Abstract
We have previously shown in a rat model that a single bout of high-intensity aerobic exercise 20h before a simulated dive reduces bubble formation and after the dive protects from lethal decompression sickness. The present study investigated the importance of these findings in man. Twelve healthy male divers were compressed in a hyperbaric chamber to 280kPa at a rate of 100kPamin−1 breathing air and remaining at pressure for 80min. The ascent rate was 9mmin−1 with a 7min stop at 130kPa. Each diver underwent two randomly assigned simulated dives, with or without preceding exercise. A single interval exercise performed 24h before the dive consisted of treadmill running at 90% of maximum heart rate for 3min, followed by exercise at 50% of maximum heart rate for 2min; this was repeated eight times for a total exercise period of 40min. Venous gas bubbles were monitored with an ultrasonic scanner every 20min for 80min after reaching surface pressure. The study demonstrated that a single bout of strenuous exercise 24h before a dive to 18 m of seawater significantly reduced the average number of bubbles in the pulmonary artery from 0.98 to 0.22 bubbles cm−2(P= 0.006) compared to dives without preceding exercise. The maximum bubble grade was decreased from 3 to 1.5 (P= 0.002) by pre-dive exercise, thereby increasing safety. This is the first report to indicate that pre-dive exercise may form the basis for a new way of preventing serious decompression sickness.
Divers and astronauts continue to be at risk of decompression illness (DCI). The term DCI encompasses both decompression sickness (DCS), which is caused by tissue bubble formation due to inert gas supersaturation, and arterial gas embolism caused by entry of gas in the arteries. DCS arises as a result of decompression in various conditions, including deep sea diving, aviation and space flights. There is growing concern that diving can lead to permanent damage to the central nervous system, even in the absence of clear signs of DCS, and one of the suspected mechanisms is silent bubble formation. Injury to the central nervous system is at present the most common decompression-related problem in sport divers requiring treatment (Brubakk & Neuman, 2003). Preventive measures to reduce the risk of DCS involve breathing oxygen and reducing decompression speed. Vascular bubbles have been used as an indicator of decompression stress. Although bubbles can be present without overt clinical symptoms, the occurrence of many bubbles is clearly linked to a high risk of DCS (Nishi, 1990).
Exercise training weeks before dives has been shown to reduce the incidence of neurological DCS in pigs (Broome et al. 1995) and rats (Rattner et al. 1979). This indicates that exercise can either influence bubble formation or can change the reaction of the organism to the bubbles. In a study on divers, LaGrue et al. (1978) noted that there was less bubble formation in divers who were in good physical shape. Recently, Carturan et al. (2002) reported that younger, slimmer, or aerobically fitter divers produced fewer bubbles than older, fatter, or poorly fit divers. A similar finding was also reported by Webb et al. (2002) for persons of either sex under hypobaric conditions. Wisløff & Brubakk (2001) have recently shown that a single bout of high-intensity aerobic exercise 20h before the dive protects rats from severe decompression and death by reducing the number of bubbles. Rather than altering nitrogen elimination, exercise may reduce the number of nuclei from which bubbles are formed (Wisløff & Brubakk, 2001).
The hypothesis of the present study is that a single bout of exercise 24h before a dive reduces bubble formation in humans.
Methods
Study population
Thirteen men aged 22–38 years (29.9 ± 5.0 years, mean ±s.d.) participated in the study. Their body mass index varied between 21.5 and 29.0 kg m−2. The subjects were all experienced divers with considerable diving experience, ranging from 90 to 8000h of diving. None of them had experienced DCS in the past. Four of the divers (Nos. 3,7,10 and 12) were smokers (3–5 cigarettes a day). At the time of the study, all had a valid medical certificate for diving. All experimental procedures were conducted in accordance with the Declaration of Helsinki, and were approved by the ethics committee of the University of Split School of Medicine. Each method and potential risks were explained to the participants in detail and they gave written informed consent before the experiment.
Measurements of maximal oxygen uptake
Maximal oxygen uptake (V̇O2max) and maximum heart rate were determined in all divers 1 week prior to the experiments, using an incremental protocol on a treadmill (Cosmed T165, Rome, Italy). The subjects ran on the motorized treadmill (3 deg inclination), starting at 8 km h−1 and increasing their running speed by 1 km h−1 each minute up to exhaustion. This occurred within 10–15min in all subjects. During the entire test, oxygen uptake and lung ventilation were measured with a cardiopulmonary exercise testing unit (Quark b2, Cosmed, Rome, Italy), and heart rate (HR) was registered continuously with Polar HR monitor (Polar Vantage, Finland) and 12-lead electrocardiogram (C12, Cosmed). Criteria for assessment of V̇O2max included: (1) HR in excess of 90% of age predicted maximum (220 – age), (2) respiratory exchange ratio (RER) ≥1.1 and (3) plateau (≤150ml increase) in V̇O2 with an increase in workload. If at least two of the three criteria were met, the highest V̇O2 measured was chosen as the subject's V̇O2max. The mean V̇O2max was 47.8 ± 4.7mlkg−1 min−1 (mean ±s.d.) and the maximum heart rate at VO2max was 195 ± 8 beats min−1 (mean ±s.d.).
Exercise procedure
A single bout of interval submaximal exercise consisted of treadmill running at 90% of maximum heart rate for 3min followed by exercise at 50% of maximum heart rate for 2min. This was repeated eight times for a total exercise session of 40min. Before the first interval, a 20min warm-up was performed. The interval training regimen used in this study was based on a recently published procedure for improving aerobic capacity in soccer players (Helgerud et al. 2001).
Simulated dive protocol and bubble analysis
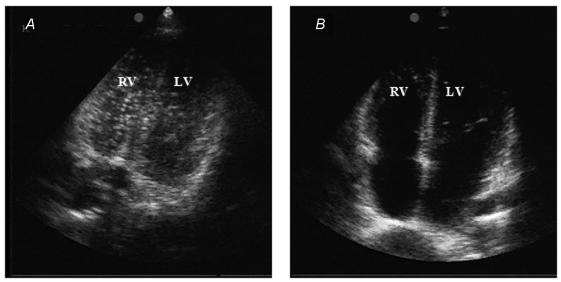
The divers were compressed in a hyperbaric chamber (Brodosplit, Split, Croatia) to 280kPa at a rate of 100kPamin−1, breathing air and remaining at pressure for 80min. They were then decompressed at a rate of 90kPamin−1 to 130kPa, where they remained for 7min before they were decompressed to the surface pressure (100kPa) at the same rate (United States Navy air decompression procedure, US Navy, 1996). This shallow, long air dive has been shown to reproducibly produce a significant amount of bubbles (Cross et al. 1987). Each diver performed two dives 7days apart, one with and one without physical exercise 24h before the dive. The order of the two dive protocols was randomly allocated. Following the dive, subjects were placed in the left supine position and an echocardiographic investigation with a phase array probe (1.5–3.3MHz) using a Vivid 3 Expert ultrasonic scanner (GE, Milwaukee, USA) was conducted. Echocardiographic investigations were performed by an experienced cardiologist (D.D.) who was not aware of the exercise regime before the dive. High quality images were obtained in all subjects. Gas bubbles were seen as high intensity echoes in the right heart and the pulmonary artery (see Fig. 1). Monitoring was performed every 20min for 80min after reaching surface pressure (first measurement completed at 20min after the dive). Images were graded according to a previously reported method (Eftedal & Brubakk, 1997). This grading system has been used extensively in several animal species, as well as in man. It has also been demonstrated that the grading system for Doppler ultrasound (Spencer, 1976) coincides with that used for images (Brubakk & Eftedal, 2001). In this study the images were graded according to a method previously described (Eftedal & Brubakk, 1997) that was very reproducible, even when inexperienced observers were used. The grading system is compatible with the grading system used for grading Doppler ultrasonic recordings (Brubakk & Eftedal, 2001). The bubble grades presented are the maximum grades observed at any of the observation points. The grading system is highly non-linear and thus single high bubble grades may influence the result. To determine if this was the case, the grades were converted into a linear scale using conversion factors previously described (Nishi et al. 2003). These conversion factors are an approximation, but are based on several thousand observations of actual bubble numbers. The bubble numbers were integrated over the whole observation period to give an indicator of the total bubble load over that time. One diver had no bubble formation on either dive and was excluded from further analysis. The data were saved on tape and grading was controlled for accuracy by one of the authors (A.O.B.), who had developed the method.
Figure 1. Decrease in venous bubble grade after strenuous exercise.
The figure depicts change in venous gas bubbles within the right heart and pulmonary artery following a dive without (A) and with (B) a previous bout of strenuous exercise in one diver (No. 8 from the Table 1). In A, there are numerous, clearly visible venous gas bubbles. After performing the exercise, bubbles are completely absent (B).
Statistical analysis
Differences in bubble grade and average number of bubbles cm−2 between groups were determined using the Wilcoxon paired test. The limit of significance was set at P < 0.05.
Results
Exercise and bubble production
None of the divers had any clinical symptoms in relation to the dives.
The complete results on formation of venous gas bubbles are presented in Table 1. Exhausting exercise 24h before the dive significantly reduced both maximum bubble grades and average bubble numbers during the 80min observation period. The maximum bubble grade decreased from 3 to 1.5 (P= 0.002) and the average number of bubbles seen during the whole observation period decreased from 0.98 to 0.22 bubbles cm−2(P= 0.006). Only one diver showed an increase in venous bubble grade after performing the described exercise and nine showed a decrease. The dramatic reduction in venous gas bubble occurrence in the right heart and pulmonary artery in one typical diver is presented in Fig. 1.
Table 1.
The effect of exercise on venous gas bubble formation in divers
| Average no. of bubbles cm−2 | Maximum bubble grade | |||
|---|---|---|---|---|
| Diver No. | Sedentary | Exercise | Sedentary | Exercise |
| 1 | 0.60 | 0.80 | 3 | 3 |
| 2 | 0.60 | 0.28 | 3 | 1 |
| 3 | 2.00 | 0.06 | 4 | 2 |
| 4 | 0.05 | 0.13 | 1 | 2 |
| 5 | 1.80 | 0.00 | 4 | 0 |
| 6 | 0.80 | 0.20 | 3 | 2 |
| 7 | 1.80 | 0.00 | 4 | 0 |
| 8 | 1.80 | 0.60 | 4 | 1 |
| 9 | 0.08 | 0.00 | 2 | 0 |
| 10 | 0.60 | 0.03 | 3 | 1 |
| 11 | 0.80 | 0.16 | 3 | 2 |
| 12 | 0.80 | 0.40 | 3 | 3 |
| Mean ±s.d. | 0.98 ± 0.69 | 0.22 ± 0.26* | — | — |
| Median | — | — | 3.0 | 1.5* |
*P < 0.05. The bubble grades shown are the maximum observed. No. of bubbles cm−2 is the mean of four observations (every 20min for 80min).
Effect of individual variables on bubble production
There was no statistically significant correlation between average bubble number or maximum bubble grade and their potential predictors: divers age, body mass index and V̇O2max (data not shown).
Discussion
This study demonstrated that a single bout of exercise 24h before performing a dive significantly reduced the number of bubbles in the right heart of divers. Both the maximum number of bubbles and the total bubble load were reduced. These results are in accordance with our previous studies in rats (Wisløff & Brubakk, 2001). However, this is the first time that such findings have been observed in a human model employing a single bout of strenuous exercise, and not an endurance training protocol. Studies have shown that even a single observation of grade 3 bubbles significantly increases the risk of DCS (Sawatzky & Nishi, 1991), thus exercise before diving can have a preventive effect on occurence of DCS.
It is generally accepted that gas bubbles grow from pre-existing gas nuclei (Yount & Strauss, 1982). The nature of such nuclei is not well defined, but they are probably small (∼1µm) gas-filled bubble precursors attached to blood vessel endothelium, where they grow into bubbles during the reduction in ambient pressure. The number of these nucleation sites may be influenced by exercise and it has been shown in cats that bubble formation can be affected by both exercise and trauma to large muscle groups (Harvey et al. 1944). Muscle contraction and tissue movement associated with exercise may activate additional micronuclei by reducing absolute tissue pressure (McElroy et al. 1944). Although these studies exploited electrically stimulated muscle contractions in anaesthetized animals, exercise has been generally considered an additional risk factor for DCS and divers and aviators were advised not to engage in unnecessary physical activity. Exercise following diving or shortly before or after decompression to altitude can promote bubble formation (McElroy et al. 1944; Dervay et al. 2002). The uptake and elimination of gas is dependent on the blood flow. Exercise during the bottom phase of a dive will increase, in theory, the amount of gas taken up (Flook, 1997) and consequently increase the decompression time required (Buhlman, 1975), while mild exercise during decompression will increase gas elimination (Flook, 1997) and reduce the number of venous bubbles detected after diving (Jankowski et al. 1997). Webb et al. (2002) have recently failed to show that moderate dynamic exercise after altitude exposure results in either delayed onset of DCS or recurring DCS. The exercise regimen used in their study was quite strenuous. Data from rats in our laboratory indicate that low-intensity exercise never inhibits bubble formation, nor does exercise up to 10h before the dive. Furthermore, if strenuous exercise is performed 48h before the dive, no effect is observed (Wisløff et al. 2003).
Hills (1992) has demonstrated that the endothelium of veins and the aorta are hydrophobic due to a coating of surface-acting substances and that bubbles can be stable more of less indefinitely on a hydrophobic surface. Since it has been shown that NO is released from the endothelial cells following exercise (Buga et al. 1991) and that NO inhibits leucocytes and platelet adhesion and aggregation (Bult, 1996), it is conceivable that an increase in NO will also reduce the hydrophobicity of the endothelial wall, thereby reducing the number of nuclei. It has been demonstrated that NO can act both as a mediator for cell injury and as a cytoprotective agent. The mechanism for the preventive effect of a single bout of exercise on bubble formation may be linked to NO production (Wisløff et al. 2003). If a NO synthase (NOS) inhibitor is given prior to the dive, a dive that does not produce any bubbles in control rats, will produce bubbles in the treated rats. Interestingly enough, this effect can only be demonstrated in sedentary rats. If exercise is performed, NOS inhibition will have no effect, suggesting that independent mechanisms are involved in the protection against the formation of vascular bubbles (Wisløff et al. 2003).
An association between bubble formation and adiposity has been previously suggested (Dembert et al. 1984; Carturan et al. 2002). The high solubility of nitrogen in fat tissue increases the total nitrogen content in the body, with consequent increased DCS risk. Dembert et al. 1984) reported a higher percentage of body fat in United States Navy divers who experienced DCS, but no association between age and DCS was found. Broome et al. (1995) hypothesized that when body weight was associated with increased DCS risk in epidemiological studies, the underlying association was really with poor aerobic fitness, where adiposity was only a surrogate indicator. Wisløff et al. 2003) have shown that higher bubble grades were present in rats weighing over 300g, supporting that view. As previously mentioned aerobically fitter divers produced fewer bubbles after field dives (Carturan et al. 2002). In the present study the bubble formation appeared to be unrelated to the diver's age, body mass index or maximal oxygen uptake. In this respect, our findings may be considered limited because of the small sample size. However, the scattergrams of bubble production versus the potential predictors showed completely dispersed data points, i.e. the absence not only of statistically significance but also of any apparent correlation.
Conclusions
This study has demonstrated that a single bout of aerobic exercise ameliorates venous gas bubble formation in man. These results have considerable implications for the development of non-pharmacological procedures for the reduction of bubble formation and thus the risk of serious DCS.
Acknowledgments
This study was supported by the Croatian National Council for Research, Grant no. 216006 and Technological Grant TP-01/0216–02, and by the Norwegian Petroleum Directorate, Norsk Hydro, Esso Norge and Statoil under the ‘Dive Contingency Contract no. 4600002328’ with Norwegian Underwater Intervention (NUI).
References
- Broome J, Dutka A, McNamee G. Exercise conditioning reduces the risk of neurologic decompression sickness in swine. Undersea Hyperb Med. 1995;22:73–85. [PubMed] [Google Scholar]
- Brubakk AO, Eftedal O. Comparison of three different ultrasonic methods for quantification of intravascular gas bubbles. Undersea Hyperb Med. 2001;28:131–136. [PubMed] [Google Scholar]
- Brubakk AO, Neuman TS. Bennett & Elliott's Physiology and Medicine of Diving. 5th edn. London: Saunders; 2003. [Google Scholar]
- Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991;17:187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- Buhlman AA. Decompression theory: Swiss practice. In: Bennett PB, Elliott DH, editors. The Physiology and Medicine of Diving and Compressed Air Work. 2nd edn. Baltimore: Williams & Wilkins; 1975. pp. 348–365. [Google Scholar]
- Bult H. Nitric oxide and atherosclerosis: possible implications for therapy. Mol Med Today. 1996;2:510–518. doi: 10.1016/s1357-4310(97)81455-4. [DOI] [PubMed] [Google Scholar]
- Carturan D, Boussuges A, Vanuxem P, Bar-Hen A, Burnet H, Gardette B. Ascent rate, age, maximal oxygen uptake, adiposity, and circulating venous bubbles after diving. J Appl Physiol. 2002;93:1349–1356. doi: 10.1152/japplphysiol.00723.1999. [DOI] [PubMed] [Google Scholar]
- Cross MR, Ritter JM, Pimlott J, Barlow S, Dollery CT. Absence of circulating PGI2 response to bubble-provoking decompression. Undersea Biomed Res. 1987;14:371–372. [PubMed] [Google Scholar]
- Davis JA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc. 1985;17:6–18. [PubMed] [Google Scholar]
- Dembert ML, Jekel JF, Mooley LW. Health risk factors for the development of decompression sickness among US Navy divers. Undersea Biomed Res. 1984;11:395–406. [PubMed] [Google Scholar]
- Dervay JP, Powell MR, Butler B, Fife CE. The effect of exercise and rest duration on the generation of venous gas bubbles at altitude. Aviat Space Environ Med. 2002;73:22–27. [PubMed] [Google Scholar]
- Eckenhoff RG, Olstad CS, Carrod G. Human dose–response relationship for decompression and endogenous bubble formation. J Appl Physiol. 1990;69:914–918. doi: 10.1152/jappl.1990.69.3.914. [DOI] [PubMed] [Google Scholar]
- Eftedal O, Brubakk AO. Agreement between trained and untrained observers in grading intravascular bubbles signals in ultrasonic images. Undersea Hyperb Med. 1997;24:293–299. [PubMed] [Google Scholar]
- Flook V. The effect of exercise on decompression bubbles. A Theoretical Study. In: Mekjavic IB, Tipton MJ, Eiken O, editors. Proceedings of the XXIII Annual Scientific Meeting of the European Underwater & Baromedical Society. Bled, Slovenia: 1997. pp. 55–61. [Google Scholar]
- Harvey EN, Barnes DK, McElroy WD, Whiteley AH, Pease DC, Cooper KW. Bubble formation in animals, I: Physical factors. J Cell Comp Physiol. 1944;24:1–22. [Google Scholar]
- Helgerud J, Engen LC, Wisloff U, Hoff J. Aerobic endurance training improves soccer performance. Med Sci Sports Exerc. 2001;33:1925–1931. doi: 10.1097/00005768-200111000-00019. [DOI] [PubMed] [Google Scholar]
- Hills BA. A hydrophobic oligolamellar lining to the vascular lumen in some organs. Undersea Biomed Res. 1992;19:107–120. [PubMed] [Google Scholar]
- Jankowski LW, Nishi RY, Eaton DJ, Griffin AP. Exercise during decompression reduces the amount of venous gas emboli. Undersea Hyperb Med. 1997;24:59–66. [PubMed] [Google Scholar]
- LaGrue D, LePechon J, Kisman K, Masurel G, Guillerm R. Etude comparative de la methode de decompression du Ministre Francais du Travail et de la methode de decompression dite du surface de l'U.S. Navy pour la plongee a lu air. Medical. Med Aero Spat Med Sub Hyp. 1978;68:353–357. [Google Scholar]
- McElroy WD, Whiteley AH, Warren GH, Harvey EN. Bubble formation in animals, IV: The relative importance of carbon dioxide concentration and mechanical tension during muscle contraction. J Cell Comp Physiol. 1944;24:133–146. [Google Scholar]
- Nishi RY. Doppler evaluation of decompression tables. In: Lin YC, Shida KK, editors. Man in the Sea. Honolulu: University of Hawaii Press; 1990. pp. 297–316. [Google Scholar]
- Nishi RY, Brubakk AO, Eftedal OS. Bubble detection. In: Brubakk AO, Neuman TS, editors. Physiology and Medicine of Diving. London: Saunders; 2003. pp. 501–529. [Google Scholar]
- Nossum V, Koteng S, Brubakk AO. Endothelial damage by bubbles in the pulmonary artery of the pig. Undersea Hyperbaric Med. 1999;26:1–8. [PubMed] [Google Scholar]
- Rattner B, Gruenau S, Altland P. Cross-adaptive effects of cold, hypoxia, or physical training on decompression sickness in mice. J Appl Physiol. 1979;47:412–417. doi: 10.1152/jappl.1979.47.2.412. [DOI] [PubMed] [Google Scholar]
- Sawatzky KD, Nishi RY. Assessment of inter-rater agreement on the grading of intravascular bubble signals. Undersea Biomed Res. 1991;18:373–396. [PubMed] [Google Scholar]
- Spencer MP. Decompression limits for compressed air determined by ultrasonically detected blood bubbles. J Appl Physiol. 1976;40:229–235. doi: 10.1152/jappl.1976.40.2.229. [DOI] [PubMed] [Google Scholar]
- US Navy. US Navy Diving Manual: Air Diving. Best Publishing, Flagstaff; 1996. [Google Scholar]
- Webb JT, Pilmanis AA, Fischer MD, Kannan N. Enhancement of preoxygenation for decompression sickness protection: effect of exercise duration. Aviat Space Environ Med. 2002;73:1161–1166. [PubMed] [Google Scholar]
- Wisløff U, Brubakk AO. Aerobic endurance training reduces bubble formation and increases survival in rats exposed to hyperbaric pressure. J Physiol. 2001;537:607–611. doi: 10.1111/j.1469-7793.2001.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisløff U, Richardson RS, Brubakk AO. NOS inhibition increases bubble formation and reduces survival in sedentary but not exercised rats. J Physiol. 2003;546:577–582. doi: 10.1113/jphysiol.2002.030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount D, Strauss R. On the evolution, generation and regeneration of gas cavitation nuclei. J Acoust Soc Am. 1982;65:1431–1439. [Google Scholar]