Abstract
Lactate is released from skeletal muscle in proportion to glucose uptake rates, and it leaves the cells via simple diffusion and two monocarboxylate transporter proteins, MCT1 and MCT4. In reponse to endurance training MCT1 – and possibly MCT4 – content in muscle increases. The MCTs have not previously been measured in patients with type 2 diabetes (Type 2), and the response to strength training is unknown. Ten Type 2 and seven healthy men (Control) strength-trained one leg (T) 3 times a week for 6 weeks while the other leg remained untrained (UT). Each session lasted no more than 30 min. After strength training, muscle biopsies were obtained and an isoglycaemic, hyperinsulinaemic clamp, combined with arterial and femoral venous catheterization of both legs, was carried out. During hyperinsulinaemia lactate release was always increased in T versus UT legs. MCT1 was lower (P<0.05) and MCT4 similar in Type 2 versus Control. With training, MCT1 content always increased, while MCT4 only increased in Control. Conclusions: MCT1 content in skeletal muscle in Type 2 is lower compared with healthy men. Strength training increases MCT1 content in healthy men and in Type 2, thus normalizing the content in Type 2.
When glucose disposal is stimulated with insulin (e.g. an hyperinsulinaemic clamp) plasma lactate concentration increases, and the concentration is positively correlated with the rate of glucose disposal (Yki-Jarvinen et al. 1990). Most likely, the increased lactate concentration during hyperinsulinaemia is the consequence of an increased glycolytic flux with subsequent conversion of pyruvate to lactate. Since the effect of insulin on glucose disposal occurs predominantly in skeletal muscle, the origin of the increased lactate in the blood is probably skeletal muscle, although extramuscular tissue may also contribute. In accordance with this, we have found increasing rates of lactate release from the legs with increasing plasma insulin concentrations both in young (Dela et al. 1992) and old (Dela et al. 1995b), healthy subjects and in patients with type 2 diabetes (Dela et al. 1995b).
In addition, we have consistently observed that the release of lactate during resting, hyperinsulinaemic conditions is significantly higher from endurance-trained compared with untrained skeletal muscle (Dela et al. 1992, 1995b). This finding is in accordance with the positive correlation to the rate of glucose disposal (Yki-Jarvinen et al. 1990), as training enhances insulin-mediated glucose uptake in skeletal muscle.
The intracellularly produced lactate leaves the cell via via simple diffusion and two monocarboxylate transporter proteins, MCT1 and MCT4 (Wilson et al. 1998). The density of MCT1 and/or MCT4 proteins in human skeletal muscle is elevated after a period of endurance training (Bonen et al. 1998; Pilegaard et al. 1999; Dubouchaud et al. 2000), although some training studies found no increase in MCT4 (Dubouchaud et al. 2000; Evertsen et al. 2001). Furthermore, lactate transport capacity is reduced in denervated (Pilegaard & Juel, 1995) and in hypokinetic (hindlimb suspension) (Dubouchaud et al. 1996) rat muscle and in humans with spinal cord injuries (Pilegaard et al. 1998). Thus, the lactate transport capacity seems to be related to the training status of the muscle.
In patients with type 2 diabetes, resting blood lactate concentrations may be elevated (Reaven et al. 1988; Chen et al. 1993; Avogaro et al. 1996) but studies on MCT1 and MCT4 content are lacking. Obese Zucker fa/fa rats with normal glucose but increased insulin plasma concentrations have increased plasma lactate concentrations and decreased fibre-type-dependent muscle MCT4 (to some extent also MCT1) content compared with control rats (Py et al. 2001). In streptozotocin (STZ)-induced diabetes (which is a model of type 1 diabetes), resting blood lactate is reported to be elevated (Py et al. 2002; Enoki et al. 2003), and STZ-induced diabetes in rats has also been found to decrease skeletal muscle lactate transport (Py et al. 2002). In these studies the STZ-induced diabetes was not associated with changes in MCT1 and MCT4 in skeletal muscle (Py et al. 2002), whereas a recent study in STZ-induced diabetic rats found a selective reduction in MCT1 and MCT4 density in some skeletal muscles and in the heart (Enoki et al. 2003). The reduction in transporter protein content was alleviated by endurance treadmill training (Enoki et al. 2003).
On the basis of this and our previous observations of increased lactate release from endurance-trained muscle during hyperinsulinaemic clamp conditions, we expected that lactate release would also be increased in strength-trained muscle. We hypothesized that strength training would increase the protein contents of MCT1 and MCT4 in human skeletal muscle – in line with the known effects of training on GLUT4 protein content (Dela et al. 1993, 1994; Holten et al. 2004). We used a one-legged training protocol, a model which is robust against biological variation and which has previously been used to demonstrate the effect of endurance training on skeletal muscle insulin sensitivity (Dela et al. 1995b). Secondly, we obtained muscle biopsies from both legs and analysed the MCT1 and MCT4 protein content.
Methods
Subjects and experimental protocol
Ten male Caucasian patients with type 2 diabetes (Type 2) and seven male Caucasian healthy control subjects (Control) without a family history of type 2 diabetes participated in the study, which was approved by the ethical committee of Copenhagen and Frederiksberg (KF 01-204/99). All of the subjects gave written, informed consent.
The time since diagnosis of type 2 diabetes ranged from 2 to 11 years. All the patients were treated with diet recommendations and in addition some patients were treated with 1000 mg day−1 tolbutamide (n=2), 7 mg day−1 glibenclamide (n=1), 1700 mg day−1 metformin (n=1), 5mg day−1 amlodipin (n=1), and 200μg day−1 cerivastatin (n=1). On the experimental day no medication was taken. None of the control subjects took any medication. The patients were similar to the control subjects with respect to age (62±2 versus 61±2 years), body weight (85±5 versus 78±3 kg), but the height (172±1 versus 178±2 cm (P<0.05)) was lower in Type 2 compared with Control. Thus, body mass index (BMI) was different (P<0.05) between Type 2 (28.3±1.2 kg m−2) and Control (24.5±0.8 kg m−2). Resting arterial blood pressure was 157±10 mmHg (systolic) and 82±6 mmHg (diastolic), and 147±6 and 74±3 mmHg, in Type 2 and Control, respectively). Other characteristics of the subjects are given in Table 1.
Table 1.
Biochemical characteristics of blood
| Type 2 diabetes (n=10) | Healthy control subjects (n=7) | |
|---|---|---|
| B-glucose (mmol l−1) | 7.9±0.9 | 4.7±0.3* |
| B-lactate (mmol l−1) | 0.77±0.08 | 0.64±0.07 |
| HbA1C (%) | 7.4±0.4 | 6.0±0.2* |
| S-insulin (pmol l−1) | 72±17 | 39±5* |
| S-C-peptide (pmol l−1) | 1019±115 | 664±63* |
B=whole blood; S=serum. Data are mean±s.e.m. and are fasting values
P<0.05. HbA1C=glycosylated haemoglobin.
In one of the healthy control subjects, femoral venous catheterization in one leg proved to be very difficult and for ethical reasons it had to be given up. Nevertheless, the experiment was continued without a venous catheter in one leg. Thus paired comparisons between the legs could not be done and therefore n=6 for all leg balance data, but n=7 for all other data (including biopsy data).
Training programme
All subjects participated in a 6 week strength-training programme. The focus of the programme was to have one leg performing strength-training exercises, while the other leg remained sedentary. The leg to be trained was chosen by drawing lots.
Training sessions were all supervised and took place three times a week, with each training session lasting no more than 30min. This included time for a warm-up, which involved light exercises for the upper body plus 10–12 repetitions with a light load on each leg followed by a 2min rest period. During the first and the last training session the subject's three-repetition maximum (3RM) was measured. One RM was calculated as 106% of the measured 3RM for each leg exercise (leg press, knee extension, hamstring curl). During the first 2 weeks of the training, the subjects performed 3 sets of 10 repetitions, utilizing a load equivalent to 50% of one RM. During weeks 3–6 the subjects performed 4 sets of 8–12 repetitions utilizing 70–80% of one RM. For the last 2 weeks of the 6 week period the load was adjusted so that all sets were exhaustive within 8–12 repetitions. The subjects rested for >90 s between sets, and for >2 min between lifting stations (different exercise equipment).
Experimental procedure
On the day after the last training session (i.e. in the trained state, ‘between’ sessions) an isoglycaemic, hyperinsulinaemic clamp, combined with arterio-venous catheterization of both legs, was performed. Having fasted since midnight (only water allowed thereafter), the subjects arrived in the laboratory in the morning. Leg volume (by water displacement) and thigh circumference (20 cm proximal to the patella) were measured. The subjects were weighed, their height was measured and then they were placed in a bed. Electrocardiogram (ECG) and heart rate were monitored by precordial electrodes. A catheter was inserted into a medial cubital vein for infusions of insulin and glucose (20%), and an arterial cannula was inserted into the radial or brachial artery for the sampling of blood and continuous monitoring of blood pressure. Teflon catheters were inserted into both femoral veins for the sampling of blood and measurement of leg blood flow (thermodilution technique) as previously described (Dela et al. 1995a).
After basal measurements, a two-step, sequential isoglycaemic, hyperinsulinaemic clamp was started. For each subject an 50 ml insulin infusate was prepared for each clamp step (clamp steps I and II) from insulin (Actrapid, Novo, Copenhagen, Denmark, 100IU ml−1), saline, and 2.5 ml of the subject's own plasma. At each clamp step insulin was given as a 2 ml bolus followed by constant infusion (rates of 28 and 480 mU min−1 m−2) for 120min each. Plasma glucose was maintained at isoglycaemia, i.e. the glucose concentration was kept at individual fasting plasma glucose concentrations throughout the clamp by frequent arterial blood samples analysed on an automatic glucose analyser (YSI 2300, Yellow Springs Instruments, USA), with subsequent adjustment of the glucose infusion rate. Arterial and femoral venous blood samples were drawn and blood flow measurements were made (3–5 single measurements in each leg at each time point) at 30 and 15min before start, and then after 90, 105 and 120min from the start of each clamp step. In the basal state – before initiation of the clamp – muscle biopsies were obtained from both legs.
Calculations and analytical procedures
Whole body glucose clearance rates during the final 30min of each clamp step were calculated as the glucose infusion rate divided by the glucose concentration in plasma. Leg balance of lactate was calculated as arterio-venous concentrations in whole blood difference multiplied by blood flow. Leg balance data are expressed relative to leg mass, assuming that the volume of one litre corresponds to one kilogram of leg. All samples were stored at –20°C until analysis, except for C-peptide and muscle biopsies which were stored at –80°C.
Blood lactate was measured on an automatic glucose/lactate analyser (YSI 2300, Yellow Springs International) with Triton-X added to the analysing buffer solution (Foxdal et al. 1992).
After excision the muscle biopsies were quickly cleaned from visible blood and fat and frozen immediately in liquid nitrogen. Biopsies were stored at –80°C until analysed. Before measurement of lactate dehydrogenase (LDH) activity (Roche/Hitachi 912 analyser) and lactate concentrations (Olsen, 1971), biopsies were freeze-dried, and connective tissue and fat tissue removed. Lactate concentrations in muscle are given as mmol (kg dry weight)−1, and thus contain both intracellular lactate and lactate molecules in the evaporated extracellular water.
MCT1 and MCT4 detection in total crude membranes (Western blotting)
The 30mg of each muscle sample was homogenized in sucrose buffer (250mm sucrose, 30mm Hepes, 2mm EGTA, 40mm NaCl, 2mm phenylmethylsulphonyl fluoride (PMSF), pH 7.4) using a Polytron 2100 and centrifuged at 1000 g for 5 min. The supernatant was spun at 190 000 g for 90min at 4°C in a high-speed centrifuge equipped with a swing-out bucket rotor. The resulting pellet (corresponding to the membrane fraction) was re-suspended in Tris-SDS (10mm Tris, 4% SDS, 1mm EDTA, 2mm PMSF, pH 7.4) and protein content determined with a bovine serum albumin (BSA) standard (DC protein assay, Bio-Rad). Samples were mixed 1 : 1 with sample buffer containing 10% SDS, 5% glycerol, 10mm Tris-HCl, 1mm EDTA, 10mm dithiothreitol, and subjected to SDS–PAGE (ExcelGel 8–18% gradient gel). The amount of protein per lane was 6μg. The separated proteins were electro-blotted onto a Millipore Immobilon-P polyvinylidene difluoride membrane. This membrane was blocked by 1% BSA, 0.5% low fat dry milk, 0.1% Tween-20, and incubated with the primary antibody diluted in a BSA-containing buffer. After treatment with the horseradish peroxidase (HRP)-coupled secondary antibody, it was repeatedly washed with distilled water, 0.05% Tween-20 and 1 m NaCl. The membrane was then incubated with ECL or ECL + reagents (Amersham) and visualized on film. Scanning the film and analysing band intensities with SigmaGel software yielded quantities of protein. Membranes were used for more than one primary antibody taking advantage of the different protein molecular weights. Some membranes were re-used after treatment with Re-Blot Plus (Chemicon Int.). The antibodies to the human lactate–H+ cotransporter isoforms MCT1 and MCT4 were provided by Professor A. P. Halestrap (Bristol, UK; Price et al. 1998).
Statistics
Results are presented as mean±s.e.m. Analysis of variance for repeated measures was used to detect differences in lactate balances across the trained and the untrained legs in the two groups. When a significant main effect was observed, the Student-Newman-Keuls test was used post hoc. Other comparisons were tested with Student's t tests, paired and unpaired as appropriate. The SigmaStat version 2.03 software package was used for all statistical calculations. P<0.05 was considered significant in two-tailed testing.
Results
Leg size and strength
After the strength-training programme, leg volumes and sizes were similar for Control and Type 2, with slightly higher values in T versus UT legs (Table 2). Muscle strength and the training-induced improvements were similar in the two groups (Table 2).
Table 2.
Leg size and strength
| Type 2 diabetes (n=10) | Healthy control subjects (n=7) | |||||
|---|---|---|---|---|---|---|
| UT | T | % difference | UT | T | % difference | |
| Leg volume (l) | 12.5±0.7 | 12.7±0.6 | 2.3±2.2 | 12.9±1.0 | 13.4±1.0 | 4.1±1.5 |
| aThigh circumference (cm) | 52.5±1.5 | 54.3±3.4* | 3.4±1.1 | 52.8±3.1 | 53.4±2.7 | 1.5±1.6 |
| Knee extension (kg) | 27±2 | 37±2* | 42±8 | 28±3 | 36±3* | 29±1 |
| Leg press (kg) | 80±7 | 135±13* | 75±7 | 74±8 | 124±5* | 77±15 |
n=8 and 4 for Type 2 and Control, respectively. Data are mean±s.e.m.
P<0.05 between untrained (UT) and trained (T) legs. No significant difference existed between corresponding measurements in the two study groups.
Insulin concentrations and glucose clearance rates during clamp
Insulin infusion elevated plasma insulin concentrations to 377±26 and 270±20 pmol l−1, in Type 2 and Control, respectively (P<0.05), in clamp step I, while at clamp step II plasma insulin concentrations were 12 453±856 and 11 066±874 pmol l−1 (P > 0.05), in Type 2 and Control, respectively.
Whole body glucose clearance rates were lower in Type 2 compared with Control (2.5±0.6 and 8.9±0.7 versus 5.7±0.9 and 14.4±0.7 ml min−1 (kg body weight)−1 in the two clamp steps, respectively; both P<0.05).
Lactate concentrations and release from trained and untrained legs
Arterial concentrations of lactate were similar between Control and Type 2 before insulin infusion began and the concentrations also increased (P<0.05) similarly in the two groups with insulin infusion (Fig. 1).
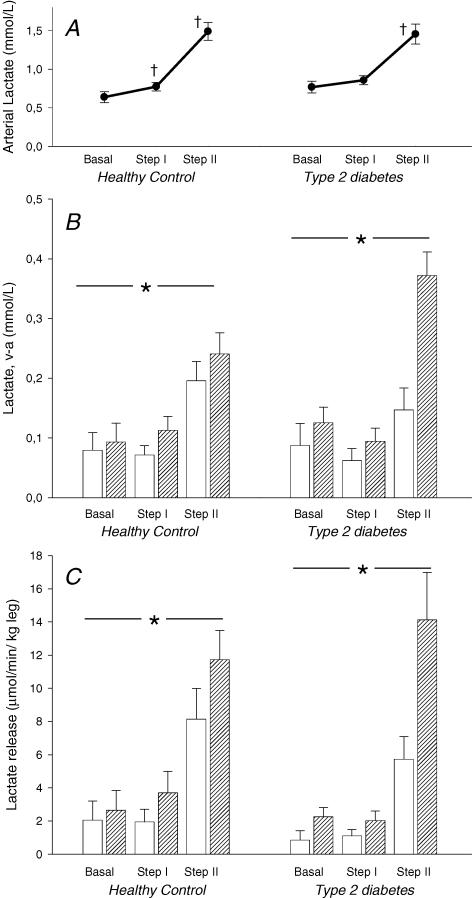
Figure 1. Blood lactate measurements from ten patients with type 2 diabetes and in six healthy control subjects after completion of 6 weeks of one-legged strength training.
Measurements were taken before and during an hyperinsulinaemic, isoglycaemic clamp combined with arterial-venous catheterization of both legs. All values are mean±s.e.m.A, arterial blood lactate concentration. † Significant difference (P<0.05) from previous value. B, venous–arterial differences in blood lactate across trained (hatched columns) and untrained (open columns) legs.*Main effect of training in both Control (P=0.014) and Type 2 (P<0.001). C, lactate balance across the legs. Positive value=release. *Main effect of training in both Control and Type 2 (both P<0.05).
Arterial–femoral venous differences in lactate were significantly increased in the trained leg in Type 2 (main effect P<0.001) and Control (main effect P=0.014), with no difference between the groups (Fig. 1).
Insulin infusion increased leg blood flow in both groups, and leg blood flow was higher in Control compared with Type 2 (main effect: P=0.034) (Table 3).
Table 3.
Leg blood flow (ml min−1 (kg leg)−1)
| Type 2 diabetes (n=10) | Healthy control subjects (n=7) | |||
|---|---|---|---|---|
| UT | T | UT | T | |
| Basal | 15±2 | 18±2 | 23±6 | 26±4 |
| Clamp step I | 18±2 | 24±3* | 26±5 | 31±6 |
| Clamp step II | 29±3 | 35±4* | 41±6 | 49±4* |
Data are mean±s.e.m.
P<0.05 between untrained (UT) and trained (T) legs. Leg blood flow was higher in Type 2 compared with Control (main effect, P=0.034).
Lacate release from the legs was increased in trained compared with untrained legs in both Type 2 (main effect P=0.004) and Control (main effect P<0.001; Fig. 1). Post hoc statistical tests showed a significant difference between T and UT legs during clamp step I (Control P=0.004) and step II (Control and Type 2, both P<0.001; Fig. 1).
Biopsy data
MCT1
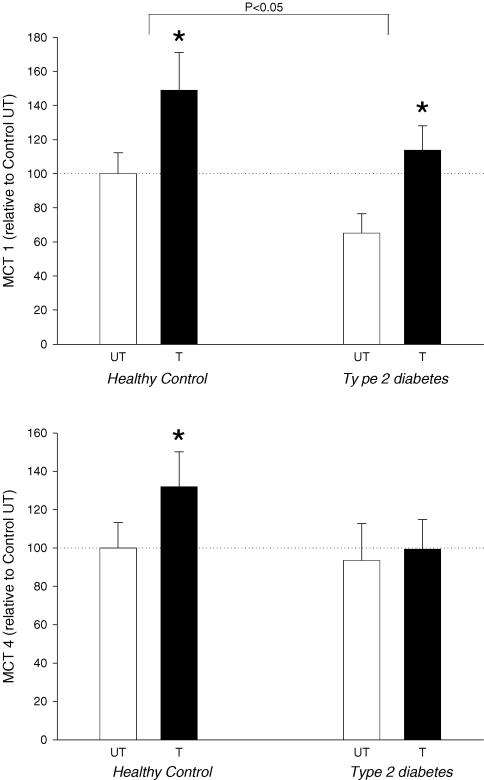
MCT1 content in the skeletal muscle was significantly lower in Type 2 compared with Control (P = 0.04; values from trained and untrained muscle pooled within each group; Fig. 2). Training resulted in +48% and +75% higher MCT1 content in Control and Type 2, respectively (both P<0.05; Fig. 2).
Figure 2. Monocarboxylase transporters (MCT) 1 and 4 measured as protein content in skeletal muscle biopsies from ten patients with type 2 diabetes and seven healthy control subjects after completion of 6 weeks of one-legged strength training.
Approximately 16 h after the last training session, muscle biopsies were obtained from both the trained (T) and untrained (UT) leg. Data are mean±s.e.m. and are expressed relative to the protein content in UT muscle from healthy, control subjects. MCT1 content was significantly lower in muscle from patients with type 2 diabetes (main effect; P<0.05). *Significant difference between trained and untrained leg (P<0.05).
MCT4
MCT4 content in the skeletal muscle was similar in Control and Type 2 (Fig. 2). In Control an increase (+32%) was seen with training (P<0.05), but in Type 2 the +6% increase did not attain statistical significance (Fig. 2).
Lactate
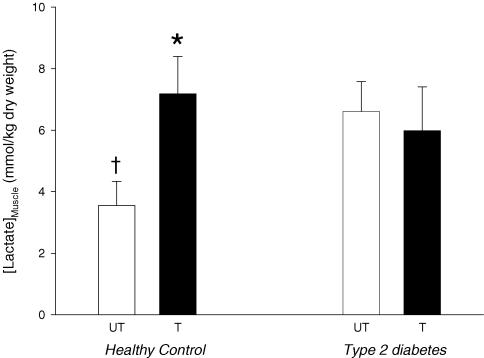
Lactate concentration in the untrained muscle was significantly lower in Control compared with Type 2 (P<0.05), but with training the intramuscular lactate concentration increased (P<0.05) to levels seen in Type 2 (Fig. 3). No effect of training was seen in Type 2 (Fig. 3).
Figure 3. Lactate concentration in skeletal muscle biopsies from ten patients with type 2 diabetes and six healthy control subjects after completion of 6 weeks of one-legged strength training.
Approximately 16 h after the last training session, muscle biopsies were obtained from both the trained (T) and untrained (UT) leg. Data are mean±s.e.m.* Significant difference (P<0.05) between trained and untrained legs; † significant difference from UT muscle in patients with type 2 diabetes.
LDH
The enzyme activity of LDH did not increase with training in either Control (UT: 840±62, T: 922±75 μmol min−1 (g dry weight of muscle tissue)−1) or Type 2 [UT: 966±93, T: 1069±185 μmol min−1 (g dry weight of muscle tissue)−1].
Discussion
The new findings in the present study are: (a) MCT1 content in skeletal muscle in patients with type 2 diabetes is lower compared with healthy men; (b) strength training increases MCT1 content in healthy men and in patients with type 2 diabetes, thus normalizing the content in the latter group; (c) the increase in MCT1 and MCT4 content occurred in response to a low training effort.
The difference in MCT1 content in the untrained skeletal muscle between the patients with type 2 diabetes and the healthy controls was substantial, 35%. MCT1 is considered to be the major monocarboxylate transporter in the oxidative slow-twitch muscle fibres, while the MCT4 isoform is found mainly in the glycolytic fast-twitch fibres (Wilson et al. 1998). However, this preferential location of the transporters cannot explain the difference in MCT1 content between Type 2 and Control in the present study, because – as reported elsewhere – the ratio of slow versus fast-twitch fibre types was similar in these subjects (and between trained and untrained muscle) (Holten et al. 2004). The lower MCT1 content in Type 2 versus Control also cannot be explained by differences in leg muscle strength between the two groups. Furthermore, the MCT1 content in the muscles (Fig. 2) does not seem to vary in parallel with the intracellular lactate concentrations (Fig. 3) or with blood lactate concentrations (Table 1). Although the diminished MCT1 content in untrained muscle in Type 2 versus Control might fit well with the increased intracellular lactate concentration in the former versus the latter, the training-induced increase in MCT1 content in Control (Fig. 2) was not accompanied by a decrease in intracellular lactate concentration (Fig. 3). The present findings in humans are not in agreement with data from known animal studies. Thus, in the insulin-resistant obese Zucker fa/fa rat the densities of muscle MCT1 and MCT4 are both decreased in the red tibialis anterior muscle, and MCT4 also in the extensor digitorum longus and soleus muscles (Py et al. 2001). In animals with STZ-induced diabetes both unchanged (Py et al. 2002) and decreased (Enoki et al. 2003) MCT1 and MCT4 content is found in skeletal muscle. However, the animal model used does not perfectly mimic human type 2 diabetes. For example in the obese Zucker fa/fa rats hyperglycaemia was not present (Py et al. 2001) and in the STZ-treated rats circulating glucose concentration was elevated by a factor of 3–4 while body weights were less than control animals (Py et al. 2002; Enoki et al. 2003). Altogether, we are unable to explain the difference in MCT1 content between Type 2 and Control subjects and this finding warrants further (human) studies.
It is known that high intensity endurance training increases MCT1 content in healthy, untrained people. Thus, Dubouchaud et al. used bicycle ergometer training for 1 h day−1, 6 days week−1 at 75% of for nine weeks + interval training and obtained a ∼90% increase (Dubouchaud et al. 2000), Bonen et al. also used bicycle ergometers but for 2 h day−1 at 60% of for 7–8 days and obtained an 18% increase (Bonen et al. 1998), and Pilegaard et al. used 3–5 sets of intermittent one-legged knee extensor exercise training for 3–5 days week−1 and obtained a 70% increase (Pilegaard et al. 1999). However, in already highly trained athletes no effect of moderate or high-intensity training was found on MCT1 content (Evertsen et al. 2001). With the present study, it can now be concluded that the type of training is not of importance for the increase in MCT1 content in skeletal muscle.
Apart from the type of training, it is remarkable that the quite low training effort used in the present study (3 sessions week−1 for 6 weeks, with each session lasting no more than 30 min), elicited increases in MCT1 content comparable to those using high intensity endurance training increase (Bonen et al. 1998; Pilegaard et al. 1999; Dubouchaud et al. 2000). In fact, the present strength-training programme was moderate in intensity, using low weights but a high number of repetitions. This was done in order to avoid exercises which would substantially increase blood pressures.
What is then the signal behind the training-induced adaptation of MCT1 content? Due to the model used in the present study, humoral blood-borne factors can be excluded. The adaptation of MCT1 content must be based upon local, contraction-induced factors. One such factor could be the local formation of lactate during the actual training bouts. Although we did not measure blood lactate concentrations during the training sessions in the present study, it is reasonable to believe that blood lactate concentrations did not reach the 5–6 mmol l−1 seen in the study by Pilegaard et al. (1999) or which must have been present in the previously used high intensity endurance training/interval training programmes (Bonen et al. 1998; Dubouchaud et al. 2000). However, substantial lactate accumulations in the trained muscle may not be necessary for mediating increases in MCT gene transcription and protein expression, but the signal basically remains unknown – as with the training-induced increase in GLUT4.
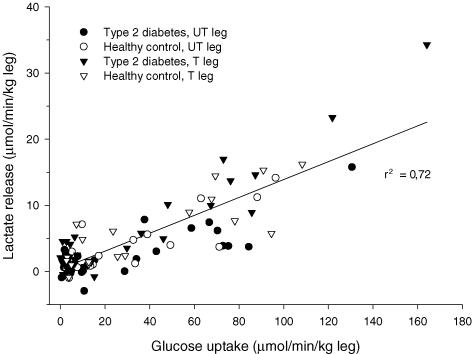
In this study we have confirmed our previous findings of increased lactate release from trained muscle during insulin stimulation (Dela et al. 1992, 1995b) (Fig. 1). The increased release was not dependent on the observed larger blood flow rates in the trained leg, as the difference between the trained and the untrained leg in arterial–femoral venous differences attained statistical significance. The reason for the enhanced lactate release from trained muscle is most likely a consequence of increased glucose uptake rates in trained muscle and subsequent enhancement of glycolysis, in agreement with earlier reports (Yki-Jarvinen et al. 1990), and further supported by the positive correlation between lactate release and glucose uptake rates found the present study (Fig. 4). The MCT1 and MCT4 densities did not correlate with leg lactate balances before or during insulin stimulation.
Figure 4. Relationship between leg lactate release and glucose uptake rates in trained (T) and untrained (UT) legs of healthy subjects and patients with type 2 diabetes.
Data taken from measurements at baseline and during an isoglycaemic, hyperinsulinaemic clamp combined with arterio-venous catheterization of both trained (T) and untrained (UT) legs. Squared value of Pearson's product moment correlation coefficient is shown.
The finding that MCT4 content increased in response to the strength-training programme in the healthy men but not in the patients is noteworthy (Fig. 2). Only one study in humans has previously reported an increase in MCT4, and the 32% increase in the present study is similar to that study (+33%) (Pilegaard et al. 1999). The reason for the lack of increase in MCT4 in the patients with type 2 diabetes is unknown to us, but the result is in line with previous indications of major disturbances in intracellular lactate metabolism in skeletal muscle of these patients (Avogaro et al. 1996). Furthermore, the difference in response illustrates that the regulation of the MCT1 and MCT4 isoforms are quite different.
In summary, in this study we report that MCT1 content is reduced in skeletal muscle from patients with type 2 diabetes, but strength training normalizes the reduction. In healthy people, but not in patients with type 2 diabetes, MCT4 increases in response to strength training. Lactate release from trained muscle is increased during hyperinsulinaemia, and lactate release rates from muscle are positively correlated with glucose uptake rates.
Acknowledgments
The financial support from the Danish National Research Foundation (J. nr. 504-14), the Danish Diabetes Association, the Novo-Nordisk Foundation, The Foundation of 1870, Jacob and Olga Madsens Foundation, The Danish Heart Foundation, and the Danish Medical Research Council is gratefully acknowledged. Regitze Kraunsøe and Jeppe Bach are thanked for excellent technical assistance.
References
- Avogaro A, Toffolo G, Miola M, Valerio A, Tiengo A, Cobelli C, Del Prato S. Intracellular lactate- and pyruvate-interconversion rates are increased in muscle tissue of non-insulin-dependent diabetic individuals. J Clin Invest. 1996;98:108–115. doi: 10.1172/JCI118754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen A, McCullagh KJ, Putman CT, Hultman E, Jones NL, Heigenhauser GJ. Short-term training increases human muscle MCT1 and femoral venous lactate in relation to muscle lactate. Am J Physiol. 1998;274:E102–E107. doi: 10.1152/ajpendo.1998.274.1.E102. [DOI] [PubMed] [Google Scholar]
- Chen YD, Varasteh BB, Reaven GM. Plasma lactate concentration in obesity and type 2 diabetes. Diabete Metab. 1993;19:348–354. [PubMed] [Google Scholar]
- Dela F, Handberg A, Mikines KJ, Vinten J, Galbo H. GLUT 4 and insulin receptor binding and kinase activity in trained human muscle. J Physiol. 1993;469:615–624. doi: 10.1113/jphysiol.1993.sp019833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela F, Larsen JJ, Mikines KJ, Galbo H. Normal effect of insulin to stimulate leg blood flow in NIDDM. Diabetes. 1995a;44:221–226. doi: 10.2337/diab.44.2.221. [DOI] [PubMed] [Google Scholar]
- Dela F, Mikines KJ, Larsen JJ, Ploug T, Petersen LN, Galbo H. Insulin stimulated muscle glucose clearance in patients with type 2 diabetes mellitus. Effects of one-legged physical training. Diabetes. 1995b;44:1010–1020. doi: 10.2337/diab.44.9.1010. [DOI] [PubMed] [Google Scholar]
- Dela F, Mikines KJ, Linstow VM, Secher NH, Galbo H. Effect of training on insulin mediated glucose uptake in human skeletal muscle. Am J Physiol. 1992;263:E1134–E1143. doi: 10.1152/ajpendo.2006.263.6.E1134. [DOI] [PubMed] [Google Scholar]
- Dela F, Ploug T, Handberg A, Petersen LN, Larsen JJ, Mikines KJ, Galbo H. Physical training increases muscle GLUT-4 protein and mRNA in patients with NIDDM. Diabetes. 1994;43:862–865. doi: 10.2337/diab.43.7.862. [DOI] [PubMed] [Google Scholar]
- Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E571–E579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- Dubouchaud H, Granier P, Mercier J, Le Peuch C, Prefaut C. Lactate uptake by skeletal muscle sarcolemmal vesicles decreases after 4 wk of hindlimb unweighting in rats. J Appl Physiol. 1996;80:416–421. doi: 10.1152/jappl.1996.80.2.416. [DOI] [PubMed] [Google Scholar]
- Enoki T, Yoshida Y, Hatta H, Bonen A. Exercise training alleviates MCT1 and MCT4 reductions in heart and skeletal muscles of STZ-induced diabetic rats. J Appl Physiol. 2003;94:2433–2438. doi: 10.1152/japplphysiol.01155.2002. [DOI] [PubMed] [Google Scholar]
- Evertsen F, Medbo JI, Bonen A. Effect of training intensity on muscle lactate transporters and lactate threshold of cross-country skiers. Acta Physiol Scand. 2001;173:195–205. doi: 10.1046/j.1365-201X.2001.00871.x. [DOI] [PubMed] [Google Scholar]
- Foxdal P, Bergvist Y, Eckerbom S, Sandhagen B. Improving lactate analysis with the YSI-2300-GL – hemolyzing blood samples makes results comparable with those for deproteinized whole blood. Clin Chem. 1992;38:2110–2114. [PubMed] [Google Scholar]
- Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JFP, Dela F. Strength training increases insulin-mediated glucose uptake, GLUT4 content and insulin signaling in skeletal muscle in patients with Type 2 diabetes. Diabetes. 2004;3:394–305. doi: 10.2337/diabetes.53.2.294. [DOI] [PubMed] [Google Scholar]
- Olsen C. An enzymatic fluorimetric micromethod for the determination of acetoacetate, [beta]-hydroxybutyrate, pyruvate and lactate. Clinica Chimica Acta. 1971;33:293–300. doi: 10.1016/0009-8981(71)90486-4. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Domino K, Noland T, Juel C, Hellsten Y, Halestrap AP, Bangsbo J. Effect of high-intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. Am J Physiol. 1999;276:E255–E261. doi: 10.1152/ajpendo.1999.276.2.E255. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Juel C. Lactate transport studied in sarcolemmal giant vesicles from rat skeletal muscles: effect of denervation. Am J Physiol. 1995;269:E679–E682. doi: 10.1152/ajpendo.1995.269.4.E679. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Mohr T, Kjaer M, Juel C. Lactate/H+ transport in skeletal muscle from spinal-cord-injured patients. Scand J Med Sports. 1998;8:98–101. doi: 10.1111/j.1600-0838.1998.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Price NT, Jackson VN, Halestrap AP. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem J. 1998;329:321–328. doi: 10.1042/bj3290321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py G, Lambert K, Milhavet O, Eydoux N, Prefaut C, Mercier J. Effects of streptozotocin-induced diabetes on markers of skeletal muscle metabolism and monocarboxylate transporter 1 to monocarboxylate transporter 4 transporters. Metabolism. 2002;51:807–813. doi: 10.1053/meta.2002.33343. [DOI] [PubMed] [Google Scholar]
- Py G, Lambert K, Perez-Martin A, Raynaud E, Prefaut C, Mercier J. Impaired sarcolemmal vesicle lactate uptake and skeletal muscle MCT1 and MCT4 expression in obese Zucker rats. Am J Physiol Endocrinol Metab. 2001;281:E1308–E1315. doi: 10.1152/ajpendo.2001.281.6.E1308. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Jackson VN, Heddle C, Price NT, Pilegaard H, Juel C, Bonen A, Montgomery I, Hutter OF, Halestrap AP. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J Biol Chem. 1998;273:15920–15926. doi: 10.1074/jbc.273.26.15920. [DOI] [PubMed] [Google Scholar]
- Yki-Jarvinen H, Bogardus C, Foley JE. Regulation of plasma lactate concentration in resting human subjects. Metabolism. 1990;39:859–864. doi: 10.1016/0026-0495(90)90133-w. [DOI] [PubMed] [Google Scholar]