Abstract
An electromyographic (EMG) activity pattern for individual muscles in the gait cycle exhibits a great deal of intersubject, intermuscle and context-dependent variability. Here we examined the issue of common underlying patterns by applying factor analysis to the set of EMG records obtained at different walking speeds and gravitational loads. To this end healthy subjects were asked to walk on a treadmill at speeds of 1, 2, 3 and 5kmh−1 as well as when 35–95% of the body weight was supported using a harness. We recorded from 12–16 ipsilateral leg and trunk muscles using both surface and intramuscular recording and determined the average, normalized EMG of each record for 10–15 consecutive step cycles. We identified five basic underlying factors or component waveforms that can account for about 90% of the total waveform variance across different muscles during normal gait. Furthermore, while activation patterns of individual muscles could vary dramatically with speed and gravitational load, both the limb kinematics and the basic EMG components displayed only limited changes. Thus, we found a systematic phase shift of all five factors with speed in the same direction as the shift in the onset of the swing phase. This tendency for the factors to be timed according to the lift-off event supports the idea that the origin of the gait cycle generation is the propulsion rather than heel strike event. The basic invariance of the factors with walking speed and with body weight unloading implies that a few oscillating circuits drive the active muscles to produce the locomotion kinematics. A flexible and dynamic distribution of these basic components to the muscles may result from various descending and proprioceptive signals that depend on the kinematic and kinetic demands of the movements.
Electromyograph (EMG) recordings have shown that the patterns of activity in various muscles that are active in locomotion may exhibit high step-by-step variability. Nevertheless, when activity is ensemble-averaged over a number of steps, each of 25 lower limb and trunk muscles has a characteristic average activity pattern over a locomotion step cycle, and it is similar across normal subjects (Winter, 1991). These average activity patterns appear to be different for each muscle (see Fig. 2), although certain features, like a burst of activity at heel strike, are common to many muscles. In fact there is analytical evidence that many muscles may share certain activity patterns (Shiavi & Griffin, 1981; Wootten et al. 1990; Winter, 1991; Yakovenko et al. 2002).
Figure 2. Activity patterns during a single locomotion step cycle.
Averaged EMG activity recorded from 18 subjects for 25 muscles during a single cycle of over-ground locomotion. EMG records were filtered with a low-pass cut-off of 3Hz. Data taken from Winter (1991); abbreviations are defined in Methods.
This was investigated by Patla (1985) and later by Vaughan and colleagues (Davis & Vaughan, 1993; Olree & Vaughan, 1995) in studies that applied principal component analysis (PCA) to determine whether the main features of the EMG patterns could be described by a few underlying components. Patla applied the technique to EMG recordings from six limb muscles and one trunk muscle. The result implied that relatively few activity patterns could, in appropriate combination, lead to the observed motor activity. Davis & Vaughan (1993) later applied the technique to the average intersubject data of Winter (1991), and showed that various combinations of four underlying basic patterns could account for the EMG activity of 16 leg muscles during locomotion. Olree & Vaughan (1995) recorded EMGs bilaterally from eight leg muscles, and showed that three basic patterns could account for the locomotion activity of these muscles. They suggested therefore that the complex muscle activity patterns observed during locomotion may be controlled by just a few underlying activity pattern generators.
This interpretation seems to support the popular idea that a central pattern generator (CPG) circuitry acting on each limb during locomotion activates the flexor and extensor muscles alternately. The underlying mechanisms of locomotion are undoubtedly more complex than this because the CPG alone cannot account for the variety of EMG patterns noted above, nor can it account for reflex or triggered activity occurring in response to peripheral stimuli during locomotion (Grillner, 1981; Winter, 1989; Rossignol, 1996; Duysens & Van de Crommert, 1998; Pearson et al. 1998; Barbeau et al. 1999; Orlovsky et al. 1999).
Another recent body of evidence supports the idea that global limb kinematics are controlled during locomotion (Shen & Poppele, 1995; Borghese et al. 1996; Grasso et al. 1998; Lacquaniti et al. 1999, 2002). Limb kinematics are relatively invariant in various modes of locomotion, while the muscle activity patterns required to produce those kinematic patterns can vary considerably (Winter & Yack, 1987; Trank et al. 1996; Grasso et al. 1998, 2000; Ivanenko et al. 2002). Such results have led to the suggestion that the neural circuitry may in some way specify the limb kinematics (Lacquaniti et al. 1999, 2002). If so, the muscle activation patterns must be derived in some way from a kinematics control signal in accordance with the kinetic requirements of the biomechanical system. That is, a basic kinematics control signal may exert its action via an appropriate ‘inverse dynamics model’ and peripheral feedback that determines the muscle torques required to achieve the kinematic goals.
There is ample evidence favouring the existence of some form of inverse model in motor control (Kawato, 1999) and there are various proposals regarding the location of the circuitry that may carry out this function. It is clear however, that if a form of inverse model is operating during locomotion, then the underlying circuitry must be located in the spinal cord, at least in the cat. Spinalized cats are capable of supported locomotion on a treadmill, while exhibiting quasi-normal kinematic movement patterns (Belanger et al. 1996; de Leon et al. 1998). Thus the same kind of kinematic invariance noted above for human locomotion is found to be a property of the spinal circuitry in the cat. There are also some indications that the isolated human spinal cord can interpret both foot loading and translation during walking (Harkema et al. 1997; Duysens et al. 2000).
The results presented in earlier human locomotion studies (Patla, 1985; Davis & Vaughan, 1993; Olree & Vaughan, 1995), and also the results regarding limb movements in frogs (Bizzi et al. 2000; Kargo & Giszter, 2000; d'Avella et al. 2003), could provide a basis for understanding the relationship between kinematics and kinetics in locomotion. The results of both types of study suggest that a few basic muscle activity patterns, referred to as motor primitives in the frog, may be distributed to the motoneurones with different weightings. In the case of locomotion, the basic patterns may be distributed to all the muscles activated during locomotion and therefore represent a global control signal. It should be noted that individual EMG patterns are likely to contain components reflecting global control variables as well as local feedback, whereas the activity patterns that are common across muscles are more likely to reflect the global aspects. If the basic patterns are also invariant under conditions in which global kinematics are invariant, and muscle activation patterns are not, then it may indicate a possible link to kinematics control.
We investigated this possibility by extending the work of Patla, and Vaughan and colleagues, to include a larger sample of muscles. We also examined muscle activation in conditions where muscle activation patterns can be different from those observed in normal walking, yet the limb kinematics are similar. We found that five component activity patterns could fully account for the patterns seen in up to 25 limb and trunk muscles during locomotion, and similar component patterns were observed with different locomotion speeds and under weight-supported conditions. A preliminary report of these findings has been presented (Poppele et al. 2002).
Methods
Subjects
Six healthy subjects (4 males and 2 females, between 26 and 42 years of age, 66 ± 12 kg (mean ±s.d.), 1.70 ± 0.08 m) volunteered for the experiments. The studies conformed to the Declaration of Helsinki, and informed consent was obtained from all participants according to the protocol of the Ethics Committee of the Santa Lucia Institute.
Experimental setup
The experiments were carried out on a treadmill (EN-Mill 3446.527, Bonte Zwolle BV, The Netherlands) at different speeds. The walking surface of the treadmill is 1.5 m long, 0.6 m wide, and 0.15 m above the ground. Body weight support (BWS) was obtained by supporting the subjects in a harness connected to a pneumatic device that applied a controlled upward force (Ivanenko et al. 2002). The overall constant error in the force applied to a subject and dynamic force fluctuations monitored by the load cell have been estimated to be less than 5% of body weight (Gazzani et al. 2000).
Kinematic data were recorded at 100Hz by means of the Vicon-612 system. The spatial accuracy of the system is better than 1 mm (root mean square). Nine TV cameras were spaced around the treadmill. Five infrared reflective markers were attached on the right side of the subject to the skin overlying the following landmarks: the midpoint between the anterior and the posterior superior iliac spine (ilium, IL), greater trochanter (GT), lateral femur epicondyle (LE), lateral malleolus (LM), and fifth metatarso-phalangeal joint (VM).
We recorded from 12–16 muscles in each of six normal subjects. We recorded from slightly different sets of muscles in the six subjects (see Table 1). The following nine muscles were recorded from all six subjects: tibialis anterior (TA), gastrocnemius lateralis (LG), peroneus longus (PERL), vastus lateralis (VL), rectus femoris (RF), sartorius (SART), biceps femoris (long head, BF), semitendinosus (ST), tensor fascia latae (TFL). The following three muscles were recorded from five subjects: adductor longus (ADD), gluteus maximus (GM), and erector spinae (ES, recorded at L1–L2). Rectus abdominis, middle and superior portions (RAM or RAS, respectively) was recorded in four subjects, and the following five muscles were recorded from three subjects: soleus (Sol), gastrocnemius medialis (MG), external oblique (OE), gluteus medius (Gmed) and latissimus dorsi (LD); trapezius (TRAP) was recorded in one subject. The activity was recorded using active Delsys electrodes (model DE2.1, Delsys Inc., Boston, MA, USA) applied to lightly abraded skin over the respective muscle belly. Electrode placement was carefully chosen so as to minimize cross-talk from adjacent muscles during maximal isometric contractions. The signals were amplified (× 10 000), filtered (20–450Hz) (Bagnoli 16, Delsys Inc.) and sampled at 1000Hz. Sampling of kinematic and EMG data were synchronized.
Table 1.
Muscles recorded for each subject
| Muscle | GB | LE | GC | GM | RL | YI |
|---|---|---|---|---|---|---|
| Tibialis anterior (TA) | X | X | X | X | X | X |
| Gastrocnemius lateralis (LG) | X | X | X | X | X | X |
| Peroneus longus (PERL) | X | X | X | X | X | X |
| Vastus lateralis (VL) | X | X | X | X | X | X |
| Rectus femoris (RF) | X | X | X | X | X | X |
| Sartorius (SART) | X | X | X | X | X | X |
| Biceps femoris (BF) | X | X | X | X | X | X |
| Semitendinosus (ST) | X | X | X | X | X | X |
| Tensor fascia latae (TFL) | X | X | X | X | X | X |
| Adductor longus (ADD) | X | X | X | X | X | |
| Gluteus maximus (GM) | X | X | X | X | X | |
| Erector spinae (ES) | X | X | X | X | X | |
| Soleus (Sol) | X | X | X | |||
| Rectus abdominis (superior) (RAS) | X | X | X | X | ||
| External oblique (OE) | X | X | X | |||
| Gastrocnemius medialis (MG) | X | X | X | |||
| Gluteus medius (Gmed) | X | X | X | |||
| Rectus abdominis (middle) (RAM) | X | X | ||||
| Latissimus dorsi (LD) | X | X | X | |||
| Trapezius (TRAP) | X |
In addition we recorded intramuscular EMGs in one subject (YI) to control the effectiveness of cross-talk rejection by our surface recordings (see below). We used a single-needle technique (Basmajian & De Luca, 1985) to record activity within the following nine muscles: LG, TA, PERL, VL, RF, SART, BF, ST and TFL. Surface EMG electrodes were also placed within 2–3 cm of the intramuscular insertion points along the direction of muscle fibres. Before the wire electrodes were inserted, the subject was instructed about how to selectively activate each muscle (Kendall et al. 1993), while EMG signals were monitored.
The intramuscular electrodes consisted of two 50µm-diameter, heavy polyamide-coated, nickel–chromium alloy wires (Stablohm 800B + ML insulation; California Fine Wire, Grover Beach, CA, USA) connected to Delsys differential preamplifiers taped to the adjacent skin. Twisted wire pairs were threaded through a 27 gauge hypodermic needle that was used to insert the wires, and subsequently withdrawn to leave the wires in place. Each wire extended several millimeters beyond the tip of the needle, and the insulation was removed from the terminal 1.5–2 mm of each wire. The wires were bent back to form hooks on the opposite sites of the shaft in order to prevent their direct contact and to provide a desirable orientation of the electrodes along the muscle fibres. The recording system bandwidth was 20–1000Hz with an overall gain of 1000; signals were digitized at 2000Hz.
Protocol
Subjects were required to walk on a treadmill, which was driven at four different speeds: 1, 2, 3 and 5kmh−1. Presentation order was randomized across experiments. They were also placed in a harness so that 35, 50, 75 or 95% of their body weight could be supported by a pneumatic lift. At 95% BWS, subjects were almost completely unloaded but still able to step on the treadmill during the stance phase though measurable contact forces could only be detected at the forefoot (Ivanenko et al. 2002). Before the recording session, subjects practiced for a few minutes in walking on the treadmill at different speeds and BWS levels. Subjects were asked to place the abducted arms on horizontal rollbars located at the side of the treadmill, at breast height. Each trial for a given condition included 10–15 consecutive gait cycles.
Data analysis
The body was modelled as an interconnected chain of rigid segments: IL–GT for the pelvis, GT–LE for the thigh, LE–LM for the shank, and LM–VM for the foot. The elevation angle of each segment in the sagittal plane corresponds to the angle between the projected segment and the vertical. These angles are positive in the forward direction (i.e. when the distal marker is located anterior to the proximal marker). The limb axis was defined as GT–LM. Gait cycle was defined as the time between two successive maxima of the limb axis elevation angle. The time of maximum and minimum elevation of the limb axis roughly corresponds to heel contact and toe-off (stance to swing transition), respectively (Borghese et al. 1996). They were used to identify ‘stance’ and ‘swing’ phases.
We determined the average, normalized EMG of each record for 10–15 consecutive step cycles. The rectified EMG records were low-pass filtered using a zero-lag Butterworth filter with a cut-off of 15Hz. Data were time-interpolated over individual gait cycles to fit a normalized 200-point time base.
Factor analysis
We applied a principal component analysis to each of several data sets consisting of normalized EMG patterns over a step cycle (using the ipsilateral heel strike as the origin). In factor analysis, the basic waveforms are not specified in advance as in a Fourier series expansion. Instead they are determined by the structure of the data waveforms. The steps involve calculation of the correlation matrix, extraction of the initial principal components, application of the varimax rotation, calculation of factor scores and interpretation of the results. The principal components (PCs) were expressed using a varimax rotation in order to minimize the number of variables with high loadings on each component factor (Kaiser, 1974). This has the effect of simplifying the interpretation of the PCs since the waveforms of the rotated factors are more similar to those of the EMGs than are the basic PC waveforms (Davis & Vaughan, 1993; Chau, 2001). While the first PC in each case accounted for between 30 and 45% of the total waveform variance in the data set, the most significant varimax factors in each case explained between 20 and 30%.
The appropriate application of factor analysis involves an initial estimate of (a) the extent to which each data waveform is composed of components common to other data waves, the communality, and (b) the extent to which activity is specific to each wave alone, the uniqueness (Glaser & Ruchkin, 1976). We can think of EMG waveforms as being dependent on two aspects. First, there are some underlying common waveforms shared by the muscles. Second, each muscle also captures a unique aspect of activation that is not addressed by any other muscle.
There are different methods to assess whether the dataset is adequate for factor analysis: communalities, the Bartlett's test of sphericity and the Kaiser-Meyer-Olkin (KMO) measure. In the language of factor analysis, the communality is the proportion of variance of a particular variable that is due to common factors shared with other variables. Therefore, a common starting point to the application of factor analysis is to use the squared multiple correlation of an item with all other items as an estimate of the communality. For the data to be adequate for factor analysis, the mean communality should be quite high. The Bartlett's test of sphericity tests the hypothesis that the correlation matrix comes from a population in which the variables are independent (i.e. an identity matrix). Rejection of the independence hypothesis is an indication that the data are adequate to factor analysis. The KMO measure compares the magnitudes of the observed correlation coefficients to the magnitudes of the partial correlation coefficients. This indicator should be 0.5 or greater. Smaller values indicate that factor analysis is not a good choice (Kaiser, 1974; Merkle et al. 1998; Sabatini, 2002).
Another aspect of factor analysis is how much of the variance in the data set has to be accounted for. Eigenvectors with the corresponding eigenvalues less than unity are usually considered to describe noise (Davis & Vaughan, 1993; Sabatini, 2002). Therefore, we can retain only factors with eigenvalues greater than 1. In essence this is like saying that, unless a factor extracts at least as much as the equivalent of one original variable, we drop it. This criterion was proposed by Kaiser (1974), and is probably the one most widely used. In practice, an additional important aspect is the extent to which a solution is interpretable. Therefore, one usually examines several solutions with more or fewer factors, and chooses the one that makes the best ‘sense’. In our study, for principal component analysis we accepted eigenvectors with the corresponding eigenvalues higher than 0.5. This criterion increased slightly the number of extracted components, but we preferred to make a final ranking and selection of the number of basic waveforms after the procedure of varimax rotation. Otherwise, we could have missed an important factor (factor 5) in some of the data sets. We will discuss this issue later in the context of the factors found by Davis & Vaughan (1993).
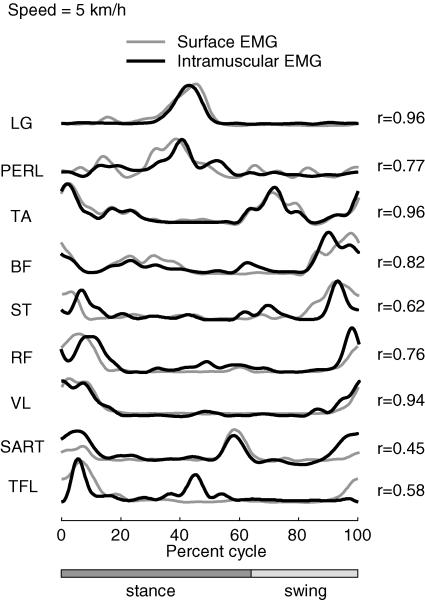
Since this analysis technique employs a cross-correlation of muscle activation patterns, it could theoretically be compromised by electrical cross-talk among adjacent muscle recordings. We controlled for this possibility in one subject by comparing the surface EMGs with intramuscularly recorded EMGs in nine muscles for which cross-talk contamination might be most likely (see above). The result presented in Fig. 1 was that the surface electrodes registered activity that was generally well correlated with the intramuscular recordings (waveform correlation coefficients ranged from 0.45 in SART to 0.96 in TA).
Figure 1. Intramuscular and surface EMG activity of 9 leg muscles recorded simultaneously in one subject stepping at 5kmh−1 on the treadmill.
Correlation coefficients between intramuscular and surface EMG waveforms are shown on the right.
Calculation of time shifts of the factors with speed
The cross-correlation function between pairs of normalized waveforms was computed to quantify temporal offsets of the varimax factors with speed by means of the following formula (Orfanidis, 1996):
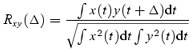 |
where x and y are the two waveforms (after subtraction of the respective means) and Δ is the time lag between the two signals. The numerator corresponds to the power of the common signal in x and y, and it is scaled to the product of total signal power (i.e. the autocovariance at 0 lag, the denominator) so that the cross-correlation ranges from −1 to 1. A peak detection algorithm was used to determine the highest positive correlation peak and its corresponding time lag. Using this method, we calculated time delays of the varimax factors at 1, 2 and 3kmh−1 with respect to those at 5kmh−1 and expressed them as a percentage of gait cycle.
Published data
Published graphs were each scanned, digitized manually (in about 30–50 points) and time-interpolated to fit a normalized 200 points time base. This included the average EMG records published in Winter (1991), referred to as the Winter data, and the PCA factors published in Davis & Vaughan (1993) and in Olree & Vaughan (1995).
The Winter data included average EMG recordings from the following muscles (Fig. 2): adductor longus (ADDL), adductor magnus (ADDM), BF, extensor digitorum longus (EDL), erector spinae (lumbar) (ES; L4), erector spinae (thoracic) (ES; T9), LG, MG, GM, Gmed, external oblique lateralis (OEL), external oblique medialis (OEM), peroneus brevis (PERB), PERL, rectus abdominus (RA), RF, SART, ST, Sol, splenius (SPLEN), TA, TFL, TRAP, VL and VM. Davis & Vaughan (1993) reported the results of a PCA of EMG recordings of 16 muscles of the Winter data list (less ES, OEL, OEM, PERB, TFL, TRAP, VM and SPLEN). Olree & Vaughan (1995) reported their results for the following muscles recorded bilaterally: ES, GM, Gmed, RF, ADDM, BF, TA, LG.
Statistics
A principal component analysis was performed using Statistica v6.0 (StatSoft Inc.) or Systat v9.0 (SPSS Inc.). Statistics on correlation coefficients were performed on the normally distributed, Z-transformed values.
Results
Factor analysis
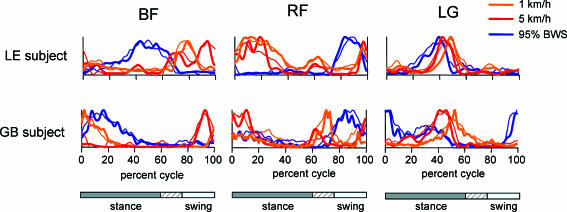
We recorded the patterns of EMG activity in 12–16 leg and trunk muscles from six normal volunteer subjects during treadmill locomotion at speeds of between 1 and 5kmh−1, and at four different levels of weight support (see Methods). We found that EMG patterns were often different for a given muscle in different subjects and that a given muscle often expressed a different pattern of activation for a different stepping velocity in the same subject. Figure 3 shows the normalized EMG records from two of our subjects (LE and GB) from three muscles (BF, RF and LG). The activity patterns recorded during 5kmh−1 locomotion (red traces) for BF were different for these subjects and different from those recorded at 1kmh−1 (orange traces) in the same subjects. The patterns for these subjects were similar at 5kmh−1 for RF, but different at 1kmh−1. The patterns recorded from the LG were similar for the two subjects at both locomotion speeds. The same muscles showed almost opposite phases of activation for the two subjects when 95% of the body weight was supported at 3kmh−1 (blue traces). However, RF showed almost the same activation pattern for these subjects, and yet the peaks of activity in BF were different with weight support. The BF activity peak for subject LE also occurred at a different phase from the activity peaks recorded during unsupported locomotion.
Figure 3. Examples of muscle activity with different locomotion speeds with and without body weight support.
Normalized EMG recordings from two subjects (LE and GB) are compared for 3 muscles (BF, RF and LG) under three conditions of treadmill locomotion: 5kmh−1 (red), 1kmh−1 (orange) and 3kmh−1 with 95% weight support (blue). Data are plotted with thick traces, and reconstruction of the data from the weighted varimax factors are plotted with thin traces. As the relative duration of stance varied with speed and BWS, a hatched region indicates an amount of variability in the stance phase duration across conditions.
We applied a principal component analysis (see Methods) to these EMG records to determine whether such variability could be accounted for by a small set of basic components. We first pooled the data for all speeds and all muscles recorded for each of the six subjects with no weight support. Bartlett's test of sphericity was always significant (P < 0.001), KMO = 0.64, and the communalities were always high (> 0.996) indicating that a factor analysis was appropriate. Results of the separate analysis for each subject are illustrated in Fig. 4A (different colour trace for each). The component factors were quite similar across subjects, although there were a number of specific differences. For example, two of the subjects have a later and less prominent peak in the initial part of factor 2. There was also a spread of about 10% of the step cycle in the timing of the peak activity among subjects in factors 3, 4 and 5.
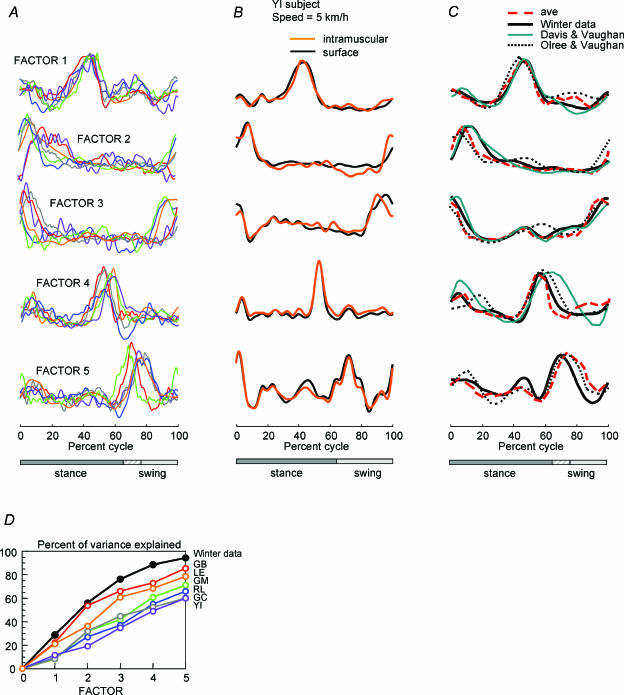
Figure 4. Varimax factors derived from muscle activity patterns by principle component analysis (PCA) during locomotion.
A, 5 varimax factors derived for each of 6 subjects (different colours) from the recordings made with 4 different speeds (1, 2, 3 and 5kmh−1). Stance phase was somewhat different across subjects and speeds as indicated by hatched area of the stance–swing bar. B, the factors derived from the recordings made from one subject for the 9 leg muscles plus 6 trunk muscles at one speed (5kmh−1) are compared using either the intramuscular or surface recordings of leg muscle activity (Fig. 1). C, red dashed traces show the factors averaged across the 6 subjects in A. Results are compared with the Winter factors (derived from the activity of 25 leg and trunk muscles illustrated in Fig. 2) (black traces) and with the result of a PCA of EMGs recorded from 16 leg muscles (Davis & Vaughan, 1993) (blue traces) and from 8 muscles in each leg (Olree & Vaughan, 1995) (dotted traces). D, the cumulative percentage of variance explained by each factor is shown for each subject and for the Winter factors.
In order to exclude the possibility that electrical cross-talk in the recordings could substantially influence this result, which is based on cross-correlation, we recorded simultaneously from intramuscular and surface electrodes in nine leg muscles in one subject (see Methods, Fig. 1). The factors derived from the recordings made from this subject for the nine leg muscles plus six trunk muscles at one speed (5kmh−1) are compared in Fig. 4B using either the intramuscular or surface leg muscle recordings. It is clear from this result that the recording site (intramuscular or surface) had no significant effect on the waveform of the principal component factors (0.91 < r < 0.99), although in some cases (e.g. TFL and SART) the intramuscular recordings may have registered components not present in the surface recordings (Fig. 1). This difference might represent some kind of intramuscular compartmentalization that was not seen at the surface (Windhorst et al. 1989; Chanaud et al. 1991; English et al. 1993).
The individual factors each explained different amounts of the total variance in each data set (Fig. 4D). The five factors together accounted for between 58 and 86% of the total variance in our data sets. In all cases there were higher order factors that tended to be idiosyncratic rather than common across muscles, but they usually accounted for less than 3–4% of the total variance.
Published data
We also examined the patterns of muscle activation from a larger set of muscle recordings published by Winter (1991), in which the average EMG activity from 25 leg and upper and lower trunk muscles was determined for a standard step cycle. Data from 18 normal subjects were pooled and averaged for the data set depicted in Fig. 2. The subjects walked over ground using a standard cadence of 106 steps min−1 (Winter, 1991). The published records included the average and standard deviation of filtered EMG activity (3Hz cut-off) for one step cycle beginning with ipsilateral heel strike.
Although the activation patterns in this data set appear muscle specific, there do appear to be preferred phases of activation in the cycle, for instance just following heel strike (see Fig. 2). The communalities ranged from 0.997 to 0.999, the Bartlett's test of sphericity was significant (P < 0.001) and the KMO measure was 0.72 (much more than 0.5), indicating that the sample was adequate for factor analysis. The results of the PCA showed that four factors or component waveforms accounted for 87% of the total waveform variance across the 25 different muscles, and five components accounted for 95% (Fig. 4C and D, black traces). This result is essentially the same as that reported by Davis & Vaughan (1993), who analysed only the 16 leg muscles from a similar data set (Winter, 1991) and reported four significant factors (Fig. 4C, blue traces). It is worth noting that Davis & Vaughan retained only eigenvectors with the corresponding eigenvalues higher than unity. As a result, they obtained only four factors: their factor 4 was somehow a combination of our factors 4 and 5 (Fig. 4C). Moreover, when we used the same criterion (minimum eigenvalue ≥ 1 instead of minimum eigenvalue ≥ 0.5) for the Winter data, we obtained the same four factors as Davis & Vaughan; that is, we failed to see the varimax factor 5 even though we included all 25 muscles in the factor analysis. Note that a unique pattern in 1 of 25 muscles would represent 4% of the variance; however, factor 5 explained 7% of the total variance in the Winter data (and from 8 to 27% of the variance in our own experimental data). Therefore, we think that this factor is significant, and the failure to detect it by Davis & Vaughan (1993) is related to both a smaller number of EMG records and to a strict application of the Kaiser criterion (see Methods). Thus, even when we included the upper and lower trunk muscles in the Winter data set we found the same basic factors. This means that any of the activation patterns in the entire set could be essentially reconstructed from an appropriate linear sum of five activation patterns.
In Fig. 4C we also compare the results of the Winter data analysis with our results from single subjects. The average factors derived from our subjects (red dashed traces) are nearly the same as the factors we derived from the Winter data (25 muscles, black traces; or 16 leg muscles (Davis & Vaughan, 1993), blue traces). They are also very similar to the factors derived from eight leg muscles recorded bilaterally by Olree & Vaughan (1995) (dotted traces).
These comparisons are further quantified in Table 2A and B. The table shows the correlation coefficients for a linear regression of the respective factor waveforms. The high levels of correlation (r) and variance (r2) explained for each of the respective factors across data sets indicate the level of similarity of the respective waveforms of these varimax factors.
A. Winter data factors compared to Davis and Vaughan factors
| Factors | r | r2 |
|---|---|---|
| Factor 1 | 0.94 | 0.90 |
| Factor 2 | 0.90 | 0.88 |
| Factor 3 | 0.95 | 0.94 |
| Factor 4 | 0.82 | 0.67 |
| Mean | 0.91 | 0.87 |
Effects of stepping speed and weight support
In addition to the individual differences we note above, there were also systematic differences that were related to stepping speed and weight support as we noted in Fig. 3. We examined this by pooling the data for all subjects by speed or by the percentage of BWS. The results of this analysis are plotted in Fig. 5. Again, Bartlett's test of sphericity (P < 0.001), KMO (ranging from 0.55 to 0.72 in the speed data set and from 0.56 to 0.75 in the BWS set), and the communalities (> 0.997 for speed and 0.998–0.999 in the BWS set) indicated that a factor analysis was appropriate in both cases.
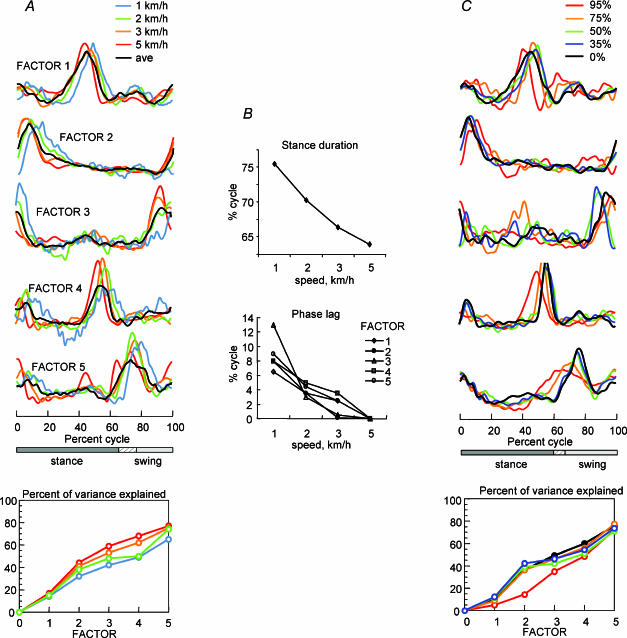
Figure 5. Effect of locomotion speed and of body weight support on component factors.
A, 5 varimax factors derived for each of 4 different speeds (coloured traces) across 6 subjects compared to the overall average across subjects (black trace). B, upper graph shows changes in the duration of the stance phase with speed. Lower graph shows the phase lag required to provide the best fit between each factor and the factor determined from the 5kmh−1 data. C, 5 varimax factors derived for each of 4 levels of body weight support (coloured traces) compared to no support (black traces; 0%) for treadmill locomotion at 3kmh−1. The cumulative percentage of variance explained by each factor is shown for each data set in the lower panels of A and C.
It can be seen in Fig. 5A that despite the 5-fold range of walking speeds, the activity patterns represented by the varimax factors are basically the same across speeds. The similarity of the waveform shapes indicates that the patterns were normalized to the duration of the step cycle, which varied from 2.4 s cycle−1 at 1kmh−1 to 1.0 s cycle−1 at 5kmh−1. However, there are systematic differences in the timing of the patterns. The waveforms are shifted to successively earlier phases in the step cycle as speed increases. The shifts are equivalent to a phase delay in the origin (heel strike reference) with increasing speed (Fig. 5B). The effect seems to be related to the change in the duration of stance as a function of speed, which decreased by 11.6% of the gait cycle from 75.5% at 1kmh−1 to 63.9% at 5kmh−1. The activity peaks in the factors had a comparable shift (about 9% on average). However, the shifted waveforms for the first three factors at least are basically indistinguishable across speeds (see Table 2C).
The analysis of speed effects shows that the activation patterns represented by the component factors remain stable even when the activation of specific muscles varies. Thus it raises the question of the extent to which the component factors may be associated with joint torques. One way to address this question is to examine the patterns of muscle activation under conditions in which the limb kinematics are similar but the joint torques are completely different. We did this by suspending the subjects in a harness that supported 35%, 50%, 75% or 95% of their body weight while they walked on the treadmill. The limb kinematics under these conditions are quite similar to those of the unsupported stepping (Ivanenko et al. 2002), although there were some specific differences in the relative durations of stance and swing. However, it is clear that the loading of the limbs and the torques produced to make the stepping movements were quite different in the two conditions (see the effect of 95% body weight support on muscle activation in Fig. 3, for example).
In order to examine the patterns of activity under these conditions, we pooled the EMG data from all six subjects for 3kmh−1 locomotion into five sets, normal walking and 4 BWS conditions. The results of the PCA are illustrated in Fig. 5C. Once again, we found five factors that were similar to those reported for normal unsupported walking (black traces). The factors obtained for 75% (orange) and 95% (red) BWS showed more variation but were still quite similar to the others. In general, factor 2 was basically indistinguishable from the corresponding factor in the other conditions, while the others were less similar but showed the same general features. The peak in factor 5 appears to be phase-shifted to an earlier time in the cycle compared to the corresponding activity peaks observed with less weight support. The five factors determined from these pooled data sets explained together about the same fraction of total variance in the EMG patterns recorded during supported and unsupported conditions, but the proportion of the variance explained by each factor could vary.
It is clear from these results that the five factors do appear to represent robust components of the EMG patterns during locomotion, and they do not seem to be highly dependent on the locomotion speed, the duration of the step cycle or on the patterns of mechanical loading in the limbs. Thus even though individual muscles might show prominent changes in their patterns of activity in various conditions, they could nevertheless all be accounted for by a weighted sum of the same five varimax factors. For example, the response patterns illustrated in Fig. 3 were mostly accounted for by a weighted sum of the five factors (thin traces in Fig. 3; range was r= 0.79 (LE, RF 1kmh−1) to r= 0.96 (GB, RF 5kmh−1)).
Weighting coefficients
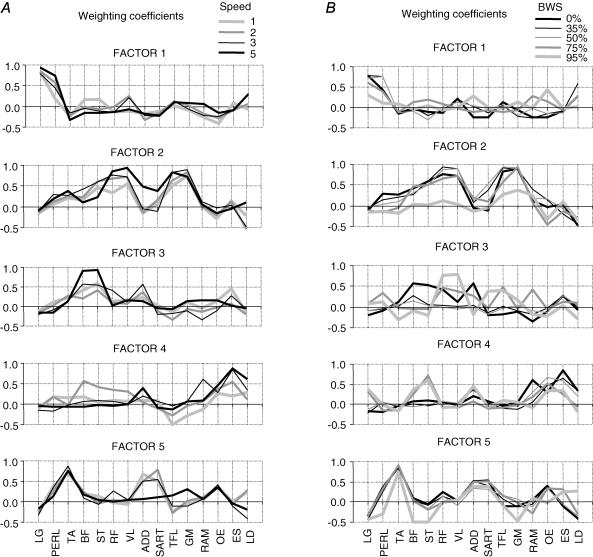
The relative strength of the effect of each factor on a given EMG pattern is given by the factor loading or weighting coefficient. The differences in loadings that we observed among subjects are illustrated in Fig. 6, which shows the value of the weighting coefficient associated with factors 1–5 for each subject for each of eight muscles during locomotion at 2kmh−1. The values are also compared with the weighting of factors for the Winter data for those muscles. There is a fairly large consistency across subjects for several distal muscles (e.g. TA, LG and PER) that is much less true for some more proximal limb muscles (e.g. ST, VL) or trunk muscles (ES). The weightings also showed some systematic changes with speed and BWS.
Figure 6. Weightings of factors in individual activity patterns for 6 subjects.
The weighting coefficients for factors 1–5 in the EMG waveforms of 8 muscles recorded from each of the 6 subjects during treadmill locomotion at 2kmh−1.
It can be seen in Fig. 7A that some muscles displayed a systematic trend in loading at different speeds (PERL, BF, ST, RF, VL, ADD, SART, TFL, ES) while others loaded more evenly (LG, TA, GM). Individual factor loadings of some muscles were more variable at low speeds (1–2kmh−1) than at high speeds (3–5kmh−1). For example, at 5kmh−1 the ST muscle loaded heavily in all subjects on factor 3 (r∼ 0.9), whereas at 1kmh−1 it loaded heavily as well but on factors 1, 2, 3 and 5 (r = 0.7–0.95) depending on the subject. A similar dispersion of highly loaded factors among subjects at low locomotion speeds was observed in RF, BF, VL and ES muscles. This explains in particular why the averaged factor loadings of these muscles were higher at 5kmh−1 than at 1kmh−1.
Figure 7. Distribution of factors across muscles.
The weighting coefficients (loadings) averaged across 6 subjects are plotted as a function of speed (A) and body weight support (BWS; B).
The effect of BWS on loadings is illustrated in Fig. 7B. Under these conditions the factor loadings changed for most of the muscles, particularly at the higher levels of BWS.
Thus, while the intrinsic structure (basic components, Figs 4 and 5) of the locomotor programme was similar, we observed a systematic redistribution of factors across speeds and BWS conditions. In part this might be accounted for by the changes in the kinetic requirements in the two cases. For instance, both the EMG patterns (Fig. 3) and the dynamics of joint moments change drastically with speed (Winter, 1991; Holden et al. 1997). Finally, it is worth noting that although some muscles (LG, TA and GM) were loaded heavily on single factors, many muscles had loadings on more than one factor.
Discussion
We found that five component factors can account for a considerable fraction of the total EMG pattern variance in a large number of muscles that are active during locomotion, including those of the legs, trunk and upper body. This finding implies that a few basic patterns may be distributed to all the muscles that are specifically activated during locomotion, and thus the activation of each muscle involves a dynamic weighting of these basic patterns.
The finding that 95% of the EMG pattern variance in the Winter data can be explained by only five component factors may result in part from the data reduction introduced by the 3Hz low-pass filtering and the averaging across subjects. In fact, while the analysis of our data from individual subjects produced the same five factors, they tended to explain a smaller fraction of the total waveform variance in each case. The higher order factors were generally not significant though, and individually they usually accounted for less than 3–4% of the variance. Note that a unique pattern in 1 of 16 muscles would represent 6% of the variance. It seems unlikely that there are other basic activation patterns, although it cannot be ruled out that other components may have been lost in the data averaging and filtering. The question remains though whether such minor components are also ‘basic’ in some sense or else idiosyncratic to particular muscles, or whether they are directly related to the locomotion, or even to ‘noise’ in the recording. What is interesting about the finding of five factors is that they are robust and apparently ubiquitous.
Another potential confound is the possibility of electrical cross-talk between recording channels, particularly between closely spaced muscles like PERL and TA (De Luca & Merletti, 1988). Since the analysis technique is based on a cross-correlation matrix derived from the individual EMG records, any cross-talk might exaggerate a positive correlation among EMG records, and therefore potentially bias the result. It was important therefore, to show that our surface EMG recordings did not have significant cross-talk as indicated by simultaneous intramuscular recordings. Nevertheless, any instance of cross-talk may be limited to only a few of the 16 muscles in total that we recorded. Thus, it is unlikely that the factors themselves could have been strongly biased. If cross-talk did exist, it would most likely have affected only the loadings or weighting coefficients assigned to each factor in accounting for the activity of a muscle that was contaminated by cross-talk.
Our basic result, namely that five component factors can account for the activation of muscles during locomotion, was reported earlier by Patla (1985), who used a PCA without varimax rotation based on averaged EMG recordings from seven muscles in six subjects. He found that the first PC accounted for 42% of the waveform variance and four PCs accounted for 92%. Based on these results, he proposed a conceptual model for human locomotion control, and developed it further in a cat model (Patla et al. 1985). He suggested that the central nervous system does not need to generate all the muscle activity patterns, rather only a few basic patterns that can be combined appropriately to produce the observed muscle activations. Even though his approach was limited by the relatively few muscles he sampled, and he was unable to show that the PCs represented the same basic patterns across conditions, our data strongly corroborate this concept. We have extended the previous PCA results (Patla, 1985; Davis & Vaughan, 1993; Olree & Vaughan, 1995) by showing that the basic patterns are conserved across subjects with quite different anthropometrical characteristics of weight, height and mass distribution, and also for locomotion at different speeds and under different gravitational loads.
The varimax factors represent bursts of activity occurring at certain points in the step cycle. The basic invariance of the factors with locomotion speed (over a 5-fold range, at least) implies that the timing and duration of the bursts are normalized with respect to step cycle kinematics. In fact there is also a phase shift with speed, that is about the same magnitude for each factor, and in the same direction as the shift in the lift-off marking the end of the stance phase. Both the lift-off of the step and the peaks in the component factors occur progressively earlier in the cycle as speed increases. The kinematic shift is approximately as large as the pattern shift over the range of speeds we reported, though there is not an exact correspondence. For instance, the duration of the swing phase at 1kmh−1 was a smaller fraction of the cycle length than its duration at 5kmh−1 (reduced by 11.6%), while the average shift of varimax factors was 8.9%. It may be that the shift in activity peaks is somehow related to the timing of force production required by the loaded limb at touch down, and this may need to be anticipated differently (i.e in a different kinematic phase) for different speeds. Nevertheless, the tendency for the factors to be timed according to the toe-off event supports the idea that the origin of the gait cycle generation is the propulsion rather than the heel strike event.
Davis & Vaughan (1993) proposed that the varimax factors have a specific relationship to the propulsion and loading events in locomotion. They suggested that the factors represent motor ‘programmes’ for groups of muscles that have to perform a given function during locomotion. Some evidence for such a functional grouping may be seen in our data, in the tendency for distal extensors to load on factor 1 (presumably for propulsion) and proximal extensors to load on factor 2 (for loading or weight acceptance). This pattern was enforced by increasing speed (Fig. 7A) and altered by changes in body weight support that altered the propulsion and loading demands (Fig. 7B). A more thorough analysis would be required however, before drawing inferences about such relationships between the factors and these specific functional demands.
In contrast with this view, the EMG analysis during supported locomotion suggests that the factors may not relate easily to specific force demands during locomotion. Propulsion and loading are not simply attenuated under body weight support conditions, but rather undergo qualitative changes as well (Ivanenko et al. 2002). Moreover, there is a pronounced differential effect of body load on leg flexor and extensor muscles (Finch et al. 1991; Ivanenko et al. 2002), and energetic aspects of walking in simulated reduced gravity are also different (Griffin et al. 1999; Cavagna et al. 2000). Thus the lack of any substantial qualitative changes in the five factors with weight support makes it seem unlikely that the factors can be easily related to strictly kinetic conditions in the limbs. The differences between the normal and weight-supported factors were primarily differences in timing (Fig. 5C), particularly at 75% and 95% BWS where there are also differences in the stepping kinematics (Finch et al. 1991; Ivanenko et al. 2002). For example the peaks in factors 1, 3 and 5 seem to be systematically phase-shifted to an earlier time in the cycle (relative to heel strike) with weight support.
In their analysis of EMG activity recorded bilaterally from eight leg muscles, Olree & Vaughan (1995) suggested that two of the five varimax factors they found (see Fig. 4C) namely factors 1 and 3 were actually the same as factors 2 and 4, but phase shifted by one-half a step cycle. According to that interpretation there may be only three basic factors that are somehow associated with each limb. Although their bilateral recordings suggested that the three component patterns may be distributed primarily to ipsilateral muscles, the extensive unilateral recording in our study showed that both the ipsilaterally and contralaterally phased patterns are significantly present in the activity patterns of the ipsilateral muscles. This finding does not necessarily contradict the interpretation of Olree & Vaughan (1995), but it does imply a more complex distribution of activity to the working muscles than one might suppose from a more straightforward pattern generator model of locomotion. While the evidence suggests there may be lateralized components that oscillate in opposite phase, the components may be distributed to many or all of the motoneurones bilaterally via a network that modulates the weighting of each component, perhaps according to the kinetic demands of the biomechanical system.
Nevertheless, it is tempting to speculate from these findings that the locomotion circuitry may consist of a few simple oscillating circuits that provide the major input to the active motoneurones during locomotion (Patla, 1985; Orlovsky et al. 1999; Yakovenko et al. 2002). This was explored by Patla et al. (1985) in a model of the pattern generator for locomotion in which they proposed that a limb pattern generator has three subsystems. They were represented by a few sinusoidal oscillators and wave-shaping circuits to produce a set of basic waveforms (corresponding to the EMG factors) and weighting functions to generate appropriate muscle activations. This idea may also relate in some way to the spinal cord motor primitives. The primitives represent specific muscle synergies that may be combined in different proportions to produce a variety of limb positions and movements (Bizzi et al. 2000; Kargo & Giszter, 2000; d'Avella et al. 2003). The EMG factors, in contrast, represent only the temporal structure of the muscle activity. Perhaps the temporal patterns are established upstream from the formation of primitives, which may be created from the component factors by the factor loadings associated with each specific task. Thus, the components (essentially bursts of activity) may be parcelled out to muscles via a network that creates the muscle synergies required to produce the appropriate locomotion kinematics. Since this process appears to occur dynamically, a major part of the proprioceptive feedback may be directed to modulate this network, along with descending information from vestibular and other balance control systems as well as more global commands that may determine speed, for example. Thus we propose that the modulations occurring within this spinal network may be a key element in a kind of inverse model that adapts the global activity patterns to the kinetic and kinematic demands of the limbs during locomotion.
Table 2.
B. Winter data factors compared to factors for all speeds (averaged across subjects)
| Factors | r | r2 |
|---|---|---|
| Factor 1 | 0.88 | 0.78 |
| Factor 2 | 0.87 | 0.76 |
| Factor 3 | 0.88 | 0.78 |
| Factor 4 | 0.71 | 0.50 |
| Factor 5 | 0.78 | 0.62 |
| Mean | 0.83 | 0.70 |
C. Factors for 5kmh−1 compared to factors for 1kmh−1*
| Factors | r | r2 |
|---|---|---|
| Factor 1 | 0.93 | 0.88 |
| Factor 2 | 0.95 | 0.90 |
| Factor 3 | 0.97 | 0.95 |
| Factor 4 | 0.83 | 0.69 |
| Factor 5 | 0.86 | 0.75 |
| Mean | 0.92 | 0.86 |
D. Winter data compared to factors for weight support (50% BWS at 3kmh−1)*
| Factors | r | r2 |
|---|---|---|
| Factor 1 | 0.64 | 0.42 |
| Factor 2 | 0.94 | 0.88 |
| Factor 3 | 0.81 | 0.66 |
| Factor 4 | 0.86 | 0.74 |
| Factor 5 | 0.59 | 0.35 |
| Mean | 0.81 | 0.66 |
Data phase shifted for best phase alignment.
Acknowledgments
We thank A. d'Avella, G. Cappellini, N. Dominici and D. Prissinotti for help with some of the experiments. The financial support of the Italian Health Ministry, Italian University Ministry (MIUR), Italian Space Agency (ASI), Telethon and C.N.R. (Progetto Strategico Neuroscienze) is gratefully acknowledged.
References
- Barbeau H, McCrea DA, O'Donovan MJ, Rossignol S, Grill WM, Lemay MA. Tapping into spinal circuits to restore motor function. Brain Res Rev. 1999;30:27–51. doi: 10.1016/s0165-0173(99)00008-9. [DOI] [PubMed] [Google Scholar]
- Basmajian JV, De Luca CJ. Muscles Alive: Their Functions Revealed by Electromyography. 5. Baltimore: Williams & Wilkins; 1985. [Google Scholar]
- Belanger M, Drew T, Provencher J, Rossignol S. A comparison of treadmill locomotion in adult cats before and after spinal transection. J Neurophysiol. 1996;76:471–491. doi: 10.1152/jn.1996.76.1.471. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Tresch MC, Saltiel P, d'Avella A. New perspectives on spinal motor systems. Nat Rev Neurosci. 2000;1:101–108. doi: 10.1038/35039000. [DOI] [PubMed] [Google Scholar]
- Borghese NA, Bianchi L, Lacquaniti F. Kinematic determinants of human locomotion. J Physiol. 1996;494:863–879. doi: 10.1113/jphysiol.1996.sp021539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna GA, Willems PA, Heglund NC. The role of gravity in human walking: pendular energy exchange, external work and optimal speed. J Physiol. 2000;528:657–668. doi: 10.1111/j.1469-7793.2000.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanaud CM, Pratt CA, Loeb GE. Functionally complex muscles of the cat hindlimb. V. The roles of histochemical fiber-type regionalization and mechanical heterogeneity in differential muscle activation. Exp Brain Res. 1991;85:300–313. doi: 10.1007/BF00229408. [DOI] [PubMed] [Google Scholar]
- Chau T. A review of analytical techniques for gait data. Part 1: Fuzzy, statistical and fractal methods. Gait Posture. 2001;13:49–66. doi: 10.1016/s0966-6362(00)00094-1. [DOI] [PubMed] [Google Scholar]
- d'Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci. 2003;6:300–308. doi: 10.1038/nn1010. [DOI] [PubMed] [Google Scholar]
- Davis BL, Vaughan CL. Phasic behavior of EMG signals during gait: Use of multivariate statistics. J EMG Kinesiol. 1993;3:51–60. doi: 10.1016/1050-6411(93)90023-P. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Merletti R. Surface myoelectric signal cross-talk among muscles of the leg. Electroencephalogr Clin Neurophysiol. 1988;69:568–575. doi: 10.1016/0013-4694(88)90169-1. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev. 2000;80:83–133. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- Duysens J, Van de Crommert HW. Neural control of locomotion; The central pattern generator from cats to humans. Gait Posture. 1998;7:131–141. doi: 10.1016/s0966-6362(97)00042-8. [DOI] [PubMed] [Google Scholar]
- English AW, Wolf SL, Segal RL. Compartmentalization of muscles and their motor nuclei: the partitioning hypothesis. Phys Ther. 1993;73:857–867. doi: 10.1093/ptj/73.12.857. [DOI] [PubMed] [Google Scholar]
- Finch L, Barbeau H, Arsenault B. Influence of body weight support on normal human gait: development of a gait retraining strategy. Phys Ther. 1991;71:842–855. doi: 10.1093/ptj/71.11.842. [DOI] [PubMed] [Google Scholar]
- Gazzani F, Fadda A, Torre M, Macellari V. WARD: a pneumatic system for body weight relief in gait rehabilitation. IEEE Trans Rehab Eng. 2000;8:506–513. doi: 10.1109/86.895954. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Ruchkin DS. Principles of Neurobiological Signal Analysis. New York: Academic Press; 1976. [Google Scholar]
- Grasso R, Bianchi L, Lacquaniti F. Motor patterns for human gait: backward versus forward locomotion. J Neurophysiol. 1998;80:1868–1885. doi: 10.1152/jn.1998.80.4.1868. [DOI] [PubMed] [Google Scholar]
- Grasso R, Zago M, Lacquaniti F. Interactions between posture and locomotion: motor patterns in humans walking with bent posture versus erect posture. J Neurophysiol. 2000;83:288–300. doi: 10.1152/jn.2000.83.1.288. [DOI] [PubMed] [Google Scholar]
- Griffin TM, Tolani NA, Kram R. Walking in simulated reduced gravity: mechanical energy fluctuations and exchange. J Appl Physiol. 1999;86:383–390. doi: 10.1152/jappl.1999.86.1.383. [DOI] [PubMed] [Google Scholar]
- Grillner S. Handbook of Physiology, section 1, The Nervous System, vol. 2, Motor Control, part 1. Vol. 2. Bethesda, MD, USA: Am Physiol Soc; 1981. Control of locomotion in bipeds, tetrapods, and fish; pp. 1179–1236. [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- Holden JP, Chou G, Stanhope SJ. Changes in knee joint function over a wide range of walking speeds. Clin Biomech. 1997;12:375–382. doi: 10.1016/s0268-0033(97)00020-x. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Grasso R, Macellari V, Lacquaniti F. Control of foot trajectory in human locomotion: role of ground contact forces in simulated reduced gravity. J Neurophysiol. 2002;87:3070–3089. doi: 10.1152/jn.2002.87.6.3070. [DOI] [PubMed] [Google Scholar]
- Kaiser HF. Analysis of factorial simplicity. Psychometrika. 1974;39:31–36. [Google Scholar]
- Kargo WJ, Giszter SF. Rapid correction of aimed movements by summation of force-field primitives. J Neurosci. 2000;20:409–426. doi: 10.1523/JNEUROSCI.20-01-00409.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–727. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Kendall FP, McCreary EK, Provance PG. Muscles. Testing and Function. Baltimore: Williams & Wilkins; 1993. [Google Scholar]
- Lacquaniti F, Grasso R, Zago M. Motor patterns in walking. News Physiol Sci. 1999;14:168–174. doi: 10.1152/physiologyonline.1999.14.4.168. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Ivanenko YP, Zago M. Kinematic control of walking. Arch Ital Biol. 2002;140:263–272. [PubMed] [Google Scholar]
- Merkle LA, Layne CS, Bloomberg JJ, Zhang JJ. Using factor analysis to identify neuromuscular synergies during treadmill walking. J Neurosci Meth. 1998;82:207–214. doi: 10.1016/s0165-0270(98)00054-5. [DOI] [PubMed] [Google Scholar]
- Olree KS, Vaughan CL. Fundamental patterns of bilateral muscle activity in human locomotion. Biol Cybern. 1995;73:409–414. doi: 10.1007/BF00201475. [DOI] [PubMed] [Google Scholar]
- Orfanidis SJ. Optimum Signal Processing. An Introduction. 2. Englewood Cliffs, NJ, USA: Prentice Hall; 1996. [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. Neural Control of Locomotion. From Mollusc to Man. Oxford: Oxford University Press; 1999. [Google Scholar]
- Patla AE. Some characteristics of EMG patterns during locomotion: implications for the locomotor control process. J Mot Behav. 1985;17:443–461. doi: 10.1080/00222895.1985.10735360. [DOI] [PubMed] [Google Scholar]
- Patla AE, Calvert TW, Stein RB. Model of a pattern generator for locomotion in mammals. Am J Physiol. 1985;248:R484–R494. doi: 10.1152/ajpregu.1985.248.4.R484. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE, Fouad K. Enhancement and resetting of locomotor activity by muscle afferents. Ann N Y Acad Sci. 1998;860:203–215. doi: 10.1111/j.1749-6632.1998.tb09050.x. [DOI] [PubMed] [Google Scholar]
- Poppele D, Ivanenko YP, Lacquaniti F. Basic components underlying EMG patterns in locomotion. The 12th Annual Neural Control of Movement Meeting, Naples, Florida. 2002 Abstracts 7, E-20. [Google Scholar]
- Rossignol S. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. Washington, DC, USA: Am Physiol Soc; 1996. Neural control of stereotypic limb movements; pp. 173–216. [Google Scholar]
- Sabatini AM. Identification of neuromuscular synergies in natural upper-arm movements. Biol Cybern. 2002;86:253–262. doi: 10.1007/s00422-001-0297-7. [DOI] [PubMed] [Google Scholar]
- Shen L, Poppele RE. Kinematic analysis of cat hindlimb stepping. J Neurophysiol. 1995;74:2266–2280. doi: 10.1152/jn.1995.74.6.2266. [DOI] [PubMed] [Google Scholar]
- Shiavi R, Griffin P. Representing and clustering electromyographic gait patterns with multivariate techniques. Med Biol Eng Comput. 1981;19:605–611. doi: 10.1007/BF02442775. [DOI] [PubMed] [Google Scholar]
- Trank TV, Chen C, Smith JL. Forms of forward quadrupedal locomotion. I. A comparison of posture, hindlimb kinematics, and motor patterns for normal and crouched walking. J Neurophysiol. 1996;76:2316–2326. doi: 10.1152/jn.1996.76.4.2316. [DOI] [PubMed] [Google Scholar]
- Windhorst U, Hamm TM, Stuart DG. On the function of muscle and reflex partitioning. Behav Brain Sci. 1989;12:629–681. [Google Scholar]
- Winter DA. Biomechanics of normal and pathological gait: implications for understanding human locomotor control. J Mot Behav. 1989;21:337–355. doi: 10.1080/00222895.1989.10735488. [DOI] [PubMed] [Google Scholar]
- Winter DA. The Biomechanics and Motor Control of Human Gait: Normal, Elderly and Pathological. Waterloo, Ontario: Waterloo Biomechanics Press; 1991. [Google Scholar]
- Winter DA, Yack HJ. EMG profiles during normal human walking: stride-to-stride and inter-subject variability. Electroencephalogr Clin Neurophysiol. 1987;67:402–411. doi: 10.1016/0013-4694(87)90003-4. [DOI] [PubMed] [Google Scholar]
- Wootten ME, Kadaba MP, Cochran GV. Dynamic electromyography. II. Normal patterns during gait. J Orthop Res. 1990;8:259–265. doi: 10.1002/jor.1100080215. [DOI] [PubMed] [Google Scholar]
- Yakovenko S, Mushahwar V, VanderHorst V, Holstege G, Prochazka A. Spatiotemporal activation of lumbosacral motoneurons in the locomotor step cycle. J Neurophysiol. 2002;87:1542–1553. doi: 10.1152/jn.00479.2001. [DOI] [PubMed] [Google Scholar]