdeljan et al. 2001). The exposure time for NGFI-A mRNA was 10 weeks. Control 45 base sense oligonucleotide probes demonstrated no hybridization when incubated with sections known to contain NGFI-A, 5-HT7 receptor and GR mRNA.
For in situ hybridization, brain sections were processed simultaneously to allow direct comparison between groups. The sections were exposed together with 14C-standards (American Radiochemical Company, St Louis, MO, USA) to ensure analysis was performed in the linear range of the autoradiographic film. The relative optical density of the signal on autoradiographic film was quantified, after subtraction of background values, using a computerized image analysis system (Imaging Research Inc., St Catharine's, ON, Canada) (Matthews, 1998). All analyses were undertaken by an operator blinded to age or treatment. Levels of GR and NGFI-A mRNA expression were measured in the hippocampus (CA1, CA2/3, CA4), dentate gyrus, and cingulate cerebral cortex. 5-HT7 receptor mRNA levels were determined in the hippocampus (CA2/3, CA4), dentate gyrus, cingulate cortex and paraventricular nucleus of the thalamus. In sections dipped in silver emulsion (NGFI-A mRNA), background labelling, as indicated by the presence of silver grains over non-expressing areas of the brain, was virtually undetectable.
Western blotting
Frozen hippocampal tissue was homogenized and assayed for total protein using the method described by Bradford (1976). NGFI-A protein expression in right fetal hippocampi was assessed by Western blotting, as previously described (Owen & Matthews, 2003). Incubation with anti-NGFI-A antibody (sc-110, Santa-Cruz Biotechnology, CA, USA), revealed three specific bands, at 102, 57 and 45kDa, respectively. The specificity of this antibody against guinea-pig tissue was characterized by preabsoprtion with a specific blocking peptide (sc-110P, Santa Cruz Biotechnology). To further test antibody specificity, anti-NGFI-A was incubated with rat hippocampal tissue, which is known to contain high levels of NGFI-A protein (Meaney et al. 2000); this produced a similar distribution of bands. The relative level of tubulin (antitubulin (T-3526), Sigma-Aldrich Corp.) in each hippocampal sample was used as an internal loading control. The relative optical density (ROD) of these bands was measured using computerized image analysis. Results are reported as the ratio of the ROD of the specific NGFI-A bands with that for the internal control, tubulin. Antibody dilutions were as follows: anti-NGFI-A and antitubulin, 1: 10000; secondary antibody (antirabbit, Perkin-Elmer), 1: 20000. Samples were analysed three times in separate Western blots; data were pooled to produce a mean for each animal. There are currently no 5-HT7 receptor-specific antibodies available for the guinea-pig.
Immunohistochemical staining
Frozen sections were thawed, postfixed in paraformaldehyde (4%) and washed in phosphate-buffered saline (PBS). Prior to incubation with primary antibody, sections were treated with hydrogen peroxide (0.3%, 30min). Immunohistochemical staining was performed using the Vectastain Elite ABC kit (Vector Laboratories Inc, CA, USA) with an anti-NGFI-A antibody (sc-110, Santa Cruz Biotechnology) at 1: 200 dilution, incubated overnight (4°C). NGFI-A protein was visualized using diaminobenzidine tetrachloride (DAB; Sigma-Aldrich). The reaction with DAB (10min) was stopped by rinsing in PBS. Sections were dehydrated prior to mounting. The specificity of the antibody was characterized by preabsorption with specific blocking peptide (sc-110P, Santa Cruz Biotechnology), which resulted in no detectable signal.
Statistical analysis
Group data are presented as means ±s.e.m. and were statistically analysed using multiple ANOVA followed by Dunnet's method of post hoc comparison (Statistica, Statsoft Inc., Tulsa, OK, USA). Statistical significance was set at P < 0.05. In separate analyses, linear regression and measures of correlation were performed to test firstly the association between hippocampal NGFI-A mRNA expression and plasma cortisol levels, and secondly the relationship between hippocampal GR mRNA relative to hippocampal NGFI-A mRNA expression in fetuses during the second half of gestation. Statistical significance for linear regression models was set at P < 0.05. The slopes and correlation coefficient for each analysis were calculated by Statistica.
Results
Development
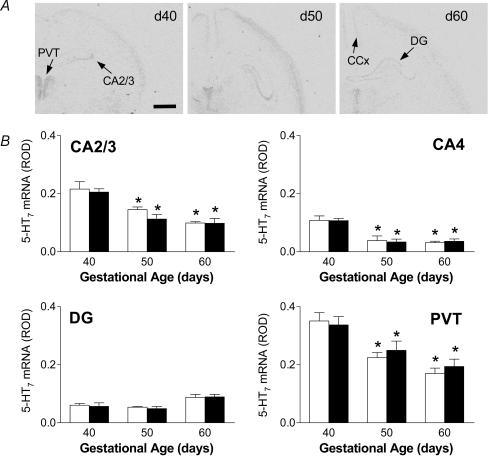
5-HT7 receptor mRNA was detected in several areas of the developing fetal guinea-pig brain, at all points of gestation examined. In the hippocampus, 5-HT7 receptor mRNA was confined to the CA2/3 and CA4 regions, with almost undetectable expression in the CA1 subfield (Fig. 1A). In both the CA2/3 and CA4 hippocampal subfields, the highest level of 5-HT7 receptor mRNA occurred at gd40. Analysis of the changes in mRNA expression using multiple ANOVA (gestational age × gender × region) revealed an overall significant (P < 0.001) effect of gestational age and region. A significant (P < 0.05) decrease in expression in both the CA2/3 and CA4 subfields was measured at gd50 and gd60 (Fig. 1B). Similar gestational changes were also observed in the thalamus, with highest levels occurring in the paraventricular thalamic nucleus (PVT). The dentate gyrus (DG) contained low levels of 5-HT7 receptor mRNA (Fig. 1A) which remained relatively constant throughout gestation without any significant changes (Fig. 1B). 5-HT7 receptor was also detected in the cingulate cortex (ROD; mean ±s.e.m., female: gd40 0.061 ± 0.01, gd50 0.047 ± 0.002, gd60 0.047 ± 0.009; male: gd40 0.058 ± 0.006, gd50 0.039 ± 0.004, gd60 0.044 ± 0.013) but levels remained low and did not change throughout late gestation. There were no sex differences in the expression of 5-HT7 receptor mRNA in late gestation.
Figure 1.
A, representative expression of 5-HT7 receptor mRNA in coronal sections of fetal guinea-pig hippocampus at gd40, 50 and 60. Specific 5-HT7 receptor mRNA expression was detected in the hippocampal CA2/3 subfields, dentate gyrus (DG), cingulate cortex (CCx) and paraventricular thalamic nucleus (PVT). Bar: 1.5mm. B, relative levels of 5-HT7 receptor mRNA expression in the hippocampus (CA2/3, CA4), dentate gyrus (DG) and paraventricular nucleus of the thalamus (PVT) in female (open bars) and male (filled bars) fetuses in the second half of gestation. *Significant (P < 0.05) differences compared to gd40 fetuses.
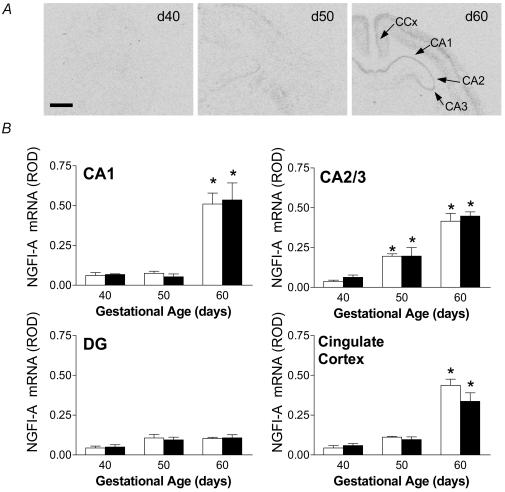
The developmental pattern of NGFI-A mRNA expression was very different from that for 5-HT7 receptor. NGFI-A mRNA was barely detectable at gd40, in all areas examined (Fig. 2A). A significant (P < 0.05) increase in levels of NGFI-A mRNA was observed in the CA2/3 region by gd50, followed by significant elevations in expression in CA1 and CA2/3 (Fig. 2B) and CA4 (ROD; mean ±s.e.m., female: gd40 0.02 ± 0.001, gd50 0.1 ± 0.04, gd60 0.2 ± 0.03; male: gd40 0.05 ± 0.02, gd50 0.05 ± 0.01, gd60 0.2 ± 0.02) by gd60. NGFI-A mRNA was detectable in the cingulate cortex at gd40 and 50, with a significant increase in expression at gd60 (Fig. 2B). In similar fashion to 5-HT7 receptor mRNA, expression of NGFI-A in the dentate gyrus was detected at gd40, and did not significantly change through late gestation. Examination of high resolution silver emulsion autoradiograms revealed up-regulation of cellular NGFI-A mRNA levels in CA1–3 subfields of the hippocampus with the progression of gestation (Fig. 3, upper panel).
Figure 2.
A, representative expression NGFI-A mRNA in coronal sections of fetal guinea-pig hippocampus at gd40, 50 and 60. NGFI-A mRNA expression is shown in hippocampal subfields (CA1, CA2 and CA3), and cingulate cortex (CCx). Bar: 1.5mm. B, relative levels of NGFI-A mRNA expression in the hippocampus (CA1, CA2/3), dentate gyrus (DG) and cingulate cortex in female (open bars) and male (filled bars) fetuses in the second half of gestation. *Significant (P < 0.05) differences compared to previous gestational age.
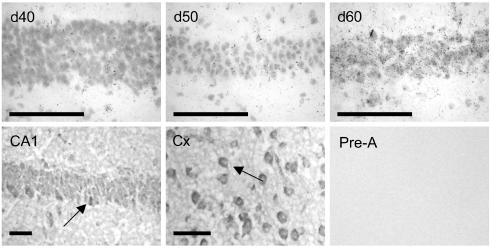
Figure 3.
Upper panel, representative expression NGFI-A mRNA in the CA1 hippocampal subfield in coronal sections of fetal guinea-pig hippocampus at gd40 (left panel), 50 (middle panel) and 60 (right panel), following high resolution silver emulsion autoradiography. Bar: 25μm. Lower panel, immunohistochemical staining of guinea-pig hippocampus (CA1, left panel) and frontal cortex (middle panel) using anti-NGFI-A antibody and DAB visualization. Arrows indicate cells that are darkly stained for NGFI-A protein. Preabsorption of the NGFI-A antibody (right panel) resulted in no detectable signal. Bar: 25μm.
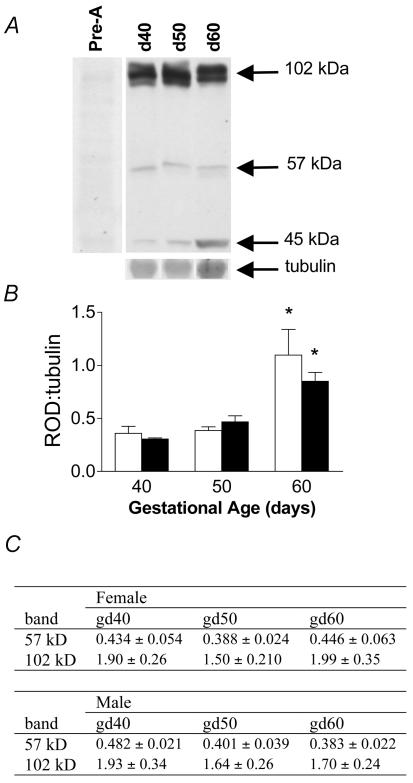
Western analysis of NGFI-A protein levels in fetal hippocampi revealed three distinct bands at approximately 102, 57 and 45kDa. All bands were preabsorbed following preincubation with the blocking peptide (Fig. 4). Neither the 102 KDa nor the 57 KDa band changed with increasing gestational age (Fig. 4). However, expression of the 45kDa band was significantly (P < 0.05) elevated near term (Fig. 4A and B) in both female and male fetuses, paralleling changes in NGFI-A mRNA. Using immunohistochemistry, we localized NGFI-A protein to the hippocampus and cingulate cortex (Fig. 3, lower panel). NGFI-A appeared to be localized in both the cytoplasm and nucleus. Staining was completely prevented by preincubation of the NGFI-A antibody with blocking peptide (Fig. 3, lower right panel).
Figure 4.
A, representative Western blot for NGFI-A protein in fetal hippocampus during late gestation. Three bands (102, 57 and 45kDa) were detected. Western analysis using preabsorbed antibody completely eliminated all bands. B, relative changes in 45kDa form of NGFI-A protein in female (open bars) and male (filled bars) fetuses in the second half of gestation, expressed as a ratio to tubulin (mean ±s.e.m.). *Significant (P < 0.05) differences compared to previous gestational age. C, relative changes in the 57 and 102kDa bands in female and male fetuses in the second half of gestation (expressed as a ratio to tubulin (mean ±s.e.m.).
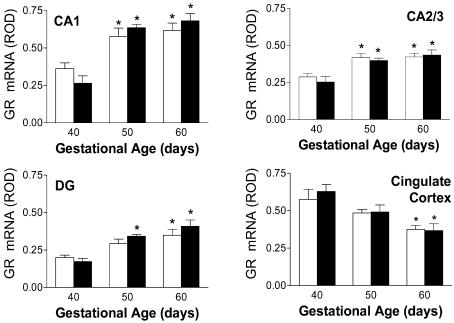
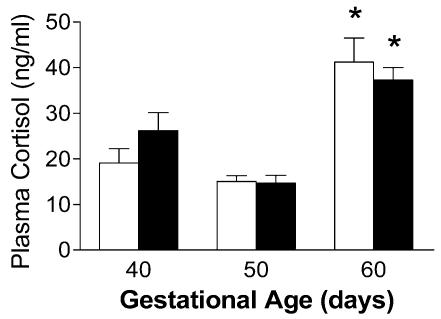
Relative expression of GR mRNA in fetal guinea-pig hippocampus (CA1, CA2/3), dentate gyrus and cingulate cortex, are shown in Fig. 5. The progressive rise of GR mRNA expression in the hippocampal formation (CA1, CA2/3), and decline in cingulate cortex are consistent with our previously published results (Matthews, 1998). GR protein expression in the fetal guinea-pig has been measured previously (Owen & Matthews, 2003). Plasma cortisol concentrations were significantly (P < 0.05) elevated in the gd60 fetuses, compared to all previous gestational ages (Fig. 6).
Figure 5.
Relative levels of GR mRNA expression in the hippocampus (CA1,CA2/3), dentate gyrus (DG) and cingulate cortex in female (open bars) and male (filled bars) fetuses in the second half of gestation. *Significant (P < 0.05) differences compared to gd40 fetuses. These data are presented to validate correlation analysis (see text; Fig. 7).
Figure 6.
Plasma cortisol concentrations in female (open bars) and male (filled bars) fetuses in late gestation. *Significant (P < 0.05) differences compared to previous gestational age.
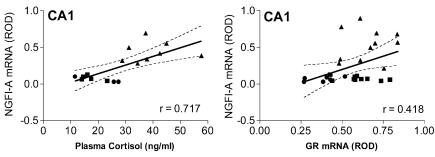
Correlation analysis indicated that a strong association existed between NGFI-A mRNA expression in the hippocampal CA1 subfield (r= 0.717, P= 0.002; Fig. 7, left panel) with fetal plasma cortisol levels. A similarly strong association between fetal cortisol levels and NGFI-A mRNA expression in the cingulate cortex (data not shown, r= 0.737, P= 0.0005) of fetal guinea-pigs was also detected. Further, a significant association between NGFI-A mRNA and GR mRNA expression in the CA1 region of the hippocampus was also observed (r= 0.418, P= 0.03; Fig. 7, right panel). Correlation analysis revealed that the relationship between circulating plasma cortisol levels and 5-HT7 receptor mRNA expression was non-significant in both the CA3 (r= (0.435, P= 0.063) and CA4 (r=−0.330, P= 0.168) hippocampal subfields.
Figure 7.
Correlation analysis of NGFI-A mRNA expression (gd40 (•), gd50 (▪) and gd60 (▴)) in CA1 hippocampal subfield to plasma cortisol (left panel) and GR mRNA expression in the CA1 region (right panel) in second half of gestation (data pooled to include both sexes). Both relationships were determined to have statistically significant (P < 0.05) correlations.
Antenatal glucocorticoid treatment
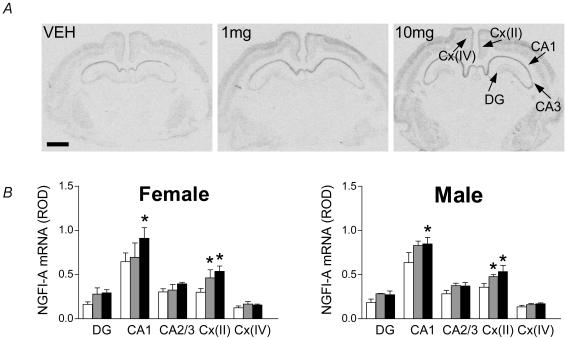
Analysis of individual regions, indicated that repeated courses of glucocorticoid did not significantly alter 5-HT7 receptor mRNA expression in the fetal brain (Table 1). Analysis of NGFI-A mRNA expression following repeated glucocorticoid exposure by three-way ANOVA (region × gender × dose) indicated an overall significant effect of treatment (P < 0.001) and region (P < 0.001). Analysis of specific brain regions indicated that levels of NGFI-A mRNA were significantly (P < 0.05) elevated in the CA1 subfields of both male and female fetuses following high dose of glucocorticoid (10mgkg−1). Both doses of glucocorticoid resulted in significant (P < 0.05) increases in NGFI-A mRNA in layer II of the cingulate cortex, in males and females. There was no significant effect of glucocorticoid treatment on NGFI-A mRNA levels in layer IV of the cingulate cortex (Fig. 8B). Though there were trends towards increased NGFI-A mRNA in the dentate gyrus and other regions of the hippocampus, these did not reach significance (Fig. 8B).
Table 1.
The effect of maternal dexamethasone (DEX1, 1mgkg−1; DEX10, 10mgkg−1) or vehicle (VEH) treatment on days 40, 41, 50, 51, 60 and 61 gestation on 5-HT7 receptor mRNA expression in the fetal hippocampus
| Female | Male | |||||
|---|---|---|---|---|---|---|
| ROD | VEH | DEX1 | DEX10 | VEH | DEX1 | DEX10 |
| CA3 | 0.187 ± 0.012 | 0.182 ± 0.019 | 0.212 ± 0.020 | 0.178 ± 0.016 | 0.199 ± 0.021 | 0.202 ± 0.014 |
| CA4 | 0.080 ± 0.005 | 0.088 ± 0.018 | 0.114 ± 0.024 | 0.093 ± 0.014 | 0.093 ± 0.014 | 0.112 ± 0.026 |
| DG | 0.161 ± 0.008 | 0.151 ± 0.025 | 0.197 ± 0.019 | 0.160 ± 0.009 | 0.179 ± 0.011 | 0.200 ± 0.011 |
| PVT | 0.131 ± 0.017 | 0.106 ± 0.015 | 0.124 ± 0.019 | 0.108 ± 0.010 | 0.112 ± 0.019 | 0.113 ± 0.011 |
Results are expressed as means ±s.e.m.
Figure 8.
A, representative expression of NGFI-A mRNA in the fetal male guinea-pig hippocampus, following treatment with repeated courses of dexamethasone. NGFI-A mRNA expression was detected in the hippocampus (CA1, CA2/3), dentate gyrus (DG), and layers II and IV of the cingulate cortex (Cx(II) and Cx(IV)). Bar: 1.5mm. B, effect of dexamethasone (1mgkg−1 (grey bar) or 10mgkg−1 (filled bar)) or vehicle (open bar) on gd40, 41, 50, 51, 60 and 61 on NGFI-A mRNA expression in the hippocampus (CA1, CA2/3) dentate gyrus (DG), and cingulate cortex (layers II and IV) of female and male fetuses in late gestation. *Significant (P < 0.05) differences compared to vehicle control.
Discussion
We report, for the first time in any species, the developmental expression of limbic and thalamic 5-HT7 receptor and NGFI-A over the second half of gestation. We have also determined the effects of repeated fetal exposure to synthetic glucocorticoid on the trajectory of this development. This is important because approximately 10% of all pregnant women in North America are treated with synthetic glucocorticoid in late gestation. We report a dramatic up-regulation of NGFI-A expression in all regions of the hippocampus and frontal cortex, but not the dentate gyrus, as fetuses approach term. The increase in hippocampal NGFI-A expression is positively correlated with hippocampal GR mRNA and plasma cortisol levels in the near-term fetus. Further, our results suggest that the synthetic glucocorticoid-mediated increase in NGFI-A mRNA expression in the hippocampus is dose-related. In contrast, 5-HT7 receptor mRNA levels are higher in the hippocampus and thalamus at gd40 than at later stages of gestation. However, this developmental profile appears to be specific to hippocampus, as 5-HT7 receptor mRNA levels in the dentate gyrus and cingulate cortex remained low throughout late gestation. Expression of the hippocampal 5-HT7 receptor does not appear responsive to glucocorticoids. Finally, development of these systems is not sex-specific.
The high levels of 5-HT7 receptor mRNA localized in the CA2/3 region of the fetal hippocampus, coupled with very low expression in the CA1 subfield and the dentate gyrus, is consistent with a previous report on the distribution of 5-HT7 receptors in the adult guinea-pig and rat hippocampus (Yau et al. 1997; Vanhoenacker et al. 2000). Though there have been reports of 5-HT7 receptor development in the early postnatal period in the rat (Vizuete et al. 1997; Muneoka & Takigawa, 2003), no previous studies have investigated developmental expression during fetal life. Guinea-pigs, unlike rats and mice, give birth to neuroanatomically mature young, and as such, their profile of fetal brain development more closely resembles that of the human (Dobbing & Sands, 1970; Darlington et al. 1999). In the young rat, 5-HT7 receptors are highly expressed in the CA2/3 regions of the hippocampus at 5 days of age, but levels appear to decrease in the CA3 after 15 days of age and remain low into adulthood. A similar pattern has been described in the paraventricular thalamic nucleus (Vizuete et al. 1997). In the developing brain, serotonin has been shown to act as a neurotrophic signal as well as a neurotransmitter (Whitaker-Azmitia, 2001). High levels of 5-HT7 receptor mRNA detected in the hippocampus and frontal cortex around mid-gestation (gd40) in the guinea-pig may facilitate a neurotrophic role of serotonin in these regions (Gould, 1999; Whitaker-Azmitia, 2001). In this regard, rates of neurogenesis are maximal around gd40 in the guinea-pig (Dobbing & Sands, 1970). The patterns of 5-HT7 receptor expression are very different in the hippocampus and dentate gyrus suggesting differential regulation in these two closely related structures.
A number of studies have shown that serotonin can act to increase the expression of glucocorticoid receptors in dispersed hippocampal neurones derived from fetal mice (Erdeljan et al. 2000), rats (Mitchell et al. 1990; Laplante et al. 2002) and guinea-pigs (Erdeljan et al. 2000), and this is thought to represent a primary mechanism for programming of HPA function (Matthews, 2002). In rats, this effect is mediated by the 5-HT7 receptor, and it is possible that the same receptor is involved in the guinea-pig and mouse. In the guinea-pig, studies in which serotonin increased hippocampal GR were undertaken in hippocampi derived from gd40 fetuses (Erdeljan et al. 2000). This is a time when high levels of 5-HT7 receptors are present in the hippocampal CA2/3 regions and in the frontal cortex. Our studies have demonstrated that in later gestation, increases in NGFI-A and GR expression are coincident with a reduction in 5-HT7 receptor expression. This suggests there may be specific windows when development of the hippocampal GR system is more susceptible to the influences of serotonin. In addition, given the distribution of 5-HT7 receptors within the hippocampus, one might predict that the influences of serotonin on GR expression in CA2/3 neurones would be greater than that in the CA1 region. Selective hippocampal cultures will need to be undertaken to establish whether this occurs.
Serotonin has been shown to alter GR expression through a cAMP-mediated mechanism (Meaney et al. 1994) involving the transcription factor NGFI-A (Meaney et al. 2000). NGFI-A is as an immediate-early gene and has been used as a marker of neuronal activation (Honkaniemi & Sharp, 1999). We have shown that NGFI-A expression is present at very low levels at gd40 and gd50, but dramatically increases in all hippocampal subfields by gd60. This is paralleled by an increase in NGFI-A protein levels, which we have localized using immunohistochemistry. This would indicate a good correlation between mRNA and immunoreactive protein during development. A similar relationship has been reported in the adult rat brain (Beckmann & Wilce, 1997). There is also a rapid up-regulation of NGFI-A mRNA in the cingulate cortex near term, positively correlating (P < 0.05) with plasma cortisol levels. The distribution of NGFI-A mRNA expression in the fetal guinea-pig hippocampus is consistent with that described in the adult rat (Schlingensiepen et al. 1991). Previous studies have failed to detect NGFI-A in the fetal rat hippocampus (McMahon et al. 1990). Low levels have been detected postnatally in this species, with levels increasing into adulthood (Watson & Milbrandt, 1990). Our data clearly indicate that there is a dramatic up-regulation of NGFI-A expression in the fetal guinea-pig hippocampus approaching term. The positive correlation between NGFI-A expression and fetal plasma cortisol, suggests that glucocorticoids may be involved in activation of NGFI-A gene expression near term.
In the present study, we identified three clearly definable bands following Western blotting for NGFI-A protein in the guinea-pig hippocampus. Previously, the antibody has been used to quantify changes in NGFI-A protein in the rat hippocampus (Meaney et al. 2000). All bands were absent following preabsorption of the antibody with peptide antigen. Developmental changes in NGFI-A were specific to the 45kDa band. A large number of publications report different band sizes following Western blot for NGFI-A indicating that the size of functional NGFI-A across species may vary (reviewed by Beckmann & Wilce, 1997). The species and study differences may relate to the high degree of post-translational modification which has been reported for NGFI-A, in addition to the presence of several open-reading frames found in the coding exon (Beckmann & Wilce, 1997). As an example, Meaney et al. (2000) report a band representing NGFI-A protein (80–88kDa) in the rat, which was much larger than the estimated weight (57kDa) based on the original sequence published by Milbrandt (1987). Furthermore, both cytoplasmic (truncated) and nuclear forms of the protein have been reported (Day et al. 1990). Our data, from whole cell fractions of fetal hippocampus, indicate robust changes in protein levels of the smallest form (45kDa), and this is entirely consistent with the developmental changes in NGFI-A mRNA that we report. Further studies will be required to establish whether differences exist in the molecular mass of NGFI-A between fetal and adult guinea-pigs.
During the second half of gestation, there are progressive increases in GR in all regions of the fetal guinea-pig hippocampus, consistent with our previous results which demonstrate that levels reach a peak at term (Owen & Matthews, 2003). The fact that 5HT7 receptor levels decrease in late gestation might suggest that activation of this receptor is not responsible for the normal maturational rise in GR at this time. In this connection, it is also unlikely that activation of the 5HT7 receptor is responsible for the dramatic increase in NGFI-A which occurs as the fetus approaches term. However, there is currently no information as to levels of serotonin in the fetal hippocampus. In adults rats, stress-induced increases in glucocorticoid are paralleled by increased hippocampal serotonin concentrations (Rueter et al. 1997), and a similar relationship may exist as the fetus approaches term. Given the significant correlation between increases in plasma cortisol and NGFI-A, it is possible that increased plasma cortisol drives the NGFI-A promoter, though further studies are required.
Repeated exposure to synthetic glucocorticoid had no effect on hippocampal 5-HT7 receptor mRNA, suggesting that glucocorticoids do not play a major role in the development of this receptor system in the hippocampus. The significance of the decrease in 5-HT7 receptor mRNA in the paraventricular thalamic nucleus in females following the lower treatment dose is not clear. In contrast, repeated high dose glucocorticoid exposure resulted in an increase in NGFI-A mRNA expression in both the CA1 region of the hippocampus and layer II of the cingulate cortex. Under normal circumstances in adults, glucocorticoids would be expected to down-regulate limbic GR. We have previously shown that this does not happen in the fetal guinea-pig at term, when endogenous glucocorticoid levels are high (Matthews, 1998; Owen & Matthews, 2003) or following repeated fetal exposure to synthetic glucocorticoid (McCabe et al. 2001). Given the fact that during specific periods of development, NGFI-A acts directly on the GR promoter to increase GR expression (Meaney et al. 2000), it is possible that the dramatic increase in hippocampal NGFI-A expression near term maintains high levels of GR expression in the presence of very high circulating concentrations of endogenous glucocorticoid.
In conclusion, 5-HT7 receptor mRNA is present at highest levels in the CA2/3 region of the hippocampus by gd40, and levels decrease progressively thereafter. Repeated exposure to glucocorticoids during the second half of gestation does not affect hippocampal 5-HT7 receptor expression; however, this does not preclude the possibility that changes in local hippocampal serotonin concentrations may occur. These data suggest that the 5-HT7 receptor plays only a limited role, if any, in normal maturation of the hippocampal GR in late gestation, though it may be important at earlier stages of development. In contrast, fetal hippocampal NGFI-A expression is elevated at term, coincident with rising fetal plasma cortisol concentrations, and increased hippocampal GR expression. Synthetic glucocorticoids, at doses that we have shown to permanently program hippocampal glucocorticoid receptor expression in the guinea-pig (Liu et al. 2001), increase hippocampal NGFI-A expression. NGFI-A is an important regulator of hippocampal transcription, and our data would suggest that there is a dramatic activation of transcription in the fetal hippocampus in the final approach to term. Mimicking the late gestation rise in cortisol, using synthetic glucocorticoid, probably prematurely activates the hippocampus and may lead to long-term modification or ‘programming’ of limbic function.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (MOP-49511) awarded to SGM.
References
- Ballard RA, Ballard PL. Antenatal hormone therapy for improving the outcome of the preterm infant. J Perinatol. 1996;16:390–396. [PubMed] [Google Scholar]
- Beckmann AM, Wilce PA. Egr transcription factors in the nervous system. Neurochem Int. 1997;31:477–510. doi: 10.1016/s0197-0186(96)00136-2. (discussion, pp. 511–516) [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brocklehurst P, Gates S, McKenzie-McHarg K, Alfirevic Z, Chamberlain G. Are we prescribing multiple courses of antenatal corticosteroids? A survey of practice in the UK. Br J Obstet Gynaecol. 1999;106:977–979. doi: 10.1111/j.1471-0528.1999.tb08440.x. [DOI] [PubMed] [Google Scholar]
- Darlington RB, Dunlop SA, Finlay BL. Neural development in metatherian and eutherian mammals: variation and constraint. J Comp Neurol. 1999;411:359–368. [PubMed] [Google Scholar]
- Day ML, Fahrner TJ, Aykent S, Milbrandt J. The zinc finger protein NGFI-A exists in both nuclear and cytoplasmic forms in nerve growth factor-stimulated PC12 cells. J Biol Chem. 1990;265:15253–15260. [PubMed] [Google Scholar]
- Dean F, Matthews SG. Maternal dexamethasone treatment in late gestation alters glucocorticoid and mineralocorticoid receptor mRNA in the fetal guinea pig brain. Brain Res. 1999;846:253–259. doi: 10.1016/s0006-8993(99)02064-8. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 1970;17:115–123. doi: 10.1016/0006-8993(70)90311-2. [DOI] [PubMed] [Google Scholar]
- Erdeljan P, MacDonald JF, Matthews SG. Do glucocorticoids and serotonin modify glucocorticoid receptor and mineralocorticoid receptor mRNA levels in fetal guinea pig neurons, in vitro? Abstr Soc Neurosci. 2000;152:153. [Google Scholar]
- Erdeljan P, MacDonald JF, Matthews SG. Glucocorticoids and serotonin alter glucocorticoid receptor (GR) but not mineralocorticoid receptor (MR) mRNA levels in fetal mouse hippocampal neurons, in vitro. Brain Res. 2001;896:130–136. doi: 10.1016/s0006-8993(01)02075-3. [DOI] [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Honkaniemi J, Sharp FR. Prolonged expression of zinc finger immediate-early gene mRNAs and decreased protein synthesis following kainic acid induced seizures. Eur J Neurosci. 1999;11:10–17. doi: 10.1046/j.1460-9568.1999.00401.x. [DOI] [PubMed] [Google Scholar]
- Keightley MC, Curtis AJ, Chu S, Fuller PJ. Structural determinants of cortisol resistance in the guinea pig glucocorticoid receptor. Endocrinology. 1998;139:2479–2485. doi: 10.1210/endo.139.5.5982. [DOI] [PubMed] [Google Scholar]
- Laplante P, Diorio J, Meaney MJ. Serotonin regulates hippocampal glucocorticoid receptor expression via a 5-HT7 receptor. Brain Res Dev Brain Res. 2002;139:199–203. doi: 10.1016/s0165-3806(02)00550-3. [DOI] [PubMed] [Google Scholar]
- Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J Physiol Endocrinol Metab. 2001;280:E729–E739. doi: 10.1152/ajpendo.2001.280.5.E729. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Dynamic changes in glucocorticoid and mineralocorticoid receptor mRNA in the developing guinea pig brain. Brain Res Dev Brain Res. 1998;107:123–132. doi: 10.1016/s0165-3806(98)00008-x. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- McCabe L, Marash D, Li A, Matthews SG. Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotropin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea-pig brain. J Neuroendocrinol. 2001;13:425–431. doi: 10.1046/j.1365-2826.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Champion JE, McMahon JA, Sukhatme VP. Developmental expression of the putative transcription factor Egr-1 suggests that Egr-1 and c-fos are coregulated in some tissues. Development. 1990;108:281–287. doi: 10.1242/dev.108.2.281. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, LaRocque S, O'Donnell D, Smythe JW, Sharma S, Tannenbaum B. Environmental regulation of the development of glucocorticoid receptor systems in the rat forebrain. The role of serotonin. Ann N Y Acad Sci. 1994;746:260–273. doi: 10.1111/j.1749-6632.1994.tb39243.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Weaver S, Yau J, Chapman K, Seckl JR. Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: the effects of thyroid hormones and serotonin. J Neurosci. 2000;20:3926–3935. doi: 10.1523/JNEUROSCI.20-10-03926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;238:797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Rowe W, Boksa P, Meaney MJ. Serotonin regulates type II corticosteroid receptor binding in hippocampal cell cultures. J Neurosci. 1990;10:1745–1752. doi: 10.1523/JNEUROSCI.10-06-01745.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneoka KT, Takigawa M. 5-Hydroxytryptamine7 (5-HT7) receptor immunoreactivity-positive ‘stigmoid body’-like structure in developing rat brains. Int J Dev Neurosci. 2003;21:133–143. doi: 10.1016/s0736-5748(03)00029-7. [DOI] [PubMed] [Google Scholar]
- NIH Consensus Development Conference. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- NIH Consensus Development Conference. Antenatal corticosteroids revisited: repeat courses. NIH Consens Statement. 2000;17:1–18. [PubMed] [Google Scholar]
- Nyirenda MJ, Welberg LA, Seckl JR. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids-fetal effect or maternal influence? J Endocrinol. 2001;170:653–660. doi: 10.1677/joe.0.1700653. [DOI] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144:2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Phillips D. Endocrine programming and fetal origins of adult disease. Trends Endocrinol Metab. 2002;13:363. doi: 10.1016/s1043-2760(02)00696-3. [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Whorwood CB, Phillips DI. Altered control of cortisol secretion in adult men with low birth weight and cardiovascular risk factors. J Clin Endocrinol Metab. 2001a;86:245–250. doi: 10.1210/jcem.86.1.7145. [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Walker BR, Syddall HE, Whorwood CB, Wood PJ, Phillips DI. Elevated plasma cortisol in glucose-intolerant men: differences in responses to glucose and habituation to venepuncture. J Clin Endocrinol Metab. 2001b;86:1149–1153. doi: 10.1210/jcem.86.3.7300. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Fornal CA, Jacobs BL. A critical review of 5-HT brain microdialysis and behavior. Rev Neurosci. 1997;8:117–137. doi: 10.1515/revneuro.1997.8.2.117. [DOI] [PubMed] [Google Scholar]
- Schlingensiepen KH, Luno K, Brysch W. High basal expression of the zif/268 immediate early gene in cortical layers IV and VI, in CA1 and in the corpus striatum – an in situ hybridization study. Neurosci Lett. 1991;122:67–70. doi: 10.1016/0304-3940(91)90195-y. [DOI] [PubMed] [Google Scholar]
- Sloboda DM, Moss TJ, Gurrin LC, Newnham JP, Challis JR. The effect of prenatal betamethasone administration on postnatal ovine hypothalamic-pituitary-adrenal function. J Endocrinol. 2002;172:71–81. doi: 10.1677/joe.0.1720071. [DOI] [PubMed] [Google Scholar]
- Smith GN, Kingdom JC, Penning DH, Matthews SG. Antenatal corticosteroids: is more better? Lancet. 2000;355:251–252. doi: 10.1016/s0140-6736(99)00448-1. [DOI] [PubMed] [Google Scholar]
- Tsou AP, Kosaka A, Bach C, Zuppan P, Yee C, Tom L, Alvarez R, Ramsey S, Bonhaus DW, Stefanich E, et al. Cloning and expression of a 5-hydroxytryptamine7 receptor positively coupled to adenylyl cyclase. J Neurochem. 1994;63:456–464. doi: 10.1046/j.1471-4159.1994.63020456.x. [DOI] [PubMed] [Google Scholar]
- Vanhoenacker P, Haegeman G, Leysen JE. 5-HT7 receptors: current knowledge and future prospects. Trends Pharmacol Sci. 2000;21:70–77. doi: 10.1016/s0165-6147(99)01432-7. [DOI] [PubMed] [Google Scholar]
- Vizuete ML, Venero JL, Traiffort E, Vargas C, Machado A, Cano J. Expression of 5-HT7 receptor mRNA in rat brain during postnatal development. Neurosci Lett. 1997;227:53–56. doi: 10.1016/s0304-3940(97)00302-9. [DOI] [PubMed] [Google Scholar]
- Watson MA, Milbrandt J. Expression of the nerve growth factor-regulated NGFI-A and NGFI-B genes in the developing rat. Development. 1990;110:173–183. doi: 10.1242/dev.110.1.173. [DOI] [PubMed] [Google Scholar]
- Weaver IC, La Plante P, Weaver S, Parent A, Sharma S, Diorio J, Chapman KE, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Mol Cell Endocrinol. 2001;185:205–218. doi: 10.1016/s0303-7207(01)00635-9. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56:479–485. doi: 10.1016/s0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Yau JL, Noble J, Widdowson J, Seckl JR. Impact of adrenalectomy on 5-HT6 and 5-HT7 receptor gene expression in the rat hippocampus. Brain Res Mol Brain Res. 1997;45:182–186. doi: 10.1016/s0169-328x(97)00026-0. [DOI] [PubMed] [Google Scholar]