Abstract
Inhibitors of mitochondrial energy metabolism have long been known to be potent stimulants of the carotid body, yet their mechanism of action remains obscure. We have therefore investigated the effects of rotenone, myxothiazol, antimycin A, cyanide (CN−) and oligomycin on isolated carotid body type I cells. All five compounds caused a rapid rise in intracellular Ca2+, which was inhibited on removal of extracellular Ca2+. Under current clamp conditions rotenone and CN− caused a rapid membrane depolarization and elevation of [Ca2+]i. Voltage clamping cells to −70 mV substantially attenuated this rise in [Ca2+]i. Rotenone, cyanide, myxothiazol and oligomycin significantly inhibited resting background K+ currents. Thus rotenone, myxothiazol, cyanide and oligomycin mimic the effects of hypoxia in that they all inhibit background K+ current leading to membrane depolarization and voltage-gated calcium entry. Hypoxia, however, failed to have any additional effect upon membrane currents in the presence of CN− or rotenone or the mitochondrial uncoupler p-trifluoromethoxyphenyl hydrazone (FCCP). Thus not only do mitochondrial inhibitors mimic the effects of hypoxia, but they also abolish oxygen sensitivity. These observations suggest that there is a close link between oxygen sensing and mitochondrial function in type I cells. Mechanisms that could account for this link and the actions of mitochondrial inhibitors are discussed.
The carotid body is the primary peripheral arterial chemoreceptor. Type I cells of the carotid body act as sensors for fluctuations in blood PO2, PCO2 and pH. Hypoxia, hypercapnia or acidosis results in an elevation of activity in the carotid sinus nerve (CSN), which ultimately innervates the respiratory centres of the brain. Thus the carotid body can initiate corrective respiratory reflexes crucial for maintaining blood PO2, PCO2 and pH within acceptable levels.
The mechanisms by which the carotid body transduces a hypoxic stimulus into elevated CSN activity remain controversial. There is a body of evidence which suggests that hypoxia leads to type I cell depolarization, calcium entry and neurosecretion (Buckler & Vaughan-Jones, 1994; Urena et al. 1994; Montoro et al. 1996). In neonatal rat type I cells this depolarization appears to be mediated, in part, via inhibition of a TASK-like background potassium channel which is active at the resting membrane potential of the type I cell (Buckler, 1997; Buckler et al. 2000). The biochemical pathways leading to the modulation of these, or other, oxygen-sensitive ion channels, however, remain obscure.
It has been known for a long time that agents that inhibit mitochondrial energy metabolism are potent stimulants of the carotid body (Heymans et al. 1931; Anichkov & Belen'kii, 1963; Krylov & Anichkov, 1968; Mills & Jobsis. 1971, Duchen & Biscoe, 1992a, b). These observations originally led to the hypothesis that oxygen sensing by the carotid body may itself be mediated through some aspect of mitochondrial function. This hypothesis has fallen from favour in the past decade, principally because it is believed that the effects of hypoxia and mitochondrial inhibitors are mediated through different calcium signalling pathways, with hypoxia promoting voltage-gated calcium entry (Buckler & Vaughan-Jones, 1994) and mitochondrial inhibitors promoting calcium release from intracellular stores (Biscoe et al. 1989; Biscoe & Duchen, 1989, 1990). More recent studies, however, have challenged this view by demonstrating that the primary effect of mitochondrial uncouplers is to inhibit type I cell background K+ channels leading to membrane depolarization and voltage gated Ca2+ entry (Buckler & Vaughan-Jones, 1998). These latter observations have prompted us to reinvestigate the effects of other inhibitors of mitochondrial energy metabolism upon type I cell calcium signalling. In this paper we show that three structurally and functionally diverse inhibitors of mitochondrial electron transport (rotenone, myxothiazol and NaCN) and an inhibitor of ATP synthase (oligomycin) all mimic the effects of hypoxia upon intracellular calcium [Ca2+]i and background K+ currents in neonatal rat type I cells.
Methods
Cell isolation
Carotid bodies were excised from anaesthetized (4% halothane) Sprague-Dawley rat pups (10–15 days old) and enzymically dispersed using collagenase (0.4 mg ml−1, type I, Worthington) and trypsin (0.15–0.2 mg ml−1, Sigma) as previously described (Buckler, 1997). The rats were then killed by decapitation whilst still anaesthetized. The isolated cells were maintained in Ham's F-12 medium (supplemented with: 10% heat-inactivated fetal calf serum, 100 i.u ml−1 penicillin, 100 μg ml−1 streptomycin and 84 U l−1 insulin, Sigma) and plated out onto glass coverslips coated with poly-d-lysine (Sigma). Cells were kept at 37°C, 5% CO2 in air until use (2–12 h).
Measurement of recording chamber oxygen levels (PO2)
The PO2 of the solutions was monitored using a needle electrode attached to a Strathkelvin instruments oxygen meter (model 781, Glasgow).
Measurement of intracellular calcium
Intracellular calcium was measured using indo-1 or fura-2. Cells were loaded with fura-2 or indo-1 by incubation in a solution of 5μm fura-2 AM or 2.5μm indo-1 AM in culture medium at room temperature (20–24°C) for 20–30 min. Indo-1 fluorescence was excited at 340 ± 5nm and measured at 405 ± 16 and 495 ± 10nm using photomultiplier tubes. Fura-2 fluorescence was excited alternately (2 Hz) at 340nm and 380nm using a Cairn monochromator (Faversham, Kent) and measured at 510 ± 20nm using a photomultiplier tube. The fluorescence emission ratio 405nm/495nm for indo-1, and the fluorescence excitation ratio 340nm/380nm for fura-2, were calibrated as previously described (Buckler & Vaughan-Jones, 1994).
Measurement of mitochondrial membrane potential
Mitochondrial membrane potential was monitored using rhodamine 123 (Duchen & Biscoe, 1992b). Cells were incubated with 10 μg ml−1 rhodamine 123 in culture medium at room temperature for 30 min. Rhodamine 123 fluorescence was excited at 490nm and measured at 525 ± 10nm. Because rhodamine 123 slowly leaks out of cells, causing a gradual decline in signal intensity, selected regions of the data were scaled by subtracting the minimum (baseline) fluorescence intensity and then normalizing to the maximum fluorescence intensity obtained in the presence of FCCP.
Measurement of cyanide concentration
The concentrations of cyanide presented in Fig. 1 were measured colourimetrically using a Hanna instruments cyanide meter HI 93714 (http://www.hannainst.com).
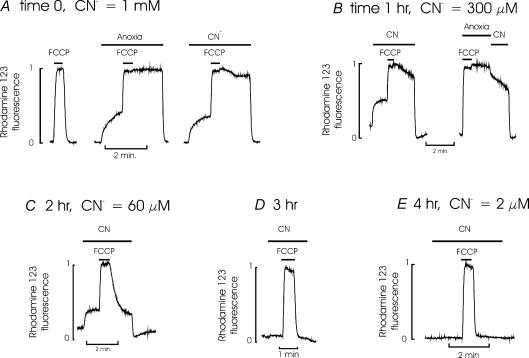
Figure 1. Effect of cyanide on mitochondrial membrane potential.
This figure shows the results of 5 successive experiments recording the effects of NaCN on mitochondrial membrane potential (Ψm) carried out over a period of 4 h using the same solution of NaCN in bicarbonate buffered medium. This solution was maintained at 37°C and gassed with a 5% CO2, 95% air mixture. Ψm was monitored using rhodamine 123 (see Methods). Note that an increase in fluorescence intensity reflects a decrease in Ψm. A, recordings obtained from a group of type I cells at time 0 (i.e. immediately after adding NaCN to the solution). The first trace shows a normal response to FCCP, a mitochondrial uncoupler, which causes a rapid mitochondrial depolarization. Upon removal of FCCP the mitochondria rapidly repolarize. The second trace, from the same group of cells, shows that anoxia (0.5 mm Na2S2O4 added to the solution and gassed with 95% N2, 5% CO2) also depolarizes mitochondria. The further addition of FCCP rapidly completes mitochondrial depolarization. Upon removal of FCCP in the continued presence of anoxia, the mitochondria remain depolarized until oxygen is reintroduced. This confirms that the rhodamine 123 signal does indeed reflect oxygen-dependant mitochondrial electron transport and not the redistribution of the dye between other cellular compartments. The third trace (again from the same cells) shows that NaCN also causes a slow mitochondrial depolarization. The further subsequent addition of FCCP again fully depolarizes the mitochondria. Upon removal of FCCP in the continued presence of CN− mitochondria remain fully depolarized indicating that the NaCN solution is completely blocking electron transport. Upon completion of this segment of the recording a sample of the NaCN solution was removed and assayed for CN− (see methods). The CN− concentration was 1.0 mm. B, 1 h later another group of cells were exposed to the same NaCN solution which caused a marked depolarization of Ψm. There was however, some evidence of a slow repolarization of Ψm following addition and removal of FCCP in the continued presence of CN− suggesting that electron transport may not be fully inhibited. This repolarization is even more evident in the second trace (same cells) where, following FCCP addition and removal, the cells are first exposed to anoxia, which prevents any repolarization and then to CN− solution in which there is a clear partial repolarization of Ψm. The concentration of CN− at this point was 300μm. C, recording of the effects of CN− of Ψm 2 h after preparation of the NaCN solution. Note that the CN− solution still produces some depolarization of the resting Ψm but only partially inhibits repolarization of Ψm following addition and removal of FCCP. The CN− concentration at this time point was 60μm which was clearly insufficient to fully block electron transport. D, recording of the effects of CN− on Ψm 3 h after the preparation of the CN− solution. Note that the CN− solution produces an only just discernable effect on resting Ψm and has little effect on repolarization of Ψm following FCCP addition and removal, indicating that the CN− solution was having only a minor effect on electron transport. E, recording of the effects of CN− on Ψm 4 h after preparation of the CN− solution. Note that the CN− solution has no effect on either resting Ψm or upon repolarization of Ψm following FCCP addition and removal. The CN− concentration at this time point was 2μm.
All cyanide solutions were replaced every 30–60 min. Cyanide is extremely volatile in buffered solutions exposed to air, or actively aerated, as it is rapidly lost as HCN. This problem is exemplified in Fig. 1, which shows a series of successive recordings of mitochondrial membrane potential (Ψm) from type I cells using the same solution of NaCN over a period of 4 h. Initially this NaCN solution causes mitochondrial depolarization, as evidenced by a rise in rhodamine 123 fluorescence and fully inhibits mitochondrial repolarization following the brief application and removal of FCCP. However, it should be noted that upon removal of CN− the mitochondria fully repolarize indicating that the effects of CN− on mitochondrial function in type I cells are fully reversible (see also Duchen & Biscoe, 1992b). The concentration of NaCN in solution at this time point (time 0) was 1 mm. Within 1 h the CN− concentration in the solution had fallen to 300μm and there was some evidence of slight repolarization of Ψm following removal of FCCP in the continuing presence of CN− (Fig. 1B, compare change in Ψm in second trace in the presence of CN versus anoxia). By 2 h the concentration of CN− had fallen to 60μm and there was a prompt partial repolarization of Ψm following FCCP removal in the presence of this same NaCN solution (Fig. 1C). By 4 h the concentration of CN− in this solution was 2μm. At this time point the NaCN solution had no detectable effect on resting Ψm or upon the rate of repolarization of Ψm following removal of FCCP (Fig. 1E). It is apparent from these studies that (a) complete inhibition of electron transport in type I cells requires quite high levels of CN− and (b) that the actual levels of CN− in a solution exposed to air for even relatively short periods of time can be substantially less than intended. It is therefore apparent that high (mm) levels of CN− need to be added to solutions if full inhibition of electron transport is to be reliably achieved and that that such solutions can only be used for relatively short periods of time. In this study we have used CN− solutions with a nominal concentration of 2 mm (i.e. 2 mmol l−1 CN− added to the solution) which were replaced every 30–60 min. These studies also indicate that the use of low concentrations of CN− in solutions exposed to, or equilibrated with, a gas phase could result in there being insufficient CN− in solution to have any significant effect upon electron transport at all.
Electrophysiology
Prior to attempting electrophysiological recordings we first confirmed that selected, indo-1-loaded, cells responded to a hypoxic challenge (6 Torr) with a rapid and robust increase in [Ca2+]i. Perforated patch whole cell recordings were then performed with simultaneous measurement of intracellular calcium as previously described (Buckler, 1997). Experiments were conducted using an Axopatch-1D amplifier (Axon Instruments). Voltage clamp commands were generated from a program written by one of the authors (K.J.B.) using an analog to digital and digital to analog converter (CED1401; Cambridge Electronic Design, Cambridge, UK). Consequently all voltage ramps are staircases with step amplitude of 0.78 mV. Membrane potential and current were filtered at 1 kHz and digitized at 2–4 kHz. Cells were subjected to repetitive voltage ramps at 0.5 Hz and the resultant current records averaged. Each value of n is the average of 10 ramp currents under one experimental condition for one individual cell.
Solutions and reagents
Filling solution for perforated patch recordings contained (mm): K2SO4, 55; KCl, 30; MgCl2, 5.0; EGTA, 1.0; Hepes, 20; glucose, 10; pH adjusted to 7.3 with NaOH at room temperature. Amphotericin B, 240 μg ml−1, was added from a stock solution of 60 mg ml−1 in DMSO. Data are presented without correction for liquid junction potentials (approximately 3 mV).
The standard HCO3−-buffered saline contained (mm): NaCl, 117; KCl, 4.5; NaHCO3, 23; MgCl2, 1.0; CaCl2, 2.5; glucose, 11; pH 7.4–7.45. Elevated K+ Tyrode solution contained 101.5 mm NaCl and 20 mm KCl but was otherwise the same as the previous solution. Ca2+-free solutions were made by not adding any Ca2+ to the solutions and including 0.25–1.0 mm EGTA. In order to make solutions hypoxic they were bubbled with 5% CO2, 95% N2 otherwise they were bubbled with 5% CO2, 95% air. Salines containing H2O2 were prepared by direct addition of 30% w/w H2O2 (sigma) prior to use and were replaced every hour.
Solutions were perfused through a recording chamber with a volume of 80 μl at 2–3 ml min−1. All experiments were conducted at 34–37°C.
Amphotericin, tetramethyl-p-phenylenediamine, methyl succinate, FCCP, rotenone, myxothiazol, oligomycin, NaCN and rhodamine 123 were obtained from Sigma. Indo-1 AM was obtained from Calbiochem and fura-2 AM was obtained from Cambridge Bioscience (Cambridge, UK).
Statistics
All data are presented as means ± standard error of the mean. Statistical significance of results was assessed using Student's paired or unpaired t test as appropriate.
Results
Effects of inhibitors of electron transport and ATP synthase on [Ca2+]i in type I cells
We have investigated the effects of four different inhibitors of electron transport, each targeting a different component of the electron transport chain, and an inhibitor of ATP synthase upon intracellular calcium in isolated rat type I cells. All type I cells were first challenged with a hypoxic stimulus (6 Torr) to confirm their oxygen sensitivity. Only cells that responded to this hypoxic stimulus with a brisk rise in intracellular Ca2+ were included in this study.
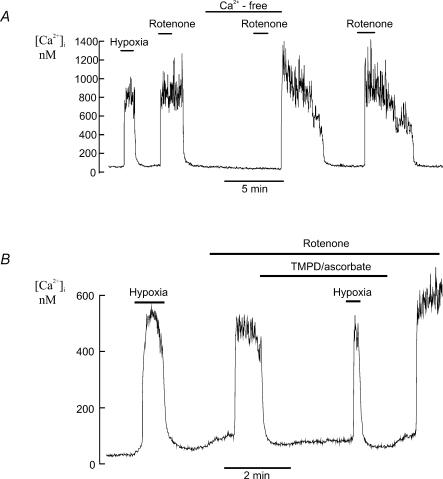
Rotenone inhibits mitochondrial electron transport by acting at complex I (NADH CoQ1 reductase) of the respiratory chain and preventing the reduction of ubiquinone (Earley et al. 1987). Application of 1.0μm rotenone caused a rapid rise in [Ca2+]i in all cells studied (Δ[Ca2+]i= 869.5 ± 91.8 nm, n= 6, P < 0.0003). Following rotenone removal [Ca2+]i recovery was slow and was often delayed by a period of several minutes (Fig. 2). Removal of extracellular Ca2+ (Ca-free + 1 mm EGTA) completely abolished the rise in [Ca2+]i (n= 6, Fig. 2A).
Figure 2. Effects of rotenone on [Ca2+]i in type I cells.
A, effects of hypoxia and rotenone (1μm) on type I cell [Ca2+]i (measured using Indo-1). Rotenone induces a rapid increase in [Ca2+]i in the presence of extracellular Ca2+ but not in the absence of extracellular Ca2+. The rotenone-induced rise in [Ca2+]i was slowly reversed upon rotenone removal with a variable delay of several minutes. Note that following the second exposure to rotenone the effects of rotenone had not fully reversed before Ca2+ was reintroduced into the medium resulting in a transient rise in [Ca2+]i. B, the rotenone-induced rise in [Ca2+]i was reversed by application of the electron donor TMPD + ascorbate (see Results). Note that in the presence of rotenone, TMPD and ascorbate, hypoxia induced an abrupt rise in [Ca2+]i (see Discussion). Intracellular calcium was measured using fura-2.
As rotenone inhibits at a proximal location in the electron transport chain, it is possible to bypass the effects of rotenone on mitochondrial energy metabolism by providing an alternative source of electrons which can feed into the electron transport chain downstream of complex I. Tetramethyl-p-phenylenediamine (TMPD) can donate electrons to cytochrome c which can then pass on electrons to complex IV which transports protons across the inner mitochondrial membrane thus maintaining mitochondrial membrane potential. The ability of TMPD (in the presence of ascorbate which maintains TMPD in the reduced state) to rapidly reverse the effects of rotenone upon mitochondrial membrane potential in type I cells has been previously demonstrated by Duchen & Biscoe (1992b). These researchers also demonstrated that TMPD could reverse the effects of rotenone upon intracellular calcium in type I cells. Figure 2B confirms this earlier result, showing that the rise in calcium in response to rotenone was reversed by TMPD (40 μg ml−1) and ascorbate (5 mm). Note however, that calcium still increased in response to hypoxia despite the presence of TMPD, ascorbate and rotenone ([Ca2+]i= 78 ± 8 nm in the presence of TMPD, ascorbate and rotenone versus 576 ± 121 nm in the presence of TMPD, ascorbate, rotenone and hypoxia; n= 6); see Discussion. We were not, however, able to antagonize the effects of rotenone upon intracellular [Ca2+]i or prevent rotenone-induced mitochondrial depolarization with exogenously applied methyl succinate (5 mm; data not shown). This probably reflects a failure of succinate to enter the cell sufficiently rapidly to provide an adequate alternative source of electrons via complex II.
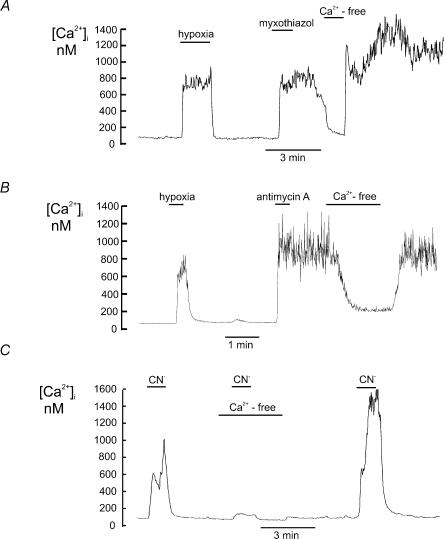
Myxothiazol inhibits electron transport at complex III (ubiquinol–cytochrome c reductase) by binding to, or near, the low potential haem b (Thierbach & Michaelis, 1982). Application of 1μm myxothiazol caused a rapid but irreversible rise in [Ca2+]i (Δ[Ca2+]i= 677.6 ± 81.9 nm, n= 7 P < 0.0003). It should be noted that myxothiazol often took approximately a 1-min perfusion before it elicited its effects. This rise in [Ca2+]i was substantially inhibited by removal of extracellular calcium (92.8 ± 1.8%, P < 0.0003, n= 7, Fig. 3A). Antimycin A also inhibits electron transport at complex III but at a more distal point than myxothiazol. It prevents the conversion of ubisemiquinone to ubiquinone. Application of 0.5μm antimycin A also caused a rapid and irreversible rise in [Ca2+]i very similar to that observed with myxothiazol (Δ[Ca2+]i= 652.6 ± 155.2 nm, P < 0.006, n= 7, Fig. 3B). This rise in [Ca2+]i was significantly inhibited by removal of extracellular calcium (89.2 ± 3.4%, n= 7, P < 0.005, Fig. 3B).
Figure 3. Effects of electron transport inhibitors on [Ca2+]i in type I cells.
Effects of electron transport inhibitors on intracellular calcium in type I cells (measured using indo-1) in the presence and absence of extracellular calcium. A, myxothiazol (1μm) caused a large increase in intracellular calcium which was slow in onset, often with a delay of 30 s to 2 min, and which was maintained even after wash out of the drug. This sustained increase in [Ca2+]i was substantially reduced by removal of extracellular Ca2+. B, antimycin A (0.5μm) caused a rapid increase in [Ca2+]i that was also maintained following wash out of the drug. The antimycin A induced increase in [Ca2+]i was largely reversed by removal of extracellular Ca2. C, cyanide (nominally 2 mm; see methods) caused a rapid and reversible increase [Ca2+]i. The cyanide induced increase in [Ca2+]i was greatly reduced in calcium free media.
Cyanide is the classical inhibitor of complex IV (cytochrome c oxidase), the final component of the electron transport chain. Complex IV catalyses the oxidation of cytochrome c and the reduction of oxygen. Cyanide causes the cytochrome oxidase to complex with cytochrome a3 (van Gelder & Muijers, 1966). Application of cyanide (nominally 2 mm, but see Methods) produced a rapid and reversible rise in [Ca2+]i (Δ[Ca2+]i= 584.3 ± 105.6 nm, n= 7, P < 0.003). The calcium response to cyanide was significantly attenuated in the absence of extracellular Ca2+ (90.6 ± 2.3%, P < 0.003, n= 7, Fig. 3C).
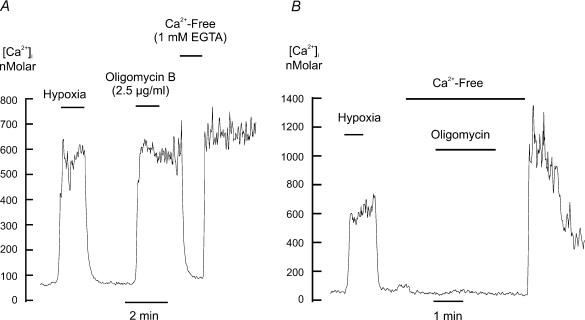
Another mechanism by which oxidative phosphorylation can be arrested is by inhibiting the F1–F0 ATP synthase using the compound oligomycin. Similar effects to those observed with the electron transport inhibitors were seen with oligomycin. Oligomycin at 2.5 μg ml−1 caused a rapid, but irreversible rise in [Ca2+]i (Δ[Ca2+]i= 675.2 ± 84.5 nm, n= 6, P < 0.001) and either removal of extracellular Ca2+ during the oligomycin response (Fig. 4A) or application of oligomycin in the absence of extracellular Ca2+ (Fig. 4B) virtually abolished this rise in [Ca2+]i (95.0 ± 1.2%, P < 0.003, n= 6).
Figure 4. Effect of ATP synthase blockade on [Ca2+]i in type I cells.
Effects of hypoxia and oligomycin on [Ca2+]i (measured using indo-1) in type I cells. A, effect of removing extracellular calcium after application of oligomycin. B, effect of removing extracellular calcium prior to and during the application of oligomycin. Note that in the presence of extracellular calcium oligomycin induced a large rise in [Ca2+]i that was maintained following oligomycin removal. Removal of extracellular calcium prevented or reversed this oligomycin induced rise in [Ca2+]i.
These results indicate that inhibition of mitochondrial electron transport or inhibition of the mitochondrial ATP synthase causes a rapid rise in intracellular Ca2+ and that this rise in [Ca2+]i is due predominantly to Ca2+ entry from the extracellular medium. The slow reversibility of the rotenone response may indicate that dissociation from complex I is slow, or that efflux from the cell is slow or that wash out of this drug from the experimental chamber is slow. The effects of oligomycin, antimycin A and myxothiazol were essentially irreversible. Moreover we found that extensive washing of the experimental chamber and perfusion lines with ethanol was necessary after each experiment utilizing myxothiazol, oligomycin or antimycin A to prevent spontaneous increases in [Ca2+]i when fresh cells were introduced into the recording chamber.
Effects of electron transport inhibitors on membrane potential and [Ca2+]i
It has been shown that hypoxia depolarizes type I cells leading to an influx of Ca2+ (Buckler, 1997) and that hypoxia and mitochondrial uncouplers inhibit a TASK-like background K+ current (Buckler, 1997; Buckler & Vaughan-Jones, 1998; Buckler et al. 2000). We therefore investigated whether other mitochondrial inhibitors would also depolarize type I cells. To this end membrane potentials were recorded from cells in the current clamp configuration and [Ca2+]i was simultaneously measured. NaCN at 2 mm depolarized cells from a resting membrane potential of −59.8 ± 1.8 mV to −41.7 ± 1.1 mV (n= 10, P < 0.0001, see Fig. 5A for an example); this depolarization was accompanied by a rapid and robust rise in intracellular Ca2+. This NaCN-induced rise in [Ca2+]i was substantially attenuated by voltage clamping the cells to −70 mV (rise in [Ca2+]i in current clamp = 496 ± 39 nm; rise in [Ca2+]i in voltage clamp = 79 ± 13 nm, n= 5, P < 0.005, see Fig. 5A and B). Rotenone at 1μm also rapidly depolarized type I cells from −49.4 ± 2.9 mV to −26.4 ± 1.1 mV (n= 9, P < 0.0002, see Fig. 6A). Again this depolarization was accompanied by a sharp rise in [Ca2+]i that was attenuated by 74.3 ± 4.8% (n= 9, P < 0.005, see Fig. 6) when the cells were clamped at −70 mV. The cells were switched to voltage clamp conditions during the depolarization rather than the method employed with the previous NaCN experiments due to the slow reversibility of the rotenone effect.
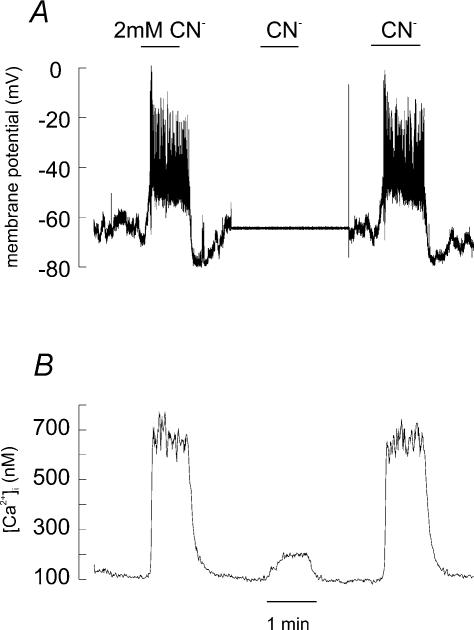
Figure 5. Effects of NaCN on resting membrane potential and [Ca2+]i in a type I cell.
A, perforated patch recording of membrane potential. Experiment starts and finishes in current clamp mode and shows a rapid membrane depolarization in response to NaCN application (nominally 2 mm). In the middle of the experiment (where the membrane potential trace is flat) the cell was voltage clamped at −70 mV and cyanide was reapplied. B, simultaneous recording of [Ca2+]i from the same cell (using indo-1) showing that the rise in [Ca2+]i in response to NaCN was greatly attenuated when the cell was voltage clamped at −70 mV.
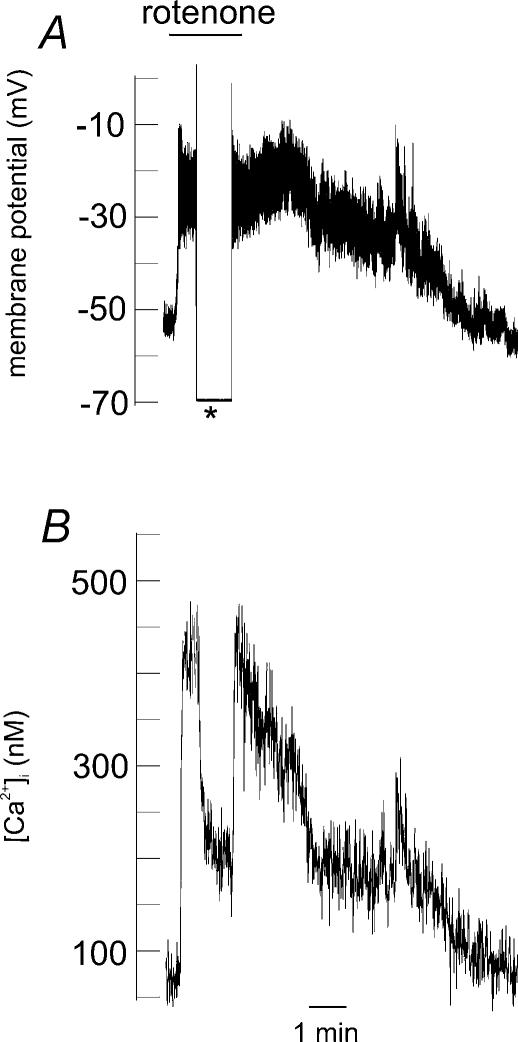
Figure 6. Effects of 1μm rotenone on resting membrane potential and [Ca2+]i in a type I cell.
A, perforated patch recording of membrane potential in a type I cell. The asterisk denotes a switch from voltage recording (current clamp) mode to voltage clamping the membrane potential to −70 mV. Application of rotenone causes an abrupt membrane depolarization. B, simultaneous recording of [Ca2+]i (using indo-1) from the same cell; note the rapid fall in [Ca2+]i upon on voltage clamping the cell to −70 mV.
Effects of electron transport inhibitors and oligomycin on membrane currents
Under voltage clamp conditions voltage ramps from −100 to −40 mV were applied to cells at 2 Hz. Generation of current–voltage relationships from the applied voltage ramps (Fig. 7A) revealed that bath application of 1.0μm rotenone significantly reduced type I cell membrane conductance. Membrane conductance, measured between −60 and −50 mV, was reduced from 285 ± 31 pS (control) to 108 ± 49 pS in the presence of rotenone (n= 8, P < 0.002). Similar results were also seen with the other two electron transport inhibitors and with oligomycin. Myxothiazol at 100 nm reduced cell conductance from 312 ± 43 pS to 30 ± 62 pS (n= 6, P < 0.01, Fig. 7B). NaCN at 2.0 mm reduced cell conductance from 359 ± 57 pS to 136 ± 34 pS (n= 7, P < 0.005, Fig. 7C). Oligomycin at 2.5 μg ml−1 reduced type I cell conductance from 324 ± 28 pS to 105 ± 24 pS (n= 8, P < 0.0001, Fig. 7D). In addition to the decrease in membrane conductance, application of mitochondrial inhibitors also resulted in the loss of any zero current potential over the voltage range tested indicating that all four mitochondrial inhibitors may be expected to depolarize type I cells (see Fig. 7).
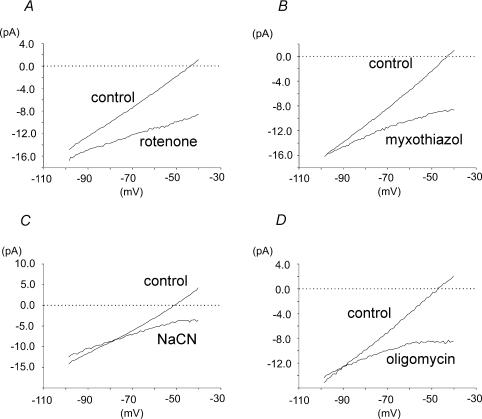
Figure 7. Effects of electron transport inhibitors and an ATP synthase inhibitor on membrane currents in type I cells.
A, mean whole cell current–voltage (I–V) relationships for type I cells in the presence and absence of rotenone 1μm (n= 8). B, mean whole cell I–V relationships in the presence and absence of myxothiazol 100 nm (n= 6). C, mean whole cell I–V relationships in the presence and absence of NaCN 2 mm (n= 7). D, mean whole cell I–V relationships in the presence and absence of oligomycin 2.5 μg ml−1 (n= 8). The slope of the above I–V plots give an indication of resting membrane conductance. The potential at which the I–V plots transect the zero current axis (the zero current potential) corresponds to the predicted resting membrane potential. Note that the I–V relationships obtained in the presence of all four mitochondrial inhibitors show not only a decrease in the resting membrane conductance but also the absence of a zero current potential within the voltage range tested (indicating that all four drugs will cause membrane depolarization).
In order to ascertain whether the reduction of membrane conductance caused by all four of these mitochondrial inhibitors was mediated via the inhibition of the TASK-like background K+ current we carried out experiments at two different concentrations of extracellular [K+]o. Control I–V relationships were first determined in the presence of 4.5 mm[K+]o and in the presence of 20 mm[K+]o. The mitochondrial inhibitor was then applied and I–V relationships were re-determined in the presence of both 4.5 mm and 20 mm[K+]o (see Fig. 8A). Inhibitor-sensitive current was then calculated by subtracting the I–V relationship observed in the presence of the mitochondrial inhibitor from the control I–V relationship at each level of extracellular K+. All four inhibitor-sensitive currents (see Fig. 8B–E) were shifted to a more negative range in 20 mm K+ compared to 4.5 mm K+ and reversal potentials, where evident (Fig. 8C and D), were shifted in a positive direction along the voltage axis indicating that a proportion of the mitochondrial inhibitor-sensitive current is carried by K+ ions. It was noted however, that the reversal potentials for a number of inhibitor-sensitive currents were negative to those expected for a pure K+ current. A reversal potential for both oligomycin and rotenone was not obtained under control conditions over the voltage range tested in this series of experiments (but see Figs 7D and 9A) and in 20 mm K+ were negative to the equilibrium potential for potassium ions (EK=−55 mV; reversal potentials for rotenone =−93.1 mV, 3 currents failed to reverse, n= 8; reversal potential for oligomycin =−77.0 ± 6.6 mV, n= 8). The reversal potential for the myxothiazol-sensitive current obtained under control conditions (−90.2 mV, n= 6, 4 currents failed to reverse) was close to the predicted EK of −96 mV, but that in 20 mm K+ (−77.8 mV) was again negative to EK. The control reversal potential for the NaCN-sensitive current was −78.7 mV (n= 7, 2 currents failed to reverse) and in 20 mm K+ was −48.0 ± 2.2 mV (n= 7).
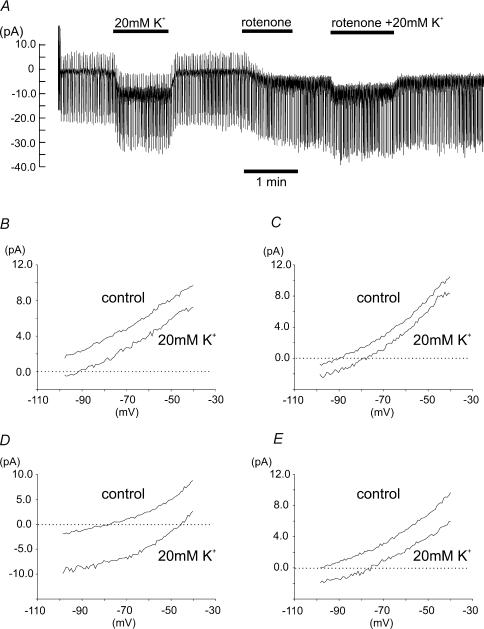
Figure 8. Current–voltage relationships of inhibitor-sensitive currents.
A, an example of membrane current recorded from a type I cell voltage clamped at a holding potential of –70 mV and subjected to repetitive voltage ramps from −100 mV to −40 mV at 0.5 Hz. The effects of high K+ (20 mm), rotenone and rotenone in the presence of high K+ are shown. B, mean I–V relationships of rotenone-sensitive currents (1μm, n= 8) obtained by subtracting the I–V relationship obtained in the presence of rotenone from the control I–V relationship in both normal extracellular K+ (4.5 mm) and at elevated extracellular K+ (20 mm). C, mean I–V relationships of myxothiazol-sensitive currents (100 nm, n= 6) at normal and elevated extracellular K+. D, mean I–V relationships of NaCN-sensitive currents (2 mm, n= 7). E, mean I–V relationships of oligomycin-sensitive currents (2.5 μg ml−1, n= 8). Note the depolarizing, inward, shift in all the inhibitor-sensitive currents when extracellular K+ was elevated to 20 mm.
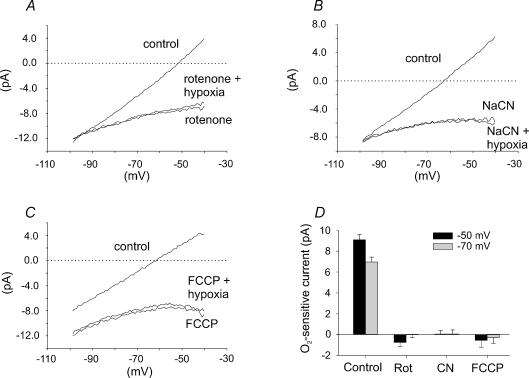
Figure 9. Effect of hypoxia, in combination with application of rotenone, NaCN and FCCP, on membrane currents in type I cells.
A, mean I–V relationships (n= 6) in control conditions, in the presence of 1μm rotenone and in the presence of 1μm rotenone and hypoxia (6 Torr). B, mean I–V relationships (n= 6) in control conditions, in the presence of 2 mm NaCN and in the presence of 2 mm NaCN and hypoxia (6 Torr). C, mean I–V relationships (n= 4) in the presence of 1μm FCCP and in the presence of 1μm FCCP and hypoxia (6 Torr). Note the absence of any additional effect of hypoxia upon membrane currents in the presence of rotenone, cyanide or FCCP. D, comparison of mean (+s.e.m.) oxygen-sensitive current measured at two different potentials −50 mV (black bars) and −70 mV (grey bars) under control conditions (control – hypoxia; n= 46) with that determined in the presence of rotenone (rotenone – hypoxia and rotenone; n= 6), cyanide (cyanide – hypoxia and cyanide; n= 6) and FCCP (FCCP – hypoxia and FCCP; n= 4). Note that the magnitude of the oxygen-sensitive current measured in the presence of mitochondrial inhibitors was negligible compared to that determined under control conditions.
We also estimated potassium conductance at −70 mV from the change in holding current upon switching from 4.5 mm K+ to 20 mm K+, i.e. gK= (I4.5−I20)/(EK20–EK4.5), in the presence and absence of inhibitor, where I4.5= membrane current at −70 mV in 4.5 mm[K+] Tyrode solution, I20= membrane current at −70 mV in 20 mm[K+] Tyrode solution, EK4.5= potassium equilibrium potential in 4.5 mm[K+] Tyrode solution, and EK20= potassium equilibrium potential in 20 mm[K+] Tyrode solution. (Note this calculation assumes that gK remains constant with change in [K]o. There is, however, evidence that background K+ channels in type I cells display GHK-type rectification (Williams & Buckler, 2004). The values for gK calculated here may therefore tend to overestimate the true gK in 4.5 mm[K+]o (and underestimate gK in 20 mm[K+]o).)
From these values it was possible to calculate the percentage inhibition of resting K+ conductance by each inhibitor. Rotenone (1.0μm) reduced resting K+ conductance by approximately 42% from 224 ± 22.9 pS to 129.5 ± 17.7 pS (n= 8, P < 0.0003). Myxothiazol (0.1μm) reduced resting K+ conductance by 31% from 225.5 ± 15.2 pS to 159.5 ± 18.3 pS (n= 8, P < 0.02). NaCN (2 mm) caused a 63% reduction in resting K+ conductance from 419.8 ± 57.6 pS to 166.9 ± 30.3 pS (n= 7, P < 0.0003) and finally oligomycin (2.5 μg ml−1) caused a 39% reduction in K+ conductance from 217.5 ± 36.9 pS to 118.6 ± 9.8 pS (n= 8, P < 0.02). Anoxia also inhibits resting membrane conductance to potassium ions at −70 mV by 55% (from 406 ± 66 pS to 181 ± 38 pS) as do mitochondrial uncouplers (by 56%, see Buckler & Vaughan-Jones, 1998). Thus both mitochondrial inhibitors and anoxia cause a comparable reduction in the resting membrane conductance to potassium ions. It should be noted, however, that none of these compounds completely inhibit resting K+ conductance. Taken together, these data show that a substantial proportion of the mitochondrial inhibitor-sensitive current is clearly carried by K+ ions although other membrane currents may also be modulated.
It was also noted during the course of these voltage clamp experiments that application of rotenone, myxothiazol, CN− or oligomycin caused only a very small rise in intracellular calcium (data not shown) compared to that seen in cells not subject to voltage-clamp control of membrane potential. This observation further supports the conclusion that the rise in [Ca2+]i normally seen with these compounds is primarily mediated via membrane depolarization and voltage gated calcium entry (the origin of the residual small rise in [Ca2+]i seen in voltage clamp will be discussed later).
Effect of hypoxia on membrane currents in combination with electron transport inhibitors and the mitochondrial uncoupler FCCP
The above, and previously published data (Buckler & Vaughan-Jones, 1998), indicate that inhibitors of oxidative phosphorylation have similar effects on type I cells to those of hypoxia. In order to determine whether metabolic inhibitors and hypoxia modulate membrane currents through a common mechanism we investigated the effects of hypoxia on membrane currents in the presence of rotenone, cyanide and FCCP. I–V relationships were constructed in control conditions, in the presence of 1μm rotenone, 2 mm NaCN and 1μm FCCP and in the presence of each compound in a hypoxic solution (6 Torr). As can be seen from the data presented in Fig. 9, hypoxia (6 Torr) had no significant effect on membrane currents in the presence of rotenone (n= 6, Fig. 9A), NaCN (n= 6, Fig. 9B) or FCCP (n= 4, Fig. 9C). In contrast, in the absence of these mitochondrial inhibitors, hypoxia substantially inhibits background K+ currents (see, e.g. Buckler, 1997; Buckler et al. 2000); Fig. 9D shows a quantitative comparison of the relative magnitudes of the O2-sensitive current recorded under control conditions with that seen in the presence of the above mitochondrial inhibitors at two potentials, −50 and −70 mV (at both potentials the difference between the control O2-sensitive current, and that recorded in the presence of any of the three mitochondrial inhibitors, was significantly different P < 0.0001). Thus inhibitors of oxidative phosphorylation abolish hypoxic sensitivity of background K+ current. It is important to note that these results contrast with the work of Searle et al. (2002) who suggested that rotenone may be a non-selective K+ channel inhibitor. However, in the study by Searle et al. (2002), the effects of rotenone were reversed by hypoxia, an effect which we failed to observe in type I cells.
Discussion
The mechanism by which carotid body type I cells transduce a fall in oxygen tension to excitatory transmitter release is not yet fully understood, although a number of hypotheses have been advanced (see Prabhakar, 2000 for a review). The data presented here support the hypothesis that mitochondria play a critical role in oxygen sensing.
The idea that the mitochondria are involved in the sensing of oxygen changes in the carotid body is by no means new. Mills & Jobsis (1971) presented evidence for a specialized cytochrome aa3 in carotid body mitochondria and subsequently elegant work by Duchen & Biscoe (1992a) demonstrated that mitochondrial function in type I cells was unusually sensitive to oxygen. Duchen & Biscoe (1992b) were also the first to demonstrate that interrupting electron transport, blocking the F1–F0-ATPase or chemically uncoupling the mitochondria resulted in a rise in intracellular Ca2+. They concluded that the rise in intracellular Ca2+ in response to both hypoxia and inhibitors of mitochondrial metabolism was mediated by a depolarization of mitochondrial membrane potential and Ca2+ release from mitochondria and other intracellular sources (Duchen & Biscoe, 1992b). It was subsequently demonstrated that the Ca2+ response to hypoxia was mediated primarily via voltage-gated calcium entry, rather than release from internal stores (Buckler & Vaughan-Jones, 1994). Consequently a common current view is that the mechanisms by which hypoxia and mitochondrial inhibitors excite the carotid body are different with hypoxia promoting voltage-gated calcium entry and metabolic inhibitors promoting calcium release (Lopez-Barneo et al. 2001). In contrast, the data we present here reveal that inhibition of mitochondrial function excites carotid body type I cells in a manner very similar to that seen with hypoxia.
Effects of Mitochondrial inhibitors on calcium signalling in type I cells
Pharmacological inhibition of mitochondrial electron transport at three different points in the electron transport chain using rotenone (complex I), myxothiazol, antimycin A (complex III) or cyanide (complex IV) caused a rapid rise in intracellular Ca2+. With all four electron transport inhibitors the calcium response was primarily dependent upon extracellular calcium. Oligomycin, which is known to excite the carotid body in vivo (Mulligan et al. 1981), also evoked a rapid increase in type I cell [Ca2+]i that was again dependent upon extracellular calcium. Thus inhibition of mitochondrial electron transport, or inhibition of the F1–F0-ATPase, or mitochondrial uncoupling (Buckler & Vaughan-Jones, 1998) have similar effects on type I cell [Ca2+]i to those observed with hypoxia, i.e. a rapid rise in [Ca2+]i that is largely mediated by calcium influx. The only significant differences we observed were in the degree of reversibility of some of these agents with the effects of uncouplers (Buckler & Vaughan-Jones, 1998) and CN− being rapidly reversible, rotenone being slowly reversible and oligomycin, antimycin A and myxothiazol being essentially irreversible.
Hypoxia and mitochondrial uncouplers evoke a rise in intracellular calcium by inducing membrane depolarization and voltage-gated calcium entry (Buckler & Vaughan-Jones, 1994, 1998). We therefore investigated the effects of both cyanide and rotenone on membrane potential in type I cells using perforated patch current clamp. Both compounds caused a rapid cell depolarization and a rapid rise in [Ca2+]i. The rise in Ca2+ was significantly attenuated when the cells were voltage clamped at −70 mV. We also observed that both myxothiazol and oligomycin caused only a very small increase in [Ca2+]i during other voltage-clamp experiments (i.e. during application of voltage ramps to determine the effects of these compounds upon membrane conductance). Thus the rise in [Ca2+]i in response to inhibition of electron transport or inhibition of the ATP synthase is also primarily mediated by voltage gated calcium entry. There may however, also be a small proportion of the rise in [Ca2+]i that is not due to voltage-gated Ca2+ entry since a small rise in [Ca2+]i persisted both in voltage clamped cells and in the absence of extracellular calcium (see Figs 3C and 5B). A similar small, voltage- and Ca2+o- independent rise in [Ca2+]i has also been observed with both anoxia and mitochondrial inhibitors (Buckler & Vaughan-Jones, 1994, 1998). This component of the Ca2+ signal presumably results from Ca2+ release from internal stores, e.g. mitochondria or the endoplasmic reticulum. A recent study, however, clearly showed that agents that empty intracellular Ca2+ stores (caffeine, ryanodine, thapsigargin and cyclopiazonic acid) were unable to elicit changes in [Ca2+]i of sufficient magnitude to initiate neurosecretion from intact carotid body or to modulate Ca2+ transients evoked by cell depolarization in isolated type I cells (Vicario et al. 2000). Consequently although both hypoxia and mitochondrial inhibitors may evoke some degree of Ca2+ release from internal stores this is likely to be of minor significance to Ca2+ signalling overall or consequent neurotransmitter release.
In conclusion, from the perspective of calcium signalling events, the mechanisms of action of a wide range of mitochondrial inhibitors are indistinguishable from that of hypoxia, i.e. membrane depolarization followed by voltage-gated calcium entry. Calcium release from internal, unidentified, stores although detectable plays only a minor and probably insignificant role.
Effects of mitochondrial inhibitors on membrane currents
Previous work in this laboratory has shown that neonatal rat type I cells possess O2-sensitive TASK-1-like K+ currents; these currents are inhibited by both hypoxia and mitochondrial uncouplers (Buckler, 1997; Buckler & Vaughan-Jones, 1998) resulting in membrane depolarization and Ca2+ entry. In order to ascertain whether the mitochondrial inhibitors used here could also be inhibiting the TASK-1-like K+ current, membrane currents were observed under voltage clamp conditions. Rotenone, myxothiazol, cyanide and oligomycin all significantly inhibited a background K+ current (see Figs 7 and 8) an effect reminiscent of that seen with hypoxia. The observation that hypoxia had no additional effect in the presence of cyanide, rotenone and the uncoupler FCCP, further argues that inhibitors of mitochondrial metabolism are inhibiting the same background K+ current as that modulated by hypoxia.
In addition to their effects upon background K+ current, inhibitors of mitochondrial metabolism may well modulate other ionic currents as well. As we have previously reported for mitochondrial uncouplers, the reversal potentials for the inhibitor-sensitive currents of some of the compounds tested here showed some deviation from the predicted reversal potentials for a pure K+ current (see results). Indeed it is known that in adult guinea-pig chromaffin cells both anoxia and cyanide can activate non-selective cation channels (Inoue et al. 1998, 1999). A similar action in the type I cells would inevitably shift the reversal potential of total inhibitor-sensitive current to more negative potentials. The identification of additional currents that may be activated by mitochondrial inhibitors is currently under investigation.
Mechanism of action of mitochondrial inhibitors
A key question posed by the data presented in this paper is how does inhibition of mitochondrial function lead to modulation of ion channel activity. In principle there are a number of facets of mitochondrial activity that could be altered by metabolic inhibitors. Here we consider some of the main ones and whether they could account for our observed results.
ATP synthesis
All inhibitors studied here would be expected to inhibit the synthesis of ATP and most (i.e. all bar oligomycin) may be expected to accelerate its consumption via reversal of the ATP synthase secondary to mitochondrial depolarization. It has recently been reported that the activity of background K+ channels in inside out patches is increased by the application of solutions containing ATP to the intracellular aspect of the membrane patch (Williams & Buckler, 2004). These observations suggest that, in principle, changes in cytosolic ATP could provide a link between background K+ channel activity and mitochondrial energy metabolism. Whether inhibition of mitochondrial energy metabolism leads directly to significant changes in cytosolic ATP (or ADP, AMP or Pi) in type I cells remains to be determined, but such studies in chromaffin cells suggest that mitochondrial inhibition does lead to rapid changes cytosolic ATP levels (Inoue et al. 2002). Changes in mitochondrial ATP levels/synthesis could also alter the production of an unknown signalling molecule that is involved in the regulation of ion channel function.
Mitochondrial depolarization
In their original papers Duchen & Biscoe (1992a, b) postulated that mitochondrial depolarization could be the key event leading to the generation of a calcium signal (originally postulated to be via Ca2+ release from internal stores). The principle argument against this is that whilst electron transport inhibitors and uncouplers depolarize mitochondrial membrane potential, oligomycin causes a hyperpolarization (Duchen & Biscoe, 1992b).
Change in mitochondrial redox NAD+/NADH
Electron transport inhibitors will decrease NAD+/NADH but uncouplers have the opposite effect. This was demonstrated in rabbit type I cells by Duchen & Biscoe (1992a) and we have observed the same effect in neonatal rat type I cells in which anoxia increases and FCCP decreases NADH autofluoresence (data not shown). Consequently we would reject changes in NAD+/NADH levels as a likely signalling mechanism.
Changes in reactive oxygen species (ROS) generation
A number of studies have suggested that modulation of mitochondrial superoxide (O2−) production plays a role in hypoxic pulmonary vasoconstriction in the lung and in the inhibition of potassium channels in pulmonary vascular myocytes. There are, however, two diametrically opposed views, (1) that hypoxia increases ROS (Waypa et al. 2001) and (2) that hypoxia decreases ROS (Archer et al. 1993). This raises the question as to whether changes in ROS production could be involved in mediating the response of type I cells to metabolic inhibitors.
O2− is generated at two points within the mitochondrial respiratory chain, complex I (Turrens & Boveris, 1980) and complex III (Cadenas et al. 1977; Turrens et al. 1985; Turrens, 1997). Various inhibitors of electron transport including cyanide, rotenone and myxothiazol promote O2− production by NADH dehydrogenase (Turrens & Boveris, 1980). Antimycin A also increases O2− production at complex III (see Turrens, 1997 and refs therein). In contrast both rotenone and myxothiazol inhibit O2− production by complex III (Turrens et al. 1985), as does cyanide (IC50= 30μm; Cadenas & Boveris, 1980). Mitochondrial uncouplers also decrease mitochondrial O2− production (Skulachev, 1996; Boveris & Chance, 1973); O2− production at complex III is believed to be highly sensitive to mitochondrial membrane potential (Demin et al. 1998). Whereas oligomycin is reported to have no effect upon O2− production (Boveris & Chance, 1973). Thus the range of mitochondrial inhibitors used in this study, all of which mediate an increase in type I cell intracellular calcium, are expected to have diverse effects upon mitochondrial O2− production. Furthermore any increase in O2− production by complex I in response to electron transport inhibition should be antagonized by a reduction in oxygen levels (O2− production at both complex I and III is a non-enzymatic process that should be governed by the laws of mass action, Turrens, 1997). We saw no such antagonism of the effects of either rotenone or cyanide when PO2 was lowered from 150 Torr to around 5 Torr (Fig. 9). Thus the effects of all the inhibitors of mitochondrial metabolism used in this study cannot be accounted for by either an increase or decrease in O2− production.
In summary the most likely candidate for mediating the effects of mitochondrial inhibitors seems to be a reduction in mitochondrial ATP synthesis. The above discussion, and our conclusion, is however, based upon two assumptions (a) that there is only one unifying mechanism responsible for mediating the effects of electron transport inhibition, uncoupling, and inhibition of ATP synthase and (b) that none of the compounds used are having ‘non-specific’ effects.
Role of mitochondria in O2 sensing
The data presented in this, and previous studies (Buckler, 1997; Buckler & Vaughan-Jones, 1998) show that hypoxia and many different inhibitors of oxidative phosphorylation have very similar effects upon isolated type I cells. If the transduction pathways for hypoxia and mitochondrial inhibitors are independent, one might expect the effects of hypoxia and mitochondrial inhibitors to be additive. It has recently been reported that the secretory response to hypoxia in carotid body slices is additive to that evoked by cyanide, myxothiazol or antimycin A; but that rotenone selectively occludes sensitivity to hypoxia (Ortega-Sáenz et al. 2003). On the basis of these observations it has been suggested that mitochondrial electron transport flow is not directly involved in acute oxygen sensing but that a rotenone-sensitive molecule is critical to oxygen sensing (Ortega-Sáenz et al. 2003). It is important to note, however, that additivity between the effects of hypoxia and of electron transport inhibitors can only be considered definitive evidence that oxygen sensing is independent from electron transport if the inhibition of electron transport is complete. We have found that the volatility of cyanide in buffered solutions requires the use of very high initial concentrations of CN− and regular replacement of such solutions if full inhibition of electron transport is to be achieved (Fig. 1). We have also observed that the effects of some electron transport inhibitors, e.g. myxothiazol, are slow in onset such that several minutes' exposure may be required before electron transport is fully inhibited (data not shown). In our study, we have found no evidence for additivity between hypoxia and the effects of cyanide, rotenone or the uncoupler FCCP on background K+ currents (see Fig. 9A, B and C). Similar observations have also been made in the intact carotid body in which the increase in chemosensory nerve discharge in response to hypoxia is abolished in the presence of antimycin A, oligomycin or FCCP (Mulligan et al. 1981; Mulligan & Lahiri, 1982; Mosqueira & Iturriaga, 2002). This mutual exclusivity between the actions of mitochondrial inhibitors and hypoxia suggests that they share some common mode of action.
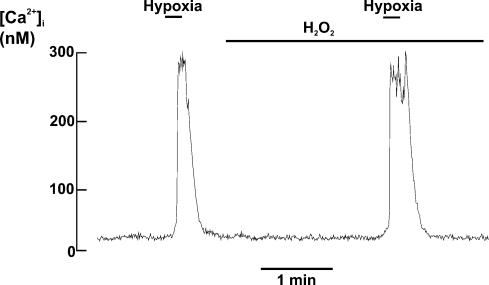
In order to explore a possible role for mitochondrial H2O2 production in mediating the response to hypoxia we investigated the effects of applying exogenous H2O2 upon oxygen sensing. Brief application of 30μm H2O2, a concentration several orders of magnitude higher than that expected to be normally present within cells (Cadenas & Davies, 2000; Chance et al. 1979), had no effect upon intracellular calcium alone ([Ca2+]i= 31 ± 2 nm, control and 30 ± 3 nm in the presence of H2O2; n= 6, n.s.) and neither did it antagonize the [Ca2+]i response to a hypoxic stimulus (hypoxia evoked rise in [Ca2+]i= 270 ± 68 nm, control, and 267 ± 56 nm in the presence of H2O2; n= 6, n.s.); see Fig. 10. Thus neither increase nor decrease in H2O2 generation would appear to play a major role in mediating the acute effects of hypoxia upon type I cells. With respect to the suggestion that a rotenone-sensitive molecule critically participates in oxygen sensing (Ortega-Sáenz et al. 2003), we have found that whilst rotenone does indeed occlude oxygen sensitivity it is not unique in this respect. Moreover, oxygen sensitivity can be restored in the continued presence of rotenone by the addition of the electron donor TMPD. These data suggest that if a rotenone-sensitive molecule does have a specific role in oxygen sensing, the site of action of rotenone must be distinct from the site of interaction of oxygen. These data are, however, consistent with the effect of rotenone being mediated through inhibition of mitochondrial electron transport as TMPD can restore electron transport by donating electrons to cytochrome c. At present therefore we can only suggest two general mechanisms which could account for the apparent role of mitochondria in oxygen sensing.
Figure 10. Effect of H2O2 on [Ca2+]i in type I cells.
Effect of hypoxia on intracellular calcium (indo-1) in the presence and absence of 30μm H2O2. Note that H2O2 alone has no effect upon [Ca2+]i nor does it antagonize the abrupt increase in [Ca2+]i seen in response to a hypoxic stimulus.
(1) The oxygen-sensing pathway may be dependent upon mitochondrial energy metabolism. For example ATP may be required to generate the signal that indicates the presence of oxygen. Thus either a lack of ATP (due to metabolic inhibition) or a lack of oxygen will result in the failure to generate this signalling molecule and the activation of a common response. In this context it is of interest to note that oxygen sensing in some prokaryotes is mediated via oxygen-sensitive kinases and phosphorylation cascades (see Bunn & Poyton, 1996, for a review).
(2) Oxygen sensing is a direct function of mitochondrial energy metabolism and ATP synthesis.
ATP synthesis and oxygen sensing
The hypothesis that oxygen sensing itself could be mediated via changes in ATP synthesis is not new but is controversial. The principle theoretical argument against a direct role for ATP in oxygen sensing is that the level of hypoxia required to significantly impair electron transport and ATP synthesis (approx <3 Torr) should be well below the physiological range for oxygen sensing. Optical measurements of mitochondrial membrane potential and NADH levels in isolated type I cells, however, have shown that type I cell mitochondria depolarize and NADH accumulates as PO2 is reduced below about 60 Torr (Duchen & Biscoe, 1992a, b). This surprising sensitivity of type I cell mitochondrial function to hypoxia is comparable to that of key events in the oxygen signalling cascade (i.e. elevation of intracellular calcium, neurosecretion and inhibition of background K+ currents; Buckler & Vaughan-Jones, 1994; Montoro et al. 1996; Buckler, 1997) and to estimates of oxygen sensitivity in the intact organ when expressed as a function of microvascular PO2, as opposed to arterial PO2 (Lahiri et al. 1993). Thus the issue of whether mitochondria sense hypoxia through a reduced oxygen affinity cytochrome oxidase and subsequent variation in mitochondrial ATP synthesis remains open.
In summary, the data presented in this paper suggests that the mitochondria play an essential role in the oxygen-dependent regulation of background K+ channels in type I cells. Whether mitochondria act in an accessory role (e.g. by supplying ATP to the real oxygen sensing pathway) or form an integral part of the oxygen sensing pathway itself remains to be determined.
Acknowledgments
The authors would like to thank K. L. Tsai for help in preparing isolated cells. This work was supported by the MRC.
References
- Anichcov SV, Belen'kii ML. Pharmacology of the Carotid Body Chemoreceptors. New York: Macmillan Publishing; 1963. [Google Scholar]
- Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- Biscoe TJ, Duchen MR. Electrophysiological responses of dissociated type I cells of the rabbit carotid body to cyanide. J Physiol. 1989;413:447–468. doi: 10.1113/jphysiol.1989.sp017663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe TJ, Duchen MR. The cellular basis of transduction in carotid chemoreceptors. Am J Physiol. 1990;258:L271–L278. doi: 10.1152/ajplung.1990.258.6.L271. [DOI] [PubMed] [Google Scholar]
- Biscoe TJ, Duchen MR, Eisner DA, O'Neill SC, Valdeolmillos M. Measurements of intracellular Ca2+ in dissociated type I cells of the rabbit carotid body. J Physiol. 1989;416:421–434. doi: 10.1113/jphysiol.1989.sp017769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J Physiol. 1997;498:649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol. 1994;476:423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. J Physiol. 1998;513:819–833. doi: 10.1111/j.1469-7793.1998.819ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Boveris A. Enhancement of hydrogen peroxide formation by protophores and ionophores in antimycin-supplemented mitochondria. Biochem J. 1980;188:31–37. doi: 10.1042/bj1880031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E, Boveris A, Ragan CI, Stoppani AOM. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef heart mitochondria. Arch Biochem Biophys. 1977;180:248–247. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJA. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Demin OV, Kholodenko BN, Skulachev VP. A model of O2− generation in the complex III of the electron transport chain. Mol Cell Biochem. 1998;184:21–33. [PubMed] [Google Scholar]
- Duchen MR, Biscoe TJ. Mitochondrial function in type I cells isolated from rabbit arterial chemoreceptors. J Physiol. 1992a;450:13–31. doi: 10.1113/jphysiol.1992.sp019114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, Biscoe TJ. Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rat carotid body. J Physiol. 1992b;450:33–61. doi: 10.1113/jphysiol.1992.sp019115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley FGP, Patel SD, Ragan CI, Attardi G. Photolabelling of a mitochondrially encoded subunit of NADH dehydrogenase with [1H]dihydrorotenone. FEBS Lett. 1987;219:108–113. doi: 10.1016/0014-5793(87)81200-0. [DOI] [PubMed] [Google Scholar]
- Heymans C, Bouckaert JJ, Dautrebande L. Sinus carodidien et reflexes respiratoires; sensibilite des sines carotidiens aux substances chimiques. Action stimulante respiratoire reflex du sulfre de sodium, du cyanure de potassium, de la nicotine et de la lobeline. Arch Int Pharmacodyn Ther. 1931;40:54–91. [Google Scholar]
- Inoue M, Fujishiro N, Imanaga I. Hypoxia and cyanide induce depolarisation and catecholamine release in dispersed guinea-pig chromaffin cells. J Physiol. 1998;507:807–818. doi: 10.1111/j.1469-7793.1998.807bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Fujishiro N, Imanaga I. Na+ pump inhibition and non-selective cation channel activation by cyanide and anoxia in guinea-pig chromaffin cells. J Physiol. 1999;519:385–396. doi: 10.1111/j.1469-7793.1999.0385m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Fujishiro N, Imanaga I, Sakamoto Y. Role of ATP decrease in secretion induced by mitochondrial dysfunction in guinea-pig adrenal chromaffin cells. J Physiol. 2002;539:145–155. doi: 10.1113/jphysiol.2001.012936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylov SS, Anichkov SV. The effect of metabolic inhibitors on carotid chemoreceptors. In: Torrance RW, editor. Arterial Chemoreceptors. Oxford: Blackwell; 1968. pp. 103–109. [Google Scholar]
- Lahiri S, Rumsey WL, Wilson DF, Iturriaga R. Contribution of in vivo microvascular PO2 in the cat carotid body chemotransduction. J Appl Physiol. 1993;75:1035–1043. doi: 10.1152/jappl.1993.75.3.1035. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Pardal R, Ortega-Saenz P. Cellular mechanism of oxygen sensing. Ann Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- Mills E, Jobsis FF. Mitochondrial respiratory chain of carotid body and chemoreceptor response to changes in oxygen tension. J Neurophysiol. 1971;35:405–428. doi: 10.1152/jn.1972.35.4.405. [DOI] [PubMed] [Google Scholar]
- Montoro RJ, Urena J, Fernandez-Chacon R, Alvarez de Toledo G, Lopez-Barneo J. Oxygen sensing by ion channels and chemotransduction in single glomus cells. J General Physiol. 1996;107:133–143. doi: 10.1085/jgp.107.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosqueiria M, Iturriaga R. Carotid body chemosensory excitation induced by nitric oxide: involvement of oxidative metabolism. Respir Physiol Neurobiol. 2002;131:175–187. doi: 10.1016/s1569-9048(02)00020-4. [DOI] [PubMed] [Google Scholar]
- Mulligan E, Lahiri S. Separation of carotid body chemoreceptor responses to O2 and CO2 by oligomycin and by antimycin A. Am J Physiol. 1982;242:C200–C206. doi: 10.1152/ajpcell.1982.242.3.C200. [DOI] [PubMed] [Google Scholar]
- Mulligan E, Lahiri S, Storey BT. Carotid body O2 chemoreception and mitochondrial oxidative phosphorylation. J Appl Physiol. 1981;250:H202–H207. doi: 10.1152/jappl.1981.51.2.438. [DOI] [PubMed] [Google Scholar]
- Ortega-Sáenz P, Pardal R, Garcia-Fernández M, López-Barneo J. Rotenone selectively occludes sensitivity to hypoxia in rat carotid body glomus cells. J Physiol. 2003;548:789–800. doi: 10.1113/jphysiol.2003.039693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol. 2000;88:2287–2295. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- Searle GJ, Hartness ME, Hoareau R, Peers C, Kemp PJ. Lack of contribution of mitochondrial electron transport to acute O2 sensing in model airway chemoreceptors. Biochem Biophys Res Commun. 2002;291:332–337. doi: 10.1006/bbrc.2002.6428. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys. 1996;29:169–202. doi: 10.1017/s0033583500005795. [DOI] [PubMed] [Google Scholar]
- Thierbach G, Michaelis G. Mitochondrial and nuclear myxothiazol resistance in sachromyces cerevisiae. Mol General Genet. 1982;196:501–506. doi: 10.1007/BF00337956. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Superoxide production by the mitochondrial respiratory chain. Biosci Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urena J, Fernandez-Chacon R, Bentot AR, Alvarez de Toledo G, Lopez-Barneo J. Hypoxia induces voltage-dependent Ca2+ entry and quantal dopamine secretion in carotid body glomus cells. Proc Natl Acad Sci U S A. 1994;91:10208–10211. doi: 10.1073/pnas.91.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder BF, Muijers AO. On cytochrome c oxidase II. The ratio of cytochrome a to cytochrome a3. Biochim Biophys Acta. 1966;118:47–57. doi: 10.1016/s0926-6593(66)80143-1. [DOI] [PubMed] [Google Scholar]
- Vicario I, Obeso A, Rocher A, Lopez-Lopez JR, Gonzales C. Intracellular Ca2+ stores in chemoreceptor cells of the rabbit carotid body: significance for chemoreception. Am J Physiol. 2000;279:C51–C61. doi: 10.1152/ajpcell.2000.279.1.C51. [DOI] [PubMed] [Google Scholar]
- Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- Williams BA, Buckler KJ. Biophysical properties and metabolic regulation of a TASK-like potassium channel in rat carotid body type-1 cells. Am J Physiol. 1994;286:L221–L230. doi: 10.1152/ajplung.00010.2003. [DOI] [PubMed] [Google Scholar]