Abstract
Peripheral conduit artery flow-mediated dilatation decreases with ageing in humans. The underlying mechanisms and efficacy of preventive strategies are unknown. Brachial artery flow-mediated dilatation was determined at baseline and after ascorbic acid (vitamin C) intravenous infusion and chronic supplementation (500 mg day−1 for 30 days) in three groups of healthy men: young sedentary (n= 11; 25 ± 1 years, mean ±s.e.m.), older sedentary (n= 9; 64 ± 2), and older endurance-exercise trained (n= 9; 64 ± 2). At baseline, flow-mediated dilatation (normalized for the hyperaemic stimulus) was ∼45% lower in the older (0.015 ± 0.001) versus young (0.028 ± 0.004) sedentary men (P < 0.01), but was preserved in older exercising men (0.028 ± 0.004). Ascorbic acid infusion increased plasma concentrations > 15-fold in all groups and restored flow-mediated dilatation in the sedentary older men (to 0.023 ± 0.002; P > 0.1 versus other groups), with no effects in the other two groups. Oral ascorbic acid supplementation did not affect flow-mediated dilatation in any group. Brachial artery endothelium-independent dilatation (sublingual nitroglycerin) did not differ among the groups at baseline nor change with ascorbic acid administration. These results provide the first evidence for an important role of oxidative stress in both the impairment in peripheral conduit artery flow-mediated dilatation with sedentary human ageing and the preservation of flow-mediated dilatation with physically active ageing.
‘Vascular ageing’ has recently been emphasized as the major risk factor for cardiovascular diseases (CVD) by combining with pathophysiological processes to produce a potentially lethal ‘age–disease interaction’ (Lakatta & Levy, 2003). The mechanisms involved and strategies that attenuate or prevent adverse age-associated changes (i.e. vascular ageing as a ‘therapeutic target’) were recognized as two key areas of future research.
In this context, arterial endothelial dysfunction is an important feature of a number of cardiovascular disorders (Moncada et al. 1991). The ability to produce vascular endothelium-dependent increases in blood flow in response to pharmacological stimulation is reduced in patients with CVD compared with age- and sex-matched healthy controls (Ludmer et al. 1986; Panza et al. 1990; Drexler et al. 1992). A smaller forearm blood flow response to acetylcholine or methacholine chloride is also observed in older compared with young healthy sedentary adults (Taddei et al. 1995; Gerhard et al. 1996). However, there is no age-associated reduction in the forearm blood flow response to other endothelium-dependent pharmacological stimuli (DeSouza et al. 2002), suggesting that reductions in endothelium-dependent vasodilatory responsiveness with primary ageing are stimulus specific.
Flow-mediated dilatation (FMD) is an endothelium-dependent dilatory response (Joannides et al. 1995) to a true physiological stimulus (acute increase in vascular shear stress or blood flow) (Rubanyi et al. 1986), and reflects the overall health and functional integrity of the vascular endothelium (Bonetti et al. 2003). FMD in peripheral (e.g. brachial) conduit arteries is reduced in patients with CVD (Hayoz et al. 1993), and is an independent predictor of future cardiac events (Neunteufl et al. 2000; Gokce et al. 2002). Brachial artery FMD is reduced with age (Celermajer et al. 1994) and therefore may contribute to the increased prevalence of CVD in older adults.
The decreased FMD in patients with congestive heart failure (Hornig et al. 1998) and coronary artery disease (Levine et al. 1996a) is restored with acute administration of the potent antioxidant ascorbic acid (vitamin C), suggesting elevated vascular oxidative stress as a key mechanism involved. It is not known if this mechanism plays a significant role in the impairment of brachial FMD observed with ageing.
Another important question is whether lifestyle behaviours such as habitual aerobic endurance exercise can attenuate or prevent the age-associated reduction in brachial artery FMD and, if so, the mechanism involved. Brachial artery FMD is greater in endurance-exercise trained than in sedentary older adults (Rywik et al. 1999; Rinder et al. 2000), but it is unknown if the augmented FMD in older exercising adults represents preserved function compared with young adults. Moreover, if increased oxidative stress contributes to a reduction in brachial artery FMD with sedentary ageing, a preservation of FMD in exercising older adults may be linked to reduced oxidative stress.
Finally, if acute administration of ascorbic acid can improve/restore brachial artery FMD in sedentary older adults, it is possible that longer-term ascorbic acid supplementation could be used therapeutically to sustain the improvement. Such an effect has been reported previously in patients with coronary artery disease and congestive heart failure (Hornig et al. 1998; Gokce et al. 1999). However, currently no information is available on the effects of ascorbic acid supplementation on brachial artery FMD in older adults without clinical disease.
In the present study we tested the hypotheses that the reduction in brachial artery FMD with sedentary ageing is mediated by increased oxidative stress, and that habitual endurance exercise preserves FMD with ageing by tonically suppressing oxidative stress.
Methods
Subjects
A total of 29 healthy men were studied: 11 young sedentary (aged 25 ± 1, 18–30 years) and 18 older (aged 60–79). For at least the previous 2 years, the older individuals were either sedentary (no regular physical activity) (n= 9, 64 ± 2 years) or endurance-exercise trained (vigorous aerobic endurance exercise > 3 times per week) (n= 9, 64 ± 2 years). Subjects were normotensive (BP < 140/90), non-smokers, non-obese, and free of CVD as assessed by medical history, physical examination, blood chemistry, and resting and exercise ECG (older men only). Candidates who had used antioxidants (vitamin C and E or any other type) within 6 weeks or were taking other medications were excluded. Subjects gave their written informed consent to participate. All procedures were approved by the Human Research Committee of the University of Colorado at Boulder.
Experimental procedures
After completion of screening procedures, two main experimental sessions were conducted ∼1 month apart. Prior to the main sessions subjects fasted for 12 h and did not participate in any physical activity on the previous day. During these sessions subjects were positioned supine and instrumented with an intravenous catheter in the left arm for ascorbic acid infusions and acquisition of blood.
Measurements
FMD and vascular endothelium-independent dilatation
Brachial artery FMD was assessed non-invasively as described originally by Celermajer et al. (1992) using an ultrasound machine (Toshiba Power Vision 6000) equipped with a 7.5MHz adjustable (6–11MHz) transducer. The guidelines for determination of FMD described recently by Corretti et al. (2002) were strictly followed.
Briefly, the right arm was adducted at heart level, placed on a foam pad, and the brachial artery was located 3–6 cm above the antecubital crease. To ensure the location of the same arterial segment with serial measurements, anatomical landmarks were noted and the distance from the antecubital crease was recorded. The ultrasound probe was then clamped to avoid any involuntary movement. After obtaining baseline diameters, reactive hyperaemia was produced by inflating a blood pressure cuff placed on the upper forearm for 5min at 250mmHg of pressure followed by a rapid deflation. Blood velocity envelopes were obtained during the first 10 arterial pulses after cuff deflation to establish the magnitude of the hyperaemic response (peak blood flow – stimulus for FMD); the brachial artery was then scanned continuously until 2 min post-occlusion to obtain the peak dilatory response. Ultrasound images were recorded on a super-VHS videocassette for later off-line manual analysis as previously described (Eskurza et al. 2001). FMD was calculated as absolute (Δmm) and percentage change in brachial artery diameter in response to the forearm hyperaemic stimulus. Because the main stimulus for FMD is an acute increase in vascular shear stress or blood flow, for proper interpretation of potential baseline group (e.g. young versus older sedentary men) or condition (e.g. baseline versus ascorbic acid infusion) differences the FMD values were normalized for the magnitude of the hyperaemic stimulus (i.e. change in diameter divided by the hyperaemic blood flow response). Blood flow was calculated as (mean blood velocity [cm s−1]) × (baseline diameter2/4 × 3.14) × (6 × 10−1) (Dinenno et al. 1999). The constant 6 × 10−1 is the conversion factor to obtain blood flow in units of ml min−1.
Vascular endothelium-independent dilatation was assessed with sublingual nitroglycerine (NTG) (0.4 mg) as previously described (Levine et al. 1996a) with brachial artery images recorded for 10 min. All ultrasound images were recorded and analysed by the same investigator (I.E.) who was blinded to subject group assignment and experimental conditions. The coefficient of variation for trial-to-trial reliability for baseline diameter, peak diameter, FMD (%), and FMD corrected for the hyperaemic response were 0.3, 0.6, 8.1 and 11.5%, respectively.
Arterial blood pressure
Resting blood pressure was measured over the brachial artery using a semiautomated device (Dynamap Pro, 100; Crifikon, Tampa, FL, USA) with subjects in supine position as previously described (Dinenno et al. 1999).
Blood measurements
Plasma samples were analysed for venous concentrations of ascorbic acid (Frei et al. 1989), cathecholamines (Peuler & Johnson, 1977), endothelin-1 (competitive radioimmunoassay) (Lerman et al. 1991), and oxidized low-density lipoproteins (Holvoet et al. 1998). Cathecholamines and endothelin-1 were measured because nitric oxide regulates their vasoconstrictor effects.
Body composition
Fat mass and fat-free mass (FFM) were measured using dual-energy X-ray absorptiometry (DXA-GE; Lunar corporation (Madison, WI, USA) software version 5.60.003).
Maximal oxygen consumption (V̇O2,max)
V̇O2,max was measured during graded treadmill exercise using open circuit spirometry as previously described (DeSouza et al. 2000).
Protocol
To determine if oxidative stress plays a mechanistic role in the age-associated decline in FMD, FMD was measured before and after intravenous administration of a pharmachological dose of ascorbic acid (American Regent Laboratories Inc., NY, USA): priming bolus of 0.06 g kg−1 FFM dissolved in 100 ml of saline infused at 5 ml min−1 for 20 min followed by a drip infusion of 0.02 g kg−1 FFM dissolved in 30 ml of saline administered over 60 min at 0.5 ml min−1. To determine if an improvement in FMD can be obtained with oral ascorbic acid supplementation, subjects ingested 500mg day−1 of ascorbic acid (timed-release capsules, Goldline Laboratories, FL, USA) for 30 consecutive days as previously described (Gokce et al. 1999). Chronic supplementation started at least 72 h after the acute administration. Compliance was established by subject logs that were completed each day and by measuring plasma ascorbic acid concentration 2 weeks after starting the treatment.
Statistical analyses
Differences in subject characteristics across the three groups were determined by ANOVA. To determine the effect of acute and long-term ascorbic acid administration on all outcome measures, repeated measures ANOVA was used. In the case of a significant F-value, a post hoc test using the Newman-Keuls method identified significant differences among mean values. Univariate correlation analyses were performed to examine relations between variables of interest.
Results
Subject characteristics
Values are shown in Table 1. The groups did not differ in body weight, body mass index, systolic blood pressure, heart rate, or plasma HDL cholesterol, insulin, glucose, or adrenaline concentrations. Body fat and plasma endothelin-1 were greater, and V̇O2,max was lower in the older sedentary men (P < 0.05). Plasma oxidized low-density lipoprotein tended to be higher in the older sedentary men (77 ± 8 U l−1) than in the young (65 ± 3 U l−1, P= 0.07) and older trained (72 ± 6 U l−1, P= 0.19) men. Diastolic blood pressure, and plasma noradrenaline and total and LDL cholesterol concentrations were (P < 0.05) or tended to be greater in the older groups compared with the young controls.
Table 1.
Subject characteristics
| Young sedentary (n= 11) | Older sedentary (n= 9) | Older endurance- trained (n= 9) | |
|---|---|---|---|
| Body mass (kg) | 79 ± 3 | 83 ± 3 | 75 ± 3 |
| Body fat (%) | 20 ± 1 | 26 ± 2* | 22 ± 2† |
| BMI (kg m−2) | 23.5 ± 0.8 | 25.9 ± 1.0 | 24.6 ± 0.6 |
| Systolic BP (mmHg) | 113 ± 2 | 116 ± 4 | 113 ± 3 |
| Diastolic BP (mmHg) | 63 ± 2 | 73 ± 4* | 71 ± 2* |
| Heart rate (b.p.m.) | 56 ± 3 | 54 ± 2 | 50 ± 4 |
| Total cholesterol (mmol l−1) | 4.5 ± 0.2 | 5.1 ± 0.2 | 5.4 ± 0.3* |
| LDL cholesterol (mmol l−1) | 2.7 ± 0.2 | 3.1 ± 3 | 3.5 ± 0.2* |
| HDL cholesterol (mmol l−1) | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.1 |
| Plasma insulin (µU ml−1) | 5.7 ± 0.6 | 4.6 ± 0.7 | 4.6 ± 0.2 |
| Plasma glucose (mmol l−1) | 5.0 ± 0.1 | 5.2 ± 0.2 | 5.4 ± 0.2 |
| Plasma noradrenaline (pg ml−1) | 151 ± 16 | 245 ± 42* | 329 ± 38* |
| Plasma adrenaline (pg ml−1) | 43 ± 13 | 37 ± 13 | 72 ± 30 |
| Plasma endothelin-1 (pg ml−1) | 5.8 ± 0.3 | 6.9 ± 0.3* | 6.4 ± 0.5 |
| V̇O2,max (ml kg−1 min−1) | 48 ± 1 | 32 ± 1* | 40 ± 2*† |
Data are means ±s.e.m. BMI, body mass index; BP, blood pressure; V̇O2,max maximal oxygen consumption.
P < 0.05 versus young;
P < 0.05 versus older sedentary.
Ascorbic acid concentrations
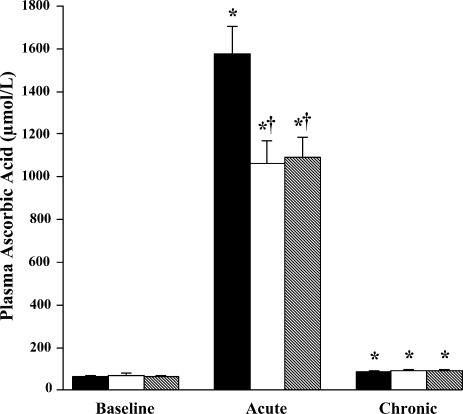
Values are presented in Fig. 1. At baseline, there were no group differences in plasma ascorbic acid concentrations. During infusion, plasma ascorbic acid concentration increased (P < 0.0001) > 15-fold above baseline to supra-physiological levels in all groups; the increases were somewhat smaller (P < 0.05) in the two older groups. At the end of the period of oral supplementation, plasma concentrations of ascorbic acid measured 12 h after ingestion were modestly elevated compared with baseline concentrations in all groups (P < 0.0001).
Figure 1. Plasma ascorbic acid concentrations.
Plasma ascorbic acid concentrations are shown at baseline, after acute ascorbic acid infusion, and after 30 days of ascorbic acid supplementation. Filled bars: young sedentary; open bars: older sedentary; and hatched bars: older endurance trained. Acute = post-infusion; Chronic = end of oral supplementation. * P < 0.0001 versus baseline within the same group; † P < 0.01 versus young within the same condition.
Vasodilatory responses to ascorbic acid infusion and oral supplementation
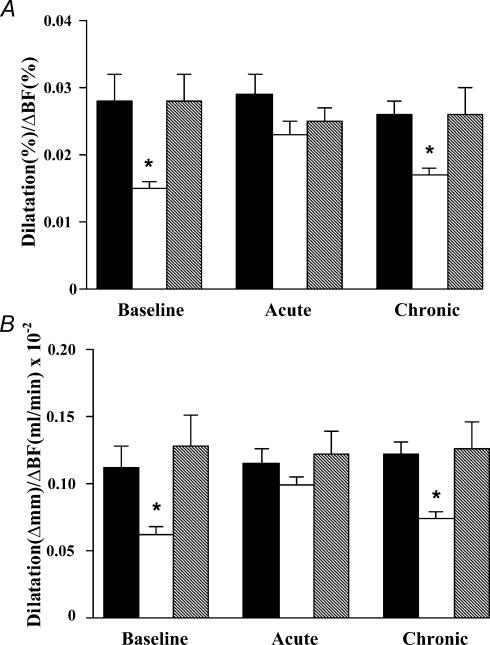
Brachial artery diameter prior to inducing the hyperaemic stimulus did not differ among the groups at baseline, and group values after acute and chronic ascorbic acid administration were not different from baseline (Table 2). Individual and group values for brachial artery diameters at baseline before and after the hyperaemic stimulus are shown in Figs 2 and 3. Mean group values for brachial artery FMD (normalized for the hyperaemic stimulus) at baseline, during ascorbic acid infusion, and after ascorbic acid supplementation are shown in Fig. 4, and absolute (Δmm) and percentage values of FMD are shown in Table 2. At baseline, normalized FMD was ∼45% lower in the older sedentary men compared with the young controls (P < 0.01). In contrast, baseline FMD was similar in the older exercise-trained and young men. Ascorbic acid infusion markedly augmented FMD in the older sedentary men (P < 0.005 versus baseline), but had no effect in the young and older exercise-trained men. Ascorbic acid infusion abolished the age- and habitual exercise-related differences in FMD (P= 0.13–0.60). At the end of the oral supplementation period, FMD was similar to baseline values in all three groups.
Table 2.
Brachial artery parameters and hyperaemic flow
| Parameter | BA diameter (mm) | FMD (Δmm) | FMD (%) | HF (% increase) | EID (%) | |
|---|---|---|---|---|---|---|
| Young sedentary | Baseline | 4.1 ± 0.1 | 0.33 ± 0.02 | 8.1 ± 0.5 | 314 ± 21 | 20.7 ± 0.9 |
| Acute | 4.1 ± 0.1 | 0.34 ± 0.02 | 8.2 ± 0.6 | 302 ± 26 | 20.8 ± 1.0 | |
| Chronic | 4.1 ± 0.1 | 0.31 ± 0.02 | 7.8 ± 0.3 | 330 ± 26 | 21.3 ± 1.0 | |
| Older sedentary | Baseline | 4.3 ± 0.1 | 0.20 ± 0.01* | 4.6 ± 0.2* | 324 ± 31 | 20.8 ± 0.8 |
| Acute | 4.3 ± 0.1 | 0.29 ± 0.02† | 6.7 ± 0.3*‡ | 291 ± 24 | 20.6 ± 0.8 | |
| Chronic | 4.3 ± 0.1 | 0.22 ± 0.03* | 5.4 ± 0.4* | 325 ± 18 | 18.8 ± 1.1 | |
| Older trained | Baseline | 4.0 ± 0.1 | 0.28 ± 0.02† | 7.0 ± 0.6† | 273 ± 17 | 21.7 ± 2.2 |
| Acute | 4.1 ± 0.1 | 0.30 ± 0.01 | 7.3 ± 0.3 | 306 ± 23 | 21.4 ± 1.7 | |
| Chronic | 4.0 ± 0.1 | 0.28 ± 0.01† | 7.0 ± 0.4† | 305 ± 38 | 22.5 ± 1.9 |
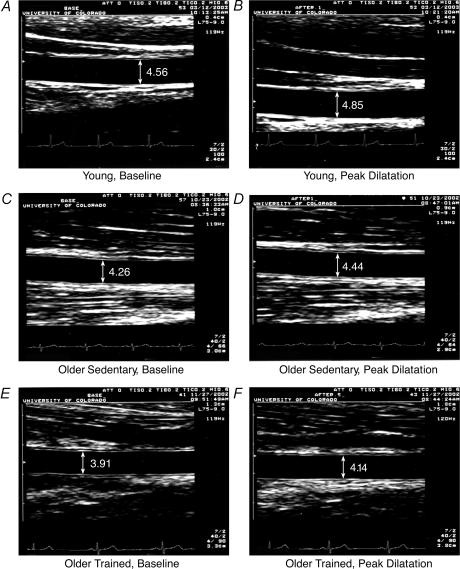
Figure 2. Brachial artery diameters in representative men.
Brachial artery diameters are shown before (baseline, A, C and E) and during peak flow-mediated dilatation (B, D and F), in representative young sedentary (A and B), older sedentary (C and D), and older exercise-trained (E and F) men.
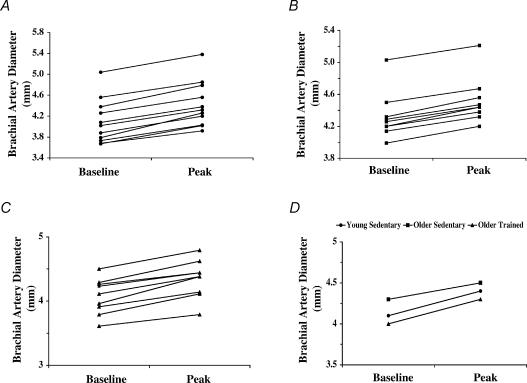
Figure 3. Brachial artery diameters before and after the hyperemic stimulus.
Brachial artery diameters before (baseline) and during (peak) flow-mediated dilatation in the young sedentary (A), older sedentary (B), and older exercise-trained (C) men. D, mean brachial artery diameters before (baseline) and during (peak) flow-mediated dilatation for the young sedentary, older sedentary, and older exercise-trained groups.
Figure 4. Brachial artery flow-mediated dilatation.
Brachial artery flow-mediated dilatation is shown (normalized for the hyperaemic flow stimulus) in young and older sedentary men and older endurance exercise-trained men at baseline, after acute ascorbic acid infusion, and after 30 days of ascorbic acid supplementation. A, percentage change in diameter divided by percentage change in blood flow; B, absolute (mm) change in diameter divided by absolute (ml min−1) change in blood flow. Mean ±s.e.m. values are shown. Filled bars: young sedentary; open bars: older sedentary; and hatched bars: older endurance trained. Acute = postinfusion; Chronic = end of oral supplementation. * P < 0.01 versus young and older trained groups.
There were no group differences in the vasodilatory response to NTG at baseline, during ascorbic acid infusion, or after chronic ascorbic acid supplementation (Table 2). Arterial blood pressure, heart rate, and plasma concentrations of catecholamines and endothelin-1 did not change from baseline levels in response to acute or chronic ascorbic acid administration (data not shown).
Physiological correlates of FMD with primary ageing
There were no significant relations between FMD normalized for the hyperaemic stimulus and any subject characteristic or baseline cardiovascular function.
Discussion
The present study produced several novel findings that extend our current understanding of the decrease in conduit artery FMD with ageing in adult humans. First, the impairment in brachial artery FMD with age in sedentary men appears to be mediated by increased vascular oxidative stress. Second, older men who regularly perform vigorous aerobic-endurance exercise demonstrate FMD similar to that of young men. Third, the preserved FMD in older exercise-trained men appears to be mediated by a reduced level of vascular oxidative stress. Finally, chronic oral ascorbic acid supplementation of 500mg day−1 does not improve the impaired baseline brachial artery FMD of older sedentary men.
Mechanism underlying impaired FMD with primary ageing in adult humans
In the present study we found that sedentary men aged 60–79 years without clinical disease have only about one-half of the capacity for peripheral conduit artery FMD observed in young adult males. This marked reduction in FMD with sedentary ageing appears to reflect a reduced ability to produce vascular endothelium-dependent vasodilatation in that: (1) the responses were normalized for the magnitude of the dilatory hyperaemic stimulus; and (2) the vascular endothelium-independent dilatation evoked by sublingual NTG did not differ in the young and older sedentary men.
Ascorbic acid is a potent antioxidant that scavenges superoxide anions and other reactive oxygen species at supraphysiological concentrations such as those produced by the infusions in the present investigation (Frei et al. 1989; Jackson et al. 1998). Consistent with this, recently (Bell et al. 2003) our laboratory demonstrated that the same intravenous infusion of ascorbic acid as used in the present study decreases plasma isoprostanes, a biomarker of lipid oxidation-associated oxidative stress, in older healthy men similar to those studied here. As such, our finding that supra-physiological concentrations of ascorbic acid reversed the baseline impairment in FMD in the older sedentary men implicates increased oxidative stress acting on the vascular endothelium as the key mechanism involved. This is the first experimental evidence indicating the involvement of this mechanism in the striking reduction in peripheral conduit artery FMD with primary ageing in adult humans. Our results suggest that the impairments in peripheral conduit artery FMD with ageing and cardiovascular disorders such as coronary artery disease (Gokce et al. 1999) and congestive heart failure (Hornig et al. 1998) may share this common mechanism. The present results also are consistent with previous observations that ascorbic acid reverses the reduced forearm blood flow response to acetylcholine, a measure of endothelium-dependent dilatation of arterial resistance vessels, in older sedentary men (Taddei et al. 2000).
In the present study baseline plasma concentrations of oxidized low-density lipoprotein, an indirect estimate of oxidative stress (Holvoet et al. 1998), were ∼20% higher in our older compared with our young sedentary men, whereas less of a difference was observed between the two older groups. It is important to emphasize the limited sensitivity of such plasma markers in establishing differences in oxidative stress among groups, or in response to oxidative stress-altering perturbations such as acute antioxidant administration. Although these markers often (although not always) demonstrate chronic differences between groups with pathophysiological levels of oxidative stress, such as patients with cardiovascular diseases or type 2 diabetes, and healthy controls (Maggi et al. 1994), it is more difficult to show quantitative differences among groups of healthy adults at baseline and/or in response to acute changes in oxidative stress.
Preservation of FMD with age in habitually exercising men
Greater brachial artery FMD has been reported in habitually exercising compared with sedentary middle-aged and older adults (Rywik et al. 1999; Rinder et al. 2000). The present results showing a 47% greater baseline FMD in our exercising than in our sedentary older men are consistent with these earlier findings. Interestingly, as in previous investigations we found that brachial artery FMD was augmented in subjects habitually performing exercise with the legs, suggesting a systemic rather than a strictly ‘local’ peripheral vascular adaptation. The increased brachial artery FMD in the older trained men may be mediated by the elevations in systemic pulsatile blood flow generated during their exercise training sessions (DeSouza et al. 2000). Such repetitive periods of augmented pulsatile flow produce corresponding increases in systemic arterial shear stress, the primary stimulus for endothelial nitric oxide synthase (eNOS) expression (Nakano et al. 2000). Up-regulation of conduit artery eNOS would presumably increase NO bioavailability and FMD. Importantly, brachial artery dilatation in response to sublingual NTG was similar in the exercising and sedentary older adults in these earlier studies as well as the present investigation, indicating that the augmented FMD in the exercising older men is mediated by the vascular endothelium.
Our results extend these prior observations in two important ways. First, we demonstrated that the brachial artery FMD of older exercising men was similar to that observed in young men, suggesting the maintenance of this key endothelium-dependent vasodilatory function with physically active ageing. This finding supports the idea that regular aerobic endurance exercise may be an effective lifestyle intervention to prevent the marked decline in FMD with primary ageing. The present observations complement our earlier findings (DeSouza et al. 2000) and those of others (Taddei et al. 2000) showing preserved forearm blood flow responses to intra-arterial acetylcholine in older exercising men.
Second, the present results indicate that the mechanism underlying the maintenance of brachial artery FMD with age in habitually exercising men involves reduced vascular oxidative stress. This is based on the fact that FMD was not augmented by ascorbic acid infusion in the older endurance-trained men. Earlier observations on the effects of ascorbic acid in the preserved forearm blood flow responses to acetylcholine in older athletes indicate a similar influence on peripheral resistance vessels (Taddei et al. 2000). The exact mechanism(s) by which habitual endurance exercise suppresses oxidative stress have not been determined. However, exercise training has been associated with both reduced production of reactive oxygen species (Leeuwenburgh & Heinecke, 2001) and augmented antioxidant defences (Sen, 1995).
Potential physiological basis for oxidative stress suppression of FMD with sedentary ageing and preservation of FMD with physically active ageing
Endothelial-derived NO (Joannides et al. 1995; Lieberman et al. 1996), vasodilator prostanoids (i.e, prostacyclins) (Koller et al. 1993), and endothelial-derived hyperpolarizing factor (EDHF) (Miura et al. 2001) all may contribute to FMD, with NO having by far the largest effect. As such, the most likely explanation as to how sedentary ageing suppresses FMD is via the development of excessive vascular superoxide anion and other ROS bioavailability (oxidative stress) (van der Loo et al. 2000; Hamilton et al. 2001; Csiszar et al. 2002), which causes increased scavenging of NO (Beckman et al. 1990) while also possibly inhibiting prostacyclin synthesis (Camacho et al. 1998). Although the nature of EDHF is still unknown, H2O2 (hydroxyl peroxide), a ROS formed primarily from superoxide anions by the action of Superoxide dismutase (SOD), appears to function as an EDHF (Matoba et al. 2000). Therefore, in the face of marked superoxide bioavailabilty the depressed activity of SOD associated with sedentary ageing could decrease the formation of H2O2 and its vasodilatory effect. In contrast, regular aerobic endurance exercise appears to be associated with reduced or absent oxidative stress in middle-aged and older adults, possibly mediated by increased SOD and other antioxidant system activity. If so, this should result in a local vascular environment characterized by less destruction of NO and possibly greater formation of prostacyclins and the EDHF H2O2, thus explaining the preserved FMD with physically active ageing.
Effects of chronic oral ascorbic acid supplementation
We found that in contrast to acute infusion, 500mg day−1 oral supplementation of ascorbic acid did not augment brachial artery FMD in older sedentary men with impaired baseline function. We believe that this is most likely explained by the differences in plasma concentrations of ascorbic acid. Our acute infusions produced plasma concentrations known to scavenge superoxide anions in vitro (Jackson et al. 1998), whereas our oral supplementation did not (Fig. 1). Previous findings of improved FMD in patients with coronary artery disease (Gokce et al. 1999) with the same supplemental dose could be the result of higher baseline oxidative stress compared with our healthy older men. However, it is also important to emphasize that similar to the present findings, 500mg day−1 supplementation of ascorbic acid has no effect on the impaired FMD in patients with essential hypertension (Duffy et al. 2001). It is unlikely that our oral dose of ascorbic acid was simply insufficient because doses above that used here do not result in greater plasma concentrations of ascorbic acid (Levine et al. 1996b).
Conclusions
The results of the present investigation provide the first evidence for an important role of oxidative stress in both the marked impairment in peripheral conduit artery FMD associated with sedentary human ageing and the preservation of FMD with physically active ageing. As such, our findings provide strong support for the concept that reducing oxidative stress through regular exercise and other approaches should be a major goal in the prevention of vascular endothelial dysfunction with ageing.
Acknowledgments
This work was supported by National Institutes of Health Awards AG06537, AG13038, AG19365, AG00828 and RR00051.
References
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C, Jones PP, Seals DR. Oxidative stress does not modulate metabolic rate or skeletal muscle sympathetic activity with primary aging in adult humans. J Clin Endocrinol Metab. 2003;88:4950–4954. doi: 10.1210/jc.2003-030454. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- Camacho M, Lopez-Belmonte J, Vila L. Rate of vasoconstrictor prostanoids released by endothelial cells depends on cyclooxygenase-2 expression and prostaglandin I synthase activity. Circ Res. 1998;83:353–365. doi: 10.1161/01.res.83.4.353. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Desouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol. 2002;542:255–262. doi: 10.1113/jphysiol.2002.019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Drexler H, Hayoz D, Munzel T, Hornig B, Just H, Brunner HR, Zelis R. Endothelial function in chronic congestive heart failure. Am J Cardiol. 1992;69:1596–1601. doi: 10.1016/0002-9149(92)90710-g. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Gokce N, Holbrook M, Hunter LM, Biegelsen ES, Huang A, Keaney JF, Jr, Vita JA. Effect of ascorbic acid treatment on conduit vessel endothelial dysfunction in patients with hypertension. Am J Physiol Heart Circ Physiol. 2001;280:H528–H534. doi: 10.1152/ajpheart.2001.280.2.H528. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Seals DR, DeSouza CA, Tanaka H. Pharmacologic versus flow-mediated assessments of peripheral vascular endothelial vasodilatory function in humans. Am J Cardiol. 2001;88:1067–1069. doi: 10.1016/s0002-9149(01)01997-x. [DOI] [PubMed] [Google Scholar]
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- Gokce N, Keaney JF, Jr, Frei B, Holbrook M, Olesiak M, Zachariah BJ, Leeuwenburgh C, Heinecke JW, Vita JA. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1999;99:3234–3240. doi: 10.1161/01.cir.99.25.3234. [DOI] [PubMed] [Google Scholar]
- Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, Mcintyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- Hayoz D, Drexler H, Munzel T. Flow-mediated arterial dilation is abnormal in congestive heart failure. Circulation. 1993;87(VII):92–96. [Google Scholar]
- Holvoet P, Vanhaecke J, Janssens S, Van De Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–1494. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- Hornig B, Arakawa N, Kohler C, Drexler H. Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation. 1998;97:363–368. doi: 10.1161/01.cir.97.4.363. [DOI] [PubMed] [Google Scholar]
- Jackson TS, Xu A, Vita JA, Keaney JF., Jr Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- Koller A, Sun D, Kaley G. Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circ Res. 1993;72:1276–1284. doi: 10.1161/01.res.72.6.1276. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a ‘set up’ for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Heinecke JW. Oxidative stress and antioxidants in exercise. Curr Med Chem. 2001;8:829–838. doi: 10.2174/0929867013372896. [DOI] [PubMed] [Google Scholar]
- Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996b;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF, Jr, Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1996a;93:1107–1113. doi: 10.1161/01.cir.93.6.1107. [DOI] [PubMed] [Google Scholar]
- Lieberman EH, Gerhard MD, Uehata A, Selwyn a P, Ganz P, Yeung AC, Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–1214. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- Maggi E, Chiesa R, Melissano G, Castellano R, Astore D, Grossi A, Finardi G, Bellomo G. LDL oxidation in patients with severe carotid atherosclerosis. A study of in vitro and in vivo oxidation markers. Arterioscler Thromb. 1994;14:1892–1899. doi: 10.1161/01.atv.14.12.1892. [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca2+-activated K+ channels. Circulation. 2001;103:1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer R, Higgs E. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Nakano T, Tominaga R, Nagano I, Okabe H, Yasui H. Pulsatile flow enhances endothelium-derived nitric oxide release in the peripheral vasculature. Am J Physiol Heart Circ Physiol. 2000;278:H1098–H1104. doi: 10.1152/ajpheart.2000.278.4.H1098. [DOI] [PubMed] [Google Scholar]
- Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–210. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- Peuler JD, Johnson GA. Simultaneous single isotope radioenzymatic assay of plasma norepinephrine, epinephrine and dopamine. Life Sci. 1977;21:625–636. doi: 10.1016/0024-3205(77)90070-4. [DOI] [PubMed] [Google Scholar]
- Rinder MR, Spina RJ, Ehsani a A. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol. 2000;88:761–766. doi: 10.1152/jappl.2000.88.2.761. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium derived relaxing factor. Am J Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Rywik TM, Blackman MR, Yataco AR, Vaitkevicius PV, Zink RC, Cottrell EH, Wright JG, Katzel LI, Fleg JL. Enhanced endothelial vasoreactivity in endurance-trained older men. J Appl Physiol. 1999;87:2136–2142. doi: 10.1152/jappl.1999.87.6.2136. [DOI] [PubMed] [Google Scholar]
- Sen CK. Oxidants and antioxidants in exercise. J Appl Physiol. 1995;79:675–686. doi: 10.1152/jappl.1995.79.3.675. [DOI] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]