Abstract
The most physiologically important sensors for systemic glucoregulation are located in extra-cranial sites. Recent evidence suggests that the carotid body may be one such site. We assessed rat carotid body afferent neural output in response to lowered glucose, indirectly by measurement of ventilation, and directly by recording single or few-fibre chemoafferent discharge, in vitro. Insulin (0.4Ukg−1min−1)-induced hypoglycaemia (blood glucose reduced by ca 50% to 3.4 ± 0.1mmoll−1) significantly increased spontaneous ventilation (V̇E) in sham-operated animals but not in bilateral carotid sinus nerve sectioned (CSNX) animals. In both groups, metabolic rate (measured as V̇O2) was almost doubled during hypoglycaemia. The ventilatory equivalent (V̇E/ V̇O2) was unchanged in the sham group leading to a maintained control level of Pa,CO2, but V̇E/V̇O2 was significantly reduced in the CSNX group, giving rise to an elevation of 6.0 ± 1.3mmHg in Pa,CO2. When pulmonary ventilation in sham animals was controlled and maintained, phrenic neural activity increased during hypoglycaemia and was associated with a significant increase in Pa,CO2 of 5.1 ± 0.5mmHg. Baseline chemoreceptor discharge frequency, recorded in vitro, was not affected, and did not increase when the superfusate [glucose] was lowered from 10 mm to 2 mm by substitution with sucrose: 0.40 ± 0.20 Hz to 0.27 ± 0.15Hz, respectively (P > 0.20). We suggest therefore that any potential role of the carotid bodies in glucose homeostasis in vivo is mediated through its transduction of some other metabolically derived blood-borne factor rather than glucose per se and that this may also provide the link between exercise, metabolic rate and ventilation.
The carotid body is the major mammalian peripheral chemoreceptor organ and is anatomically well placed for sampling carotid arterial blood. It is a polymodal sensor, being activated by a variety of stimuli, including hyperosmolarity, hyperkalaemia and increased temperature as well as by its more recognized natural stimuli of hypoxia, hypercapnia and acidaemia (Gonzalez et al. 1994). In addition, a role for the carotid body in glucose sensing was proposed, based upon the observation that carotid chemoreceptor discharge was reduced, in vivo, by intracarotid glucose infusion (Alvarez-Buylla & de Alvarez-Buylla, 1988). Although these authors subsequently proposed a more specific role for the carotid body in brain glucose regulation (Alvarez-Buylla & de Alvarez-Buylla, 1994), a more generalized role has been re-proposed for the carotid body in systemic glucose regulation (Koyama et al. 2000). This was based on the finding that carotid sinus nerve (CSN)-denervated animals were less able to mount adequate, counterregulatory, neuroendocrine responses to mild, insulin-induced hypoglycaemia when compared to sham-operated controls. Further, a lowering of the superfusate extracellular glucose to an in vitro thin-slice preparation of the carotid body leads to a dose-dependent increase in catecholamine secretion from type I cells, subsequent to K+ channel inhibition and voltage-gated Ca2+ entry (Pardal & Lopez-Barneo, 2002). This suggests that the mechanism for the initiation of chemoafferent discharge in hypoglycaemia, in vivo, would be a direct consequence of the lowered glucose concentration, acting like hypoxia to depolarize type I cells and, presumably, to initiate chemoafferent discharge.
Although relatively little studied, there is no evidence to date to suggest that the pattern or frequency of CSN afferent discharge retains specific information regarding the modality that generated it and certainly all chemoreceptor fibres tested for this appear to be able to respond both to hypoxia and hypercapnia (Hornbein et al. 1961; Kumar et al. 1988). Each stimulus, once of sufficient magnitude to generate afferent discharge, can therefore elicit a stereotypical set of cardiorespiratory reflexes that would be graded according to stimulus intensity. These reflexes include increased ventilation and sympathetic-mediated alterations in blood flow (Marshall, 1994) as well as adrenomedullary catecholamine secretion (Critchley et al. 1980). Thus, for example, carotid body stimulation by hypoxia can produce a similar hormonal response to that observed during systemic hypoglycaemia (Zinker et al. 1994), although hypoglycaemia would elicit a relatively greater adrenaline : noradrenaline response than hypoxia.
We hypothesized that carotid body stimulation by insulin-induced hypoglycaemia should, like hypoxia, also induce hyperventilation in vivo and have tested this in anaesthetized rats. In addition, the direct effect of low glucose upon chemodischarge has been determined in an in vitro carotid body preparation. Our data suggest that insulin-induced hypoglycaemia induces a hyperpnoea in anaesthetized rats that is appropriate for the induced increase in metabolic rate and that the stimulus to the carotid body is not hypoglycaemia per se but some other as yet undetermined product(s) of metabolism.
Some of this work has been published previously in abstract form (Bin-Jaliah & Kumar, 2003; Bin-Jaliah et al. 2003).
Methods
In vivo and in vitro studies were used to investigate the carotid body response to low glucose. Experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1986.
In vivo studies
Animals and surgical preparation
Anaesthesia of adult male Wistar rats (334.2 ± 3.7 g, n= 37) was induced with halothane (3–4% in oxygen). The right femoral vein was cannulated for i.v. insulin infusion and anaesthesia maintained with 650mgkg−1 of 25% w/v ethyl carbamate (urethane, Sigma). The femoral artery was cannulated for continuous recording of arterial blood pressure (NL108T2 Neurolog system, Digitimer Research Instrumentation, Hertfordshire, England). In some animals, the left femoral vein was also cannulated for glucose infusion. The trachea was exposed and cannulated.
The carotid artery bifurcations were located bilaterally and carotid sinus nerves (CSNs) were identified and either sectioned bilaterally (CSNX) at their junction with the glossopharyngeal (IXth cranial) nerve or left intact (sham). Denervation was confirmed by absence of hypoxia-induced hyperventilation prior to experiments. Rectal temperature was monitored throughout the study and kept ca 37°C using a small homoeothermic blanket system (Harvard Apparatus Ltd, Kent, UK). At the end of the experiment, animals were killed with a urethane overdose and decapitation.
Experimental protocols
After a surgical recovery period of 60 min, when baseline cardiovascular and respiratory variables were stable, experimental protocols consisted of an initial basal sampling period of 40min followed by either a hypoglycaemic period (intravenous insulin (from bovine pancreas, 28.6 USP units mg−1, Sigma, St Louis, MO, USA) infusion 0.4Ukg−1min−1) or a euglycaemic clamp period (concomitant 50% glucose solution infusion (14mgmin−1kg−1) with insulin infusion to clamp the blood glucose (BG) at ≥6mmoll−1) lasting up to 180min. In all groups of animals, partial pressures of O2 and CO2 as well as pH and glucose concentration of the arterial blood and ventilation (see below) were sampled at 40, 30, 20 and 10min prior to insulin infusion and at 10, 20, 40, 60, 90 and 120min after the start of insulin infusion. Arterial blood samples were taken in 150μl glass capillary tubes for blood gas and pH analysis (1604 Blood Gas Analyser; Instrumentation Laboratory Ltd, Warrington, UK). Arterial blood glucose was measured with glucose meter cards (MediSense G2 Sensor electrodes, Abbott Laboratories, Maidenhead, UK). The MediSense glucometer has a small deviation from laboratory reference values (<10%) and a high concurrence with results obtained by laboratory methods (Solnica et al. 2003).
Recording of spontaneous ventilation
Spontaneous minute ventilation (V̇E) in air was derived from integrated tracheal airflow. The tracheal cannula was connected to a respiratory flow head (F10L, gm instruments Ltd, Kilwinning, UK), which was connected to a pneumotachometer (electrospirometer CS8, Mercury Electronics Ltd, Glasgow, UK). Tidal volume calibration of the pneumotachometer was performed regularly using known volumes.
Measurement of oxygen consumption
The anaesthetized rat was placed into an airtight, temperature-regulated metabolic chamber; expired CO2 was absorbed by soda lime placed in the bottom of the chamber. The chamber was connected to a respiratory flow head (F10L, gm instruments Ltd) that was in turn connected to a spirometer (CS8, Mercury Electronics). The slope of the flow recording, resulting from the pressure gradient, was taken as a measure of oxygen consumption (V̇O2).
Phrenic nerve recordings
Urethane-anaesthetized (650mgkg−1; i.v.) animals were vagotomized, neuromuscularly blocked with pancuronium bromide (David Bull Laboratories, Warwick, UK; 3mgkg−1, i.v.) and artificially ventilated with O2-enriched air (30% O2 in N2) using a positive pressure ventilator (Harvard rodent ventilator, model 683; Harvard Apparatus, Holliston, MA, USA). The right phrenic nerve was dissected via a dorsolateral approach. The nerve was identified, freed from nearby tissues and cut as distal as possible. The central end of the nerve was submerged in liquid paraffin and placed on a recording electrode. The raw phrenic nerve discharge was amplified, filtered, rectified and integrated to derive an index of ventilation. Adequacy of anaesthesia was confirmed throughout the procedure by continuous measurement of arterial blood pressure and lack of cardiovascular response to a strong paw pinch. Supplementary anaesthesia, urethane (50mgkg−1; i.v.) was given as required.
Data and statistical analysis
All data were recorded online via a MICRO 1401 connected to a computer running Spike2 software (Cambridge Electronics Design, Cambridge, UK). Mean arterial blood pressure (MABP) and heart rate (HR) were calculated from the blood pressure trace. A CED Spike2 script was used to determine tidal volume (VT), inspiratory time (Ti) and expiratory time (Te) breath by breath from the integrated airflow. These variables were used to calculate V̇E. Another CED Spike2 script was used to determine phrenic burst frequency (f) and peak amplitude of integrated phrenic activity (∫Phr) from the phrenic trace. The minute phrenic activity then was calculated and corrected for body weight.
Data are expressed as means ± s.e.m. and significance (P < 0.05) tested with regression analysis, one or two factor ANOVA with post hoc Bonferroni/Dunn test and paired t tests as appropriate, using StatView (SAS Institute Inc., Cary, NC, USA) software.
In vitro studies
Animals, surgical preparation and tissue dissection
Adult rats (114.5 ± 17.0 g, n= 5) were anaesthetized with halothane (3–4% in oxygen for induction and maintained at 2%). Left and right carotid bifurcations were removed as previously described (Pepper et al. 1995). Animals were killed by halothane overdose and decapitation.
Each carotid bifurcation was pinned on Sylgard (184 Silicone Elastomer, Farnell Electronic Companies Ltd, Leeds, UK) in a small volume, ca 0.2 ml, tissue bath. A gassed (95% O2 and 5% CO2), bicarbonate-buffered saline solution (composition (mm): 125 NaCl, 3 KCl, 1.25 NaH2PO4, 5 Na2SO4, 1.3 MgSO4, 24 NaHCO2, 2.4 CaCl2, and either 10 glucose or 2 glucose and 8 sucrose, pH ∼7.38) was superfused at 3mlmin−1 into the tissue bath. The perfusate was equilibrated to PO2 and PO2 levels of 40mmHg and 400mmHg, respectively, by use of precision flow valves (Cole-Parmer Instrument Company) and calibrated against a blood gas analyser (IL 1640, Instrumentation Laboratory). The temperature was continually monitored in the superfusion line immediately before entering the bath by a thermocouple (871 A, Tegam Inc., Madison, OH, USA) and maintained at 36–37°C. The excess connective tissue around the bifurcation was removed and the carotid body and its attached sinus nerve identified before sectioning the nerve at its junction with the glossopharyngeal nerve. The preparation was partially digested in a gassed enzyme solution (0.06mgml−1 collagenase (Sigma Type II), 0.03mgml−1 protease (Sigma Type IX)) for 20–30min at 37°C to facilitate the recording of neuronal activity.
Data acquisition and analysis
Extracellular recordings of afferent single fibre activity were made from the cut end of the carotid sinus nerve using glass suction electrodes (GC150T-10, Harvard Apparatus) and standard Neurolog modules (Digitimer). Afferent spike activity was monitored on an oscilloscope (Gould 1604) and recorded throughout each experiment on a VHS video recorder via a DC-modified PCM digital unit (Applegarth Electronics). Chemoreceptor discharge was discriminated as activity exceeding a level which was at least 25% of the amplitude of the baseline noise above the noise and which responded to a decrease in superfusate PO2 with a reversible increase in discharge frequency. Single fibres were discriminated on the basis of amplitude and shape. Action potentials were sampled digitally after conversion to TTL pulses via a window discriminator (Neurolog NL200) by a computer (Macintosh IIci with NB-MIO-16 DA and NB-MOI-8G DMA cards; National Instruments Co., Austin, TX, USA) running customised LabVIEW 2 (National Instruments Co.) software. TTL pulses were counted and binned into predetermined time periods.
Data are expressed as means ±s.e.m. and significance (P < 0.05) tested with paired t tests using StatView (SAS Institute Inc.) software.
Results
Infusion of insulin reduces blood [glucose] similarly in sham and CSNX
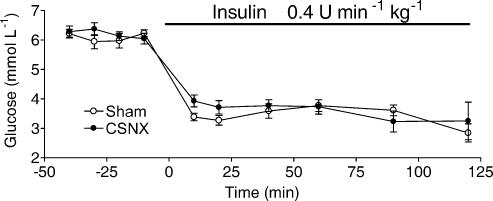
Blood glucose (BG) levels prior to insulin infusion were not significantly different between sham (n = 7) and CSNX (n = 7) animals (6.0 ± 0.1 and 6.3 ± 0.1mmoll−1, respectively; P > 0.15, ANOVA). With venous infusion of insulin at a rate of 0.4Ukg−1min−1, BG was lowered significantly in both sets of animals to the same mean level of 3.5 ± 0.1mmoll−1 (P < 0.0001, ANOVA) within 30min of the start of insulin infusion. This steady level of BG was maintained for up to 120min (Fig. 1). In the euglycaemic clamp protocol, blood glucose was maintained at 7.7 ± 0.2mmoll−1 (n = 7) by the contemporaneous intravenous infusion of insulin (0.4Ukg−1min−1) with glucose (14mgmin−1kg−1). Arterial blood potassium levels were slightly but significantly reduced during insulin infusion from a basal control level of 4.1 ± 0.1mmoll−1 to 3.8 ± 0.1mmoll−1 (P < 0.04, ANOVA; n= 4).
Figure 1. Insulin infusion decreases blood glucose concentration in sham and carotid sinus nerve-sectioned (CSNX) adult rats.
Data points represent means ± s.e.m. of the blood glucose concentration in sham (n = 7) and CSNX (n = 7) animals. Insulin infusion, represented by the horizontal bar, began at time 0, was maintained for 120min and caused significant (P < 0.0001, ANOVA) and similar (P > 0.15, ANOVA) amplitude falls from preinfusion levels of blood glucose in both groups of animals.
Insulin-induced hypoglycaemia increased ventilation in sham but not in CSNX and induced respiratory acidosis in CSNX only
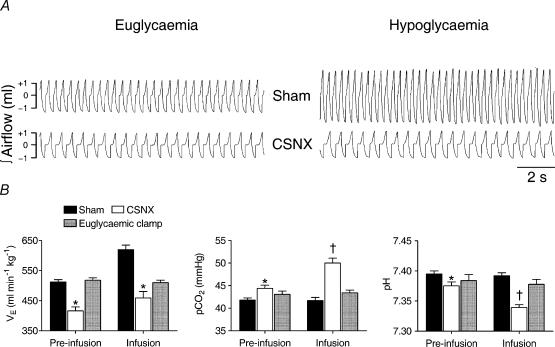
Basal V̇E in CSNX animals was significantly reduced compared to sham and basal Pa,CO2 was, correspondingly, slightly higher. Insulin-induced hypoglycaemia in the sham operated rats was associated with an ca 25% increase in V̇E from basal levels (P < 0.0001, ANOVA; Fig. 2), but, in contrast, V̇E was not increased in CSNX animals during hypoglycaemia (P > 0.19, ANOVA; Fig. 2). The increased V̇E in sham operated rats did not cause any significant change in Pa,CO2 or pH (P > 0.90 and P > 0.65, respectively) which remained around the basal levels of ca 41mmHg and 7.39, respectively (Fig. 2). In contrast, Pa,CO2 in CSNX rats increased significantly during insulin-induced hypoglycaemia by ca 6mmHg P < 0.0001, ANOVA; Fig. 2) and was correlated with a significant fall in pH of ca 0.04 units (P < 0.0002, ANOVA; Fig. 2). The basal Pa,O2 was lower in CSNX than in sham animals (80 ± 2mmHg versus 84 ± 1mmHg, P < 0.02, ANOVA) and did not alter significantly from these levels during hypoglycaemia (77 ± 2mmHg versus 86 ± 1mmHg). Three CSNX animals, but no sham animals, died during the postmeasurement period of insulin infusion (120–180min after insulin infusion began) presumably due to the severity of the uncorrected respiratory acidosis. During the euglycaemic clamp period, no changes in V̇E or blood gas status were observed.
Figure 2. Effect of hypoglycaemia on spontaneous ventilation (V̇E), Pa,CO2 and pH.
A, representative traces of integrated tracheal airflow from a sham (above) and a CSNX (below) animal, taken 20min before insulin infusion (left) and at 60min after the infusion began (right). Inspiratory and expiratory flows were separately integrated and depicted in a single trace with + indicating inspiratory volume and – indicating expiratory volume. B, means ±s.e.m. of V̇E, Pa,CO2 and pH in sham (n= 7), CSNX (n = 7) and euglycaemic clamped animals (n = 7) before (10–40min prior) and during (20–90min post) insulin infusion at 0.4Umin−1kg−1. * and † indicate significant difference (P < 0.05) from sham and CSNX preinfusion levels, respectively.
CSNX rats have lower basal MABP and HR than sham but insulin-induced hypoglycaemia did not affect these variables
Mean arterial blood pressure (MABP) did not change significantly from its basal levels during the hypoglycaemic/euglycaemic testing period in all groups; sham (from 127.1 ± 2.9mmHg to 128.5 ± 4.4mmHg, P > 0.77), CSNX (from 90.4 ± 1.7mmHg to 86.0 ± 2.9mmHg, P > 0.19) and euglycaemic clamped animals (from 113.2 ± 3.1mmHg to 118.9 ± 2.2mmHg, P > 0.15). Nevertheless, MABP was substantially lower in CSNX than in sham animals during the basal (90.4 ± 1.7mmHg versus 127.1 ± 2.9mmHg, respectively, P < 0.01) and the hypoglycaemic (86.0 ± 2.9mmHg versus 128.5 ± 4.4mmHg, respectively, P < 0.01) periods. In addition, CSNX basal heart rate (HR) was significantly lower than the sham basal HR (348 ± 6 b.p.m. versus 393 ± 5 b.p.m., respectively, P < 0.01). Mean HR during the hypoglycaemic/euglycaemic testing period were not significantly different from basal levels in sham animals (402 ± 7 b.p.m., P > 0.25) and CSNX animals (361 ± 6 b.p.m., P > 0.14). Mean HR also did not vary in euglycemic clamped animals (from 382 ± 5 to 380 ± 5 b.p.m., P > 0.25).
Oxygen consumption was increased in both sham and CSNX by insulin-induced hypoglycaemia and ventilatory equivalent was decreased only in CSNX
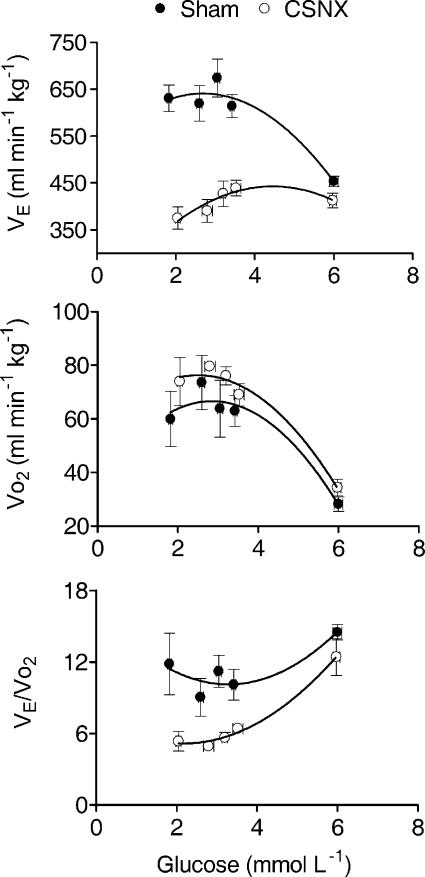
The hyperpnea observed in the sham animals during insulin-induced hypoglycaemia indicated that the ventilatory response was appropriate for an increased metabolism. In a separate series of experiments, basal levels of oxygen consumption (V̇O2) were measured and found to be not significantly different between sham (n = 4) and CSNX (n = 4) animals; P > 0.18, ANOVA). As previously described, in these animals, V̇E was also found to increase significantly in response to decreasing glucose concentrations in sham operated rats with no increase in V̇E observed in CSNX rats (Fig. 3). V̇O2 increased considerably in both sham (P < 0.02, ANOVA) and CSNX (P < 0.001, ANOVA) rats with decreasing glucose concentration (Fig. 3). The ventilatory equivalent (V̇E/V̇O2) was calculated and no changes were observed in sham operated rats (P > 0.08, ANOVA) but a significant and ca 50% decrease in the V̇E/V̇O2 ratio was found in CSNX rats (P < 0.001, ANOVA; Fig. 3). In control experiments using a euglycaemic clamp protocol, neither V̇E nor V̇O2 was significantly altered from their basal values of 531.9 ± 19.3 ml min−1kg−1 (P > 0.95, ANOVA) and 39.29 ± 4.59mlmin−1kg−1 (P > 0.08, ANOVA), respectively, during the insulin/glucose infusion period and the V̇E/V̇O2 was therefore not altered from its basal value of 13.92 ± 1.12 (P > 0.09, ANOVA). These findings indicate that the carotid body is stimulated during insulin-induced hypoglycaemia to increase ventilation in proportion to the elevation in metabolic rate. Thus the V̇E/V̇O2 ratio is maintained and therefore blood gas tensions and pH remain at control levels. This effect is independent of any direct effect of insulin.
Figure 3. Changes in minute ventilation (V̇E), oxygen consumption (V̇O2) and ventilatory equivalent (V̇E/V̇O2) at varying blood glucose concentrations.
Data points represent means ±s.e.m. in sham (n = 4) and CSNX (n = 4) animals. Glucose concentrations were measured at fixed times during the protocol period. During insulin-induced hypoglycaemia (blood glucose < 4 mmoll−1), V̇O2 increased significantly in sham and CSNX groups (P < 0.02, ANOVA) whilst V̇E increased only in sham (P < 0.002, ANOVA). V̇E/V̇O2 therefore decreased only in CSNX (P < 0.001, ANOVA) and remained unchanged in sham. Data are shown fitted by second order polynomials.
Insulin-induced hypoglycaemia increases the minute phrenic activity in artificially ventilated rats
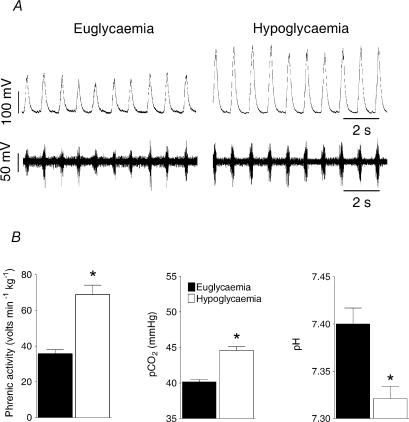
To test the hypothesis that ventilation was matched to metabolism in the sham operated animals during hypoglycaemia, phrenic nerve activity was measured in anaesthetized, vagotomised, paralysed and artificially ventilated rats. The level of pulmonary ventilation was adjusted, for each animal, during the preinfusion period to establish a Pa,CO2 between 39 and 41mmHg and the ventilator tidal volume and frequency settings were then left unchanged throughout the insulin infusion period. Insulin infusion (0.4Ukg−1min−1) induced the same level of hypoglycaemia described above. Minute phrenic activity was increased approximately twofold (n = 4; P < 0.006, ANOVA; Fig. 4), primarily by an increase in peak amplitude (∫Phr) from 0.15 ± 0.02 V to 0.26 ± 0.01 V (P < 0.001, ANOVA) than in burst frequency (f; preinfusion 90.6 ± 11.4 burst min−1 and postinfusion 99.4 ± 7.0 bursts min−1; P > 0.55, ANOVA). The fixed pulmonary ventilation revealed a significant elevation of Pa, CO2 (P < 0.001, ANOVA; Fig. 4) and a reduction of pH (P < 0.012, ANOVA; Fig. 5) during hypoglycaemia that were absent in the spontaneously breathing, sham operated animals, suggesting that the increased V̇E could account for the isocapnia.
Figure 4. Phrenic nerve activity response to hypoglycaemia during controlled ventilation.
Phrenic nerve discharge, blood gases and pH were recorded during insulin-induced hypoglycaemia in paralysed and artificially ventilated, sham animals (n= 4) in which the Pa,CO2 was set at 39–41mmHg before infusion and maintained at this level of ventilation throughout the infusion period. A, example traces from a single experiment showing raw phrenic nerve discharge (lower trace) and integrated discharge (100 ms time constant; upper trace) during euglycaemia (6.1mmoll−1) and hypoglycaemia (3.2mmoll−1). B, phrenic nerve discharge, Pa, CO2 and pH data expressed as means ±s.e.m.* indicates significant difference (P < 0.05, Student's t test) from euglycaemic levels.
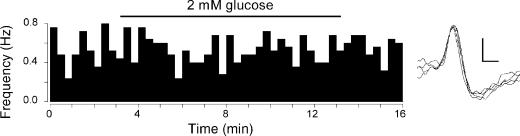
Figure 5. In vitro carotid sinus nerve afferent discharge is unaffected by decreasing glucose.
Single-fibre chemoafferent discharge recorded from one animal during control (10mm glucose) and low glucose superfusion (2mm glucose; bar). Discharge was binned into 20-s periods and frequency calculated as impulses s−1 (Hz). Discharge did not change significantly from control during the period of low glucose superfusion. On the right are shown five superimposed afferent action potentials from this recording. The vertical scale bar is 100 mV, horizontal scale bar 0.4 ms.
Low glucose has no effect upon baseline discharge in the in vitro carotid body preparation
The direct effect of low glucose was tested on the in vitro isolated rat carotid body. Chemoafferent recordings from single or few-fibre preparations of the carotid sinus nerve showed that, whilst all fibres responded to falls in PO2 or elevations in PCO2, lowering the superfusate glucose from 10mm to 2mm by substitution with sucrose, did not increase the baseline chemoreceptor discharge frequency (Fig. 5): 0.40 ± 0.20Hz to 0.27 ± 0.15Hz, respectively (P > 0.20, paired t test; n= 7) In addition, in a separate series of experiments, lowering superfusate glucose to 0mm by complete substitution with sucrose also did not increase baseline discharge and in most cases led to reversible decreases in discharge frequency with prolonged exposure of up to 20min (data not shown).
Discussion
These studies were designed to use direct and indirect assessment of carotid body output to test the hypothesis that this organ is able to transduce low blood glucose in vivo into afferent CSN discharge suitable for initiating counterregulatory responses to hypoglycaemia. We hypothesized that if the hypoglycaemic stimulus was of sufficient amplitude it should evoke hyperventilation, as would occur in response to hypoxia or hypercapnia/acidosis. Our data shows that, whilst insulin-induced hypoglycaemia is indeed correlated with an increased ventilation, the maintenance of Pa,CO2 during hypoglycaemia indicates that the elevated ventilation is an appropriate hyperpnoeic response to a raised metabolic rate. In addition, the failure of in vitro CSN discharge to be elevated by lowered superfusate glucose suggests strongly that the mediator of the hyperpnoea in vivo is most likely not hypoglycaemia per se. As the ventilatory and metabolic effects observed in our in vivo study were absent during the euglycaemic clamp protocol this suggests that the stimulus is also not likely to be insulin itself, but is instead some other factor(s) associated with hypermetabolism.
Insulin acts similarly in both anaesthetized and awake animals (Haynes et al. 1988), at least with regard to reductions in blood glucose, free fatty acids and β-hydroxybutyrate concentration although the rate of glucose oxidation may be slightly reduced in anaesthetized animals as a consequence of the reduced rate of V̇O2 during anaesthesia. Urethane, a long-lasting anaesthetic giving a more stable level of anaesthesia, was used in all our in vivo studies as it causes less depression than pentobarbital (Eager et al. 1994). Urethane, at doses lower than we used, can inhibit chemoreflex excitation of medullary vasomotor neurones without affecting chemoafferents (Sun & Reis, 1995). It can also stimulate both neurosympathetic and adrenomedullary functions (Carruba et al. 1987), which might counteract this central inhibitory effect. Daly (1997) has reported that urethane can also prevent activation of brainstem defence areas from peripheral input and that this is more similar to the response obtained in conscious animals than with some other anaesthetics. It is possible therefore that urethane could shift the relative influences of peripheral inputs to central respiratory neurones but, clearly, the resultant effect, in vivo, would be complex. Although the finding that basal V̇E was reduced by almost 25% by CSNX might imply that a significant peripheral chemoreceptor drive was apparent in our anaesthetized animals, it is interesting to note that the reduction of normoxic V̇E by a 100% O2 challenge (Dejours test), when measured in conscious rats, was also around 25% (Bamford & Carroll, 1999). Rectal temperature was maintained during anaesthesia as anaesthetized hypothermic, but not temperature maintained, rats show altered glucose kinetics (Lang et al. 1987) and strong metabolic acidosis (Alfaro & Palacios, 1997).
In addition to loss of chemosensory input, carotid sinus nerve section would also remove carotid baroreceptor sensory input from the sinus receptors. Loss of baroreceptor input could affect blood pressure, cardiac output, peripheral resistance and/or heart rate – indeed loss of chemoafferents could also affect these variables in a ventilation- and anaesthetic-dependent manner (Marshall, 1994). It was not our aim to study these reflexes in detail but, in practice, we found that both basal arterial blood pressure and basal heart rate were decreased after CSNX. It is difficult to interpret these findings fully, given that we did not control ventilation and had vagi and aortic nerves intact which could confound interpretation. Clearly, CSNX would not only lead to a loss of baroreceptor influence upon baseline cardiovascular variables but would also abolish any contribution that the baroreflex might make to the response to hypoglycaemia. However, hypoglycaemia did not induce any significant change in blood pressure or heart rate in either sham or CSNX groups. We believe therefore, that the effect of loss of carotid chemoreceptors and/or baroreceptors upon the cardiovascular response to hypoglycaemia is negligible – at least within the constraints of our model. Given that the ventilatory response to hypoglycaemia was profoundly different between sham and CSNX groups, this would suggest (given the greater contribution to ventilation by chemo- rather than baroreceptor stimulation) that the ventilatory response during hypoglycaemia was primarily, if not solely, determined by chemoexcitation.
Previous studies, using conscious dogs, had revealed that CSNX animals were less able to mount a counterregulatory hormonal response to insulin-induced hypoglycaemia as, in these denervated animals, plasma glucagon and cortisol levels were reduced when compared to sham-operated animals (Koyama et al. 2000). As a consequence, the rate of exogenous glucose infusion required to clamp plasma glucose at a fixed level, during hyperinsulinaemia, was 2.5 times greater in CSNX animals than sham. Although not measured directly by Koyama et al. (2000), the implication of their study was that CSNX animals should have a lower blood glucose concentration for a fixed dose of insulin when compared to sham animals. Our study involved an infusion of insulin at a dose higher than used by Koyama et al. (2000) and one which was selected deliberately to induce a rapid, significant and sustained hypoglycaemia that could not easily be counterregulated. We found therefore, as we expected, that blood glucose in the sham animals during insulin infusion was not different from that in the denervated animals. Our results are therefore not in disagreement with a probable glucoregulatory role of the carotid bodies (Koyama et al. 2000) but we would argue that the stimulus driving the carotid body-mediated, counterregulatory responses to insulin-induced hypoglycaemia is not the hypoglycaemia but a product of the associated increase in metabolic rate. Insulin increases energy expenditure in the rat as a consequence of its hypoglycaemic effect rather than, for example, any change in motor activity (Menendez & Atrens, 1989) and insulin-induced hypoglycaemia also increases the metabolic rate in non-diabetic human subjects (Swaminathan et al. 1986). The study by Koyama et al. (2000) was not directed towards the stimulus acting upon the carotid body and they did not measure metabolic rate in their animals, but it is unlikely, that metabolic rate was not elevated in these dogs during insulin-induced hypoglycaemia. In agreement with their study, we also found that basal Pa,O2 was decreased and Pa,CO2 increased in CSNX animals compared to sham animals as a consequence of the decreased basal ventilation. In addition, the respiratory acidosis observed during hypoglycaemia, in our CSNX animals, did not induce hyperventilation. This is somewhat surprising and may indicate that insulin and/or an absence of peripheral chemoreceptor input alters the central threshold or sensitivity to CO2 in urethane-anaesthestised rats. The decreased pH we observed most likely reflects a lack of full renal compensation in our relatively short protocol period. Certainly the effects upon ventilation of insulin-induced hypoglycaemia would appear to be mediated solely through the carotid, rather than the aortic, bodies as also noted previously (Koyama et al. 2000).
Firm control of arterial blood glucose levels is crucial to prevent neuronal damage by hypoglycaemia. The most important physiological glucose-sensitive receptors appear to be located in extra-cranial sites (Cane et al. 1986, 1988) including the pancreas (Matschinsky, 1990; Heimberg et al. 1996), liver (Donovan et al. 1991; Burcelin et al. 2000) and portal vein (Hevener et al. 1997, 2001). Whilst central neurones sensitive to glucose concentrations have been described in different brain areas, particularly in the lateral and ventromedial hypothalamus (Oomura et al. 1969; Ono et al. 1982; Silver & Erecinska, 1998; Garcia et al. 2003) and nucleus of the solitary tract (Mizuno & Oomura, 1984; Yettefti et al. 1997), it remains unclear whether these are capable of detecting glucose concentration at physiological levels (Levin et al. 2002) or whether some related metabolic factor, such as leptin, is sensed (Spanswick et al. 1997). The action of these metabolic factors in central neurones may be linked to KATP channel activation and hyperpolarization with falling ATP concentration (Levin et al. 2001). A similar action in carotid body type I cells would also, presumably, lead to a decrease rather than an increase in excitability, and there is evidence that KATP channels play no role in the type I cell's response to lowered [glucose] (Pardal & Lopez-Barneo, 2002).
The carotid body is recognized as a peripheral chemoreceptor, responding with increased afferent neural discharge to falls in Pa,O2 or pH or to elevations in Pa,CO2. Whilst there is no doubt that an in vitro thin slice preparation of the carotid body can also respond to decreased glucose concentrations by increasing catecholamine secretion (Pardal & Lopez-Barneo, 2002), we found no increased chemoafferent discharge when glucose concentration was reduced to 2 or 0mm for periods of up to 20 min. This is in agreement with a previous in vitro study, using the cat carotid body, in which chemoafferent discharge, during superfusion with hyperoxia and 0mm glucose, did not increase until after at least 1–2 h (Almaraz et al. 1984). We cannot explain why an increased catecholamine secretion does not translate into increased afferent neural discharge. Perhaps a requirement for catecholamines in the generation of afferent discharge is not essential for hypoxia transduction, at least in some models. For example, discharge during hypoxia could be abolished by a combination of cholinergic and purinergic receptor block in a reconstituted glomus cell–petrosal ganglion neuronal co-culture system (Zhang et al. 2000). In addition, the carbon monoxide-induced discharge in a rat, in vitro, whole carotid body preparation could also be abolished by purinoceptor block (Barbe et al. 2002). Alternatively, the differences observed may reflect the methodologies employed. For example, the carotid body thin slice preparation is kept in culture for 48–96h prior to experimentation (Pardal et al. 2000) which may make it become more dependent upon glucose metabolism than the more acutely prepared whole carotid body preparation. The response to low glucose in the carotid body thin-slice preparation can be augmented by hypoxia (Pardal & Lopez-Barneo, 2002) but as a true systemic glucosensor should be able to mount an adequate response to hypoglycaemia even in normoxia and to avoid complications arising from stimulus interaction, we did not perform experiments in hypoxia. Indeed, the optimal glucose concentration required to maintain cat chemosensory neural responsiveness to either hypoxia (induced by interruption of perfusate flow), or to nicotine stimulation, was 11mm and the response was severely attenuated by removal of the glucose substrate with no evidence of any initial stimulatory effect (Spergel et al. 1992). The evidence does not therefore support the notion that the carotid body might function as a ‘metabolic integrator’, responding to intracellular ATP production in a substrate-independent manner as described for certain hypothalamic neurones (Dunn-Meynell et al. 2002; Levin et al. 2002; Routh, 2002). Indeed, the effect of low glucose on carotid body type I cells appeared to be unrelated to changes in intracellular ATP concentration (Pardal & Lopez-Barneo, 2002). This differs, however, to some extent, with the finding that 2-deoxy-d-glucose (2-DG), when used to deplete ATP content in cat carotid bodies (Obeso et al. 1986) caused an increase in sinus nerve activity, which in this situation was correlated with catecholamine release. The reason(s) for these various discrepancies is not known.
Ventilation is precisely related to CO2 flux during exercise such that Pa,CO2 remains little or unchanged (Haldane & Priestley, 1905; Wasserman et al. 1967). A combination of neural and humoral control is certainly involved with an appreciable degree of redundancy existing between the various systems (Paterson, 1992) such that no single mechanism appears to be predominant. This regulation, however, requires intact carotid bodies, at least at the onset of the exercise (Wasserman et al. 1975; Phillipson et al. 1981; Whipp, 1994) presumably acting through a feedforward but, as yet, undetermined mechanism that might involve oscillations in blood gas tensions and/or pH (Yamamoto, 1962; Goodman et al. 1974; Band & Wolff, 1978; Kumar et al. 1988). Although an elevated arterial K+ concentration has also been proposed as a mediator of exercise hyperpnoea (Band & Linton, 1986; Burger et al. 1988), this is not a universally accepted hypothesis (Casaburi et al. 1995) but, in any case, cannot explain our findings as we observed a decrease in arterial K+ concentration during insulin infusion. Insulin infusion is well known to induce hypokalaemia (Strakosch et al. 1976) through an α-adrenoceptor mechanism (Fisher et al. 1991). That the feedforward mechanism controlling exercise hyperpnoea might be a learned phenomenon, which improves with repeated ‘practice’ in a similar way perhaps to learning motor skills (Marr, 1969), was hypothesized by Somjen (1992). Although the testing of this hypothesis has led to conflicting findings, a recent study (Wood et al. 2003) does provide strong evidence that, in humans at least, learning and memory are required to maintain PET,CO2 during exercise. That our data, obtained in anaesthetized rats, also demonstrates a precise matching of ventilation to metabolism would suggest either that such higher processes are not found in mammals other than humans, or that, in the absence of a learned component, a blood-borne factor(s) can account for the entire response. The carotid bodies also appear to play a key role in maintaining blood glucose levels during exercise, presumably by initiating sympathetic-mediated elevations in blood noradrenaline and glucagon (Koyama et al. 2001). Again, the precise stimulus is not known but there is no reason to believe that it is not the same one that relates ventilation to metabolism where there may be no necessity for a feedback error signal. The anaesthetized model of insulin-mediated increased metabolic rate may, however, provide a useful new model to investigate further the means by which ventilation and glucoregulation are matched to metabolism during exercise.
In conclusion, our results demonstrate that insulin-induced hypoglycaemia can produce a carotid body-dependent increase in ventilation. In addition, it is shown that this response is directly proportional to the increased metabolism induced and thus, the increased ventilation observed is an appropriate hyperpnoea. In vitro data demonstrates that a lowered [glucose] does not increase the baseline carotid body chemoafferent discharge during hyperoxia and this suggests that the in vivo effects of insulin-induced hypoglycaemia upon ventilation and arterial blood gas tensions are more likely induced by the associated increase in systemic metabolic rate rather than the hypoglycaemia per se. We suggest that the role of the carotid bodies in glucose homeostasis in vivo is mediated through its transduction of a metabolically derived blood-borne factor that may also provide the link between exercise and ventilation. The nature and mechanism of action of this factor is not known.
Acknowledgments
We thank the British Heart Foundation and the Wellcome Trust for their financial support of this research. We thank Dr Teresa Thomas for her support in recording phrenic nerve discharge. I.B.-J. is sponsored by King Khalid University, Saudi Arabia.
References
- Alfaro V, Palacios L. Components of the blood acid-base disturbance that accompanies urethane anaesthesia in rats during normothermia and hypothermia. Clin Exp Pharmacol Physiol. 1997;24:498–502. doi: 10.1111/j.1440-1681.1997.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Almaraz L, Obeso A, Gonzalez C. Metabolic dissociation of carotid body chemoreceptors responses to different types of stimulation: preliminary findings. In: Pallot DJ, editor. The Peripheral Arterial Chemoreceptors. London: Croom-Helm; 1984. pp. 141–151. [Google Scholar]
- Alvarez-Buylla R, de Alvarez-Buylla ER. Carotid sinus receptors participate in glucose homeostasis. Respir Physiol. 1988;72:347–359. doi: 10.1016/0034-5687(88)90093-x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla R, de Alvarez-Buylla ER. Changes in blood glucose concentration in the carotid body-sinus modify brain glucose retention. Brain Res. 1994;654:167–170. doi: 10.1016/0006-8993(94)91585-7. [DOI] [PubMed] [Google Scholar]
- Bamford OS, Carroll JL. Dynamic ventilatory responses in rats: normal development and effects of prenatal nicotine exposure. Respir Physiol. 1999;117:29–40. doi: 10.1016/s0034-5687(99)00054-7. [DOI] [PubMed] [Google Scholar]
- Band DM, Linton RAF. The effect of potassium on carotid-body chemoreceptor discharge in the anesthetized cat. J Physiol. 1986;381:39–47. doi: 10.1113/jphysiol.1986.sp016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band DM, Wolff CB. Respiratory oscillations in discharge frequency of chemoreceptor afferents in sinus nerve of anaesthetised cats at normal and low arterial oxygen tensions. J Physiol. 1978;282:1–6. doi: 10.1113/jphysiol.1978.sp012443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe C, Al-Hashem F, Conway AF, Dubuis E, Vandier C, Kumar P. A possible dual site of action for carbon monoxide-mediated chemoexcitation in the rat carotid body. J Physiol. 2002;543:933–945. doi: 10.1113/jphysiol.2001.015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin-Jaliah I, Kumar P. The carotid body-mediated ventilatory response to insulin-induced hypoglycaemia in anaesthetized rats. J Physiol. 2003;551.P:C44. [Google Scholar]
- Bin-Jaliah I, Maskell PD, Kumar P. Low glucose and carotid body-mediated CO2 chemosensitivity in the rat. J Physiol. 2003;552P:C47. [Google Scholar]
- Burcelin R, Dolci W, Thorens B. Glucose sensing by the hepatoportal sensor is GLUT2-dependent: in vivo analysis in GLUT2-null mice. Diabetes. 2000;49:1643–1648. doi: 10.2337/diabetes.49.10.1643. [DOI] [PubMed] [Google Scholar]
- Burger RE, Estavillo JA, Kumar P, Nye PC, Paterson DJ. Effects of potassium, oxygen and carbon dioxide on the steady-state discharge of cat carotid body chemoreceptors. J Physiol. 1988;401:519–531. doi: 10.1113/jphysiol.1988.sp017176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane P, Artal R, Bergman RN. Putative hypothalamic glucoreceptors play no essential role in the response to moderate hypoglycemia. Diabetes. 1986;35:268–277. doi: 10.2337/diab.35.3.268. [DOI] [PubMed] [Google Scholar]
- Cane P, Haun CK, Evered J, Youn JH, Bergman RN. Response to deep hypoglycemia does not involve glucoreceptors in carotid perfused tissue. Am J Physiol Endocrinol Metab. 1988;255:E680–E687. doi: 10.1152/ajpendo.1988.255.5.E680. [DOI] [PubMed] [Google Scholar]
- Carruba MO, Bondiolotti G, Picotti GB, Catteruccia N, Da Prada M. Effects of diethyl ether, halothane, ketamine and urethane on sympathetic activity in the rat. Eur J Pharmacol. 1987;134:15–24. doi: 10.1016/0014-2999(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Casaburi R, Stringer WW, Singer E. Comparison of arterial potassium and ventilatory dynamics during sinusoidal work rate variation in man. J Physiol. 1995;485:571–580. doi: 10.1113/jphysiol.1995.sp020753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley JA, Ellis P, Ungar A. The reflex release of adrenaline and noradrenaline from the adrenal glands of cats and dogs. J Physiol. 1980;298:71–78. doi: 10.1113/jphysiol.1980.sp013067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MDB. Peripheral Arterial Chemoreceptors and Respiratory-Cardiovascular Integration. Oxford: Oxford University Press; 1997. [Google Scholar]
- Donovan C, Halter J, Bergman R. Importance of hepatic glucoreceptors in sympathoadrenal response to hypoglycemia. Diabetes. 1991;40:155–158. doi: 10.2337/diab.40.1.155. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes. 2002;51:2056–2065. doi: 10.2337/diabetes.51.7.2056. [DOI] [PubMed] [Google Scholar]
- Eager KR, Robinson BJ, Galletly DC, Miller JH. Endogenous opioid modulation of hypercapnic-stimulated respiration in the rat. Respir Physiol. 1994;96:13–24. doi: 10.1016/0034-5687(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Fisher BM, Thomson I, Hepburn DA, Frier BM. Effects of adrenergic blockade on serum potassium changes in response to acute insulin-induced hypoglycemia in nondiabetic humans. Diabetes Care. 1991;14:548–552. doi: 10.2337/diacare.14.7.548. [DOI] [PubMed] [Google Scholar]
- Garcia MdIA, Millan C, Balmaceda-Aguilera C, Castro T, Pastor P, Montecinos H, Reinicke K, Zuniga F, Vera JC, Onate SA, Nualart F. Hypothalamic ependymal-glial cells express the glucose transporter GLUT2, a protein involved in glucose sensing. J Neurochem. 2003;86:709–724. doi: 10.1046/j.1471-4159.2003.01892.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Goodman NW, Nail BS, Torrance RW. Oscillations in the discharge of single carotid chemorecptor fibers of the cat. Respir Physiol. 1974;20:251–269. doi: 10.1016/0034-5687(74)90023-1. [DOI] [PubMed] [Google Scholar]
- Haldane JS, Priestley JG. The regulation of the lung-ventilation. J Physiol. 1905;32:225–266. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes FJ, Cheema-Dhadli S, Halperin RM, Robinson L, Halperin ML. Effect of anaesthesia on insulin-induced hypoglycemia in rabbits. Can J Physiol Pharmacol. 1988;66:1531–1537. doi: 10.1139/y88-250. [DOI] [PubMed] [Google Scholar]
- Heimberg H, De Vos A, Moens K, Quartier E, Bouwens L, Pipeleers D, Van Schaftingen E, Madsen O, Schuit F. The glucose sensor protein glucokinase is expressed in glucagon-producing alpha -cells. Proc Natl Acad Sci U S A. 1996;93:7036–7041. doi: 10.1073/pnas.93.14.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevener AL, Bergman RN, Donovan CM. Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes. 1997;46:1521–1525. doi: 10.2337/diab.46.9.1521. [DOI] [PubMed] [Google Scholar]
- Hevener AL, Bergman RN, Donovan CM. Hypoglycemic detection does not occur in the hepatic artery or liver: findings consistent with a portal vein glucosensor locus. Diabetes. 2001;50:399–403. doi: 10.2337/diabetes.50.2.399. [DOI] [PubMed] [Google Scholar]
- Hornbein TF, Griffo ZJ, Roos A. Quantitation of chemoreceptor activity: interrelation of hypoxia and hypercapnia. J Neurophysiol. 1961;24:561–568. doi: 10.1152/jn.1961.24.6.561. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Coker RH, Denny JC, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Role of carotid bodies in control of the neuroendocrine response to exercise. Am J Physiol Endocrinol Metab. 2001;281:E742–E748. doi: 10.1152/ajpendo.2001.281.4.E742. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes. 2000;49:1434–1442. doi: 10.2337/diabetes.49.9.1434. [DOI] [PubMed] [Google Scholar]
- Kumar P, Nye PCG, Torrance RW. Do oxygen-tension variations contribute to the respiratory oscillations of chemoreceptor discharge in the cat. J Physiol. 1988;395:531–552. doi: 10.1113/jphysiol.1988.sp016933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CH, Bagby GJ, Hargrove DM, Hyde PM, Spitzer JJ. Alterations in glucose kinetics induced by pentobarbital anesthesia. Am J Physiol Endocrinol Metab. 1987;253:E657–E663. doi: 10.1152/ajpendo.1987.253.6.E657. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Routh VH. Brain glucosensing and the K(ATP) channel. Nat Neurosci. 2001;4:459–460. doi: 10.1038/87405. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Routh VH. CNS sensing and regulation of peripheral glucose levels. Int Rev Neurobiol. 2002;51:219–258. doi: 10.1016/s0074-7742(02)51007-2. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev. 1994;74:543–593. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes. 1990;39:647–652. doi: 10.2337/diab.39.6.647. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Atrens DM. Insulin increases energy expenditure and respiratory quotient in the rat. Pharmacol Biochem Behav. 1989;34:765–768. doi: 10.1016/0091-3057(89)90272-4. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Oomura Y. Glucose responding neurons in the nucleus tractus solitarius of the rat: in vitro study. Brain Res. 1984;307:109–116. doi: 10.1016/0006-8993(84)90466-9. [DOI] [PubMed] [Google Scholar]
- Obeso A, Almaraz L, Gonzalez C. Effects of 2-deoxy-D-glucose on in vitro cat carotid body. Brain Res. 1986;371:25–36. doi: 10.1016/0006-8993(86)90806-1. [DOI] [PubMed] [Google Scholar]
- Ono T, Nishino H, Fukuda M, Sasaki K, Muramoto K-I, Oomura Y. Glucoresponsive neurons in rat ventromedial hypothalamic tissue slices in vitro. Brain Res. 1982;232:494–499. doi: 10.1016/0006-8993(82)90295-5. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- Pardal R, Lopez-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci. 2002;5:197–198. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- Pardal R, Ludewig U, Garcia-Hirschfeld J, Lopez-Barneo J. Secretory responses of intact glomus cells in thin slices of rat carotid body to hypoxia and tetraethylammonium. Proc Natl Acad Sci U S A. 2000;97:2361–2366. doi: 10.1073/pnas.030522297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DJ. Potassium and ventilation in exercise. J Appl Physiol. 1992;72:811–820. doi: 10.1152/jappl.1992.72.3.811. [DOI] [PubMed] [Google Scholar]
- Pepper DR, Landauer RC, Kumar P. Postnatal development of CO2–O2 interaction in the rat carotid body in vitro. J Physiol. 1995;485:531–541. doi: 10.1113/jphysiol.1995.sp020749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson EA, Bowes G, Townsend ER, Duffin J, Cooper JD. Carotid chemoreceptors in ventilatory responses to changes in venous CO2 load. J Appl Physiol. 1981;51:1398–1403. doi: 10.1152/jappl.1981.51.6.1398. [DOI] [PubMed] [Google Scholar]
- Routh VH. Glucose-sensing neurons: are they physiologically relevant. Physiol Behav. 2002;76:403–413. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J Neurophysiol. 1998;79:1733–1745. doi: 10.1152/jn.1998.79.4.1733. [DOI] [PubMed] [Google Scholar]
- Solnica B, Naskalski JW, Sieradzki J. Analytical performance of glucometers used for routine glucose self-monitoring of diabetic patients. Clin Chim Acta. 2003;331:29–35. doi: 10.1016/s0009-8981(03)00079-2. [DOI] [PubMed] [Google Scholar]
- Somjen GG. The missing error signal-regulation beyond negative feedback. News Physiol Sci. 1992;7:184–185. [Google Scholar]
- Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- Spergel D, Lahiri S, Wilson DF. Dependence of carotid chemosensory responses on metabolic substrates. Brain Res. 1992;596:80–88. doi: 10.1016/0006-8993(92)91535-m. [DOI] [PubMed] [Google Scholar]
- Strakosch CR, Stiel JN, Gyory AZ. Hypokalaemia occurring during insulin-induced hypoglycaemia. Aust N Z J Medical. 1976;6:314–316. doi: 10.1111/imj.1976.6.4.314. [DOI] [PubMed] [Google Scholar]
- Sun MK, Reis DJ. Urethane directly inhibits chemoreflex excitation of medullary vasomotor neurons in rats. Eur J Pharmacol. 1995;293:237–243. doi: 10.1016/0926-6917(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Swaminathan R, Anderson E, Dean H, Wales JK. Metabolic response to insulin induced hypoglycaemia in lean and obese subjects. Horm Metab Res. 1986;18:45–48. doi: 10.1055/s-2007-1012222. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Van Kessel AL, Burton GG. Interaction of physiological mechanisms during exercise. J Appl Physiol. 1967;22:71–85. doi: 10.1152/jappl.1967.22.1.71. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Whipp BJ, Koyal SN, Cleary MG. Effect of carotid body resection on ventilatory and acid-base control during exercise. J Appl Physiol. 1975;39:354–358. doi: 10.1152/jappl.1975.39.3.354. [DOI] [PubMed] [Google Scholar]
- Whipp BJ. Peripheral chemoreceptor control of exercise hyperpnea in humans. Med Sci Sports Exerc. 1994;26:337–347. [PubMed] [Google Scholar]
- Wood HE, Fatemian M, Robbins PA. A learned component of the ventilatory response to exercise in man. J Physiol. 2003;553:967–974. doi: 10.1113/jphysiol.2003.047597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto WS. Transmission of information by the arterial blood stream with particular reference to carbon dioxide. Biophys J. 1962;2:143–159. doi: 10.1016/s0006-3495(62)86846-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yettefti K, Orsini J-C, Perrin J. Characteristics of glycemia-sensitive neurons in the nucleus tractus solitarii: Possible involvement in nutritional regulation. Physiol Behav. 1997;61:93–100. doi: 10.1016/s0031-9384(96)00358-7. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhong H, Vollmer C, Nurse C. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol. 2000;525:143–158. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinker BA, Namdaran K, Wilson R, Lacy DB, Wasserman DH. Acute adaptation of carbohydrate metabolism to decreased arterial PO2. Am J Physiol Endocrinol Metab. 1994;266:E921–E929. doi: 10.1152/ajpendo.1994.266.6.E921. [DOI] [PubMed] [Google Scholar]