Abstract
In the heart ischaemic conditions induce metabolic changes known to have profound effects on Ca2+ signalling during excitation–contraction coupling. Ischaemia also affects the redox state of the cell. However, the role of cytosolic redox couples, such as the NADH/NAD+ redox system, for the regulation of Ca2+ homeostasis has remained elusive. We studied the effects of NADH and NAD+ on sarcoplasmic reticulum (SR) Ca2+ release in permeabilized rat ventricular myocytes as well as on Ca2+ uptake by SR microsomes and ryanodine receptor (RyR) single channel activity. Exposure of permeabilized myocytes to NADH (2 mm; [Ca2+]cyt = 100nm) decreased the frequency and the amplitude of spontaneous Ca2+ sparks by 62% and 24%, respectively. This inhibitory effect was reversed by NAD+ (2 mm) and did not depend on mitochondrial function. The inhibition of Ca2+ sparks by NADH was associated with a 52% decrease in SR Ca2+ load. Some of the effects observed with NADH may involve the generation of superoxide anion (O2−·) as they were attenuated to just a transient decrease of Ca2+ spark frequency by superoxide dismutase (SOD). O2−· generated in situ from the xanthine/xanthine oxidase reaction caused a slowly developing decrease of Ca2+ spark frequency and SR Ca2+ load by 44% and 32%, respectively. Furthermore, in studies with cardiac SR microsomes NADH slowed the rate of ATP-dependent Ca2+ uptake by 39%. This effect also appeared to depend on O2−· formation. Single channel recordings from RyRs incorporated into lipid bilayers revealed that NADH (2 mm) inhibited the activity of RyR channels by 84%. However, NADH inhibition of RyR activity was O2−·-independent. In summary, an increase of the cytoplasmic NADH/NAD+ ratio depresses SR Ca2+ release in ventricular cardiomyocytes. The effect appears to be mediated by direct NADH inhibition of RyR channel activity and by indirect NADH inhibition (O2−· mediated) of SR Ca2+-ATPase activity with a subsequent decrease in SR Ca2+ content.
Contraction of cardiac myocytes is activated by Ca2+ entry through L-type Ca2+ channels in the sarcolemma. During an action potential, this relatively small Ca2+ influx triggers the opening of sarcoplasmic reticulum (SR) Ca2+ release channels (ryanodine receptors, RyRs), resulting in a massive Ca2+ release from these stores. This mechanism is known as Ca2+-induced Ca2+ release (CICR; Fabiato, 1983) and generates the bulk of Ca2+ required to mediate the process of excitation–contraction (E-C) coupling. The global Ca2+ release is the result of spatial and temporal summation of many localized elementary Ca2+ release events, Ca2+ sparks (Cheng et al. 1993). Cardiac relaxation occurs upon termination of Ca2+ release, Ca2+ extrusion and by SR Ca2+-ATPase (SERCA)-mediated sequestration of cytosolic Ca2+. It has been shown that cardiac ischaemia induces alterations of action potential-induced [Ca2+]i transients, causes Ca2+ oscillations due to spontaneous SR Ca2+ release, and leads to a rise of diastolic [Ca2+]i (Lee & Allen, 1992). Ischaemia is associated with complex cellular metabolic changes including intracellular acidification, decrease in [ATP] and increase of free [Mg2+], [ADP] and inorganic phosphate ([Pi]; Allen & Orchard, 1987; Headrick & Willis, 1991; Elliott et al. 1992; Cross et al. 1995). All these agents are known to modulate both RyRs and the SR Ca2+-ATPase (Mandel et al. 1982; Xiang & Kentish, 1995; Xu et al. 1996; Balnave & Vaughan-Jones, 2000; Yang & Steele, 2000; Copello et al. 2002). Thus, these metabolic changes, which affect [Ca2+]i regulation, are likely to play a role in the generation of ischaemia-related arrhythmias (Opie & Clusin, 1990).
During ischaemia there are also important changes in the redox potential of myocytes due to changes in the relative ratios of redox couples such NADH/NAD+. There is evidence that some of the mechanisms participating in E-C coupling can be affected by redox modulation. For example, RyRs contain highly reactive sulfhydryl moieties that may play a redox-sensing function (Abramson & Salama, 1989; Pessah et al. 2002). Oxidation of these thiol-groups activates RyRs, whereas their reduction inhibits the channels (Trimm et al. 1986; Boraso & Williams, 1994; Marengo et al. 1998). Redox agents are also known to modulate the SR Ca2+-ATPase (Scherer & Deamer, 1986; Moreau et al. 1998). It has been found that during cardiac ischaemia the cytosolic NADH/NAD+ ratio increases (Park et al. 1998). As NADH mediates many cellular processes, these changes can have a broad impact on cell physiology, including the generation of reactive oxygen species (ROS). It has been shown previously that a major source of superoxide anion (O2−·) production in bovine cardiac myocytes is derived from the membrane-associated NADH oxidase, an enzyme controlled by cytosolic NADH/NAD+ levels and PO2 (Mohazzab et al. 1997). There is evidence that O2−· affects cardiac RyR function and SR Ca2+ uptake (for review see Suzuki & Ford, 1999). Nevertheless, the possible role of O2−· generated from NADH oxidase in the regulation of SR Ca2+ release in cardiac tissue remains unclear. Recently, we reported that cytosolic NADH depressed the activity of cardiac RyRs incorporated in lipid bilayer (Zima et al. 2003a). Thus, NADH levels may play an important role in modulating cardiac E-C coupling.
The goal of the present study was therefore to investigate the effects of NADH on SR Ca2+ release and uptake in rat ventricular myocytes. To this end, we studied the effects of NADH and NAD+ on spontaneous Ca2+ release from the SR (Ca2+ sparks) in permeabilized myocytes, Ca2+ uptake by SR microsomes and activity of RyR channels reconstituted into planar lipid bilayer. Our results indicate that an increase of cytosolic NADH/NAD+ levels can modulate SR Ca2+ release in cardiac myocytes through an inhibition of RyR channels and the SR Ca2+ pump.
Methods
General
Single ventricular myocytes were enzymatically isolated from adult male Sprague-Dawley rats as previously described (Zima et al. 2003b). The procedure for cell isolation was approved by the Institutional Animal Care and Use Committee of Loyola University Chicago, Stritch School of Medicine. Briefly, rats were anaesthetized by intraperitoneal injection of sodium pentobarbital (100mgkg−1). Hearts were removed quickly and flushed with nominally Ca2+-free Tyrode solution containing (mm): NaCl 140; KCl 4; MgCl2 1; glucose 10; Hepes 10; pH 7.4 (NaOH). Hearts were mounted on a Langendorff apparatus and perfused with Ca2+-free Tyrode solution for 5min at 37°C. Perfusion was then switched to Ca2+-free Tyrode solution containing 1mgml−1 collagenase (type I, Sigma) and 0.16mgml−1 protease (type XIV, Sigma) for 7min. Subsequently hearts were washed with 50mlCa2+-free Tyrode solution. The digested ventricular tissue was minced, triturated and filtered. Isolated cells were re-suspended in normal Tyrode solution (1mm CaCl2) and kept at room temperature (22–24°C) until used for experimentation. All chemicals were obtained from Sigma (St Louis, MO, USA) unless specified otherwise. Experiments were carried out at room temperature.
Confocal microscopy
Changes in [Ca2+]i and flavoprotein (FAD) autofluorescence were measured using a laser scanning confocal microscope (Radiance 2000 MP, Bio-Rad, UK) equipped with a × 40 oil-immersion objective lens (N.A. = 1.3).
Measurements of Ca2+-sparks in permeabilized cells.
The sarcolemma was permeabilized with saponin (0.005% for 30s; for details see Zima et al. 2003b). After permeabilization cells were placed in an experimental solution composed of (mm): potassium aspartate 100; KCl 15; KH2PO4 5; MgATP 5; EGTA 0.4; CaCl2 0.12; MgCl2 0.75; phosphocreatine 10; Hepes 10; fluo-3 pentapotassium salt (Molecular Probes, Eugene, OR, USA) 0.04; the solution also contained creatine phosphokinase 5Uml−1; dextran (Mr: 40 000) 8%, and was titrated to pH 7.2 (KOH). Free [Ca2+] and [Mg2+] of this solution were 100nm and 1mm, respectively (calculated using WinMAXC 2.05, Stanford University, CA, USA). During experiments, the solutions were changed by simple replacement. Fluo-3 was excited with the 488nm line of an argon ion laser and fluorescence was measured at wavelengths >515nm. Ca2+ sparks were recorded in the linescan mode (3 or 6 ms per scan; pixel size 0.1μm). Ca2+ sparks were detected and quantified in terms of amplitude (ΔF/F0) and frequency using an automated detection algorithm (Cheng et al. 1999). The algorithm detects sparks as areas of elevated fluorescence (F) intensity relative to the standard deviation (s.d.) of background noise of the fluorescence image. The detection threshold was set to 3.5 × s.d., which translated to the detection of sparks with an amplitude of ΔF/F0 ≥ 0.3. F0 is the initial fluorescence recorded under steady-state conditions at the beginning of an experiment, and ΔF = F − F0. Ca2+ spark frequencies are expressed as number of observed sparks per second and per 100μm of scanned distance (sparks s−1 (100μm)−1). No corrections were made for missed events.
Measurements of endogenous FAD fluorescence.
The method for measuring FAD autofluorescence was described in an earlier report (Zima et al. 2003b). Briefly, FAD autofluorescence was excited at 488nm and fluorescence was measured at wavelengths > 515nm. Two-dimensional images were acquired at 15s intervals. The relative changes in FAD fluorescence are presented as background-subtracted normalized fluorescence (F/F0) where F is the fluorescence intensity and F0 is basal FAD autofluorescence recorded under steady-state conditions at the beginning of an experiment. Fluorescence intensity was integrated over subcellular regions of interest (10μm × 10μm), excluding the nuclei and the edges of the cell.
Measurements of Ca2+ uptake by SR microsomes
Heavy SR membrane vesicles were isolated from rat ventricle as previously described (Zima et al. 2003b). SR Ca2+ uptake was measured with a spectrophotometer (Cory 50, Varian) using the Ca2+-sensitive dye antipyrylazo III (APIII). SR membrane vesicles (50μgml−1) were added to 1ml phosphate buffer containing (mm): KH2PO4 100; MgCl2 3; ATP 2; ruthenium red 0.01; APIII 0.2; pH 7.0. Ca2+ uptake was initiated by addition of 10μm of Ca2+ to the medium and measured as changes in absorbance of APIII between 710nm and 790nm. Ruthenium red (10μm) was used to block the Ca2+ leak from SR.
Recordings of ryanodine receptor channel currents
Planar lipid bilayers were formed from a lipid mixture containing 50% phosphatidylethanolamine, 40% phosphatidylserine and 10% phosphatidylcholine (Avanti Polar-Lipid Inc., Alabaster, AL, USA) dissolved in n-decane at a final lipid concentration of 45mgml−1. RyR channel reconstitution was started by adding 2–5μg of SR microsomes to the cis-chamber. During fusion, the cis- and trans-chambers contained solutions of the following composition (mm): CsCH3SO3 400 (cis) and 40 (trans); CaCl2 0.1; Hepes 20; pH 7.2 (CsOH). After channel incorporation the concentration of CsCH3SO3 in the trans-chamber was increased to 400mm and free [Ca2+] in the cis-chamber was adjusted to 3μm by addition of EGTA. Single channel currents were recorded using an Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA). All recordings were made at a holding potential of −20 mV. Currents were filtered at 1 kHz and digitized at 5 kHz.
Data analysis
Data are presented as means ± s.e.m. of n measurements. Statistical comparisons between groups were performed with Student's t test. Differences were considered statistically significant at P < 0.05.
Results
Effects of NADH and NAD+ on Ca2+ sparks in permeabilized myocytes
Local spontaneous events of RyR-mediated Ca2+ release (Ca2+ sparks) were studied in saponin-permeabilized ventricular myocytes. Permeabilization allows experiments to be performed under controlled cytoplasmic conditions regarding ion concentrations and energy supply (for details see Methods). Figure 1A shows representative confocal linescan images of Ca2+ sparks and plots of F/F0 from selected subcellular regions. Images and plots are shown under control conditions, at various times after addition of NADH and following washout of NADH. After NADH addition the frequency of Ca2+ sparks gradually decreased (Fig. 1A). On average, 2mm NADH decreased the frequency of Ca2+ sparks from 7.5 ± 0.4 to 3.0 ± 0.2 sparks s−1 (100μm)−1, or by 62 ± 6% from control values (n = 20; P < 0.001; measured after 8min of NADH exposure). The inhibition of Ca2+ sparks was apparent after 1min of NADH application, but required 5–8min to reach a maximum. The effect was only partially reversed upon washout of NADH. During high-frequency spark activity some events occurred as larger Ca2+ sparks (‘macrosparks’). Mini-Ca2+ waves were also observed, consistent with multiple sparks occurring in close spatial vicinity and/or temporal succession. Figure 1B presents average data of the effect of 2mm NADH on Ca2+ spark frequency and basal [Ca2+] as a function of time. Both parameters were normalized to the levels recorded in control conditions at the beginning of the experiment. Basal [Ca2+] was relatively stable during the course of the experiment, suggesting that laser illumination did not cause significant damage to the cells. Furthermore, the inhibition of spark frequency was accompanied by a decrease of Ca2+ spark amplitude (ΔF/F0) from 1.23 ± 0.08 to 0.93 ± 0.06 (P < 0.001; n = 20 cells; number of sparks analysed: control 2146; NADH 1295) or by 24% (Fig. 1C). Occasionally (11 of 20 cells; 2.3% of all sparks) long-lasting events (>100 ms) were observed as seen in Fig. 1A (second image), however, the frequency of such events was not significantly affected by NADH.
Figure 1. Effect of NADH on Ca2+ sparks in saponin-permeabilized rat ventricular myocytes.
A, top, confocal linescan images of Ca2+ sparks in control conditions, at various times after addition of NADH (2mm) and following washout of NADH. Bottom, local F/F0 profiles of Ca2+ release events. F/F0 plots were obtained by averaging fluo-3 fluorescence from 1μm wide region marked by boxes. B, average data of NADH effect on Ca2+ spark frequency (filled circles) and basal [Ca2+]i (open circles). Values were normalized to the levels recorded under control conditions (C, control). C, average Ca2+ spark amplitude in control conditions, in the presence of NADH (2mm; averaged over 2min from 6 to 8min after the beginning of NADH application) and after washout of NADH. Statistically different at *P < 0.05 and **P < 0.001.
As shown in Fig. 1B the inhibitory effect of NADH on Ca2+ spark frequency was only partially reversible upon washout of NADH. In contrast, addition of 2mm NAD+ (the oxidized form of NADH) fully restored Ca2+ spark activity (Fig. 2A). In the presence of NADH, NAD+ increased the spark frequency from 3.3 ± 0.3 to 6.1 ± 0.5 sparks s−1 (100μm)−1, a value similar to that of control conditions (6.7 ± 0.5 sparks s−1 (100μm)−1, n = 7). In a separate set of experiments the effect of NAD+ was studied alone (no NADH present). NAD+ per se did not change significantly Ca2+ spark frequency (6.4 ± 0.7 in control versus 7.0 ± 0.4 sparks s−1 (100μm)−1 with 2mm NAD+, n = 6 paired observations). Therefore, it is apparent that NAD+ neutralized the inhibitory effect of NADH rather than had an independent stimulatory effect on Ca2+ sparks. Furthermore, we also investigated the effect of different NADH/NAD+ ratios on local SR Ca2+ release. As illustrated in Fig. 2B only reducing conditions (NADH/NAD+ > 1) resulted in an inhibition of Ca2+ spark frequency, whereas oxidizing conditions (NADH/NAD+ ≤ 1) did not affect significantly these spontaneous local SR Ca2+ release events.
Figure 2. Effect of different NADH/NAD+ ratios on Ca2+ spark frequency.
A, top, confocal linescan images of Ca2+ sparks in control conditions, after addition of NADH (2mm) and after the subsequent addition of NAD+ (2mm). Bottom, local F/F0 profiles of Ca2+ release events. F/F0 plots were obtained by averaging fluo-3 fluorescence from 1μm-wide region marked by boxes. B, average data of effects of different NADH/NAD+ ratios on Ca2+ spark frequency. Statistically different at *P < 0.05 and **P < 0.001.
Role of the mitochondria in the inhibitory effect of NADH on Ca2+ sparks
It has been reported that spontaneous Ca2+ release from SR is dependent on the metabolic activity of mitochondria (Pacher et al. 2002; Isaeva & Shirokova, 2003). In cardiac cells the redox potential accumulated in the form of cytosolic NADH can be transferred into the mitochondria by two shuttle systems (malate–aspartate and α-glycerol phosphate shuttles). This, in turn, can change the metabolic status of mitochondria. The following experiments were designed to determine a possible role of mitochondria in the observed effect of NADH on Ca2+ sparks.
We first tested in permeabilized myocytes whether NADH addition to the cytosol changed the mitochondrial redox state. If NADH redox equivalents are transferred to mitochondria, they will induce the reduction of FAD to FADH2. We monitored FAD levels by measuring changes in FAD-linked autofluorescence originating from mitochondria (Hassinen & Chance, 1968). Figure 3A shows confocal images of FAD fluorescence from a permeabilized myocyte and the time course of the FAD signal after NADH addition. As shown in Fig. 3B, the application of NADH (2mm) did not produce any significant changes in FAD fluorescence (97 ± 2% of control; n = 8). After NADH removal we added 1mm CN−, which blocks the electron transfer chain at the level of cytochrome oxidase. CN− stops consumption of mitochondrial NADH, which accumulates and promotes the reduction of FAD to FADH2. As expected, FAD levels fell significantly (Fig. 3A). Figure 3B shows that FAD autofluorescence decreased 44 ± 3% (n = 8; P < 0.001). Furthermore, we also tested succinate (2mm), which is converted by complex II (succinate dehydrogenase) of the electron transport chain to fumarate thereby consuming FAD. Succinate also decreased FAD autofluorescence, by 49 ± 4% (n = 5; P < 0.001; Fig. 3B). The CN− and succinate experiments indicated that the mitochondrial electron transfer chain was functional under our experimental conditions. The lack of effect of cytosolic NADH would suggest, however, that during permeabilization cytosolic components of NADH shuttle systems were washed out, rendering mitochondria unable to oxidize cytosolic NADH.
Figure 3. Effect of NADH on flavoprotein (FAD) autofluorescence in saponin-permeabilized rat ventricular myocytes.
A, confocal images of FAD fluorescence from a permeabilized myocyte and the time course of changes in FAD signal. NADH (2mm) and CN− (1mm) were added as indicated by horizontal bars above the trace. Downward deflection in FAD fluorescence trace denotes reduction. B, summarized data of relative changes of FAD fluorescence during the application of NADH (2mm), CN− (1mm) and succinate (Suc, 2mm). Data were normalized to the FAD signal recorded at the beginning of the experiment. **Statistically different at P < 0.001 versus control.
We confirmed that the NADH effects described in Figs 1 and 2 were not related to mitochondrial modulation. For that, we studied the effect of NADH on Ca2+ sparks after inhibiting mitochondrial function with CN−. We found that in CN−-treated myocytes NADH inhibited Ca2+ sparks similarly to control (data not shown). Application of CN− alone did not affect the Ca2+ spark frequency. Spark frequency in the absence and presence of CN− (1mm) was 6.2 ± 0.4 and 6.5 ± 0.2 sparks s−1 (100μm)−1, respectively. The Ca2+ spark frequency in the presence of CN− decreased from 6.5 ± 0.2 to 3.3 ± 0.5 sparks s−1 (100μm)−1 (n = 5; P < 0.005) after subsequent addition of 2mm NADH. These experiments indicate that the observed inhibitory effects of NADH on SR Ca2+ release are mainly the consequence of its cytosolic action and do not require active mitochondrial metabolism.
Effect of NADH on SR Ca2+ load
It has been reported that Ca2+ spark frequency depends on SR Ca2+ load (Cheng et al. 1993; Lukyanenko et al. 1996; Satoh et al. 1997). Therefore, we studied the effect of NADH on the amplitude of the caffeine-induced [Ca2+]i transient, which was used as an index of SR Ca2+ load. Figure 4A shows representative confocal linescan images and F/F0 plots of Ca2+ release induced by application of 20mm caffeine. Images are shown under control conditions, in the presence of NADH and after washout of NADH. Initially, exposure to NADH appeared to increase SR Ca2+ load. After 2min of NADH (2mm) application, the amplitude of the caffeine-induced Ca2+ release increased by 8 ± 4% (n = 12; not significantly different from control). A larger increase of SR content would be consistent with the long-term inhibition of Ca2+ sparks, i.e. a decrease of spontaneous SR Ca2+ release. In contrast, exposure of cells to 2mm NADH for a longer period (∼8min) decreased the amplitude of the caffeine-induced Ca2+ release by 52 ± 8% (n = 12; P < 0.001). This effect on SR Ca2+ content was completely reversible upon washout of NADH. Figure 4B summarizes the NADH effect on the magnitude of caffeine-induced Ca2+ release. These data suggest a temporal disparity between NADH effects on Ca2+ sparks and on SR Ca2+ load. The rapid effect of NADH on Ca2+ spark frequency (Fig. 1) cannot be explained simply on the basis of a reduced SR Ca2+ load.
Figure 4. Effect of NADH on caffeine-induced Ca2+ release.
A, confocal linescan images and corresponding F/F0 profiles of Ca2+ release induced by application of 20mm caffeine under control conditions, after addition of 2mm NADH (at 2 and 8min) and following washout of NADH. F/F0 profiles were obtained by averaging the entire cellular fluorescence signal from the linescan images. B, average amplitudes of caffeine-induced Ca2+ release in control, in the presence of NADH and after washout of NADH. **Statistically different at P < 0.001.
Role of superoxide anion in the inhibitory effect of NADH on Ca2+ sparks
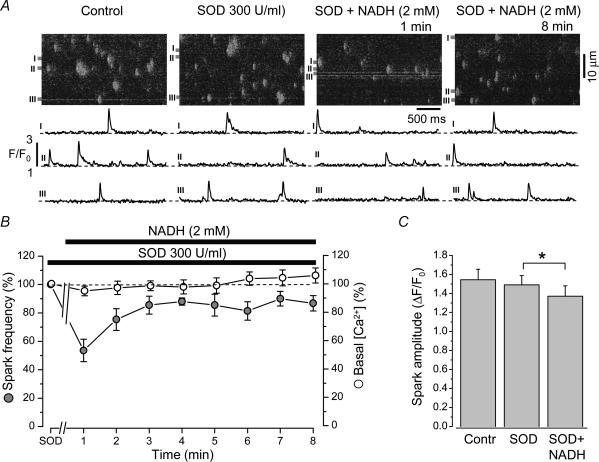
It has been shown previously (Mohazzab et al. 1997) that cardiac myocytes contain a cytosolic NADH-dependent oxidase, an enzyme capable of producing O2−·. Furthermore, O2−· can modulate SR Ca2+ release channels and the SR Ca2+-ATPase (Suzuki & Ford, 1999). Therefore, we tested whether NADH effects on Ca2+ sparks and SR Ca2+ load can be counteracted by superoxide dismutase (SOD). This enzyme, which transforms O2−· to H2O2, should decrease basal O2−· levels and impair NADH-mediated O2−· accumulation.
Figure 5A shows confocal images of Ca2+ sparks and selected plots of subcellular changes of F/F0 under control conditions, after addition of SOD and at various times after subsequent NADH addition. Pretreatment of cells with SOD (300Uml−1) for 3min did not change significantly Ca2+ spark frequency (7.5 ± 0.4 in control versus 7.9 ± 0.3 sparks s−1 (100μm)−1 in the presence of SOD; n = 7). The subsequent addition of NADH (2mm) decreased Ca2+ spark frequency by 46 ± 8% (n = 7; P < 0.001). Different from NADH alone (Fig. 1B), the inhibitory effect of NADH in the presence of SOD was maximal within the first minute. Also different from NADH alone, spark activity in NADH plus SOD displayed a partial, but incomplete, recovery. After 8min of exposure to NADH the spark frequency remained 14 ± 4% below control values (n = 7; P < 0.05). This degree of spark inhibition, however, was significantly smaller than with NADH alone (Fig. 1C; 62 ± 6%; P = 0.0001). Figure 5B shows the time course of the effect of NADH on the average Ca2+ spark frequency and basal [Ca2+] in the presence of SOD. The values were normalized to their respective levels in the presence of SOD alone. In addition, pretreatment of cells with SOD partially prevented the inhibitory effect of NADH on Ca2+ spark amplitude. The mean spark amplitude changed from 1.47 ± 0.10 to 1.37 ± 0.11 (P < 0.05; n = 7 cells; number of sparks analysed: SOD 596; SOD + NADH 515) or by 7% only (Fig. 5C). This effect was significantly less than in control condition (24%; Fig. 1C).
Figure 5. Effect of NADH on Ca2+ sparks in the presence of superoxide dismutase (SOD).
A, top, confocal linescan images showing Ca2+ sparks under control conditions, after addition of SOD (300Uml−1) and at 1 and 8min after the subsequent addition of NADH (2mm). Bottom, local F/F0 profiles of Ca2+ release events. F/F0 plots were obtained by averaging fluo-3 fluorescence from 1μm-wide region marked by boxes. B, average data of NADH effect on Ca2+ spark frequency (filled circles) and basal [Ca2+]i (open circles) in the presence of SOD. Values were normalized to the levels recorded in the presence of SOD. C, average Ca2+ spark amplitude in control conditions, in the presence of SOD and after the subsequent addition of NADH (averaged over 2min from 6 to 8min after the beginning of NADH application). *Statistically different at P < 0.05.
Figure 6A shows a typical experiment for estimating SR Ca2+ load under control conditions, after addition of SOD (300Uml−1), after subsequent addition of 2mm NADH for 8min and, finally, after washout of SOD. Pretreatment of cells with SOD did not significantly change SR Ca2+ load. In the presence of SOD, NADH (2mm) decreased the amplitude of the caffeine-induced Ca2+ release by 19 ± 3% (n = 7; P < 0.05). After removal of SOD, the inhibitory effect of NADH on SR Ca2+ load was significantly higher (56 ± 10%, n = 6; P < 0.05 versus SOD + NADH). The average data of the effects NADH on caffeine-induced Ca2+ release in the presence of SOD are summarized in Fig. 6B.
Figure 6. Effect of NADH on caffeine-induced Ca2+ release in the presence of superoxide dismutase (SOD).
A, confocal linescan images and corresponding F/F0 profiles of Ca2+ release induced by application of 20mm caffeine under control conditions, after addition of SOD (300Uml−1), after subsequent addition of 2mm NADH and after washout of SOD. F/F0 profiles were obtained by averaging the entire cellular fluorescence signal from the linescan images. B, average amplitudes of caffeine-induced Ca2+ release in control, in the presence of SOD, after subsequent addition of NADH, followed by washout of SOD (with NADH still present). *Statistically different at P < 0.05.
These results suggest that there were rapid NADH effects on spontaneous Ca2+ release from the SR that appeared to be O2−·-independent and were not associated with any depletion of the SR. We also found that NADH-dependent O2−· production may contribute to the decrease in Ca2+ spark activity by less Ca2+ uptake into the SR, and that this effect requires time to develop.
To explore the possibility of SR Ca2+ release being modulated by O2−·, we studied the effect of xanthine oxidase on Ca2+ sparks. In the presence of xanthine, this enzyme can generate high levels of O2−· (Kimura et al. 2002). Figure 7A shows confocal images of Ca2+ sparks and selected plots of subcellular changes of F/F0 under control conditions, after addition of xanthine and at various times after the subsequent addition of xanthine oxidase. Addition of xanthine (0.4mm) did not significantly change Ca2+ spark frequency (7.1 ± 0.5 in control versus 7.5 ± 0.8 sparks s−1 (100μm)−1 in the presence of xanthine; n = 8). After subsequent application of xanthine oxidase (0.2Uml−1) a gradual inhibition of Ca2+ sparks was observed. Ten minutes after application of xanthine oxidase the spark frequency was decreased by 44 ± 9% (n = 8; P < 0.005). In some cases, xanthine oxidase initially increased slightly (by 11 ± 8%; n = 8) the spark frequency (Fig. 7A, third image), but overall this effect was not statistically different from control. Figure 7B compares averaged Ca2+ spark frequency and basal [Ca2+] in xanthine plus xanthine oxidase normalized to their respective levels in control conditions (xanthine alone). Xanthine oxidase plus xanthine also significantly decreased Ca2+ spark amplitude (recorded after 8min of xanthine oxidase application) from 1.46 ± 0.05 to 1.07 ± 0.08 (P < 0.05; n = 8; number of sparks analysed: xanthine 959; xanthine + xanthine oxidase 501) or by 28% (Fig. 7C). Furthermore, exposure of cells to 0.2Uml−1 xanthine oxidase (for 10min in the presence of 0.4mm xanthine) decreased the amplitude of the caffeine-induced Ca2+ release by 32 ± 5% (n = 5; P < 0.05; data not shown), indicative of a diminished SR Ca2+ load.
Figure 7. Effect of superoxide anion generated by xanthine/xanthine oxidase reaction on Ca2+ sparks.
A, top, confocal linescan images showing Ca2+ sparks under control conditions, after addition of xanthine (0.4mm) and at various times after the subsequent addition of xanthine oxidase (0.2Uml−1). Bottom, local F/F0 profiles of Ca2+ release events. F/F0 plots were obtained by averaging fluo-3 fluorescence from 1μm-wide regions marked by boxes. B, average data of xanthine oxidase effect on Ca2+ spark frequency (filled circles) and basal [Ca2+]i (open circles) in the presence of xanthine. Values were normalized to the levels recorded in the presence of xanthine (X). C, average Ca2+ spark amplitude in control conditions, in the presence of xanthine and after the subsequent addition of xanthine oxidase. Statistically different at *P < 0.05 and **P < 0.001.
Thus, these data suggest that NADH inhibition of SR Ca2+ release is mediated by an increase in cytosolic O2−· levels. Yet, the rapid phase of spontaneous Ca2+ spark inhibition by NADH (Fig. 1) suggests that this agent also acts through a more direct and O2−· -independent mechanism (Fig. 5).
Effects of NADH and superoxide anion on single RyR channels activity
We investigated direct effects of NADH on the activity of cardiac RyR type-2 (RyR2) channels incorporated into lipid bilayer. As before (Zima et al. 2003a) we found that NADH inhibited RyR2 function. Figure 8A shows representative recordings of RyR channels under control condition (cis[Ca2+] = 3μm) and after addition of 2mm NADH to the cytosolic side of the channels. On average NADH decreased RyR channel activity by 84% (n = 10). Po of the channel decreased from 0.120 ± 0.017 to 0.019 ± 0.008 (P < 0.001; Fig. 8B). The effect of NADH was noticed seconds after its addition and was fully established in < 1min. NADH did not change significantly the mean open time of the channel (2.4 ± 0.6 ms in control versus 2.2 ± 0.5 in the presence of NADH; Fig. 8C). Pretreatment of the channels with SOD (300Uml−1) did not prevent the inhibitory effect of NADH (Fig. 8C). In the presence of SOD, Po decreased from 0.108 ± 0.021 to 0.021 ± 0.009 when of 2mm NADH was added (n = 3, P < 0.001). Thus, there are rapid and direct inhibitory effects of NADH on RyR2 channels both in the presence and absence of SOD. These results suggest that NADH inhibition of RyR channels does not require generation of O2−·.
Figure 8. Effect of NADH and superoxide anion on single ryanodine receptor (RyR) channel activity.
A, representative RyR channel currents recorded under control conditions and after addition of 2mm NADH. B, effect of NADH on Po and mean open time of single RyR channels. C, RyR channel currents recorded under control conditions, after addition of SOD (300Uml−1) and subsequent addition of 2mm NADH. D, RyR channel currents recorded in the presence of xanthine (0.4mm; X), after addition of xanthine oxidase (0.2Uml−1; XO) and subsequent addition of 2mm NADH. All recordings were made at a holding potential of −20 mV and in the presence of 3μm cis[Ca2+]. The dotted line indicates the open channel level. **Statistically different at P < 0.001.
As indicated, NADH can stimulate O2−· production in cellular systems, which may also affect RyR2 function. We then tested the effect of NADH on RyR activity in the presence of high levels of O2−·, generated by using the xanthine/xanthine oxidase protocol (see Fig. 7). Figure 8D shows that xanthine oxidase (0.2Uml−1) plus xanthine (0.4mm) increased Po of the RyR channels from 0.092 ± 0.010 to 0.213 ± 0.032 (n = 5, P < 0.05) or by 132%, suggesting that high levels of O2−· actually might activate the RyR. This is in line with our observation that xanthine/xanthine oxidase occasionally caused a transient increase in Ca2+ spark frequency (Fig. 7A). More importantly, however, in the presence of xanthine/xanthine oxidase, NADH (2mm) remained highly effective for inhibiting RyR channel activity (Fig. 8D). Po decreased to 0.034 ± 0.011 (n = 5, P < 0.001) or by 94%. This result supports the hypothesis that NADH has a direct O2−·-independent inhibitory effect on RyRs and is consistent with the rapid effect on Ca2+ sparks (Fig. 1).
NADH inhibits SR Ca2+ uptake by superoxide anion production
We tested whether NADH and O2−· affect the activity of SR Ca2+-ATPase. For that we measured Ca2+ uptake by SR microsomes isolated from rat ventricle. The net Ca2+ uptake is the sum of the SR Ca2+ influx (which reflects the activity of the SR Ca2+-ATPase) and the Ca2+ leak rate (the Ca2+ leak reflects the loss of Ca2+ from the vesicles via RyRs). The experiments were carried out in the presence of ruthenium red (10μm), which fully inhibits the cardiac type-2 RyR Ca2+ release channel. Therefore, under these conditions, the net Ca2+ uptake by the SR vesicles closely correlates with the SR Ca2+-ATPase pumping rate. Experimental data were fitted by a single exponential function from which the rate of uptake (expressed as the time constant τ of the decay of the APIII signal) was derived. Figure 9A illustrates that NADH (4mm) slowed down the Ca2+ uptake rate by 39%. On average, the time constant for the uptake changed from 78 ± 5s (n = 18) in control to 109 ± 4s (n = 12; P < 0.05) in the presence of NADH (Fig. 9B). The inhibition of Ca2+ uptake was prevented by SOD (300Uml−1) when added together with NADH. In the presence of SOD and NADH, the Ca2+ uptake time constant was 89 ± 6s (n = 5), which was not significantly different from control values. Furthermore, we studied the effect of O2−· generated by the xanthine/xanthine oxidase reaction on SR Ca2+ uptake. Xanthine (0.4mm) itself did not change the Ca2+ uptake rate (τ = 82 ± 4s; n = 26). However, pretreatment of the microsomes with xanthine together with xanthine oxidase (0.2Uml−1) decreased the rate of uptake (τ increased to 116 ± 14s or by 43%; P < 0.05; n = 10; Fig. 8B). These results show that NADH inhibits SR Ca2+ uptake and that this NADH effect may be mediated by O2−· production.
Figure 9. Effect of NADH and superoxide anion generated by xanthine/xanthine oxidase reaction on Ca2+ uptake by cardiac SR vesicles.
A, top, SR Ca2+ uptake measured in control conditions, in the presence of NADH (4mm) and in the presence of NADH + SOD (300Uml−1; preincubated for > 2min). Bottom, SR Ca2+ uptake measured in the presence of xanthine (0.4mm; X) and in the presence of xanthine/xanthine oxidase (0.2Uml−1; X + XO). Ca2+ uptake was initiated by addition of CaCl2 (10μm). Experimental data were fitted by a single exponential function from which the time constant (τ) of Ca2+ uptake was derived. B, average time constants of SR Ca2+ uptake: control, NADH, NADH + SOD, xanthine, and xanthine + xanthine oxidase. *Statistically different at P < 0.05.
Discussion
Cardiac ischaemia is associated with complex changes of cellular metabolism, intracellular acidification, depletion of energy reserves and impairment of intracellular Ca2+ regulation (e.g. Allen & Orchard, 1987) which ultimately can result in cardiac arrhythmias. Ischaemia also leads to an increase of cytosolic NADH/NAD+ reducing levels (Opie, 1991). Little is known, however, about how the cytosolic NADH/NAD+ ratio may affect CICR and E-C coupling in the heart. In this study, we investigated the possible role of NADH in modulating RyR-mediated calcium signalling in heart. We found that an increase of cytosolic NADH/NAD+ ratio caused inhibition of spontaneous SR Ca2+ release events (Ca2+ sparks) in rat ventricular myocytes. The underlying mechanisms of this NADH effect include: (i) a direct inhibition of SR Ca2+ release by decreasing the activity of RyRs, and (ii) an indirect inhibition of SR Ca2+ release by O2−· -mediated depression of SR Ca2+-ATPase activity with subsequent depletion of the SR Ca2+ levels. Cytosolic NAD+, the oxidized form of NADH with a similar chemical structure, by itself had no significant effect on Ca2+ sparks. However, the inhibition of sparks by NADH was reversed by equimolar concentrations of NAD+. Applying different NADH/NAD+ ratios revealed that only reducing conditions (NADH/NAD+ > 1) produced an inhibition of Ca2+ sparks, whereas oxidizing conditions (NADH/NAD+ ≤ 1) did not affect significantly spontaneous SR Ca2+ release. These findings suggest that, under certain metabolic conditions, cytosolic NADH redox potential can be an important factor for the modulation of SR Ca2+ release.
Ca2+ spark inhibition by cytosolic NADH does not appear to involve mitochondria
Mitochondria have been shown to play an important role for the regulation of intracellular Ca2+ homeostasis in many types of cells (for review see Duchen, 1999). Mitochondria and SR Ca2+ release sites are in close physical association. Therefore, Ca2+ released during Ca2+ sparks can be sequestered into the mitochondria via the electrogenic Ca2+ uniporter. Thus, energized mitochondria can inhibit spontaneous SR Ca2+ release, whereas the collapse of mitochondrial membrane potential stimulates Ca2+ sparks (Pacher et al. 2002; Isaeva & Shirokova, 2003). The reducing equivalent of cytosolic NADH can be transferred via NADH shuttle systems into mitochondria where NADH is oxidized by dehydrogenases of the electron transport chain. This could increase the mitochondrial membrane potential and the driving force for Ca2+ uptake, thus inhibiting Ca2+ sparks. However, the measurement of FAD autofluorescence indicated that mitochondrial redox potential did not change in the presence of NADH, suggesting that mitochondria did not oxidize cytosolic NADH in our experimental conditions. Moreover, the inhibition of mitochondrial respiration by CN− did not abolish the effect of NADH on Ca2+ sparks. This finding suggests that the inhibitory effect of cytosolic NADH on spontaneous SR Ca2+ release observed here was based on mechanisms that were independent of mitochondrial metabolism.
Does NADH inhibit SR Ca2+ release or Ca2+ uptake?
The inhibition of spontaneous SR Ca2+ release by NADH can be the result of diminished SR Ca2+ release through RyR channels, a decrease in SR Ca2+ load, or a combination of both. It has been shown that interventions that specifically inhibit RyRs (such as tetracaine or Mg2+) cause only a transient and reversible inhibition of Ca2+ release from SR (Györke et al. 1997; Overend et al. 1997; Eisner et al. 1998; Lukyanenko et al. 2001). This can be explained by an increase in SR Ca2+ content with subsequent Ca2+-dependent activation of RyRs from the luminal side of the channel. In contrast to RyR inhibitors, NADH caused a maintained decrease in Ca2+ sparks frequency. Furthermore, caffeine applications showed that SR Ca2+ load even decreased after NADH application. Thus, the inhibitory effect of NADH on Ca2+ sparks could not be explained solely based on the depression of RyR activity. On the other hand, depletion of the SR alone could not fully explain this effect of NADH either. It has been shown that thapsigargin at concentrations, which completely inhibit the SR Ca2+ pump, did not acutely affect SR Ca2+ release. Thapsigargin was found to cause a very gradual inhibition of Ca2+ sparks, which correlated well with the decrease in SR Ca2+ load (Lukyanenko et al. 2001). Here, however, NADH produced a significant decrease in Ca2+ spark frequency within the first minute (Fig. 1) and this effect was not associated with any SR depletion at this point. Thus, we suggest that the inhibition of Ca2+ sparks by NADH can be explained only by a mechanism which involves a direct inhibition of Ca2+ release through RyR channels and an indirect inhibition of Ca2+ release through subsequent depletion of the SR, possibly through inhibition of SR Ca2+ uptake.
NADH-dependent inhibition SR Ca2+ uptake may involve superoxide anion formation
A growing body of evidence documents the important role of ROS in many disorders of the cardiovascular system such as cardiac ischaemia–reperfusion injury. ROS interact with different target molecules, including ion transport systems that participate in E-C coupling like the RyR and SR Ca2+-ATPase (Suzuki & Ford, 1999).
We found that the inhibitory effect of NADH on spontaneous SR Ca2+ release was, in part, mediated by a decrease of SR Ca2+ uptake and this effect was associated with an augmentation of O2−· production. First, SOD partially attenuated the decrease of Ca2+ spark frequency and amplitude, and SR Ca2+ load by NADH. Furthermore, O2−· generated from the xanthine/xanthine oxidase reaction caused a gradual inhibition of Ca2+ spark frequency and amplitude which was associated with a decrease in SR Ca2+ load. The slow onset of Ca2+ spark inhibition is reminiscent of the effect of thapsigargin, a specific inhibitor of the SR Ca2+-ATPase (Lukyanenko et al. 2001). Indeed, direct measurement of ATP-dependent SR Ca2+ pump activity revealed that NADH inhibited Ca2+ uptake into the SR, an effect that was prevented by SOD. In addition, O2−· generated from the xanthine/xanthine oxidase reaction also caused inhibition of SR Ca2+ uptake.
The mechanisms through which ROS inhibit SR Ca2+-ATPase are not clear. Current hypotheses suggest that oxygen free radicals can directly modify enzyme activities by oxidation of specific amino acids and catalytic sites. For example, it has been shown that oxidation of sulfhydryl groups depressed the cardiac SR Ca2+ pump (Scherer & Deamer, 1986). In addition, ROS may inhibit the activity of membrane-bound enzymes by peroxidation of membrane phospholipids (Kaneko et al. 1989). Nonetheless, our data clearly indicate that NADH-dependent ROS formation contributes to SR Ca2+ pump inhibition with subsequent reduction of SR Ca2+ load and Ca2+ spark activity.
Mechanisms of RyR modulation by NADH
The transient inhibition of Ca2+ sparks by NADH in the presence of SOD suggested additional direct and O2−· -independent effects of NADH on spontaneous Ca2+ release from the SR. Indeed, we found direct inhibitory effects of NADH on RyR channels incorporated into lipid bilayers. In the presence of SOD, NADH decreased the Po of channels by the same degree as in control condition. Thus, this inhibitory effect did not appear to depend on O2−· production. Contrarily, in our hands O2−· per se had a stimulatory effect on RyRs. In single cell experiments O2−· generated from the xanthine/xanthine oxidase reaction initially increased Ca2+ spark frequency (Fig. 7A, third image) and stimulated RyR channel activity in bilayer experiments. This finding is consistent with previous observations (Kawakami & Okabe, 1998) which suggested that O2−· stimulates cardiac RyRs. Thus, according to our data, an augmentation of cytosolic NADH may have a dual effect on RyR channel activity, i.e. a direct inhibition and O2−· -mediated stimulation. It seems, however, that the direct inhibitory effect outweighs the stimulation of RyR by O2−·
Our previous study has shown that NADH acts on cardiac RyRs only from the cytosolic side of the channel and does not interact with the adenine nucleotide binding site (Zima et al. 2003a). The most likely mechanism involves a redox modulation of the RyR, since the NADH effect was reversed by NAD+. It is well established that chemical reducing agents (e.g. dithiothreitol or cysteine) depress channel function (Trimm et al. 1986; Marengo et al. 1998; Boraso & Williams, 1994). At this moment, however, we do not know whether NADH directly interacts with cardiac RyRs or with some ancillary SR proteins that are incorporated into the bilayers together with the RyR channels. Skeletal muscle RyR type-1, for example, contains an oxidoreductase-like domain that could function as a redox sensor (Baker et al. 2002); however, whether such a domains also exists in the heart and with what other binding site on the cardiac RyR NADH interacts remain to be determined.
Physiological and pathophysiological relevance
Myocytes contain ∼2–3mm of NADH + NAD+ in their cytoplasm. Under physiological aerobic conditions, the cytosolic NADH/NAD+ ratio is <0.1 (Park et al. 1998). This results from low glycolytic flux and the ability of mitochondria to oxidize cytosolic NADH. Our data suggest that such levels of cytosolic NADH/NAD+ would not significantly affect SR Ca2+ release (Fig. 2). During severe ischaemia, however, blood flow is poor and levels of O2 fall. Consequently, accumulation of intracellular lactate occurs. High levels of lactate will force the lactate dehydrogenase (LDH) reaction toward pyruvate production. This can cause a rise of cytosolic NADH/NAD+ ratio up to 30-fold in the ischaemic heart (Park et al. 1998). Our results suggest that a high cytosolic NADH level would cause a negative inotropic effect in the ischaemic heart by effectively inhibiting RyR-mediated SR Ca2+ release. The inhibition of RyRs by high NADH could lead to SR Ca2+ overload, an effect that may contribute to arrhythmias as they occur during ischaemia (Opie & Clusin, 1990) by acting in synchrony with changes of levels of other cytosolic factors (ATP, Mg2+ and H+, e.g. Allen & Orchard, 1987). The indirect effect of NADH on Ca2+ homeostasis, which involves O2−· production, is likely to be less significant during severe ischaemia, because it requires certain levels of PO2 (Mohazzab et al. 1997). However, it seems likely that O2−· plays a crucial role in the dramatic changes of Ca2+ homeostasis and E-C coupling that occur immediately after restoration of blood flow and O2 supply (reperfusion). As cytosolic NADH/NAD+ levels would be still high, the increased availability of O2 now stimulates O2−· production. During reperfusion, the inhibition of RyR channels will decline as a result of a gradual decrease in NADH/NAD+ levels and the increase in O2−· production. These changes would be favourable to pro-arrhythmogenic spontaneous Ca2+ release which can occur in the form of enhanced Ca2+ spark activity, Ca2+ waves and Ca2+ alternans (Kockskämper & Blatter, 2002).
In conclusion, our data show that NADH is an important modulator of RyR function and SR Ca2+ release in the heart. Significant changes of NADH/NAD+ levels which occur during ischaemia and reperfusion injury are likely to play a crucial role in the impairment of CICR and E-C coupling observed under these pathological conditions.
Acknowledgments
We would like to thank Dr D. M. Bers and his staff for the cell preparations. This work was supported by the NIH (R01HL62231 to L.A.B) and the American Heart Association (0130142 N to J.A.C).
References
- Abramson JJ, Salama G. Critical sulfhydryls regulate calcium release from sarcoplasmic reticulum. J Bioenerg Biomembr. 1989;21:283–294. doi: 10.1007/BF00812073. [DOI] [PubMed] [Google Scholar]
- Allen DG, Orchard CH. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987;60:153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Baker ML, Serysheva II, Sencer S, Wu Y, Ludtke SJ, Jiang W, Hamilton SL, Chiu W. The skeletal muscle Ca2+ release channel has an oxidoreductase-like domain. Proc Natl Acad Sci U S A. 2002;99:12155–12160. doi: 10.1073/pnas.182058899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balnave CD, Vaughan-Jones RD. Effect of intracellular pH on spontaneous Ca2+ sparks in rat ventricular myocytes. J Physiol. 2000;528:25–37. doi: 10.1111/j.1469-7793.2000.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraso A, Williams AJ. Modification of the gating of the cardiac sarcoplasmic reticulum Ca2+-release channel by H2O2 and dithiothreitol. Am J Physiol. 1994;267:H1010–H1016. doi: 10.1152/ajpheart.1994.267.3.H1010. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Rios E, Stern MD. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J. 1999;76:606–617. doi: 10.1016/S0006-3495(99)77229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copello JA, Barg S, Sonnleitner A, Porta M, Diaz-Sylvester P, Fill M, Schindler H, Fleischer S. Differential activation by Ca2+, ATP and caffeine of cardiac and skeletal muscle ryanodine receptors after block by Mg2+ J Membr Biol. 2002;187:51–64. doi: 10.1007/s00232-001-0150-x. [DOI] [PubMed] [Google Scholar]
- Cross HR, Clarke K, Opie LH, Radda GK. Is lactate-induced myocardial ischaemic injury mediated by decreased pH or increased intracellular lactate? J Mol Cell Cardiol. 1995;27:1369–1381. doi: 10.1006/jmcc.1995.0130. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner DA, Trafford AW, Diaz ME, Overend CL, O'Neill SC. The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovasc Res. 1998;38:589–604. doi: 10.1016/s0008-6363(98)00062-5. [DOI] [PubMed] [Google Scholar]
- Elliott AC, Smith GL, Eisner DA, Allen DG. Metabolic changes during ischaemia and their role in contractile failure in isolated ferret hearts. J Physiol. 1992;454:467–490. doi: 10.1113/jphysiol.1992.sp019274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Györke S, Lukyanenko V, Györke I. Dual effects of tetracaine on spontaneous calcium release in rat ventricular myocytes. J Physiol. 1997;500:297–309. doi: 10.1113/jphysiol.1997.sp022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassinen I, Chance B. Oxidation–reduction properties of the mitochondrial flavoprotein chain. Biochem Biophys Res Commun. 1968;31:895–900. doi: 10.1016/0006-291x(68)90536-6. [DOI] [PubMed] [Google Scholar]
- Headrick JP, Willis RJ. Cytosolic free magnesium in stimulated, hypoxic, and underperfused rat heart. J Mol Cell Cardiol. 1991;23:991–999. doi: 10.1016/0022-2828(91)91635-5. [DOI] [PubMed] [Google Scholar]
- Isaeva EV, Shirokova N. Metabolic regulation of Ca2+ release in permeabilized mammalian skeletal muscle fibres. J Physiol. 2003;547:453–462. doi: 10.1113/jphysiol.2002.036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Beamish RE, Dhalla NS. Depression of heart sarcolemmal Ca2+-pump activity by oxygen free radicals. Am J Physiol. 1989;256:H368–H374. doi: 10.1152/ajpheart.1989.256.2.H368. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Okabe E. Superoxide anion radical-triggered Ca2+ release from cardiac sarcoplasmic reticulum through ryanodine receptor Ca2+ channel. Mol Pharmacol. 1998;53:497–503. doi: 10.1124/mol.53.3.497. [DOI] [PubMed] [Google Scholar]
- Kimura C, Cheng W, Hisadome K, Wang YP, Koyama T, Karashima Y, Oike M, Ito Y. Superoxide anion impairs contractility in cultured aortic smooth muscle cells. Am J Physiol. 2002;283:H382–H390. doi: 10.1152/ajpheart.00574.2001. [DOI] [PubMed] [Google Scholar]
- Kockskämper J, Blatter LA. Subcellular Ca2+ alternans represents a novel mechanism for the generation of arrhythmogenic Ca2+ waves in cat atrial myocytes. J Physiol. 2002;545:65–79. doi: 10.1113/jphysiol.2002.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Allen DG. Changes in intracellular free calcium concentration during long exposures to simulated ischemia in isolated mammalian ventricular muscle. Circ Res. 1992;71:58–69. doi: 10.1161/01.res.71.1.58. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Györke I, Györke S. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflügers Arch. 1996;432:1047–1054. doi: 10.1007/s004240050233. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Viatchenko-Karpinski S, Smirnov A, Wiesner TF, Györke S. Dynamic regulation of sarcoplasmic reticulum Ca2+ content and release by luminal Ca2+-sensitive leak in rat ventricular myocytes. Biophys J. 2001;81:785–798. doi: 10.1016/S0006-3495(01)75741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel F, Kranias EG, Grassi de Gende A, Sumida M, Schwartz A. The effect of pH on the transient-state kinetics of Ca2+Mg2+ATPase of cardiac sarcoplasmic reticulum. A comparison with skeletal sarcoplasmic reticulum. Circ Res. 1982;50:310–317. doi: 10.1161/01.res.50.2.310. [DOI] [PubMed] [Google Scholar]
- Marengo JJ, Hidalgo C, Bull R. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys J. 1998;74:1263–1277. doi: 10.1016/S0006-3495(98)77840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohazzab HKM, Kaminski PM, Wolin MS. Lactate and pO2 modulate superoxide anion production in bovine cardiac myocytes: potential role of NADH oxidase. Circulation. 1997;96:614–620. doi: 10.1161/01.cir.96.2.614. [DOI] [PubMed] [Google Scholar]
- Moreau VH, Castilho RF, Ferreira ST, Carvalho-Alves PC. Oxidative damage to sarcoplasmic reticulum Ca2+-ATPase at submicromolar iron concentrations: evidence for metal-catalyzed oxidation. Free Radic Biol Med. 1998;25:554–560. doi: 10.1016/s0891-5849(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Opie LH. The Heart: Physiology and Metabolism. New York: Raven Press; 1991. pp. 425–450. [Google Scholar]
- Opie LH, Clusin WT. Cellular mechanism for ischemic ventricular arrhythmias. Annu Rev Med. 1990;41:231–238. doi: 10.1146/annurev.me.41.020190.001311. [DOI] [PubMed] [Google Scholar]
- Overend CL, Eisner DA, O'Neill SC. The effect of tetracaine on spontaneous Ca2+ release and sarcoplasmic reticulum calcium content in rat ventricular myocytes. J Physiol. 1997;502:471–579. doi: 10.1111/j.1469-7793.1997.471bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Thomas AP, Hajnoczky G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci U S A. 2002;99:2380–2385. doi: 10.1073/pnas.032423699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Chun YS, Kim MS, Park YC, Kwak SJ, Park SC. Metabolic modulation of cellular redox potential can improve cardiac recovery from ischemia-reperfusion injury. Int J Cardiol. 1998;65:139–147. doi: 10.1016/s0167-5273(98)00117-x. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Kim KH, Feng W. Redox sensing properties of the ryanodine receptor complex. Front Biosci. 2002;7:a72–a79. doi: 10.2741/A741. [DOI] [PubMed] [Google Scholar]
- Satoh H, Blatter LA, Bers DM. Effects of [Ca2+]i, SR Ca2+ load, and rest on Ca2+ spark frequency in ventricular myocytes. Am J Physiol. 1997;272:H657–H668. doi: 10.1152/ajpheart.1997.272.2.H657. [DOI] [PubMed] [Google Scholar]
- Scherer NM, Deamer DW. Oxidative stress impairs the function of sarcoplasmic reticulum by oxidation of sulfhydryl groups in the Ca2+-ATPase. Arch Biochem Biophys. 1986;246:589–601. doi: 10.1016/0003-9861(86)90314-0. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Ford GD. Redox regulation of signal transduction in cardiac and smooth muscle. J Mol Cell Cardiol. 1999;31:345–353. doi: 10.1006/jmcc.1998.0872. [DOI] [PubMed] [Google Scholar]
- Trimm JL, Salama G, Abramson JJ. Sulfhydryl oxidation induces rapid calcium release from sarcoplasmic reticulum vesicles. J Biol Chem. 1986;261:16092–16098. [PubMed] [Google Scholar]
- Xiang JZ, Kentish JC. Effects of inorganic phosphate and ADP on calcium handling by the sarcoplasmic reticulum in rat skinned cardiac muscles. Cardiovasc Res. 1995;29:391–400. [PubMed] [Google Scholar]
- Xu L, Mann G, Meissner G. Regulation of cardiac Ca2+ release channel (ryanodine receptor) by Ca2+, H+, Mg2+, and adenine nucleotides under normal and simulated ischemic conditions. Circ Res. 1996;79:1100–1109. doi: 10.1161/01.res.79.6.1100. [DOI] [PubMed] [Google Scholar]
- Yang Z, Steele DS. Effects of cytosolic ATP on spontaneous and triggered Ca2+-induced Ca2+ release in permeabilised rat ventricular myocytes. J Physiol. 2000;523:29–44. doi: 10.1111/j.1469-7793.2000.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima AV, Copello JA, Blatter LA. Differential modulation of cardiac and skeletal muscle ryanodine receptors by NADH. FEBS Lett. 2003a;547:32–36. doi: 10.1016/s0014-5793(03)00664-1. [DOI] [PubMed] [Google Scholar]
- Zima AV, Kockskämper J, Mejia-Alvarez R, Blatter LA. Pyruvate modulates cardiac sarcoplasmic reticulum Ca2+ release in rats via mitochondria-dependent and -independent mechanisms. J Physiol. 2003b;550:765–783. doi: 10.1113/jphysiol.2003.040345. [DOI] [PMC free article] [PubMed] [Google Scholar]