Abstract
In decerebrate cats, changes in the monosynaptic reflex (MSR) of gastrocnemius–soleus (G–S) motoneurones were studied after fatiguing stimulation (FST) of the G–S muscles. Monosynaptic reflexes were evoked by stimulation of Ia fibres in the G–S nerve and recorded from a filament of ventral root (VR) L7. FST (intermittent 40 s−1 stimulation for 10–12 min) was applied to the distal part of the cut VR S1. FST reduced MSR amplitudes to 0.64 ± 0.04 (mean ±s.e.m.) of the prefatigue values. The suppression remained stable for approximately 25 min and then MSR amplitudes gradually returned towards the normal. To test for the involvement of presynaptic and recurrent inhibition, MSRs were conditioned by stimulation of the nerve to the posterior biceps and semitendinosus (PBSt) muscles or a filament of VR L7, respectively. The intensity of presynaptic inhibition (reduction of the normalized value of MSR amplitude during conditioning) increased from 0.19 ± 0.02 in prefatigue to 0.44 ± 0.04 within a 5.3–18.2 min interval after FST, followed by a recovery. In contrast, the intensity of recurrent inhibition first diminished from 0.23 ± 0.02 in prefatigue to 0.15 ± 0.01 within 15.6–30.1 min after FST and then gradually recovered. Both primary afferent depolarization and the intensity of antidromic discharges in primary afferents increased with the presynaptic inhibition intensity. These results demonstrate a fatigue-related suppression of Ia excitation of synergistic motoneurones, probably arising from the activation of group III and IV afferents. The effects could in part be due to increased presynaptic inhibition, while recurrent inhibition plays a minor role.
Muscle fatigue results from a number of cellular processes in fatiguing muscle fibres and at the neuromuscular junction (Fitts, 1994; Gandevia et al. 1995). It is also associated with widespread changes in the central nervous system at the spinal and supraspinal levels, the former level having been investigated more extensively (Gandevia et al. 1995; Gandevia, 2001). Changes in spinal cord circuits during muscle fatigue are complex for a number of reasons. The problems become more apparent when considering the following experimental protocol that has instigated a number of subsequent studies in humans and animals. During sustained maximal voluntary isometric muscle contraction, motor unit discharge rates decline over several tens of seconds from an initially high value (reviewed in Gandevia, 2001). Several underlying mechanisms have been proposed.
Firstly, the firing-rate drop may, in part, result from intrinsic properties of spinal α-motoneurones, such as adaptation during long-lasting maintained activation (Kernell & Monster, 1982; Spielmann et al. 1993; Binder et al. 1996).
Secondly, it has been shown in human experiments, albeit for submaximal contractions, that the mean discharge rates of muscle spindle afferents from the fatiguing muscles decline over time, thus disfacilitating synergistic α-motoneurones (Macefield et al. 1991). However, this finding is at variance with data obtained in cats, which demonstrate a fatigue-dependent increase in muscle spindle afferent activity (Ljubisavljevic & Anastasijevic, 1994) that is assumed to result from a fatigue-evoked reflex activation of fusimotor motoneurones (Ljubisavljevic et al. 1992). This explanation already suggests a third mechanism, namely reflex actions of sensory afferents that are activated during muscle fatigue.
Thirdly, it has been proposed, and substantiated by indirect evidence, that the decline of α-motoneurone firing rate in humans results from an inhibitory spinal reflex arising in group III and IV muscle afferents that are activated during muscle fatigue (Bigland-Ritchie et al. 1986; Woods et al. 1987; Garland et al. 1988). While group III and IV muscle afferents activated by muscle fatigue may thus inhibit at least extensor α-motoneurones, the inhibitory interneurones involved are potentially manifold because many spinal interneurones receive inputs from group III and IV muscle afferents (Schomburg, 1990; Jankowska, 1992). Consequently, the inhibitory reflex effects might be mediated, at least partially, by known inhibitory interneurones intercalated in other spinal pathways, such as neurones mediating presynaptic inhibition, non-reciprocal group I inhibition or recurrent inhibition (Windhorst & Boorman, 1995).
Indeed, experimental data from humans indicate a strong fatigue-related suppression of monosynaptic excitation exerted by group Ia muscle spindle afferents on motoneurones supplying the exercising muscles. H-reflex amplitudes and short-latency responses to muscle stretch were decreased after fatiguing contractions irrespective of whether fatigue was evoked by direct muscle or muscle nerve stimulation (Garland & McComas, 1990; Butler & Thomas, 2003), or by voluntary contractions (Enoka et al. 1980; Balestra et al. 1992; Duchateau et al. 2002). Although some of these changes suggest a decrease in muscle spindle sensitivity, they could also be due to presynaptic modulation of the afferent input from spindles. Segmental presynaptic inhibition of Ia terminals, that is known to be an important gain regulator at premotoneuronal level, might be one of the mechanisms that would decrease both H-reflex and the short-latency stretch responses. Thus, an enhancement of presynaptic inhibition was suggested to occur in humans during muscle fatigue (Avela et al. 2001) and during chemical activation of group III and IV muscle afferents (Rossi et al. 1999b). In rats, presynaptic inhibition was also assumed to take place during muscle fatigue (Pettorossi et al. 1999), where the observed effects were ascribed to the activation of capsaicin-sensitive group III and IV afferents.
As to recurrent inhibition, the reported results are more diverse. In humans, recurrent inhibition was suggested to be enhanced during sustained maximal voluntary contraction (Kukulka et al. 1986) and upon chemical activation of group III and IV muscle afferents (Rossi et al. 2003). However, it is decreased during sustained submaximal muscle contraction (Löscher et al. 1996). A mixed excitation/depression of Renshaw cells was found upon chemical activation of muscle group III and IV afferents in decerebrate, spinalized cats (Windhorst et al. 1997). These differences may result from differences in the preparations used (see Discussion).
As emphasized by Gandevia (2001), it is notoriously difficult in human experiments to unequivocally dissect the multiple causes of the decrease in firing rate and drop in excitability of motoneurones. It is thus reasonable to try to reduce the number of potentially contributing mechanisms. One approach is to record monosynaptic reflexes (MSRs) in animal experiments, in which it is possible to separate the fatiguing stimulation of the efferents supplying the muscle under study from the undesirable stimulation of low-threshold afferents and antidromic activation of motoneurones, and to concomitantly probe into some potential mechanisms. This approach was adopted in the present study in order to achieve the following goals. The first was to determine the polarity, degree and time course of changes in the gastrocnemius–soleus (G–S) MSRs after the development of strong fatigue in the G–S muscles. The second was to investigate to what extent interneuronal networks at the spinal input stage of the MSR could contribute to its changes. Presynaptic inhibition controls the synaptic transmission from, among others, Ia afferents to motoneurones, and its modulation by activation of group III and IV muscle afferents would thus influence MSR size. The third goal was to examine to what extent the interneuronal networks at the motor output stage (the recurrent inhibition system) could contribute to this process.
Methods
Preparation
The experiments were carried out on 17 adult cats of both sexes, weighing between 3.4 and 4.2 kg. The animals were purchased from state-controlled animal farms via the common animal facility of the University of Umeå. The experiments were performed according to the NIH guidelines for the use of experimental animals and with the approval of the local Animal Ethics Committee (Umeå Djurförsöksetiska Nämnd, proj. 1997/0337). Animals were initially anaesthetized by inhalation of a mixture of oxygen and nitrous oxide (1 : 2) with halothane (2.5%). After insertion of a catheter into an external jugular vein, the inhalation anaesthesia was replaced with intravenous injections of pentobarbital sodium (Sigma) – 1 ml of a 10 mg ml−1 solution was administered intravenously every 15 min during the subsequent surgical procedures. For blood pressure monitoring, a catheter was inserted into the common carotid artery. A laminectomy was performed in the region of the lumbar enlargement of the spinal cord. The animal was suspended in a firm frame and additional pins were inserted into the femur and tibia to rigidly fix the knee joint of the right leg or both legs (in 5 experiments). Then an intercollicular decerebration was performed and the anaesthesia was discontinued. The G–S muscles of the right (12 experiments) or both (5 experiments) hindlimbs were separated from the surrounding tissues. The tendons were detached at the distal insertions, leaving a small bone chip on the heel, which was used to attach the tendon via a steel cable to a servo-controlled muscle stretcher. The muscles were held isometric near the resting length. All nerves except those innervating the G–S were cut. The hindlimb muscles were placed in pools formed by surrounding skin flaps. A similar pool was formed around the exposed spinal cord. Both pools were filled with mineral oil whose temperature was kept close to 37–37.5°C by means of radiant heat. The rectal temperature was kept at a constant physiological level by heating the body using a controlled heating pad. If necessary, the animals were artificially ventilated, keeping the end-tidal CO2 concentration at 3.8–4.5%. At the end of all experiments, death was ensured by the administration of a large overdose of pentobarbital sodium (5 ml of 60 mg ml−1).
Stimulation and recording
An IBM personal computer was used to create test and conditioning stimulation patterns. The DAC channels in the input–output interface card had 12-bit resolution and 1 kHz sampling rate. Standard isolation units (DS2A, Digitimer Ltd, UK) were used and stimulus pulses were of 0.2 ms duration. In order to record monosynaptic reflexes and elicit recurrent inhibition, the ventral root (VR) L7 was cut at the maximal possible distance from the spinal cord and divided into 5–7 filaments. From one of these filaments the MSRs were recorded, while the others were used for stimulation. MSRs were evoked by two stimuli at 2 ms intervals, applied to the G–S nerve. The duration of the stimuli was 0.2 ms; the current intensity was chosen to evoke MSRs in the range of 60–70% of their maximal amplitude and usually did not exceed 1.3–1.4 times the threshold current for appearance of the cord dorsum potential after single pulse stimulation. To record dorsal root potentials (DRPs), a small filament was carefully dissected from the most caudal rootlet of L7 dorsal root up to its entry into the cord and transected 20 mm from the entry zone. The central end of the filament was placed on a pair of platinum hook electrodes placed 10 mm apart, one on the cut end of the root and the other at 1 mm distance from the cord. The signals of the MSRs, the dorsal surface potential and DRPs were amplified by Brownlee model 440 amplifiers (Brownlee Precision Co, USA). The frequency bandwidth was set to a range of 10 Hz to 5 kHz for the recording of MSRs and dorsal surface potentials and 0.1 Hz to 10 kHz for the recording of DRPs. To study the effects of presynaptic and recurrent inhibition on the MSRs, in some experiments, G–S MSRs were conditioned by stimulating (1) the nerve to posterior biceps and semitendinosus muscles (PBSt) and (2) a VR L7 filament adjacent to the one from which the MSR was recorded. In both cases, pairs of stimuli at 2 ms intervals were used, and the interval between the conditioning and the test pairs of stimuli was set at 20 ms. Conditioning PBSt stimulation evokes no postsynaptic effects in G–S motoneurones and has therefore been used previously in studies of presynaptic inhibition (Frank & Fuortes, 1957; Eccles et al. 1961). The timing of both conditioning stimuli closely corresponds to the maximum intensities of presynaptic and recurrent inhibition (Brooks & Wilson, 1959; Manjarrez et al. 2000). The intensity of PBSt stimulation was set so as to evoke about 20% inhibition of the test MSR under control conditions. In this series of experiments, the current intensities were in the range of 1.3–1.5 times the threshold value for the afferent wave in the cord dorsum potential.
Fatiguing stimulation
Fatiguing contractions were evoked by long-lasting intermittent stimulation of the whole ipsilateral VR S1 that was cut proximally; the current strength was set to 1.3 times twitch threshold. Stimulation periods of 20 s, or in several experiments 10 s duration, with repetitive regular stimuli occurring at a rate of 50 s−1, were separated by rest intervals of 10 s duration. The entire duration of the fatiguing stimulation (FST) was 10 or 12 min. At the very beginning of the contractions, the peak tension ranged between 15 and 25 N. During the entire time of fatiguing stimulation, the peak values of tension within the individual stimulation periods fell to 25–30% of the initial value (Fig. 1D) and the amplitude of the twitch contractions evoked by the paired stimuli applied to the G–S nerve to evoke MSR decreased by more than 50%. The relative drop in tension during the individual stimulation periods increased noticeably with time (see Fig. 1D).
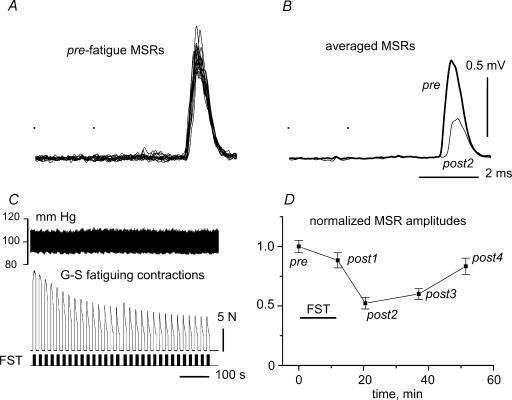
Figure 1. Changes in monosynaptic reflexes of gastrocnemius–soleus motoneurones (G–S MSRs)after fatiguing G–S stimulation.
A, superposition of 12 individual MSRs recorded in prefatigue (pre) test. MSRs were evoked by two electrical stimuli to the G–S nerve (dots) with current strength 1.4 times the threshold value. B, averages of the pre (thick) and post2 (thin line) MSRs. C, fatiguing stimulation (FST), with stimulus marks sketched in the bottom trace, isometric muscle force in the middle trace and blood pressure at the top. D, change of MSR amplitude over time. The means ± s.e.m. of 12 MSRs within each test period were determined, normalized to the mean prefatigue amplitude and plotted as a function of time. The period of FST is indicated by a horizontal bar.
Data acquisition and analysis
The signals recorded from filaments of the ventral and dorsal roots (DR), muscle tension, blood pressure, and stimulation markers were sampled by CED Power 1401 (Cambridge Electronic Design (CED), UK) while the Spike 2 program (CED) was used for data acquisition and further processing. The input signals were digitized with 12-bit resolution at rates of 1 kHz (blood pressure, muscle length and tension), 10 kHz (MSR, dorsal surface potential), and 15 kHz (DRPs). The data analysis was performed using the Origin 7.0 program (OriginLab Corporation, USA).
Statistical analysis
The statistical significance of postfatigue changes in MSR amplitude and in the intensity of presynaptic and recurrent inhibitions was determined by repeated-measures analysis of variance (ANOVA). The time interval of measurement (prefatigue (pre) test versuspost1 test versuspost2, etc.) was used as a within-subject factor. Whenever the sphericity assumption was not met, the Huynh-Feldt correction was applied. Differences were considered significant at P < 0.05. Provided that changes of the parameter under study were significant in successive time intervals, post hoc comparisons were performed using the Bonferroni adjustment for multiple comparisons. Statistical analyses were performed using SPSS 10.0 for Windows (SPSS Inc., USA).
Results
Monosynaptic reflex changes after fatiguing stimulation
Although it was possible to evoke clear MSRs with only one stimulus to the intact G–S nerve, they were often unstable during the long-lasting test procedures lasting up to 70 min. More reliable and stable MSRs could be evoked by pairs of stimuli at an interval of 2 ms. This pattern was therefore used throughout. The general procedure is described in Fig. 1. Individual test periods consisted of 12 MSRs elicited at intervals of 7 s. A typical experiment is displayed in Fig. 1A which shows the superposition of all 12 MSRs recorded before fatiguing stimulation (FST). Figure 1B shows the average of the MSRs before FST (thick line) together with the corresponding average MSR obtained in the post2 test interval (thin line). FST was applied just after the prefatigue (control) MSRs as illustrated in Fig. 1C. Arterial blood pressure (upper trace) changed only slightly (see also below), and the muscle tension (middle trace) in response to the bouts of stimuli (lower trace) declined substantially. Both of these occurred within each stimulation period and throughout the entire FST epoch. Series of 12 MSRs were elicited before FST (pre) and at different intervals after its cessation: post1–post4. The post1 series was evoked just after the end of FST, with a delay of about 30 s. The following tests were usually applied consecutively at 10–15 min intervals. The thin line labelled post2 in Fig. 1B shows the averaged MSR of the second test series (post2) occurring approximately 10 min after FST. Both averaged reflexes (Fig. 1B) and statistical parameters of the amplitudes of individual MSRs were further analysed. The mean ±s.e.m. values of individual MSR amplitudes were determined for all test periods. These values were normalized to the mean amplitude of the MSRs in the prefatigue period and plotted as a function of time, as illustrated in Fig. D. This typical plot shows a substantial and long-lasting reduction of MSR amplitude after FST. The initial drop in the amplitude just after FST (post1) was small with the mean value reduced by about 0.12. The main reduction in the MSR amplitude occurred later, reaching a maximal value of nearly 0.48 (post2) approximately 10 min after FST. Thereafter, the reflex amplitude recovered. In some experimental trials blood pressure changed markedly during FST due to activation of the exercise pressor reflex (compare Figs 1C and 6A). However, the intensity of the pressor reflex was apparently not correlated with the action of FST on the MSRs.
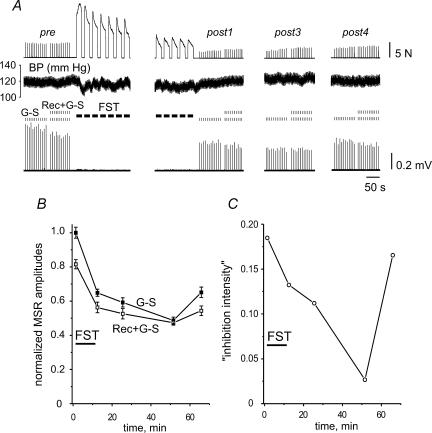
Figure 6. Fatigue-dependent changes in the intensity of recurrent inhibition.
A, muscle force (upper trace), blood pressure (BP; second trace), stimulus marks and evoked MSRs (lower two traces; G–S, unconditioned MSRs; Rec + G–S, recurrently conditioned MSRs). The precise timing of the test periods can be derived from the graph in B. Duration of FST was 10 min. B, amplitudes of the unconditioned (G–S) and conditioned (Rec + G–S) MSRs in different test periods, normalized to the mean unconditioned prefatigue MSR amplitude. C, ‘Inhibition intensity index’, as defined for presynaptic inhibition (see text), plotted versus time.
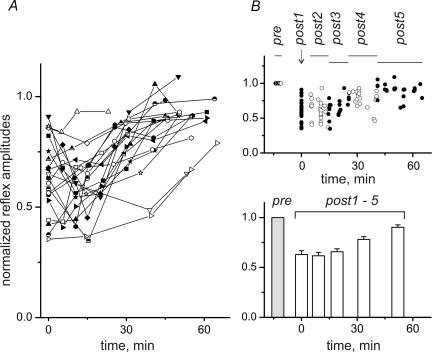
For the comparison of data from different experiments, all curves such as the one in Fig. 1D were plotted on a time axis whose zero point (t = 0) was set at the midpoint of the post1 period as shown in Fig. 2A. As in the example presented in Fig. 1, MSRs dropped maximally just after FST or somewhat later, and then slowly recovered in the following 10–15 min (Fig. 2A). There was no steady increase in MSRs after FST in any experiment. For statistical analysis, all data points from different trials across different experiments were sorted into six contiguous time slots so that each contained the same number of data points. As illustrated in the upper plot of Fig. 2B, this process yielded time slots of different duration as indicated by the horizontal lines labelled pre and post1–post5. For easier distinction, the data points falling into adjacent slots are shown as open and filled circles. The consecutive slots covered the following time intervals: post2, 5.0–14.4 min; post3, 14.5–25.6 min; post4, 25.7–40.6 min; post5, 41.1–65.2 min. The means ±s.e.m. determined for the data in each slot are shown in the bottom graph of Fig. 2B, with the prefatigue control shown as a shaded column.
Figure 2. Postfatigue changes of MSRs.
A, 21 fatigue tests were run in 16 different experiments; in 5 experiments the test procedure was repeated in the opposite hindlimb. For comparison of data from different experiments, all curves, such as the one in Fig. 1D, were plotted on a time axis whose zero point (t = 0) was set at the midpoint of the post1 period. B, for statistical analysis, all data points from different trials across different experiments were sorted into six contiguous time slots, so that each contained approximately the same number of data points. As illustrated in the upper plot, this process yielded time slots of different durations, as indicated by the horizontal lines labelled pre and post1–post5. For easier distinction, the data points falling into adjacent slots are shown as open and filled circles. The consecutive slots covered the following time intervals: post2, 5.0–14.4 min; post3, 14.5–25.6 min; post4, 25.7–40.6 min; post5, 41.1–65.2 min. The means ±s.e.m. determined for the data in every slot are shown in the bottom graph, with the prefatigue control shown as a grey column. Statistical significance of the differences of MSRs belonging to different time intervals (repeated-measures ANOVA with post hoc Bonferroni adjustment for multiple comparisons) is shown in Table 1.
Immediately after FST (post1), the MSR amplitudes were strongly reduced, with an average of 0.64 ± 0.04 of the pre values. The changes in MSR amplitude were analysed using repeated-measures ANOVA. Since the sphericity assumption was not met, the Huynh-Feldt correction was applied (see Methods). The changes in MSR amplitude in successive time intervals were significant (F = 32.594; P < 0.001). Post hoc comparisons were performed using the Bonferroni adjustment for multiple comparisons. Differences were considered significant at P < 0.05. The results of the statistical analysis are presented in Table 1. Significant differences were observed between the pre and each of the post groups. No differences were found for pairs within the first three postfatigue groups and for the post1–post4 and post3–post4 pairs. At the same time, there was a distinctive tendency toward restoration of the MSR amplitude in time. A statistically significant difference occurred for the pairs post2–post4, post1–post5, post2–post5, post3–post5 and post4–post5. It should be pointed out that only long-lasting and rather strong fatiguing contractions were effective in evoking the pronounced and steady depression of the G–S MSRs following FST. Durations of FST shorter than 5 min did not usually evoke discernible and stable reflex depression.
Table 1.
Statistical significance of the differences between monosynaptic reflexes (MSRs) belonging to different time intervals
| Compared time intervals | Significance level | Direction of change |
|---|---|---|
| pre–post1 | P < 0.001 | ↓ |
| pre–post2 | P < 0.001 | ↓ |
| pre–post3 | P < 0.001 | ↓ |
| pre–post4 | P < 0.001 | ↓ |
| pre–post5 | P < 0.005 | ↓ |
| post1–post2 | — | |
| post1–post3 | — | |
| post1–post4 | — | |
| post1–post5 | P < 0.001 | ↑ |
| post2–post3 | — | |
| post2–post4 | P < 0.01 | ↑ |
| post2–post5 | P < 0.001 | ↑ |
| post3–post4 | — | |
| post3–post5 | P < 0.001 | ↑ |
| post4–post5 | P < 0.001 | ↑ |
Methods for the MSR grouping and statistical characteristics of the means ±s.e.m. in each group are presented in Fig. 2.
Fatigue-dependent changes in the strength of presynaptic inhibition
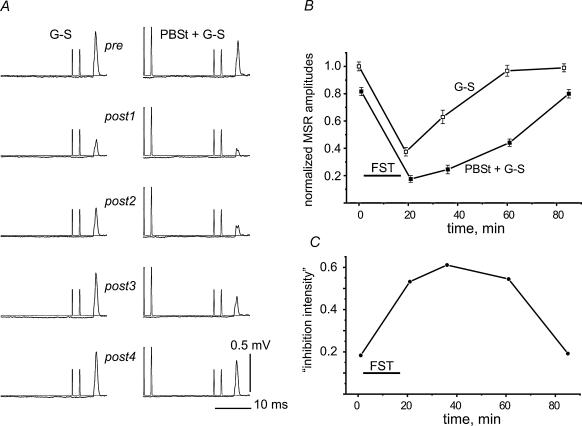
In order to test whether changes in presynaptic inhibition could contribute to the fatigue-related depression of the G–S MSRs, two approaches were used. In the first, the G–S MSRs were conditioned by stimulating the PBSt nerve (for justification see Methods). In the experiments chosen for analysis the PBSt stimulation did not evoke MSRs in the VR L7 filament from which the G–S MSRs were recorded. Both without (Fig. 3A, left column) and with (Fig. 3A, right column) conditioning PBSt stimulation, the G–S MSRs were strongly suppressed after FST of the G–S muscle. Figure 3B shows the amplitudes of the unconditioned (G–S) and conditioned (PBSt + G–S) reflexes in different pre- and postfatigue test periods; the data are normalized to the mean values of the prefatigue G–S MSR. In this experiment both curves showed a maximal drop just after FST, followed by a slow recovery in the subsequent 40–60 min. The inhibition of the conditioned MSRs was more pronounced and its recovery was slower (Fig. 3B). In order to quantify this difference, an ‘inhibition intensity index’ was defined as I = 1 – Mc/Mu, where Mu and Mc were the mean amplitudes of the unconditioned (G–S) and conditioned (PBSt + G–S) MSRs, respectively. This index is equal to 1 upon full suppression of the reflex (Mc = 0), and 0 upon lack of inhibition (Mc = Mu). When plotted over time as in Fig. 3C, the ‘inhibition intensity’ first increased to a maximum at around 40 min after FST and then decreased back to the prefatigue value.
Figure 3. Postfatigue changes in the presynaptic inhibition of G–S MSRs induced by conditioning stimulation of the nerve to the posterior biceps and semitendinosus muscle (PBSt).
A, fatigue-evoked changes in G–S MSRs (averages of 12 records in each test interval) without (left column) and with (right column) preceding stimulation of the PBSt nerve. The conditioned reflexes were obtained around 30 s after the control reflexes. The intensity of the PBSt stimulation was set to evoke approximately 20% inhibition of the G–S MSR in the prefatigue tests. B, amplitudes of the unconditioned (G–S) and conditioned (PBSt + G–S) reflexes in different pre- and postfatigue test periods, normalized to the mean value of the prefatigue G–S MSR. C, ‘Inhibition intensity’, as defined in the text, plotted against time.
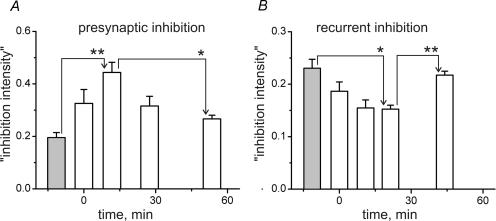
The precise shape of the fatigue-dependent changes in ‘inhibition intensity’ varied in different experiments. However, statistical analysis of the data from nine experiments demonstrated that the ‘inhibition intensity’ tended first to increase after FST and then to decline again (see Fig. 7A). The methods of grouping the data into different postfatigue time slots and the statistical procedures (repeated-measures ANOVA) were the same as described in the previous section. Statistically significant differences in ‘inhibition intensity’ occurred for two pairs of test groups i.e. pre–post2 (increase of ‘inhibition intensity’, P < 0.01), and post2–post4 (decrease of ‘inhibition intensity’, P < 0.05) (Fig. 7A).
Figure 7. Summary of the postfatigue changes in presynaptic and recurrent inhibition.
Statistical characteristics of the ‘inhibition intensity indexes’ for presynaptic inhibition (A) and recurrent inhibition (B). The left-hand grey columns in A and B represent the prefatigue tests and the following open columns represent the post1–post4 groups sequentially. The time intervals for the different data groups in A(n = 9) and B(n = 8) were, respectively: 5.3–18.2 and 8.7–13.9 min (post2); 21.2–35.0 and 15.6–30.1 min (post3); 36.5–59.8 and 30.5–56.7 min (post4). The bar graphs present means ± s.e.m. defined within each group. Repeated-measures ANOVA with post hoc Bonferroni adjustment for multiple comparisons revealed significant differences as indicated (*P < 0.05, **P < 0.01). Refer to text for a more detailed description.
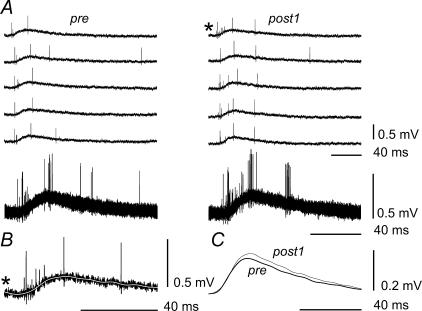
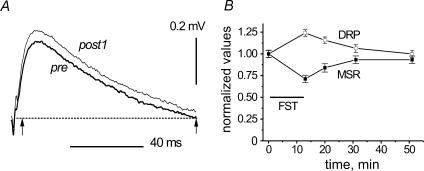
As independent additional evidence for changes in presynaptic inhibition, primary afferent depolarization (PAD) was assessed in four experiments during G–S MSR test periods by recording DRPs in filaments of dorsal root (DR) L7. Figures 4 and 5 show results from two of these experiments. Figure 4A demonstrates five examples of 12 single DRPs (five upper traces), which were recorded in parallel with MSRs in pre and post1 tests (left and right columns, respectively). The superposition of all the traces is shown in the bottom row. It can be seen that the DRPs included well-discernible antidromic spikes. Comparison of the pre- and postfatigue DRPs suggests that the frequency of these spikes increased after FST. The slow components of the DRPs changed as well. To demonstrate this, individual DRP records were subjected to off-line digital filtering using a fast Fourier transformation (FFT) procedure in order to eliminate the spikes. The efficiency of this procedure is demonstrated in Fig. 4B. The dark line marked by an asterisk reproduces the individual record marked similarly in the first row of the right column in Fig. 4A. The superimposed white line in Fig. 4B is the same record but low-pass-filtered with a 0.01 Hz cut-off frequency. As seen, the filter extracted the slow component and removed the spikes. The 12 individual filtered DRPs from each period (pre and post1) were then averaged and these averages were superimposed in Fig. 4C. The post1 average was clearly larger than the pre average. A quantitative analysis of postfatigue DRP changes is shown for another experiment in Fig. 5. The same procedures of smoothing and averaging were applied to DRPs recorded in the pre and post1–post4 periods, and the pre and post1 averages were superimposed in Fig. 5A. Again, the post1 DRP was larger than the pre DRP. The individual filtered DRP records in all five test periods were integrated over the time interval indicated by the arrows in Fig. 5A and the means ±s.e.m. (n = 12) of the integrals were determined for each test period. These parameters were normalized to the mean value of the pre test and plotted as a function of time (□) in Fig. 5B together with the normalized MSR amplitudes (▪). MSRs and slow components of DRPs changed in opposite directions. The maximal increase of the DRP area occurred just after FST, reaching nearly 1.24 of the prefatigue value, while the MSR amplitude dropped to 0.71 of its prefatigue value. Thus, both the PAD and the intensity of antidromic discharges increased after the development of fatigue, which is consistent with an assumption that presynaptic inhibition could participate in the postfatigue depression of G–S MSRs.
Figure 4. Analysis of the dorsal root potential (DRP) changes during fatigue.
A, five examples of 12 single DRPs (five upper traces), recorded in pre and post1 tests (left and right columns, respectively), and superposition of upper traces in the bottom row. B, comparison of individual original (black trace) and off-line-filtered (white trace) records of a DRP. The individual DRP record marked by an asterisk in the first row of the right column in A is reproduced by the black line with the asterisk in B. This record was then subjected to off-line digital filtering using a fast Fourier transformation (FFT) procedure with a 0.01 Hz cut-off frequency in order to eliminate the spikes. This procedure yielded the superimposed white line, with the spikes extinguished. C, superimposed averages of the 12 individual filtered DRPs from each period (pre and post1).
Figure 5. Fatigue-dependent changes of slow components in DRPs.
Quantitative analysis of postfatigue DRP changes. The same procedures of smoothing and averaging as in Fig. 4 were applied to DRPs recorded in another experiment. A, superimposed examples of the averaged records (pre and post1 tests), the dotted line indicating zero potential. B, for further quantitative analysis, the individual filtered DRP records in all five test periods were integrated over the time interval indicated by the arrows in Fig. 5A. The time interval chosen for integration approximately coincided with the duration of the averaged prefatigue wave; initial fast transients that could fluctuate more intensely were excluded from analysis. The means ±s.e.m.(n = 12) of the integrals were determined for each test period, normalized to the mean value of the pre test and plotted as a function of time (□), together with the normalized MSR amplitudes (▪).
Fatigue-dependent changes in the strength of recurrent inhibition
Recurrent inhibition was elicited by conditioning stimulation of a VR L7 filament lying close to the filament used for the recording the G–S MSR. Figure 6 shows representative examples of the changes occurring after FST. Figure 6A displays raw data, with muscle force on the top, blood pressure in the middle, and MSRs at the bottom. The amplitudes of unconditioned and conditioned MSRs, labelled G–S and Rec + G–S, respectively, decreased after FST and then recovered slowly. Plots of means ±s.e.m. of the MSRs over time are shown in Fig. 6B. Again, as in the previous section, an ‘inhibition intensity index’ was computed and plotted in Fig. 6C. The recurrent ‘inhibition intensity’ had a temporal profile which was the opposite of that of the presynaptic inhibition shown in Fig. 3C. It initially decreased, and then, after reaching the minimum at around 50 min, increased rather quickly towards the prefatigue level (Fig. 6C).
These general features were seen in eight different experiments. Figure 7B shows the mean results. The methods of grouping the data into different postfatigue time slots and the statistical procedures (repeated-measures ANOVA) were the same as described in the previous section. Statistically significant differences emerged for two pairs of test groups i.e. pre–post3 (decrease of ‘inhibition intensity’, P < 0.05), and post3–post4 (increase of ‘inhibition intensity’, P < 0.01) (Fig. 7B).
Discussion
This study was designed to quantify the time course of changes in monosynaptic extensor reflexes after fatiguing the homonymous and synergistic muscles and to check for the possible involvement of presynaptic and recurrent inhibition. Long-lasting FST of the G–S muscles reduced the amplitudes of MSRs evoked by G–S group Ia afferents. The intensity and duration of suppression of the MSR amplitudes varied in different experiments; in many cases it could be observed nearly 1 h after FST. The strength of presynaptic inhibition, as assessed by the ‘inhibition intensity index’, increased with a time course that approximately mirrored the decrease in MSR amplitudes. This increase in presynaptic inhibition could thus potentially contribute to the MSR depression. By contrast, the strength of recurrent inhibition diminished with approximately the same time course as the depression of MSR amplitudes. This decrease in recurrent inhibition intensity could disinhibit motoneurones. On the other hand, this effect seemed to be too weak to counteract the opposing increase in presynaptic inhibition. A major goal of the present study was to trace the complete time course of G–S MSR changes after fatiguing contractions of the homonymous and synergistic muscles. This required stable recordings of the MSRs over long time spans. The present preparation provided these conditions, enabling not only a quantitative analysis of motoneuronal excitability, but also an evaluation of possible contributions of presynaptic and recurrent inhibitions. Another requirement was to reduce, as far as possible, potential complicating factors. One such factor would have been the activation of motoneurones and large-diameter muscle afferents during fatiguing contractions (see below). This activation was minimized by cutting the VRs supplying the contracting muscles and stimulating their distal ends, which prevented antidromic stimulation of motoneurones and direct activation of the fast conducting afferent fibres.
An important question as to the effects of long-lasting muscle activation is whether the observed fatigue is attributable entirely to factors within muscle fibres or whether it includes a component of neuromuscular transmission failure. In humans, the role of neuromuscular block seems to be controversial. Some authors deny a substantial contribution of neuromuscular block to fatigue even during maximal voluntary contractions (Bigland-Ritchie et al. 1982; Bellemare & Bigland-Ritchie, 1987; McKenzie et al. 1992), while others attest to the contrary (Stephens & Taylor, 1972; Sieck & Prakash, 1994). In cat experiments, it was shown that fatigue effects evoked by long-lasting distributed stimulation of the muscle nerve included neuromuscular components (Kostyukov et al. 2000; Wise et al. 2001). However, while possible fatigue-dependent impairments in neuromuscular transmission are relevant for H-reflex studies in humans, they are not for the study of MRSs in animal experiments.
It should be pointed out that long-lasting fatiguing stimulation was intense enough to potentially damage the muscle tissue. Thus, muscle damage or soreness (for review, see Miles & Clarkson, 1994) could play a role in the entire fatigue process and might be one of the possible reasons for the spreading of fatigue-related effects from active to inactive parts within muscle tissue (Kostyukov et al. 2002).
Long-lasting changes in monosynaptic reflexes
As mentioned above, only long-lasting and strong fatiguing contractions effectively depressed the G–S MSR. This is in line with our recent data (Pilyavskii et al. 2001) showing that long-lasting fatiguing stimulation (exceeding 10 min) was necessary to induce c-fos expression in rat spinal interneurones. These results are at variance with those of Pettorossi et al. (1999), who observed a noticeable depression of the rat lateral gastrocnemius MSR even after 30–60 s of intermittent 85 Hz FST. This difference could be due to differences in preparations. Pettorossi et al. (1999) electrically stimulated the isolated nerve supplying the muscle under study. Since both dorsal and ventral roots were intact in this case, (i) motoneurones were activated antidromically, and (ii) low-threshold afferents were stimulated. So these effects could have influenced the MSR amplitudes. By contrast, in the present study, in order to minimize these side-effects, FST was applied to the cut VR S1. This procedure still left the possibility of some fusimotor activation through stimulation of β-axons (Emonet-Denand et al. 1975; Scott et al. 1995). However, it seems unlikely that this could have evoked changes in muscle spindle discharge that were potent and long-lasting enough to induce the powerful and durable changes in MSRs described here.
The MSR depression lasted up to an hour after the cessation of FST. This delayed and long-lasting effect would be expected assuming that it resulted from reflex effects of small-diameter (group III and IV) muscle afferents activated by the accumulation of metabolites and/or inflammatory substances in the intramuscular interstitial space. The idea of such an inhibitory reflex is supported by diverse data from animal studies. Fatiguing stimulation of hindlimb skeletal muscles enhances the Fos protein expression in spinal neurones in the cat (Williams et al. 2000) and the rat (Pilyavskii et al. 2001). The lamellar distributions of the Fos-labelled neurones overlap almost completely with the known termination patterns of high-threshold muscle afferents (Mense & Prabhakar, 1986). During persistent, fatiguing muscle contractions, afferents of groups III and IV are activated (Cleland et al. 1982; Kaufman et al. 1983; Mense, 1993; Darques & Jammes, 1997). Many group III and IV afferents are chemically activated and/or sensitized by muscle metabolites and/or inflammatory substances (Mense, 1993; Le Bars & Adam, 2002; Decherchi & Dousset, 2003). Importantly, the fatigue-induced activation of group IV muscle afferents appears to be mediated by interstitial release of lactic acid and inflammatory substances (Darques et al. 1998). Chemical activation of group III and IV muscle afferents via intra-arterial injection of metabolic/inflammatory substances into the circulation of calf muscles has inhibitory effects on extensor α-motoneurones, but usually facilitatory effects on flexor α-motoneurones (Kniffki et al. 1981), excitatory effects on extensor and flexor γ-motoneurones (Schmidt et al. 1981; Jovanovic et al. 1990), as well as various effects on spinal interneurones. However, whether the discharge of group III and IV muscle afferents is elevated over such long time spans and whether peripheral and/or central sensitization plays a role remains to be elucidated.
Modulation of presynaptic inhibition
In this study, postfatigue increments of presynaptic inhibition were assessed in two ways. Firstly, G–S MSRs were conditioned by stimuli to PBSt group Ia afferents preceding the stimuli to the G–S nerve. This protocol has been used in studies of spinal presynaptic inhibition because of the absence of postsynaptic effects in G–S motoneurones during PBSt group Ia afferent stimulation (Frank & Fuortes, 1957; Eccles et al. 1961). The time course of fatigue-related changes in the presynaptic ‘inhibition intensity’ (Figs 3C and 7A) corresponded well with the time course of postfatigue MSR changes, which suggested a causal relationship. The maximal drop of MSR amplitudes occurred within 25–30 min of FST (Fig. 2B), while the presynaptic ‘inhibition intensity’ attained its maximum within 10–15 min of FST, but was noticeably elevated over even longer time intervals (Fig. 7A). Secondly, to substantiate the postfatigue enhancement of presynaptic inhibition, DRPs were recorded alongside changes in G–S MSRs. In fact, DRPs increased and were often accompanied by higher frequencies of antidromic spikes in the cut DR filaments (Fig. 4A), known to be evoked in the depolarized afferent terminals (Eccles et al. 1961; Rudomin & Schmidt, 1999). Again, the time course of enhancement of the slow DRP components mirrored that of MSR depression (Fig. 5B), which is consistent with an assumption of their close functional interaction. The role of presynaptic inhibition in modulating the signal transfer in the group Ia–motoneurone pathway has been demonstrated during different kinds of voluntary contractions in humans (Hultborn et al. 1987; Avela et al. 2001; Aymard et al. 2001). It has also been shown in man that muscle nociceptive activity evoked by injection of levo-ascorbic acid into the foot extensor digitorum brevis muscle depresses, in soleus motoneurones, the excitation resulting from large-diameter group Ia fibres, the depression supposedly resulting from enhanced presynaptic inhibition (Rossi et al. 1999a). It is possible that the enhancement of presynaptic inhibition observed in our experiments could be even more pronounced in natural conditions because decerebration could have attenuated facilitatory descending signals. Another potential mechanism for long-lasting MSR depression was described by Hultborn et al. (1996) and Wood et al. (1996). Both groups of authors supposed that homosynaptic postactivation depression (a phenomenon caused by reduced transmitter release from previously activated nerve fibres) might contribute to the reduction in MSR amplitude during passive dorsiflexion of the ankle joint. In addition, in parallel experiments on decerebrate cats, Hultborn et al. (1996) showed that a similar long-lasting depression of triceps surae MSRs was evoked by a preceding conditioning stimulation of the triceps surae group Ia afferents. However, the postfatigue MSR depression described in the present study most probably results from different mechanisms. It appears predominantly associated with long-lasting activation of high-threshold muscle afferents of groups III and IV, because the activation of low-threshold muscle afferents during FST was minimized (see Methods).
Modulation of recurrent inhibition
As suggested by the diverse results reported in the literature, the modulation of recurrent inhibition by muscle fatigue and activation of group III and IV muscle afferents appears to be more variable than that of presynaptic inhibition. Two major reasons may be that Renshaw cells receive their main excitatory input from motor axon collaterals and that this coupling depends on descending motor commands (e.g. Hultborn & Pierrot-Deseilligny, 1979). This combination may make the operation of recurrent inhibition more highly dependent on the motor task. For example, in the human experiments of Rossi et al. (2003), the activation of group III and IV muscle afferents did not alter recurrent inhibition at rest (without muscle contraction), but did enhance recurrent inhibition with weak muscle contraction when motoneurones excited Renshaw cells and descending motor commands potentially facilitated them (Hultborn & Pierrot-Deseilligny, 1979). However, this interpretation is not compatible with the suggestion of Kukulka et al. (1986) that recurrent inhibition can be enhanced during sustained maximal voluntary contractions because, in this case, Renshaw cells should be suppressed by descending motor commands (Hultborn & Pierrot-Deseilligny, 1979). By contrast, in the case of sustained fatiguing submaximal contractions, which are associated with increasing muscle activation and descending commands, and potentially increasing inhibition of Renshaw cells, recurrent inhibition was depressed (Löscher et al. 1996). These examples show that the strength of recurrent inhibition, and its function in motor tasks, may crucially depend on the fine balance between excitation from motoneurones and modulatory signals from descending and segmental afferent sources. In the present experiments, the elimination of excitation from motoneurones and descending modulatory signals simplified the study of modulation of recurrent inhibition by muscle fatigue and activation of group III and IV muscle afferents. The time course of the postfatigue modification of the recurrent ‘inhibition intensity’ was almost opposite to that of presynaptic inhibition (Fig. 7).
Conclusions
The depression of G–S MSRs after FST most probably originates from neurochemical activation of group III and IV muscle afferents (Pettorossi et al. 1999), which would exert various effects at the spinal level. One effect could be the well-known polysynaptic inhibition of extensor motoneurones (e.g. Kniffki et al. 1981; Schmidt et al. 1981). Another could be an enhancement of presynaptic inhibition, which would depress the transmission of monosynaptic group Ia excitation to motoneurones. In contrast, the depression of recurrent inhibition described here would have an effect on MSR excitability only if the Renshaw cells had substantial spontaneous discharge, which would be depressed in parallel with their responses to synchronous motor-axon stimulation. While Renshaw cell background activity might be substantial in decerebrate preparations (Benecke et al. 1974), the depression of recurrent inhibition described here would result in motoneurone disinhibition rather than increased inhibition. Hence, in this case, changes in Renshaw cell discharge were not effective enough to overcome other effects leading to MSR depression. Thus, the reduction in the recurrent inhibition intensity lowers its contribution to the fatigue-related alteration of motor output and enhances a possible role of presynaptic inhibition in the central fatigue processing.
Acknowledgments
This work was supported by the Swedish Agency for Innovation Systems, the Swedish Council for Working Life and Social Research; by the INTAS grant 01-2130; and Dr Ljubisavljevic was partly supported by the Serbian Ministry for Science, Technology and Development Grant 1737.
References
- Avela J, Kyröläinen H, Komi PV. Neuromuscular changes after long-lasting mechanically and electrically elicited fatigue. Eur J Appl Physiol. 2001;85:317–325. doi: 10.1007/s004210100455. [DOI] [PubMed] [Google Scholar]
- Aymard C, Baret M, Katz R, Lafitte C, Penicaud A, Raoul S. Modulation of presynaptic inhibition of la afferents during voluntary wrist flexion and extension in man. Exp Brain Res. 2001;137:127–131. doi: 10.1007/s002210000662. [DOI] [PubMed] [Google Scholar]
- Balestra C, Duchateau J, Hainaut K. Effects of fatigue on the stretch reflex in a human muscle. Electroencephalogr Clin Neurophysiol. 1992;85:46–52. doi: 10.1016/0168-5597(92)90101-g. [DOI] [PubMed] [Google Scholar]
- Bellemare F, Bigland-Ritchie B. Central components of diaphragm fatigue assessed by phrenic nerve stimulation. J Appl Physiol. 1987;62:1307–1316. doi: 10.1152/jappl.1987.62.3.1307. [DOI] [PubMed] [Google Scholar]
- Benecke R, Hellweg C, Meyer-Lohmann J. Activity and excitability of Renshaw cells in non-decerebrate and decerebrate cats. Exp Brain Res. 1974;21:113–124. doi: 10.1007/BF00234383. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OC. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Kukulka CG, Lippold OCJ, Woods JJ. The absence of neuromuscular transmission failure in sustained maximal voluntary contractions. J Physiol. 1982;330:265–278. doi: 10.1113/jphysiol.1982.sp014340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD, USA: Am Physiol Soc; 1996. The physiological control of motoneuron activity; pp. 3–53. section 12, chap. 2. [Google Scholar]
- Brooks VB, Wilson VJ. Recurrent inhibition in the cat's spinal cord. J Physiol. 1959;146:380–391. doi: 10.1113/jphysiol.1959.sp006199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Thomas CK. Effects of sustained stimulation on the excitability of motoneurons innervating paralyzed and control muscles. J Appl Physiol. 2003;94:567–575. doi: 10.1152/japplphysiol.01176.2001. [DOI] [PubMed] [Google Scholar]
- Cleland CL, Rymer WZ, Edwards FR. Force-sensitive interneurons in the spinal cord of the cat. Science. 1982;217:652–655. doi: 10.1126/science.7089586. [DOI] [PubMed] [Google Scholar]
- Darques JL, Decherchi P, Jammes Y. Mechanisms of fatigue-induced activation of group IV muscle afferents: the roles played by lactic acid and inflammatory mediators. Neurosci Lett. 1998;257:109–112. doi: 10.1016/s0304-3940(98)00816-7. [DOI] [PubMed] [Google Scholar]
- Darques JL, Jammes Y. Fatigue-induced changes in group IV muscle afferent activity: differences between high- and low-frequency electrically induced fatigue. Brain Res. 1997;750:147–154. doi: 10.1016/s0006-8993(96)01341-8. [DOI] [PubMed] [Google Scholar]
- Decherchi P, Dousset E. Le rôle joué par les fibres afférentes métabosensibles dans les mécanismes adaptifs neuromusculaires. Can J Neurol Sci. 2003;30:91–97. doi: 10.1017/S0317167100053348. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Balestra C, Carpentier A, Hainaut K. Reflex regulation during sustained and intermittent submaximal contractions in humans. J Physiol. 2002;541:959–967. doi: 10.1113/jphysiol.2002.016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Magni F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol. 1961;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Denand F, Jami L, Laporte Y. Skeleto-fusimotor axons in the hind-limb muscles of the cat. J Physiol. 1975;249:153–166. doi: 10.1113/jphysiol.1975.sp011008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Hutton RS, Eldred E. Changes in excitability of tendon tap and Hoffmann reflexes following voluntary contractions. Electroencephalogr Clin Neurophysiol. 1980;48:664–672. doi: 10.1016/0013-4694(80)90423-x. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Frank K, Fuortes MGF. Presynaptic and postsynaptic inhibition of monosynaptic reflexes. Fed Proc. 1957;16:39–40. [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Enoka RM, McComas AJ, Stuart DG, Thomas CK. Neurobiology of muscle fatigue: advances and issues. In: Gandevia SC, Enoka RM, McComas AJ, Stuart DG, Thomas CK, editors. Fatigue. Neural and Muscular Mechanisms. New York, London: Plenum Press; 1995. pp. 515–527. [Google Scholar]
- Garland SJ, Garner SH, McComas AJ. Reduced voluntary electromyographic activity after fatiguing stimulation of human muscle. J Physiol. 1988;401:547–556. doi: 10.1113/jphysiol.1988.sp017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland SJ, McComas AJ. Reflex inhibition of human soleus muscle during fatigue. J Physiol. 1990;429:17–27. doi: 10.1113/jphysiol.1990.sp018241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol. 1987;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Pierrot-Deseilligny E. Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. J Physiol. 1979;297:229–251. doi: 10.1113/jphysiol.1979.sp013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jovanovic K, Anastasijevic R, Vuco J. Reflex effects on gamma fusimotor neurons of chemically induced discharges in small-diameter muscle afferents in decerebrate cats. Brain Res. 1990;521:89–94. doi: 10.1016/0006-8993(90)91528-o. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Motoneurone properties and motor fatigue. An intracellular study of gastrocnemius motoneurones of the cat. Exp Brain Res. 1982;46:197–204. doi: 10.1007/BF00237177. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Schomburg ED, Steffens H. Synaptic effects from chemically activated fine muscle afferents upon alpha-motoneurons in decerebrate and spinal cats. Brain Res. 1981;206:361–370. doi: 10.1016/0006-8993(81)90537-0. [DOI] [PubMed] [Google Scholar]
- Kostyukov AI, Day S, Hellstrom F, Radovanovic S, Ljubisavljevic M, Windhorst U, Johansson H. Fatigue-related changes in electromyogram activity of the cat gastrocnemius during frequency-modulated efferent stimulation. Neuroscience. 2000;97:801–809. doi: 10.1016/s0306-4522(00)00064-6. [DOI] [PubMed] [Google Scholar]
- Kostyukov AI, Kalezic I, Serenko SG, Ljubisavljevic M, Windhorst U, Johansson H. Spreading of fatigue-related effects from active to inactive parts in the medial gastrocnemius muscle of the cat. Eur J Appl Physiol. 2002;86:295–307. doi: 10.1007/s00421-001-0550-8. [DOI] [PubMed] [Google Scholar]
- Kukulka CG, Moore MA, Russell AG. Changes in human alpha-motoneuron excitability during sustained maximum isometric contractions. Neurosci Lett. 1986;68:327–333. doi: 10.1016/0304-3940(86)90511-2. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Adam F. Nocicepteurs et médiateurs dans la douleur aiguë inflammatoire. Ann Fr Anesth Réanim. 2002;21:315–335. doi: 10.1016/s0750-7658(02)00592-0. [DOI] [PubMed] [Google Scholar]
- Ljubisavljevic M, Anastasijevic R. Fusimotor-induced changes in muscle spindle outflow and responsiveness in muscle fatigue in decerebrate cats. Neuroscience. 1994;63:339–348. doi: 10.1016/0306-4522(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Ljubisavljevic M, Jovanovic K, Anastasijevic R. Changes in discharge rate of fusimotor neurones provoked by fatiguing contractions of cat triceps surae muscles. J Physiol. 1992;445:499–513. doi: 10.1113/jphysiol.1992.sp018936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher WN, Cresswell AG, Thorstensson A. Recurrent inhibition of soleus alpha-motoneurons during a sustained submaximal plantar flexion. Electroencephalogr Clin Neurophysiol. 1996;101:334–338. doi: 10.1016/0924-980x(96)95670-2. [DOI] [PubMed] [Google Scholar]
- Macefield G, Hagbarth KE, Gorman R, Gandevia SC, Burke D. Decline in spindle support to α-motoneurones during sustained voluntary contractions. J Physiol. 1991;440:497–512. doi: 10.1113/jphysiol.1991.sp018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie DK, Bigland-Ritchie B, Gorman RB, Gandevia SC. Central and peripheral fatigue of human diaphragm and limb muscles assessed by twitch interpolation. J Physiol. 1992;454:643–656. doi: 10.1113/jphysiol.1992.sp019284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjarrez E, Rojas-Piloni JG, Jimenez I, Rudomin P. Modulation of synaptic transmission from segmental afferents by spontaneous activity of dorsal horn spinal neurons in the cat. J Physiol. 2000;529:445–460. doi: 10.1111/j.1469-7793.2000.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54:241–289. doi: 10.1016/0304-3959(93)90027-M. [DOI] [PubMed] [Google Scholar]
- Mense S, Prabhakar NR. Spinal termination of nociceptive afferent fibers from deep tissues in the cat. Neurosci Lett. 1986;66:169–174. doi: 10.1016/0304-3940(86)90185-0. [DOI] [PubMed] [Google Scholar]
- Miles MP, Clarkson PM. Exercise-induced muscle pain, soreness, and cramps. J Sports Med Phys Fitness. 1994;34:203–216. [PubMed] [Google Scholar]
- Pettorossi VE, Della Torre G, Bortolami R, Brunetti O. The role of capsaicin-sensitive muscle afferents in fatigue-induced modulation of the monosynaptic reflex in the rat. J Physiol. 1999;515:599–607. doi: 10.1111/j.1469-7793.1999.599ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilyavskii AI, Maisky VA, Kalezic I, Ljubisavljevic M, Kostyukov AI, Windhorst U, Johansson H. c-fos expression and NADPH-d reactivity in spinal neurons after fatiguing stimulation of hindlimb muscles in the rat. Brain Res. 2001;923:91–102. doi: 10.1016/s0006-8993(01)03049-9. [DOI] [PubMed] [Google Scholar]
- Rossi A, Decchi B, Dami S, Della Volpe R, Groccia V. On the effect of chemically activated fine muscle afferents on interneurones mediating group I non-reciprocal inhibition of extensor ankle and knee muscles in humans. Brain Res. 1999b;815:106–110. doi: 10.1016/s0006-8993(98)01111-1. [DOI] [PubMed] [Google Scholar]
- Rossi A, Decchi B, Ginanneschi F. Presynaptic excitability changes of group Ia fibres to muscle nociceptive stimulation in human. Brain Res. 1999a;818:12–22. doi: 10.1016/s0006-8993(98)01253-0. [DOI] [PubMed] [Google Scholar]
- Rossi A, Mazzocchio R, Decchi B. Effect of chemically activated fine muscle afferents on spinal recurrent inhibition in humans. Clin Neurophysiol. 2003;114:279–287. doi: 10.1016/s1388-2457(02)00334-6. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Schmidt RF, Kniffki K-D, Schomburg ED. Der Einfluß kleinkalibriger Muskelafferenzen auf den Muskeltonus. In: Bauer HJ, Koella WP, Struppler A, editors. Therapie der Spastik. München: Verlag für angewandte Wissenschaften; 1981. pp. 71–84. [Google Scholar]
- Schomburg ED. Spinal sensorimotor systems and their supraspinal control. Neurosci Res. 1990;7:265–340. doi: 10.1016/0168-0102(90)90008-3. [DOI] [PubMed] [Google Scholar]
- Scott JJ, Kummel H, Illert M. Skeletofusimotor (beta) innervation of proximal and distal forelimb muscles of the cat. Neurosci Lett. 1995;190:1–4. doi: 10.1016/0304-3940(95)11485-f. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Prakash YS. Fatigue at the neuromuscular junction. In: Gandevia SC, Enoka RM, McComas AJ, Stuart DG, Thomas CK, editors. Fatigue. Neural and Muscular Mechanisms. New York, London: Plenum Press; 1994. pp. 83–100. [Google Scholar]
- Spielmann JM, Laouris Y, Nordstrom MA, Robinson GA, Reinking RM, Stuart DG. Adaptation of cat motoneurones to sustained and intermittent extracellular activation. J Physiol. 1993;464:75–120. doi: 10.1113/jphysiol.1993.sp019625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JA, Taylor A. Fatigue of maintained voluntary muscle contractions in man. J Physiol. 1972;220:1–18. doi: 10.1113/jphysiol.1972.sp009691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CA, Loyd SD, Hampton TA, Hoover DB. Expression of c-fos-like immunoreactivity in the feline brainstem in response to isometric muscle contraction and baroreceptor reflex changes in arterial pressure. Brain Res. 2000;852:424–435. doi: 10.1016/s0006-8993(99)02217-9. [DOI] [PubMed] [Google Scholar]
- Windhorst U, Boorman G. Overview: potential role of segmental motor circuitry in muscle fatigue. In: Gandevia SC, Enoka RM, McComas AJ, Stuart DG, Thomas CK, editors. Fatigue. Neural and Muscular Mechanisms. New York, London: Plenum Press; 1995. pp. 241–258. [DOI] [PubMed] [Google Scholar]
- Windhorst U, Meyer-Lohmann J, Kirmayer D, Zochodne D. Renshaw cell responses to intra-arterial injection of muscle metabolites into cat calf muscles. Neurosci Res. 1997;27:235–247. doi: 10.1016/s0168-0102(97)01157-7. [DOI] [PubMed] [Google Scholar]
- Wise AK, Morgan DL, Gregory JE, Proske U. Fatigue in mammalian skeletal muscle stimulated under computer control. J Appl Physiol. 2001;90:189–197. doi: 10.1152/jappl.2001.90.1.189. [DOI] [PubMed] [Google Scholar]
- Wood SA, Gregory JE, Proske U. The influence of muscle spindle discharge on the human H-reflex and the monosynaptic reflex in the cat. J Physiol. 1996;497:279–290. doi: 10.1113/jphysiol.1996.sp021767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J Neurophysiol. 1987;58:125–137. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]