Abstract
Nurr1, a transcription factor belonging to the family of nuclear receptors, is expressed at high levels immediately after birth. Gene-targeted mice lacking Nurr1 fail to develop midbrain dopaminergic neurones and do not survive beyond 24 h after birth. Dopamine (DA) levels may be regulated by Nurr1, and as DA is involved in both central and peripheral respiratory control, we hypothesized that lack of Nurr1 may impair breathing and cause death by respiratory failure. We demonstrate herein that Nurr1 newborn knockout mice have a severely disturbed breathing pattern characterized by hypoventilation, numerous apnoeas and failure to increase breathing when challenged with hypoxia. In heterozygote Nurr1 mice the response to hypoxia is also altered. Furthermore, the central respiratory rhythm, generated from isolated brainstem–spinal cord preparations, exhibits impaired response to hypoxia in mice lacking Nurr1. Moreover, Nurr1 is expressed in several respiratory-related regions of the nervous system, including the nucleus of the solitary tract, the nucleus ambiguus and the dorsal motor nucleus of the vagus nerve, and in the carotid bodies. The prominent Nurr1 expression in these areas, involved in respiratory control, along with the severe respiratory phenotype, indicates that Nurr1 plays a major role in the extrauterine adaption of respiratory control and the response to hypoxia.
The transition of the mammalian fetus to extrauterine life requires onset of continuous breathing and independent oral feeding. Relatively few genes involved in such adaptive responses have been identified. One such gene may be Nurr1 (NR4A2). Nurr1 belongs to the family of nuclear receptors that function as ligand-activated transcription factors (Law et al. 1992). Nuclear receptors are activated by binding steroid hormones, retinoids, vitamin D and other lipophilic signalling molecules (Aranda & Pascual, 2001). However, in the case of Nurr1 and several other members of this gene family, ligands have not yet been identified (Giguère, 1999). Nurr1 is expressed in the central nervous system both during development and in the adult (Zetterström et al. 1996). Nurr1 is essential for the development of midbrain dopamine (DA) neurones (Zetterström et al. 1997; Castillo et al. 1998; Saucedo-Cardenas et al. 1998; Wallén et al. 1999), with a complete agenesis of midbrain dopamine cells apparent at birth in Nurr1 knockout mice.
At birth, newborn Nurr1 mutant mice exhibit no gross morphological abnormalities but seem to adapt poorly to the extrauterine environment as they die within 24 h after birth (Zetterström et al. 1997). This early lethality is not likely to be a result of DA deficiency since DA deficient mice survive several weeks after birth (Zhou & Palmiter, 1995). Expression of Nurr1 is first detected at embryonic day (E) 10.5 in the mouse. At this stage, its expression is confined to the developing midbrain DA neurones and the dorsal motor nucleus of the vagus nerve (DMN X) (Zetterström et al. 1997; Wallén et al. 2001). Nurr1 expression subsequently expands to involve most levels of the neuraxis during development and adulthood. Nurr1 is also expressed in mature dopaminergic neurones during adulthood, suggesting that the protein is required for normal function of adult dopaminergic neurones. Moreover, along with the closely related orphan receptors Nur77 and Nor1, Nurr1 is classified as an immediate-early gene of the NGFI-B subfamily. These genes are rapidly but transiently induced by a variety of stimuli, e.g. brain ischaemia and other stressful insults (Maruyama et al. 1998). Nurr1 may thus also be involved in acute responses to external stimuli and adaptive responses essential at birth.
The receptor tyrosine kinase Ret, essential for the maturation of the respiratory network (Dauger et al. 2001b), is absent in the Nurr1−/− midbrain DA precursor cells as well as in the DMN X regions where Nurr1 and Ret are normally colocalized (Wallén et al. 2001). Ret, the signal transducing component for the glial cell line-derived neurotrophic factor (GDNF) family of ligands, thus appears to be regulated by Nurr1. GDNF and the brain-derived neurotrophic factor (BDNF) promote survival of dopaminergic sensory neurones in vivo (Erickson et al. 2001). BDNF−/− and GDNF−/− newborn mice display similar respiratory phenotypes characterized by a depressed and irregular respiratory frequency (Erickson et al. 1996; Erickson et al. 2001). In addition, mice lacking genes involved in the Ret–MASH pathway die within 24 h after birth and lack gastric milk. These mice have been shown to possess deficiencies in breathing and respiratory control due to dysfunction of brainstem respiratory regions (Shirasawa et al. 2000; Dauger et al. 2001b; Qian et al. 2001). We hypothesized that Nurr1 mutant mice might display disturbances in respiratory control.
To analyse respiration in Nurr1 mutant mice we measured the respiratory activity during normoxic and hypoxic conditions in vivo and in isolated brainstem–spinal cord preparations. We also examined the expression pattern of Nurr1 in respiratory-related regions in the brainstem and in carotid bodies, the major peripheral chemoreceptors which are rich in DA, assumed to be a major transmitter in hypoxic sensitivity (Finley & Katz, 1992; Bianchi et al. 1995). The results firmly establish that Nurr1 is involved in regulating vital extrauterine functions related to respiratory control.
Methods
Animals
Nurr1 knockout mice were generated and genotyped as previously described (Zetterström et al. 1997). Plugged females were housed individually, with a normal 12 h-light cycle, and provided with food and water ad libitum. All newborn mice used for in vivo experiments (n= 75), were observed during the end of pregnancy, born spontaneously between E18.5 and E19.5 and studied 12 h after birth. Mice used for in vitro experiments were either born spontaneously (n = 16) or at E18.5, in which case pregnant mice were killed by cervical dislocation and pups delivered within 2min by caesarean section (n = 30). Each pup delivered by caesarean section was gently stimulated by compressing the thorax and pinching the tail and the face was stimulated using a brush to mimic maternal care. Newborn mice delivered by caesarean section were kept with foster mothers after their own litters had been killed, or if spontaneously born were kept with their own mother. For immunohistochemical analyses, some dams were anaesthetized with pentobarbiturates before pups were removed by caesarean section. All animals were killed by decapitation. The regional animal ethics committee approved the experiments, which conformed to European Community regulations.
Plethysmography
Breath duration (TTOT, s), tidal volume (VT, μl g−1), and ventilation (VE, calculated as VT/TTOT and expressed in μl s−1 g−1) were measured non-invasively in unrestrained mice using whole-body flow barometric plethysmography (Epstein & Epstein, 1978). The plethysmograph was composed of two Plexiglas cylinders serving as animal (40 ml) and reference (100 ml) chambers connected to each other by a catheter (time constant, 2 s). The chambers were immersed in a thermoregulated water-bath that maintained their temperature constant at 30.5°C. A 50 ml min−1 flow of dry air (Bronkhorst Hi-Tec airflow stabilizer, Uurlo, the Netherlands) was divided into two 25 ml min−1 flows through the chambers, thus avoiding CO2 and water accumulation. Body temperature was assumed to be stable at 32°C. The differential pressure between the animal and the reference chambers (EFFA transducer, Asnières, France; range ± 0.1 mbar) was filtered (bandwidth, 0.05–15Hz at –3 dB), converted to a digital signal (MacAdios A/D 12-bits converter, GW Instruments, Somerville, MA, USA) at a sample rate of 100Hz, and processed by custom-written software (Superscope softwares II, GW Instruments). Calibration was performed before each session by injecting 2 μl of air into the animal chamber using a Hamilton syringe. The pressure rise induced by this injection was similar in magnitude to that induced by the VT of a newborn mouse.
After a familiarization period (1.3 ± 0.6 min), the pups were calm and baseline ventilation was recorded for 3 min. The airflow through the plethysmograph was subsequently changed to a hypoxic flow (5% O2, 95% N2) at the same flow rate (25 ml min−1) for 3 min, followed by a return to air for 6 min. We measured mouth temperature and body weight after each test. Then each newborn was killed by neck section, and tail tissue fragments were taken for Nurr1 genotyping. The brainstem was removed and frozen in liquid nitrogen for future analyses of Nurr1 expression (Dauger et al. 2001b).
Artefact-free periods of the ventilatory recordings were analysed. We discarded parts of the records in which large non-respiratory movements occurred; segments without individualized breaths or with large drifts exceeding twice the mean amplitude of the volume signal. Respiratory frequency (Rf, breaths min−1), TTOT, VT and VE were averaged over successive 30-s periods. Apnoea was defined as a pause in breathing lasting more than twice the duration of the immediately preceding breath. Ventilation (excluding periods of apnoea) was analysed over three periods: baseline in normoxia (the first 3min of air), the period of the peak ventilatory response (from 1min 30 s following the onset of the hypoxic stimulus to 1 min 30 s later), and the posthypoxic period (the final 3min of normoxia), see Fig. 2B, based on our previous analyses of the hypoxic response in newborn mice (Dauger et al. 2001a; Renolleau et al. 2001). Behaviour was scored continuously throughout the period of hypoxia. Behavioural arousal in response to hypoxia was defined as a stereotypic motor response characterized by sudden neck and forepaw extension (Dauger et al. 2001a). Arousal latency was the delay to arousal following the switch from air to hypoxia. All recordings and analyses were performed before genotype was known.
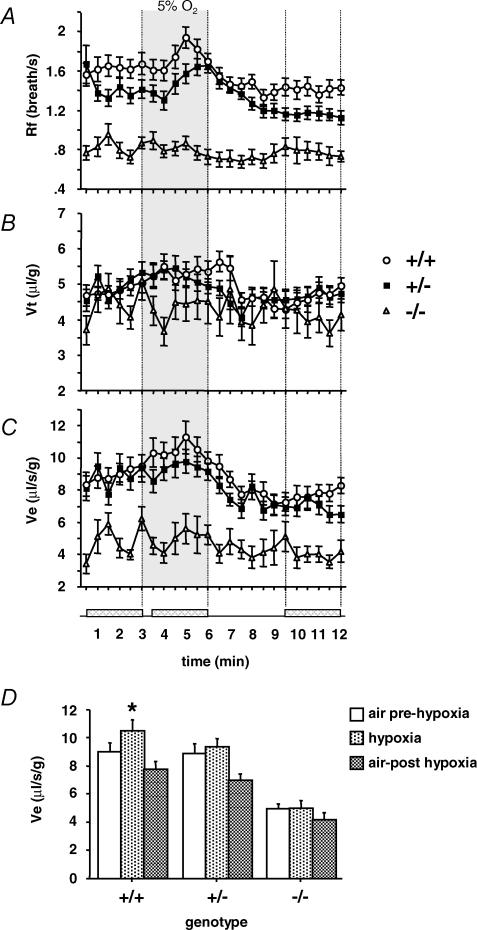
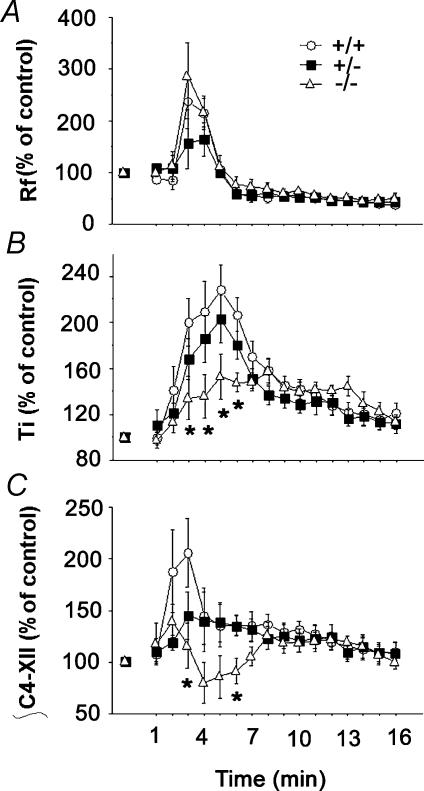
Figure 2. Impaired response to hypoxia in Nurr1 mutant pups.
The breathing variables frequency (Rf) (A), tidal volume (VT) (B) and ventilation (VE) (C),averaged over successive 30 s periods during air prehypoxia (3 min), hypoxia (5% O2, 3 min, grey area) and air posthypoxia (6 min) in Nurr1+/− pups (n= 32), Nurr1+/+ pups (n= 29) and Nurr1−/− pups (n= 14). D, summary of VE profile during prehypoxic normoxia, hypoxia and posthypoxic normoxia (averaged over the shaded areas indicated in the time axis). VE increased in response to hypoxia in Nurr1+/+ (+19%, *P<0.0001), less in Nurr1+/− (+12%, n.s.) and Nurr1−/− (6%, n.s.), and was depressed during posthypoxia irrespective of genotype. Values are means ±s.e.m.
Brainstem–spinal cord preparation
Postnatal age of pup in minutes was noted before the brainstem and spinal cord were isolated as previously described (Herlenius & Lagercrantz, 1999; Johansson et al. 2001). Briefly, the brainstem was rostrally decerebrated between the VIth cranial nerve roots and the lower border of the trapezoid body. The preparation was transferred to a 2 ml recording chamber continuously superfused at a rate of 3mlmin−1 with artificial cerebrospinal fluid (aCSF) containing: 130mm NaCl, 5.4mm KCl, 0.8mm KH2PO4, 0.8mm CaCl2, 1.0mm MgCl2, 26mm NaHCO3, 30mm glucose. The aCSF was continuously equilibrated with 95% O2–5% CO2 to pH 7.4 at 28°C and the pH was monitored using a membrane pH meter (HI 8314, Hanna Instruments). The temperature was measured indirectly in the water-heating bath (Julabo UC, Julabo 5B, Julabo Labortechnik, Seelbach, Germany) after calibration by direct measurement. The solutions were equilibrated at room temperature and pH and temperature measured directly before entering the water-heater and subsequently perfused the recording chamber. During hypoxic stimulation the aCSF was equilibrated with 95% N2–5% CO2 (hypoxic aCSF, pH 7.4). aCSF and the brainstem respiratory regions were not considered to be completely anoxic during perfusion with hypoxic aCSF, because of the nature of the open perfusion system used (see, e.g. Brockhaus et al. 1993). The preparation was stabilized with aCSF perfusion for at least 30 min. It was then perfused with aCSF for another 10min followed by hypoxic aCSF perfusion for 16–20min and finally 15min washout with aCSF. All experiments were performed before the genotype was known.
Respiratory-related activity was recorded using a suction electrode applied to the proximal end of a ventral root (C4) or hypoglossal nerve (XII). The C4/XII activity was amplified, band-pass filtered (3 or 10kHz filter setting), and recorded via an analog–digital converter (Digidata 1320, Axon Instruments, Union City, CA, USA) and data acquisition software (Axoscope, Axon Instruments) at (1–10kHz) to a computer for off-line analysis (Johansson et al. 2001). Analysis of rhythmic respiratory activity was performed using Datapac 2K2 (Run Technologies Inc, Laguna Hills, CA, USA). The respiratory burst characteristics were analysed in integrated and smoothed (time constant 50 ms) recordings. An inspiratory burst activity was defined as integrated burst activity, clearly distinguished above background activity, during > 100 ms. The program parameters to define the inspiratory bursts were adjusted for each recording under visual inspection; then the burst characteristics were measured. Figures of integrated and smoothed activity were prepared in Origin (OriginLab Corp., MA, USA) by averaging 500 adjacent data points, to calculate each averaged result. The inspiratory time (Ti, ms) was defined as the interval during which a continuous discharge occurred in the ventral or cranial roots. The duration between the onset of two consecutive bursts (TTOT) was used to calculate the frequency in respiratory bursts permin (Rf, B min−1). Integrated respiratory burst activity (∫C4/XII) referred to the baseline integrated activity during control, the tonic activity occurring during the initial 1–4min of hypoxic exposure not being subtracted from (∫C4/XII). Respiratory variables were averaged over successive 1-min periods and total ∫C4/XII min−1 were calculated.
Histology
Cryosections (14 μm) were prepared and stained with haematoxylin–eosin using standard procedures.
Cell death analysis
For in vivo detection of apoptotic cells with a TUNEL assay, the ApopTag in situ apoptosis detection kit (Oncor, USA) was used. Analyses were performed on cryosections according to the manufacturer's protocol, except that sections were fixed in 4% paraformaldehyde.
Immunohistochemistry
Embryos and pups killed by neck section (brainstem), or with pentobarbital (carotid bodies) were fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS), followed by immersion in 30% sucrose. Cryosections were prepared at 10–14 μm thickness and adhered to SuperfrostPlus glass slides (Menzel-Gläser, Germany).
For brainstem staining, 14 μm cryosections were air-dried and incubated at 4°C overnight with a polyclonal rabbit anti-Nurr1 antibody (E-20, Santa Cruz Biotech., Santa Cruz, CA, USA) diluted 1: 5000 in PBS containing 0.3% Triton X-100 and 10% fetal calf serum. Following rinses in PBS, slides were incubated with biotinylated goat antirabbit IgG (1: 200, Vector, Burlingame, CA, USA) for 1 h at room temperature. Detection was via horseradish peroxidase using DAB as substrate (ABC-kit PK-6101, Vector). Neurokinin receptor type 1 (NKR1) was detected with a guinea-pig anti-NK1R antibody (Chemicon Int., Temecula, CA, USA) diluted 1 : 100 in PBS containing 0.3% Triton X-100 and 0.5% fetal calf serum, and bound antibody detected by FITC-conjugated antiguinea-pig IgG (Jackson ImmunoResearch, West Grove, PA, USA). Slides were mounted in Vectashield mounting medium (Vector).
In the newborn mouse, several nuclei important for control of breathing can be identified based on their cholinergic properties. One way of detecting cholinergic neurones is by staining for acetylcholine esterase activity (AChEA). For analysis of AChEA, brainstem cryosections (14 μm) were immersed in ice-cold 4% PFA for 15 min, washed in PBS and incubated in staining solution (38mm sodium acetate, 0.012% acetic acid, 4.8mm sodium citrate, 3mm copper sulphate, 0.08mm tetraisopropyl pyrophosphoramide, 0.5mm potassium ferricyanide, 0.87mm acetylthiocholine iodide) as described. (Simon et al. 1998). Anatomical structures were identified using published atlases (Jacobowitz & Abbott, 1998) and sections were analysed by at least two persons where one person was ‘blind’ to the genotype of the animals to ensure non-subjective identification of structural abnormalities.
For carotid body staining, 10–12 μm cryosections were air-dried and permeabilized with 0.1% saponin, blocked with 7% normal rabbit (NRS) or donkey (NDS) serum, then incubated at 4°C overnight with a primary antibody in PBS containing 3% serum and 0.1% saponin. Nurr1 was probed with an affinity-purified rabbit polyclonal antibody diluted 1 : 1000 (Wallén et al. 2001). Tyrosine hydroxylase (TH) was probed with a polyclonal sheep antibody (Calbiochem-Novabiochem, USA) diluted 1 : 1000. Following rinses in PBS, slides were incubated with an alkaline phosphatase (ALP)-conjugated goat antirabbit antibody (Sigma) diluted 1 : 200 or with an (ALP)-conjugated polyclonal rabbit antisheep antibody (Abcam, Cambridge, UK) diluted 1 : 200 for 1 h at room temperature. Both staining protocols utilized the Fast Red kit (Sigma) for detection. Goat-antirabbit Alexa 546 (1 : 200) and donkey-antisheep Alexa 488 (1 : 200) from Molecular Probes were used as secondary antibodies and analysis was performed using confocal microscopy (Leica DM IRBE, Leica Mikroskopie und Systeme GmbH, Wetzlar, Germany).
Data analysis
After analysis of the variance using the F test values statistical analyses were performed with parametric or non-parametric Wilcoxon's signed rank tests when variances were unequal. Spearman's rank non-parametric correlation and Chi-square test were performed on non-respiratory activity.
Respiratory variables from plethysmographic recordings were examined by analyses of variance (ANOVA) with genotype as a between-subject factor and the period (normoxia, hypoxia, and posthypoxia) as a within-subject factor (Superanova software, Abacus Concepts, Berkeley, CA, USA). Respiratory variables from brainstem–spinal cord preparations were analysed with ANOVA, repeated measures design followed by Fisher's PLSD post hoc test. In brainstem–spinal cord experiments the genotype was defined as between-subjects factor and the time,min 1–7 in normoxia,min 1–16 in hypoxia andmin 11–15 in the washout period, as a within-subject factor. To evaluate the responsiveness of the central respiratory system to decreased oxygen tension, and to compensate for different recording qualities, values were normalized (100 × hypoxia/control), i.e. changes during hypoxia were expressed as percentage of control.
As an index of the regularity of the respiratory pattern, the coefficient of variation, i.e. (the ratio of the s.d. and the mean cycle duration) × 100, during control conditions, was calculated. For digital image analysis of the DMN X, the program ImagePro was used with calibrations according to microscopical analyses. Area (polygonal), perimeter and roundness were calculated, where roundness is defined as the perimeter-to-area ratio (normalized so that a circular object should have a roundness of 1.0). Values are presented in the text and tables as means ±s.d. and means ±s.e.m. in the figures. A value of P <0.05 was considered statistically significant.
Results
Dysfunctional breathing during normoxic conditions in Nurr1 deficient mice
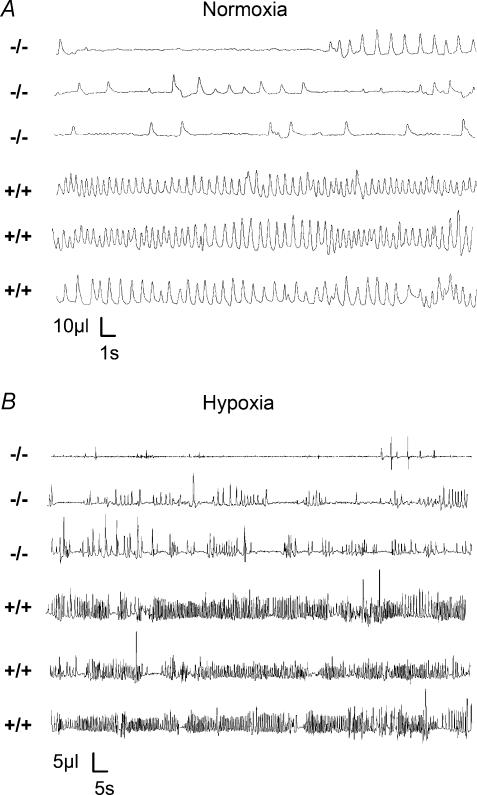
Mice were examined in vivo 12 h after birth before genotype was known. Whole-body barometric plethysmographic recordings demonstrate that Nurr1 homozygote knockout (Nurr1−/−) mice breathe abnormally compared to wild-type mice (Nurr1+/+), as illustrated in Fig. 1A. Nurr1−/− pups had more frequent apnoeas and longer total duration of apnoeas in normoxia than did heterozygote (Nurr1+/−) and Nurr1+/+ mice (see also Table 1 and Fig. 3). Apnoea characteristics were not different between Nurr1+/+ and Nurr1+/− mice. In addition, Nurr1−/− mice had significantly longer breath duration, TTOT, than Nurr1+/− and Nurr1+/+ pups, which were not different from each other. The mean in vivo breathing frequency in each group was: Nurr1+/+: 94.6 ± 32.5 min−1; Nurr1+/−: 82.1 ± 26.6 min−1; Nurr1−/−: 47.2 ± 14.5 min−1 (P<0.0001). As a consequence, ventilation (VE), was less in the Nurr1 knockout pups. The tidal volume, VT, was not different between genotypes; furthermore, Nurr1−/− mice had a more irregular breathing rhythm than Nurr1+/+ or Nurr1+/− mice (P<0.05 and P<0.01, respectively; coefficient of variance analysis of TTOT; Nurr1+/+ CV: 22.4 ± 16.6; Nurr1+/− CV: 19.7 ± 12.2; and Nurr1−/− CV: 34.4 ± 18.6). Body weights were smaller in 12-h-old Nurr1−/− pups (n= 14, 1.2 ± 0.1 g) compared to Nurr1+/−(n= 32, 1.3 ± 0.1 g) and Nurr1+/+ mice (n= 29, 1.3 ± 0.1 g) (P<0.01). Mouth temperatures measured after ventilatory tests were not different between groups (Nurr1+/+: 30.6 ± 0.8°C; Nurr1+/−: 30.6 ± 0.7°C; and Nurr1−/−: 30.6 ± 0.7°C).
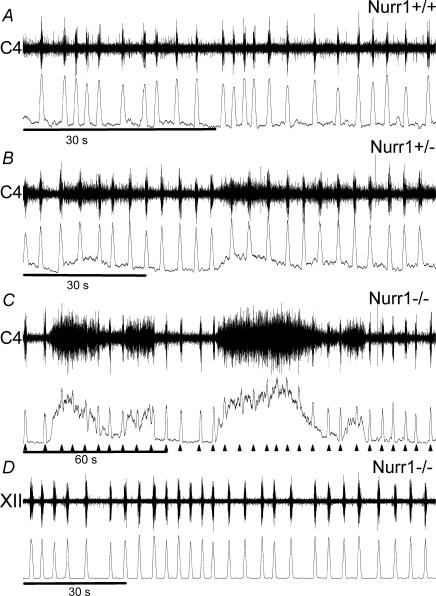
Figure 1. Illustrative respiratory traces of Nurr−/− and Nurr1+/+ mice.
A, ventilatory recordings during normoxia demonstrate irregular breathing patterns with long apnoeic pauses in Nurr1−/− pups and smooth, regular breathing in Nurr1+/+ pups. B, during hypoxia (5% O2) Nurr1−/− mice do not change their breathing pattern with irregular breathing intercepted with frequent apnoeas of long duration while wild-type mice increase their ventilation (VE). Three traces are presented for Nurr1−/− and Nurr1+/+ mice, respectively, during normoxia and hypoxia.
Table 1.
Severe hypoventilation and apnoea during normoxia in Nurr11−/− mice
| Nurr1+/+ (n = 29) | Nurr1+/− (n = 32) | Nurr1−/− (n = 14) | |
|---|---|---|---|
| TTOT (s) | 0.77 ± 0.46 | 0.84 ± 0.39 | 1.39 ± 0.45** |
| Rf (breaths min−1) | 95 +32 | 82 +27 | 47 +14** |
| VT (μl g−1) | 4.8 ± 1.1 | 4.9 ± 1.3 | 4.5 ± 0.9 |
| VE (μl s−1 g−1) | 9,0 ± 3.3 | 8.8 ± 3.8 | 4.9 ± 1.5** |
| Apnea number/3 min | 1.8 ± 3.2 | 2.4 ± 2.4 | 4.9 ± 3.5**/* |
| Apnea duration(s)/3 min | 9.7 ± 19.6 | 13.3 ± 17.9 | 42.3 ± 32.4** |
In normal air Nurr1−/− mice hypoventilate and have frequent and long apnoeas. TTOT, breath duration; Rf, breathing frequency (breaths min−1); VT, tidal volume; VE, ventilation. Baseline breathing recordings in normoxia showed that Nurr1−/− pups had longer TTOT than Nurr1+/− and Nurr1+/+ pups, while the VT was not altered. The longer TTOT (slower Rf) results in smaller VE in the mutant pups. The number of apnoeas were higher and the total duration of the apnoeas longer in the Nurr1−/−, compared to Nurr1+/+ and Nurr1+/− mice. Apnoea number and duration: mean values of apnoea number and total apnoea duration over the 3min baseline recording. *P<0.05; **P<0.01; values are means ±s.d.
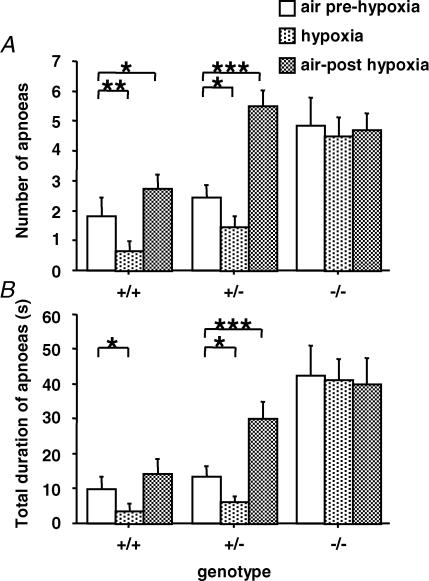
Figure 3. Nurr1−/− mice experience frequent apnoeas and heterozygote Nurr1 mice have altered hypoxic response.
The number (A) and duration (B) of apnoeas in Nurr1+/− and Nurr1+/+ pups decreased during hypoxia (5% O2) and then increased during posthypoxic recovery in normoxia. Note that during posthypoxia apnoeas were more numerous and their duration longer in Nurr1+/− compared to Nurr1+/+ mice (P <0.0001). Nurr1−/− did not show any variations of the number or duration of apnoeas during or after hypoxia. During air and hypoxia the Nurr1−/− pups displayed more apnoeas with longer total duration than the other genotype groups (P <0.0001), and compared to Nurr1+/+ (P <0.05), but not compared with Nurr+/−, after hypoxia. *P<0.05; **P<0.01; ***P<0.001. Values are means ±s.e.m.
Nurr1 mutant mice have an impaired response to hypoxia
The three genotype groups displayed a biphasic ventilatory response to hypoxia characterized by an initial increase in VE followed by a decrease below the prehypoxic baseline level (Fig. 2A–D), although these changes significantly depended on genotype (genotype by period interaction: P <0.02). The initial increase in VE was only significant in Nurr1+/+ pups (19%, P<0.0001), compared to Nurr1+/− and Nurr1−/− pups (12% and 6%, NS, respectively). Post-hypoxic ventilatory decline (P<0.0001) was not significantly different between groups. Hypoxia-induced changes in VE were primarily due to changes in Rf (genotype by period interaction: P<0.02, Fig. 2A); whereas the changes in VT were not significantly different between genotypes (Fig. 2B). The number and total duration of apnoea differed between genotypes (genotype by period interaction: P<0.0001, Fig. 3). In Nurr1+/+ and Nurr1+/− pups, number and duration of apnoeas decreased during hypoxia compared to normoxia (Fig. 3A). The number of apnoea increased again during posthypoxic recovery, slightly in Nurr1+/+ (P<0.05), but more than doubled in Nurr1+/− mice (P <0.001) compared to prehypoxic levels (Fig. 3A and B). Likewise, the posthypoxic increase in duration of apnoea was significantly more pronounced in Nurr1+/− compared to Nurr1+/+ pups (P <0.01, Fig. 3A and B). In Nurr1−/− mice, the high number of apnoeas during normoxia was not altered with changes in oxygenation. There was no difference between genotypes in arousal response to hypoxia (Dauger et al. 2001a) (data not included).
Nurr1 is expressed in brainstem respiration-related areas
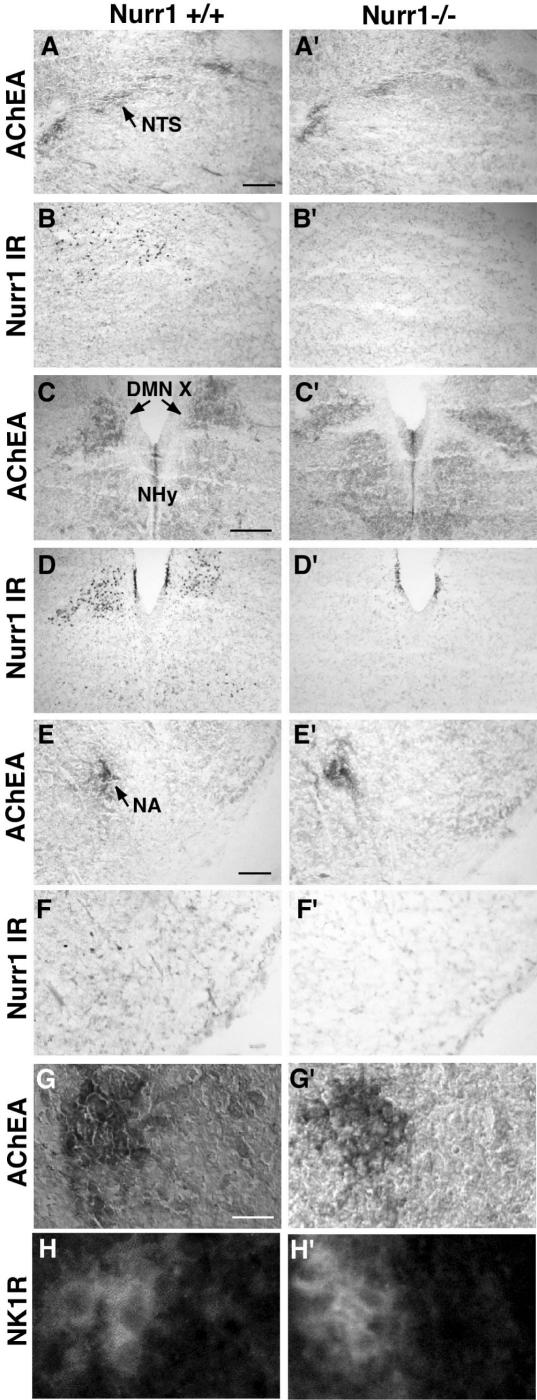
We investigated Nurr1 expression in the brainstem by immunohistochemistry. The nucleus of the solitary tract (NTS) (Zhang & Ashwell, 2001) (Fig. 4A and A′), DMN X (Fig. 4C and C′) and nucleus ambiguous (Fig. 4E and E′) were identified by acetylcholine esterase activity (AChEA). The most extensive Nurr1 expression in the brainstem was in the DMN X (Fig. 4D). Although not exclusively located to these areas, scattered Nurr1 expression was also observed both in the regions of the NTS (Fig. 4B) and the nucleus ambiguous (Fig. 4F). In the DMN X, prominent Nurr1 expression was detected in the motor neurones from E10.5. In the nucleus ambiguous, a weak but distinct Nurr1 signal was seen from E13.5 (data not shown and see also Wallén et al. 2001).
Figure 4. Ample Nurr1 expression in the brainstem of newborn pups.
A–F′, using an AChEA assay, the cholinergic neurones of the NTS (A), DMN X (C) and the nucleus ambiguous (E) in the dorsolateral, dorsomedial and ventrolateral medulla, respectively, were detected. Scattered Nurr1 immunoreactivity (IR) is evident in the area of the NTS (B) and nucleus ambiguous (F), and strong localized Nurr1 IR is detected in the DMN X (D). In Nurr1−/− pups, the AChEA assay reveals the presence of these cholinergic nuclei (A′, C′, E′), and, as expected, no staining is observed using the Nurr1 antibody (B′, D′, F′). Note the elongated shape of the DMN X in the Nurr1−/− DMN X (C′), which differs from that of the wild-type (C) (n= 4 wild-type and 4 Nurr1 knockout pups, see Results for quantification). G–H′, in the anterior part of the ventromedial medulla, the pre-Bötzinger complex can be visualized by IR for the substance P receptor, NK1R, within the area of the NA. In both wild-type and Nurr1 null mutant pups, AChEA assay shows the nucleus ambiguous (close-up of nucleus ambiguous in G and G′) and NK1R IR in the pre-Bötzinger (close-up in H and H′) areas. Nurr1 IR was not detected in the nuclei of the NK1R-expressing cells (not shown). The bars in A, C and E show 400 μm and in G 1500 μm.
The NTS (Fig. 4A′), DMN X (Fig. 4C′) and nucleus ambiguous (Fig. 4E′) could all be identified in mutant newborn mice by AChEA immunostaining. Interestingly, the shape of the DMN X appeared elongated in the Nurr1 mutant pups, possibly demonstrating a defective cell migration of the DMN X cells in the absence of Nurr1 (4/4 mutant and 0/4 wild-type pups exhibited this phenotype). To further analyse this anomaly, the perimeter, area and roundness of the DMN X in wild-type and Nurr1 knockout mice were digitally quantified. Although the perimeter of DMN X was similar in the two genotypes (Nurr1+/+: 1.544 ± 0.274 mm; Nurr1−/−: 1.492 ± 0.367 mm; P= 0.448), the area and roundness (perimeter-to-area ratio, where a greater number indicates a stronger deviation from a circular shape) were significantly different. As such, the area in the Nurr1+/+ mice was 0.139 ± 0.047mm2 and that in Nurr1−/− mice was 0.105 ± 0.039mm2(P = 0.012), the roundness being calculated to 1.417 ± 0.191 mm in Nurr1+/+ and 1.736 ± 0.412 mm in Nurr1−/− mice (P= 0.035). As expected no Nurr1 expression was detected in Nurr1−/− mice (Fig. 4B′, D′ and F′). A general histological examination of the area around the DMN X was performed after haematoxylin–eosin staining. No morphological abnormalities were observed in Nurr1 mutant pups. To analyse if the loss of Nurr1 in the DMN X leads to increased cell death, a TUNEL assay was performed on P0 pups. No difference was observed between Nurr1 knockout pups (n= 2) compared to wild-type littermates (n= 2).
The pre-Bötzinger complex important for respiratory rhythmogenesis can be visualized through staining of the receptor for substance P neurokinin-1 (NK1R) (Gray et al. 2001). Using an NK1R-specific antibody we detected a small NK1R-expressing area that stretched over several sections in the anterior–posterior direction within the AChEA region in the ventrolateral medulla (Fig. 4G and H). However, Nurr1 was not expressed in these cells (data not included), and normal NK1R expression was observed in Nurr1−/− mice (Fig. 4G′ and H′), indicating that Nurr1 is not involved in genesis of the pre-Bötzinger complex.
Brainstem generation of respiratory rhythm is preserved in Nurr1−/− mice
All preparations examined displayed a rhythmic respiratory output (see Figs 5 and 6). During normoxic conditions there were no significant differences between genotypes in any respiratory-dependent variable examined (Nurr1+/+, n= 17: Ti, 447 ± 198 ms; Rf, 10.9 ± 0.96 B min−1; and ∫C4/XII, 37 ± 28 a.u; Nurr1+/−, n= 19: Ti, 503 ± 267 ms; Rf, 10.08 ± 0.91 B min−1; and ∫C4/XII, 49 ± 28 a.u.; and Nurr1−/−, n= 10: Ti, 514 ± 235; Rf, 8.92 +1.26 B min−1; and ∫C4/XII, 43 ± 29; ANOVA: Ti, P= 0.71; Rf, P= 0.45; and ∫C4/XII, P= 0.48). The regularity of respiratory rhythm, quantified as coefficient of variation (CV) of burst interval, was similar in all groups (Nurr1+/+ CV: 48 ± 19; Nurr1+/− CV: 48 ± 20; and Nurr1−/− CV: 56 ± 22). In addition to the respiratory rhythm, a secondary, non-respiratory-like discharge activity, defined as more than twice amplitude of mean background activity, was identified in a few Nurr1+/+, some Nurr1+/− and most Nurr1−/− mice (2/17, 9/19, and 7/10, respectively, Chi-square test: P<0.05); see Figs 5 and 6. This non-respiratory activity did not seem to perturb the respiratory rhythm. Brainstem spinal cord preparations taken from younger pups tended to have more non-respiratory discharges but this result was not significant (Spearman's rank non-parametric correlation, P<0.08). The non-respiratory-like discharge activity was present in some ventral root but not in XII cranial nerve recordings (Fig. 5). Recording sites, XII nerve or ventral root, were similarly distributed between genotypes (Nurr1+/+, 6 and 11; Nurr1+/−, 6 and 13; Nurr1−/−, 3 and 7). There were no differences in the distribution between genotypes in mode of delivery. Independent of genotype, pups delivered by caesarean section weighed less than those delivered spontaneously (Caesarean section: 1.13 ± 0.03 g, n = 30; and spontaneous delivery: 1.39 ± 0.04 g, n= 16; ANOVA, P<0.001). The body weight and postnatal age of pups before experiments was similar between genotypes (Nurr1+/+: 1.19 ± 0.05 g, 1024 ± 410 min, n= 17; Nurr1+/−: 1.28 ± 0.04 g, 789 ± 423 min, n = 19; and Nurr1−/−: 1.16 ± 0.06 g, 724 ± 381 min n= 10; ANOVA, P = 0.19 and P = 0.12 for weight and age, respectively).
Figure 5. Similar extracellular respiratory activity in wild-type and Nurr1−/− brainstem–spinal cord preparations.
Illustrated here are raw and integrated recordings from wild-type (A), heterozygote (B) and Nurr1−/− (C) preparations during perfusion with control aCSF. Note the similar respiratory rhythm and that cervical non-rhythmic discharge activity in the preparations, even of high amplitude as in C, does not affect the rhythm of respiratory burst activity (▴). D, in recordings from XII nerve roots (n = 15), respiratory activity was observed but non-respiratory like activity was not detected, illustrated with a recording from a Nurr1−/− preparation.
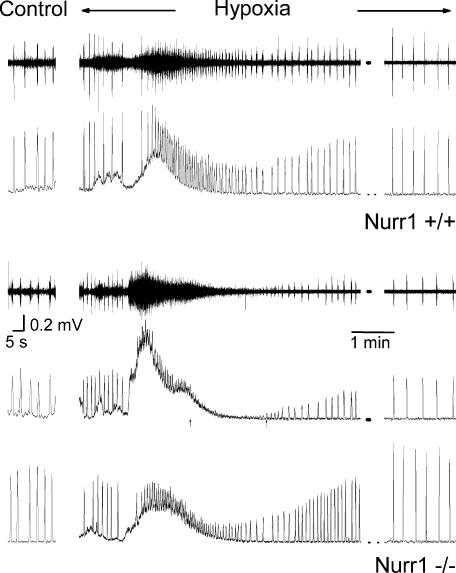
Figure 6. Response to perfusion with hypoxic aCSF in brainstem–spinal cord preparations.
C4 respiratory activity recorded during aCSF control perfusion (far left) and during perfusion with hypoxic aCSF (right). Changing to perfusion with hypoxic aCSF (95% N2) induced a tonic discharge activity and an initial increase in the frequency of respiratory bursts in wild-type (upper raw and integrated trace) as well as Nurr1−/− preparations (lower traces, taken from the same two preparations as in Fig. 5C and D). In some preparations respiratory burst activity was difficult to distinguish from background activity, during a short period (<2 min), at the end of or after tonic discharge activity had occurred, exemplified here in a Nurr1−/− preparation, with last and subsequent first measurable respiratory activity during hypoxia, indicated by an arrow. Note that in preparations, which displayed respiratory activity but also a non-respiratory-like discharge activity during normoxia, only respiratory activity remained after the first minutes of hypoxia. All preparations continued generating respiratory activity during the perfusion with hypoxic aCSF, illustrated with recording from the lastmin of hypoxic exposure (far right).
Nurr1−/− mice have an impaired central respiratory response to hypoxia
To examine central respiratory rhythm generation and its innate ability to respond to hypoxia, aCSF was exchanged with hypoxic aCSF in 24 preparations, see Table 2 for respiratory variables, which were similar during control in these preparations. Perfusion with hypoxic aCSF resulted in an initial increase in respiratory discharge frequency and tonic activity followed by a decrease in respiratory activity in all preparations (Fig. 6). The inspiratory time (Ti) and the integrated C4/XII displayed a genotype-by-time interaction during the hypoxic period (P < 0.0001, Fig. 7). Post hoc analysis revealed that Nurr1−/− preparations reacted less to hypoxic aCSF (P <0.01). The non-respiratory-like discharge activity disappeared in all preparations that had it during control conditions after the first minutes of exposure to hypoxic aCSF (Fig. 6). Analysis of the last 5min of the washout period revealed that all the preparations had recovered from hypoxia. There was no difference between pups born by caesarean section or spontaneously delivered or recording site (C4 or XII nerve), and data were thus pooled according to the genotype.
Table 2.
Respiratory activity during control aCSF perfusion in brainstem–spinal cord preparations exposed to hypoxic aCSF
| Genotype | Ti (ms) | Integrated burst (a.u) | Rf (min−1) |
|---|---|---|---|
| Nurr1+/+ | 448 ± 177 | 42 ± 32 | 10.1 ± 4.4 |
| Nurr1+/− | 456 ± 184 | 53 ± 34 | 10.4 ± 3.2 |
| Nurr1−/− | 591 ± 241 | 47 ± 40 | 8.5 ± 2.6 |
Inspiratory time (Ti), integrated burst activity (∫C4/XII, arbitrary units) and frequency (Rf, B min−1) were not significantly altered between the genotypes. Values are means ±s.d.(n= 8 Nurr1+/+, 9 Nurr1+/− and 7 Nurr1−/− mice).
Figure 7. Effect of hypoxia on inspiratory respiratory discharge.
A, Rf, mean frequency; B, Ti, mean inspiratory time; and C, ∫C4/XII, total integrated C4 and XII activities per minute. Ti was shorter in Nurr1−/− than Nurr1+/+ mice duringmin 3–6. ∫C4/XII was smaller in Nurr1−/− than Nurr1+/+ mice duringmin 3 and smaller in Nurr1−/− than Nurr1+/− and Nurr1+/+ mice duringmin 6. No differences between genotypes were found in frequency response. *P<0.05. Values are group means ±s.e.m.
Nurr1 is expressed in the carotid bodies
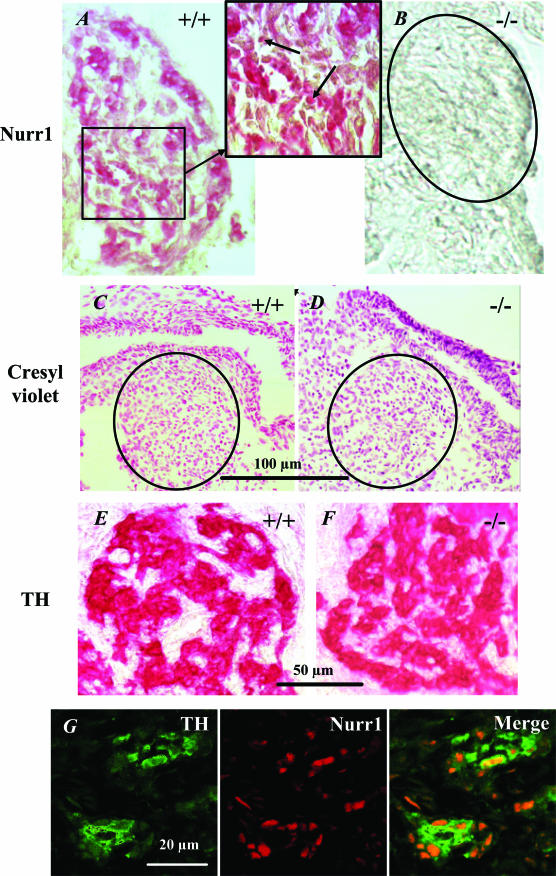
Nurr1 was markedly expressed in the carotid body of Nurr1+/+ mice at birth (Fig. 8). Nurr1 expression was strongest in the nuclei of carotid body cells, the majority of which were identified as dopaminergic by combined Nurr1 and TH staining (Fig. 8G). Nurr1 was not detected in carotid bodies from Nurr1−/− mice. However, TH immunoreactivity was preserved in Nurr1−/− mice, and cresyl violet staining was similar in Nurr1+/+ and Nurr1−/− mice (Fig. 8). Neither the maximum diameter of the carotid body nor the number of TH-cells differed between genotypes (Table 3).
Figure 8. Carotid bodies have ample expression of Nurr1.
A and B, Nurr1 immunoreactivity (IR) is detected in the carotid body of wild-type mice with a high nuclear level of expression (arrow) and absent in Nurr1−/− mice. C, D, E and F, TH immunoreactivity and cresyl violet staining in the carotid body of wild-type and Nurr1−/− mice indicate that the carotid body organization is preserved in wild-type and Nurr1−/−. G, double immunolabelling of wild-type mice carotid body stained with TH (green) and Nurr1 (red). Merged image shows that TH and Nurr1 present a cellular segregation; Nurr1 is mainly located in the nucleus of TH-positive cells. (Internal scale bars = 25 μm).
Table 3.
TH expression and morphology of carotid bodies similar in Nurr1 mutant newborns
| Maximum diameter (μm) | No. TH cells mm−2 | |
|---|---|---|
| Nurr1+/+ | 116.8 ± 42.5 | (79.8 ± 31.2) × 102 |
| Nurr1+/− | 147.1 ± 25.6 | (70.1 ± 8.5) × 102 |
| Nurr1−/− | 127.2 ± 48.7 | (72.4 ± 10.4) × 102 |
Morphometric analysis of carotid bodies from Nurr1+/+, Nurr1+/− and Nurr1−/− mice. The genotype groups did not differ in diameter or TH expression. Data expressed as means ±s.d.(n= 6 Nurr1+/+, 3 Nurr1+/− and 4 Nurr1−/− mice).
Discussion
Nurr1 was found to be expressed in all the constituents of the peripheral chemosensitive pathways, as well as in other respiration-related areas in the brainstem. Nurr1 knockout mice hypoventilated and failed to respond to hypoxia. Moreover, Nurr1 heterozygotes displayed a disturbed hypoxic response.
Hypoventilation in Nurr1−/− mice
The abnormal respiratory phenotype of the Nurr1−/− mouse does not seem to be due to defects in the thoracic muscles, lungs or upper airways, as no gross morphological abnormalities have been demonstrated (Zetterström et al. 1997; Wallén et al. 2001). Neuronal innervation of smooth muscle walls of lung bronchi develop and appear normal (Wallén et al. 2001). Furthermore, Nurr1−/− mice are able to inhale with similar VT as littermates and are indistinguishable from wild-type littermates in their general movement pattern, although Nurr1−/− have difficulties in turning from the supine position (Zetterström et al. 1997). Deficits in the lungs or upper airways are thus probably not the cause of the severe respiratory dysfunction observed in Nurr1 knockouts.
Even mice with severe airway obstruction due to tracheal malformations may survive until adulthood (Regnier et al. 2002). The decreased frequency in vivo of Nurr1−/− mice does not seem to be due to disturbances in the central respiratory rhythm generator as similar respiratory rhythm was found in the isolated brainstem–spinal cord preparations from all genotype groups. Notably, the unusual breathing phenotype of Nurr1−/− pups is reminiscent of carotid body denervation in newborn rodents, which also hypoventilate due to a reduced Rf and frequent apnoeas (Forster et al. 2000). We thus investigated if Nurr1 was expressed in afferent pathways modulating the central respiratory rhythm generator.
Altered chemosensitivity in mice lacking Nurr1
Ample Nurr1 expression was detected in the carotid bodies of newborn wild-type mice indicating it is likely involved in the chemosensitive process. Carotid body-denervated newborn pups have a reduced (–70%) response to hypoxia and high mortality (50%) (Forster et al. 2000). The hypoxic response of the Nurr1−/− mice thus resembles the response of carotid body-denervated rats. In carotid body-denervated rodents it is primarily the frequency response to hypoxia that is decreased or abolished whereas the VT response is less disturbed (Roux et al. 2000). The differences between the in vivoRf response to hypoxia, which showed no response in the Nurr1−/−, and the in vitroRf response, which was similar between genotypes, may be explained by a dysfunctional afferent input.
In Nurr1 mutant pups the hypoxic response is almost totally abolished and all animals die. The deficiency is thus most likely not solely located in the carotid bodies. The aortic chemoreceptors were not examined in the present study and we cannot exclude that a deficiency in their function may contribute to the severe respiratory phenotype observed in vivo. Nurr1 is also expressed in the NTS and petrosal ganglia (Brosenitsch & Katz, 2001), which are both parts of the O2-chemosensitive pathway from the carotid bodies. O2 sensing-cells also exist in the pre-Bötzinger complex (Solomon et al. 2000). We demonstrated that as well in the isolated brainstem–spinal cord preparation of knockout mice, an impaired respiratory response to hypoxia exists. However, the pre-Bötzinger complex, containing propriobulbar neurones crucial for respiratory rhythm generation (Smith et al. 1991; Gray et al. 2001), was not different in Nurr1−/− compared with wild-type mice and the respiratory rhythm was similar. Moreover, the regularity of respiratory rhythm was similar in vitro and the initial increase in frequency during exposure to hypoxic aCSF unaltered. This further supports the concept that the pre-Bötzinger complex, considered to be important for frequency modulation, is functional in the knockout mice. The deficiencies in Ti and ∫C4/XII responses to hypoxia could be due to dysfunction of respiration-related neurones outside the pre-Bötzinger complex (e.g. in the periambiguual area or phrenic, XII or ambigual motoneurones). Frequency and amplitude modulation during hypoxia may be regulated in a differential manner within the respiratory network (Feldman et al. 1990), but future studies will have to be performed to determine these mechanisms, which are currently not well explained in mammals.
Central respiratory activity and hypoxic response
All brainstem–spinal cord preparations responded to hypoxic aCSF perfusion with an increase in respiratory frequency that subsequently declined to below baseline. The biphasic response to hypoxia with an initial increase followed by a decrease of respiratory activity, described in the present and other studies (see Ballanyi et al. 1999 for review), has also been demonstrated in the reduced in vitro slice preparation (Thoby-Brisson & Ramirez, 2000). This biphasic hypoxic response is reminiscent of the response to severe hypoxia in vivo. It has been proposed that the early onset of IPSP suppression and simultaneous increase in tonic activity, preceding the hypoxic depression of Rf, might contribute to the initial frequency increase apparent during hypoxic exposure in several in vitro studies (Ballanyi et al. 1999). Other authors report a decrease of respiratory activity during hypoxia without an initial increase using the brainstem-spinal cord preparation (Viemari et al. 2003). The discrepancies in hypoxic response, including the absence of a biphasic response and tonic discharge activity reported by Viemari et al. may be explained by different responses to hypoxia depending on age of preparation, metabolic state or nervous structures that remain. As reported by Bodineau et al. (2000), the initial frequency increase during hypoxia is attenuated if structures between the 5th and 6th cranial nerves remain; in the present study, structures rostral to the 6th nerve were removed while they remained in the preparations studied by Viemari et al.
Nurr1 is thus not required for neurogenesis, or for the establishment of functional connections in the rostral ventral respiratory group (rVRG), or in other respiration-related structures examined with the exception of the DMN X (see below). Despite this, functional impairments are clearly present both in the central and peripheral responses to hypoxia. Nurr1 mutant pups at 12h of age are probably already hypoxic given that their minute ventilation is about half that of controls. This may decrease their ability to respond to hypoxia. However, even severe postnatal hypoxia (Bermingham et al. 1996), does not eliminate the hypoxic response. Chronic hypoxia may even increase the acute hypoxic response (Thomas & Marshall, 1995). Moreover, Nurr1 heterozygotes also displayed a disturbed hypoxic response without any signs of breathing disturbances during normoxia. In addition, no difference in the oxygenation of the preparations in vitro was evident and yet the response to hypoxia was still diminished in preparations from Nurr1−/− mice. This suggests that deficiencies in the hypoxic response are not secondary due to hypoxic depression but are associated with Nurr1 gene dosage effects on hypoxic respiratory control.
Non-respiratory like discharges in vitro
The irregular spike discharges that occurred in some preparations disappeared during hypoxic conditions and did not seem to affect rhythmic respiratory output. The non-respiratory-like spike discharges during normoxic conditions may be attributed to less-hyperpolarized motoneurones in young mice that generate non-specific or motor associated burst activity. The brainstem–spinal cord preparations examined in the present study were from late fetal (E18.5) or newborns (less than 12-h-old mice). The non-respiratory activity tended to be observed more often in preparations from younger animals. This is in agreement with previous reports of non-respiratory-like activities occurring in rat fetuses before E20 (Di Pasquale et al. 1992). The increased incidence of non-respiratory activity in Nurr1−/− during normoxic conditions does not seem to be caused by age differences as similar ages were used in all groups. It is probably caused by more depolarized spinal non-respiratory motoneurones and our speculation is that development of these non-respiratory motoneurones could be slightly altered, although this was not further examined in the present study.
Due to depolarization of non-respiratory motoneurones, α1-adrenoreceptor activation can generate non-respiratory motor discharges from cervical ventral roots of the spinal cord but not in inspiratory cranial nerves or phrenic nerves (Morin et al. 2000), similar to the present data. These discharges do not affect the pattern of phrenic inspiratory bursts. Thus if a similar cervical root discharge exists in vivo, it may participate in the slight hypotonicity observed in Nurr1−/− compared to wild-type mice, but its role in the decreased respiratory frequency and ensuing hypoventilation demonstrated in this study should be minor. This is further supported by the fact that non-respiratory discharges in cervical roots are present in several in vitro preparations from heterozygote and some homozygote mice without any apparent respiratory phenotype during in vivo normoxia.
Possible involvement of DMN X disturbance in Nurr1 phenotype
Marker gene analyses of the DMN X revealed that Ret expression is decreased in Nurr1 mutant mice (Wallén et al. 2001). We also observed an elongated shape of the DMN X, possibly due to a deficiency in cell migration. These abnormalities of the DMN X may alter development of somatosensory neurones. The DMN X cholinergic target area innervation appeared normal, except for a slight decreased choline acetyltransferase immunostaining in the abdominal part of the oesophagus mucosa (Wallén et al. 2001). It seems unlikely, however, that this subtle difference in the lower oesophagus could explain the respiratory phenotype, especially since all target organs appeared normal.
A TUNEL assay was performed on P0 pups to assess an apoptotic phenotype. Although no difference was observed between the genotypes, this experiment cannot completely rule out that there is a difference in cell survival. As the TUNEL assay detects cells that are actively apoptosing, a more precise analysis would include several different developmental stages to more definitely rule out an occurrence of increased cell death. However even such an elaborate analysis may not rule out increased apoptosis as any narrow window of cell death could easily be missed. Such analyses might be addressed in further studies.
The DMN X is involved both in the response to hypoxia (Rodier et al. 2001) and in the motor act of swallowing. In NTS, ‘chemoreceptive’ neurones receive carotid body and pharyngoesophageal afferents. These neurones then project towards the rVRG and the DMN X (Paton et al. 1999, 2001). Stimulation of peripheral chemoreceptors by hypoxia evokes a coordinated response involving swallowing and activation of multiple respiratory motor outflows. This is a defensive reflex that prevents airway obstruction and facilitates autoresuscitation from hypoxic apnoea (Khurana & Thach, 1996). In addition, a feedback loop seems to exist between the rVRG, the peripheral chemoreceptors and the upper airways through the DMN X that may facilitate or antagonize one another (Jordan, 2001) and be important for respiratory control. Nurr1 is highly expressed in DMN X and the carotid bodies and DMN X morphology is disturbed in Nurr1−/− mice. It is therefore likely that the absence of gastric milk, ensuing weight difference, and some of the respiratory deficiencies observed in Nurr1−/− mice are due to dysfunction of the DMN X.
It is worth noting that infants born with congenital central hypoventilation syndrome (CCHS) lack chemosensitivity and have life-threatening hypoxia after birth. A subgroup of these patients have dyscoordination of swallowing and breathing and carry mutations in the Ret–GDNF pathway (Amiel et al. 1998; Sakai et al. 1998). We did not test the sensitivity and response to hypercapnia (reduced in several CCHS patients). Nonetheless, the phenotype of the Nurr1 mice shows striking resemblances to the symptoms observed in infants with CCHS implicating Nurr1 abnormality in this syndrome. Further studies are needed to test the hypothesis and to determine whether Nurr1 disturbances could be involved in CCHS pathophysiology.
Is Nurr1 an immediate early gene necessary at birth?
Although Nurr1 expression is not necessary for development of dopaminergic neurones in the carotid bodies, as shown in Fig. 8, Nurr1 is involved in the regulation of the dopaminergic response to environmental cues (Iwawaki et al. 2000) by activating the tyrosine hydroxylase promoter (Kim et al. 2003). Tyrosine hydroxylase is the initial and rate-limiting enzyme of catecholamine synthesis. Its initial expression and ongoing regulation may be important for the function of dopaminergic neurones.
The stress of being born and the concomitant surge of catecholamine at birth are important for physiological adaptation to neonatal life (Lagercrantz & Slotkin, 1986). Nurr1 is an immediate-early gene of the NGFI-B subfamily. Genes of this subfamily are rapidly but transiently induced by a variety of stimuli. Notably, Nurr1 has a peak expression at birth or soon thereafter (Law et al. 1992). Depolarization of sensory neurones from the petrosal ganglia, e.g. by changes in oxygenation, induces Nurr1 expression (Brosenitsch & Katz, 2001). Furthermore, stress, e.g. during birth (Lagercrantz & Slotkin, 1986), rapidly induces Nurr1 (Honkaniemi et al. 2000) and increases the expression of tyrosine hydroxylase (Iwawaki et al. 2000). Nurr-1 could therefore participate in regulation of the dopaminergic system, i.e. in the carotid chemoafferent pathway. By acting as a stress inductor it could play a critical role at birth or during hypoxic stimulation. A deficit in the ability to respond to environmental changes (Iwawaki et al. 2000), such as the transition to extrauterine life or hypoxia, is a credible explanation for the postnatal hypoventilation, impaired response to hypoxia and early death of pups lacking Nurr1. Impaired ventilatory responses to hypoxia may play a critical role in Parkinson's disease, sleep-related breathing disorders, CCHS and sudden infant death syndrome (Cutz et al. 1997; Onodera et al. 2000). Whether the critical role of Nurr1 is via developmental processes, such as the maturation of the chemoreflex mechanisms in which Nurr1 is abundantly expressed, or via its properties as an immediate-early gene required during stress responses, or via both, is currently not known. Modification of Nurr1 activity and identification of downstream target genes for this orphan receptor might thus be of therapeutic importance not only for Parkinson's disease but also for respiratory deficiencies such as CCHS. It is noteworthy that Nurr1 heterozygous mice also exhibit impaired ventilation with more frequent apnoeas than wild-type mice, implying that further studies of these mice, which show no other apparent deficiencies, could provide valuable insights in the role of this nuclear receptor in normal physiology and disease.
In conclusion, this study provides evidence that Nurr1 deficiency results in severe respiratory dysfunction and an inadequate response to hypoxia. As Nurr1 is expressed in brainstem areas (NTS, DMN X and nucleus ambiguous) and peripheral sensory ganglia that are important for the generation and maintenance of respiratory pattern, we suggest a vital role for Nurr1 in postnatal breathing and the response to hypoxia.
Acknowledgments
We thank Jorge Gallego and Marie Wahren for critical reading and suggestions and Robert A. Harris for linguistic advice. Support for this study was provided by grants from Swedish Medical Research Council (5234 and 22901), National Heart and Lung, Göran Gustafsson, Human Frontiers of Science, Freemason Children's House, Sven Jerring, Child Care, AGA Medical Research, Wiberg, Gösta Fraenckels and Jeanssonska foundations and a personal fellowship for Dr E. Nsegbe from the Université Paris VII (Legs Poix).
References
- Amiel J, Salomon R, Attie T, Pelet A, Trang H, Mokhtari M, Gaultier C, Munnich A, Lyonnet S. Mutations of the RET-GDNF signaling pathway in Ondine's curse. Am J Hum Genet. 1998;62:715–717. doi: 10.1086/301759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Jr, Scherer SS, O'Connell S, Arroyo E, Kalla KA, Powell FL, Rosenfeld MG. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10:1751–1762. doi: 10.1101/gad.10.14.1751. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Bodineau L, Cayetanot F, Frugiere A. Possible role of retrotrapezoid nucleus and parapyramidal area in the respiratory response to anoxia: an in vitro study in neonatal rat. Neurosci Lett. 2000;295:67–69. doi: 10.1016/s0304-3940(00)01590-1. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K, Smith JC, Richter DW. Microenvironment of respiratory neurons in the in vitro brainstem-spinal cord of neonatal rats. J Physiol. 1993;462:421–445. doi: 10.1113/jphysiol.1993.sp019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosenitsch TA, Katz DM. Physiological patterns of electrical stimulation can induce neuronal gene expression by activating N-type calcium channels. J Neurosci. 2001;21:2571–2579. doi: 10.1523/JNEUROSCI.21-08-02571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo SO, Baffi JS, Palkovits M, Goldstein DS, Kopin IJ, Witta J, Magnuson MA, Nikodem VM. Dopamine biosynthesis is selectively abolished in substantia nigra/ventral tegmental area but not in hypothalamic neurons in mice with targeted disruption of the Nurr1 gene. Mol Cell Neurosci. 1998;11:36–46. doi: 10.1006/mcne.1998.0673. [DOI] [PubMed] [Google Scholar]
- Cutz E, Ma TK, Perrin DG, Moore AM, Becker LE. Peripheral chemoreceptors in congenital central hypoventilation syndrome. Am J Respir Crit Care Med. 1997;155:358–363. doi: 10.1164/ajrccm.155.1.9001336. [DOI] [PubMed] [Google Scholar]
- Dauger S, Aizenfisz S, Renolleau S, Durand E, Vardon G, Gaultier C, Gallego J. Arousal response to hypoxia in newborn mice. Respir Physiol. 2001a;128:235–240. doi: 10.1016/s0034-5687(01)00303-6. [DOI] [PubMed] [Google Scholar]
- Dauger S, Guimiot F, Renolleau S, Levacher B, Boda B, Mas C, Nepote V, Simonneau M, Gaultier C, Gallego J. MASH-1/RET pathway involvement in development of brain stem control of respiratory frequency in newborn mice. Physiol Genomics. 2001b;7:149–157. doi: 10.1152/physiolgenomics.00056.2001. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Monteau R, Hilaire G. In vitro study of central respiratory-like activity of the fetal rat. Exp Brain Res. 1992;89:459–464. doi: 10.1007/BF00228263. [DOI] [PubMed] [Google Scholar]
- Epstein MA, Epstein RA. A theoretical analysis of the barometric method for measurement of tidal volume. Respir Physiol. 1978;32:105–120. doi: 10.1016/0034-5687(78)90103-2. [DOI] [PubMed] [Google Scholar]
- Erickson J, Brosenitsch T, Katz D. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J Neurosci. 2001;21:581–589. doi: 10.1523/JNEUROSCI.21-02-00581.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, Katz DM. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J Neurosci. 1996;16:5361–5371. doi: 10.1523/JNEUROSCI.16-17-05361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Smith JC, Ellenberger HH, Connelly CA, Liu GS, Greer JJ, Lindsay AD, Otto MR. Neurogenesis of respiratory rhythm and pattern: emerging concepts. Am J Physiol. 1990;259:R879–R886. doi: 10.1152/ajpregu.1990.259.5.R879. [DOI] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, Martino P. Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol. 2000;119:199–208. doi: 10.1016/s0034-5687(99)00115-2. [DOI] [PubMed] [Google Scholar]
- Giguère V. Orphan nuclear receptors: from gene to function. Endocr Rev. 1999;20:689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, Mccrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Adenosinergic modulation of respiratory neurones in the neonatal rat brainstem in vitro. J Physiol. 1999;518:159–172. doi: 10.1111/j.1469-7793.1999.0159r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkaniemi J, Zhang JS, Longo FM, Sharp FR. Stress induces zinc finger immediate early genes in the rat adrenal gland. Brain Res. 2000;877:203–208. doi: 10.1016/s0006-8993(00)02673-1. [DOI] [PubMed] [Google Scholar]
- Iwawaki T, Kohno K, Kobayashi K. Identification of a potential nurr1 response element that activates the tyrosine hydroxylase gene promoter in cultured cells. Biochem Biophys Res Commun. 2000;274:590–595. doi: 10.1006/bbrc.2000.3204. [DOI] [PubMed] [Google Scholar]
- Jacobowitz DM, Abbott LC. Chemoarchitectonic Atlas of the Developing Mouse Brain. Boca Raton, FL, USA: CRC Press LLC; 1998. [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci U S A. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. Central nervous pathways and control of the airways. Respir Physiol. 2001;125:67–81. doi: 10.1016/s0034-5687(00)00205-x. [DOI] [PubMed] [Google Scholar]
- Khurana A, Thach BT. Effects of upper airway stimulation on swallowing, gasping, and autoresuscitation in hypoxic mice. J Appl Physiol. 1996;80:472–477. doi: 10.1152/jappl.1996.80.2.472. [DOI] [PubMed] [Google Scholar]
- Kim KS, Kim CH, Hwang DY, Seo H, Chung S, Hong SJ, Lim JK, Anderson T, Isacson O. Orphan nuclear receptor Nurr1 directly transactivates the promoter activity of the tyrosine hydroxylase gene in a cell-specific manner. J Neurochem. 2003;85:622–634. doi: 10.1046/j.1471-4159.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H, Slotkin TA. The ‘stress’ of being born. Sci Am. 1986;254:100–107. doi: 10.1038/scientificamerican0486-100. [DOI] [PubMed] [Google Scholar]
- Law SW, Conneely OM, Demayo FJ, Malley BW. Identification of a new brain-speicific transcription factor, NURR1. Mol Endocrinol. 1992;6:2129–2135. doi: 10.1210/mend.6.12.1491694. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Tsukada T, Ohkura N, Bandoh S, Hosono T, Yamaguchi K. The NGFI-B subfamily of the nuclear receptor superfamily (review) Int J Oncol. 1998;12:1237–1243. doi: 10.3892/ijo.12.6.1237. [DOI] [PubMed] [Google Scholar]
- Morin D, Bonnot AES, Ballion BERER, Viala D. alpha1-Adrenergic receptor-induced slow rhythmicity in nonrespiratory cervical motoneurons of neonatal rat spinal cord. Eur J Neurosci. 2000;12:2950–2966. doi: 10.1046/j.1460-9568.2000.00154.x. [DOI] [PubMed] [Google Scholar]
- Onodera H, Okabe S, Kikuchi Y, Tsuda T, Itoyama Y. Impaired chemosensitivity and perception of dyspnoea in Parkinson's disease. Lancet. 2000;356:739–740. doi: 10.1016/S0140-6736(00)02638-6. [DOI] [PubMed] [Google Scholar]
- Paton JF, Deuchars J, Li YW, Kasparov S. Properties of solitary tract neurones responding to peripheral arterial chemoreceptors. Neuroscience. 2001;105:231–248. doi: 10.1016/s0306-4522(01)00106-3. [DOI] [PubMed] [Google Scholar]
- Paton JF, Li YW, Kasparov S. Reflex response and convergence of pharyngoesophageal and peripheral chemoreceptors in the nucleus of the solitary tract. Neuroscience. 1999;93:143–154. doi: 10.1016/s0306-4522(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Qian Y, Fritzsch B, Shirasawa S, Chen CL, Choi Y, Ma Q. Formation of brainstem (nor) adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes Dev. 2001;15:2533–2545. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier CH, Masson R, Kedinger V, Textoris J, Stoll I, Chenard MP, Dierich A, Tomasetto C, Rio MC. Impaired neural tube closure, axial skeleton malformations, and tracheal ring disruption in TRAF4-deficient mice. Proc Natl Acad Sci U S A. 2002;99:5585–5590. doi: 10.1073/pnas.052124799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renolleau S, Dauger S, Autret F, Vardon G, Gaultier C, Gallego J. Maturation of baseline breathing and of hypercapnic and hypoxic ventilatory responses in newborn mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1746–R1753. doi: 10.1152/ajpregu.2001.281.5.R1746. [DOI] [PubMed] [Google Scholar]
- Rodier ME, Laferriere A, Moss IR. Effects of age and clustered hypoxia on [125I] substance P binding to neurotachykinin-1 receptors in brainstem of developing swine. Brain Res Dev Brain Res. 2001;127:31–39. doi: 10.1016/s0165-3806(01)00109-2. [DOI] [PubMed] [Google Scholar]
- Roux JC, Peyronnet J, Pascual O, Dalmaz Y, Pequignot JM. Ventilatory and central neurochemical reorganisation of O2 chemoreflex after carotid sinus nerve transection in rat. J Physiol. 2000;522:493–501. doi: 10.1111/j.1469-7793.2000.t01-4-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Wakizaka A, Matsuda H, Nirasawa Y, Itoh Y. Point mutation in exon 12 of the receptor tyrosine kinase proto- oncogene RET in Ondine–Hirschsprung syndrome. Pediatrics. 1998;101:924–926. doi: 10.1542/peds.101.5.924. [DOI] [PubMed] [Google Scholar]
- Saucedo-Cardenas O, Quintana-Hau JD, Le W-D, Smidt MP, Cox JJ, De Mayo F, Burbach JPH, Conneely OM. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci U S A. 1998;95:4013–4018. doi: 10.1073/pnas.95.7.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasawa S, Arata A, Onimaru H, Roth KA, Brown GA, Horning S, Arata S, Okumura K, Sasazuki T, Korsmeyer SJ. Rnx deficiency results in congenital central hypoventilation. Nat Genet. 2000;24:287–290. doi: 10.1038/73516. [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr Biol. 1998;8:295–298. doi: 10.1016/s0960-9822(98)70113-7. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, Neubauer JA. Pre-Botzinger complex functions as a central hypoxia chemosensor for respiration in vivo. J Neurophysiol. 2000;83:2854–2868. doi: 10.1152/jn.2000.83.5.2854. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM. Role of inspiratory pacemaker neurons in mediating the hypoxic response of the respiratory network in vitro. J Neurosci. 2000;20:5858–5866. doi: 10.1523/JNEUROSCI.20-15-05858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Marshall JM. A study on rats of the effects of chronic hypoxia from birth on respiratory and cardiovascular responses evoked by acute hypoxia. J Physiol. 1995;487:513–525. doi: 10.1113/jphysiol.1995.sp020896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Burnet H, Bevengut M, Hilaire G. Perinatal maturation of the mouse respiratory rhythm-generator: in vivo and in vitro studies. Eur J Neurosci. 2003;17:1233–1244. doi: 10.1046/j.1460-9568.2003.02561.x. [DOI] [PubMed] [Google Scholar]
- Wallén Å, Castro DS, Zetterstrom RH, Karlen M, Olson L, Ericson J, Perlmann T. Orphan nuclear receptor Nurr1 is essential for Ret expression in midbrain dopamine neurons and in the brain stem. Mol Cell Neurosci. 2001;18:649–663. doi: 10.1006/mcne.2001.1057. [DOI] [PubMed] [Google Scholar]
- Wallén Å, Zetterstrom RH, Solomin L, Arvidsson M, Olson L, Perlmann T. Fate of mesencephalic AHD2-expressing dopamine progenitor cells in NURR1 mutant mice. Exp Cell Res. 1999;253:737–746. doi: 10.1006/excr.1999.4691. [DOI] [PubMed] [Google Scholar]
- Zetterström RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- Zetterström RH, Solomin L, Mitsiadis T, Olson L, Perlmann T. Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1 and Nor1. Mol Endocrinol. 1996;10:1656–1666. doi: 10.1210/mend.10.12.8961274. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Ashwell KW. Development of the cyto- and chemoarchitectural organization of the rat nucleus of the solitary tract. Anat Embryol (Berl) 2001;203:265–282. doi: 10.1007/s004290000151. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]