Abstract
The thermogenic response of humans depends critically on the coordination of muscle fibre recruitment and oxidative fuel metabolism. The primary goal of this study was to determine whether the electromyographic (EMG) pattern of muscle recruitment could provide metabolic information on oxidative fuel selection during high-intensity shivering. EMG activity (of 8 large muscles) and fuel metabolism were monitored simultaneously in non-acclimatized adult men during high-intensity shivering. Even though acute cold exposure elicited similar changes in metabolic rate among subjects, lipid and carbohydrate use was very different. Depending on the subject, the cold-induced increase in carbohydrate (CHO) oxidation ranged between 2- and 8-fold, with CHO accounting for 33–78% of total heat production (Ḣprod), and lipids for 14–60% Ḣprod. This high variability in fuel selection was primarily explained by differences in ‘burst shivering’ rate, indicating that the recruitment of type II fibres plays a key role in orchestrating fuel selection. This study is the first to show that the pattern of muscle recruitment can provide quantitative information on energy metabolism. Future work should focus on the study of shivering bursts that may provide essential clues on what limits human survival in the cold.
Shivering is essential for human survival in the cold and this thermogenic response depends critically on coordinating muscle fibre recruitment and oxidative fuel metabolism. Research on cold exposure has included electromyographic (EMG) studies (Bell et al. 1992; Tikuisis et al. 2000; Meigal, 2002) and measurements of energy metabolism in shivering muscles (Jacobs et al. 1994; Haman et al. 2002), but these two complementary approaches have not been traditionally integrated (Haman et al. 2004a, b). In active muscles, fuel selection can either be achieved by mobilizing different metabolic pathways within the same fibres or by recruiting distinct fibre populations specialized for different fuels. Two EMG patterns associated with the recruitment of specific motor units (MUs) have been identified during shivering: (i) continuous, low-intensity shivering at 4–8Hz (or thermogenic muscle tone) and (ii) bursts of high-intensity shivering at much lower frequencies (0.1–0.2Hz) (Petajian & Williams, 1972; Israel & Pozos, 1989; Meigal et al. 1993; Meigal, 2002). While continuous, low-intensity shivering is linked to low-threshold MUs (type I, slow-oxidative, fatigue resistant), high-intensity bursts are associated with high-threshold MUs (type II, fast-glycolytic, fatigable). No information is available on the physiological significance of this dual pattern in relation to fuel selection or thermogenic rate. Adjusting the relative importance of low-intensity shivering and shivering bursts may be a key mechanism to modify oxidative fuel mix or total heat production.
Through simultaneous measurements of EMG and fuel metabolism during mild shivering, we have recently shown that glycogen-depleted and glycogen-loaded humans can sustain the same thermogenic rate by oxidizing widely different fuel mixtures within the same muscle fibres (Haman et al. 2004a). Drastic changes in fuel metabolism were achieved predominantly within type I, slow oxidative fibres (28 versus 65% of total heat production (Ḣprod) from carbohydrates (CHO) for glycogen-depleted and glycogen-loaded subjects, respectively; 53 versus 23% Ḣprod from lipids, and 19 versus 12% Ḣprod from proteins). However, it is not clear whether this fuel selection strategy is also used during high-intensity shivering, rather than recruiting different populations of fuel-specific fibres. For example, slow-oxidative fibres used predominantly during mild shivering may not be able to produce enough heat for intense shivering, and higher heat production rates could require the recruitment of fast fibres specialized for CHO oxidation.
The primary goal of this study was to determine whether the EMG pattern of muscle recruitment could provide metabolic information on oxidative fuel selection during high-intensity shivering. More specifically, we have simultaneously monitored burst shivering activity (in 8 large muscles representing >90% of total shivering muscle mass) and fuel metabolism during intense shivering. We hypothesized that burst shivering rate would be correlated with the oxidation of CHO for thermogenesis.
Methods
Subjects
Eight healthy, trained men volunteered for this study, approved by the Health Sciences Ethical Committee of the University of Ottawa, and written consent was obtained from the participants. The study conformed with the declaration of Helsinki. The participants were not acclimatized to cold exposure. Physical characteristics of the subjects are presented in Table 1. Percentage body fat (underwater weighing; Brosek et al. 1963) and maximal oxygen consumption (using a progressive treadmill protocol) were measured 5–7 days before the experiments.
Table 1. Metabolic rate (V̇O2), shivering intensity (%Shivpeak), CHO (%CHO) and lipid (%FAT) oxidation rate in men during sustained high-intensity shivering (∼60%Shivpeak). Physical characteristics including body mass (Mb), percent body fat (BF), body surface area (BSA), aerobic capacity (V̇O2max) and shivering capacity (Shivpeak) are also presented.
| Subjects | Mb (kg) | Height (cm) | Age (years) | BF (%) | BSA (m2) | V̇O2max (ml kg−1min−1) | Shivpeak* (mlkg−1min−1) | V̇O2 (ml kg−1min−1) | %Shivpeak | %CHO | %FAT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 81.0 | 178 | 23 | 13.4 | 1.99 | 59.0 | 22.4 | 10.6 | 47.2 | 49.5 | 38.6 |
| 2 | 59.5 | 176 | 23 | 13.4 | 1.73 | 59.9 | 28.5 | 14.0 | 49.1 | 78.0 | 14.1 |
| 3 | 68.8 | 170 | 23 | 15.7 | 1.79 | 46.6 | 19.7 | 10.9 | 55.0 | 39.8 | 50.3 |
| 4 | 83.4 | 180 | 23 | 15.1 | 2.03 | 45.6 | 17.6 | 13.4 | 75.9 | 71.5 | 25.3 |
| 5 | 64.7 | 170 | 22 | 10.9 | 1.74 | 43.2 | 20.1 | 11.1 | 55.6 | 65.1 | 25.9 |
| 6 | 73.4 | 172 | 25 | 12.7 | 1.86 | 52.9 | 20.5 | 12.1 | 59.2 | 40.6 | 48.3 |
| 7 | 77.0 | 174 | 22 | 10.2 | 1.91 | 58.0 | 23.0 | 13.1 | 56.9 | 61.8 | 32.5 |
| 8 | 65.9 | 173 | 25 | 9.9 | 1.78 | 59.5 | 25.4 | 15.3 | 60.2 | 32.5 | 59.2 |
| Mean | 71.7 | 174 | 23.3 | 12.7 | 1.85 | 53.1 | 22.2 | 12.6 | 57.4 | 54.8 | 36.8 |
| s.e.m.(n= 8) | 3.0 | 1.3 | 0.4 | 0.8 | 0.04 | 2.5 | 1.2 | 0.6 | 3.1 | 5.9 | 5.4 |
Shivpeakwas calculated as described by Eyolfson et al. (2001).
Experimental protocol
Experiments were conducted between 08.00 and 12.00 h, following 36 h without heavy physical activity. The last evening meal was standardized (∼950 kJ, ∼51% CHO, ∼27% lipids, ∼22% proteins) and subjects were asked to report to the laboratory the next morning (08.00 h) after a 12–14 h fast. Care was taken to minimize thermal stimuli between awakening and the start of the experiment. Upon arrival in the laboratory, subjects were instrumented with thermal probes and fitted with a liquid conditioned suit (LCS; One-piece Coretec, Delta Temax, Inc., Pembroke, ON, Canada). EMG collection sites, located on the right side of the body, were shaved and cleaned using an ethanol swab. Following skin preparation, disposable surface electrodes (Blue Sensor, Medicotest Inc., USA) were placed 2 cm apart over the bellies of each muscle – parallel to the direction of muscle fibres – and secured in place with medical transpore tape (3M Canada, London, ON, Canada). Shivering EMG was collected from eight muscles: trapezius (TR), latissimus dorsi (LA), pectoralis major (PE), rectus abdominis (RA), vastus lateralis (VL), rectus femoris (RF), vastus medialis (VM) and adductor magnus (AD). Pre-amplified and grounded EMG wires (375 ×) connected the surface electrode of each muscle to a ME-3000 Professional EMG system (Mega Electronics, Kuopio, Finland). Subjects were then asked to empty their bladder (t= 0 min) and sit quietly for 2 h at 25.5 ± 0.2°C (759 ± 2mmHg, 45 ± 4% relative humidity). Following this habituation period, they were transferred to an environmental chamber (5.7 ± 0.1°C, 759 ± 2mmHg, 69 ± 2% relative humidity) and a 5°C water perfusion was started through the LCS using a temperature-controlled circulation bath (Endocal, NESLAB and Model 200-00, Micropump, Vancouver, WA, USA). Thermal, metabolic and electrophysiological parameters were quantified at 26°C (baseline) and during 90 min at 5°C.
Thermal response
Central body temperature (Tes) was monitored continuously using a paediatric oesophageal temperature probe (Mon-a-therm general purpose, Mallinckrodt Medical Inc, St Louis, MO, USA) which was inserted through the nose to a depth placing the tip of the thermocouple at the level of the left atrium, or one-quarter of the standing height of the subject (Mekjavic & Rempel, 1990). Heat flux transducers (Concept Engineering, Old Saybrook, CT, USA) were used to estimate skin temperature from the forehead, chest, biceps, forearm, abdomen, lower and upper back, front and back calf, quadriceps, hamstrings and finger. Mean skin temperature (T¯skin) and mean heat flux were calculated using an area-weighted equation (Dubois & Dubois, 1916).
Metabolic rate and fuel selection
Oxygen consumption (V̇O2) and carbon dioxide production (V̇CO2) were determined by open-circuit spirometry (250 l, chain-compensated gasometer, Warren Collins Inc., Braintree, MA, USA). Expired gases were collected for 5 min, every 15min before and during cold exposure. Oxygen and carbon dioxide concentrations in dry expired gases were determined using calibrated electrochemical gas analysers (Ametek Model S-3 A/1 and CD 3A, Applied Electrochemistry, Pittsburg, PA, USA).
Total protein (PROTox), carbohydrate (CHOox) and lipid (FATox) oxidation rates (in g min−1) were calculated using the following equations (Livesey & Elia, 1988):
| (1) |
| (2) |
| (3) |
where V̇CO2 (l min−1) and V̇O2 (l min−1) were corrected for the volumes of O2 and CO2 corresponding to protein oxidation (1.010 and 0.843 l g−1, respectively). PROTox was estimated from urinary urea excretion (UREAurine) in urine samples collected for 90min at 26°C and 5°C. Urinary concentrations were determined on a Synchron Clinical System (CX7, Beckman, Anaheim, CA, USA). Energy potentials of 16.3, 40.8 and 19.7 kJ g−1 were used to calculate the relative contributions of CHO (%CHO), lipid (%FAT) and protein (%PROT) oxidation to total heat production, respectively (Elia, 1991; Péronnet & Massicotte, 1991).
The work presented in this paper deals exclusively with shivering thermogenesis because the contribution of non-shivering thermogenesis (brown adipose tissue and futile cycles) is known to be minimal in adult humans (< 5% of total heat produced) (Weber et al. 1990; Wolfe et al. 1990; Himms-Hagen & Ricquier, 1998; Vallerand et al. 1999).
EMG analysis
Raw EMG signals were collected at 1000Hz, filtered to remove below 20Hz and above 500Hz as well as 60Hz contamination and analysed using custom-designed MATLAB algorithms (Mathworks, Natick, MA, USA). EMG amplitude of individual muscles was determined based on root-mean-square (RMS) values calculated from EMG signals using a 50 ms overlapping-window (50%). Baseline RMS values (RMSbaseline: 15min RMS average measured prior to cold exposure) were subtracted from shivering RMS values.
Burst shivering rate (BR) was determined as described in a previous paper where an example of EMG signal showing the two shivering patterns is illustrated (see Fig. 5 in Haman et al. 2004a). A shivering burst was defined as an EMG interval with a duration >0.2s, an interburst interval > 0.75 s and an amplitude higher than the amplitude threshold of the given recording period. Amplitude threshold for the identification of shivering bursts was done by: (i) averaging shivering intensity (A¯EMG) over the entire recording period, (ii) averaging the remaining values above A¯EMG (B¯EMG), and (iii) setting the amplitude threshold at B¯EMG. At each sampling interval, burst rate was calculated by dividing the number of bursts by total interval recording time.
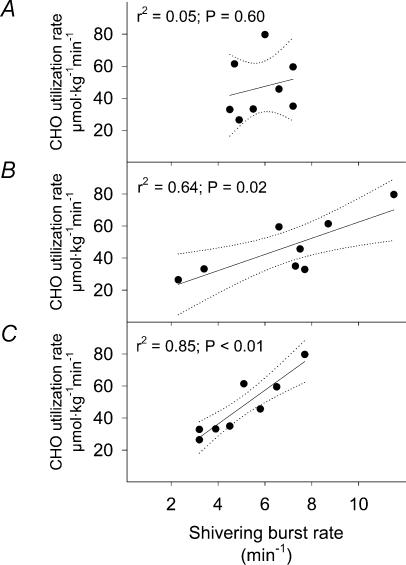
Figure 5. Relationship between burst shivering rate (BR) and the absolute CHO oxidation rate (CHOox) for the upper trunk (A), lower trunk (B) and upper leg muscles (C) in non-acclimatized men exposed to 5°C for 90 min.
Dotted lines indicate the 95% confidence interval. Values were averaged in the last 15min of cold exposure (t = 75–90 min).
Calculations and statistical analysis
Overall changes in Tes, T¯skin and Ḣprod were assessed using a one-way analysis of variance (ANOVA) with replication. For each sampling time, a Bonferroni t test was used to detect potential differences from control values observed at 26°C. Stepwise regression analyses were performed to determine the best predictors of changes in rates of CHO and lipid utilization. Statistical differences were considered significant when P < 0.05. Values presented are means ±s.e.m.(n = 8).
Results
Thermal response
Changes in Ḣprod, Tes and T¯skin are presented in Fig. 1. Ḣprod increased progressively from 89.5 ± 3.5 W at 26°C to 292.1 ± 18.2 W by the end of cold exposure. A transient increase in Tes was observed in the first 60 min of cold exposure (36.6 ± 0.1°C at 26°C to 37.0 ± 0.1°C) before stabilizing at a value not different from baseline levels (36.8 ± 0.2°C). T¯skin decreased by 25% averaging 34.2 ± 0.2°C at 26°C and 25.8 ± 0.4°C by the end of cold exposure.
Figure 1. Changes in heat production rate (A) and oesophageal (Tes) and mean skin(T¯skin) temperature (B) in non-acclimatized men at 26°C and at 5°C.
*Significantly different from baseline values at 26°C.
Fuel selection: interindividual variability
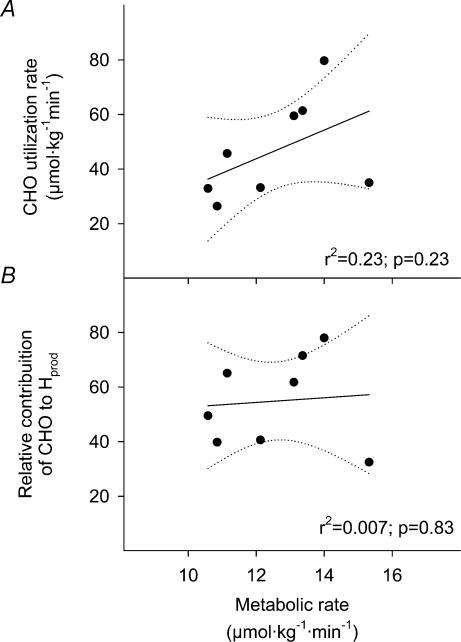
Table 1 summarizes individual values for metabolic rate (V̇O2, %Shivpeak) and relative contributions of CHO and lipids to total heat production measured in the last 15min at 5°C (t = 75–90 min). A large variability was observed in the relative changes in CHO and lipid oxidation rates from baseline values to the end of cold exposure. Depending on the subject, CHO oxidation rate increased 1.9- to 8.3-fold whereas lipid oxidation did not change or increased by as much as 23.4-fold. Average CHO and lipid oxidation were, respectively, 2.0 ± 0.2 and 0.6 ± 0.1 mg kg−1 min−1 at 26°C and 8.9 ± 1.3 and 2.3 ± 0.4 mg kg−1 min−1. Total amount of CHO used over the entire cold exposure (in mmol kg−1 for t= 0–90 min) for each subject are presented in Fig. 2. Total CHO use averaged 3.1 ± 0.5 mmol kg−1 but was extremely variable among subjects ranging between 1.5 and 5.1 mmol kg−1 (Fig. 2). Relationships between metabolic rate (based on V̇O2 measurements) and absolute utilization as well as relative contribution of CHO to total heat production are presented in Fig. 3. The large variation in CHO oxidation observed during high-intensity shivering was not correlated with differences in metabolic rate.
Figure 2. Mean and individual CHO utilization calculated over the entire 90 min at 5°C in non-acclimatized men.
Subject numbers are the same as in Table 1.
Figure 3. Relationship between shivering intensity (V̇O2) and the absolute oxidation (CHOox) (A) and relative contribution of CHO to total heat production (%CHOox) (B) in non-acclimatized men exposed to 5°C for 90 min.
Dotted lines indicate the 95% confidence interval. Values were averaged in the last 15min of cold exposure (t = 75–90 min).
EMG pattern and fuel selection
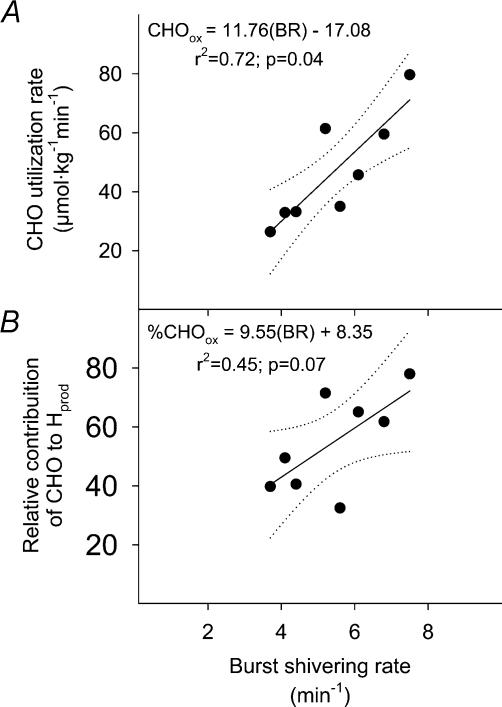
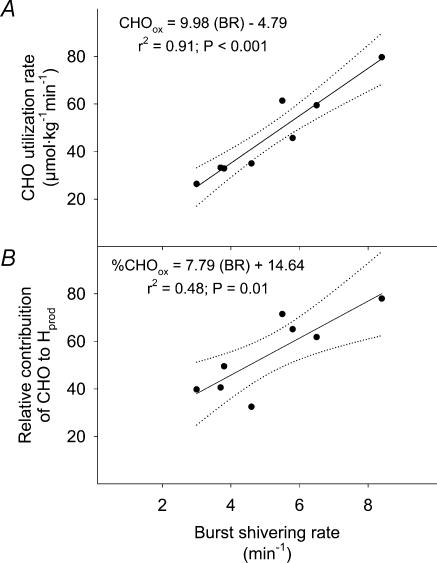
The relationship between mean burst shivering rate (averaged for the 8 muscles) and CHOox is presented in Fig. 4. While mean burst shivering rate covaried with CHOox (Fig. 4A), no significant correlation could be established for percentage CHOox (P= 0.07; Fig. 4B). Table 2 summarizes the correlation coefficients between BR and CHOox and FATox for individual muscles. While no relationship between BR and FATox was observed, close correlations between BR and CHOox were found for RA, VL, RF and VM, but not TR, LA, PE and AD. Correlations between mean BR averaged by body regions (upper trunk, lower trunk and upper leg) and CHOox are presented in Fig. 5. While mean BR of upper trunk (TR, PE and LA; Fig. 5A) was not related with CHOox, BR for lower trunk (RA; Fig. 5B) and upper leg muscles (average of VL, RF, VM and AD; Fig. 5C) were correlated with differences in CHOox. Correlations between mean BR for muscles showing the highest correlation coefficients with CHOox (RA, VL, RF and VM, Table 2) or percentage CHOox are presented in Fig. 6.
Figure 4. Relationship between burst shivering rate (BR) and the absolute oxidation (CHOox) (A) and relative contribution of CHO to total heat production (%CHOox) (B) in non-acclimatized men exposed to 5°C for 90 min.
BR is the average of the 8 sampling sites. Dotted lines indicate the 95% confidence interval. Values were averaged in the last 15min of cold exposure (t = 75–90 min).
Table 2.
Pearson correlations between burst shivering rate and CHO and lipid (FAT) utilization rates for individual muscles
| EMG sites | CHO | FAT |
|---|---|---|
| Trapezius | 0.14 | 0.09 |
| Lattissimus dorsi | 0.58 | 0.09 |
| Pectoralis major | −0.27 | 0.42 |
| Rectus abdominis† | 0.79* | −0.52 |
| Vastus lateralis | 0.84* | −0.65 |
| Rectus femoris | 0.79* | −0.43 |
| Vastus medialis† | 0.87* | −0.67 |
| Adductor magnus | 0.09 | −0.23 |
Correlation is significant at P < 0.05.
Best predictors of CHO utilization.
Figure 6. Relationship between burst shivering rate (BR) and the absolute oxidation (CHOox) (A) and relative contribution of CHO to total heat production (%CHOox) (B) in non-acclimatized men exposed to 5°C for 90 min.
BR is the average of only the best predictors of CHOox and percentage CHOox from Table 2: rectus abdominis, vastus lateralis, rectus femoris and vastus medialis. Dotted lines indicate the 95% confidence interval. Values were averaged in the last 15min of cold exposure (t = 75–90 min).
Discussion
This study is the first to show that the pattern of muscle recruitment (EMG) can provide quantitative information on energy metabolism. Acute cold exposure was used as a tool to investigate muscle activity and oxidative fuel selection simultaneously. We reasoned that shivering thermogenesis would provide a better model than exercise for this purpose, because it eliminates electrical noise caused by limb movements. During high-intensity shivering, the relative use of lipids and carbohydrates was very different among subjects (Table 1), and this high variability in fuel selection was primarily explained by differences in burst shivering rate (Fig. 4). In contrast, during low-intensity shivering, our previous work had shown that no such correlation between fuel selection and burst shivering rate was apparent (Haman et al. 2004a).
Shivering pattern reflects fuel selection
We were particularly careful to minimize interindividual variability in the response to cold. The group of non-acclimatized male subjects selected for this study was normalized as much as possible (Table 1) for age, morphology (surface to volume ratio), body composition (percentage body fat) and diet. These precautions were sufficient to elicit similar changes in metabolic rate among subjects: a 3.3-fold increase in the cold. However, surprisingly large interindividual differences in fuel selection were observed. Depending on the subject, the cold-induced increase in CHO oxidation ranged between 2- and 8-fold, with CHO oxidation accounting for 33–78% Ḣprod, and lipid oxidation for 14–60% Ḣprod (Table 1). In exercising muscles, such large differences in substrate metabolism are usually explained by differences in exercise intensity but here, during shivering, there was no significant correlation between fuel selection and metabolic rate (Fig. 3). Therefore, we turned our attention to the possibility that differences in fuel metabolism were related to the selective recruitment of different fibre populations; a detailed characterization of shivering patterns was performed to quantify the relationship between EMG signatures and fuel selection. EMG signals were analysed by separating the patterns for continuous, low-intensity shivering (type I fibres) and burst shivering (type II fibres). Distinguishing the two types was based on large differences in intensity and rate of occurrence (8–10 versus 0.1–0.2Hz). Results clearly show that mean burst shivering rate (averaged among subjects and for 8 muscles in each subject) covaries closely with the rate of CHO oxidation (Fig. 4). This finding indicates that, during high-intensity shivering, the recruitment of type II fibres is linked to an increase in CHO use and plays a key role in orchestrating fuel selection.
During exercise, the relationship between type II fibre recruitment and CHO oxidation is generally accepted, even though no direct experimental evidence is available because no simultaneous measurements of EMG and fuel selection has been performed. However, indirect support is strong (Armstrong, 1988; Roberts et al. 1996; Brooks et al. 1999). At low exercise intensity, the recruitment of type I fibres is related to an increase in lipid use. When exercise intensity is increased, CHO oxidation becomes progressively more important with the gradual recruitment of more type II fibres. Through simultaneous monitoring of EMG signals and fuel metabolism in shivering muscles, this study shows that higher rates of CHO oxidation are achieved by recruiting more type II fibres.
Shivering pattern of individual muscles
After relating mean burst shivering rate to CHO oxidation at the whole-organism level, the same relationship was examined individually for each muscle. While the burst shivering rates of abdominal and quadriceps muscles were closely correlated with whole-body CHO use (r2= 0.79–0.87), no such relationship could be established for the upper trunk (pectoralis major, trapezius and lattissimus dorsi) or for adductor magnus (Table 2). Therefore, we found that burst shivering activity was not synchronized among muscles, and these results are not consistent with the only two previous studies dealing with this issue in humans (Bawa et al. 1987; Israel & Pozos, 1989). The exact reasons for this discrepancy are unclear, but they may be related to differences in the fibre composition of the subjects used in the three studies. Interestingly, changes in burst shivering pattern have been associated with differences in fibre composition in birds. While more aerobic muscles shiver continuously (high in type I fibres), those depending more on anaerobic metabolism (high in type II fibres) have the tendency to shiver in bursts (Hohtola & Stevens, 1986). In humans, fibre composition varies significantly among muscles (Gardiner, 2001) and even more so between individuals (Simoneau & Bouchard, 1989). Unfortunately, because little attention has been dedicated to shivering bursts (Israel & Pozos, 1989), no information is available on their rate of occurrence in relation to fibre composition.
Another important factor that could explain the lack of synchronization in burst shivering rate between muscles of the upper trunk, lower trunk and upper leg may be related to regional differences in skin temperature. While research on animals suggests that continuous low-intensity shivering (tonic) is controlled by the hypothalamic shivering centre, burst shivering (phasic) appears to be generated within the spinal cord (Simon et al. 1966; Kosaka & Simon, 1968; Herdman, 1978; Gorke & Pierau, 1979). Whether burst shivering activity is controlled by a non-hypothalamic site in humans is still unclear. However, some evidence indicates that burst shivering frequency is modulated by thermosensitive segmental influences (i.e. fluctuations in skin temperature detected by cutaneous cold receptors) (Burton & Edholm, 1969) and/or by efferent signals from the hypothalamic centre (Martin & Cooper, 1981). In our experiments, oesophageal temperatures were similar amongst subjects, but significant differences in regional skin temperature gradients were observed (upper trunk 5.0 ± 0.4°C, lower trunk 7.0 ± 0.7°C, upper leg 11.6 ± 0.6°C). In view of our results, additional work on shivering bursts is clearly needed. This seemingly minor component of the overall EMG signal appears to play the most prominent role in modulating fuel metabolism. In particular, close attention will have to be given to differences in fibre composition and regional skin temperature.
Changing fuel selection in the cold
Modifying fuel selection of contracting muscles can be achieved in two ways: (i) by mobilizing different metabolic pathways within the same fibres, or (ii) by recruiting distinct fibre populations specialized for different fuels. Previous results for low-intensity shivering have shown that glycogen-depleted and glycogen-loaded humans are able to sustain the same rate of heat production by oxidizing broadly different fuels within the same muscle fibres (Haman et al. 2004a). Here, during high-intensity shivering, the alternative mechanism of fuel selection is observed: large differences in fuel use are achieved by recruiting different ‘fuel-specific’ fibres. Depending on the individual, thermogenesis was sustained by oxidizing mostly lipids (up to 59% Ḣprod) within predominantly type I fibres (4 bursts min−1) or by recruiting more type II fibres (8 bursts min−1) and using mostly CHO (up to 78% Ḣprod). Whether these large intersubject differences in fibre recruitment offer a selective advantage for cold survival is unknown and more research is needed to establish the physiological significance of individual shivering patterns. What are the possible consequences of differences in burst shivering rate (and related CHO use) on cold endurance? Over the last few decades, CHO metabolism has often been considered as a limiting factor for heat production because the availability of this critical fuel is very low (∼1% of total energy reserves) (Jacobs et al. 1994; Haman et al. 2002). However, it is still unclear whether CHOs are essential for maintaining such a high thermogenic rate (Martineau & Jacobs, 1989; Young et al. 1989; Haman et al. 2004a). If CHOs are essential, our results imply that cold endurance varies greatly among morphologically similar individuals shivering at the same relative intensity. Consequently, the normalization of burst shivering rate will be required in future studies to decrease intersubject variability. If CHOs are not essential, lipids and proteins will have to compensate for varying contributions from CHOs and survival time in the cold will be substantially increased.
Shivering versus exercise
Our study reveals interesting differences between exercise and shivering, further supporting the notion that these two processes are not analogous (Tipton et al. 1997). For the same metabolic rate, mean recruitment of type II fibres and variability in fuel selection are much higher during shivering than exercise. This suggests that the ‘crossover point’ (i.e. the metabolic rate for which CHO and lipid oxidation contribute equally to oxygen consumption; see Brooks & Mercier, 1994) is significantly lower during shivering than exercise. Also, interindividual differences in type II fibre recruitment are sufficient to affect the relative use of CHO (from 33 to 78% Ḣprod, greatly exceeding the expected range of values for exercise at such low metabolic rates, see Table 1). Patterns of fibre recruitment have received a lot of attention during exercise (see Gardiner, 2001; Wakeling et al. 2002; Linnamo et al. 2003 for review), but very few studies have investigated them during shivering (Petajian & Williams, 1972; Israel & Pozos, 1989; Meigal et al. 1993; Meigal et al. 1995; Meigal, 2002). In exercise, fibres are recruited according to the ‘size principle’ (Henneman et al. 1965) which stipulates that type I fibres are activated for low-force contractions and increasingly larger and faster fibres (type II) are then activated to supply greater force (Milner-Brown et al. 1973; Freund et al. 1975; De Luca et al. 1982; Moritani & Muro, 1987; Brooks et al. 1999; Wakeling et al. 2002; Linnamo et al. 2003). Clearly, further research is needed to establish what principle governs fibre recruitment as shivering intensity is modified.
Conclusion
Traditionally, EMG signals have provided information on overall muscle activity, on the recruitment of specific motor units, and on the onset of fatigue. Here, we show for the first time that the EMG signature of active muscles also includes important quantitative information on metabolic fuel utilization. During intense cold exposure, differences in burst shivering rate are directly linked to differences in CHO oxidation. All subjects have the ability to sustain the same high thermogenic rate, but each individual can do so by recruiting different combinations of ‘fuel-specific’ fibres that oxidize widely different fuel mixtures. Further research should focus on understanding burst shivering because it plays a central role in orchestrating fuel selection and may provide essential clues on what limits human survival in the cold.
Acknowledgments
This project was funded through a Natural Sciences and Engineering Research Council of Canada (NSERC) grant to J.-M. Weber. F. Haman was the recipient of a NSERC scholarship. We particularly thank an anonymous reviewer for pointing out the possible relationship between local skin temperature and burst shivering rate.
References
- Armstrong RB. Muscle fiber recruitment patterns and their metabolic correlates. In: Horton ES, Terjung RL, editors. Exercise, Nutrition and Energy Metabolism. New York: Macmillan; 1988. pp. 9–26. [Google Scholar]
- Bawa P, Mathews PBC, Mekjavic IB. Electromyographic activity during shivering of muscles acting at the human elbow. J Therm Biol. 1987;12:1–4. [Google Scholar]
- Bell DG, Tikuisis P, Jacobs I. Relative intensity of muscular contraction during shivering. J Appl Physiol. 1992;72:2336–2342. doi: 10.1152/jappl.1992.72.6.2336. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Fahey TD, White TP, Baldwin KM. Exercise Physiology – Human Bioenergetics and its Applications. Toronto: Mayfield Publishing Co; 1999. [Google Scholar]
- Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the ‘crossover’ concept. J Appl Physiol. 1994;76:2253–2261. doi: 10.1152/jappl.1994.76.6.2253. [DOI] [PubMed] [Google Scholar]
- Brosek JF, Grande JT, Andersen JT, Keys A. Densiometric analysis of body composition: review of some quantitative assumptions. Ann N Y Acad Sci. 1963;110:113–140. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- Burton AC, Edholm OG. Physiological Society Monograph 2. New York: Hafner; 1969. Man In A Cold Environment; pp. 149–153. [Google Scholar]
- De Luca C, Lefever M, McCue M, Xenakis A. Behavior of human motor units in different muscles during linearly varying concentrations. J Physiol. 1982;329:113–128. doi: 10.1113/jphysiol.1982.sp014293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois D, Dubois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Inter Med. 1916;17:863–871. [Google Scholar]
- Elia M. Energy equivalents of CO2 and their importance in assessing energy expenditure when using tracer techniques. Am J Physiol Endocrinol Metab. 1991;23:E75–E88. doi: 10.1152/ajpendo.1991.260.1.E75. [DOI] [PubMed] [Google Scholar]
- Eyolfson DA, Tikuisis P, Xu X, Weseen G, Giesbrecht GG. Measurement and prediction of peak shivering intensity in humans. Eur J Appl Physiol. 2001;84:100–106. doi: 10.1007/s004210000329. [DOI] [PubMed] [Google Scholar]
- Freund H, Büdingen H, Dietz V. Activity of single motor units from human forearm muscles during voluntary isometric contractions. J Neurophysiol. 1975;38:933–946. doi: 10.1152/jn.1975.38.4.933. [DOI] [PubMed] [Google Scholar]
- Gardiner P. Neuromuscular Aspects of Physical Activity. Windsor, ON, Canada: Human Kinetics; 2001. [Google Scholar]
- Gorke K, Pierau FK. Initiation of muscle activity in spinalized pigeons during spinal cord cooling and warming. Pflugers Arch. 1979;381:47–52. doi: 10.1007/BF00582331. [DOI] [PubMed] [Google Scholar]
- Haman F, Legault SR, Rakobowchuk M, Ducharme MB, Weber J-M. Effects of carbohydrate availability on sustained shivering II. Relating muscle recruitment to fuel selection. J Appl Physiol. 2004a;96:41–49. doi: 10.1152/japplphysiol.00428.2003. [DOI] [PubMed] [Google Scholar]
- Haman F, Peronnet F, Kenny GP, Doucet E, Massicotte D, Lavoie C, Weber J-M. Effects of carbohydrate availability on sustained shivering I. Oxidation of plasma glucose, muscle glycogen, and proteins. J Appl Physiol. 2004b;96:32–40. doi: 10.1152/japplphysiol.00427.2003. [DOI] [PubMed] [Google Scholar]
- Haman F, Péronnet F, Kenny GP, Massicotte D, Lavoie C, Scott C, Weber J-M. Effect of cold exposure on fuel utilization in humans: plasma glucose, muscle glycogen, and lipids. J Appl Physiol. 2002;93:77–84. doi: 10.1152/japplphysiol.00773.2001. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter D. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Herdman SJ. Recovery of shivering in spinal cats. Exp Neurol. 1978;59:177–189. doi: 10.1016/0014-4886(78)90148-6. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J, Ricquier D. Brown adipose tissue. In: Bray GA, Bouchard C, James WPT, editors. Handbook of Obesity. New York: Marcel Dekker, Inc.; 1998. pp. 415–441. [Google Scholar]
- Hohtola E, Stevens ED. The relationship of muscle electrical activity, tremor and heat production to shivering thermogenesis in Japanese quail. J Exp Biol. 1986;125:119–135. doi: 10.1242/jeb.125.1.119. [DOI] [PubMed] [Google Scholar]
- Israel DJ, Pozos RS. Synchronized slow-amplitude modulations in the electromyograms of shivering muscles. J Appl Physiol. 1989;66:2358–2363. doi: 10.1152/jappl.1989.66.5.2358. [DOI] [PubMed] [Google Scholar]
- Jacobs I, Martineau L, Vallerand AL. Thermoregulatory thermogenesis in humans during cold stress. Exerc Sport Sci Rev. 1994;22:221–250. [PubMed] [Google Scholar]
- Kosaka M, Simon E. Central nervous spinal mechanism of cold shivering. Pflugers Arch. 1968;302:357–373. doi: 10.1007/BF00592733. [DOI] [PubMed] [Google Scholar]
- Linnamo V, Moritani T, Nicol C, Komi PV. Motor unit activation patterns during isometric, concentric and eccentric actions at different force levels. J Electromyo Kinesio. 2003;13:93–101. doi: 10.1016/s1050-6411(02)00063-9. [DOI] [PubMed] [Google Scholar]
- Livesey G, Elia M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr. 1988;47:608–628. doi: 10.1093/ajcn/47.4.608. [DOI] [PubMed] [Google Scholar]
- Martin S, Cooper KE. Factors which affect shivering in man during cold water immersion. Pflugers Arch. 1981;391:81–83. doi: 10.1007/BF00580700. [DOI] [PubMed] [Google Scholar]
- Martineau L, Jacobs I. Muscle glycogen availability and temperature regulation in humans. J Appl Physiol. 1989;66:72–78. doi: 10.1152/jappl.1989.66.1.72. [DOI] [PubMed] [Google Scholar]
- Meigal A. Gross and fine neuromuscular performance. Int J Circumpolar Health. 2002;61:163–172. doi: 10.3402/ijch.v61i2.17449. [DOI] [PubMed] [Google Scholar]
- Meigal A, Lupandin V, Kuzmina GI. Electromyographic patterns of thermoregulatory activity of motor units in the course of body cooling. Fiziol Cheloveka. 1993;19:106–114. (in Russian) [PubMed] [Google Scholar]
- Meigal A, Pavlova I, Lupnadin Y, Sokolov A, Antonen E. Thermoregulatory activity of motor units during human development. Arctic Med Res. 1995;54:192–200. [PubMed] [Google Scholar]
- Mekjavic IB, Rempel ME. Determination of oesophageal probe insertion length based on standing and sitting height. J Appl Physiol. 1990;69:376–379. doi: 10.1152/jappl.1990.69.1.376. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H, Stein R, Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol. 1973;230:359–370. doi: 10.1113/jphysiol.1973.sp010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritani T, Muro M. Motor unit activity and surface electromyogram power spectrum during increasing force of contraction. Eur J Appl Physiol. 1987;56:260–265. doi: 10.1007/BF00690890. [DOI] [PubMed] [Google Scholar]
- Péronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Spt Sci. 1991;16:23–29. [PubMed] [Google Scholar]
- Petajian JH, Williams DD. Behavior of single motor units during preshivering tone and shivering tremor. Am J Phys Med. 1972;51:16–23. [PubMed] [Google Scholar]
- Roberts TJ, Weber J-M, Hoppeler H, Weibel ER, Taylor CR. Design of the oxygen and substrate pathways. II. Defining the upper limits of carbohydrate and fat oxidation. J Exp Biol. 1996;199:1651–1658. doi: 10.1242/jeb.199.8.1651. [DOI] [PubMed] [Google Scholar]
- Simon E, Klussmann FW, Rautenberg W, Kosaka M. Shivering from cold in anesthetized spinal dogs. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;291:187–204. [PubMed] [Google Scholar]
- Simoneau J-A, Bouchard C. Human variations in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol Endocrinol Metab. 1989;257:E567–E572. doi: 10.1152/ajpendo.1989.257.4.E567. [DOI] [PubMed] [Google Scholar]
- Tikuisis P, Jacobs I, Moroz D, Vallerand A, Bell D. Female vs male shivering EMG responses to 10°C air. In: Hexamer JWaM., editor. The 9th International Conference on Environmental Ergonomics; Shaker Verlag, Dortmund, Germany. 2000. pp. 149–152. [Google Scholar]
- Tipton MJ, Franks GM, Meneilly GS, Mekjavic IB. Substrate utilisation during exercise and shivering. Eur J Appl Physiol. 1997;76:103–108. doi: 10.1007/s004210050220. [DOI] [PubMed] [Google Scholar]
- Vallerand AL, Zamenick J, Jones PJH, Jacobs I. Cold stress increases lipolysis, FFA Ra and TG/FFA cycling in humans. Aviat Space Environ Med. 1999;70:42–50. [PubMed] [Google Scholar]
- Wakeling JM, Kaya M, Temple GK, Johnston IA, Herzog W. Determining patterns of motor recruitment during locomotion. J Exp Biol. 2002;205:359–369. doi: 10.1242/jeb.205.3.359. [DOI] [PubMed] [Google Scholar]
- Weber J-M, Klein S, Wolfe RR. Role of the glucose cycle in control of net glucose flux in exercising humans. J Appl Physiol. 1990;68:1815–1819. doi: 10.1152/jappl.1990.68.5.1815. [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Klein S, Carraro F, Weber JM. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Physiol. 1990;258:E382–E389. doi: 10.1152/ajpendo.1990.258.2.E382. [DOI] [PubMed] [Google Scholar]
- Young AJ, Sawka MN, Neufer PD, Muza SR, Askew EW, Pandolf KB. Thermoregulation during cold water immersion is impaired by low glycogen levels. J Appl Physiol. 1989;66:1806–1816. doi: 10.1152/jappl.1989.66.4.1809. [DOI] [PubMed] [Google Scholar]