Abstract
We studied the role of nitric oxide (NO) in blunting sympathetically evoked muscle vasoconstriction during acute and chronic systemic hypoxia. Experiments were performed on anaesthetized normoxic (N) and chronically hypoxic (CH) rats that had been acclimated to 12% O2 for 3–4 weeks. The lumbar sympathetic chain was stimulated for 1 min with bursts at 20 or 40 Hz and continuously at 2 Hz. In N rats, acute hypoxia (breathing 8% O2) reduced baseline femoral vascular resistance (FVR) and depressed increases in FVR evoked by all three patterns of stimulation, but infusion of the NO donor sodium nitroprusside (SNP), so as to similarly reduce baseline FVR, did not affect sympathetically evoked responses. Blockade of NO synthase (NOS) with l-NAME increased baseline FVR and facilitated the sympathetically evoked increases in FVR, but when baseline FVR was restored by SNP infusion, these evoked responses were restored. Acute hypoxia after l-NAME still reduced baseline FVR and depressed evoked responses. In CH rats breathing 12% O2, baseline FVR was lower than in N rats breathing air, but l-NAME had qualitatively similar effects on baseline FVR and sympathetically evoked increases in FVR. SNP similarly restored baseline FVR and evoked responses. Inhibition of neuronal NOS or inducible NOS did not affect baselines, or evoked responses. We propose that in N and CH rats sympathetically evoked muscle vasoconstriction is modulated by tonically released NO, but not depressed by additional NO released on sympathetic activation. The present results suggest that hypoxia-induced blunting of sympathetic vasoconstriction in skeletal muscle is not mediated by NO.
During acute systemic hypoxia in the rat, as in human subjects, there is vasodilatation in skeletal muscle (Marshall, 2000), even though the sympathetic nerve activity to muscle is increased (Saito et al. 1988; Hudson et al. 2002). This suggests the vasoconstrictor influence of sympathetic nerve activity is impaired during acute systemic hypoxia. Consistent with this, Heistad & Wheeler (1970) showed that the reflex increases in vascular resistance evoked in the forearm of human subjects by lower body negative pressure (LBNP) or by infusion of noradrenaline were reduced when they breathed 12 or 10% O2 rather than air. Further, Rowell & Seals (1990) showed that the increases in muscle sympathetic nerve activity evoked by graded levels of LBNP were similar when subjects breathed air or 12 or 10% O2, although the absolute increases in forearm vascular resistance were smaller in hypoxia. Moreover, others have provided evidence that the release of noradrenaline from sympathetic fibres is inhibited during systemic hypoxia (Rowell et al. 1989; Rowell & Seals, 1990) and that noradrenaline clearance is elevated (Leuenberger et al. 1991). In some contrast, in studies involving the use of near-infrared spectroscopy, the decrease in tissue oxygenation evoked in forearm by LBNP was preserved during hypoxia (10% O2), indicating preserved vasoconstriction of arterial vessels within muscle (Hansen et al. 2000), while vasoconstrictor responses evoked in the forearm by noradrenaline released from sympathetic varicosities by tyramine were well maintained during graded levels of hypoxia (85–75% arterial O2 saturation; Dinenno et al. 2003).
In a recent study, on the rat, we directly tested the hypothesis that vasoconstrictor responses evoked in hindlimb muscle by stimulation of sympathetic fibres is impaired by graded levels of systemic hypoxia (breathing 12, 10 or 8% O2). We showed that increases in FVR evoked by three different patterns of sympathetic nerve stimulation chosen to reflect the low frequency tonic activity and bursts of high frequency activity that occur naturally were considerably blunted when applied during severe systemic hypoxia (8% O2), while only the response to low frequency stimulation was blunted in mild or moderate hypoxia (Coney & Marshall, 2003). Since the muscle vasodilatation that occurs during systemic hypoxia is largely attributable to adenosine (Neylon & Marshall, 1991; Bryan & Marshall, 1999a; Edmunds & Marshall, 2001a), it was a reasonable hypothesis that adenosine is responsible for impairing sympathetically mediated vasoconstriction. However, even though exogenous adenosine infused during normoxia substantially blunted sympathetically evoked vasoconstriction, we obtained no evidence that the local release of adenosine during systemic hypoxia contributed to hypoxia-induced depression of sympathetic vasoconstriction (Coney & Marshall, 2003).
From other experiments performed on the rat, it was concluded that during normoxia, vasoconstriction in small intestinal arterioles and increases in vascular resistance evoked by sympathetic nerve stimulation in skeletal muscle are blunted, not only by tonically released NO, but by additional NO released by sympathetic nerve activation (Nase & Boegehold, 1996; Habler et al. 1997a,b). It was suggested in the intestine that the local fall in arteriolar wall partial pressure of O2 (PO2) resulting from sympathetically evoked vasoconstriction acted as the stimulus for endothelial NO release (Sauls & Boegehold, 2000). Subsequently, Sauls & Boegehold (2001) concluded that the fall in arteriolar wall, or tissue, PO2 induced by sympathetic vasoconstriction leads to production of adenosine which in turn stimulates NO synthesis.
In skeletal muscle, the component of hypoxia-induced vasodilatation that is attributable to adenosine is NO-dependent (Bryan & Marshall, 1999b; Edmunds & Marshall, 2001b, 2003). However, inhibition of NO synthesis with l-NAME attenuated the hypoxia-induced muscle vasodilatation to a much greater extent than inhibition of adenosine receptors (Skinner & Marshall, 1996; Bryan & Marshall, 1999a; Edmunds & Marshall, 2001a), suggesting that NO makes a contribution to hypoxia-induced vasodilatation over and above its involvement in the adenosine-mediated vasodilatation. Moreover, hypoxia can directly release NO from endothelial cells (Xu et al. 1995). Thus, the primary aim of the present study was to establish whether tonically synthesized NO, or additional NO synthesized during acute systemic hypoxia, depresses sympathetically evoked vasoconstriction.
Chronically hypoxic patients were reported to show blunted reflex forearm vasoconstriction in response to LBNP relative to normoxic subjects (Heistad et al. 1972). Further, healthy subjects who had acclimatized to 4 weeks at high altitude showed a considerable increase in muscle sympathetic nerve activity relative to the activity at sea level (∼3-fold), but only a modest (2-fold) increase in calf vascular resistance, again suggesting the vasoconstrictor influence of sympathetic fibres is blunted in chronic hypoxia (Hansen & Sander, 2003). Vasoconstrictor responses evoked by noradrenaline are also depressed in CH rats relative to normoxic rats (Doyle & Walker, 1991). Moreover, we recently showed that the depressed noradrenaline-evoked responses in isolated iliac arteries of CH rats were made comparable to those of normoxic rats, by l-NAME or by removing the endothelium, suggesting additional NO released by the endothelium depressed noradrenaline responsiveness in the CH rats (Bartlett & Marshall, 2003). In the pulmonary circulation of CH rats, chronic hypoxia up-regulates the synthesis of inducible NOS (iNOS), as well as endothelial NOS (LeCras et al. 1996). Thus, the second aim of the present study was to directly test whether muscle vasoconstriction evoked by sympathetic nerve activation is depressed in CH rats relative to normoxic rats and to establish whether any such depression is attributable to NO synthesized by eNOS or iNOS. Some of these results have been reported in brief (Bishay et al. 2000; Coney & Marshall, 2000).
Methods
Experiments were performed on four groups of male Wistar rats: control, normoxic (N) rats (Group 1: n= 12, 257 ± 10 g) and on chronically hypoxic (CH) rats (Group 2: n= 14, 244 ± 22 g; Group 3: n= 4, 204 ± 14 g; Group 4: n= 6, 228 ± 28 g). All experiments were approved under the Home Office Animals (Scientific Procedures) Act 1986. The N rats were kept in standard cages in a room comparable to that of the CH rats, but they breathed air. The CH rats were kept in a normobaric chamber at 12% O2 for 3–4 weeks prior to the acute experiment. Details of the chamber have been published previously (Thomas & Marshall, 1995). In brief, O2 levels were maintained in the range 11.75–12.25% and the build-up of CO2, humidity and ammonia was prevented by appropriate scrubbing procedures. All rats had free access to food and water and the hypoxic chamber was opened to air twice weekly for 15–20 min whilst the cages were cleaned and the animals weighed. At the time of the acute experiments, the CH rats were removed from the chamber and taken to the laboratory. The anaesthetic procedures and preparation of the animals has been described in detail (Coney & Marshall, 2003). In brief, both N and CH rats were initially anaesthetized with halothane (3.5% in O2) to allow cannulation of a jugular vein. Halothane anaesthesia was then withdrawn and the steroid anaesthetic Saffan (Schering-Plough Animal Health, Welwyn Garden City, UK) was continuously infused at 7–12 mg kg−1 h−1i.v. The depth of anaesthesia was judged by the stability of the arterial blood pressure (ABP) and respiratory movements and by the absence of a withdrawal response to a paw pinch. At the end of the experiments all the animals were humanely killed by anaesthetic overdose. The trachea was cannulated with a T-shaped cannula to maintain a patent airway. The side-arm of the cannula was connected to a system of rotameters in a gas proportioner frame (CP Instruments Co. Ltd) which allowed N rats to routinely breathe 21% O2 and CH rats to routinely breathe 12% O2 (to which they were acclimated). Both brachial arteries were cannulated to allow monitoring of ABP and to allow 150 μl samples to be taken anaerobically for blood gas analysis. Blood gases were measured periodically throughout the protocol on a blood gas machine (IL1640; Instrumentation Laboratories, Warrington, UK). The caudal ventral tail artery was cannulated retrogradely to allow infusion of the NO donor sodium nitroprusside (SNP).
A bipolar silver wire stimulating electrode was attached to the lumbar sympathetic chain between L3 and L4 (see Coney & Marshall, 2003), access being gained via a laparotomy while the great vessels were retracted to expose the sympathetic chains. The electrode tips were embedded in dental impression material (President, Light Body, Coltène, Switzerland) to mechanically fix and electrically isolate them. The electrodes were used to deliver three different patterns of nerve stimulation via an isolated stimulator (DS2A; Digitimer Ltd, UK). The patterns were chosen to comprise the same number of 1 ms pulses at 1 mA in a 1 min period. The three patterns were: (i) bursts at 40 Hz for 0.5 s, repeated every 10 s (ii) bursts at 20 Hz for 1 s, repeated every 10 s and (iii) continuous stimulation at 2 Hz. This resulted in 120 impulses being delivered over the 60 s stimulation period. Femoral blood flow (FBF) was recorded via a perivascular flowprobe (0.7 V; Transonic Systems Inc., Ithaca, NY, USA) connected to a flowmeter (T106; Transonic Systems Inc.). ABP and FBF were acquired into Chart software (AD Instruments Ltd) via a MacLab/8 s (AD Instruments Ltd) at a sampling frequency of 100 Hz. Heart rate (HR) was derived online from the ABP signal, and femoral vascular resistance (FVR) was calculated online by the division of ABP by FBF.
Protocols
Group 1: Responses to sympathetic nerve stimulation in N rats.
Following surgery, a period of at least 20 min was allowed for the recorded variables to stabilize. The responses to the three different patterns of stimulation were tested in random order, with a minimum of 5 min between successive stimulation periods to allow baselines to stabilize. The inspirate was then switched to 8% O2 to achieve systemic hypoxia by appropriate adjustment of the rotameters (see above). When a steady baseline had been achieved, the three patterns were repeated. The inspirate was then returned to 21% O2 and baselines were allowed to stabilize again. Then, SNP was infused via the tail artery at a rate chosen to reduce baseline FVR to a level similar to that induced by acute hypoxia (∼10–20 μg kg−1 min−1): the general rate was established in pilot studies and adjusted within individual experiments. The three patterns of stimulation were repeated once more during infusion and following the third period of stimulation the infusion was stopped.
After recovery of the baselines following the SNP infusion, a single bolus dose of the NO synthase inhibitor l-NAME (10 mg kg−1i.v.) was administered. After ∼10 min, when new steady baselines were achieved, the three patterns of sympathetic stimulation were repeated as above during normoxia (21% O2), hypoxia (8% O2) and then during infusion of SNP at a rate chosen to restore baseline FVR to the level preceding l-NAME administration (generally ∼20 μg kg−1 min−1).
Group 2: Responses to sympathetic nerve stimulation in CH rats before and after l-NAME.
The protocol was similar to that for Group 1, except these CH rats breathed 12% O2 throughout: responses were evoked by the three different patterns of sympathetic stimulation before and after l-NAME and then during SNP infusion at a rate chosen to restore baseline FVR to the pre-l-NAME value.
Group 3: Responses to sympathetic nerve stimulation in CH rats before and after iNOS inhibition.
Responses to the three different patterns of nerve stimulation were tested as in Group 2, before and after administration of the iNOS inhibitor aminoguanidine (AG) given as a bolus dose of 17.5 mg kg−1i.v. This dose of AG abolished the increase in iNOS activity induced in the aorta during sepsis (Scott & McCormack, 1999). AG did not affect baseline FVR (see Results), thus the patterns of sympathetic stimulation were not repeated during SNP infusion.
Group 4: Responses to nerve stimulation in CH rats before and after nNOS inhibition.
The protocol used was similar to that in Group 3, except that the vehicle for the nNOS inhibitor, 1-(2-trifluoromethylphenyl)imidazole (TRIM) was administered first (see below) and the responses evoked by sympathetic stimulation were tested. TRIM was then administered at 30 mg kg−1i.v.: this dose was shown to inhibit NO modulation of vagally induced bradycardia (Conlon & Kidd, 1999) and the responses evoked by sympathetic stimulation were retested.
Drugs
l-NAME and SNP were purchased from Sigma (Poole, UK), and aminoguanidine was purchased from Tocris Cookson (Bristol, UK). All drugs were dissolved in physiological saline. TRIM was also purchased from Tocris Cookson and dissolved in DMSO and diluted with physiological saline to 10% v/v DMSO.
Data analysis
All data are expressed as mean ±s.e.m. Data were analysed as described in Coney & Marshall (2003). Thus, FVR (mmHg. ml−1. min) was computed on-line by the division of ABP by FBF and the size of the constrictor response to sympathetic stimulation was calculated by subtracting the integrated baseline FVR, calculated from the preceding 1 min, from the integrated FVR measured during the 1 min stimulus. The integrated constrictor response was expressed in arbitrary resistance units (RU). This allowed comparison between responses evoked by different patterns of stimulation as well as between responses evoked from different values of baseline FVR. Differences in baseline and in integrated FVR before and after any drug administrations were determined by repeated measures ANOVA followed by Student-Newman-Keuls post hoc test if P < 0.05. The reasons for expressing our results in terms of vascular resistance rather than vascular conductance are given in our previous study (Coney & Marshall, 2003). Briefly, calculation of vascular resistance allows us to use the variable that changed least (FBF) as the denominator. Further, vasoconstrictor responses can increase vascular resistance to infinity, but can only decrease vascular conductance to zero. Thus, vasoconstrictor responses are maximized when expressed as resistance so facilitating statistical comparison between responses evoked under different conditions. We previously showed that systemic hypoxia still significantly blunted sympathetically evoked vasoconstrictor responses when the data were presented as vascular conductance rather than resistance. This is also the case in the present study and our major conclusions would not be altered if we presented the data as vascular conductance.
Results
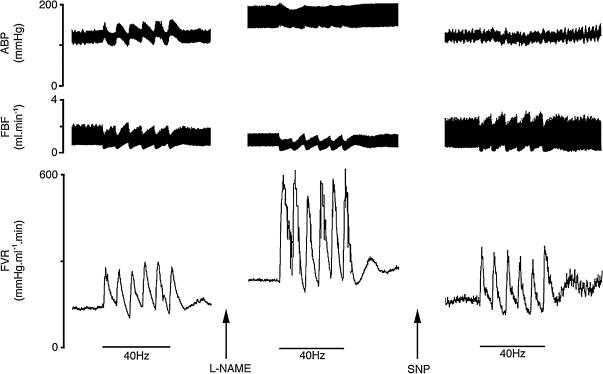
Typical responses elicited in N rats by bursts at 40 Hz during normoxia, after l-NAME and during an SNP infusion after l-NAME are illustrated in Fig. 1. Baseline values for each group are shown in Table 1 and the corresponding arterial blood gas values are shown in Table 2.
Figure 1. Original recordings of responses in N rats evoked by a bursting pattern (40 Hz) of sympathetic nerve stimulation during air breathing throughout, before and after l-NAME and during SNP infusion to restore baseline FVR to pre-l-NAME value.
The period of stimulation is indicated by the bars. ABP, arterial blood pressure; FBF, femoral blood flow; FVR, femoral vascular resistance.
Table 1.
Baseline values of cardiovascular variables in all groups and in different conditions (mean ±s.e.m.)
| MAP (mmHg) | FBF (ml min−1) | HR (beats min−1) | Baseline FVR (mmHg ml−1 min) | ||
|---|---|---|---|---|---|
| Group 1 (N rats) | Control | 107 ± 3 | 1.25 ± 0.13 | 395 ± 12 | 94.5 ± 10.0 |
| H | 82 ± 3††† | 1.50 ± 0.17 | 408 ± 24 | 61.9 ± 9.4† | |
| SNP | 79 ± 4††† | 1.39 ± 0.13† | 397 ± 21 | 55.9 ± 6.8††† | |
| l-NAME | 138 ± 4††† | 0.88 ± 0.08†† | 324 ± 12††† | 163.5 ± 14.9††† | |
| l-NAME + H | 99 ± 6 | 0.80 ± 0.10† | 337 ± 19† | 124.0 ± 11.0 | |
| l-NAME + SNP | 87 ± 6 | 1.00 ± 0.07 | 366 ± 30 | 85.7 ± 9.3 | |
| Group 2 (CH rats) | Control | 105 ± 3 | 2.11 ± 0.19** | 386 ± 10 | 53.4 ± 4.0*** |
| l-NAME | 141 ± 2††† | 1.87 ± 0.16*** | 336 ± 10††† | 82.4 ± 6.3***††† | |
| l-NAME + SNP | 86 ± 3 | 1.67 ± 0.17** | 372 ± 16 | 56.6 ± 4.3** | |
| Group 3 (CH rats) | Control | 101 ± 1 | 2.12 ± 0.18 | 408 ± 21 | 54.8 ± 5.1 |
| AG | 101 ± 4 | 1.61 ± 0.19 | 420 ± 12 | 65.4 ± 6.6 | |
| Group 4 (CH rats) | Control | 83 ± 5 | 2.45 ± 0.26 | 424 ± 15 | 35.5 ± 3.7 |
| Vehicle for TRIM | 100 ± 5† | 2.17 ± 0.29 | 437 ± 15 | 50.0 ± 6.1† | |
| TRIM | 97 ± 5†† | 1.81 ± 0.21 | 398 ± 24 | 56.9 ± 6.0† |
Control: breathing air in N rats, breathing 12% O2 in CH rats; H: breathing 8% O2 in N rats; SNP: during SNP infusion to restore baseline FVR; l-NAME: after l-NAME; l-NAME + H: N rats breathing 8% O2 after l-NAME; l-NAME + SNP: after l-NAME and during SNP infusion to restore baseline FVR; AG: after aminoguanidine; Vehicle for TRIM: after vehicle for TRIM; TRIM: after TRIM in vehicle.
Significant difference from control values.
Significant difference from N rats. 1, 2 and 3 symbols indicate P < 0.05, P < 0.01 and P < 0.001, respectively.
Table 2.
Arterial blood gas values in all groups in different conditions (mean ±s.e.m.)
| PaO2(mmHg) | PaCO2(mmHg) | pH | ||
|---|---|---|---|---|
| Group 1 | Control | 87.5 ± 1.8 | 39.8 ± 1.7 | 7.359 ± 0.012 |
| H | 33.6 ± 1.0††† | 29.2 ± 1.3††† | 7.445 ± 0.030†† | |
| SNP | 88.3 ± 2.5 | 39.4 ± 1.0 | 7.384 ± 0.012 | |
| l-NAME | 86.5 ± 2.3 | 38.8 ± 1.4 | 7.347 ± 0.021 | |
| l-NAME + H | 33.5 ± 0.8*** | 31.4 ± 1.7** | 7.411 ± 0.028*** | |
| l-NAME + SNP | 89.5 ± 2.7 | 36.7 ± 1.7 | 7.388 ± 0.023* | |
| Group 2 | Control | 48.7 ± 1.4 | 33.1 ± 1.0 | 7.427 ± 0.013 |
| l-NAME | 53.0 ± 1.4 | 31.0 ± 2.4 | 7.434 ± 0.021 | |
| l-NAME + SNP | 56.6 ± 1.6† | 32.5 ± 1.1 | 7.425 ± 0.019 | |
| Group 3 | Control | 44.8 ± 1.3 | 31.9 ± 1.4 | 7.460 ± 0.015 |
| AG | 51.2 ± 0.9†† | 30.2 ± 0.8 | 7.429 ± 0.009 | |
| Group 4 | Control | 48.4 ± 1.8 | 32.6 ± 1.5 | 7.454 ± 0.013 |
| TRIM | 52.2 ± 1.1† | 31.3 ± 2.2 | 7.446 ± 0.018 |
Abbreviations for conditions as in Table 1.
Significant difference from Control values.
Significant difference from pre-l-NAME. N rats breathing air; CH rats breathing 12% O2.
Group 1: Sympathetic stimulation in N rats before and after l-NAME
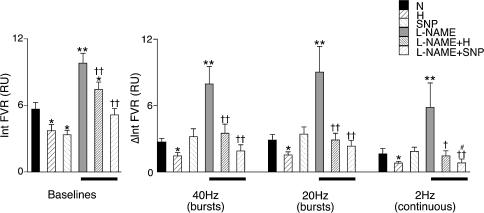
During air breathing, sympathetic stimulation with the bursts at 40 and 20 Hz evoked an increase in integrated FVR of 2.7 ± 0.3 and 2.9 ± 0.5 RU, respectively (see Fig. 2). However, continuous stimulation at 2 Hz only evoked an increase in integrated FVR of 1.6 ± 0.5 RU (see Fig. 2 and Coney & Marshall, 2003).
Figure 2. Effect of hypoxia, SNP and l-NAME on vasoconstrictor responses evoked in hindlimb muscle by different patterns of sympathetic stimulation in N rats.
Panel on left shows baseline values from which responses to sympathetic stimulation were evoked, as the integral of FVR (Int FVR) over 1 min. Panel to the right shows the evoked responses to the three patterns of stimulation as indicated below panel; responses are shown as change in the integral of FVR from baseline (ΔInt FVR) over 1 min (columns show mean ±s.e.m.). Conditions under which baselines and responses were recorded are shown by the different shading (see key): N, breathing 21% O2; H, breathing 8% O2; SNP, during SNP infusion; l-NAME, following l-NAME; l-NAME + H, breathing 8% O2 after l-NAME; l-NAME + SNP, SNP infusion after l-NAME administration. Bars below panel indicate responses after l-NAME. *Significant difference from values recorded in N. †Significant difference from values recorded after l-NAME administration. #Significant difference from value recorded during SNP infusion before l-NAME. In each case 1 and 2 symbols indicate P < 0.05 and P < 0.01, respectively.
Hypoxia induced a significant fall in baseline FVR reflecting hypoxia-induced vasodilatation, and also significantly attenuated the constrictor responses to all three patterns of sympathetic stimulation (Fig. 2, right-hand panel). As intended, SNP infusion reduced baseline FVR to a similar extent as hypoxia, but the responses evoked by sympathetic stimulation were fully comparable with those evoked during air breathing (Fig. 2).
Administration of the NO synthesis inhibitor l-NAME during air breathing produced an increase in ABP of ∼30 mmHg with a concomitant fall in HR of ∼70 beats min−1 (Table 1), whilst integrated baseline FVR rose from 5.7 ± 0.6 to 9.8 ± 0.9 RU (Figs 1 and 2). Under these conditions, there was a significant enhancement of the constrictor responses evoked by all patterns of sympathetic stimulation (e.g. the response to bursts at 40 Hz increased to 7.9 ± 1.6 from 2.7 ± 0.3 RU; see Figs 1 and 2). After l-NAME, hypoxia still induced a fall in baseline FVR as well as depressing the responses evoked by all patterns of stimulation (Fig. 2).
As intended, SNP infusion after l-NAME restored baseline FVR to pre-l-NAME levels (Figs 1 and 2). When responses evoked by sympathetic stimulation were re-tested after restoration of baseline FVR, the response evoked by bursts at 40 and 20 Hz returned to magnitudes not significantly different from control (1.9 ± 0.6 and 3.2 ± 1.1 RU, respectively; see Figs 1 and 2), but the response evoked by continuous stimulation at 2 Hz was smaller than the original control response (0.7 ± 0.4 RU; see Fig. 2).
Group 2: Sympathetic stimulation in CH rats before and after l-NAME
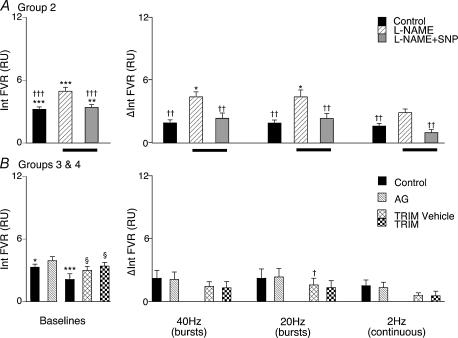
CH rats breathing 12% O2, had similar levels of ABP and HR to N rats breathing air. However, FBF was significantly higher in the CH rats as a consequence of a lower baseline FVR (see Table 1 and Fig. 2). The bursting patterns of stimulation produced increases in integrated FVR that were similar to each other (1.92 ± 0.29 and 1.90 ± 0.30, respectively, for bursts at 40 and 20 Hz), but were generally depressed compared with those evoked in N rats (compare Figs 2 and 3): this trend just failed to reach statistical significance (P = 0.08). In contrast, the response to 2 Hz in CH rats was very similar to that evoked in N rats (compare Figs 2 and 3).
Figure 3. Effect of NOS inhibition on vasoconstrictor responses evoked in hindlimb muscle by different patterns of sympathetic stimulation in CH rats.
Panels on left in A and B show baseline values for the integral of FVR recorded under the different experimental conditions and panels on right show changes from baseline of the integral of FVR evoked by the three patterns of sympathetic nerve stimulation, as described for Fig. 2. Conditions are indicated by the key. Control, breathing 12% O2. Abbreviations in A: l-NAME, following l-NAME; l-NAME + SNP, during SNP infusion to restore baseline (Group 2). Abbreviations in B: AG, following administration of aminoguanidine (Group 3); TRIM vehicle, following administration of vehicle for TRIM (Group 4); TRIM, following administration of TRIM in vehicle (Group 4). *Significant difference from values recorded in N rats. †Significant difference from values recorded after antagonist administration. §Significant difference from own control. In each case 1, 2 and 3 symbols indicate P < 0.05, P < 0.01 and P < 0.001, respectively.
Following administration of l-NAME, ABP increased and HR decreased as in N rats (see Table 1). Baseline FVR was also increased by l-NAME in CH rats but was still significantly lower than in N rats (P < 0.001:see Table 1) – the increase in baseline FVR in CH rats was smaller in absolute terms, but the percentage increase was not significantly different from that seen in N rats (59 ± 11% in CH versus 86 ± 18% in N).
The vasoconstrictor responses evoked in CH rats by all patterns of sympathetic stimulation were enhanced following l-NAME as in N rats (compare Figs 2 and 3). Further, as in N rats, infusion of SNP after l-NAME returned baseline FVR to control levels (Table 1) and under this condition the increases in FVR evoked by bursts at 40 or 20 Hz were not different from those evoked under control conditions (Fig. 3). However, the constrictor response evoked by continuous stimulation at 2 Hz tended to be depressed compared with the control response (see Fig. 3) as it was in N rats under this experimental condition, this effect just failing to reach statistical significance (P = 0.0506).
(Group 3) Sympathetic stimulation in CH rats before and after aminoguanidine
In this group of CH rats, when breathing 12% O2, the baseline cardiovascular variables were comparable to the CH rats of Group 2 (see Table 1): they had a lower baseline FVR and a higher baseline FBF than Group 1 rats breathing air. The increases in integrated FVR evoked by bursts at 40 and 20 Hz were also similar to those of Group 2 and continuous stimulation at 2 Hz evoked a constrictor response that tended to be smaller than the response to burst stimulation (see Fig. 3). Administration of the iNOS inhibitor AG did not significantly affect the baseline FVR. The increases in integrated FVR evoked by all patterns of sympathetic stimulation were not affected by the administration of AG.
(Group 4) Sympathetic stimulation in CH rats before and after TRIM
As in Groups 2 & 3, the CH rats of Group 4 had lower baseline FVR than Group 1. The increases in integrated FVR evoked by all patterns of sympathetic stimulation tended to be smaller than in Groups 2 & 3: there is no obvious explanation for this discrepancy (see Discussion for further comment). Administration of the vehicle for TRIM induced an increase in ABP & FVR (see Table 1), but only to the baseline levels observed in Groups 2 & 3 (Tables 1 and Fig. 3). TRIM given in its vehicle had no effect on baseline FVR or ABP compared to vehicle alone and the increases in integrated FVR evoked by sympathetic stimulation at 40 and 2 Hz were not significantly different from those evoked after vehicle alone; those evoked by 20 Hz were only slightly but significantly different (Fig. 3).
Discussion
The results of the present study allow us to consider the role of NO in modulating vasoconstriction evoked in skeletal muscle by sympathetic nerve activation with bursts at high frequency and with continuous stimulation at low frequency, during normoxia, acute systemic hypoxia and after acclimation to chronic hypoxia.
Modulation by NO in normoxia
Stimulation of the sympathetic chain with bursts at 40 or 20 Hz produced phasic increases in FVR, while continuous stimulation at 2 Hz produced a tonic increase in FVR. The integral of the evoked change in FVR was greater in response to the bursting patterns of stimulation than constant frequency stimulation, even though the same number of pulses were delivered in the 1 min stimulation period in all three cases (see Coney & Marshall, 2003). Inhibition of NOS activity with l-NAME produced the expected increase in baseline FVR consistent with blockade of the tonic dilator action of NO as reported many times before for muscle vasculature (Skinner & Marshall, 1996; Habler et al. 1997b; Thomas & Victor, 1998). Under this condition, the increases in FVR evoked by all three patterns of sympathetic stimulation were greatly enhanced.
These results are consistent with those reported for skeletal muscle vasculature by Habler et al. (1997b) who stimulated the lumbar sympathetic chain at constant frequencies of 0.5–20 Hz and calculated changes in vascular resistance. They are also consistent with those reported by Nase & Boegehold (1996, 1997) and by Sauls & Boegehold (2000) who stimulated the sympathetic supply to the microcirculation of intestinal wall at constant frequencies of 3 or 8 Hz before and after topical application of the NOS inhibitor NG-monomethyl-l-arginine and measured changes in arteriolar diameter. By contrast, Thomas & Victor (1998) reported that l-NAME had no effect on the vasoconstrictor responses expressed as decreases in femoral vascular conductance (FVC), evoked in hindlimb muscles of the rat by stimulation of the sympathetic chain at a constant frequency of 2.5 or 5 Hz. The reasons for the disparity between the latter results and those of the present and other published studies are not clear. The experimental conditions were apparently similar to those of the present study except that Thomas & Victor (1998) gave l-NAME at a dose of 5 mg kg−1 whereas we used 10 mg kg−1. It seems unlikely that Thomas & Victor (1998) did not give sufficient l-NAME to block NOS activity, for they reported a decrease in baseline vascular conductance (see above) and were able to show effects of l-NAME on responses evoked by sympathetic nerve stimulation during muscle contraction (see below). Direct qualitative comparison between the two sets of results is difficult because Thomas & Victor (1998) presented their results as the peak percentage change in FVC evoked by sympathetic stimulation from the relevant baseline FVC, whereas we show an absolute change in integrated FVR from the relevant baseline FVR. However, we note that if our results are calculated as changes in integrated FVC, rather than FVR, l-NAME decreased baseline FVC whilst any change in the decrease in the integral of FVC evoked by the three patterns of sympathetic stimulation does not reach statistical significance (A. M. Coney & J. M. Marshall, unpublished results). This probably reflects the fact that vasoconstrictor responses are mathematically compressed when expressed as vascular conductance (see Methods).
In the present study, in contrast to the other similar studies mentioned above, we restored baseline FVR after l-NAME by continuous infusion of the NO donor SNP. Thus, we hoped to mimic the tonic dilator influence of tonically synthesized NO. Under this condition, the effect of l-NAME on the magnitude of the sympathetically evoked responses was reversed. It is very unlikely that SNP infusion after l-NAME had this effect simply by restoring the baseline to the pre-l-NAME control value, because our results indicate that whether or not a change in baseline FVR is associated with a change in the sympathetically evoked vasoconstrictor responses is dependent on the mechanisms by which the baseline is changed rather than by the change in baseline FVR per se. Thus, before l-NAME, SNP and hypoxia decreased baseline FVR to the same extent, but only hypoxia reduced the magnitude of sympathetically evoked responses, whereas after l-NAME, both hypoxia and SNP decreased baseline FVR and the evoked increases in FVR, albeit to different extents. Similarly, in our previous study the effects of graded levels of hypoxia and adenosine infusion on baseline FVR and sympathetically evoked increases in FVR showed no correlation (Coney & Marshall, 2003). Rather, the simplest explanation for the effects of SNP infusion after l-NAME is that tonically synthesized NO limits the vasoconstriction evoked in skeletal muscle by the low frequency sympathetic nerve activity that is present under resting conditions (see Habler et al. 1994; Macefield et al. 1994; Johnson et al. 2001) and by the high frequency bursts that occur when muscle sympathetic nerves are naturally activated (see Johnson et al. 2001).
Importantly, there is no reason to suggest that increases in FVR were additionally limited by NO synthesized as a direct, or indirect, consequence of sympathetic nerve activity as proposed by others (Habler et al. 1997b; Nase & Boegehold, 1997; Sauls & Boegehold, 2000, 2001). If this had been the case, then the increases in FVR evoked by sympathetic stimulation after l-NAME would have been expected to remain greater than those evoked before l-NAME, even during SNP infusion. As indicated in the Introduction, Sauls & Boegehold (2000) concluded that in the small intestine, additional NO was synthesized during sympathetic stimulation by the action of adenosine released as a consequence of the associated fall in intestinal blood flow and local tissue hypoxia, which limited the constriction of intestinal arterioles. Our recent study showed that in skeletal muscle, endogenously released adenosine did not modulate the increase in FVR evoked by sympathetic stimulation during systemic normoxia, even though muscle blood flow was considerably reduced by sympathetic stimulation and even though adenosine contributes to the vasodilatation induced by systemic hypoxia in skeletal muscle (Coney & Marshall, 2003).
Modulation by NO in acute systemic hypoxia
Acute systemic hypoxia produced the expected decrease in baseline FVR in N rats, indicating vasodilatation, and depressed the increases in FVR evoked by all three patterns of sympathetic stimulation (see Coney & Marshall, 2003). When baseline FVR was decreased by SNP infusion during normoxia, to the same extent as that induced by systemic hypoxia, the increases in FVR evoked by sympathetic stimulation were not altered. This finding strongly suggests that any additional NO that is released during systemic hypoxia as a consequence of adenosine receptor stimulation or other mechanisms (see Ray et al. 2002) is not responsible for, or does not contribute significantly to, the hypoxia-induced depression of sympathetic vasoconstriction. This conclusion is supported by the finding that hypoxia still reduced baseline FVR when NO synthesis was blocked with l-NAME and is consistent with our previous finding that hypoxia still increased FVC after l-NAME (Edmunds & Marshall, 2001b; Edmunds et al. 2003). Moreover, hypoxia still greatly reduced the increases in FVR evoked by all three patterns of sympathetic stimulation after l-NAME even though, under these conditions, hypoxia could no longer increase the synthesis of NO (see Edmunds & Marshall, 2001b).
In our previous study (Coney & Marshall, 2003), we commented that the depression of sympathetic vasoconstriction that occurs during acute systemic hypoxia resembles the apparently similar phenomenon that occurs during muscle contraction (Thomas et al. 1997; Thomas & Victor, 1998). However, we argued that the mechanisms are likely to be different: for in systemic hypoxia the hypoxic stimulus originates in the blood, whereas during muscle contraction hypoxia originates in the contracting muscle. The present results add further support to this proposal, for the depression of sympathetic vasoconstriction that occurs during muscle contraction has been largely attributed to NO, on the grounds that the vasoconstriction is largely restored by l-NAME (Thomas & Victor, 1998). Further studies indicated that the NO that modulates the sympathetic vasoconstriction, is released from contracting skeletal muscle fibres and is synthesized by nNOS (Hansen et al. 2000).
Modulation by NO in chronic systemic hypoxia
Baseline FVR was lower and FBF was higher in CH rats breathing 12% O2, the level of O2 to which they had acclimated for 3 weeks, than in N rats breathing air. The simplest explanation might seem to be that the muscle resistance vessels of the CH rats simply had lower tonic vascular tone. However, we know from previous studies that by 3 weeks of acclimation, CH rats show increased haematocrit such that O2 delivery to hindlimb muscle, when they are breathing 12% O2, is comparable to that of N rats breathing air, with no evidence of resting hypoxic dilatation (Marshall & Davies, 1999; Thomas & Marshall, 1997). Further, substantial remodelling and angiogenesis occurs in skeletal muscle vasculature during acclimation to chronic hypoxia such that there is an increase in the number of arteriolar and capillary branches (Smith & Marshall, 1999; Deveci et al. 2002): an increase in the number of resistances in parallel would be expected to reduce gross vascular resistance. Thus, seen in this context, it is possible that the tone of individual vessels in the vasculature of CH rats was in fact increased, rather than decreased. It may be noted that in human subjects who were acclimatized to high altitude, muscle vascular resistance was raised relative to the values measured in the same individuals before ascent (Hansen & Sander, 2003).
Starting from the recorded baseline values of FVR, the increases in FVR evoked by the bursting patterns of sympathetic stimulation were generally smaller in CH rats than in N rats, though not quite reaching statistical significance (P = 0.08). Because there is angiogenesis in the muscle of CH rats (see above), it is difficult to deduce whether the constrictor responses evoked in individual arterioles were blunted in CH rats relative to N rats. Nevertheless, the present study provides direct evidence for the proposal made by Heistad et al. (1972), that sympathetically evoked changes in muscle vascular resistance are blunted in chronic hypoxia: this would be expected to lead to impaired ability to maintain arterial pressure (see Introduction).
Inhibition of NO with l-NAME caused an increase in baseline FVR in CH as in N rats. Again, because of angiogenesis, it is difficult to judge whether individual arterioles of CH rats were affected more or less by removal of tonic NO-induced dilatation than those of N rats. There certainly seems no reason to suggest that there was greater tonic influence of NO in CH rats. After NOS inhibition, the increases in FVR evoked by all three patterns of sympathetic stimulation were potentiated as in N rats. Moreover, when the baseline level of FVR was restored after NOS inhibition by SNP infusion, then the sympathetically evoked increases in FVR were restored to the same size as before l-NAME. Thus, in CH rats as in N rats, we can draw the conclusion that the vasoconstrictor responses evoked by sympathetic stimulation were limited by tonically produced NO, but there is no reason to suggest that additional NO produced by sympathetic stimulation caused further blunting of the responses.
The fact that the potentiation of the increases in FVR evoked by sympathetic stimulation after l-NAME in CH rats was proportionally similar to that seen in N rats (cf. Figs 1 and 2) means there is no reason to suggest that additional, tonic generation of NO is responsible for blunting the vasoconstrictor response to sympathetic stimulation in CH rats relative to N rats. Superficially, this may seem to contrast with our recent finding that l-NAME normalized the depressed responses evoked by noradrenaline in isolated iliac arteries of CH rats and in arterioles of CH rats in vivo, such that they were comparable to responses evoked in vessels of N rats (Marshall, 2002; Bartlett & Marshall, 2003). However, noradrenaline that is exogenously applied to arteries and arterioles has access to extrajunctional adrenoreceptors on vascular smooth muscle and endothelium, as well as to the junctional receptors that are stimulated by nerve-released noradrenaline. Further, the responses evoked by nerve-released noradrenaline can be modulated, not just by changes in the postjunctional receptors, but by changes in prejunctional receptors that modulate transmitter release and by changes in the uptake of the released noradrenaline by sympathetic fibres: we have not followed the effect of chronic hypoxia on either of these processes. Moreover, vasoconstriction evoked by sympathetic stimulation is not simply dependent on the actions of noradrenaline, but reflects the influences of the cotransmitters ATP and NPY (see Johnson et al. 2001). Thus, the blunting of sympathetically evoked vasoconstriction in chronic hypoxia may reflect modulation of any of these processes: all we can say is that NO does not seem to make a major contribution to such modulation in CH rats.
As there is previous evidence of up-regulation of iNOS activity in the pulmonary circulation of CH rats (LeCras et al. 1996) and because there is evidence that NO produced by nNOS modulates sympathetically evoked vasoconstriction in N rats (Thomas & Victor, 1998; Hansen et al. 2000), it was of interest to establish whether NO produced by either of these isoforms affected baseline values, or sympathetically evoked responses in the CH rats. Assuming aminoguanidine, given at 17.5 mg kg−1 inhibited the activity of any iNOS (Scott & McCormack, 1999), our results indicated NO synthesized by iNOS did not significantly modulate baseline FVR, nor the increases in FVR evoked by sympathetic stimulation. Thus, up-regulation of iNOS cannot be held responsible for blunting sympathetic vasoconstriction in CH rats. The results obtained with the nNOS inhibitor TRIM in Group 4, are more difficult to interpret because the baseline values of Group 4 were different from the other groups of CH rats and because the vehicle for TRIM itself caused an increase in baseline FVR. Again, assuming TRIM given at 30 mg kg−1 inhibited nNOS activity (Conlon & Kidd, 1999), our finding that TRIM administered in the vehicle had no effect on baseline FVR or sympathetically evoked responses relative to vehicle alone suggests that NO produced by nNOS did not blunt sympathetically evoked increases in FVR in CH rats.
In summary, the present results indicate that vasoconstrictor responses evoked in skeletal muscle under normoxic conditions, by patterns of sympathetic stimulation that represent low frequency, resting activity and the high frequency bursts that occur when fibres are naturally activated, are limited by the tonic dilator influence of NO, but not by additional NO released by sympathetic nerve activity. Secondly, they provide direct evidence that increases in muscle vascular resistance evoked after acclimation to chronic hypoxia are blunted relative to those seen in normoxia. However, these responses are also modulated by tonically released NO in a similar manner to responses evoked under normoxic conditions in N rats; there is no reason to implicate additional NO produced by eNOS, iNOS or nNOS in modulating vascular tone or blunting muscle sympathetic vasoconstriction in CH rats. Importantly, the present results, taken in conjunction with our previous results (Coney & Marshall, 2003), indicate that the blunting of sympathetically evoked vasoconstriction that occurs in acute hypoxia is not solely mediated by either NO or adenosine. It is possible that there is a level of redundancy between the effects of adenosine and NO on sympathetically evoked vasoconstriction, which we have not tested in this preparation yet, or that some other unidentified substance or mechanism is responsible for blunting sympathetically mediated vasoconstriction in skeletal muscle during systemic hypoxia.
Acknowledgments
We gratefully acknowledge the support of the BHF for this work.
References
- Bartlett IS, Marshall JM. Effects of chronic systemic hypoxia on contraction evoked by noradrenaline in the rat iliac artery. Exp Physiol. 2003;88:497–507. doi: 10.1113/eph8802564. [DOI] [PubMed] [Google Scholar]
- Bishay M, Coney AM, Johnson CD, Marshall JM. Role of nitric oxide in vasoconstriction evoked in skeletal muscle of the rat by different patterns of sympathetic stimulation. J Physiol. 2000;523 P, 256 P. [Google Scholar]
- Bryan PT, Marshall JM. Adenosine receptor subtypes and vasodilatation in rat skeletal muscle during systemic hypoxia: a role for A1 receptors. J Physiol. 1999a;514:151–162. doi: 10.1111/j.1469-7793.1999.151af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan PT, Marshall JM. Cellular mechanisms by which adenosine induces vasodilatation in rat skeletal muscle: significance for systemic hypoxia. J Physiol. 1999b;514:163–175. doi: 10.1111/j.1469-7793.1999.163af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coney AM, Marshall JM. Effect of different patterns of sympathetic stimulation on skeletal muscle vasculature in chronically hypoxic rats. J Physiol. 2000;523.b:249–250P. [Google Scholar]
- Coney AM, Marshall JM. Contribution of adenosine to the depression of sympathetically evoked vasoconstriction induced by systemic hypoxia in the rat. J Physiol. 2003;549:613–623. doi: 10.1113/jphysiol.2003.042267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon K, Kidd C. Neuronal nitric oxide facilitates vagal chronotropic and dromotropic actions on the heart. J Auton Nerv Syst. 1999;75:136–146. doi: 10.1016/s0165-1838(98)00185-4. [DOI] [PubMed] [Google Scholar]
- Deveci D, Marshall JM, Egginton S. Chronic hypoxia induces prolonged angiogenesis in skeletal muscles of rat. Exp Physiol. 2002;87:287–291. doi: 10.1113/eph8702377. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ, Halliwill JR. Failure of systemic hypoxia to blunt α-adrenergic vasoconstriction in human forearm. J Physiol. 2003;549:985–994. doi: 10.1113/jphysiol.2003.042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MP, Walker BR. Attenuation of systemic vasoreactivity in chronically hypoxic rats. Am J Physiol. 1991;260:R1114–R1122. doi: 10.1152/ajpregu.1991.260.6.R1114. [DOI] [PubMed] [Google Scholar]
- Edmunds NJ, Marshall JM. Oxygen delivery and oxygen consumption in rat hindlimb during systemic hypoxia: role of adenosine. J Physiol. 2001a;536:927–935. doi: 10.1111/j.1469-7793.2001.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds NJ, Marshall JM. Vasodilatation, oxygen delivery and oxygen consumption in rat hindlimb during systemic hypoxia: roles of nitric oxide. J Physiol. 2001b;532:251–259. doi: 10.1111/j.1469-7793.2001.0251g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds NJ, Moncada S, Marshall JM. Does nitric oxide allow endothelial cells to sense hypoxia and mediate hypoxic vasodilatation? In vivo and in vitro studies. J Physiol. 2003;546:521–527. doi: 10.1113/jphysiol.2002.023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habler H-J, Janig W, Krummel M, Peters OA. Reflex patterns in postganglionic neurons supplying skin and skeletal muscle of the rat hindlimb. J Neurophysiol. 1994;72:2222–2236. doi: 10.1152/jn.1994.72.5.2222. [DOI] [PubMed] [Google Scholar]
- Habler H-J, Wasner G, Bartsch T, Janig W. Responses of rat postganglionic sympathetic vasoconstrictor neurons following blockade of nitric oxide synthesis in vivo. Neurosci. 1997a;77:899–909. doi: 10.1016/s0306-4522(96)00504-0. [DOI] [PubMed] [Google Scholar]
- Habler H-J, Wasner G, Janig W. Attenuation of neurogenic vasoconstriction by nitric oxide in hindlimb microvascular beds of the rat in vivo. Hypertens. 1997b;30:957–961. doi: 10.1161/01.hyp.30.4.957. [DOI] [PubMed] [Google Scholar]
- Hansen J, Sander M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol. 2003;546:921–929. doi: 10.1113/jphysiol.2002.031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Sander M, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in exercising skeletal muscle. Acta Physiol Scand. 2000;168:489–503. doi: 10.1046/j.1365-201x.2000.00701.x. [DOI] [PubMed] [Google Scholar]
- Heistad DD, Abboud FM, Mark AL, Schmid PG. Impaired reflex vasoconstriction in chronically hypoxemic patients. J Clin Invest. 1972;51:331–337. doi: 10.1172/JCI106818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad DD, Wheeler RC. Effect of acute hypoxia on vascular responsiveness in man. I. Responsiveness to lower body negative pressure and ice on the forehead. II. Responses to norepinephrine and angiotensin. III. Effect of hypoxia and hypocapnia. J Clin Invest. 1970;49:1252–1265. doi: 10.1172/JCI106338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson S, Johnson CD, Coney AM, Marshall JM. Changes in sympathetic nerve activity recorded from skeletal muscle arteries of the anaesthetised rat during graded levels of systemic hypoxia. J Physiol. 2002;544 P, 28 P. [Google Scholar]
- Johnson CD, Coney AM, Marshall JM. Roles of norepinephrine and ATP in sympathetically evoked vasoconstriction in rat tail and hindlimb in vivo. Am J Physiol. 2001;281:H2432–H2440. doi: 10.1152/ajpheart.2001.281.6.H2432. [DOI] [PubMed] [Google Scholar]
- LeCras TD, Xue C, Rengasamy A, Johns RA. Chronic hypoxia upregulates endothelial and inducible NO synthase gene and protein expression in rat lung. Am J Physiol. 1996;14:L164–L170. doi: 10.1152/ajplung.1996.270.1.L164. [DOI] [PubMed] [Google Scholar]
- Leuenberger U, Gleeson K, Wroblewski K, Prophet S, Zelis R, Zwillich C, Sinoway L. Norepinephrine clearance is increased during acute hypoxemia in humans. Am J Physiol. 1991;261:H1659–H1664. doi: 10.1152/ajpheart.1991.261.5.H1659. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG, Vallbo AB. The discharge behavior of single vasoconstrictor motoneurons in human muscle nerves. J Physiol. 1994;481:799–809. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM. Adenosine and muscle vasodilatation in acute systemic hypoxia. Acta Physiol Scand. 2000;168:561–573. doi: 10.1046/j.1365-201x.2000.00709.x. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Roles of adenosine in skeletal muscle during systemic hypoxia. Clin Exp Pharmacol Physiol. 2002;29:843–849. doi: 10.1046/j.1440-1681.2002.03734.x. [DOI] [PubMed] [Google Scholar]
- Marshall JM, Davies WR. The effects of acute and chronic systemic hypoxia on muscle oxygen supply and oxygen consumption in the rat. Exp Physiol. 1999;84:57–68. doi: 10.1111/j.1469-445x.1999.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Nase GP, Boegehold MA. Nitric oxide modulates arteriolar responses to increased sympathetic nerve activity. Am J Physiol. 1996;271:H860–H869. doi: 10.1152/ajpheart.1996.271.3.H860. [DOI] [PubMed] [Google Scholar]
- Nase GP, Boegehold MA. Endothelium-derived nitric oxide limits sympathetic neurogenic vasoconstriction in intestinal microcirculation. Am J Physiol. 1997;273:H426–H433. doi: 10.1152/ajpheart.1997.273.1.H426. [DOI] [PubMed] [Google Scholar]
- Neylon M, Marshall JM. The role of adenosine in the respiratory and cardiovascular response to systemic hypoxia in the rat. J Physiol. 1991;440:529–545. doi: 10.1113/jphysiol.1991.sp018723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray CJ, Abbas MR, Coney AM, Marshall JM. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol. 2002;544:195–209. doi: 10.1113/jphysiol.2002.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB, Johnson DG, Chase PB, Comess KA, Seals DR. Hypoxemia raises muscle sympathetic activity but not norepinephrine in resting humans. J Appl Physiol. 1989;66:1736–1743. doi: 10.1152/jappl.1989.66.4.1736. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Seals DR. Sympathetic activity during graded central hypovolemia in hypoxemic humans. Am J Physiol. 1990;259:H1197–H1206. doi: 10.1152/ajpheart.1990.259.4.H1197. [DOI] [PubMed] [Google Scholar]
- Saito M, Mano T, Iwase S, Koga K, Abe H, Yamazaki Y. Responses in muscle sympathetic activity to acute-hypoxia in humans. J Appl Physiol. 1988;65:1548–1552. doi: 10.1152/jappl.1988.65.4.1548. [DOI] [PubMed] [Google Scholar]
- Sauls BA, Boegehold MA. Arteriolar wall PO2 and nitric oxide release during sympathetic vasoconstriction in the rat intestine. Am J Physiol. 2000;279:H484–H491. doi: 10.1152/ajpheart.2000.279.2.H484. [DOI] [PubMed] [Google Scholar]
- Sauls BA, Boegehold MA. Reduced PO2 and adenosine formation preserve arteriolar nitric oxide synthesis during sympathetic constriction in the rat intestine. J Vasc Res. 2001;38:104–112. doi: 10.1159/000051037. [DOI] [PubMed] [Google Scholar]
- Scott JA, McCormack DG. Selective in vivo inhibition of inducible nitric oxide synthase in a rat model of sepsis. J Appl Physiol. 1999;86:1739–1744. doi: 10.1152/jappl.1999.86.5.1739. [DOI] [PubMed] [Google Scholar]
- Skinner MR, Marshall JM. Studies on the roles of ATP, adenosine and nitric oxide in mediating muscle vasodilatation induced in the rat by acute systemic hypoxia. J Physiol. 1996;495:553–560. doi: 10.1113/jphysiol.1996.sp021615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Marshall JM. Physiological adjustments and arteriolar remodelling within skeletal muscle during acclimation to chronic hypoxia in the rat. J Physiol. 1999;521:261–272. doi: 10.1111/j.1469-7793.1999.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. ATP-sensitive potassium channels mediate contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Clin Invest. 1997;99:2602–2609. doi: 10.1172/JCI119448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Marshall JM. A study on rats of the effects of chronic hypoxia from birth on respiratory and cardiovascular responses evoked by acute hypoxia. J Physiol. 1995;487:513–525. doi: 10.1113/jphysiol.1995.sp020896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Marshall JM. The roles of adenosine in regulating the respiratory and cardiovascular systems in chronically hypoxic, adult rats. J Phsyiol. 1997;501:439–447. doi: 10.1111/j.1469-7793.1997.439bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. 1998;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XP, Pollock JS, Tanner MA, Myers PR. Hypoxia activates nitric oxide synthase and stimulates nitric oxide production in porcine coronary resistance arteriolar endothelial cells. Cardiovas Res. 1995;30:841–847. [PubMed] [Google Scholar]