Abstract
This study focused on the hypothesis that KCNA genes (which encode KVα1 voltage-gated K+ channels) have enhanced functional expression in smooth muscle cells of a primary determinant of peripheral resistance – the small mesenteric artery. Real-time PCR methodology was developed to measure cell type-specific in situ gene expression. Profiles were determined for arterial myocyte expression of RNA species encoding KVα1 subunits as well as KVβ1, KVα2.1, KVγ9.3, BKCaα1 and BKCaβ1. The seven major KCNA genes were expressed and more readily detected in endothelium-denuded mesenteric resistance artery compared with thoracic aorta; quantification revealed dramatic differential expression of one to two orders of magnitude. There was also four times more RNA encoding KVα2.1 but less or similar amounts encoding KVβ1, KVγ9.3, BKCaα1 and BKCaβ1. Patch-clamp recordings from freshly isolated smooth muscle cells revealed dominant KVα1 K+ current and current density twice as large in mesenteric cells. Therefore, we suggest the increased RNA production of the resistance artery impacts on physiological function, although there is quantitatively less K+ current than might be expected. The mechanism conferring up-regulated expression of KCNA genes may be common to all the gene family and play a functional role in the physiological control of blood pressure.
Voltage-gated K+ channels (KV channels) are encoded by the large KCNx gene set and have a wide range of properties such that the precise expression profile is instrumental in governing the electrical phenotype of a cell and its response to extrinsic factors. Specific KV channel subtypes have established functional roles in shaping action potentials in cardiac muscle, neurones and other cell types (Coetzee et al. 1999; Nerbonne et al. 2001; MacDonald et al. 2001; Catterall et al. 2002; Song, 2002).
The roles of KV channels in shaping action potentials may make them surprising functional elements of arterial smooth muscle cells because these cells often exhibit only tonic electrical activity. However, there is substantial evidence to support a major role for this channel type. As early as 1980 it was observed that 4-aminopyridine (a blocker of many KV channels) caused tonic depolarization of guinea-pig pulmonary artery (Hara et al. 1980). Although effects of low concentrations of 4-aminopyridine were prevented by the α-adrenoceptor antagonist phentolamine (suggesting an effect via noradrenaline release from nerve terminals) effects of higher concentrations showed resistance to phentolamine, consistent with the hypothesis that KV channels also have a direct inhibitory effect in vascular smooth muscle cells – acting as a tonic physiological break on activation of voltage-gated Ca2+ channels (Knot & Nelson, 1995; Cheong et al. 2001a, b, 2002). Numerous patch-clamp studies have shown KV channel activity is common in freshly isolated contractile vascular smooth muscle cells in the physiological voltage range of −50 to 0mV (Okabe et al. 1987; Beech & Bolton, 1989; Gelband & Hume, 1992; Robertson & Nelson, 1994).
As well as KV channels, contractile vascular smooth muscle cells contain large-conductance Ca2+-activated K+ channels – the BKCa channels, which have α-subunits encoded by the Slo1 gene. Expression of BKCa channels is modulated in vascular development, ageing and hypertension (Marijic et al. 2001; Cox & Rusch, 2002) and there is an established role in hyperpolarizing signals in response to elementary intracellular Ca2+ release events (Patterson et al. 2002). Suppression of BKCa channel function by disruption of the gene encoding its β1-subunit causes elevation of blood pressure (Patterson et al. 2002). Physiologically, KV channels also have an important role. In terminal arterioles under basal conditions KV channels activate with a more negative threshold than BKCa channels and inhibition of KV, but not BKCa, channels evokes vasoconstriction; induction of pretone with endothelin-1 is necessary to confer sensitivity to blockers of BKCa and KV channels (Cheong et al. 2002). There are changes in KV channel gene expression and associated K+ currents in rat models of hypertension, and KV channels are modulated by many vasoactive substances (Martens & Gelband, 1996; Smirnov & Aaronson, 1996; Berger et al. 1998; Platts et al. 1998; Ren et al. 1999; Cox et al. 2001; Hayabuchi et al. 2001; Liu et al. 2001; Shimoda et al. 2001; Gupte et al. 2002; Heaps & Bowles, 2002; Michelakis et al. 2002; Platoshyn et al. 2002; Irvine et al. 2003).
There are 12 families of KCNx genes encoding KV channel subunits (Coetzee et al. 1999; Nerbonne et al. 2001; Catterall et al. 2002). Reverse transcriptase PCR studies indicate a large number of these genes are transcribed in blood vessels. Whether these RT-PCR signals originate from smooth muscle cells, endothelial cells, neurones or other cell types is not always completely clear. Nevertheless, the expressed genes include eight KCNA genes encoding KVα1.1–1.8, KCNB genes encoding KVα2.1–2.2, KCNC genes encoding KVα3.1–3.4, KCND genes encoding KVα4.1–4.3, the KCNH2 gene encoding KVα11.1, the KCNQ1 gene encoding KVα7.1, and the KCNS3 gene encoding KVγ9.3 (Patel et al. 1997; Archer et al. 1998; Yuan et al. 1998; Xu et al. 1999; Lang et al. 2000; Osipenko et al. 2000; Cheong et al. 2001a, b; Thorneloe et al. 2001; Ohya et al. 2002; Fergus et al. 2003; Ohya et al. 2003). Patch-clamp studies indicate heterogeneity in KV currents of vascular smooth muscle cells (Archer et al. 1996; Smirnov et al. 2002). Thus KCNx genes may be expressed differently in different blood vessels, and presumably for some purpose. It may not be enough to ask if there is all-or-nothing expression – quantitative differences may be critical. Methodological limitations have hindered progress in this area and so we have developed real-time RT-PCR assays to quantify physiological expression of RNA species encoding K+ channel subunits in arterial smooth muscle cells in situ. These assays were used to determine expression profiles of KCNx genes in a conduit artery and a resistance artery. We focused particularly on KVα1-encoding genes because of data indicating these genes have an important function in arterioles and other resistance arteries (Cheong et al. 2001a, b; Lu et al. 2002; Pozeg et al. 2003; Albarwani et al. 2003). Thus we sought to test the hypothesis that discrete KCNx genes, especially KCNA genes, are differentially expressed in resistance arteries – not in absolute terms, but quantitatively – and that this translates to functional signals. The mesenteric artery used in this study contributes a resistance at least a thousand times higher than the conduit of the thoracic aorta.
Methods
Dissection of tissues
Eight-week-old male C57/BL6 mice were killed by CO2 asphyxiation and cervical dissociation in accordance with the Code of Practice, UK Animals (Scientific Procedures) Act 1986. The thoracic aorta and mesenteric artery (approximately 0.75 and 0.2mm external diameter, respectively) were removed and placed in ice-cold Hanks' solution. Fat was removed completely by dissection and blood cells were flushed from the lumen with Hanks' solution. In some cases endothelium was removed by brief luminal perfusion with 0.1% (v/v) Triton X-100 in water, and the adventitia were removed (‘medial layer only’) by fine dissection. For the isolation of brain RNA, small cubes of cerebral cortex were cut in ice-cold Hanks' solution and snap-frozen.
RNA isolation and RT-PCR
Individual aortae or six mesenteric arteries were snap frozen on liquid N2 immediately after dissection. RNA was extracted using Tri reagent (Sigma), 250 μg glycogen added and cells disrupted using a homogenizer. RNA precipitates were re-suspended in water and digested with 6 units DNase I (Ambion) for 60 min at 37°C. RNA was quantified using Ribogreen (Molecular Probes). In all experiments 0.6 μg total RNA was reverse transcribed at 65°C for 30 min in 20 μl reactions using 6 units C. therm polymerase (Roche) and gene-specific primers (1μm). From the same sample, 0.6 μg RNA was used as a genomic DNA control in a reaction without reverse transcriptase. cDNA templates were purified on QIAquick columns (Qiagen) and quantitative real-time PCR (Bustin, 2000; Peirson et al. 2003) performed using SYBR Green I on a Roche Lightcycler. DNA amplification was for 35–40 cycles with an initial 10 min at 95°C followed by 10 s at 95°C, 6 s at 55°C, and 14 s at 72°C. All PCR primers are given in Table 1. Fluorescence was acquired at 72°C. After PCR cycling, melt-curves were generated by temperature ramps from 65 to 95°C. PCR cycle crossing-points (CP) were determined by fit-points methodology (Lightcycler software 3.5). PCR efficiency (E) was calculated as shown in Fig. 1, where E= 10(−1/slope). Relative abundance of target RNA was calculated from (Eβ−actinCp)/(EtargetCp). β-Actin RNA abundances were nevertheless not different between samples (CP values were: aorta intact, 22.2 ± 0.5; endothelium-denuded aorta, 22.9 ± 0.3; media layer of aorta, 21.9 ± 0.3; endothelium-denuded mesenteric artery, 22.2 ± 0.5; n= 4, P > 0.05, ANOVA, e.g. Fig. 1E). All quantified amplicons had identity confirmed by direct sequencing (Lark, UK).
Table 1.
PCR primer pairs and PCR amplicons
| Gene | Accession no | Primer 5′–3′ | Amplicon size (bp) | Amplicon Tm (°C) | Efficiency* |
|---|---|---|---|---|---|
| β-Actin | NM_007393 | F1 CACTATTGGCAACGAGC | 126 | 83.1 | 1.82 ± 0.05 |
| R1CGGATGTCAACGTCAC | |||||
| KCNA1 | NM_010595 | F1TCTAGCGCAGTGTACTT | 378 | 88.3 | 1.76 ± 0.05 |
| (KV α1.1) | R1GGCTATGCTATTGTTCATA | ||||
| KCNA2 | NM_008417 | F1TCGATCCCCTCCGAAA | 192 | 86.5 | 1.81 ± 0.03 |
| (KV α1.2) | R1CTAAGGGCACGTTCACA | ||||
| F2CACCCACAAGACACCT | 114 | 85.1 | 1.96 ± 0.02 | ||
| R2GGCGGTTGCGATCAAA | |||||
| KCNA3 | NM_008418 | F1GCTTCCCGAGTTTCGC | 298 | 88.9 | 1.89 ± 0.06 |
| (KV α1.3) | R1CCCATTACCTTGTCGTTC | ||||
| F2AGGACAGACGCTGAAG | 287 | 87.6 | 1.86 ± 0.03 | ||
| R2AGTTGGAAACAATCACAGG | |||||
| KCNA4 | NM_021275 | F1CCCTAAGAGCCAGCAT | 245 | N.D. | N.D. |
| (KV α1.4) | R1GGTTAAGACACCCGCA | ||||
| KCNA5 | NM_008419 | F1GAGCCGTTGAAGTGGT | 215 | 81.5 | 2.10 ± 0.05 |
| (KV α1.5) | R1AAATGCACTCGTCAGC | ||||
| KCNA6 | NM_013568 | F1CGCTGTCTACTTCGCAG | 380 | N.D. | N.D. |
| (KV α1.6) | R1CTCGATGTGGAGTCGG | ||||
| KCNA7 | NM_010596 | F1CCTAAGGGTCATCCGA | 259 | N.D. | N.D. |
| (KV α1.7) | R1CCCATAGCCAACCGTG | ||||
| KCNAB1 | NM_010597 | F1AAATGACGGTGTGAGT | 127 | 84.8 | 2.17 ± 0.01 |
| (KV β1) | R1CAGTATGTTATCAATCTCG | ||||
| KCNB1 | NM_008420 | F1AGCAATAGCGTTCAACTT | 239 | 87.0 | 1.78 ± 0.06 |
| (KV α2.1) | R1GTAACCCTTCTGAGGCG | ||||
| KCNS3 | XM_137966 | F1CCAGTCACCTTAGCTGG | 208 | 84.6 | 1.98 ± 0.03 |
| (KV γ9.3) | R1GCTGTGCATAAACGTCC | ||||
| KCNMA1 | NM_010610 | F1GGACCAAGACGATGAC | 293 | 88.3 | 1.78 ± 0.02 |
| (BKCa α1) | R1CTGCAAAGGGACCATC | ||||
| F2ATGAGCGCGACATACT | 287 | 88.4 | 1.85 ± 0.02 | ||
| R2TCTCTCAGCCGGTAAATTC | |||||
| KCNMB1 | BC_013338 | F1AAGACACTCGGGATCAAA | 167 | 84.8 | 1.87 ± 0.02 |
| (BKCa β1) | R1CTGGTACACGACGCTG |
Mean ±s.e.m. efficiency determined for ≥ 4 independent cDNA templates.
N.D., not determined. F, forward; R, reverse.
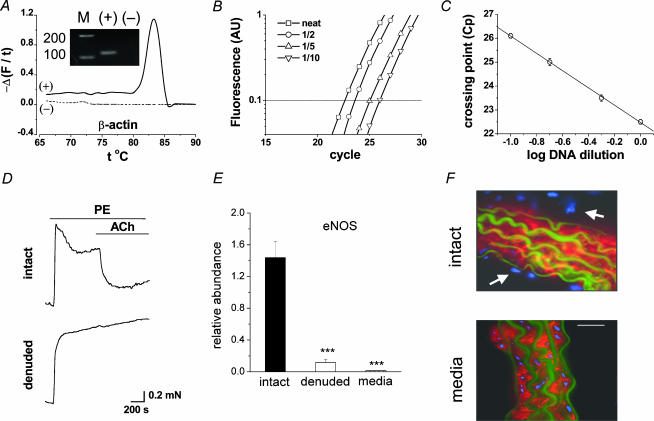
Figure 1. Expression profiling by real-time RT-PCR.
A–C, the method using aortic β-actin as an example. A, end-point PCR ‘melt-curve’ showing change of SYBR Green fluorescence with temperature and (inset) the PCR products on an agarose gel (predicted size, Table 1); + and – indicates plus and minus reverse transcriptase reaction; M, DNA markers (bp). B, SYBR Green fluorescence against PCR cycle for the reaction in A and dilutions of cDNA as indicated. C, for triplicate reactions on a cDNA sample, mean ±s.e.m. CP (i.e. intersection with the horizontal dotted line shown in B). The fitted line has a correlation coefficient of 0.99. D, tension in parallel aortic rings – one with endothelium removed. PE, phenylephrine (10 μm); and ACh, acetylcholine (1 μm). E, real-time PCR for aortic RNA encoding eNOS relative to β-actin (***P < 0.01). F, sections of intact aorta and medial-layer stained with smooth muscle α-actin antibody (red) and DAPI (blue, showing all cell nuclei). Autofluorescence of elastic laminae is in green. Scale bar, 20 μm.
Western blotting and immunostaining
Protocols were standard and largely as previously described (Cheong et al. 2001b). Lysates for Western blots were prepared from intact aortae. Antibodies were rabbit polyclonal anti-rat KVα1.1(458–475) or anti-rat KVα1.3(456–474) (1: 500; Cheong et al. 2001b) or mouse monoclonal Cy3-conjugated smooth muscle α-actin antibody (1: 200; Sigma). Control reactions were run in parallel in the absence of primary antibody. Sections were mounted in Vectashield (Vector Laboratories) with or without 4′,6′-diamino-2-phenyindole hydrochloride (DAPI). Staining was viewed using a Plan Apochromat × 63 objective (NA 1.4) on a Zeiss Axiovert microscope equipped with a 12-bit charge-coupled device camera (Orca-ER, Hamamatsu, Japan). Images were sampled at five focal planes separated by 0.5 μm, background subtracted, and haze removed by a deconvolution algorithm (Openlab software, Improvision; Coventry, UK).
Wire myography
Vessels were mounted on two 40-μm diameter wires for isometric tension recording in a 410A dual wire myograph system (Danish Myo Technology, Denmark). The bath solution was at 36°C and gassed continuously with air–5% CO2. It contained (mm): NaCl, 125; KCl, 3.8; NaHCO3, 25; MgSO4 1.5; KH2PO4, 1.2; d-glucose, 8; CaCl2, 1.2; EDTA, 0.02.
Patch-clamp recording
Aortae were incubated in Hanks' solution containing 0.15 mg ml−1 collagenase, 0.1 mg ml−1 protease, 0.13 mg ml−1 hyaluronidase and 475 Units elastase for 1 h at 4°C followed by 15 min at 37°C. Mesenteric arteries were incubated for 30 min at 37°C in 1 mg ml−1 collagenase, 0.5 mg ml−1 protease and 1 mg ml−1 hyaluronidase. Enzymes were removed and tissue agitated with a Pasteur pipette. Myocytes were used within 8 h. Recordings were made at room temperature using conventional whole-cell recording. Signals were amplified and sampled using an Axopatch 200B amplifier and pCLAMP 8 software (Axon Instruments). Signals were filtered at 1 kHz and sampled at 2 kHz. Patch pipettes had resistance of 3–5 MΩ. Hanks' solution contained (mm): NaCl, 137; KCl, 5.4; CaCl2, 0.01; NaH2PO4, 0.34; K2HPO4, 0.44; d-glucose, 8; Hepes, 5. The bath solution for KV current contained (mm): NaCl, 135; KCl, 5; d-glucose, 8; Hepes, 10; MgCl2, 4 (for BKCa current, 1.5mm CaCl2 and 1.2mm MgCl2 replaced 4mm MgCl2). The patch pipette solution contained (mm): NaCl, 5; KCl, 130; Hepes, 10; Na2ATP, 3; MgCl2, 2; EGTA, 5 (for BKCa current, 0.05 EGTA replaced 5mm EGTA). The pH of all solutions was titrated to pH 7.4 using NaOH.
Heterologous expression of KV channels
Xenopus laevis were killed by an overdose of tricaine anaesthetic followed by destruction of the brain and spinal cord. cRNA was prepared from linearized cDNA templates encoding human KVα1.6 (accession number NP002226), rat KVα2.1 (accession number P15387) and rat KVγ9.3 (accession number O88759). Oocytes were prepared and injected with cRNA as previously described (Cheong et al. 2001a). For KVγ9.3 and KVα2.1 coexpression studies cRNAs were injected in a ratio of 3: 1. Recordings were made 2 days after injection using a GeneClamp 500 amplifier (Axon Instruments) for two-electrode voltage clamp (TEVC). The extracellular bathing solution during recordings was Ringer solution (115mm NaCl, 2mm KCl, 1.8mm CaCl2, 10mm Hepes, pH 7.4).
Chemicals
Correolide Compound C (‘Correolide C’, Merck) and penitrem A (Sigma) were prepared as 10mm stocks in 100% DMSO. Arachidonic acid (Sigma) was prepared as a 100mm stock in 100% ethanol. The concentration of DMSO/ethanol in experiments did not exceed 0.01% (v/v). All other chemicals were from Sigma (Poole, UK).
Statistics
All data are given as means ±s.e.m. and significance was determined by Student's unpaired t test and ANOVA where P < 0.05 was accepted as significant. The n values indicate the number of independent experiments from separate animals except for the patch-clamp experiments where n is the number of cells from which recordings were made.
Results
Irrespective of the amplicon detection method, quantitative PCR depends on a highly specific reaction. Co-amplification of other products adversely affects the specific reaction, altering the PCR cycle cross-point (CP) that is the basis of the quantification. Specificity of all reactions was confirmed by melt-curve and gel electrophoresis (Figs 1A and 2). PCR efficiency also has major impact on quantification. Efficiency was determined for each experiment by dilution of the cDNA sample (Fig. 1B). Only straight-line fits of data with correlation coefficients ≥ 0.99 were accepted, showing the efficiency values were constant over the ranges of cDNA concentrations studied (e.g. Fig. 1C).
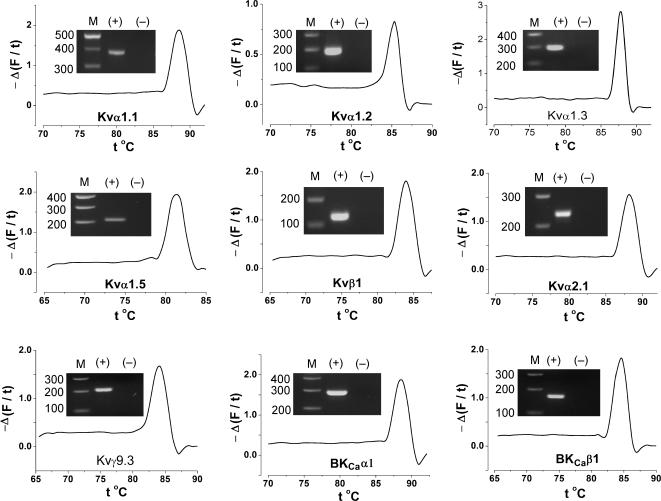
Figure 2. Specificity of detection in aorta samples using F1/R1 PCR primer sets (Table 1).
Melt curve analyses are shown with inset agarose gels.
To determine if signals originated from endothelial cells (Cheong et al. 2001a) we adopted a method for endothelium denudation (see Methods), which completely abolished relaxant responses to 1–100μm acetylcholine without damaging contractile function (Fig. 1D, n > 10 intact and denuded aortae). This was combined with real-time PCR on the assumption that a shift in the CP value will occur if RNA originated from the endothelial cells. Analysis of RNA from vessels tested by myography showed that endothelium-denudation reduced the mean abundance of RNA encoding endothelial nitric oxide synthase (eNOS) by 92% (n= 3) (Fig. 1E). Isolation of the aortic media (i.e. removal of the adventitia as well as endothelium) reduced eNOS abundance by a further 7%(n= 3) (Fig. 1E). Staining of tissue sections confirmed endothelium and adventitia had been deleted (Fig. 1F).
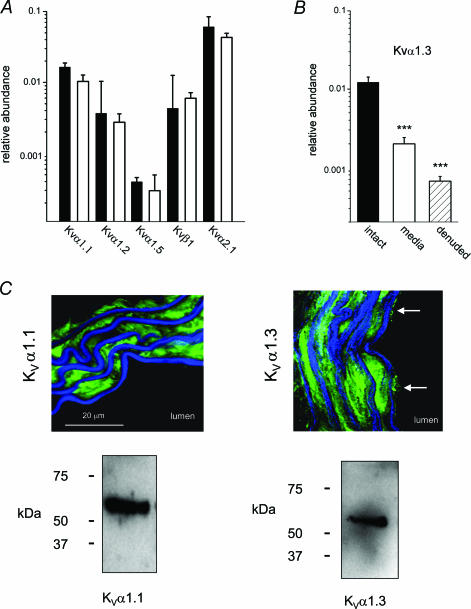
Specific quantitative PCR reactions were developed for a range of RNA species (Fig. 2). RNAs encoding KVα1.4, KVα1.6 and KVα1.7 were detected but abundances were low in aorta and quantitative reactions could not be developed (see below). Expression of protein was confirmed for KVα1.1–6, KVα2.1 and BKCaα1, BKCaβ1 (Fig. 3, or data not shown). KV1.4 and KV1.6 proteins signals were weak, consistent with the RT-PCR analysis. RNA abundances for species encoding KVα1.1, KVα1.2, KVα1.5, KVβ1 and KVα2.1 were not significantly different between intact aorta and medial layer of aorta (Fig. 3A). In other words, removal of the endothelium and adventitia did not cause a shift in the CP values, suggesting RNA for these genes was not present at significant levels in endothelial or adventitial cells. Therefore, the measurements give the in situ RNA amounts in physiological aortic smooth muscle cells. By contrast, the abundance of RNA encoding KVα1.3 was lower in the medial layer-only samples, as well as in samples with only the endothelium removed (Fig. 3B). Therefore, KVα1.3 RNA occurs in endothelial cells and presumably at a higher concentration compared with the smooth muscle cells. It follows that KVα1.3 protein should be in endothelial and smooth muscle cells. This was confirmed by antibody labelling experiments (Fig. 3C). The RT-PCR data also indicate that KVα1.1 is in the smooth muscle. Consistent with this, KVα1.1 protein was only detected in the smooth muscle cells (Fig. 3C).
Figure 3. Smooth muscle origin of signals and protein localization.
F1/R1 PCR primer sets were used (Table 1). A, mean ±s.e.m. relative RNA abundances for species encoding KVα1.1, KVα1.2, KVα1.5, KVβ1 and KVα2.1 comparing intact aorta (black bars) and medial layer only (white bars). ***P < 0.01, n= 4. B, mean ±s.e.m. relative RNA abundances for KVα1.3, comparing medial layer-only (white bar), endothelium-denuded adventitia intact (hatched bar) and intact aorta (black bar). ***P < 0.01, n= 4. C, cross-sections of aorta labelled with anti-KVα1.1 antibody (left, green) or anti-KVα1.3 antibody (right, green) with autofluorescence of elastic laminae in blue. Endothelial cells were positive for KVα1.3 (arrows) but not KVα1.1. The scale bar applies to both images. Lower panels show Western blots for aorta lysates stained for KVα1.1 (predicted mass, 57 kDa) or KVα1.3 (predicted mass, 58 kDa).
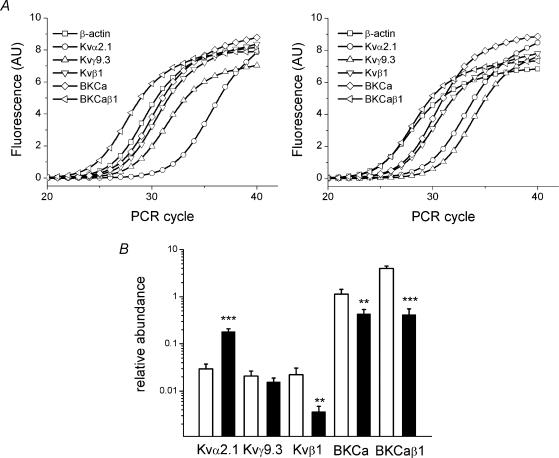
Comparisons were made between the abundances of RNA species encoding KVα1.1–3 and KVα1.5 in aorta and mesenteric artery (Fig. 4A and B). There was 20–140 times more RNA encoding KVα1.1–3 and KVα1.5 in the mesenteric artery. RNA encoding KVα1.4, 6 and 7 was more readily detected in mesenteric artery, but not quantified (Fig. 4C). Therefore, seven KCNA gene family members are more expressed at the RNA level in the resistance artery. KVα1 subunits heteromultimerize and so the sum of these RNA species is relevant. Assuming abundances of RNAs encoding KVα1.4, 6 and 7 were half those of RNA encoding KVα1.5 the sum of the KVα1-encoding RNAs in mesenteric artery was 55 times that of the aorta (the difference is 43 times if KVα1.4, -6 and -7 are excluded). There were also differences for other K+ channel genes but not as marked as for KVα1 (Fig. 5A and B). RNA encoding KVα2.1 was four times more abundant in mesenteric artery but RNA encoding its associated γ-subunit (KVγ9.3) was not proportionately increased. Abundances of RNA species encoding KVβ1, BKCaα1 and BKCaβ1 were significantly lower in mesenteric artery (Fig. 5A and B).
Figure 4. Conduit versus resistance artery for RNA encoding KVα1.
Data are for endothelium-denuded vessels. Adventitia were intact. F1/R1 PCR primer sets were used (Table 1). A, SYBR Green fluorescence plotted against PCR cycle for typical reactions. B, mean ±s.e.m. abundances for aorta (white bars) and mesenteric artery (black bars) (***P < 0.01 mesenteric versus aorta, n= 4). C, end-point PCR products on agarose gels for RNA species indicated. DNA standards were run on the left side of each gel. Templates were from aorta (‘A’), mesenteric artery (‘M’) and mouse brain samples. Presence (+) or absence (−) of reverse transcriptase reaction.
Figure 5. Expression of RNAs encoding non-KVα1 subunits.
Protocols and labelling are as for Fig. 4A and B. F1/R1 PCR primer sets were used (Table 1). B, **P < 0.05, ***P < 0.01 mesenteric versus aorta, n= 4.
Our technical approach for quantifying RNA expression in different blood vessels was validated using additional PCR primers sets for KVα1.2, KVα1.3 and BKCaα1 (F2 and R2, Table 1). Using these primer sets, KVα1.2 expression relative to β-actin was 0.0053 ± 0.0008 in aorta compared with 0.0674 ± 0.0109 in mesenteric artery, 13 times more in mesenteric artery (P < 0.01, n= 4). KVα1.3 expression was 0.001 ± 0.0001 in aorta compared with 0.064 ± 0.0133 in mesenteric artery, 64 times more in mesenteric artery (P < 0.01, n= 4), and BKCaα1 expression was 0.827 ± 0.1428 in aorta compared with 0.315 ± 0.0302 in mesenteric artery, 3 times more in aorta (P < 0.01, n= 4). These data sets are not significantly different from those shown in Figs 4 and 5. Therefore, variables such as PCR efficiency (Table 1) do not impact substantially on the outcome.
The above data show RNA levels of KVα1 genes are up-regulated in mesenteric artery smooth muscle cells. It follows that there might be more functional KVα1 channel protein in these cells. To test this idea the amplitudes of functional KVα1 signals in aortic and mesenteric smooth muscle cells must be quantified under the same conditions. Although studies of contractile function can reveal roles of KVα1 channels (e.g. Cheong et al. 2001a, b) the two vessels cannot be compared under conditions giving the same membrane potential and intracellular Ca2+ concentration, factors that determine the amplitude of KVα1 signals. Therefore, we chose to measure ionic currents through KVα1 channels in freshly isolated smooth muscle cells under voltage-clamp. To separate KVα1 current from other signals we required a method for specific block of all KVα1 channels. We previously used correolide but found its effect occurred slowly, hampering the generation of quantitative data (Cheong et al. 2001a). Therefore, we explored the effects of Compound C, a derivative of correolide (Koo et al. 1999), favouring this over the use of toxin inhibitors of KVα1 because KVα1.5 is ‘toxin-resistant’, compromising toxin-block of other KVα1 subunits (Cheong et al. 2001a, b). We refer to Compound C as ‘correolide-C’. Correolide-C blocks KVα1.6 channels expressed in Xenopus oocytes (Fig. 6A and B). To explore the specificity of correolide-C we tested it against KVα2.1 and KVα2.1 + KVγ9.3, which are expressed in the mouse blood vessels (see above). There was no effect of correolide-C (Fig. 6C and D). The data suggest correolide-C is a fast-acting and specific inhibitor of KVα1 channels.
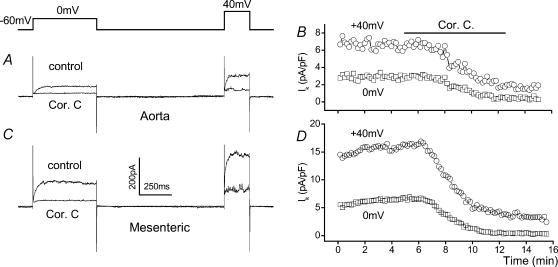
Figure 6. Specificity of correolide-C (Cor. C, 1 μm) as a blocker of KVα1.
A, current–voltage (I–V) relationships for KVα1.6–expressing Xenopus oocytes before (○) and after (•) application of 1μm Cor. C. Cells were held at −80mV and I–V relationships generated by applying 0.5 s incremental 10mV depolarizing pulses at 0.1 Hz. Mean ±s.e.m. normalized currents (n= 4 for each data set). Currents for control water-injected oocytes are also shown (□), n= 4 for each. The smooth curves are fitted modified Boltzmann functions (Cheong et al. 2001a). B, time series for KVα1.6 current at 0mV. Typical current traces are inset with the voltage step above. C–D, lack of effect of Cor. C on KVα2.1- or KVα2.1 + KVγ9.3-mediated currents in Xenopus oocytes. C, comparison of KVα2.1 alone (○) with coinjected KVα2.1 + KVγ9.3 (•) conductance–voltage relationships verifying expression of KVγ9.3 (n= 4 for each). The fitted Boltzmann functions indicated the half-activation voltage shifted significantly (P < 0.05) from +3.1 ± 1.0mV to −10.5 ± 0.5mV (n= 4), consistent with previous studies (e.g. Kerchensteiner & Stocker, 1999). D, upper panels show representative currents elicited by steps to 0mV for KVα2.1 and KVα2.1 + KVγ9.3-injected oocytes with and without Cor. C. Lower panels show mean ±s.e.m. data (n= 4, ***P < 0.01) for currents in the presence of Cor. C or 5mm tetraethylammonium (TEA) normalized to the amplitude of current before application of the blocker.
Correolide-C had a marked inhibitory effect on voltage-dependent K+ current in isolated smooth muscle cells, causing steady-state block of 88.0 ± 6.23% and 68.8 ± 9.17% at 0mV and +40mV in mesenteric artery myocytes, and 79.0 ± 6.8% and 66.9 ± 8.82% at 0mV and +40mV in aortic myocytes (n= 8 for each, 5–6 animals) (Fig. 7A–D). Correolide-C-sensitive current density was approximately twice as large in myocytes from mesenteric artery compared with aorta (4.25 ± 0.71 pA pF−1 cf. 2.05 ± 0.6 pA pF−1 at 0mV and 10.0 ± 1.93 pA pF−1 cf. 4.58 ± 1.02 pA pF−1 at +40mV, n= 8 cells for each; P < 0.05). Therefore, higher KVα1 RNA levels in mesenteric artery are associated with greater, but more modest, functional KVα1 protein expression.
Figure 7. Amplitude of functional KVα1 signals in smooth muscle cells from aorta and mesenteric artery.
Correolide-C was applied at 1 μm.A–D, whole-cell patch-clamp recordings from smooth muscle cells using the ‘KV’ recording solutions and a holding potential of −60mV. A, typical current traces from an aortic cell. B, time-series plot for experiment in A and showing current densities measured at the ends of the voltage steps to 0 and +40mV. C and D, as in A and B, except for mesenteric artery.
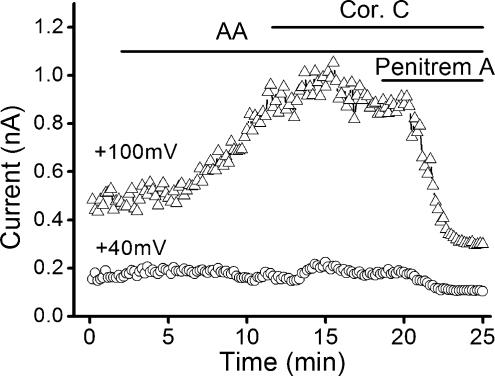
Although, in the above experiments, we used a Ca2+-free bath solution and a relatively high Ca2+ buffering capacity in the patch pipette, current through BKCa channels may contribute when recording from smooth muscle cells (e.g. Cheong et al. 2002). Therefore, we checked whether correolide-C has an effect on the smooth muscle BKCa current. The basal BKCa current was small but could be induced strongly by the addition of arachidonic acid (Fig. 8). The signal was sensitive to the BKCa inhibitor penitrem A (Cheong et al. 2002) but completely resistant to correolide-C (Fig. 8).
Figure 8. Lack of effect of correolide-C on Ca2+-activated K+ channels.
Whole-cell recording from an aortic myocyte using the ‘BKCa’ recording solutions. The cell was held at 0mV and depolarized to +40mV for 0.5 s and to +100mV during a 0.5 s ramp. Arachidonic acid (AA, 10μm) was applied to enhance BKCa current. Correolide-C (1μm) had no effect. Presence of BKCa current was confirmed by application of penitrem A (100 nm).
Discussion
Real-time quantitative RT-PCR methodology has been developed and validated for specific RNAs expressed physiologically in smooth muscle cells of murine arteries. Expression of all of the KCNA gene family members, with the exception of one distantly related member (Lang et al. 2000), was shown to be markedly up-regulated in mesenteric resistance artery compared with the conduit of the thoracic aorta. In contrast, expression of other K+ channel genes was down-regulated such that, for example, there was more than a 100-fold increase in the ratio of total KVα1 to BKCaα1. Use of a novel specific inhibitor of KVα1 channels enabled measurement of the total KVα1 signal in freshly isolated smooth muscle cells and this showed enhanced RNA expression translates partially to physiological function.
A critical issue in RT-PCR studies is the cell type from which the signal originates – in this case the arterial smooth muscle cell in its physiological contractile state. To achieve this focus we adopted a novel deletion approach. This involves validated removal of the endothelium and adventitia followed by quantitative measurement of gene expression. If RNA from the endothelium or adventitia contributes to the RT-PCR signal a shift of the CP value is expected to occur if either layer is removed. For some RNA species we observed such a shift and for others we did not. A criticism of this method might be that it is uncertain whether medial layer samples are devoid of contamination from non-smooth muscle cell types. For example, although isolation of aortic media reduced the eNOS signal by 99%, the RNA species was still detected. This might be due to expression of eNOS in smooth muscle cells (Teng et al. 1998). However, the key factor in the deletion method is quantification. If the concentration of a specific RNA species were greater in endothelial cells there would be a rightward shift in the PCR crossing-point (CP) after removal of the endothelium. This was strikingly the case for KVα1.3-encoding RNA, but not other RNA species encoding K+ channels. For genes encoding channels such as KVα1.2 our deletion approach enables highly quantitative in situ RT-PCR with RNA isolated from freshly dissected snap-frozen tissue in which the smooth muscle cells are ‘in situ’ within their elastic laminae. There is no significant contamination from endothelial or adventitial cells and there is the significant advantage that multiple genes can be analysed and compared in the same sample.
KVα1.2 and 1.5 coimmunoprecipitate, and heterologous coexpression confers properties comparable to those of a component of the native KV current in portal vein myocytes (Kerr et al. 2001). Expression data have indicated these two subunits dominate in vascular smooth muscle (Cox et al. 2001; Cox & Rusch, 2002; Albarwani et al. 2003). However, there is also expression of other KVα1 subunits in vascular smooth muscle (reviewed in Cheong et al. 2001b) and our new quantitative data reveal in situ expression of RNA species encoding seven of the KVα1 subunits in murine aorta and mesenteric artery. Although KVα1.2- and 1.5-encoding RNAs are expressed, they do not stand out, and RNA encoding KVα1.5 is difficult to detect in aorta. We have also detected KVα1.1–1.6 in the medial layer of human saphenous vein and thus the expression of several KVα1 subunits is not peculiar to mouse (S. J. Fountain, N. Quinton, C. Munsch & D. J. Beech, unpublished observations). Consistent with the expression of KVα1.1 we observe that 5mm tetraethylammonium (TEA+) inhibits the correolide-C-sensitive current (A. Cheong, P. Jackson & D. J. Beech, unpublished observations). KVα1.1 is the most TEA+ sensitive of the KVα1 subunits (Coetzee et al. 1999). Dendrotoxin-K inhibits KVα1.1 but has only a weak effect on the correolide-C-sensitive current (A. Cheong & D. J. Beech, unpublished observations). We presume this is because the coexpression of many other KVα1 subunits – especially KVα1.5 – inhibits toxin binding (Hatton et al. 2001).
In a quantitative analysis of the impact of KVα1, all subunits should be considered because they heteromultimerize into channel complexes. It follows that disruption of only one KCNA gene may have little effect on the vasculature because all the KVα1 subunits have similar electrophysiological properties. This may be why mice with a single disruption of a KCNA gene are not obviously hypertensive (Nerbonne et al. 2001). Although at this general level KCNA genes may have equivalent functions in vascular smooth muscle it is also apparent that there is differential expression between different blood vessels. The functional relevance of such differences is, however, unknown. We can only speculate that there may be regulatory implications in terms of effects of phosphorylation, subunit assembly, implications for fine-tuning of the membrane potential, or impact on cell-cycle progression as well as contractile function (Chittajallu et al. 2002; Zhu et al. 2003).
We show that there is not only up-regulation of RNA levels in resistance artery but that this translates to an increase in functional KVα1 protein at the plasma membrane (i.e. K+ current). Therefore, we assume the up-regulated RNA production is physiologically important in resistance artery function. A doubling in the amplitude of K+ current will have major inhibitory impact on voltage-dependent Ca2+ entry, especially in the context of a high resistance plasma membrane such as that of the vascular smooth muscle cell (Nelson & Quayle, 1995; Quinn et al. 2000). It is also striking, however, that there is the quantitative discrepancy between RNA and K+ current; the sum of the KVα1-encoding RNAs in resistance artery was about 50 times that of the conduit, where as the correolide C-sensitive (KVα1) current amplitude was about two-times bigger. There should not be surprise at this quantitative discrepancy. Studies of yeast and bacteria have previously revealed similar differences between RNA levels, protein levels and protein function (Gygi et al. 1999; Glanemann et al. 2003). A static correlation between levels of mRNA, protein and active protein is highly unlikely given the existence of such an array of complex post-translation mechanisms. It is only now, as we start to measure RNA levels accurately and relate them to proteins and their function, that we are can see such relationships. Ours is amongst the first of such studies, although a recent real-time PCR study of BKCa channels in myometrial smooth muscle has also revealed a perhaps surprising relationship between RNA and protein (Eghbali et al. 2003). At this stage we can only speculate on why there should be an apparent inefficiency in translation or suppressive post-translational effect in the mesenteric artery. One explanation could relate to our observation that there is lower expression of KVβ1 RNA because KVβ1 protein is a chaperone for KVα1-subunits (Manganas & Trimmer, 2000; Campomanes et al. 2002). But this may only be part of a large system in which there is regulation also at the levels of transcription, translation, protein trafficking to the membrane and protein degradation. Our data indicate there may be a stop-tap at the higher levels, restricting the impact of higher transcriptional activity. However, it is also premature to rule out the impact of acute regulation on channel activity because α-subunits could be in the membrane but non-functional. This type of effect was striking for the BKCa channel (Fig. 8).
Thyrotropin-releasing hormone, insulin-like growth factor-1, Bcl-2 oncoprotein, c-Jun immediate early gene, chronic hypoxia, cyclic-adenosine-monophosphate and glucocorticoids all affect expression of genes encoding KVα1 channels of vascular myocytes or other cell types (Mori et al. 1993; Allen et al. 1998; Levitan & Takimoto, 1998; Zhang et al. 2000; Ekhterae et al. 2001; Platoshyn et al. 2001; Yu et al. 2001; Gamper et al. 2002). Whether any of these underlie the up-regulated expression in mesenteric artery is unknown. Chronic hypoxia, however, seems unlikely from a physiological perspective and because it affected KCNA gene expression in pulmonary but not mesenteric myocytes (Platoshyn et al. 2001). Our study firmly establishes the principle that in situ expression of genes encoding KVα1 channels in smooth muscle is physiologically up-regulated upon progression to resistance mesenteric artery. The effect is dramatic and consistent with a ‘competitive’ RT-PCR study of two KVα1-encoding genes in endothelium-intact rat arteries (Cox et al. 2001). Importantly we now show this is a smooth muscle phenomenon, that it extends to essentially the whole family of KVα1-encoding genes, and that it translates to a physiological signal. The fact that up-regulation is common across the family of KVα1-encoding genes should facilitate efforts to elucidate the underlying mechanism. Intriguingly, KCNA genes are clustered primarily in loci of two chromosomes in mouse and human: KCNA6, -1 and -5 in series on human chromosome 12, and KCNA8, -2 and -3 in series on human chromosome 1. A consequence might be that elements of their transcriptional control are common. The findings of this study support this supposition and indicate that such a mechanism would have a role in the physiological control of blood pressure and local tissue perfusion.
Acknowledgments
The work was supported by the Medical Research Council (UK) and the British Heart Foundation. We thank G. Kaczorowski (Merck, USA) for the gift of correolide compound C, H. G. Knaus (Innsbruck, Austria) for anti-KVα1 antibodies, and G. Bentley (Leeds, UK), J. B. C. Findlay (Leeds, UK) and A. J. Patel (Valbonne, France) for KVα2.1-, KVα1.6- and KVγ9.3-encoding cDNAs, respectively.
References
- Albarwani S, Nemetz LT, Madden JA, Tobin AA, England SK, Pratt PF, Rusch NJ. Voltage-gated K+ channels in rat small cerebral arteries: molecular identity of the functional channels. J Physiol. 2003;551:751–763. doi: 10.1113/jphysiol.2003.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen ML, Koh DS, Tempel BL. Cyclic AMP regulates potassium channel expression in C6 glioma by destabilizing Kv1.1 mRNA. Proc Natl Acad Sci U S A. 1998;95:7693–7698. doi: 10.1073/pnas.95.13.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL, Huang JM, Reeve HL, Hampl V, Tolarova S, Michelakis E, Weir EK. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ Res. 1996;78:431–442. doi: 10.1161/01.res.78.3.431. [DOI] [PubMed] [Google Scholar]
- Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ, Bolton TB. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger MG, Vandier C, Bonnet P, Jackson WF, Rusch NJ. Intracellular acidosis differentially regulates KV channels in coronary and pulmonary vascular muscle. Am J Physiol. 1998;275:H1351–H1359. doi: 10.1152/ajpheart.1998.275.4.H1351. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Campomanes CR, Carroll KI, Manganas LN, Hershberger ME, Gong B, Antonucci DE, Rhodes KJ, Trimmer JS. KV beta subunit oxidoreductase activity and KV1 potassium channel trafficking. J Biol Chem. 2002;277:8298–8305. doi: 10.1074/jbc.M110276200. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Chandy KG, Gutterman GA. The IUPHAR Compendium of Voltage-Gated Ion Channels. UK: Nightingale Press; 2002. [Google Scholar]
- Cheong A, Dedman AM, Beech DJ. Expression and function of native potassium channel (KVα1) subunits in terminal arterioles of rabbit. J Physiol. 2001a;534:691–700. doi: 10.1111/j.1469-7793.2001.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong A, Dedman AM, Xu SZ, Beech DJ. KVα1 channels in murine arterioles: differential cellular expression and regulation of diameter. Am J Physiol Heart Circ Physiol. 2001b;281:H1057–H1065. doi: 10.1152/ajpheart.2001.281.3.H1057. [DOI] [PubMed] [Google Scholar]
- Cheong A, Quinn K, Dedman AM, Beech DJ. Activation thresholds of KV, BK and Cl(Ca) channels in smooth muscle cells in pial precapillary arterioles. J Vasc Res. 2002;39:122–130. doi: 10.1159/000057761. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Chen Y, Wang H, Yuan X, Ghiani CA, Heckman T, McBain CJ, Gallo V. Regulation of KV1 subunit expression in oligodendrocyte progenitor cells and their role in G1/S phase progression of the cell cycle. Proc Natl Acad Sci U S A. 2002;99:2350–2355. doi: 10.1073/pnas.042698399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz dM, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Cox RH, Lozinskaya I, Dietz NJ. Differences in K+ current components in mesenteric artery myocytes from WKY and SHR. Am J Hypertens. 2001;14:897–907. doi: 10.1016/s0895-7061(01)02145-8. [DOI] [PubMed] [Google Scholar]
- Cox RH, Rusch NJ. New expression profiles of voltage-gated ion channels in arteries exposed to high blood pressure. Microcirculation. 2002;9:243–257. doi: 10.1038/sj.mn.7800140. [DOI] [PubMed] [Google Scholar]
- Eghbali M, Toro L, Stefani E. Diminished surface clustering and increased perinuclear accumulation of large conductance Ca2+-activated K+ channel in mouse myometrium with pregnancy. J Biol Chem. 2003;278:45311–45317. doi: 10.1074/jbc.M306564200. [DOI] [PubMed] [Google Scholar]
- Ekhterae D, Platoshyn O, Krick S, Yu Y, McDaniel SS, Yuan JX. Bcl-2 decreases voltage-gated K+ channel activity and enhances survival in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C157–C165. doi: 10.1152/ajpcell.2001.281.1.C157. [DOI] [PubMed] [Google Scholar]
- Fergus DJ, Martens JR, England SK. Kv channel subunits that contribute to voltage-gated K+ current in renal vascular smooth muscle. Pflugers Arch. 2003;445:697–704. doi: 10.1007/s00424-002-0994-7. [DOI] [PubMed] [Google Scholar]
- Gamper N, Fillon S, Huber SM, Feng Y, Kobayashi T, Cohen P, Lang F. IGF-1 up-regulates K+ channels via PI3-kinase, PDK1 and SGK1. Pflugers Arch. 2002;443:625–634. doi: 10.1007/s00424-001-0741-5. [DOI] [PubMed] [Google Scholar]
- Gelband CH, Hume JR. Ionic currents in single smooth muscle cells of the canine renal artery. Circ Res. 1992;71:745–758. doi: 10.1161/01.res.71.4.745. [DOI] [PubMed] [Google Scholar]
- Glanemann C, Loos A, Gorret N, Willis LB, O'Brien XM, Lessard PA, Sinskey AJ. Disparity between changes in mRNA abundance and enzyme activity in Corynebacterium glutamicum: implications for DNA microarray analysis. Appl Microbiol Biotechnol. 2003;61:61–68. doi: 10.1007/s00253-002-1191-5. [DOI] [PubMed] [Google Scholar]
- Gupte SA, Li KX, Okada T, Sato K, Oka M. Inhibitors of pentose phosphate pathway cause vasodilation: involvement of voltage-gated potassium channels. J Pharmacol Exp Ther. 2002;301:299–305. doi: 10.1124/jpet.301.1.299. [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Kitamura K, Kuriyama H. Actions of 4-aminopyridine on vascular smooth muscle tissues of the guinea-pig. Br J Pharmacol. 1980;68:99–106. doi: 10.1111/j.1476-5381.1980.tb10704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton WJ, Mason HS, Carl A, Doherty P, Latten MJ, Kenyon JL, Sanders KM, Horowitz B. Functional and molecular expression of a voltage-dependent K+ channel (Kv1.1) in interstitial cells of Cajal. J Physiol. 2001;533:315–327. doi: 10.1111/j.1469-7793.2001.0315a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayabuchi Y, Standen NB, Davies NW. Angiotensin II inhibits and alters kinetics of voltage-gated K+ channels of rat arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2001;281:H2480–H2489. doi: 10.1152/ajpheart.2001.281.6.H2480. [DOI] [PubMed] [Google Scholar]
- Heaps CL, Bowles DK. Gender-specific K+-channel contribution to adenosine-induced relaxation in coronary arterioles. J Appl Physiol. 2002;92:550–558. doi: 10.1152/japplphysiol.00566.2001. [DOI] [PubMed] [Google Scholar]
- Irvine JC, Favaloro JL, Kemp-Harper BK. NO activates soluble guanylate cyclase and Kv channels to vasodilate resistance arteries. Hypertension. 2003;41:1301–1307. doi: 10.1161/01.HYP.0000072010.54901.DE. [DOI] [PubMed] [Google Scholar]
- Kerchensteiner D, Stocker M. Hetermeric assembly of Kv2.1 with Kv9.3: Effect on the state dependence of inactivation. Biophys J. 1999;77:248–257. doi: 10.1016/S0006-3495(99)76886-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr PM, Clement-Chomienne O, Thorneloe KS, Chen TT, Ishii K, Sontag DP, Walsh MP, Cole WC. Heteromultimeric Kv1.2-Kv1.5 channels underlie 4-aminopyridine- sensitive delayed rectifier K+ current of rabbit vascular myocytes. Circ Res. 2001;89:1038–1044. doi: 10.1161/hh2301.100803. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol. 1995;269:H348–H355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- Koo GC, Blake JT, Shah K, Staruch MJ, Dumont F, Wunderler D, Sanchez M, McManus OB, Sirotina-Meisher A, Fischer P, Boltz RC, Goetz MA, Baker R, Bao J, Kayser F, Rupprecht KM, Parsons WH, Tong XC, Ita IE, Pivnichny J, Vincent S, Cunningham P, Hora D, Jr, Feeney W, Kaczorowski G. Correolide and derivatives are novel immunosuppressants blocking the lymphocyte Kv1.3 potassium channels. Cell Immunol. 1999;197:99–107. doi: 10.1006/cimm.1999.1569. [DOI] [PubMed] [Google Scholar]
- Lang R, Lee G, Liu W, Tian S, Rafi H, Orias M, Segal AS, Desir GV. KCNA10: a novel ion channel functionally related to both voltage-gated potassium and CNG cation channels. Am J Physiol Renal Physiol. 2000;278:F1013–F1021. doi: 10.1152/ajprenal.2000.278.6.F1013. [DOI] [PubMed] [Google Scholar]
- Levitan ES, Takimoto K. Dynamic regulation of K+ channel gene expression in differentiated cells. J Neurobiol. 1998;37:60–68. doi: 10.1002/(sici)1097-4695(199810)37:1<60::aid-neu5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Liu Y, Terata K, Rusch NJ, Gutterman DD. High glucose impairs voltage-gated K+ channel current in rat small coronary arteries. Circ Res. 2001;89:146–152. doi: 10.1161/hh1401.093294. [DOI] [PubMed] [Google Scholar]
- Lu Y, Hanna ST, Tang G, Wang R. Contributions of Kv1.2, Kv1.5 and Kv2.1 subunits to the native delayed rectifier K+ current in rat mesenteric artery smooth muscle cells. Life Sci. 2002;71:1465–1473. doi: 10.1016/s0024-3205(02)01922-7. [DOI] [PubMed] [Google Scholar]
- MacDonald PE, Ha XF, Wang J, Smukler SR, Sun AM, Gaisano HY, Salapatek AM, Back PH, Wheeler MB. Members of the Kv1 and Kv2 voltage-dependent K+ channel families regulate insulin secretion. Mol Endocrinol. 2001;15:1423–1435. doi: 10.1210/mend.15.8.0685. [DOI] [PubMed] [Google Scholar]
- Manganas LN, Trimmer JS. Subunit composition determines Kv1 potassium channel surface expression. J Biol Chem. 2000;275:29685–29693. doi: 10.1074/jbc.M005010200. [DOI] [PubMed] [Google Scholar]
- Marijic J, Li Q, Song M, Nishimaru K, Stefani E, Toro L. Decreased expression of voltage- and Ca2+-activated K+ channels in coronary smooth muscle during aging. Circ Res. 2001;88:210–216. doi: 10.1161/01.res.88.2.210. [DOI] [PubMed] [Google Scholar]
- Martens JR, Gelband CH. Alterations in rat interlobar artery membrane potential and K+ channels in genetic and nongenetic hypertension. Circ Res. 1996;79:295–301. doi: 10.1161/01.res.79.2.295. [DOI] [PubMed] [Google Scholar]
- Michelakis ED, Rebeyka I, Wu X, Nsair A, Thebaud B, Hashimoto K, Dyck JR, Haromy A, Harry G, Barr A, Archer SL. O2 sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Re. 2002;91:478–486. doi: 10.1161/01.res.0000035057.63303.d1. [DOI] [PubMed] [Google Scholar]
- Mori Y, Matsubara H, Folco E, Siegel A, Koren G. The transcription of a mammalian voltage-gated potassium channel is regulated by cAMP in a cell-specific manner. J Biol Chem. 1993;268:26482–26493. [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM, Nichols CG, Schwarz TL, Escande D. Genetic manipulation of cardiac K+ channel function in mice: what have we learned, and where do we go from here. Circ Res. 2001;89:944–956. doi: 10.1161/hh2301.100349. [DOI] [PubMed] [Google Scholar]
- Ohya S, Horowitz B, Greenwood IA. Functional and molecular identification of ERG channels in murine portal vein myocytes. Am J Physiol Cell Physiol. 2002;283:C866–C877. doi: 10.1152/ajpcell.00099.2002. [DOI] [PubMed] [Google Scholar]
- Ohya S, Sergeant GP, Greenwood IA, Horowitz B. Molecular variants of KCNQ channels expressed in murine portal vein myocytes: a role in delayed rectifier current. Circ Res. 2003;92:1016–1023. doi: 10.1161/01.RES.0000070880.20955.F4. [DOI] [PubMed] [Google Scholar]
- Okabe K, Kitamura K, Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987;409:561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- Osipenko ON, Tate RJ, Gurney AM. Potential role for Kv3.1b channels as oxygen sensors. Circ Res. 2000;86:534–540. doi: 10.1161/01.res.86.5.534. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Lazdunski M, Honore E. Kv2.1/Kv9.3, a novel ATP-dependent delayed-rectifier K+ channel in oxygen-sensitive pulmonary artery myocytes. EMBO J. 1997;16:6615–6625. doi: 10.1093/emboj/16.22.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AJ, Henrie-Olsen J, Brenner R. Vasoregulation at the molecular level: a role for the β1-subunit of the calcium-activated potassium (BK) channel. Trends Cardiovasc Med. 2002;2:78–82. doi: 10.1016/s1050-1738(01)00146-3. [DOI] [PubMed] [Google Scholar]
- Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucl Acid Res. 2003;31:1–7. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JX. Chronic hypoxia decreases Kv channel expression and function in pulmonary artery myocytes. Am J Physiol. 2001;280:L801–L812. doi: 10.1152/ajplung.2001.280.4.L801. [DOI] [PubMed] [Google Scholar]
- Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Cytochrome c activates K+ channels before inducing apoptosis. Am J Physiol Cell Physiol. 2002;283:C1298–C1305. doi: 10.1152/ajpcell.00592.2001. [DOI] [PubMed] [Google Scholar]
- Platts SH, Mogford JE, Davis MJ, Meininger GA. Role of K+ channels in arteriolar vasodilation mediated by integrin interaction with RGD-containing peptide. Am J Physiol. 1998;275:H1449–H1454. doi: 10.1152/ajpheart.1998.275.4.H1449. [DOI] [PubMed] [Google Scholar]
- Pozeg ZI, Michelakis ED, McMurtry MS, Thebaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- Quinn K, Guibert C, Beech DJ. Sodium potassium ATPase electrogenicity in cerebral precapillary arterioles. Am J Physiol Heart Circ Physiol. 2000;279:H351–H360. doi: 10.1152/ajpheart.2000.279.1.H351. [DOI] [PubMed] [Google Scholar]
- Ren J, Zhang L, Benishin CG. Parathyroid hypertensive factor inhibits voltage-gated K+ channels in vascular smooth muscle cells. Can J Physiol Pharmacol. 1999;77:860–865. [PubMed] [Google Scholar]
- Robertson BE, Nelson MT. Aminopyridine inhibition and voltage dependence of K+ currents in smooth muscle cells from cerebral arteries. Am J Physiol. 1994;267:C1589–C1597. doi: 10.1152/ajpcell.1994.267.6.C1589. [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Sylvester JT, Booth GM, Shimoda TH, Meeker S, Undem BJ, Sham JS. Inhibition of voltage-gated K+ currents by endothelin-1 in human pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1115–L1122. doi: 10.1152/ajplung.2001.281.5.L1115. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Aaronson PI. Modulatory effects of arachidonic acid on the delayed rectifier K+ current in rat pulmonary arterial myocytes. Structural aspects and involvement of protein kinase C. Circ Res. 1996;79:20–31. doi: 10.1161/01.res.79.1.20. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Beck R, Tammaro P, Ishii T, Aaronson PI. Electrophysiologically distinct smooth muscle cell subtypes in rat conduit and resistance pulmonary arteries. J Physiol. 2002;538:867–878. doi: 10.1113/jphysiol.2001.013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WJ. Genes responsible for native depolarization-activated K+ currents in neurons. Neurosci Res. 2002;42:7–14. doi: 10.1016/s0168-0102(01)00305-4. [DOI] [PubMed] [Google Scholar]
- Teng B, Murthy KS, Kuemmerle JF, Grider JR, Sase K, Michel T, Makhlouf GM. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol. 1998;275:G342–G351. doi: 10.1152/ajpgi.1998.275.2.G342. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. Molecular composition of 4-aminopyridine-sensitive voltage-gated K+ channels of vascular smooth muscle. Circ Res. 2001;89:1030–1037. doi: 10.1161/hh2301.100817. [DOI] [PubMed] [Google Scholar]
- Xu C, Lu Y, Tang G, Wang R. Expression of voltage-dependent K+ channel genes in mesenteric artery smooth muscle cells. Am J Physiol. 1999;277:G1055–G1063. doi: 10.1152/ajpgi.1999.277.5.G1055. [DOI] [PubMed] [Google Scholar]
- Yu Y, Platoshyn O, Zhang J, Krick S, Zhao Y, Rubin LJ, Rothman A, Yuan JX. c-Jun decreases voltage-gated K+ channel activity in pulmonary artery smooth muscle cells. Circulation. 2001;104:1557–1563. doi: 10.1161/hc3801.095662. [DOI] [PubMed] [Google Scholar]
- Yuan XJ, Wang J, Juhaszova M, Golovina VA, Rubin LJ. Molecular basis and function of voltage-gated K+ channels in pulmonary arterial smooth muscle cells. Am J Physiol. 1998;274:L621–L635. doi: 10.1152/ajplung.1998.274.4.L621. [DOI] [PubMed] [Google Scholar]
- Zhang TT, Gealy R, Lu X, Heasley LE, Takimoto K, Levitan ES. TRH regulates Kv1.5 gene expression through a Gαq-mediated PLC- independent pathway. Mol Cell Endocrinol. 2000;165:33–39. doi: 10.1016/s0303-7207(00)00268-9. [DOI] [PubMed] [Google Scholar]
- Zhu J, Watanabe I, Gomez B, Thornhill WB. Heteromeric Kv1 potassium channel expression: Amino acid determinants involved in processing and trafficking to the cell surface. J Biol Chem. 2003;278:25558–25567. doi: 10.1074/jbc.M207984200. [DOI] [PubMed] [Google Scholar]