Abstract
N-type voltage-dependent Ca2+ channels (N-VDCCs) play important roles in neurotransmitter release and certain postsynaptic phenomena. These channels are modulated by a number of intracellular factors, notably by Gβγ subunits of G proteins, which inhibit N-VDCCs in a voltage-dependent (VD) manner. Here we show that an increase in intracellular Na+ concentration inhibits N-VDCCs in hippocampal pyramidal neurones and in Xenopus oocytes. In acutely dissociated hippocampal neurones, Ba2+ current via N-VDCCs was inhibited by Na+ influx caused by the activation of NMDA receptor channels. In Xenopus oocytes expressing N-VDCCs, Ba2+ currents were inhibited by Na+ influx and enhanced by depletion of Na+, after incubation in a Na+-free extracellular solution. The Na+-induced inhibition was accompanied by the development of VD facilitation, a hallmark of a Gβγ-dependent process. Na+-induced regulation of N-VDCCs is Gβγ dependent, as suggested by the blocking of Na+ effects by Gβγ scavengers and by excess Gβγ, and may be mediated by the Na+-induced dissociation of Gαβγ heterotrimers. N-VDCCs may be novel effectors of Na+ion, regulated by the Na+ concentration via Gβγ.
Na+ ions are crucial for neuronal activity as carriers of depolarization, and play important roles in maintaining the water balance of the body, cardiac contraction, and transport processes. In neurones, short periods of synaptic activity produce large increases in intracellular Na+ concentration ([Na+]i), mainly due to Na+ influx via N-methyl-d-aspartate (NMDA) receptor channels. During such periods of activity [Na+]i rises from a resting level of 9−13mm, to ∼30mm in apical dendrites, and to 30–100mm in dendritic spines (Rose & Ransom, 1997; Rose & Konnerth, 2001).
Changes in [Na+]i regulate a variety of intracellular targets. Many membrane proteins that are engaged in the transport of Na+ itself (the Na+−K+-ATPase and Na+-coupled transporters and exchangers) are regulated by changes both in [Na+]i and extracellular [Na+] ([Na+]o) (e.g. Kanner, 1994). In mammalian neurones, elevated [Na+]i and/or Na+ influx via NMDA or AMPA/kainate channels enhances the opening of NMDA receptor channels by a Src protein-dependent mechanism (Yu & Salter, 1998), inhibits voltage-dependent K+ channels by an unknown mechanism (Van Damme et al. 2002) and activates K+ channels of large conductance (Egan et al. 1992). Na+ also binds to and activates the G protein-gated inward rectifier K+ channels GIRK (Ho & Murrell-Lagnado, 1999; Petit-Jacques et al. 1999), which are normally gated by direct binding of Gβγ subunits (Clapham & Neer, 1997).
Recently, we have described a novel regulatory effect of intracellular sodium (Rishal et al. 2003): it promotes the dissociation of heterotrimeric G proteins, Gαβγ, into free GαGDP and Gβγ, increasing free cellular Gβγ concentration ([Gβγ]). This causes a slow, Gβγ-dependent activation of GIRK channels (Rishal et al. 2003). The physiological impact of the newly described modulation of G proteins by Na+ is yet to be established.
One candidate effector for Gβγ-mediated regulation by Na+ is the neuronal N-type voltage-dependent Ca2+ channel, N-VDCC (Cav2.2). N-VDCC, and a closely related P/Q-VDCC, play a crucial role in neurotransmitter release (Catterall, 1998). Both N- and P/Q Ca2+ channels are widely regulated, usually inhibited, by neurotransmitters acting on G protein-coupled receptors (GPCRs); this is considered to be an important mechanism of neuronal presynaptic inhibition (Miller, 1998). N-VDCCs are also abundant in soma and dendrites (Westenbroek et al. 1992); postsynaptic N-VDCCs appear to be critical for associative long-term depression in the hippocampus (Normann et al. 2000). A ubiquitous modulation of N- and P/Q-VDCCs is their inhibition by direct binding of Gβγ released from the heterotrimeric Gi/o proteins, usually Go (reviewed by Dolphin, 1998; Zamponi & Snutch, 1998a; Ikeda & Dunlap, 1999; Catterall, 2000). This Gβγ-mediated inhibition is voltage dependent (VD), because the binding of Gβγ is reduced by depolarization.
Here, we demonstrate that intracellular Na+ inhibits N-VDCC in a Gβγ-dependent manner. While the influx of Na+ inhibits N-VDCC, the depletion of intracellular Na+‘disinhibits’ them, suggesting a possible bidirectional regulation by [Na+]i. These findings reveal a previously unrecognized regulation of neuronal Ca2+ channels, and support the idea that Na+, acting via Gβγ, may regulate many membrane proteins.
Methods
cDNA constructs and RNA
RNA was synthesized in vitro, using standard methods, from the following DNAs: Na+ channel subunit NaIIA-α (Dascal & Lotan, 1991); myristoylated cβARK, muscarinic m2 receptor, Gαo, Gβ1, Gγ2 subunits (Peleg et al. 2002); N-VDCC α1B subunit (Wakamori et al. 1998). The coding parts of various G protein subunits were subcloned into the pGEM-HE or its derivative, pGEM-HJ vectors (Peleg et al. 2002). Standard amounts of injected RNA, when not indicated otherwise in the text, were (ng per oocyte−1): N-VDCC α1B, 1.5–2.5; Gαo, 0.5–1; cβARK, 5; NaIIA α subunit, 5; Gβ1, 5; Gγ2, 1; Gαo, 0.5; m2 receptor, 0.5.
Xenopus oocyte preparation and electrophysiology
The care of the Xenopus laevis frogs, and the collection of oocytes, which were defolliculated and injected with RNA, was as described previously (Dascal & Lotan, 1991). Briefly, female frogs, maintained at 20 ± 2°C on an 11 h light/13 h dark cycle, were anaesthetized in a 0.15% solution of procaine methanesulphonate (MS222), and portions of ovary were removed through a small incision on the abdomen. The incision was sutured, and the animal was returned to a separate tank until it had fully recovered from the anaesthesia, and afterwards was returned to a large tank where, together with the other postoperational animals, it was allowed to recover for at least 4 weeks until the next surgery. The animals did not show any signs of postoperational distress. All the experiments were carried out in accordance with the Tel Aviv University Institutional Animal Care and Use Committee (permit no. 11-99-47). Oocytes were injected with RNA and incubated in ND96 solution (mm: NaCl, 96; KCl, 2; CaCl2, 1; MgCl2, 1; Hepes/NaOH, 5). Ca2+ channel currents were studied using two-electrode voltage clamp as described previously (Ivanina et al. 2000) in a high-Na+ solution (mm: Ba(OH)2, 20; NaOH, 85; KOH, 2; Hepes, 5) or a Na+-free solution (mm: Ba(OH)2, 20; N-methyl-d-glucamine (NMDG), 85; KOH, 2; Hepes, 5). These solutions were titrated with methanesulphonic acid to pH = 7.5. (In one experiment, solutions with 40mm Ba2+ and 50mm Na+ or NMDG+ were used.) In most experiments, the oocytes were injected with the Ca2+ chelator BAPTA (20 nl of 50mm solution). The injection of BAPTA had no influence on Na+-induced changes in IBa. Agar-cushion electrodes filled with 3mm KCl with resistances of 0.1–0.4 MΩ were used in all experiments. Data acquisition and analysis were done using the pCLAMP software (Axon Instruments). I–V curves were fitted to a standard Boltzmann equation in the form IBa=Gmax(Vm−Vrev)/(1 + exp(−(Vm−Va/Ka))), where Ka is the slope factor, Va is the voltage that causes half-maximal activation, Gmax is the maximal conductance, Vm is membrane voltage, IBa is the current measured at the same voltage, and Vrev is the reversal potential of IBa.
Hippocampal neurones in QEHA peptide experiments
Freshly dispersed hippocampal pyramidal CA1–CA3 neurones from 12- to 14-day-old Wistar rats (killed under ether anaesthesia according to Bogomoletz Institute of Physiology, Kiev, guidelines) were prepared as described previously (Panchenko et al. 1996) and identified by their characteristic form and partially preserved dendritic arborization. The cells lacked most of their long projections, allowing high-quality voltage control. N-VDCC currents in neurones were recorded with Ba2+ as the charge carrier in whole-cell configuration. L- and P/Q-type channels were blocked by 10 μm nitrendipine and 200 nmω-agatoxin IVA, respectively. The pipette solution contained (mm): Tris-phosphate 70, tetraethylammonium chloride (TEA-Cl) 40, Tris-Cl 20, EGTA 5, Mg-ATP 5, GTP-Tris salt 0.5 (adjusted to pH 7.3 with Tris-OH). When the Na+ concentration in the pipette solution was elevated, TEA-Cl was reduced accordingly. The external solution contained (mm): TEA-Cl 40, NaCl 100, BaCl2 10, Tris-Cl 20.
Hippocampal neurones in NMDA experiments
Slices of the hippocampus were incubated in solution containing 5mgml−1 protease Aspergillus orizae (Type XXIII, Sigma) for 30–40 min at 34°C. After enzyme treatment, the slices were rinsed in the same solution without enzyme, but with the addition of 0.5mm CaCl2 and 0.5mm MgCl2. The high Na+ solution and the pipette solution were the same as in QEHA experiments. Na+-free external solution solutions contained (mm): TEA-Cl 40, NMDG 100, BaCl2 10, Tris-Cl 20.
Statistics
Data are presented as mean ± s.e.m. Comparison between treatments in the same cells (e.g. effect of NMDA or Na+ depletion) was done using paired t tests. Comparisons between two groups of treatments (e.g. cells with or without coexpressed m-cβARK) were done using two-tailed t tests. Multiple group comparisons were done using one-way ANOVA followed by Dunnett's or Student–Newman–Keuls tests.
Results
Regulation of N-VDCCs in Xenopus oocytes by coexpressed Gβγ, a Gβγ scavenger, and a Gi/o-coupled neurotransmitter
Initially, we utilized Xenopus oocytes to explore the idea that intracellular Na+ might regulate N-VDCCs. ‘Classical’ Gβγ-dependent regulation of N-VDCCs expressed in Xenopus oocytes has been shown in the past (e.g. Roche et al. 1995; Bourinet et al. 1996). However, we felt it necessary to examine the quantitative aspects of Gβγ-dependent regulation, with an emphasis on the effects of moderate changes in free cellular Gβγ concentration ([Gβγ]), since only mild changes in [Gβγ] are expected to be produced by intracellular Na+ acting on G proteins (Rishal et al. 2003). To achieve this goal, we used two methods: heterologous expression of various amounts of Gβγ by injecting different amounts of RNA, and activation of a coexpressed Gi/o-coupled GPCR by low and high doses of a neurotransmitter.
Gβγ-dependent inhibition of N-type Ca2+ channels is relieved by depolarizing prepulses applied just before the standard ‘test’ pulse used to elicit the current. This phenomenon is called voltage-dependent (VD) facilitation. Overexpression of Gβγ or activation of a neurotransmitter acting via Gβγ causes VD inhibition accompanied by VD facilitation, and usually slows down the kinetics of activation of IBa (‘kinetic slowing’) and shifts the current–voltage (I–V) curve in the positive direction (Bean, 1989; Ikeda, 1991, 1996; Herlitze et al. 1996). The oocytes contain a relatively high resting [Gβγ] (Sheng et al. 2001), and expressed N-VDCCs formed by their main α1B subunit often exhibit a tonic inhibition by basal levels of Gβγ, and, accordingly, a substantial VD facilitation (Roche et al. 1995; Roche & Treistman, 1998; see Fig. 1). Tonic VD facilitation is sometimes observed in neuronal cells, for instance in sympathetic neurones (Ikeda, 1991). The presence of tonic Gβγ-induced inhibition is best seen in the absence of a coexpressed β subunit of N-VDCC, as in our conditions (Roche & Treistman, 1998), which makes it possible to examine the consequences of bidirectional changes in [Gβγ]: a mild increase in resting [Gβγ] will reduce IBa and increase VD facilitation; a decrease in [Gβγ] will have an opposite effect.
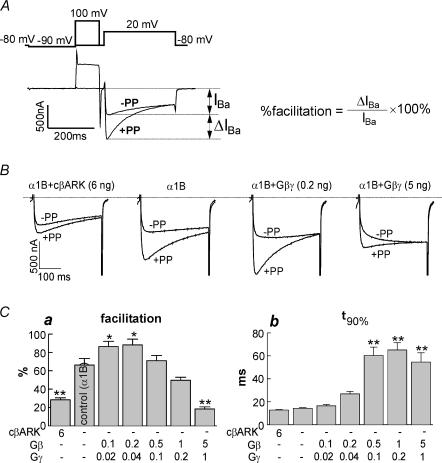
Figure 1. Effects of coexpressed cβARK and Gβγ on N-VDCCs in Xenopus oocytes.
A, IBa (lower trace) measured by the double pulse protocol (upper trace). Percentage facilitation was calculated as ΔIBa/IBa× 100%. B, effects of coexpressed cβARK and Gβγ on facilitation of α1B. Amounts of coexpressed RNAs are indicated (ng oocyte−1; RNAs of Gβ and Gγ were always injected at 5: 1 ratio). C, summary of effects of cβARK and Gβγ on facilitation (a) and t90% (b). Each column represents a group of oocytes injected with the indicated RNAs; n= 10 in each group. *P < 0.05, **P < 0.01 by one-way ANOVA.
Figure 1A shows a typical experimental protocol. N-VDCCs, formed by α1B subunits, were studied using the whole-cell, two-electrode voltage clamp method. Control (high-Na+) solution contained 85mm Na+ and 20mm Ba2+. A standard double-pulse protocol (Ikeda, 1991) was used to measure VD facilitation (Fig. 1A). A voltage step from a holding potential of −80 mV to 20 mV (‘test pulse’) was issued to measure the basal IBa. Fifteen seconds later, the second test pulse was applied again, this time preceded by a 100ms prepulse to +100mV. In most oocytes a prominent tonic VD facilitation was observed: IBa elicited after a depolarizing prepulse (+PP) was 30–100% larger than when the test pulse was given without the prepulse (–PP; Fig. 1A).
We next characterized the effects of expression of Gβγ and m-cβARK (C-terminal part of the β-adrenergic receptor kinase). The latter is a high-affinity Gβγ scavenger targeted to the membrane by a myristoyl moiety (Petit-Jacques et al. 1999; Peleg et al. 2002). The tonic VD facilitation was reduced by m-cβARK from 66.2 ± 7.2% to 28.4 ± 2.1% (P < 0.01, Fig. 1B; summarized in Fig. 1C), confirming the Gβγ-dependent character of tonic facilitation. We denote the net change in facilitation caused by a treatment as Δfacilitation (in this case 37.8%).
Coexpression of Gβγ at low doses usually enhances VD facilitation (Ikeda & Dunlap, 1999). This was also the case in our experiments (0.2 ng Gβ RNA oocyte−1; Fig. 1B and Ca). In contrast, an overwhelming excess of [Gβγ] is known to overcome the relieving effects of the depolarizing prepulse, to occlude the GPCR-induced modulation of N-VDCCs, and to reduce the extent of VD facilitation (Ikeda, 1996). In our experiments, heavy overexpression of Gβγ (5 ng Gβ RNA oocyte−1) strongly reduced the extent of facilitation and caused kinetic slowing of IBa (Fig. 1B and C). To assess kinetic slowing quantitatively, we calculated the time from the beginning of the depolarizing pulse to 90% of peak amplitude (t90%) at +20mV. In the experiment shown in Fig. 1, t90% was 14.3 ± 0.7ms in cells that expressed the channel alone (n= 10). Prominent kinetic slowing was seen at high doses of Gβγ (0.5 ng RNA oocyte−1 and more): t90% increased to 55–60ms (Fig. 1Cb). However, at 0.1 and 0.2 ng Gβ RNA oocyte−1, the kinetic slowing was very mild and did not reach statistical significance. For instance, at 0.1 ng Gβγ RNA oocyte−1, t90% was 16.5 ± 1.2ms (n= 10), a change of 15.4% only, despite the prominent and statistically significant change in VD facilitation caused by this dose of Gβγ (from 66% to 86%; Δfacilitation= 20%. See Fig. 1C). The strong reduction in the extent of facilitation caused by the scavenging of Gβγ by cβARK was accompanied by only a very mild, statistically insignificant acceleration of activation (t90%= 12.7 ± 0.5 ms, an 11% change; P > 0.05).
Despite the expectation that coexpression of Gβγ will reduce the average amplitude of IBa and cβARK will increase IBa, this did not happen. The amplitude of IBa was either slightly decreased or increased up to 40% by coexpression of Gβγ, but these changes did not reach statistical significance (data not shown). Coexpression of cβARK decreased average IBa– in the experiment of Fig. 1C, from 833 ± 132 nA to 368 ± 76 nA (P < 0.05). We assume that coexpressed Gβγ may increase the plasma membrane levels or slow down the degradation of N-type VDCCs, as often happens among interacting proteins; this issue has not been studied any further.
To examine a classical GPCR-induced, Gβγ-dependent inhibition of N-VDCCs under our experimental conditions, we looked at the inhibition caused by an agonist (acetylcholine; ACh) activating a typical Gi/o-coupled GPCR, m2 muscarinic receptor (Fig. 2). The oocytes expressed the m2 receptor, α1B, and Gαo (coexpression of Gαo proved important to obtain reproducible inhibition of IBa by ACh). Addition of ACh reduced IBa within less than 30 s. The concentrations of ACh were adjusted to produce either mild inhibition (1 nm ACh) or a maximal effect (10μm ACh, inhibition by 71.5 ± 3.8%, n= 23) (Fig. 2A and B). Inhibition was accompanied by an increase in percentage facilitation, kinetic slowing of activation, and a rightward shift in I–V curves (Fig. 2B). All these effects were very prominent at the saturating concentration of ACh. However, at 1 nm ACh, despite substantial changes in peak IBa (inhibition by 42.8 ± 3.8%; n= 19) and facilitation (from 8.5 ± 3.4% to 42.3 ± 7.0%), only very mild changes were observed in t90% (from 9.5 ± 0.3 to 11.6 ± 0.8 ms) and in the I–V curve (a shift to the right by less than 2.5mV; Fig. 2C). These results, together with those of Fig. 1, imply that mild changes in [Gβγ], that suffice to cause a substantial inhibition of IBa and a measurable increase in facilitation, cause only marginal kinetic slowing and I–V curve shift.
Figure 2. Inhibition of N-VDCCs expressed in Xenopus oocytes by a Gi/o-coupled neurotransmitter.
A, typical records of the effects of ACh (10−9 or 10−5m) on IBa. B, summary of the effects of ACh on amplitude (a), facilitation (b), and t90% (c) of IBa. Numbers above columns correspond to the number of oocytes tested. **P < 0.01, ***P < 0.001. C, normalized average current–voltage (I–V) curves of Ba2+ currents in 8 oocytes of one donor, and the effect of 10−9 and 10−5m ACh.
Note that despite the strong facilitation of ACh-inhibited IBa produced by the prepulse, the facilitated (+PP) IBa in the presence of ACh still remained smaller than the facilitated IBa measured before ACh (Fig. 2A). The depolarizing prepulse relieved only 24.5% of inhibition produced by 10μm ACh, and about 39% of inhibition produced by 1 nm ACh.
Regulation of N-VDCCs in Xenopus oocytes by depletion or influx of Na+
According to our model (Rishal et al. 2003), Na+ enhances the dissociation of Gαβγ in the reaction:
An increase in [Na+]i should increase [Gβγ], inhibit N-VDCCs and produce VD facilitation; a decrease in [Na+]i should have opposite effects. The following phenomena may be expected. (1) Influx of Na+ into the cell should inhibit N-VDCCs and increase VD facilitation. (2) Resting [Na+]i in amphibian oocytes is about 10mm (Dascal, 1987), close to the EC50 of the Na+ effect on Gαβγ (Rishal et al. 2003). Partial depletion of submembrane [Na+]i by incubation in Na+-free extracellular solution should reduce [Gβγ] and remove part of the tonic inhibition of N-VDCCs. (3) m-cβARK should strongly attenuate all effects of Na+. (4) An excess of Gβγ should attenuate (occlude) changes caused by Na+ depletion.
These predictions have been tested experimentally. To study the effect of Na+ influx, we coexpressed the neuronal voltage-dependent Na+ channel (type IIA). The level of expression of Na+ channels was adjusted to give Na+ currents of 10–50μA (Fig. 3A, upper trace). Satisfactory quality of voltage control during INa was confirmed by measuring the actual membrane voltage with an independent voltage electrode inserted into the oocyte in addition to the two standard electrodes (Fig. 3A, lower trace). Na+ influx was forced by tetanic stimulation (TS: a train of 2ms voltage steps from −80 to 0mV at 100Hz for 100 s), applied under voltage clamp conditions, in Ca2+- and Ba2+-free ND96 solution containing 96mm Na+. IBa was measured before and after TS in a Na+-free, high-Ba2+ solution (Fig. 3B). Although the TS protocol included repetitive depolarizations, the parameters were chosen such as not to inactivate IBa. This was confirmed by applying TS in the presence of 0.1μm tetrodotoxin (TTX), which blocked the Na+ currents by > 90%. This TS did not change IBa (98.2 ± 3.0% of control; n = 9).
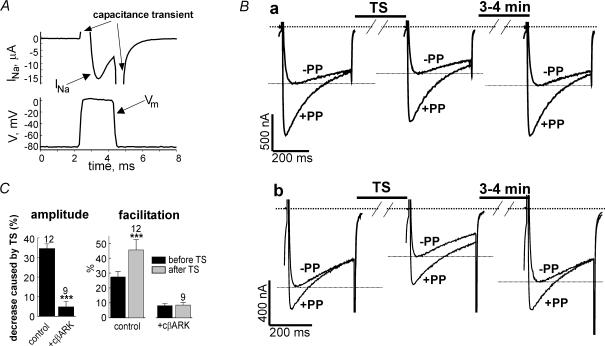
Figure 3. Inhibition of N-VDCCs expressed in Xenopus oocytes by sodium influx, and the effect of cβARK.
A, quality of voltage control during a tetanic stimulation (TS) protocol. Membrane voltage (Vm) was measured using an additional electrode and a separate amplifier. Upper panel shows the sodium current measured in the high-Na+ solution; lower panel shows the measured Vm. B, effect of TS-induced Na+ entry in an oocyte that was not preincubated in Na+-free solution (a) and in an oocyte that was incubated in Na+-free solution for 7 min before the recording (b). IBa was recorded in Na+-free solution, TS was applied in Ba2+- and Ca2+-free, high-Na+ (ND96) solution. After TS, the oocytes were again kept in Na+-free solution. C, summary of the effects of TS-induced Na+ entry on amplitude and facilitation of IBa, and the effect of cβARK in oocytes that were exposed to Na+-free solution for 7–10 min before the experiment. ***P < 0.001.
In the absence of TTX, the TS protocol reduced IBa and enhanced the facilitation. Figure 3Ba shows a representative experiment. The first record (left trace) was done about 1 min after the transfer of the oocyte from normal ND96 to the Na+-free, high-Ba2+ solution; the TS was performed in 96mm Na+, and the solution was switched again to Na+-free. IBa was measured 30s after the switch (middle trace), and in most oocytes also 3–4min later to monitor the recovery of IBa (right trace). On average, TS reduced IBa by 17.9 ± 2.9%, and facilitation became greater than before TS by 7.7 ± 1.7%(n= 10). Three minutes later IBa fully recovered to 101.7 ± 2.6%(n= 10) of initial amplitude.
When internal Na+ was depleted by prior 7–10min incubation in Na+-free solution (see below for details), the effects of Na+ influx were amplified (Fig. 3Bb). On average, TS reduced IBa by 34.5 ± 2.4% (n= 12, P < 0.001; Fig. 3B); facilitation that was 27.4 ± 3.6% before TS, became 45.5 ± 6.9% after TS (n= 12, P < 0.001; Fig. 3C). IBa partially recovered after 3–4min (to 85.7 ± 3.8% of control; n= 9; see Fig. 3Bb; longer recovery periods have not been tested). In this series of experiments, only minor changes in activation kinetics have occurred that did not reach statistical significance (data not shown).
Coexpression of the Gβγ scavenger m-cβARK fully prevented the decrease in IBa and the change in facilitation caused by TS (Fig. 3C). Thus, the effects of Na+ influx were Gβγ dependent. The changes caused by the TS were not due to alterations in [Na+]o, because both before and after TS, IBa was measured in Na+-free solution.
As in the case of ACh-induced inhibition, the depolarizing prepulse did not fully remove the inhibition caused by Na+ influx; the PP-facilitated IBa was still inhibited by TS-induced Na+ influx by 25.8 ± 2.5%(n= 12). This is still 0.75 of the 34.5% inhibition of the non-facilitated IBa. Thus, the PP relieved only about 25% of inhibition caused by Na+ influx. Although the activation of IBa after TS tended to be slower than before (by about 10%; data not shown), the difference did not reach statistical significance.
To reduce [Na+]i, which we expected to reduce [Gβγ], we used a depletion protocol that included a 7min incubation in a Na+-free solution (Vorobiov et al. 1998). Depletion procedures of this kind are known to reduce [Na+]i. In neurones, 10min incubation in Na+-free solution leads to a decrease of [Na+]i to undetectable levels (Rose & Ransom, 1997). The depletion procedure caused a gradual increase in IBa by 22.6 ± 3.0%(n= 23), and a parallel decrease in the extent of facilitation from 66.4 ± 6.3% to 48.2 ± 4.2%(P= 0.02; n= 23). Seven minutes incubation in control high-Na+ solution did not cause any changes in IBa and facilitation (Fig. 4A; summarized in Fig. 4C). In support of a Gβγ-dependent mechanism, m-cβARK strongly attenuated the changes caused by Na+ depletion, both in IBa amplitude and in facilitation (Fig. 4C). Heavy overexpression of Gβγ abolished changes in amplitude and in facilitation caused by Na+ depletion (Fig. 4B and C).
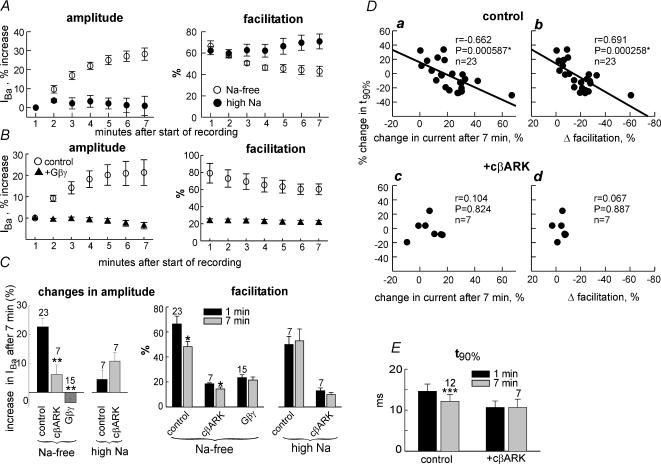
Figure 4. Modulation of N-VDCCs in Xenopus oocytes by Na+ depletion and its Gβγ dependency.
A, time course of changes in IBa (left panel) and in facilitation (right panel), caused by 7min incubation in Na+-free solution (○) or high-Na+ solution (•), in a representative experiment. IBa was: in high-Na+ solution, 849 ± 97 nA at t= 1min and 862 ± 122 nA at t= 7min (n= 4); in Na+-free solution, 845 ± 98 nA at t= 1min and 1075 ± 99 nA at t= 7min (n= 4). In each cell, changes in IBa with time were monitored relative to IBa measured 1min after the beginning of voltage clamp. B, the effect of Na+ depletion on IBa amplitude (left panel) and facilitation (right panel) is altered by overexpression of Gβγ (▴; 5 ng Gβ RNA oocyte−1). ○, channel expressed alone. n= 11–15 oocytes in each group. C, summary of the effects of 7min incubation of oocytes in low- or high-Na+ solutions, in oocytes that expressed N-VDCCs alone (control), with m-cβARK, or with Gβγ. Left panel summarizes the changes in amplitude of IBa. **P < 0.01 compared to control group. Right panel summarizes the changes in facilitation. *P < 0.05 for the difference between 1st and 7th minute. D, t90% of activation shows correlation with the change in IBa (a) and in Δfacilitation (b) after 7min of Na+ depletion. This correlation is absent in oocytes that coexpress m-cβARK (c and d). The value of P was calculated using the Pearson correlation test. E, summary of the effects of depletion on t90% of activation in oocytes that expressed N-VDCCs alone (control) or with m-cβARK. In the control group, only results from cells with more than a 20% increase in IBa after 7min of Na+ depletion (n= 12) are summarized. ***P < 0.001.
In most experiments Na+ depletion did not cause a significant shift in the I–V curve, except one experiment in which the increase in IBa was exceptionally large (data not shown). The average t90% in all cells did not significantly change after 7min of Na+ depletion (not shown). Furthermore, slight spontaneous slowing of IBa activation was often observed in the course of these long-lasting experiments under all conditions, such as high or low-Na+ solutions, the presence of cβARK or Gβγ, etc. (data not shown). The reason for this phenomenon is not known, but we suspect that it acted to offset the acceleration of activation and the decrease in t90% that we expected to see as a result of Na+ depletion. Indeed, the greater was the increase in IBa caused by the depletion procedure, the clearer became the decrease in t90%. Figure 4Da demonstrates a highly significant correlation (P < 0.001) between the change in t90% and the change in IBa caused by the 7min Na+ depletion procedure in the same cell. Accordingly, the change in t90% caused by Na+ depletion was also correlated with the change in the extent of facilitation (Fig. 4Db). Both correlations were absent in the presence of cβARK (Fig. 4Dc and d), and no correlation of any kind between these parameters has been observed when oocytes were incubated in high-Na+ solution for 7–10min (data not shown).
We have calculated the change in t90% in all cells that showed a 20% or more increase in current amplitude after Na+ depletion (12 out of 23 oocytes). The decrease in t90% in these cells was highly significant (P < 0.001, paired t test), and did not occur in oocytes that expressed cβARK (Fig. 4E). Though mild (17%), this change is comparable to that caused by Gβγ depletion by cβARK (Fig. 1C). Taken together, these results confirm that, in parallel with an increase in current amplitude and a decrease in the extent of tonic facilitation, the Na+ depletion procedure causes an acceleration in activation kinetics of IBa, in line with the hypothesis that all these effects are caused by a decrease in [Gβγ].
We stress that the effects of Na+ in the depletion experiments were not due to changes in [Na+]o. The slow (minutes) increase in IBa that developed after shifting to Na+-free solution could not be due to removal of a block caused by external Na+ (Polo-Parada & Korn, 1997), because the latter effect is immediate (seconds). Also, the direct inhibitory effect of Na+ on N-VDCCs is negligible (< 5%) at 20mm Ba2+ used in these experiments (Polo-Parada & Korn, 1997).
Intracellular Na+ causes VD facilitation of N-VDCCs in hippocampal neurones
To estimate the possible physiological impact of Na+ regulation of N-VDCCs, the effects of intracellular Na+ and of Na+ influx on native neuronal N-VDCCs were studied in freshly dispersed hippocampal pyramidal neurones. IBa via the N-VDCCs was recorded using the whole-cell patch clamp technique, by a double-pulse protocol (Fig. 5A). Other Ca2+ channels were blocked by the appropriate inhibitors (see Methods).
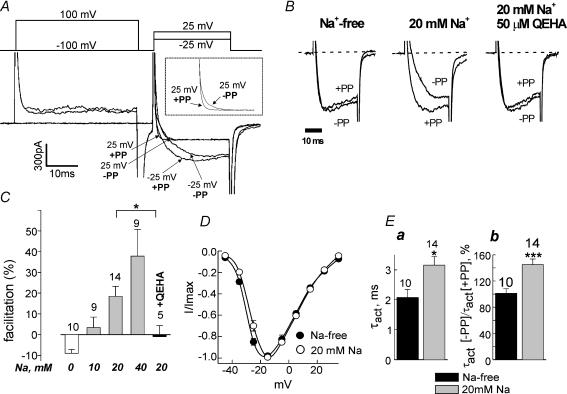
Figure 5. Intracellular Na+ induces voltage-dependent facilitation of N-VDCCs in hippocampal neurones.
A, IBa in the double pulse protocol. The first test pulse was from −100 to +25mV or −25mV without a prepulse (−PP). The second test pulse was applied after a 40ms prepulse to +100mV (+PP). Percentage facilitation was calculated as described in Fig. 1A. The inset shows on an expanded scale currents measured at +25mV (see text). B, QEHA peptide prevents the Na+-induced facilitation. Ba2+ currents measured at −25mV in three representative neurones are shown. C, dose dependency of Na+ effect on facilitation and summary of the effect of QEHA peptide at 20mm Na+. *P < 0.05. D, I–V curves averaged from 6 neurones with 20mm of Na+ inside the cell (○) and 6 neurones with Na+-free intracellular medium (•). Holding voltage was −100mV. E, effect of intracellular sodium concentration on the activation kinetics of N-type calcium channels. The time constant of activation, τact, was calculated by fitting the rising phase of IBa to a single exponential equation. Calcium currents were elicited by depolarization from −100 to −25mV. Ea, comparison of the absolute values of τact in neurones perfused with Na+-free or Na+= 20mm solutions. Eb, depolarizing prepulse accelerates the activation kinetics in the presence of 20mm Na+. Y-axis shows the ratio between τact after and before the prepulse. *P < 0.05, ***P < 0.001.
First, we examined the effect of intracellular Na+ on N-VDCCs in a Na+-free extracellular solution. The pipettes were filled with a solution that contained either 10, 20 or 40 mm Na+, or no Na+ (replaced by tetraethylammonium). The currents were recorded 15–20min after breaking into the cell, to let the intracellular Na+ equilibrate with the pipette solution. The amplitudes of Ba2+ currents varied greatly between different neurones, making the comparison of current amplitudes among different groups meaningless. However, the VD facilitation changed very consistently depending on [Na+]i. In Na+-free conditions, no VD facilitation was observed; in fact, the prepulse reduced IBa by 9% on average (Fig. 5B and C). This ‘inhibition’ probably reflected incomplete recovery from voltage-dependent inactivation caused by the prepulse. In contrast, already in 20mm Na+, a clear facilitation of 18.4 ± 4.9% was observed (P < 0.001). The extent of facilitation was stronger at negative than at positive potentials (compare the differences between −PP and +PP traces at −25 and at +25mV in Fig. 5A). In fact, at +25mV the prepulse did not increase the peak amplitude of IBa, but the differences in activation kinetics with and without the prepulse were still apparent (Fig. 5A, inset). This feature is typical for the classical voltage-dependent, Gβγ-mediated process. The facilitation, used here as the most accurate measure of Na+ effect, was dose dependent, reaching almost 40% at 40mm Na+ (Fig. 5C).
Other features of the Na+ effect were also compatible with a voltage- and Gβγ-dependent mechanism, and comparable with changes caused by a low dose of ACh in the oocyte experiments. The I–V curve showed a small shift of 3.0 ± 0.12mV to positive potentials (Fig. 5D; P < 0.05; N-VDCCs activated at more negative potentials in the neurones than in the oocytes, probably due to the presence of the β subunit in these cells and the lower Ba2+ concentration in the external solution). The kinetics of activation of IBa were assessed from the time constant of activation τact. As also expected for a Gβγ-dependent phenomenon, Na+ slowed down the activation of IBa (Fig. 5B and Ea), and the kinetics of the facilitated IBa after the prepulse were faster than those of non-facilitated current in the presence of 20mm Na+, but not in Na+-free solution (Fig. 5B and Eb).
Finally, we utilized a soluble Gβγ chelator, the QEHA peptide which, at 50–100μm, blocks the effects of Gβγ on adenylyl cyclase and GIRK channels (Chen et al. 1995; Weng et al. 1996). A separate group of neurones was perfused with a pipette solution containing 20mm Na+ and 50μm of QEHA. In these neurones, facilitation was absent (−1 ± 5.3%, P < 0.05 compared to 20mm Na+ without QEHA; Fig. 5C), supporting the involvement of Gβγ in Na+-induced facilitation.
Na+ influx inhibits N-VDCCs in hippocampal neurones
Initially, Na+ influx was produced using a tetanic stimulation (TS) protocol similar to that employed in the oocytes, which was found to inhibit N-VDCCs and to cause the appearance of VD facilitation (data not shown). However, several lines of evidence suggested imperfect voltage control during TS, that could lead to stronger depolarization than desired, causing inactivation of N-VDCCs. We have therefore examined the effect of influx of Na+ via NMDA receptor channels, probably the most relevant source of increased [Na+]i during periods of electrical activity in neurones (Schiller et al. 2000; Rose & Konnerth, 2001). The membrane potential was kept constant, −100mV, and NMDA was applied in the presence of Na+ (100mm) or in the absence of Na+ (100mm NMDG+ substituted for Na+) in the external solution. The external solution was Mg2+- and Ca2+-free and contained Ba2+ (10mm), glycine and TTX. IBa was constantly stimulated and monitored. In this series of experiments a small tonic VD facilitation was usually observed, probably due to a different cell isolation procedure (see Fig. 6Aa and D, and Methods). Note that measurement of N-VDCCs before and after Na+ influx was done at constant [Na+]o.
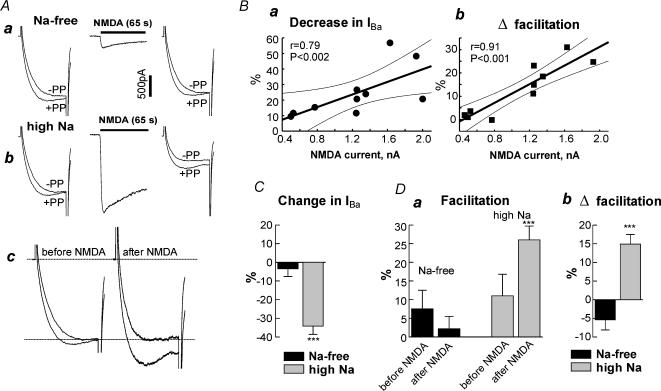
Figure 6. Na+ influx through NMDA receptor channels inhibits N-VDCCs in hippocampal neurones.
A, effect of Na+ entry through NMDA receptor channels. Aa, IBa was measured in a Na+-free solution. The double pulse protocol was as in Fig. 5. NMDA (100μm) was applied in the Na+-free solution for 65 s, washed out, and immediately IBa was measured again. Only the first second of the NMDA record is shown. Ab, continuation of the experiment in the same cell, after shifting to high Na+-solution. The currents measured before and after NMDA are shown again in Ac after the scaling up of IBa recorded after NMDA to that recorded before NMDA. B, correlation of changes in IBa (a) and in facilitation (b) induced by Na+ influx through NMDA receptors with the amplitude of NMDA responses. The straight line is a least squares fit, the dashed lines show 95% confidence intervals. C and D, summary of the effects of Na+ entry through NMDA receptors on amplitude (C) and facilitation (D) of IBa. n= 6 in Na+-free solution and n= 7 in high-Na+ solution. ***P < 0.001.
Application of NMDA (300μm) in the absence of Na+ in extracellular solution caused a small inward current, most probably carried by Ba2+, and induced no significant changes in the amplitude of IBa or in VD facilitation (Fig. 6Aa). Then the extracellular solution was exchanged to high-Na+, and the same protocol was conducted. Addition of NMDA caused a large inward current, presumably carried largely by Na+. After NMDA washout, IBa was reduced and the VD facilitation increased (Fig. 6Ab) in 7 out of 11 cells. The differences in facilitation are better illustrated in Fig. 6Ac, in which IBa values before and after NMDA are directly compared after scaling.
In four cells in which NMDA caused very small changes in IBa and facilitation, the NMDA currents themselves, measured in the high-Na+ solution, were smaller (<0.8nA) than in the other seven cells (> 1.2nA). Figure 6B shows that both the decrease in IBa and the change in VD facilitation, caused by NMDA, strictly correlated with the amplitude of NMDA-induced inward current: larger NMDA-induced currents corresponded to greater changes in IBa and facilitation. This result, and the inability of NMDA to modulate IBa in the absence of extracellular Na+, imply that the changes in IBa and in VD facilitation were caused by Na+ influx caused by NMDA, rather than by the activation of the NMDA receptor itself.
Figure 6C and D summarizes the NMDA effects in the cells that showed NMDA currents above 1.2nA. Figure 6C shows that the decrease in IBa caused by NMDA in high-Na+ solution was 34.3 ± 4.4%(n= 7). This is significantly (P < 0.001) greater than in Na+-free solution, where practically no change was observed (3.5 ± 4.1%, n= 6; in 1 out of the 7 cells, the records were done only in high-Na+ solution). Five to ten minutes after the washout of NMDA, IBa partially recovered to 85.7 ± 5.3% of its initial amplitude. The absolute values of percentage facilitation under different conditions are summarized in Fig. 6Da, and Δfacilitation is shown in Fig. 6Db. The tonic facilitation observed in Na+-free solution was actually slightly reduced by NMDA from 7.5% to 2.2%(P= 0.1). Incubation in high-Na+ solution by itself appeared to increase the facilitation slightly, to 11.2%, but this change did not reach statistical significance (P= 0.24 compared to Na+-free solution before NMDA, but P= 0.016 compared to Na+-free solution after NMDA). Most importantly, in high-Na+ solution, after NMDA the facilitation increased from 11.2 ± 3.7% to 26.1 ± 5.8%(P < 0.001). Kinetic slowing was observed in 6 out of 7 cells; the increase in τact was only 11% (from 2.84 ± 0.57ms to 3.13 ± 0.56 ms), but highly significant (P= 0.005 by paired t test). Again, the depolarizing prepulse did not fully relieve the NMDA-induced inhibition of IBa; the facilitated IBa was still reduced by 24.7 ± 4.5% after NMDA application. This corresponds to a 28% relief of inhibition by the prepulse.
Discussion
Our findings demonstrate a previously unknown modulation of neuronal Ca2+ channels: inhibition by intracellular Na+. It occurs within the physiological range of Na+ concentrations, and exhibits hallmarks of a Gβγ-mediated process: it is accompanied by voltage-dependent facilitation and is attenuated by scavengers of Gβγ and by depolarization. Our results are in line with the hypothesis that the molecular mechanism of this modulation is the regulation, by Na+ ions, of the dynamic equilibrium between Gα-bound and free Gβγ.
Intracellular Na+ inhibits N-VDCCs
Our results suggest that increased [Na+]i inhibits N-VDCCs. First, inhibition of IBa by Na+ influx caused by depolarizing tetanic stimulation (TS) in oocytes or activation of NMDA channels in neurones, did not take place when Na+ influx was eliminated. Second, depletion of Na+ in the oocytes increasedIBa. Third, intracellular perfusion of hippocampal neurones with Na+ was accompanied by the appearance of VD facilitation, which was dose dependent and absent at [Na+]i= 0. Although direct comparison of amplitudes of IBa with and without Na+ was impossible, the VD facilitation, which was always highly correlated with inhibition of IBa throughout this study, attests to an inhibitory effect of Na+.
Molecular mechanism
We believe that the mechanisms of Na+ effects on N-VDCCs were identical in oocytes and in neurones, because the effects of lowering [Na+] (by depletion in oocytes, by perfusion with Na+-free solution in neurones), and of increasing [Na+]i by Na+ influx, caused qualitatively identical changes in both cell types. Na+-induced changes in IBa were paralleled by changes in VD facilitation and in activation kinetics. The extent and time course of changes in these three parameters were strongly correlated (Figs 4 and 6), suggesting a connection between these phenomena. All changes caused by increased or decreased [Na+]i were in the direction predicted by the hypothesis that Na+ regulates N-VDCCs via changes in [Gβγ]. Additional lines of evidence suggest that a large part of Na+-induced inhibition, and all of Na+-induced VD facilitation, were Gβγ dependent. First and foremost, all changes in IBa and VD facilitation caused by both influx and depletion of Na+ in oocytes, and the VD facilitation caused by 20mm[Na+]i in hippocampal neurones, were blocked by Gβγ scavengers. Second, the changes in IBa and facilitation caused by Na+ depletion in the oocytes were fully blocked by overexpressed Gβγ. The latter maximally activates Gβγ-mediated processes, occluding additional modulations via this pathway (Herlitze et al. 1996; Ikeda, 1996).
Hence, our results suggest that Na+ regulation of N-VDCCs is Gβγ dependent; in the simplest case, it may be mediated by changes in [Gβγ]. However, more complex schemes are possible. It is notable that the consequences of Na+- and GPCR-induced activation of G proteins are similar only in terms of changes in [Gβγ], but not Gα. Whereas activation of GPCRs results in the appearance of free GαGTP (Gilman, 1987), Na+ appears to facilitate the dissociation of GαGDP from Gβγ (Rishal et al. 2003). The effects of Gα on N-VDCCs are not well understood although they are clearly less robust than those caused by Gβγ (Herlitze et al. 1996; Ikeda, 1996); we cannot exclude the possibility that GαGDP or GαGTP may additionally regulate the channel. A synergistic effect of Gβγ and some unknown direct action of Na+ on N-VDCCs also cannot be excluded at present.
Changes in [Gβγ] caused by changes in [Na+]i are probably very mild. Biochemical measurements suggest that Na+ causes only a 2- to 3-fold decrease in the affinity of binding between Gα and Gβγ (Rishal et al. 2003). Kinetic modelling based on Na+-induced activation of GIRK channels suggests that the Na+-induced rise in [Gβγ] is about 15–30% over the basal level, much less than that typically induced by activation of GPCRs (data not shown). While using N-VDCCs as ‘biosensors’ of [Gβγ], we found that current amplitude and VD facilitation are more sensitive indicators of moderate changes in [Gβγ] than the kinetic slowing and the shift in the I–V curve (Figs 1 and 2). One nanomolar ACh caused a 40% inhibition of IBa, correlated with a substantial change in facilitation (Δfacilitation of ∼20%), whereas the kinetic slowing and the shift in the I–V curve, though measurable, were on the verge of detection. Low doses of coexpressed Gβγ, which caused an increase in facilitation of 20–22%, also caused only slight acceleration of activation of IBa. These phenomena are qualitatively and quantitatively similar to those caused by increased [Na+]i. The effects of depletion of intracellular Gβγ by cβARK on facilitation and t90% in oocytes were also comparable to those induced by depletion of Na+. We propose that Na+ entry causes only small changes in free [Gβγ] which cause little kinetic slowing or shift in I–V curve, but already suffice to cause a meaningful inhibition of N-VDCCs, by 20–40% in Xenopus oocytes and in hippocampal neurones. A parallel clear change in the extent of VD facilitation (Δfacilitation of 15–30%) is always observed.
If all the Na+-induced inhibition of N-VDCCs in oocytes is mediated by Gβγ, should not the Gβγ-induced inhibition be fully removed by depolarizing prepulses? Not necessarily. The extent of removal of inhibition depends on the parameters of depolarizing prepulse and interpulse interval, and on the concentration of Gβγ (Zamponi & Snutch, 1998b; Herlitze et al. 2001). Incomplete relief of Go-coupled, GPCR-induced inhibition of N-VDCCs by prepulses is often observed both in neurones and in Xenopus oocytes (Ikeda, 1991; Canti et al. 2000). In the oocytes, the depolarizing prepulse relieved only 25–40% of inhibition caused by ACh. Very similar relief of inhibition, of 25–33%, was attained by depolarizing prepulses after TS-induced Na+ influx in oocytes, and after NMDA-induced Na+ influx in neurones (Figs 3 and 6). These results further support the hypothesis that a similar mechanism (i.e. an increase in [Gβγ]) underlies Na+- and GPCR-induced inhibition of N-VDCCs.
Potential physiological significance
The physiological impact of Na+-induced modulation of N-VDCCs will crucially depend on two parameters: the dynamic range of [Na+]i needed for regulation of N-VDCCs, and the time course of modulation. In hippocampal neurones, the VD facilitation, absent at 0mm[Na+]i, was well developed at 20mm[Na+]i. The extent of facilitation, ∼18%, was close to that caused by NMDA (∼14%). It appears that in Xenopus oocytes, the pathway that leads to inhibition of N-VDCCs is already operational at resting [Na+]i because: (1) depletion of resting [Na+]i affected N-VDCCs, and (2) the TS-induced Na+ influx caused a significantly stronger inhibition of IBa when Na+ was previously depleted from the cell, than on the background of resting [Na+]i. Therefore, we estimate that the resting [Na+]i, ∼10mm, lies close to the midpoint of the dynamic range of [Na+]i that inhibits N-VDCCs. The dose dependency of the Na+ effect in perfused neurones (Fig. 5C) supports this notion; the net change in facilitation in 10mm Na+ is above 10% (compared to a ‘negative’ facilitation in 0mm Na+) and becomes highly significant at 20mm Na+. The Na+-dependent dissociation of Gαβγ and the slow Na+ regulation of GIRK channels both occur in the same range, between 5 and 40mm[Na+]i (Rishal et al. 2003).
Such changes in [Na+]i are common in neurones. After short periods of synaptic activity (stimulation at 50–100Hz for 0.1–1 s), [Na+]i reached ∼30mm in most dendritic spines. In apical dendrites of CA1 hippocampal pyramidal cells, a train of three to five postsynaptic action potentials led to an elevation of 8–13mm above basal [Na+]i (Rose & Konnerth, 2001), doubling the resting [Na+]i. These relatively mild changes are well within the dynamic range of Na+ concentrations that regulate N-VDCCs. Less information is available regarding changes in presynaptic [Na+]i. However, measurable increases in Na+ levels have been reported in nerve termini of cerebellar granule cells, following short periods of activity, although the absolute values of [Na+]i have not been estimated (Regehr, 1997).
The regulation of Gαβγ dissociation by Na+ is much slower than receptor-induced dissociation, taking several tens of seconds (Rishal et al. 2003). Such long-lasting changes in [Na+]i most probably do take place in neurones. In presynaptic nerve termini, following 5–40 action potentials (at 100Hz), increased [Na+]i slowly decayed to resting levels, with two time constants of 6–7 s and 2–3min (Regehr, 1997). Dendritic [Na+]i transients caused by a mere 200ms train of five postsynaptic action potentials last for 5–10 s, far longer than the electrical activity itself (Rose & Konnerth, 2001). The presence of glutamate in the synaptic cleft following periods of extensive activity may last for tens of seconds; Na+ influx via glutamate-gated channels and neurotransmitter transporters is expected to last at least as long as the transmitter is present. Recent work shows that changes in [Na+]i caused by trains of presynaptic action potentials in dendrites of cerebellar Purkinje neurones last for tens of seconds and spread over substantial areas (Kuruma et al. 2003). We conclude that both the magnitude and the duration of changes in [Na+]i, caused by electrical activity or by certain neurotransmitters in neurones, are sufficient to modulate N-VDCCs via the Gβγ-dependent mechanism. Since this regulation is slow, it is probably not involved in robust signal transduction, but in the fine-tuning of cellular processes. It is tempting to speculate that the Na+-induced changes in [Gβγ] might affect long-lasting changes in excitability, or those forms of neuronal plasticity that are crucially dependent on postsynaptic spikes, especially paired with activation of NMDA receptors (reviewed by Linden, 1999).
Many effectors of Na+ (Na+-coupled transporters and pumps, NMDA receptor channels, voltage- and Na+-activated K+ channels, GIRK channels by the direct Na+-binding pathway) show little response to resting levels of Na+, because the EC50 for Na+ is in the range of 30–40mm. In these cases, Na+ may be acting as a second messenger: its concentration, tightly controlled in the resting cell by the Na+–K+-ATPase, transiently rises upon the appearance of a specific signal (excitatory neuronal activity), to regulate such effectors. In comparison, the regulation of N-VDCCs described here, and the slow, Gβγ-mediated activation of GIRK channels (Rishal et al. 2003), may already be operational at resting [Na+]i. Other examples of similar ‘high affinity’ modulations by Na+ (EC50 of about 10mm) are inhibition of nucleoside diphosphate kinase (Marshall et al. 1999) and inhibition of the epithelial amyloride-sensitive Na+ channels by a G protein- and ubiquitin-dependent mechanism (Ishibashi et al. 1999). Such reactions may operate in servo-type regimes, that sensitively respond to bidirectional changes in intracellular Na+ concentration.
Acknowledgments
We thank the colleagues who kindly provided the materials and the original cDNA constructs: Ravi Iyengar (QEHA), Y. Mori (α1B), E. Reuveny (cβARK), M. I. Simon (G protein subunits), H. A. Lester (NaIIA-α) and E. Liman (pGEM-HE). We are grateful to Ilana Lotan, Bernard Attali, Wolfgang Schreibmayer, Cristine Rose and Eitan Reuveny for helpful suggestions. This work was supported by grants from Israel Basic Research Fund and the US-Israel Binational Science Foundation to N.D.
References
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Bourinet E, Soong TW, Stea A, Snutch TP. Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci U S A. 1996;93:1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canti C, Bogdanov Y, Dolphin AC. Interaction between G proteins and accessory subunits in the regulation of 1B calcium channels in Xenopus oocytes. J Physiol. 2000;527:419–432. doi: 10.1111/j.1469-7793.2000.t01-1-00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1998;24:307–323. doi: 10.1016/s0143-4160(98)90055-0. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Chen J, DeVivo M, Dingus J, Harry A, Li J, Sui J, Carty DJ, Blank JL, Exton JH, Stoffel RH, et al. A region of adenylyl cyclase 2 critical for regulation by G protein βγ subunits. Science. 1995;268:1166–1169. doi: 10.1126/science.7761832. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Neer EJ. G protein βγ subunits. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22:317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Dascal N, Lotan I. Activation of protein kinase C alters voltage dependence of a Na+ channel. Neuron. 1991;6:165–175. doi: 10.1016/0896-6273(91)90131-i. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Mechanisms of modulation of voltage-dependent calcium channels by G proteins. J Physiol. 1998;506:3–11. doi: 10.1111/j.1469-7793.1998.003bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan TM, Dagan D, Kupper J, Levitan IB. Na+-activated K+ channels are widely distributed in rat CNS and in Xenopus oocytes. Brain Res. 1992;584:319–321. doi: 10.1016/0006-8993(92)90913-t. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Zhong H, Scheuer T, Catterall WA. Allosteric modulation of Ca2+ channels by G proteins, voltage-dependent facilitation, protein kinase C, and Cavβ subunits. Proc Natl Acad Sci U S A. 2001;98:4699–4704. doi: 10.1073/pnas.051628998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho IH, Murrell-Lagnado RD. Molecular mechanism for sodium-dependent activation of G protein-gated K+ channels. J Physiol. 1999;520:645–651. doi: 10.1111/j.1469-7793.1999.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol. 1991;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Dunlap K. Voltage-dependent modulation of N-type calcium channels: role of G protein subunits. Adv Sec Mess Phosphoprot Res. 1999;33:131–151. doi: 10.1016/s1040-7952(99)80008-1. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Dinudom A, Harvey KF, Kumar S, Young JA, Cook DI. Na+-H+ exchange in salivary secretory cells is controlled by an intracellular Na+ receptor. Proc Natl Acad Sci U S A. 1999;96:9949–9953. doi: 10.1073/pnas.96.17.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanina T, Blumenstein Y, Shistik E, Barzilai R, Dascal N. Modulation of L-type Ca2+ channels by Gβγ and calmodulin via interactions with N- and C-termini of α1C. J Biol Chem. 2000;275:39846–39854. doi: 10.1074/jbc.M005881200. [DOI] [PubMed] [Google Scholar]
- Kanner BI. Sodium-coupled neurotransmitter transport: structure, function and regulation. J Exp Biol. 1994;196:237–249. doi: 10.1242/jeb.196.1.237. [DOI] [PubMed] [Google Scholar]
- Kuruma A, Inoue T, Mikoshiba K. Dynamics of Ca2+ and Na+ in the dendrites of mouse cerebellar Purkinje cells evoked by parallel fibre stimulation. Eur J Neurosci. 2003;18:2677–2689. doi: 10.1111/j.1460-9568.2003.02977.x. [DOI] [PubMed] [Google Scholar]
- Linden DJ. The return of the spike: postsynaptic action potentials and the induction of LTP and LTD. Neuron. 1999;22:661–666. doi: 10.1016/s0896-6273(00)80726-6. [DOI] [PubMed] [Google Scholar]
- Marshall LJ, Muimo R, Riemen CE, Mehta A. Na+ and K+ regulate the phosphorylation state of nucleoside diphosphate kinase in human airway epithelium. Am J Physiol. 1999;276:C109–C119. doi: 10.1152/ajpcell.1999.276.1.C109. [DOI] [PubMed] [Google Scholar]
- Miller RJ. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- Normann C, Peckys D, Schulze CH, Walden J, Jonas P, Bischofberger J. Associative long-term depression in the hippocampus is dependent on postsynaptic N-type Ca2+ channels. J Neurosci. 2000;20:8290–8297. doi: 10.1523/JNEUROSCI.20-22-08290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchenko VA, Pintor J, Tsyndrenko AY, Miras-Portugal MT, Krishtal OA. Diadenosine polyphosphates selectively potentiate N-type Ca2+ channels in rat central neurons. Neuroscience. 1996;70:353–360. doi: 10.1016/0306-4522(95)00340-1. [DOI] [PubMed] [Google Scholar]
- Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N. Gαi controls the gating of the G-protein-activated K+ channel, GIRK. Neuron. 2002;33:87–99. doi: 10.1016/s0896-6273(01)00567-0. [DOI] [PubMed] [Google Scholar]
- Petit-Jacques J, Sui JL, Logothetis DE. Synergistic activation of G protein-gated inwardly rectifying potassium channels by the βγ subunits of G proteins and Na+ and Mg2+ ions. J General Physiol. 1999;114:673–684. doi: 10.1085/jgp.114.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo-Parada L, Korn SJ. Block of N-type calcium channels in chick sensory neurons by external sodium. J General Physiol. 1997;109:693–702. doi: 10.1085/jgp.109.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG. Interplay between sodium and calcium dynamics in granule cell presynaptic terminals. Biophys J. 1997;73:2476–2488. doi: 10.1016/S0006-3495(97)78276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rishal I, Keren-Raifman T, Yakubovich D, Ivanina T, Dessauer CW, Slepak VZ, Dascal N. Na+ promotes the dissociation between Gα-GDP and Gβγ, activating G-protein-gated K+ channels. J Biol Chem. 2003;278:3840–3845. doi: 10.1074/jbc.C200605200. [DOI] [PubMed] [Google Scholar]
- Roche JP, Anantharam V, Treistman SN. Abolition of G protein inhibition of α1A and α1B calcium channels by co-expression of the β3 subunit. FEBS Lett. 1995;371:43–46. doi: 10.1016/0014-5793(95)00860-c. [DOI] [PubMed] [Google Scholar]
- Roche JP, Treistman SN. The Ca2+ channel β3 subunit differentially modulates G-protein sensitivity of α1A and α1B Ca2+ channels. J Neurosci. 1998;18:878–886. doi: 10.1523/JNEUROSCI.18-03-00878.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Konnerth A. NMDA receptor-mediated Na+ signals in spines and dendrites. J Neurosci. 2001;21:4207–4214. doi: 10.1523/JNEUROSCI.21-12-04207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Regulation of intracellular sodium in cultured rat hippocampal neurones. J Physiol. 1997;499:573–587. doi: 10.1113/jphysiol.1997.sp021951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XM, Salter MW. Gain control of NMDA-receptor currents by intracellular sodium. Nature. 1998;396:469–474. doi: 10.1038/24877. [DOI] [PubMed] [Google Scholar]
- Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature. 2000;404:285–289. doi: 10.1038/35005094. [DOI] [PubMed] [Google Scholar]
- Sheng Y, Tiberi M, Booth RA, Ma C, Liu XJ. Regulation of Xenopus oocyte meiosis arrest by G protein βγ subunits. Curr Biol. 2001;11:405–416. doi: 10.1016/s0960-9822(01)00123-3. [DOI] [PubMed] [Google Scholar]
- Van Damme P, Van Den Bosch L, Van Houtte E, Eggermont J, Callewaert G, Robberecht W. Na+ entry through AMPA receptors results in voltage-gated K+ channel blockade in cultured rat spinal cord motoneurons. J Neurophysiol. 2002;88:965–972. doi: 10.1152/jn.2002.88.2.965. [DOI] [PubMed] [Google Scholar]
- Vorobiov D, Levin G, Lotan I, Dascal N. Agonist-independent inactivation and agonist-induced desensitization of the G protein-activated K+ channel (GIRK) in Xenopus oocytes. Pflugers Arch. 1998;436:56–68. doi: 10.1007/s004240050604. [DOI] [PubMed] [Google Scholar]
- Wakamori M, Strobeck M, Niidome T, Teramoto T, Imoto K, Mori Y. Functional characterization of ion permeation pathway in the N-type Ca2+ channel. J Neurophysiol. 1998;79:622–634. doi: 10.1152/jn.1998.79.2.622. [DOI] [PubMed] [Google Scholar]
- Weng G, Li J, Dingus J, Hildebrandt JD, Weinstein H, Iyengar R. Gβ subunit interacts with a peptide encoding region 956–982 of adenylyl cyclase 2. Cross-linking of the peptide to free Gβγ but not the heterotrimer. J Biol Chem. 1996;271:26445–26448. doi: 10.1074/jbc.271.43.26445. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, Catterall WA. Biochemical properties and subcellular distribution of an N-type calcium channel α1 subunit. Neuron. 1992;9:1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. Modulation of voltage-dependent calcium channels by G proteins. Curr Opin Neurobiol. 1998a;8:351–356. doi: 10.1016/s0959-4388(98)80060-3. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. Decay of prepulse facilitation of N type calcium channels during G protein inhibition is consistent with binding of a single Gβ subunit. Proc Natl Acad Sci U S A. 1998b;95:4035–4039. doi: 10.1073/pnas.95.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]