Abstract
Recently characterized selective agonists and developed antagonists for the corticotropin releasing factor (CRF) receptors are new tools to investigate stress-related functional changes. The influence of mammalian CRF and related peptides injected intracerebroventricularly (i.c.v.) on gastric and colonic motility, and the CRF receptor subtypes involved and their role in colonic response to stress were studied in conscious mice. The CRF1/CRF2 agonists rat urocortin 1 (rUcn 1) and rat/human CRF (r/h CRF), the preferential CRF1 agonist ovine CRF (oCRF), and the CRF2 agonist mouse (m) Ucn 2, injected i.c.v. inhibited gastric emptying and stimulated distal colonic motor function (bead transit and defecation) while oCRF9–33OH (devoid of CRF receptor affinity) showed neither effects. mUcn 2 injected peripherally had no colonic effect. The selective CRF2 antagonist astressin2-B (i.c.v.), at a 20 : 1 antagonist: agonist ratio, blocked i.c.v. r/hCRF and rUcn 1 induced inhibition of gastric transit and reduced that of mUcn 2, while the CRF1 antagonist NBI-35965 had no effect. By contrast, the colonic motor stimulation induced by i.c.v. r/hCRF and rUcn 1 and 1h restraint stress were antagonized only by NBI-35965 while stimulation induced by mUcn 2 was equally blocked by both antagonists. None of the CRF antagonists injected i.c.v. alone influenced gut transit. These data establish in mice that brain CRF1 receptors mediate the stimulation of colonic transit induced by central CRF, urocortins (1 and 2) and restraint stress, while CRF2 receptors mediate the inhibitory actions of these peptides on gastric transit.
Genes encoding a series of peptides related to the corticotropin releasing factor (CRF) family, known as urocortin 1 (Ucn 1), urocortin 2 (Ucn 2 or stresscopin-related peptide) and urocortin 3 (Ucn 3 or stresscopin), have been recently cloned (Vaughan et al. 1995; Lewis et al. 2001; Hauger et al. 2003). Rat Ucn 1 is a 40-amino acid (aa) peptide that shares 45% homology with the 41-aa peptide, rat/human (r/h) CRF (Vaughan et al. 1995). Mouse (m) Ucn 2 and mUcn 3 are 38-amino acid peptides with 34% and 26% sequence homology with r/hCRF and 42% and 18% identity with rUcn 1, respectively (Dautzenberg & Hauger, 2002; Zorrilla et al. 2003). These endogenous CRF ligands display distinct affinities for the seven-transmembrane domain, G protein-coupled CRF receptor subtypes 1 and 2 (CRF1 and CRF2) (Perrin & Vale, 1999; Lewis et al. 2001; Reyes et al. 2001). In vitro binding studies established that r/hCRF and, to a greater extent, oCRF both exhibit preferential affinity to CRF1 receptors while Ucn 1 displays equal high affinity for both CRF receptor subtypes (Perrin & Vale, 1999). Human/mouse Ucn 2 has a binding affinity equal to Ucn 1 at the CRF2 but very low potency at CRF1 receptors (Lewis et al. 2001; Reyes et al. 2001). Ucn 3 exhibits the highest degree of selectivity in binding to CRF2 receptors, but is less potent than mUcn 2 in activating adenylate cyclase in cells expressing endogenous CRF2(b) receptors (Lewis et al. 2001). Recently, antagonists selective for CRF1 or CRF2 receptors have also became available (Ruhmann et al. 1998; Higelin et al. 2001; Rivier et al. 2002; Zorrilla et al. 2003). These selective CRF agonists and antagonists are powerful tools for investigating the CRF receptor subtype mediating the physiological responses to exogenous and endogenous CRF and CRF-related peptides.
In rats, centrally administered CRF inhibits gastric emptying and contractility while simultaneously increasing colonic motility, transit and defecation, mimicking the gastrointestinal motor alterations observed in response to various stressors (Williams et al. 1987; Lenz et al. 1988; Mönnikes et al. 1992; Martinez et al. 1997; Tachéet al. 2001). Recent pharmacological studies in rats suggest that there is CRF receptor subtype selectivity in the central actions of exogenously administered CRF or Ucn 1 on gastrointestinal motor function. In particular, intracisternal injection of Ucn 1-induced inhibition of gastric motility and emptying is prevented by a selective CRF2 receptor antagonist (Chen et al. 2002). By contrast, the intracerebroventricular (i.c.v.) injection of CRF-induced stimulation of colonic motor function is blocked by i.c.v. injection of CRF1 receptor antagonists (Martinez et al. 1998; Martinez & Taché, 2001). The central actions of recently discovered members of the CRF family Ucn 2 and Ucn 3 on gastrointestinal function are still unknown.
Pharmacological blockade with non-selective CRF1/CRF2 receptor antagonists or selective CRF1 antagonists injected centrally suggest a physiological role for brain CRF receptor signalling in stress-related alterations of gastrointestinal motor function (Martinez & Taché, 2001; Tachéet al. 2001, 2002). However, these findings have been largely derived from studies in rats. Some reports indicate species-specific differential patterns in the response to i.c.v. CRF. In particular, the peptide was found to lower grooming activity and oxygen consumption in mice while opposite effects were observed in rats (Momose et al. 1999). The characterization of the central actions of CRF and novel CRF-related peptides on gut motor function in mice also provides a basis for the use of genetically modified mice. Mice deficient in CRF ligands and receptors have proved to be valuable to gain insight into the CRF signalling pathways involved in the endocrine and behavioural responses to stress (Smith et al. 1998; Timpl et al. 1998; Bale et al. 2002).
In the present study, we first investigated the differential effects of r/hCRF, oCRF, rUcn 1, mUcn 2 and mUcn 3 injected i.c.v. on gastric emptying and propulsive colonic motility in conscious mice. Two separate measures of colonic motility were used: fecal pellet output and distal colonic transit time. The latter was coupled to the measurement of gastric emptying of a solid nutrient meal in the same animals (Martinez et al. 2002). We compared the colonic motor response to mUcn 2 injected intraperitoneally and i.c.v. to ascertain the central action of the peptide. The brain CRF receptor subtypes mediating the effects of CRF and urocortins on gastric and colonic motor function were also characterized using i.c.v. injection of the CRF1/CRF2 receptor antagonist astressin (Gulyas et al. 1995), the newly developed selective water-soluble CRF1 receptor antagonist NBI-35965 (Hoare et al. 2003; Million et al. 2003) and the selective CRF2 receptor antagonist astressin2-B (Rivier et al. 2002). Lastly, the role of brain CRF receptors in restraint stress-induced fecal pellet output was assessed in mice.
Methods
Animals
Adult male C57BL/6 mice (6–8 weeks of age; Harlan, San Diego, CA, USA) were maintained on a 12h: 12h light–dark cycle with controlled temperature (21–23°C) and humidity (30–35%). Animals were group-housed in direct bedding cages with free access to food (Prolab RMH 2500) and tap water. Depending on the experimental protocols, mice were deprived of food for 18–20h in single housing conditions, with free access to water (in simultaneous measurement of gastric emptying and distal colonic transit time) or maintained with food and water ad libitum up to the beginning of the experiments (in measurement of fecal pellet output). All protocols were conducted under the Veterans Affairs Animal Component of the Research Protocol number 99-092-05; reviewed and approved by the Animal Care Research Committee of the Veterans Affairs (VA) (VA Greater Los Angeles Health Care System).
Compounds and treatments
Compounds
R/hCRF, oCRF, oCRF9–33OH, rUcn 1, mUcn 2, mUcn 3, astressin and astressin2, -B, [d-Phe11,His12,CαMeLeu13,39,Nle17,Glu31,Lys34]Ac-sauvagine(8–40) (Clayton Foundation Laboratories for Peptide Biology, Salk Institute, La Jolla, CA, USA) were synthesized using the solid-phase approach, purified using high pressure liquid chromatography and fully characterized using capillary zone electrophoresis, high pressure liquid chromatography and mass spectrometry, as previously described (Gulyas et al. 1995; Lewis et al. 2001; Reyes et al. 2001; Rivier et al. 2002). The non-peptide CRF1 antagonist NBI-35965 was supplied by Neurocrine Biosciences (San Diego, CA, USA). Immediately before use, compounds were weighed and dissolved in sterile saline, except astressin, astressin2-B and NBI-35965, which were dissolved in double-distilled water (∼pH 7.6). Either sterile saline or double-distilled water, as appropriate, served as vehicle controls. The total volume injected i.c.v. was 5.0μl per animal, either as a single 5.0μl injection or two consecutive injections of 2.5μl each. All doses of compounds are expressed in μg (mice)−1. The in vitro receptor selectivity of the different CRF receptor agonists and antagonists used in this study is indicated in Table 1.
Table 1.
Inhibitory binding constant for CRF, CRF-related peptides and CRF receptor antagonists used in this study
| Ki (nm)a | |||||
|---|---|---|---|---|---|
| CRF1 | CRF2(a) | CRF2(b) | References | ||
| CRF (rat/human) | 2 | 44 | 30.7 | Behan et al. (1995); Perrin et al. (1999) | |
| CRF (ovine) | 1 | 184 | 162.4 | Behan et al. (1995) | |
| Ucn 1 (rat) | 1.3 | 1.5 | 0.97 | Perrin et al. (1999) | |
| Ucn 2 (mice) | >100 | 2.1 | 0.66 | Lewis et al. (2001) | |
| Ucn 3 (mice) | >100 | 5.0 | 1.8 | Lewis et al. (2001) | |
| Astressin | 2.0 | 1.5 | 1.0 | Perrin et al. (1999) | |
| Astressin2-B | >500 (IC50) | 1.3 (IC50)b | — | Rivier et al. (2002) | |
| NBI-35965 | 1.4 | >1000 | — | Hoare et al. (2003) | |
See original references for experimental conditions.
No differentiation between a and b variants.
Intracerebroventricular injections
The method used was similar to that previously described by Pelleymounte et al. (2000) with minor modifications. Mice were acutely anaesthetized with enflurane (Ethrane, Anaquest, Madison, WI, USA), the head was carefully hand-restrained on a gauze and the injection site localized by visualizing an equilateral triangle between the eyes and the back of the head, with the apex of the triangle being the injection site. The injection was performed manually using a 10μl Hamilton syringe fitted with a 30-gauge needle. At the injection site, the skull was gently pressure-penetrated at the least resistance point after carefully searching with the tip of the needle. The needle was shortened by adding a ‘sleeve’ made from peristaltic pump tubing so that the actual needle length was 4–4.5 mm. The procedure lasted in all 1.5–2min and the mice regained consciousness 1–2min later and were monitored in their home cages. If any behavioural alteration was observed that could be attributed to inadequacy of the injection procedure (rotating behaviour or incoordination after recovery from anaesthesia), the animals were excluded from the experiment and killed by cervical dislocation. In total, three animals were excluded because of behavioural changes after the i.c.v. injection. At the end of the experiments, mice were killed by cervical dislocation followed by thoracotomy. Cresyl violet dye was injected i.c.v. in 50 mice to ascertain that the injections had been successful. The success rate was 96% based on the visualization of the dye.
Restraint stress
Psychological stress was induced by maintaining mice for 60min in a plastic tube (falcon type; 2.7 cm diameter, 7 cm long) with perforated holes for adequate ventilation. The dimensions of the tube effectively restrained the mice, preventing them from turning around and moving forward or back.
Gastric and distal colonic motor function measurements
Defecation score
The number of fecal pellets excreted was determined at 15min intervals for 60min after treatment and cumulative pellet output calculated at 60min.
Gastric and distal colonic transit
Gastric emptying of a solid nutrient meal and distal colonic transit were simultaneously monitored in conscious mice following a method recently described (Martinez et al. 2002). Briefly, fasted mice had free access to water and preweighed standard chow for a 1h period, and were then briefly anaesthetized with enflurane (1–2min; Ethrane-Anaquest) in order to insert a single 2 mm glass bead into the distal colon, to a distance of 2 cm from the anus. Bead insertion was accomplished using a glass rod with a fire-polished end to avoid tissue damage. After bead insertion, the mice were placed individually in their home cages without food and water. Mice regained consciousness within 1–2min of removal of anaesthetic and thereafter showed normal behaviour. Distal colonic transit was determined to the nearest 0.1min by monitoring the time required for the expulsion of the glass bead (bead latency). The percentage of gastric emptying of the ingested meal was assessed 2h after the end of food exposure. Mice were killed by cervical dislocation followed by thoracotomy. The abdominal cavity was opened, the pylorus and cardia clamped and the stomach removed. The stomach was weighed, opened and the gastric content was washed out with tap water. The gastric wall was wiped dry and weighed. The amount of food (g) contained in the stomach was calculated as the difference between the total weight of the stomach with content and the weight of the stomach wall after the content was removed. The solid food ingested by each animal before any treatment was determined by the difference between the food weight before and 1h after the feeding period. The percentage of gastric emptying for the 2h period was calculated according to the equation:
It is worth noting that the gastric content of the stomach includes both food and any secretion associated with digestion, and that any treatment that increases gastric secretion may impact on the estimation of the food that is left in the stomach. However, in our studies, this is unlikely to be a confounding factor in the assessment of gastric emptying since i.c.v. injection of CRF and related peptides inhibit gastric acid secretion (Taché et al. 1983; Improta & Broccardo, 1988).
Experimental protocols
In each daily experiment, vehicle control and several peptide doses, with or without CRF antagonists, were included and repeated on multiple days. The doses of CRF agonists and the ratio CRF antagonists: CRF agonists were selected based on our previous data in rats and mice (Martinez et al. 1997; Martinez & Taché, 2001) and adjusted according to the results of preliminary data. To avoid circadian variations, all experiments were performed during the morning, finishing no later than 2.00 p.m.
Effects of i.c.v. r/hCRF and CRF-related peptides on fecal pellet output
Mice fed ad libitum were injected i.c.v. under brief enflurane anaesthesia with either r/hCRF (0.01, 0.1, 0.5 or 1.0μg), oCRF (0.01, 0.1 or 0.5μg), rUcn 1 (0.01, 0.1 or 0.5μg), mUcn 2 (0.01, 0.1 or 0.5μg), mUcn 3 (0.1, 0.5 or 1.0μg), oCRF9–33OH (0.5μg) or vehicle (sterile saline solution, 5μl). In a separate experiment, either r/hCRF (0.5μg), mUcn 2 (0.5μg) or vehicle (sterile saline) was administered intraperitoneally (i.p., 0.1ml) in conscious mice. After i.c.v. or i.p. peptide or vehicle injection, pellet output was monitored for a 60min period. The CRF peptide agonists used share significant structural homology (Lewis et al. 2001) and the doses administered represent molar concentrations from 2.1 to 120pmol (mouse)−1 with no more than a 14% variation in pmol between peptides for a given dose.
Effects of i.c.v. CRF receptor antagonists on i.c.v. r/hCRF-, rUcn 1- or mUcn 2-induced changes in fecal pellet output
Fed mice under brief enflurane anaesthesia were injected i.c.v. with either astressin (10μg), NBI-35965 (1.5, 50 or 100μg), astressin2-B (10μg) or vehicle (distilled water, 2.5μl). Immediately thereafter, r/hCRF (0.5μg), rUcn 1 (0.5μg), mUcn 2 (0.5μg) or vehicle (saline, 2.5μl) was administered and the mice were returned to their home cages. Pellet output was monitored for the 60min period thereafter.
Effects of i.c.v. r/hCRF and CRF-related peptides on gastric and distal colonic transit
Before any treatment, fasted mice were re-fed for 1h and then, under brief enflurane anaesthesia, they were injected i.c.v. with r/hCRF (0.01, 0.03, 0.1 or 0.5μg), oCRF (0.1 or 0.5μg), rUcn 1 (0.5μg), mUcn 2 (0.01, 0.03, 0.1 or 0.5μg), mUcn 3 (0.5μg) or vehicle (saline, 5μl). Immediately thereafter, a glass bead was inserted into the distal colon. Animals were returned to their home cages, without food or water, and the bead expulsion time was monitored. Gastric emptying of the nutrient solid meal was determined 2h after peptide or saline administration.
Effects of i.c.v. CRF receptor antagonists on i.c.v. r/hCRF- and CRF-related peptides-induced alterations of gastric emptying and distal colonic transit
Before any treatment, fasted mice were re-fed for 1 h; thereafter, under brief enflurane anaesthesia, NBI-35965 (50μg), astressin2-B (10μg) or vehicle (distilled water, 2.5μl) was injected i.c.v. immediately before the i.c.v. injection of r/hCRF (0.5μg), rUcn 1 (0.5μg), mUcn 2 (0.5μg) or vehicle (saline, 2.5μl). A glass bead was then inserted into the distal colon. Animals were returned to their home cages, without food or water, and the bead expulsion time and gastric emptying of the solid meal were determined as described above.
Effects of i.c.v. CRF receptor antagonists on psychological stress-induced defecation
Fed mice were injected i.c.v. with either astressin (10μg), NBI-35965 (50 or 100μg), astressin2-B (10μg) or vehicle (distilled water, 5μl). After regaining consciousness, mice were either restrained in a tube for 1h or left undisturbed in their home cages. Pellet output was determined at 15min intervals for the following hour.
Statistical analysis
Results are expressed as mean ± s.e.m. ED50 values were calculated using non-linear regression. Comparisons within multiple groups were performed using one-way ANOVA followed by a Student–Newman–Keuls multiple comparison test. Comparisons between two groups were performed using a Student's t test. P values<0.05 were considered statistically significant.
Results
Effects of i.c.v. r/hCRF and CRF-related peptides on pellet output
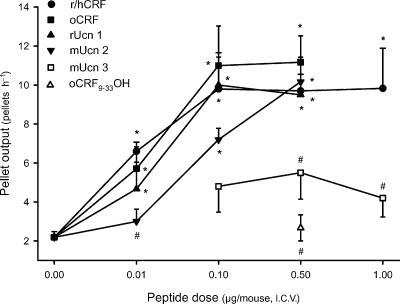
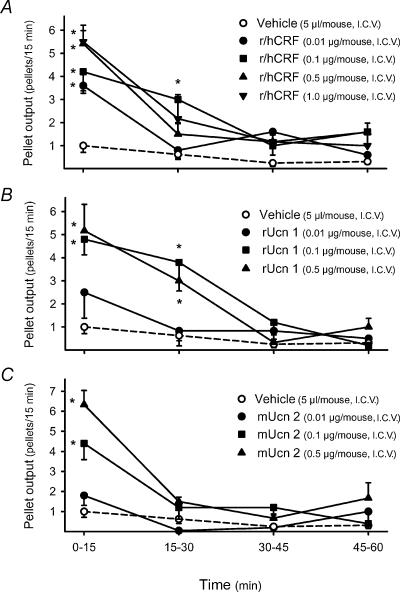
In i.c.v. vehicle-treated mice, pellet output was low over the 1h experimental period (2.2±0.3 pellets h−1, n=16). The i.c.v. injection of r/hCRF and oCRF at 0.01μg increased significantly fecal pellet output to 6.6±0.5 pellets h−1 (n=5) and 5.7±1.1 pellets h−1 (n=7), respectively. Higher i.c.v. doses of r/hCRF (0.1, 0.5 and 1.0μg) and oCRF (0.1 and 0.5μg), resulted in a sustained increase in pellet output (r/h CRF: 9.8±1.9, 9.7±0.9 and 9.8±2.0 pellets h−1, respectively; oCRF: 11.0±2.0 and 11.2±1.3 pellets h−1, respectively, n=5–12 per group; all P<0.05 versus vehicle; Fig. 1). A dose-related peak response occurred during the first 15min after i.c.v. injection of r/h CRF, with values of 3.6±0.2, 4.2±0.9, 5.2±0.6 and 5.5.±0.7 pellets (15min)−1 for doses of 0.01, 0.1, 0.5 and 1.0μg, respectively [P<0.05 compared with 1.1±0.5 pellets (15min)−1 in the i.c.v. vehicle group] (Fig. 2A). Thereafter, the defecation score returned toward basal levels, although the response to the submaximal dose of 0.1μg remained significantly elevated for 30min (Fig. 2A). Similar time courses were obtained with oCRF at 0.01, 0.1 and 0.5μg (data not shown).
Figure 1. Dose-related effects of i.c.v. r/hCRF and CRF-related peptides on fecal pellet output in conscious mice.
Under short-duration enflurane anaesthesia, mice fed ad libitum were injected i.c.v. with vehicle (saline solution, 5μl), r/hCRF (0.01, 0.1, 0.5 or 1.0μg), oCRF (0.01, 0.1 or 0.5μg), rat urocortin 1 (rUcn 1: 0.01, 0.1 or 0.5μg), mouse urocortin 2 (mUcn 2: 0.01, 0.1 or 0.5μg), mouse urocortin 3 (mUcn 3: 0.1, 0.5 or 1.0μg) or oCRF9–33OH (0.5μg). Each point represents the mean ±s.e. of cumulative number of pellets for 1h after i.c.v. injection (n= 5–12mice/group). *P<0.05 versus vehicle-treated group (ANOVA); #P<0.05 versus r/hCRF at the same dose.
Figure 2. Time course of i.c.v. r/hCRF- (A), rUcn 1- (B) and mUcn 2- (C) induced fecal pellet output in mice.
Under enflurane anaesthesia, mice fed ad libitum were injected i.c.v. with vehicle (saline solution, 5μl), r/hCRF (0.01, 0.1, 0.5 or 1.0μg), rat urocortin 1 (rUcn 1: 0.01, 0.1 or 0.5μg) or mouse urocortin 2 (mUcn 2: 0.01, 0.1 or 0.5μg). Each point represents the mean ±s.e. of number of pallets monitored at each 15min interval for 60min (n= 5–12mice/group). *P<0.05 versus vehicle-treated group (ANOVA).
Rat Ucn 1 injected i.c.v. (0.01, 0.1 and 0.5μg) induced a dose-related stimulation of pellet output similar to that induced by r/hCRF, with a significant increase observed at 0.01μg (4.7±1.3 pellets h−1) and a maximal response at 0.1 and 0.5μg (10.0±1.4 pellets h−1,n=5, and 9.5±1.9 pellets h−1, n=6, respectively; P<0.05 versus vehicle; Fig. 1). Time course data revealed that the peak increase in the number of fecal pellets was also reached during the first 15min after peptide i.c.v. injection at all doses while the duration of the colonic response was dose related (15min at 0.01μg and 30min at the highest doses, Fig. 2B).
Mouse Ucn 2 (0.1 and 0.5μg, i.c.v.) stimulated pellet output per hour in a dose-dependent manner, with values of 7.2±0.6 pellets h−1 (n=6) and 10.2±1.3 pellets h−1 (n=5), respectively, while the lowest dose (0.01μg) had no effect (3.0±0.6 pellets h−1, n=5; Fig. 1). The action of the peptide was short lasting, with a dose-related peak response at 15min; thereafter values returned to basal levels (Fig. 2C). Estimated ED50 values indicated that r/hCRF, oCRF and rUcn 1 had a higher potency than mUcn 2 for stimulating pellet output (Table 2). Mouse Ucn 3 (0.1, 0.5, 1.0μg, i.c.v.) and the fragment, oCRF9–33OH (0.5μg, i.c.v.) had no significant effect on pellet output compared with vehicle-treated animals (Fig. 1). Based on these results, in further studies, oCRF, r/hCRF, rUcn 1 and mUcn 2 were injected i.c.v. at doses of 0.5μg (107, 105, 106 and 120pmol, respectively), which induce similar maximal responses after i.c.v. injection.
Table 2.
ED50 (μg (mouse)−1) of CRF ligands to alter gastric and colonic transit after i.c.v. injection in micea
| Peptides | Pellet output (number h−1) | Distal colonic transit (bead latency time) | Gastric emptying (% in 2h) |
|---|---|---|---|
| r/hCRF | 0.0096±0.0037 (2.0)b | 0.078±0.034 (16.0) | 0.010±0.002 (2.1) |
| oCRF | 0.011±0.0036 (2.4) | — | — |
| rUcn 1 | 0.011±0.0015 (2.3) | — | — |
| mUcn 2 | 0.087±0.012 (20.9) | 0.105±0.045 (25.2) | 0.009±0.002 (2.2) |
ED50 was determined by non-linear regression and is expressed as mean±95% confidence interval.
Values in parentheses represent the ED50 values expressed inpmol (mouse)−1.
Rat/human CRF (0.5μg) injected intraperitoneally significantly increased pellet output to 8.2±1.5 pellets h−1 (n=5) compared with 4.4±0.7 pellets h−1 (n=8) in the i.p. vehicle-treated group (P<0.05; F2,15=4.999, P=0.022). By contrast, mUcn 2 (0.5μg, i.p.) did not modify pellet output (3.2±0.9 pellets h−1, n=5; P>0.05 versus vehicle; P<0.05 versusi.p. r/hCRF).
Effects of i.c.v. CRF receptor antagonists on defecation stimulated by i.c.v. r/hCRF, rUcn 1 and mUcn 2
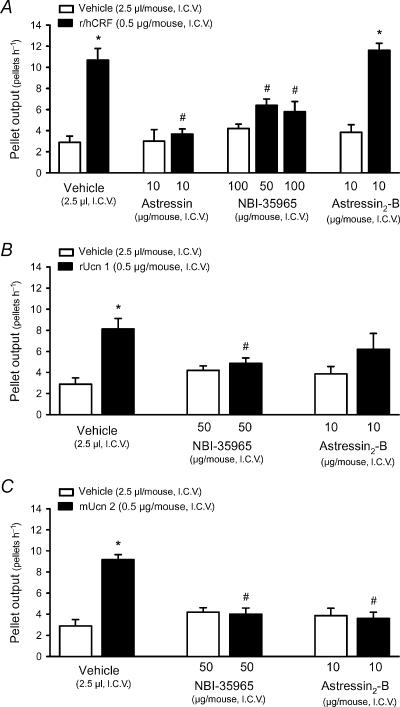
None of the CRF receptor antagonists injected i.c.v. had any significant effect by themselves on pellet output compared with vehicle-treated animals (Fig. 3).
Figure 3. Effects of i.c.v. CRF receptor antagonists on i.c.v. r/hCRF- (A), rUcn 1- (B) and mUcn 2- (C) induced stimulation of fecal pellet output in conscious mice.
Under enflurane anaesthesia, mice fed ad libitum were injected i.c.v. with vehicle (distilled water, 2.5μl), the non-selective CRF1/CRF2 antagonist astressin (10μg), the selective CRF1 antagonist NBI-35965 (50 or 100μg), or the selective CRF2 antagonist astressin2-B (10μg). Immediately thereafter vehicle (saline solution, 2.5μl), r/hCRF (0.5μg), rat urocortin 1 (rUcn 1, 0.5μg) or mouse urocortin 2 (mUcn 2, 0.5μg) was administered i.c.v. Each point represents the mean ±s.e. of cumulative number of pellets for 1h after i.c.v. injection (n= 4–6mice/group). *P<0.05 versus vehicle+vehicle- or antagonist+vehicle-treated groups; #P<0.05 versus vehicle+respective peptide-treated groups (ANOVA).
In vehicle-pretreated mice, i.c.v. r/hCRF (0.5μg) increased pellet output to 10.7±1.1 pellets h−1 (n=6; P<0.05 versus vehicle-treated animals: 2.9±0.6 pellets h−1, n=8; F3,21=20.744, P<0.001; Fig. 3). The colonic motor response to i.c.v. r/hCRF was prevented by pretreatment with the non-selective CRF1/CRF2 antagonist astressin injected i.c.v. at 10μg (3.7±0.5 pellets h−1, n=6) and the selective CRF1 antagonist NBI-35965 injected i.c.v. at 50 or 100μg kg−1 (6.4±0.6 and 5.8±0.9 pellets h−1, respectively, n=5 for each dose; P< 0.05 versus vehicle+r/hCRF; F5,32=15.099, P<0.001; Fig. 3A). At the lowest dose (1.5μg, i.c.v.), NBI-35965 had no effect (9.3±1.3 pellets h−1, n=4). Pretreatment with the selective CRF2 antagonist astressin2-B (10μg) did not modify the stimulatory effects of r/hCRF on pellet output (11.6±0.7 pellets h−1, n=5; P>0.05 versus vehicle+r/hCRF; F3,22=32.227, P<0.001, Fig. 3A).
The colonic response to rUcn 1 (0.5μg, i.c.v.) was also blocked by i.c.v. NBI-35965 at 50μg (4.9±0.5 pellets h−1, n=7; P<0.05 versus vehicle+rUcn 1: 8.1±1.0 pellets h−1, n=8; F4.32=10.892, P<0.001; Fig. 3B), but not at 1.5μg (7.8±0.2 pellets h−1, n=4). Pretreatment with astressin2-B, reduced the effects of rUcn 1 on pellet output by 23% and values (6.2±1.5 pellets h−1, n=5) were no longer significantly different from those in vehicle- or astressin2-B+vehicle-treated animals (Fig. 3B). The stimulatory effect of mUcn 2 on pellet output was equally blocked by NBI-35965 (4.0±0.6 pellets h−1, n=6; P<0.05 versus vehicle+mUcn 2: 9.2±0.5 pellets h−1, n=6; F3.26=24.629, P<0.001; Fig. 3) and astressin2-B (3.6±0.6 pellets h−1, n=5; F3.22=20.692, P<0.001; Fig. 3C).
Differential actions of i.c.v. r/hCRF, rUcn 1 and mUcn 2 on gastric emptying and distal colonic transit
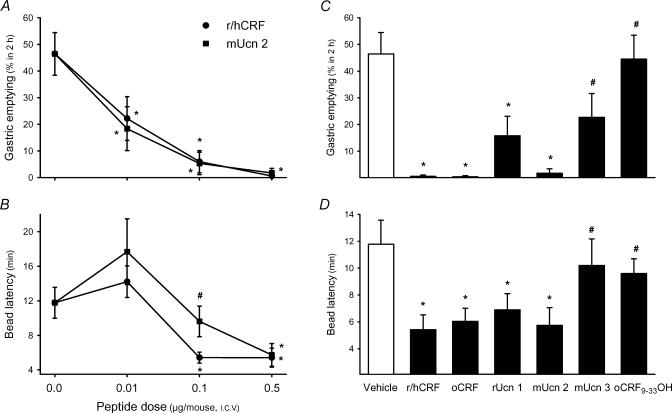
In mice fasted for 18–20 h, the amount of food ingested for the 1h feeding period before treatments was 0.55±0.05 g and not different between groups. The percentage of ingested food cleared from the stomach after 2h was 46.5±8.0% and the time for colonic bead expulsion was 11.8±1.8min in groups injected with vehicle at the end of the feeding period (n=14, Fig. 4). r/hCRF at 0.01, 0.1 or 0.5μg i.c.v. (n=5–11 for each dose) induced a dose-related suppression of gastric emptying to 22.2±8.2%, 6.0±4.2% and 0.6±0.6%, respectively (P<0.05 versus vehicle at all doses; Fig. 4A). Simultaneously, r/hCRF at 0.1 and 0.5μg i.c.v. stimulated distal colonic transit as shown by the decrease in the time latency for bead expulsion to 5.4±0.6min, and 5.4±1.1min, respectively (both P<0.05 versus vehicle), while the lowest dose (0.01μg) had no effect (14.2± 1.8min; Fig. 4B). Ovine CRF affected both gastric emptying and colonic propulsion with similar potency to r/hCRF. A maximal reduction in percentage of gastric emptying was induced by oCRF at 0.1 and 0.5μg (3.6±3.6%, n=8; and 0.4±0.4%n=5, respectively, both P<0.05 versus vehicle; Fig. 4C and data not shown) while distal colonic transit times were significantly reduced to 7.5±1.1min and 6.4±1.0min, respectively (both P<0.05 versus vehicle; Fig. 4D and data not shown).
Figure 4. Effects of i.c.v. injection of r/hCRF and CRF-related peptides on gastric emptying of a solid nutrient meal (A, C) and distal colonic transit time (B, D) monitored simultaneously in conscious mice.
Groups of fasted mice were given chow ad libitum for 1 h, then under short-duration enflurane anaesthesia were injected i.c.v. with either saline (5μl), r/hCRF or mouse urocortin 2 (mUcn 2, 0.01–0.5μg), oCRF, rat urocortin 1 (rUcn 1), mouse urocortin 3 (mUcn 3) or oCRF9-33 0H (0.5μg) and a glass bead was inserted into the distal colon 2 cm proximal from the anus. Gastric emptying of the ingested meal 2h after peptide administration (A, C) and the time for bead expulsion (B, D) were monitored in the same animal. *P<0.05 versus vehicle-treated group (ANOVA); #P<0.05 versus r/hCRF at the same dose.
Rat Ucn 1, tested at the maximal effective dose for r/hCRF (0.5μg, i.c.v.), shortened the bead latency time to a similar extent as r/hCRF (6.9±1.2min, n=6; P<0.05 versus vehicle, Fig. 4D). However, it was slightly less effective than r/hCRF in inhibiting gastric emptying (15.8±7.3%, n=6; P<0.05 versus vehicle; P=0.064 versus r/hCRF; Fig. 4C). i.c.v. injections of mUcn 2 at 0.01, 0.1, and 0.5μg dose dependently reduced gastric emptying of a solid meal to 18.4±8.2%, 5.3±4.2% and 1.7±1.7%, respectively (n=6–8; all P<0.05 versus vehicle; F3.28=11.815, P<0.001; Fig. 4A). By contrast, the bead latency time was significantly shortened to 5.7±1.3min by i.c.v. injection of mUcn 2 at 0.5μg only but not at lower doses (17.7±3.8 and 9.6±1.8min at 0.01 and 0.1μg, respectively). The estimated ED50 for r/hCRF and mUcn 2 indicated that the two peptides inhibited gastric emptying with similar potencies, while r/hCRF was more potent than mUcn 2 at stimulating distal colonic transit (Table 2). Mouse Ucn 3 (0.5μg, i.c.v.) showed a trend towards a reduction of gastric emptying (22.8±9.0%, P=0.076 versus vehicle; n=5) without any effect on the bead latency time (10.2±2.0min; P=0.207 versus vehicle; Fig. 4C and D). The CRF fragment, oCRF9–33OH (0.5μg, i.c.v.) had no effect either on gastric emptying (44.5±9.0%, n=5) or bead latency (9.6 ± 1.1min; Fig. 4C and D).
Effects of i.c.v. CRF receptor antagonists on i.c.v. r/hCRF-, rUcn 1- and mUcn 2-induced changes in gastric emptying and distal colonic transit
In vehicle treated-mice (n=9), gastric emptying (50.3±6.0%) and bead latency time (10.5±1.1min) was similar to that observed in previous experiments. The i.c.v. injection of either NBI-35965 (50μg, n=5), or astressin2-B (10μg, n=5), did not modify postprandial gastric emptying or distal colonic transit time. Therefore, for the sake of clarity and to reduce the number of animals used, vehicle (water)+vehicle (saline) and antagonist (NBI-35965 or astressin2-B)+vehicle (saline) groups were pooled in a common control group (n=19) with a gastric emptying value of 41.2±5.0% and a bead expulsion time of 11.8±1.2min (Fig. 5). In i.c.v. water-pretreated mice, r/hCRF (0.5μg, i.c.v., n=5) inhibited gastric emptying of the solid meal (2.9±2.9%; P<0.05 versus control; F3,29=9.995, P<0.001; Fig. 5) and shortened the bead latency to 6.4±1.9min (P<0.05 versus control, F3,29=3.937, P=0.018). Pretreatment with NBI-35965 (50μg, i.c.v.) prevented i.c.v. r/hCRF-induced acceleration of distal colonic transit (10.9±0.9min, n=5) without affecting the inhibitory effects on gastric emptying (2.6±2.6%; Fig. 5). However, astressin2-B (10μg, i.c.v.) partially prevented the inhibitory effect of i.c.v. r/hCRF on gastric emptying (26.2±5.5%, n=4; P>0.05 versus control) while the concomitant reduction in the distal colonic transit time was not influenced (7.0±0.4min; Fig. 5).
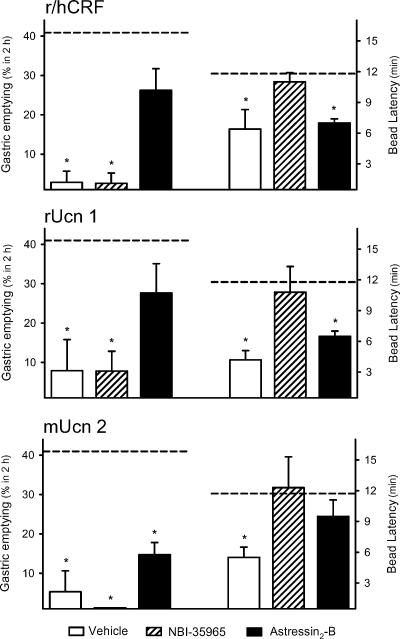
Figure 5. Effects of i.c.v. CRF receptor antagonists on i.c.v. r/hCRF-, rUcn 1- and mUcn 2-induced inhibition of gastric emptying and stimulation of distal colonic transit in conscious mice.
Groups of fasted mice were given chow ad libitum for 1 h, then under short-duration enflurane anaesthesia were injected i.c.v. with distilled water (2.5μl), the selective CRF1 antagonist NBI-35965 (50μg) or the selective CRF2 antagonist astressin2-B (10μg). Immediately thereafter, saline (2.5μl), r/hCRF, rat urocortin 1 (rUcn 1) or mouse urocortin 2 (mUcn 2, 0.5μg) was injected i.c.v. and a glass bead was inserted into the distal colon 2 cm proximal from the anus. Gastric emptying of the ingested meal 2h after peptide administration (left axis) and the time for bead expulsion (right axis) were monitored in the same animal. The dashed lines represent the mean gastric emptying rate (41.2±5.0%) and bead latency time (11.8±1.2min) in animals treated with vehicles or antagonists+vehicle. *P<0.05 versus vehicle-treated group (ANOVA).
Similar results were obtained when gastric emptying and distal colonic transit were altered by i.c.v. rUcn 1 (0.5μg). In water-pretreated mice, rUcn 1 inhibited gastric emptying to 7.9±7.9% (n=5; P<0.05 versus control group; F3.32=6.707, P=0.001) and reduced the colonic bead latency time to 4.2±0.9min (P<0.05 versus control group; F3.32=4.601, P=0.009; Fig. 5). Pretreatment with NBI-35965 (50μg) significantly prevented the effects of i.c.v. rUcn 1 on colonic propulsion (10.8±2.5min, n=6) without affecting the reduced gastric emptying (7.8±5.0%; Fig. 5). On the other hand, astressin2-B (10μg) partially prevented the inhibitory effect of i.c.v. rUcn 1 on gastric emptying (27.6±7.5%, n=6; P>0.05 versus control) while the concomitant reduction in the distal colonic transit time was not influenced (6.5±0.5min; Fig. 5).
Mouse Ucn 2 (0.5μg, i.c.v.) induced a concomitant inhibition of gastric emptying (5.3±5.3%, n=6; P<0.05 versus control group; F3,32=12.065, P<0.001) and a reduction in bead latency time in water-pretreated mice (5.5±1.0min; P<0.05 versus control group; F3,32=3.008, P=0.046; Fig. 5). Pretreatment with NBI-35965 (50μg) blocked the stimulatory effect of i.c.v. mUcn 2 on distal colonic propulsion (12.3±3.5min, n=5) without affecting the gastric inhibitory effect (0.0±0.0%; Fig. 5). Astressin2-B (10μg, i.c.v.) showed only a trend towards preventing the effect of i.c.v. mUcn 2 on gastric emptying (14.7±3.1%, n=6) while antagonizing the reduction in bead latency time to a value (9.4±1.6min not significantly different from that of the vehicle group; Fig. 5).
Effects of i.c.v. CRF receptor antagonists on restraint stress-induced defecation
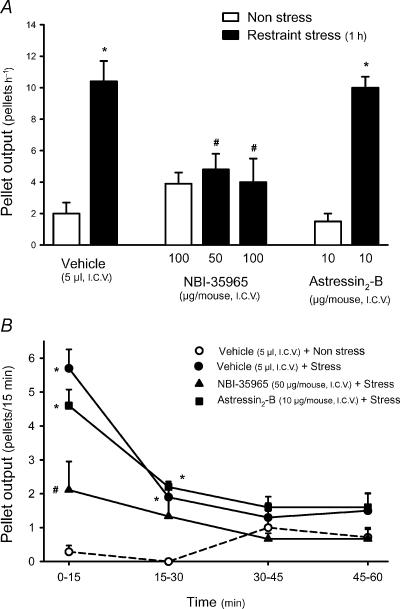
In mice maintained in non-stressful conditions, pellet output was low (2.0±0.7 pellets h−1, n=7). Restraint stress for 1h increased defecation to 10.4±1.3 pellets h−1 (n=10, P<0.05; Fig. 6A). The peak defecatory response occurred during the first 15min of stress (5.7±0.6 pellets h−1; P<0.05 versus non-stress: 0.3±0.2 pellets h−1), thereafter values decreased, although at 30min, values were still significantly elevated (Fig. 6B). NBI-35965 at 50 or 100μg reduced stress-induced defecation to 4.8±1.0 and 4.0±1.5 pellets h−1, respectively (n=9 and 5; both P<0.05 versus vehicle+stress; F4,33=10.025, P<0.001) while astressin2-B (10μg, i.c.v.), did not modify the colonic motor response to restraint stress (10.0±0.7 pellets h−1, n=5; Fig. 6). None of the CRF receptor antagonists tested by themselves (NBI-35965, n=7; astressin2-B, n=4), had a significant effect on pellet output in non-stressed mice.
Figure 6. Effects of i.c.v. CRF receptor antagonists on restraint stress-induced defecation in mice.
Groups of mice fed ad libitum were injected i.c.v., under short-duration enflurane anaesthesia, with distilled water (5μl), the non-selective CRF1/CRF2 antagonist astressin (10μg), the selective CRF1 antagonist NBI-35965 (50 or 100μg) or the selective CRF2 antagonist astressin2-B (10μg). Thereafter mice were subjected to a 1h session of stress (restraint in a cylinder) or left undisturbed in their home cages (non-stress). Pellet output was monitored at 15min intervals for the following 60min. A, cumulative pellet output for the 1h experimental time. B, time course changes in fecal pellet output at 15min intervals. *P<0.05 versus non-stress; #P<0.05 versus vehicle+stress group (ANOVA).
Discussion
In the present study, we showed that the i.c.v. injection of r/hCRF and oCRF (0.1–0.5μg), dose-dependently inhibited gastric emptying of a solid nutrient meal while stimulating distal colonic transit and defecation in conscious mice. Likewise, one previous study showed that r/hCRF injected i.c.v. into mice acts centrally to inhibit gastric emptying of a non-nutrient liquid solution (Sheldon et al. 1990). The alterations in gut transit induced by i.c.v. r/hCRF in mice are CRF receptor mediated. The potent CRF1/CRF2 receptor antagonist astressin (Miranda et al. 1997) injected i.c.v. completely prevented the increase in fecal pellet output induced in mice by i.c.v. r/hCRF at an antagonist: agonist ratio of 20: 1, similar to results previously reported in rats (Martinez et al. 1997; Martinez & Taché, 2001). In mice, i.c.v. r/hCRF-induced delayed gastric emptying of a liquid non-nutrient meal has been reported to be blocked by the CRF1/CRF2 antagonist α-helical CRF9-41 injected i.c.v. but not i.p. (Sheldon et al. 1990). The specificity of the effects observed is also strengthened by the demonstration that the CRF analog oCRF9–33OH, which has structural homology with CRF and no affinity for either CRF1 or CRF2 receptors (Behan et al. 1995), affected neither gastric nor distal colonic transit when injected i.c.v. at a higher molecular concentration (approximately 170pmol) than that maximally effective for r/hCRF (105pmol). Taken together, these observations corroborate in mice data from rats showing that CRF acts centrally to induce a CRF receptor-mediated simultaneous antipropulsive effects on the proximal (stomach) and propulsive effects on the distal (colon) segments of the gastrointestinal tract (Williams et al. 1987; Lenz et al. 1988; Mönnikes et al. 1992; Martinez et al. 1997; Taché et al. 2001).
The present data provide the first evidence that urocortins also act centrally to inhibit gastric motor function. The i.c.v. injection of rUcn 1 delays gastric emptying of an ingested solid meal in mice similarly as reported in rats after intracisternal injection (Chen et al. 2002). In addition, we found that i.c.v. injection of mUcn 2 at low doses of 0.01 and 0.1μg (2 and 20pmol) induced a potent dose-related suppression of postprandial gastric emptying, by 60% and 88%, respectively, 2h after the injection. However, mUcn 3 injected i.c.v. at a 60-fold higher concentration (120pmol) than mUcn 2 results only in a non-significant 51% reduction in gastric emptying. The lower potency of Ucn 3 in activating signal transduction mechanisms at CRF2 receptors may explain such a difference. In a CRF2(b)-expressing cell line (A7r5 rat aortic smooth muscle cells), mUcn 3 is 20-fold less potent than mUcn 2 at inducing cAMP accumulation (EC50 values of 0.18 nm for Ucn 2 and 3.7 nm for mUcn 3) (Lewis et al. 2001).
The inhibition of gastric emptying induced by i.c.v. CRF and urocortins is mediated through the activation of CRF2 receptors. The selective CRF1 antagonist NBI-35965 injected i.c.v. did not block the delayed gastric emptying induced by r/hCRF, rUcn 1 or mUcn 2. NBI-35965 was active under these conditions, since in the same animal, it prevented the stimulatory action of the peptides on colonic motor function. By contrast, the CRF2 receptor antagonist astressin2-B (Rivier et al. 2002) effectively blocked i.c.v. r/hCRF- or rUcn 1-induced inhibition of gastric emptying of a solid meal at an antagonist: agonist ratio of 20 : 1 in mice. However, the same antagonist : agonist ratio was only partially effective in reversing mUcn 2-induced inhibition of gastric emptying, probably reflecting the high affinity of the peptide for CRF2(b) receptors compared with r/hCRF (Reyes et al. 2001). We have previously shown in rats that the inhibition of gastric emptying induced by intracisternal injection of r/hCRF and the non-mammalian CRF-related peptide sauvagine was blocked at antagonist : agonist ratios varying from 3 : 1 for CRF to 16 : 1 for sauvagine (Martinez et al. 1998). In rats, astressin2-B blocked intracisternal rUcn 1-induced inhibition of gastric emptying at a 10: 1 antagonist: agonist ratio (Chen et al. 2002). It is apparent that i.c.v. injection of r/hCRF, mUcn 1 and mUcn 2 exert a potent inhibition of gastric motor function through brain CRF2-dependent signalling pathways in rodents.
This contrasts with the role of CRF1 receptors in mediating the effects of CRF on colonic motor function. Both r/hCRF and oCRF, which has a preferential affinity for the CRF1 receptor (Dieterich et al. 1997), injected i.c.v. shortened the distal colonic transit time and increased fecal pellet output in mice, as previously observed in rats (Lenz et al. 1988; Martinez et al. 1997; Martinez & Taché, 2001). The dose (0.1μg), at which i.c.v. r/hCRF induced a maximal stimulation of distal colonic motor function is similar to that inducing maximal anxiogenic behaviour in the ‘elevated plus maze’ in mice (Momose et al. 1999). Urocortin 1, which has high affinity for both CRF1 and CRF2 receptors (Vaughan et al. 1995), injected i.c.v. displays a similar potency to r/hCRF in inducing defecation and accelerating distal colonic transit. The central action of rUcn 1 on the gut has previously been assessed only on gastric motor function in rats (Kihara et al. 2001; Chen et al. 2002). Moreover, the highly selective CRF2 agonist Ucn 3 did not alter distal colonic transit and defecation when injected i.c.v. at a dose 100-fold higher than that required for r/hCRF to stimulate fecal output. Likewise, mUcn 2 injected at doses (0.01 and 0.1μg), which inhibited gastric emptying by 60% and 89%, did not significantly influence distal colonic transit monitored simultaneously. Moreover, astressin2-B, injected i.c.v. at a dose antagonizing peptide-induced inhibition of gastric emptying, did not alter r/hCRF- or rUcn 1-induced colonic motor stimulation, monitored simultaneously. Lastly, the selective CRF1 antagonist NBI-35965 (Hoare et al. 2003), injected i.c.v., blocked the distal colonic motor response to r/hCRF and Ucn 1. In rats, another selective CRF1 antagonist, NBI-27914 (Hoare et al. 2003), injected i.c.v., also prevented i.c.v. r/h CRF-induced defecation (Martinez & Taché, 2001). Collectively, these data support the involvement of brain CRF1 receptors in the stimulation of colonic propulsive motor function induced by i.c.v. CRF and Ucn 1 in rodents.
These observations may have physiological relevance during stress. The i.c.v. injection of the CRF1 selective antagonist NBI-35965 completely blocked defecation in response to a 1h exposure to restraint in mice. In contrast, astressin2-B did not alter the colonic response to restraint stress when injected i.c.v. at a dose that completely prevented the action of Ucn 2 on defecation. These results are complemented by recent observations showing that CRF1 knockout female mice produced fewer fecal pellets in the open-field test than the wild-type controls (Bale et al. 2002). In rats, i.c.v. injection of NBI-27914 reduced water avoidance stress-induced defecation (Martinez & Taché, 2001), supporting the prevailing view that CRF1 receptors participate in the colonic motor response to acute stress (Tachéet al. 2002). Previous studies established that the activation of brain CRF1 receptors contributes to the anxiogenic response to stress in rodents and humans (Steckler & Holsboer, 1999). Taken together, these findings are consistent with the hypothesis that activation of brain CRF1 signalling pathways may be part of the underlying mechanisms linking anxiogenic behaviour and defecation. This also provides biochemical support for the use of the defecation score as one parameter of emotionality in mice (Hall, 1934; Flint et al. 1995). The present data strengthens the pivotal role of CRF1 receptors in integrating the physiological endocrine, behavioural, autonomic, and visceral responses to stress (Turnbull & Rivier, 1997; Steckler & Holsboer, 1999; Tachéet al. 2002). However, none of the CRF receptor antagonists, injected i.c.v. at doses preventing exogenous CRF actions, influenced gastric emptying or distal colonic propulsion on their own, indicating that central CRF signalling pathways do not modulate postprandial gastric transit and basal colonic motor activity in non-stressed mice, as previously shown in rats (Tachéet al. 2001).
Interestingly, the selective CRF2 receptor agonist, mUcn 2, injected i.c.v. at 5- to 10-fold higher doses than those effective for r/hCRF, mimicked the CRF1-mediated colonic response in mice. The action of mUcn 2 is centrally mediated since an i.c.v. dose of 0.5μg causes a similar colonic motor response to i.c.v. r/hCRF, but was ineffective when injected i.p. In addition, mUcn 2 injected i.p. in mice at doses ranging from 6 to 50μg kg−1 (approximately 0.1–1.2μg per animal) did not modify distal colonic transit monitored by bead expulsion time (Martinez et al. 2002). A recent report showed that Ucn 2 injected i.c.v. at similar doses to those used in the present study produced a dose-dependent increase in anxiety-like behaviour in the ‘plus maze’ test in mice (Pelleymounter et al. 2002). Other recent work also supports a role for central CRF2 receptors in the anxiogenic behaviour in mice (Takahashi, 2001, 2002) that may have a bearing on the stress-related colonic response induced by i.c.v. injection of Ucn 2 in our studies. Ucn 2, which displays a low potency for stimulation of cAMP in cells expressing endogenous CRF1 receptors (Lewis et al. 2001), may have produced a CRF1-like colonic response through interaction with CRF1 receptors at the highest dose used. However, this explanation is doubtful because both astressin2-B and NBI-35965 injected i.c.v. completely blocked the stimulatory action of mUcn 2 on the colonic motility, while the gastric effects were only partially antagonized by astressin2-B. These observations suggest cross-talk between CRF1- and CRF2-dependent mechanisms at a central level. It is possible that neuronal pathways primarily activated via CRF2 receptors could lead to the activation of CRF1-dependent pathways. In this case, the same biological effects could be elicited by the independent activation of either one of the CRF receptor subtypes and, similarly, they could be blocked independently with either CRF1 or CRF2 selective antagonists. An explanation of this hypothesis implies that the activation of central CRF2 receptors leads to the release of endogenous CRF, which in turn will activate CRF1 receptors. So far the biochemical coding of CRF2 expressing neurones is still to be characterized. In addition, the possibility that mUcn 2 might act through a CRF receptor subtype that is yet to be described and is sensitive to the antagonists currently available cannot be discarded. The simultaneous participation of both CRF1 and CRF2 receptors in mediating the wide array of neuroendocrine and behavioural responses to stress has also been recently suggested (Takahashi, 2001; Bakshi et al. 2002; Reul & Holsboer, 2002).
In summary, we have shown that r/hCRF injected i.c.v. dose-dependently inhibited gastric emptying of a solid nutrient meal while stimulating distal colonic propulsion and defecation through CRF receptor activation in conscious mice. Similar effects were induced by i.c.v. injection of oCRF and rUcn 1. The use of the selective CRF1 and CRF2 receptor antagonists NBI-35965 and astressin2-B, respectively, shows that colonic effects are mediated through CRF1 receptors while the inhibition of gastric emptying depends on the activation of CRF2 receptors. The role of central CRF1 receptors in the activation of colonic motor function was established in a model of restraint stress in mice. The newly identified selective ligand for the CRF2 receptor, mUcn 2, injected i.c.v. potently inhibited gastric emptying and is 10-fold less potent than r/hCRF or rUcn 1 at stimulating defecation. The latter effect could not be demonstrated when mUcn 2 was injected peripherally, showing that mUcn 2 acts in the brain. While the gastric effects of i.c.v. mUcn 2 were blocked selectively by astressin2-B, the colonic responses were antagonized completely by either NBI-35965 or astressin2-B. These observations suggest cross-talk between central CRF2- and CRF1-dependent pathways modulating colonic motor activity in mice.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, Grants DK33061 (Y.T.), DK-57238 (Y.T.), DK-41301 (Animal Core, Y.T.) and DK-26741 (J.R.). V. Martínez was partially supported by the ‘Conselleria de Cultura Educació i Ciència’ de la Generalitat Valenciana (Spain).
References
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer's disease. Nature. 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- Chen CY, Million M, Adelson DW, Martinez V, Rivier J, Taché Y. Intracisternal urocortin inhibits vagally stimulated gastric motility in rats: role of CRF(2) Br J Pharmacol. 2002;136:237–247. doi: 10.1038/sj.bjp.0704713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Dieterich KD, Lehnert H, De Souza EB. Corticotropin-releasing factor receptors: an overview. Exp Clin Endocrinol Diabetes. 1997;105:65–82. doi: 10.1055/s-0029-1211730. [DOI] [PubMed] [Google Scholar]
- Flint J, Corley R, DeFries JC, Fulker DW, Gray JA, Miller S, Collins AC. A simple genetic basis for a complex psychological trait in laboratory mice. Science. 1995;269:1432–1435. doi: 10.1126/science.7660127. [DOI] [PubMed] [Google Scholar]
- Gulyas J, Rivier C, Perrin M, Koerber SC, Sutton S, Corrigan A, Lahrichi SL, Graig AG, Vale W, Rivier J. Potent, structurally constrained agonists and competitive antagonists of corticotropin-releasing factor. Proc Natl Acad Sci U S A. 1995;92:10575–10579. doi: 10.1073/pnas.92.23.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CS. Emotional behavior in the rat. 1. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol. 1934;18:385–403. [Google Scholar]
- Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- Higelin J, Py-Lang G, Paternoster C, Ellis GJ, Patel A, Dautzenberg FM. 125I-Antisauvagine-30: a novel and specific high-affinity radioligand for the characterization of corticotropin-releasing factor type 2 receptors. Neuropharmacology. 2001;40:114–122. doi: 10.1016/s0028-3908(00)00105-2. [DOI] [PubMed] [Google Scholar]
- Hoare SR, Sullivan SK, Ling N, Crowe PD, Grigoriadis DE. Mechanism of corticotropin-releasing factor type I receptor regulation by nonpeptide antagonists. Mol Pharmacol. 2003;63:751–765. doi: 10.1124/mol.63.3.751. [DOI] [PubMed] [Google Scholar]
- Improta G, Broccardo M. Sauvagine: effects on gastric acid secretion in rats. Peptides. 1988;9:843–846. doi: 10.1016/0196-9781(88)90131-3. [DOI] [PubMed] [Google Scholar]
- Kihara N, Fujimura M, Yamamoto I, Itoh E, Inui A, Fujimiya M. Effects of central and peripheral urocortin on fed and fasted gastroduodenal motor activity in conscious rats. Am J Physiol. 2001;280:G406–G419. doi: 10.1152/ajpgi.2001.280.3.G406. [DOI] [PubMed] [Google Scholar]
- Lenz HJ, Burlage M, Raedler A, Greten H. Central nervous system effects of corticotropin-releasing factor on gastrointestinal transit in the rat. Gastroenterology. 1988;94:598–602. doi: 10.1016/0016-5085(88)90229-6. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropinreleasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Barquist E, Rivier J, Taché Y. Central CRF inhibits gastric emptying of a nutrient solid meal in rats: the role of CRF2 receptors. Am J Physiol. 1998;274:G965–G970. doi: 10.1152/ajpgi.1998.274.5.G965. [DOI] [PubMed] [Google Scholar]
- Martinez V, Rivier J, Wang L, Taché Y. Central injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks CRF- and stress-related alterations of gastric and colonic motor function. J Pharmacol Exp Ther. 1997;280:754–760. [PubMed] [Google Scholar]
- Martinez V, Taché Y. Role of CRF receptor 1 in central CRF-induced stimulation of colonic propulsion in rats. Brain Res. 2001;893:29–35. doi: 10.1016/s0006-8993(00)03277-7. [DOI] [PubMed] [Google Scholar]
- Martinez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther. 2002;301:611–617. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- Million M, Grigoriadis DE, Sullivan S, Crowe PD, McRoberts JE, Zhou CY, Saunders PR, Maillot C, Mayer AE, Taché Y. A novel water-soluble selective CRF1 receptor antagonist, NBI 35965, blunts stress-induced visceral hyperalgesia and colonic motor function in rats. Brain Res. 2003;985:32–42. doi: 10.1016/s0006-8993(03)03027-0. [DOI] [PubMed] [Google Scholar]
- Miranda A, Lahrichi SL, Gulyas J, Koerber SC, Craig AG, Corrigan A, Rivier C, Vale W, Rivier J. Constrained corticotropin-releasing factor antagonists with i-(i+3) Glu-Lys bridges. J Med Chem. 1997;40:3651–3658. doi: 10.1021/jm970311t. [DOI] [PubMed] [Google Scholar]
- Momose K, Inui A, Asakawa A, Ueno N, Nakajima M, Fujimiya M, Kasuga M. Intracerebroventricularly administered corticotropin-releasing factor inhibits food intake and produces anxiety-like behaviour at very low doses in mice. Diabetes Obes Metab. 1999;1:281–284. doi: 10.1046/j.1463-1326.1999.00033.x. [DOI] [PubMed] [Google Scholar]
- Mönnikes H, Schmidt BG, Raybould HE, Taché Y. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am J Physiol. 1992;262:G137–G143. doi: 10.1152/ajpgi.1992.262.1.G137. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Carmouche M, Cullen MJ, Brown B, Murphy B, Grigoriadis DE, Ling N, Foster AC. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. J Pharmacol Exp Ther. 2000;293:799–806. [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Pharmacological evidence supporting a role for central corticotropin-releasing factor (2) receptors in behavioral, but not endocrine, response to environmental stress. J Pharmacol Exp Ther. 2002;302:145–152. doi: 10.1124/jpet.302.1.145. [DOI] [PubMed] [Google Scholar]
- Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann N Y Acad Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropinreleasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martinez V, Wang L, Taché Y, Vale W. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- Ruhmann A, Bonk I, Lin CR, Rosenfeld MG, Spiess J. Structural requirements for peptidic antagonists of the corticotropin-releasing factor receptor (CRFR): development of CRFR2β-selective antisauvagine-30. Proc Natl Acad Sci U S A. 1998;95:15264–15269. doi: 10.1073/pnas.95.26.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon RJ, Qi JA, Porreca F, Fisher LA. Gastrointestinal motor effects of corticotropin-releasing factor in mice. Regul Pept. 1990;28:137–151. doi: 10.1016/0167-0115(90)90013-m. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Steckler T, Holsboer F. Corticotropin-releasing hormone receptor subtypes and emotion. Biol Psychiatry. 1999;46:1480–1508. doi: 10.1016/s0006-3223(99)00170-5. [DOI] [PubMed] [Google Scholar]
- Taché Y, Goto Y, Gunion MW, Vale W, Rivier J, Brown M. Inhibition of gastric acid secretion in rats by intracerebroventricular injection of corticotropin-releasing factor. Science. 1983;222:935–937. doi: 10.1126/science.6415815. [DOI] [PubMed] [Google Scholar]
- Taché Y, Martinez V, Million M, Maillot C. Role of corticotropin releasing factor receptor subtype 1 in stress-related functional colonic alterations: implications in irritable bowel syndrome. Eur J Surg. 2002;168(suppl. 587):16–22. [PubMed] [Google Scholar]
- Taché Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol. 2001;280:G173–G177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Role of CRF(1) and CRF(2) receptors in fear and anxiety. Neurosci Biobehav Rev. 2001;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. CRF(2) receptors: an emerging role in anxiety. Drug News Perspect. 2002;15:97–101. doi: 10.1358/dnp.2002.15.2.668333. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine response to stress: CRF receptors, binding protein, and related peptides. Proc Soc Exp Biol Medical. 1997;215:1–10. doi: 10.3181/00379727-215-44108. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Williams CL, Peterson JM, Villar RG, Burks TF. Corticotropin-releasing factor directly mediates colonic responses to stress. Am J Physiol. 1987;253:G582–G586. doi: 10.1152/ajpgi.1987.253.4.G582. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Taché Y, Koob GF. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci. 2003;24:421–427. doi: 10.1016/S0165-6147(03)00177-9. [DOI] [PubMed] [Google Scholar]