Abstract
We investigated the dendritic relationship between starburst amacrine cells (SAs) and morphologically and physiologically characterized ON and ON-OFF direction-selective ganglion cells (DSGCs) in the rabbit retina. ON and ON-OFF DSGCs were found to exhibit tight dendritic cofasciculation with the SA plexus, visualized by immunolabelling of the vesicular acetylcholine transporter (VAChT). The degree of cofasciculation of both types of DSGC dendrites and SA plexus was found to be significant, unlike the relationship between non-DS cells and the SA plexus, which was close to chance distribution. No difference in the degree of cofasciculation in different regions of the DS dendritic field was observed. Individual SAs intracellularly injected both on the ‘preferred’ and ‘null’ side of the DSGCs showed the same degree of cofasciculation with the DSGCs. Therefore, the computation of motion direction is unlikely to result from apparent asymmetry in geometric proximity between SAs and DSGCs. Highly selective synaptic connections between SAs and DSGCs are necessary.
A class of retinal ganglion cells code the direction of stimulus motion by generating a strong response to one of the four cardinal directions (the preferred direction) and virtually no response to the opposite (null) direction (see reviews by Vaney & Taylor, 2002; He et al. 2003; Taylor & Vaney, 2003). Starburst amacrine cells (SAs), a type of interneurone in the retina, have recently been shown to play a critical role in retinal direction selectivity (DS). Ablation of the SA population has been shown to abolish retinal DS (Yoshida et al. 2001; Amthor et al. 2002). Imaging experiments using two-photon confocal microscopy have shown that SA processes exhibit a stronger Ca2+ signal to centrifugal motion than to centripetal motion (Euler et al. 2002). If the SA branches connect selectively to underlying DSGCs, the differential signal could be used to generate directional responses. Furthermore, simultaneous patch clamp recording from SAs and overlapping DSGCs has shown that SAs on the null side inhibit the DSGC whereas the SAs on the preferred side do not (Fried et al. 2002). Labelling both recorded cells with markers and subsequent analysis of the relationship between SA processes and DSGC dendrites have shown that the degree of dendritic cofasciculation of SAs on the null side is three times higher than that on the preferred side (Fried et al. 2002). As simultaneous patch clamp recording is technically very demanding, only three pairs of cells were examined. However, the spatial asymmetry is very important, and could provide the anatomical foundation for DS computation, if proved to be correct.
On the other hand, there is conflicting evidence in the literature. Dendrites of overlapping SAs and DSGCs have been shown to cofasciculate extensively with one another, and physiological recordings have shown that the cofasciculating DSGCs have different preferred directions (Tauchi & Masland, 1985; Vaney, 1994; Amthor & Oyster, 1995; He & Masland, 1998; Vaney & Pow, 2000). When dendrites of DSGCs with different preferred directions cofasciculate, and one of them in turn cofasciculates with an SA process, how can the dendrites of other DSGCs avoid cofasciculating with the same SA process? A recent report showed that boutons of a single SA contacted three adjacent ON-OFF DSGCs (Famiglietti, 2002), challenging simple DS models of spatially asymmetrical connections.
In this study, we investigated the relationship between the cholinergic plexus and morphologically and physiologically characterized ON and ON-OFF DSGCs. We show that there is no discernable anatomical asymmetry at the light microscopic level between the cholinergic plexus and dendrites of both types of DSGCs. Examination of individual SAs on the preferred or null side of the DSGCs reveals no difference in cofasciculation. We conclude that the computation of motion direction in the retina does not result from geometrically asymmetrical contacts between processes of DSGCs and SAs. For SAs to induce DS, they have to synapse selectively to an appropriate partner within the fascicule of DSGC processes.
Methods
Adult New Zealand White rabbits weighing 2–4kg were used. Use and handling of animals were strictly in accordance with the institutional guidelines and the Society for Neuroscience's policies on the use of animals and human subjects in neuroscience research. The procedures of prelabelling retinal ganglion cells (RGCs) and isolating and maintaining the retina in vitro have previously been described in detail (Ames & Nesbett, 1981; Yang & Masland, 1994). Briefly, 10μl (1μgμl−1) 4,6-diamidino-2-phenylindole (DAPI, Sigma) was injected intraocularly under anaesthesia (50mgkg−1 ketamine and 10mgkg−1 xylazine) to label retinal neurones about 12 h prior to each experiment. At the start of the experiment, animals were overdosed with ketamine and xylazine, eyes enucleated and hemisected. The retinas were carefully isolated and maintained in Ames' medium saturated with 95% O2 and 5% CO2. A small piece of retina was then transferred to a recording chamber situated on the stage of a Nikon E600FN microscope. This preparation was superfused at 2.5–3.5mlmin−1 with oxygenated Ames' medium at 33–35°C.
Identification of DSGCs
ON and ON-OFF DSGCs were identified by either morphological characteristics or electrophysiological responses. Both ON and ON-OFF DSGCs can be visually selected in the DAPI-labelled retina after some practice. A micropipette (impedance ∼150 MΩ) containing 1% Lucifer Yellow (Sigma) and 4% Neurobiotin (Vector Laboratory) was used to impale the selected cells and fill the cells with tracers (1nA, 1–3min). The identity of injected cells was readily revealed by their unique dendritic morphology. For physiological identification, light responses to a bar (100×500μm) drifting in 12 directions were recorded in whole-cell current clamp mode, using a standard Axopatch 200B amplifier and Clampex 8.1 (Axon Instruments). The patch pipette contained 0.4% Neurobiotin. SAs on either the preferred side or the null side were injected using a sharp micropipette containing 4% Lucifer Yellow after the recorded DSGCs had been characterized.
Immunohistochemical staining
For cryosections
Retinas were fixed with 4% paraformaldehyde for 10min, and balanced in a 10% sucrose solution followed by a 20% sucrose solution. Vertical cryosections of 16μm were cut using a Leica CM1900 cryostat. Sections were incubated overnight at 4°C with goat anti-choline acetyltransferase (ChAT) antibodies (1 : 1000, Chemicon) and rabbit anti-VAChT antibodies (1 : 4000, Sigma) diluted in PBS (pH 7.4) solution containing 0.3% Triton X-100 and 1% bovine serum albumin (Sigma). After washing 3 times with PBS, sections were incubated for 2h in antigoat-TRITC (tetramethyl rhodamine) and antirabbit-FITC (fluorescein) (1 : 100, Jackson Laboratory), coverslipped with Vectorshield (Vector lab), and sealed with nail polish.
For whole-mount preparations
Retinas were fixed with 4% paraformaldehyde for 1 h, washed 3 times and incubated with rabbit anti-VAChT antibodies (1 : 100, Sigma) for 7 days at room temperature. The preparation was then incubated in antirabbit-TRITC (1 : 100, Jackson Laboratory) for 1 day to label VAChT. Neurobiotin injected into the DS cells or SAs was visualized with streptavidin-FITC or streptavidin-Texas Red (Vector lab). Single SAs on the preferred or null side were injected with 4% Lucifer Yellow, incubated with rabbit anti-Lucifer Yellow antibodies (1 : 100, Molecular Probes, 2 days of incubation), visualized with antirabbit-FITC antibodies (1 : 100, Jackson Laboratory, 1 day of incubation). Negative controls were carried out by omitting the primary antibodies.
Quantitative analysis
Images were collected using a Zeiss LSM510 confocal microscope with a × 40 Plan Fluor (NA 1.3), a × 60 Plan Apo (NA 1.4), or a × 100 Plan Fluor (NA 1.45) for high power images. Intensity and contrast were adjusted with Photoshop 6.0 (Adobe). The labelled SA processes (either single cell injection or population staining by VAChT) is shown in one colour channel (red) and the DS dendrites in another (green). To quantify the degree of cofasciculation, the most stringent case was considered. A threshold was applied to each channel using MetaMorph 5.0 (Universal Imaging), and pixels in two channels had to overlap or touch in order to be considered as cofasciculated. In our case, the pixel size was 0.2–0.3μm and the estimated voxel height was 0.5μm. Pixels separated by more than 0.2μm could still be cofasciculated according to the definitions used in many studies, but the possibility of synaptic contacts between these pixels is quite unlikely, therefore were excluded in our analysis. A cofasiculation index (CI) was defined as follows:
where A is the total number of pixels in the area we analysed, R is the number of pixels in the red channel (either VAChT staining or single SA injection), Y is the number of contacting pixels (yellow), and G is the number of pixels in the green channel (RGC, usually DSGC). So, if 30% of pixels are positive for VAChT (red), and if these pixels are randomly distributed, then 30% of DS pixels (green) should be contacted by red pixels, yielding a CI of 1. A larger percentage of contacting pixels in the DS channel (green) signifies a tendency towards cofasciculation. Calculating the ratio of percentages has the advantage of excluding variation in the absolute number of pixels in the area analysed.
Results
VAChT-labelled plexus is composed of distal process of SAs
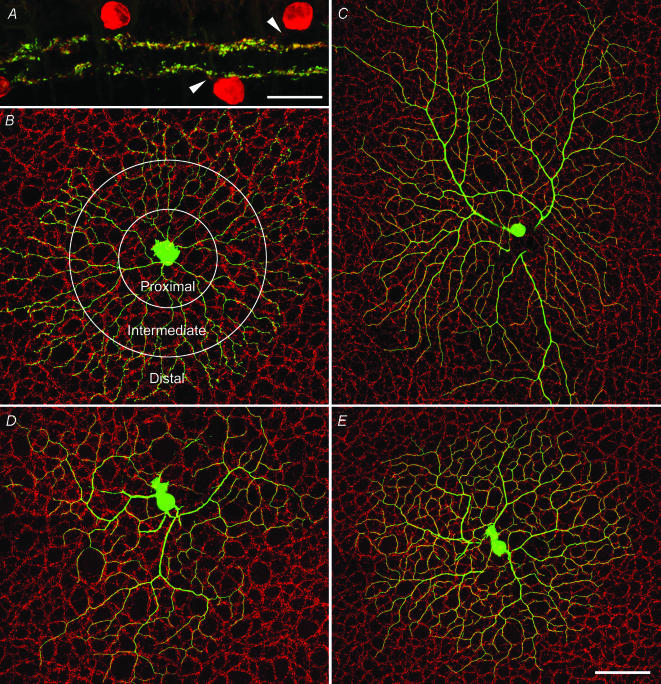
VAChT signal almost completely colocalized in the processes positive for ChAT. Confocal images of VAChT-positive processes in sublamina a and b can be seen in Fig. 1A, showing extensive double labelling with ChAT on cross-sections. On the other hand, ChAT-positive somas and proximal processes did not show any VAChT staining (Fig. 1A). This observation was further substantiated by quantitative analysis in the whole-mount retinas. An individual SA injected with Neurobiotin is shown in Fig. 1B against a background of VAChT staining. Overall, the SA processes showed cofasciculation with the VAChT-positive plexus with a CI of 1.7. Furthermore, when SA processes were divided and separately analysed at proximal, intermediate and distal regions, the degree of cofasciculation was found to increase from proximal (CI 1.3) to distal (CI 1.9) (Fig. 1B and Table 1). When the SAs were rotated by 180 deg, the CIs dropped to 1 (see Fig. S1 in Supplementary material, available online only). Even the smallest CI for the proximal region showed a statistically significant difference from the CIs of rotated SAs (P < 0.01). These results demonstrated that the VAChT-positive plexus is largely made of distal processes of SAs.
Figure 1. Relationship between the cholinergic plexus of SAs and the dendrites of ON and ON-OFF DSGCs.
A, a confocal micrograph of a retinal section double labelled with ChAT (red) and VAChT (green). ChAT-positive somas and primary processes (arrowheads) are not labelled with VAChT. B, an intracellularly injected SA on a background of VAChT staining; circles indicate proximal, intermediate and distal zones. C, an intracellularly injected ON DSGC, showing extensive dendritic cofasciculation with the cholinergic plexus. D and E, ON and OFF arbor of an intracelullarly injected ON-OFF DSGC, showing extensive dendritic cofasciculation with the cholinergic plexus. Scale bars: A, 20μm; E, 100 μm, also applies to B, C and D.
Table 1.
Summary of CI
| Cell type | No. | CI (mean ± s.d.) |
|---|---|---|
| ON of ON-OFF | 12(6) | 1.9 ± 0.3 |
| OFF of ON-OFF | 12(6) | 2.5 ± 0.2 |
| ON DS | 10(6) | 2.2 ± 0.2 |
| SA (overall) | 7 | 1.7 ± 0.2 |
| SA (proximal) | 7 | 1.3 ± 0.1 |
| SA (intermediate) | 7 | 1.6 ± 0.2 |
| SA (distal) | 7 | 1.9 ± 0.1 |
| Non-DS | 10 | 1.1 ± 0.1 |
| Rotated DS (180 deg) | 22 | 1.0 ± 0.1 |
Numbers in parenthesis are cells physiologically identified.
Both ON and ON-OFF DSGC dendrites show tight cofasciculation with SA plexus
Tight cofasciculation was obvious when quantitatively analysing processes of SAs and dendrites of morphologically or physiologically identified ON and ON-OFF DSGCs. Panels C, D and E of Fig. 1 show the cofasciculation of, respectively, an ON DSGC, and the ON and OFF arbor of an ON-OFF DSGC. Tight cofasciculation with the VAChT-labelled plexus was not ubiquitous: dendrites of several types of non-DSGCs showed close to chance distribution with the overlapping VAChT-labelled plexus (Table 1) (Fig. S2, Supplementary material). Furthermore, when the DS cell arbors were rotated by 180 degrees, the CI declined to 1 (Fig. S3, Supplementary material). Quantitative data were summarized in Table 1.
Different regions of DSGC dendritic field show same degree of cofasciculation
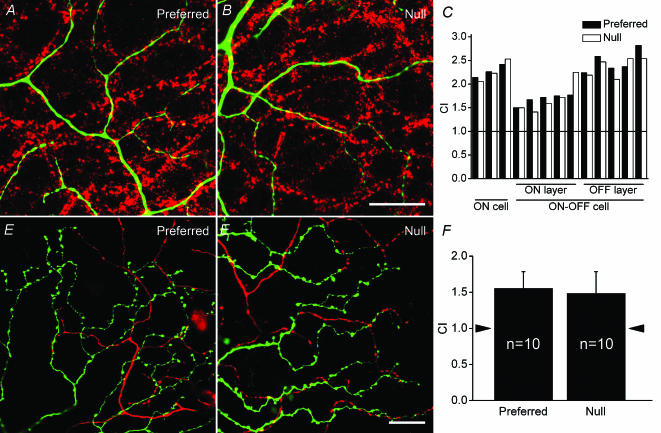
If one hypothesizes that the geometrically asymmetrical relationship between SAs and DSGCs is the sole cause of DS, one would expect less tight cofasciculation on the preferred side of the dendritic field because this region has been shown to be less directional (Barlow & Levick, 1965; He et al. 1999). The dendritic fields of DSGCs were divided into two halves perpendicular to the preferred–null axis, with each field containing an equal length of dendrites. An analysis of the extent of dendritic cofasciculation with the VAChT-positive plexus was carried out for each individual half-field. High power confocal micrographs of the DSGC dendrites on the preferred and null side are shown in Fig. 2A and B, respectively. A histogram of the CIs of eight DSGCs (1–3 ON DS, 4–8 ON layer of ON-OFF DS and 9–13 OFF layer of ON-OFF DS) is shown in Fig. 2C. A paired t test showed no statistically significant difference in cofasciculation between the preferred and null sides of the DS dendritic field (P= 0.23). Similar analyses were carried out along the preferred–null axis, with dendritic fields again divided into two halves. No significant difference in the degree of cofasciculation was found (P= 0.27).
Figure 2. Relationship between dendrites of DSGCs and the cholinergic plexus, and between dendrites of DSGCs and individual SAs.
A, a high power confocal micrograph of dendrites on the preferred side of a DS cell (green) and the cholinergic plexus (red). B, dendrites of the same DS cell as in A on the null side. C, a histogram summarizing CIs for the preferred and null halves of ON and ON-OFF DSGC dendritic fields; horizontal line indicates CI = 1.0. D, an SA extending into a DS dendritic field from the preferred direction. E, an SA extending into a DS dendritic field from the null direction. F, a histogram showing CIs between individual SA and DSGCs in the preferred and null directions; arrowheads indicate CI = 1.0. Scale bars: 20μm, also apply to A and D.
Individual SAs on different sides show same degree of cofasciculation with DSGCs
Examining the relationship between the dendrites of DSGCs and all SA processes did not permit the identification of any potential asymmetric connection between a DSGC and SAs on different sides. Therefore, it was necessary to investigate the relationship between individual SAs and DSGCs with overlapping dendritic fields. SAs injected on the preferred and null sides of physiologically characterized DSGCs (Fig. S4, Supplementary material) are shown in Fig. 2D and E, respectively. Quantitative analyses revealed no discernable differences in the degree of cofasciculation between dendrites of the DS cells and the distal processes of SAs on the preferred side or on the null side (Fig. 2F). The CI was 1.6 ± 0.2 for SAs on the preferred side and 1.5 ± 0.3 for those on the null side.
Discussion
In this study, we demonstrated that the VAChT-labelled plexus is largely composed of SA distal processes. Both ON and ON-OFF DSGC dendrites show extensive cofasciculation with SA plexus. The degree of cofasciculation between dendrites of ON DSGCs and SAs is just as good as that of ON-OFF DSGC dendrites. These findings extend, in a quantitative fashion, previous observations that both types of DSGCs costratify and cofasciculate with the SA plexus (Vaney, 1990; Famiglietti, 1991, 1992, 2002; Vaney et al. 2001). Furthermore, we demonstrated that the degree of cofasciculation does not vary across the dendritic field, and SAs extending into the DSGC dendritic field from the preferred or the null side show similar degrees of cofasciculation with the DSGC dendrites. We conclude that the computation of motion direction in the retina cannot result from a simple geometrically asymmetrical contacts between SAs and DSGCs. The terminal processes of a particular SA have to selectively synapse onto the dendrites of a particular DSGC within a fascicule containing dendrites of several DSGCs. This conclusion is obviously different from a recent observation (Fried et al. 2002). Four factors may account for the difference. First, the dendritic cofasciculation is assessed in a very stringent fashion in this study; only touching and overlapping pixels were taken into consideration. Secondly, different regions of SA processes exhibit different degrees of cofascicualtion with the VAChT-labelled plexus, with the proximal region exhibiting the lowest level and the distal region the highest, so when analysing dendritic relationships, care should be taken to investigate distal processes of SAs only. Thirdly, there are large individual differences among SA cells. In our sample, for the SAs extending into DSGC dendritic field from the preferred direction the CIs ranged from 1.4 to 2.2, and for SAs from the null direction they range from 1.1 to 2.1 (Fig. S5, Supplementary material). Finally, there might be a discrepancy between the critical GABA release sites responsible for DS and the processes marked by antibodies against VAChT.
SAs control both types of DS cells?
Although both ON and ON-OFF DSGCs code motion direction, they have very different response characteristics. The distribution of the preferred direction is different. ON DSGCs have three subtypes, with the preferred directions being anterior, superior (slightly towards posterior), and inferior (slightly towards posterior too). There are four subtypes of ON-OFF DSGCs, with the preferred directions being anterior, posterior, superior and inferior (Oyster, 1968). ON DSGCs respond optimally to slowly moving stimuli whereas ON-OFF DSGCs prefer medium-to-fast velocity stimuli (Oyster, 1968; Wyatt & Daw, 1975; He & Masland, 1998). When stimulated with a flashing spot, ON-OFF DSGCs respond transiently to both the onset and offset of the stimulus, whereas ON DSGCs exhibit a sustained response lasting as long as the receptive field is lit (He & Masland, 1998).
In the sublamina b, dendrites of ON DSGCs have been shown to exhibit a similar, if not a higher, degree of cofasciculation with the cholinergic plexus to those of the ON-OFF DSGCs. The observation of tight cofasciculation suggests that SAs play an important role in generating the direction selectivity of ON DSGCs. To control the slower response dynamics, the inhibition must be sustained. A sustained inhibitory current has been observed in DSGCs when currents were injected into SAs on the null side in a dual patch experiment (Fried et al. 2002), supporting the idea of sustained inhibition. Therefore, SAs could potentially control both the fast mechanism of ON-OFF DSGCs and the slow mechanism of ON DSGCs. Golgi-stained SAs have been observed to contact both ON and ON-OFF DSGCs in the rabbit retina (Famiglietti, 2002), although it is not clear if synapses exist within contacts.
Effective coverage factor and selective connection
This study has shown that the cofasciculated cholinergic plexus is predominantly made of distal processes of SAs. This finding reduces the effective coverage factor of SAs by 44.4%, from 30–70 to 17–39. Supposing that ON and ON-OFF DSGCs are controlled by different SAs in sublamina b, there are still sufficient SAs (2–6) to cover each of the three ON and four ON-OFF DS subtypes. In the sublamina a, there are only four subtypes of ON-OFF DSGCs, which would leave 4–10 SAs controlling each DSGC.
A sufficiently large coverage factor does not necessarily rule out the possibility that a single SA provides input to both ON and ON-OFF DS cells. The extraordinarily symmetrical morphology of SAs provides a convenient way to innervate DSGCs with different preferred directions, and as long as a DSGC selectively connects to a certain section of the SA processes, it could achieve the appropriate preferred direction.
Acknowledgments
This project was supported by a MOST Major State Basic Research Program Grant to the Institute of Neuroscience (G2000077800), and an NSFC Outstanding Young Researcher Award (39925010), NSFC project grants (30170305, 30270460), and a Shanghai Commission of Science and Technology grant (01JC14042) to S.H. We thank Weiqi Xu for technical support, and Melissa Barber for improving the English of the manuscript.
Supplementary material
The online version of this paper can be found at: DOI: 10.1113/jphysiol.2004.060715 and contains five supplementary figures: Figs S1–S5.
This material can also be found at http://www.blackwellpublishing.com/products/journals/suppmat/tjp/tjp210/tjp210sm.htm
References
- Ames AIII, Nesbett FB. In vitro retina as an experimental model of the central nervous system. J Neurochem. 1981;37:866–877. doi: 10.1111/j.1471-4159.1981.tb04473.x. [DOI] [PubMed] [Google Scholar]
- Amthor FR, Keyser KT, Dmitrieva NA. Effects of the destruction of starburst-cholinergic amacrine cells by the toxin AF64A on rabbit retinal directional selectivity. Vis Neurosci. 2002;19:495–509. doi: 10.1017/s0952523802194119. [DOI] [PubMed] [Google Scholar]
- Amthor FR, Oyster CW. Spatial organization of retinal information about the direction of image motion. Proc Natl Acad Sci U S A. 1995;92:4002–4005. doi: 10.1073/pnas.92.9.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Levick WR. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965;178:477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Synaptic organization of starburst amacrine cells in rabbit retina: analysis of serial thin sections by electron microscopy and graphic reconstruction. J Comp Neurol. 1991;309:40–70. doi: 10.1002/cne.903090105. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Dendritic co-stratification of ON and ON-OFF directionally selective ganglion cells with starburst amacrine cells in rabbit retina. J Comp Neurol. 1992;324:322–335. doi: 10.1002/cne.903240303. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. A structural basis for omnidirectional connections between starburst amacrine cells and directionally selective ganglion cells in rabbit retina, with associated bipolar cells. Vis Neurosci. 2002;19:145–162. doi: 10.1017/s0952523802191139. [DOI] [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- He S, Dong W, Deng Q, Weng S, Sun W. Seeing more clearly: recent advances in understanding retinal circuitry. Science. 2003;302:408–411. doi: 10.1126/science.1085457. [DOI] [PubMed] [Google Scholar]
- He S, Jin ZF, Masland RH. The nondiscriminating zone of directionally selective retinal ganglion cells: comparison with dendritic structure and implications for mechanism. J Neurosci. 1999;19:8049–8056. doi: 10.1523/JNEUROSCI.19-18-08049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Masland RH. ON direction-selective ganglion cells in the rabbit retina: dendritic morphology and pattern of fasciculation. Vis Neurosci. 1998;15:369–375. doi: 10.1017/s095252389815215x. [DOI] [PubMed] [Google Scholar]
- Oyster CW. The analysis of image motion by the rabbit retina. J Physiol. 1968;199:613–635. doi: 10.1113/jphysiol.1968.sp008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi M, Masland RH. Local order among the dendrites of an amacrine cell population. J Neurosci. 1985;5:2494–2501. doi: 10.1523/JNEUROSCI.05-09-02494.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. New directions in retinal research. Trends Neurosci. 2003;26:379–385. doi: 10.1016/S0166-2236(03)00167-X. [DOI] [PubMed] [Google Scholar]
- Vaney DI. The mosaic of amacrine cells in the mammalian retina. Prog Retinal Res. 1990;9:49–100. [Google Scholar]
- Vaney DI. Territorial organization of direction-selective ganglion cells in rabbit retina. J Neurosci. 1994;14:6301–6316. doi: 10.1523/JNEUROSCI.14-11-06301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI, He S, Taylor WR, Levick WR. Direction-selective ganglion cells in the retina. In: Zanker JM, Zeil J, editors. Motion Vision. Berlin: Springer; 2001. pp. 13–56. [Google Scholar]
- Vaney DI, Pow DV. The dendritic architecture of the cholinergic plexus in the rabbit retina: selective labeling by glycine accumulation in the presence of sarcosine. J Comp Neurol. 2000;421:1–13. [PubMed] [Google Scholar]
- Vaney DI, Taylor WR. Direction selectivity in the retina. Curr Opin Neurobiol. 2002;12:405–410. doi: 10.1016/s0959-4388(02)00337-9. [DOI] [PubMed] [Google Scholar]
- Wyatt HJ, Daw NW. Directionally sensitive ganglion cells in the rabbit retina: specificity for stimulus direction, size, and speed. J Neurophysiol. 1975;38:613–626. doi: 10.1152/jn.1975.38.3.613. [DOI] [PubMed] [Google Scholar]
- Yang G, Masland RH. Receptive fields and dendritic structure of directionally selective retinal ganglion cells. J Neurosci. 1994;14:5267–5280. doi: 10.1523/JNEUROSCI.14-09-05267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron. 2001;30:771–780. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online version of this paper can be found at: DOI: 10.1113/jphysiol.2004.060715 and contains five supplementary figures: Figs S1–S5.
This material can also be found at http://www.blackwellpublishing.com/products/journals/suppmat/tjp/tjp210/tjp210sm.htm