Abstract
That muscular blood flow may reach 2.5 l kg−1 min−1 in the quadriceps muscle has led to the suggestion that muscular vascular conductance must be restrained during whole body exercise to avoid hypotension. The main aim of this study was to determine the maximal arm and leg muscle vascular conductances (VC) during leg and arm exercise, to find out if the maximal muscular vasodilatory response is restrained during maximal combined arm and leg exercise. Six Swedish elite cross-country skiers, age (mean ± s.e.m.) 24 ± 2 years, height 180 ± 2 cm, weight 74 ± 2 kg, and maximal oxygen uptake (V̇O2,max) 5.1 ± 0.1 l min−1 participated in the study. Femoral and subclavian vein blood flows, intra-arterial blood pressure, cardiac output, as well as blood gases in the femoral and subclavian vein, right atrium and femoral artery were determined during skiing (roller skis) at ∼76% of V̇O2,max and at V̇O2,max with different techniques: diagonal stride (combined arm and leg exercise), double poling (predominantly arm exercise) and leg skiing (predominantly leg exercise). During submaximal exercise cardiac output (26–27 l min−1), mean blood pressure (MAP) (∼87 mmHg), systemic VC, systemic oxygen delivery and pulmonary V̇O2 (∼4 l min−1) attained similar values regardless of exercise mode. The distribution of cardiac output was modified depending on the musculature engaged in the exercise. There was a close relationship between VC and V̇O2 in arms (r = 0.99, P < 0.001) and legs (r = 0.98, P < 0.05). Peak arm VC (63.7 ± 5.6 ml min−1 mmHg−1) was attained during double poling, while peak leg VC was reached at maximal exercise with the diagonal technique (109.8 ± 11.5 ml min−1 mmHg−1) when arm VC was 38.8 ± 5.7 ml min−1 mmHg−1. If during maximal exercise arms and legs had been vasodilated to the observed maximal levels then mean arterial pressure would have dropped at least to 75–77 mmHg in our experimental conditions. It is concluded that skeletal muscle vascular conductance is restrained during whole body exercise in the upright position to avoid hypotension.
It has been reported that muscular blood flow may reach maximal values around 2.5 l kg−1 min−1 in the quadriceps muscle during maximal knee extension exercise in untrained healthy humans (Andersen et al. 1985; Radegran et al. 1999; Radegran & Saltin, 2000), while values between 3 and 4 l kg−1 min−1 have been observed in well trained subjects (Richardson et al. 1995). Since the mean muscle mass of the quadriceps muscle is about 2.5 kg (Radegran et al. 1999), it was suggested that this magnitude of perfusion would overwhelm the pumping capacity of the heart if a similar level of hyperaemia could be elicited during whole body exercise in the majority of active muscles (Andersen & Saltin, 1985). However, at least during submaximal exercise, the level of hyperaemia during dynamic arm exercise in the arm muscles is much lower (Ahlborg & Jensen-Urstad, 1991; Volianitis et al. 2003). But when a similar amount of muscle mass is recruited by performing two-leg knee extension exercise the leg blood flow increases to the same extent in both quadriceps (Roach et al. 1999). Together these data suggest that the regulation of exercise blood flow is different in the muscles of upper and lower extremities, but conclusive experimental evidence is still lacking.
During combined arm and leg exercise in humans, leg perfusion may have priority since when arm cranking has been superimposed on leg knee extension exercise no reduction in leg blood flow has been observed (Richardson et al. 1995). In contrast, the addition of leg cycling exercise to on-going arm exercise has resulted in a 5% lower arm vascular conductance (Volianitis et al. 2003). However, it remains unknown whether the level of exercise hyperaemia is differently regulated in arm and leg muscles, since no simultaneous measurements of leg and arm blood flow have been performed yet in the exercising human. In answering this question there are two main difficulties. First, arm and leg muscles differ in their level of daily use, and cross-sectional data indicate that the hyperaemic response of the quadriceps (Andersen & Saltin, 1985; Richardson et al. 1995) and calf muscles (Snell et al. 1987) to exercise is 40–60% higher in well trained subjects than in physically active subjects. This difficulty could be circumvented by studying humans who have highly trained arm and leg muscles, for example cross-country skiers. Second, there is uncertainty about the amount of muscle mass really recruited during dynamic knee extension exercise, since it is possible that not all the quadriceps mass is recruited (Ray & Dudley, 1998). During arm exercise the situation is even more complicated as several muscles are activated during arm cranking (Ahlborg & Jensen-Urstad, 1991; Volianitis et al. 2003). Since there is a linear relationship between muscle flow and muscle oxygen uptake (V̇O2) (Andersen et al. 1985), a reasonable way to compare the level of perfusion in upper and lower extremities during dynamic exercise is to normalize the observed flow values as a function of the V̇O2 achieved during exercise.
Therefore this study has been conceived to determine, first, if arm and leg vascular conductances are regulated depending on the local V̇O2, second, if the vasodilatory response to exercise is similar in the arm and leg muscles of humans with well trained arm and leg muscles, and third, if the combined maximal vascular conductance of legs and arms may overwhelm the maximal pumping capacity of the heart.
Methods
Subjects
Six Swedish elite cross-country skiers, age 24 ± 2 years, height 180 ± 2 cm, and weight 74 ± 2 kg, volunteered to participate in the study. The subjects had a maximal oxygen uptake V̇O2,max of 5.1 ± 0.1 l min−1 or 72 ± 2 ml kg−1 min−1, assessed during an incremental intensity test to exhaustion. The incremental exercise test was carried out using the diagonal stride technique while skiing uphill with roller skis on a modified treadmill (Refox, Falun, Sweden). All subjects were informed about the possible risks and discomfort involved before giving their written consent to participate. This study was carried out according to the Declaration of Helsinki and was approved by the Ethical Committee of the Karolinska Institute, Stockholm, Sweden.
Experimental preparation
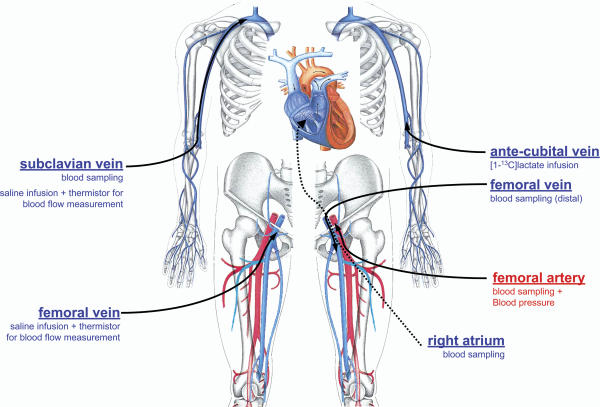
All subjects were familiar with the use of roller skis, which they use in their training activities during the part of the year without snow. All were also familiar with roller skiing on the treadmill. One week before the experiment their V̇O2,max was measured while skiing uphill using the diagonal stride technique. On the experimental day the subjects reported to the laboratory at 08.00 h, and catheters were placed under local anaesthesia (2% lidocaine) in the final position depicted in Fig. 1. An 18 gauge catheter (Hydrocath, Ohmeda, Swindon, UK) was inserted percutaneously using the Seldinger technique into either the left or right femoral artery, 2–5 cm below the inguinal ligament, and advanced 5–10 cm in the proximal direction. This catheter was connected to a blood pressure transducer positioned at the height of the fourth intercostal space (T100209A, Baxter, Unterschleissheim, Germany) and was also used to sample arterial blood. A 20 gauge catheter was inserted in the left femoral vein, 2 cm below the inguinal ligament, and advanced 5–7 cm in the distal direction for femoral venous blood sampling. In the right femoral vein, a venous catheter with side holes (Radiopack TFE, Cook, Bjaerverskov, Denmark) was inserted and advanced ∼5 cm proximal to the inguinal ligament for the injection of iced physiological saline solution. A thin polyethylene-coated thermistor (model 94-030-2.5F T.D. Probe, Edwards Edslab, Baxter, Irvine, CA, USA) was inserted through the venous catheter for blood flow measurements by the constant infusion thermodilution technique (Andersen & Saltin, 1985). An additional 18 gauge catheter was also inserted into the left femoral vein, 2–3 cm below the inguinal ligament, and under fluoroscopic guidance advanced until the tip was positioned in the centre of the right atrium, to sample blood from the right atrium. The last catheter, a Swan-Ganz triple-lumen catheter, was inserted into an antecubital vein and under fluoroscopic guidance was advanced through the basilic and axillary vein into the subclavian vein 5 cm before the merger with the jugular vein. One lumen was used for blood sampling and another for infusion of iced saline solution for blood flow measurements. Infusate temperature was measured with a thermistor set in a flow-through chamber (model 93-505, Edslab) connected to the venous catheters. All sampling catheters were connected to a three-way stopcock and, along with the thermistor, sutured to the skin to minimize the risk of movement during exercise.
Figure 1. Position and function of the catheters.
All catheters were sutured to the skin.
Once the catheterization was finished the subjects where moved from the catheterization laboratory to the experimental room where they lied in the supine position for 180 min. A three-lead electrocardiogram (ECG) was displayed on a monitor during catheterization and the rest of the experimental procedures (Dialogue 2000, Danica, Copenhagen, Denmark). The ECG, blood pressure and the temperatures registered by the thermistor, as well as the infusate temperatures were recorded simultaneously with the data acquisition system (MacLab 16/s ADInstruments, Sydney, Australia).
Two hours later resting parameters were measured and blood samples were obtained three times, 15 min apart. Femoral and subclavian venous blood flows were measured just before blood sampling and again after sampling.
Respiratory variables
Pulmonary V̇O2, CO2 production (V̇CO2), and expired minute ventilation (V̇E) were measured continuously using an ergo-spirometry system AMIS 2001 (Innovision A/S, Odense, Denmark). Before each test ambient conditions were measured, and then the gas analyser and the flowmeter were calibrated with high precision gases (16.00 ± 0.04% O2 and 4.00 ± 0.1% CO2, Air Liquide, Kungsängen, Sweden). During submaximal exercise the V̇O2 values obtained during the last 4 min were averaged. The V̇O2,max was calculated as the average of the three highest 10 s consecutive measurements of O2 uptake.
Blood flow
Femoral and subclavian venous blood flow were measured by constant-infusion thermodilution, as described in detail elsewhere (Andersen & Saltin, 1985). Briefly, iced saline was infused through the femoral and subclavian vein simultaneously at flow rates sufficient to decrease blood temperature at the thermistor by 0.5–1°C. Infusate and blood temperature were measured continuously during saline infusion (Harvard pump, Harvard Apparatus, Millis, MA, USA) via thermistors connected to the data acquisition system (MacLab 16/s ADInstruments). Infusate temperature was measured with a thermistor set in a flow-through chamber (model 93-505, Edslab) connected to the venous catheter. At rest, saline infusions were continued for at least 60 s, while during exercise 15–20 s long infusions were used until femoral vein temperature had stabilized at its new lower value. Blood flow was calculated on thermal balance principles, as detailed by Andersen et al. (1985). Resting blood flow and pressure were measured 6 times and averaged. During submaximal exercise, blood flow measurements were performed in duplicate. The reported submaximal blood flow values represent the average of at least two measurements. At peak effort, the measurements were made within 1 min of exhaustion, and repeated again whenever possible until exhaustion every 30–40 s.
Vascular conductances
Systemic vascular conductance was calculated as the cardiac output divided by the difference between mean arterial pressure and the estimated right atrium pressure. The blood pressure in right atrium was considered to be 5 mmHg during submaximal exercise and 10 mmHg at maximal exercise (Reeves et al. 1990). Leg vascular conductance was calculated as the quotient between leg blood flow and the pressure difference between the femoral artery and the femoral vein. Femoral vein pressures were assumed to be close to 4 mmHg during submaximal exercise, as during combined leg cycling and arm cranking at 60–70% of V̇O2,max (authors' unpublished observations). During maximal exercise with the diagonal technique, the femoral vein pressure was assumed to be 14 mmHg, that is, similar to that observed during maximal leg cycling in the upright position (authors' unpublished observations). Arm vascular conductance was calculated as the subclavian vein blood flow divided by the pressure gradient between the subclavian artery and the subclavian vein. During submaximal exercise subclavian vein pressure was assumed to be close to 7 mmHg, as observed during combined upright leg cycling and arm cranking at 60–70% of V̇O2,max as well as during isolated leg cycling in the upright position at 70% of V̇O2,max (authors' unpublished observations). At maximal exercise, a subclavian vein pressure of 11 mmHg was assumed, since this is the mean value observed during either maximal leg cycling in the upright position or maximal arm cranking in the upright position (authors' unpublished observations). The femoral and subclavian vein pressures used to calculated leg and arm vascular conductances were obtained in seven active subjects of similar age to the cross-country skiers included in the present investigation.
Blood samples and analytical procedures
Blood was sampled anaerobically in heparinized syringes and immediately analysed for haemoglobin (Hb), oxygen saturation (OSM3 haemoxymeter, Radiometer, Copenhagen, Denmark), blood pH, CO2 and O2 tension (ABL5, Radiometer). Blood gases were corrected for measured femoral vein blood temperature (femoral venous and arterial blood gases) and subclavian vein temperature (subclavian venous blood gases). Blood O2 content (Ca,O2 and Cfv,O2) was computed from the saturation and [Hb], i.e. (1.34[Hb] − SO2) + (0.003 – PO2). Another blood sample was taken, and the blood was collected in ice-cold tubes that contained 10 µl of 0.33 m EDTA per ml of blood and was immediately centrifuged at 4°C for 10 min and stored at –50°C until analysis. Plasma was analysed enzymatically for lactate (Roche Unikit, Neuss, Germany) on an automatic analyser (Cobas Fara, Roche, Basel, Switzerland). Arterial plasma noradrenaline and adrenaline concentrations were measured by HPLC with electrochemical detection (Hallman et al. 1978). Haematocrit was determined by microcentrifugation on triplicate samples.
Exercise protocol
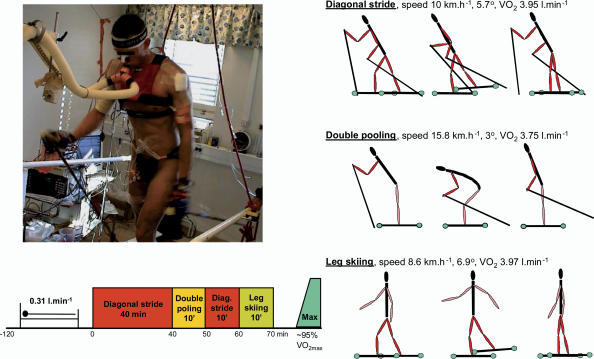
Classical skiing involves different techniques. The diagonal stride technique involves both the arms and the legs and is used uphill (Fig. 2). The double poling technique mainly involves the upper body and is used on flat terrain and slightly uphill. Leg skiing is diagonal skiing without poles which means that, in contrast to diagonal skiing, all the propulsive forces are generated by the legs. The protocol always consisted of 40 min of continuous diagonal style (Continuous diagonal), followed without breaks by 10 min of double poling, 10 min of diagonal stride, and 10 min of leg skiing. Then, the speed of the treadmill was reduced and after 3 min of active recovery while skiing with the diagonal technique at 30–40% of V̇O2,max the speed and slope of the treadmill were increased every minute until exhaustion. Blood samples were taken after 21, 24 and 36 min of continuous diagonal, and then ∼5–7 min after the start of double poling, diagonal and leg skiing. Before and after each blood sample femoral and subclavian vein blood flows and arterial blood pressure were simultaneously measured. After the study was finished, the subjects were moved to the catheterization laboratory, and the positions of the subclavian vein and right atrium catheters were checked with fluoroscopy. No catheter was found displaced during the study.
Figure 2. Experimental protocol.
Speed and inclination of the treadmill during roller skiing. The order in which the different skiing techniques were applied is illustrated in the lower part of the figure.
Statistical analysis
Descriptive statistics were performed on each variable to confirm the assumptions of normality and homoscedasticity. The effect of the kind of ski technique on the dependent variables was assessed using a one-way repeated measures analysis of variance. The Mauchly's test of sphericity was run before the ANOVA and in case of violation of the sphericity assumption the degrees of freedom were adjusted according to the Huynh and Feldt test. Pairwise comparisons were carried out with Tukey'stest. The relationship between vascular conductance and V̇O2 was determined by linear regression. Repeated measures analysis of variance of vascular conductance with V̇O2 as a covariate was used to determine if there was any difference between arms and legs in the vasodilatory response to exercise for a given V̇O2. The same approach by adding O2 extraction as a covariate was applied to find out if differences in vascular conductance for a given V̇O2 could be explained by differences in O2 extraction. The significance level was set at P < 0.05. Data are expressed as means ± standard error of the mean (s.e.m.), unless otherwise stated.
Results
Systemic oxygen delivery and pulmonary V̇O2
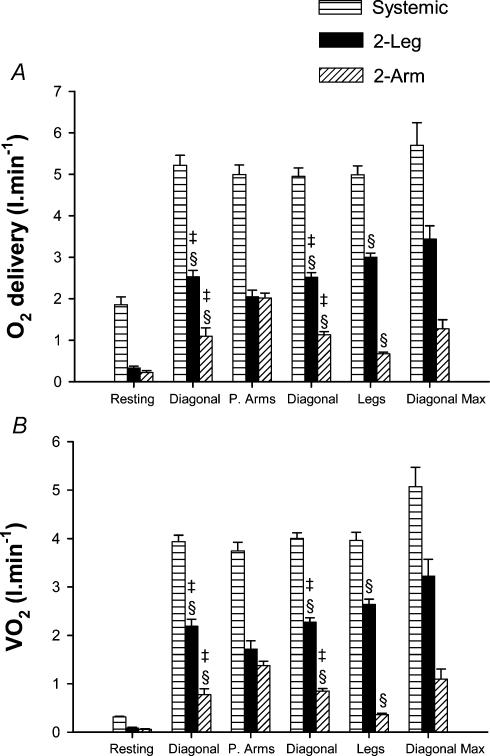
During submaximal exercise systemic oxygen delivery and, hence, pulmonary V̇O2 were close to 4 l min−1 (∼76% V̇O2,max) regardless of exercise mode (Fig. 3A and B). Arterial blood lactate concentration was 2.6 ± 0.4 mmol l−1 during the first submaximal exercise with the diagonal technique and increased to 5.0 ± 0.8 and 7.5 ± 0.7 mmol l−1 during leg skiing and double poling (P < 0.05).
Figure 3. Oxygen delivery and oxygen uptake.
Systemic (horizontally hatched bars), leg (filled bars) and arm (diagonally hatched bars) O2 delivery and V̇O2 during exercise with arms and legs (Diagonal), with double poling (predominantly arms: P. Arms), with only legs (Legs) and maximal exercise with the diagonal technique (Diagonal Max). §P < 0.05 compared to double poling; ‡P < 0.05 compared with leg skiing.
Haemodynamics
Cardiac output
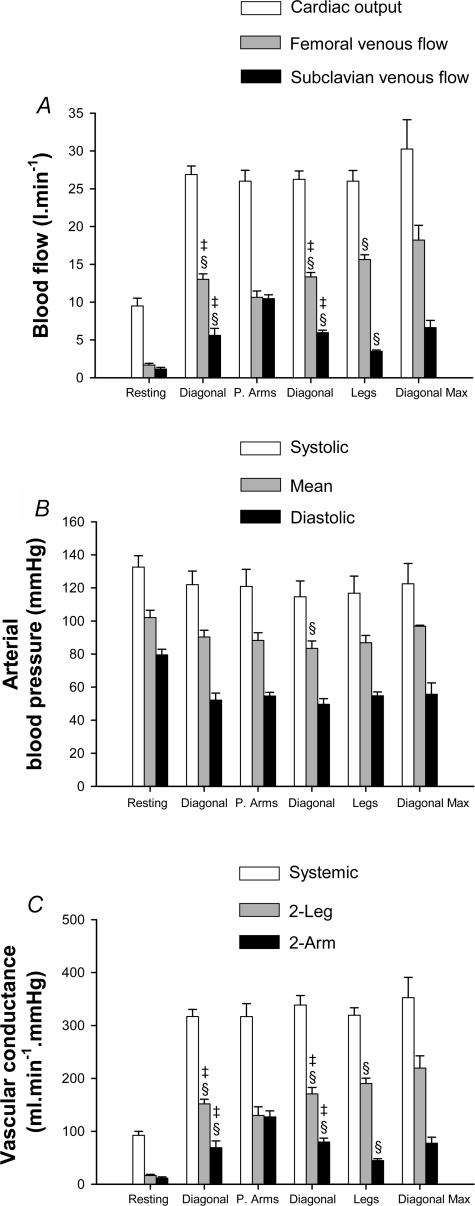
Skiers displayed similar cardiac outputs (the mean ranged from 26 to 27 l min−1) during submaximal exercise regardless of the skiing technique. This cardiac output was achieved with the same stroke volume (mean range from 147 to 155 ml beat−1) and heart rate (mean range from 173 to 177 beat min−1) (Fig. 4A).
Figure 4. Blood flow, arterial pressure and vascular conductances.
A, cardiac output (white bars), leg blood flow (grey bars) and arm blood flow (black bars). B, arterial blood pressures at the level of the right atrium: systolic (white bars), mean (grey bars) and diastolic (black bars). C, systemic vascular conductance (white bars), leg vascular conductance (grey bars) and arm vascular conductance (black bars), during exercise with arms and legs (diagonal), with double poling (predominantly arms: P. Arms), with only legs (legs) and maximal exercise with the diagonal technique (Diagonal Max). §P < 0.05 compared to double poling; ‡P < 0.05 compared with leg skiing.
Blood pressure and vascular conductances
Compared to resting conditions, mean arterial blood pressure was 14% lower, while systolic blood pressure was reduced by 11% and diastolic pressure by 34% (all P < 0.05). During submaximal exercise the mean blood pressure remained around 87 mmHg, without being affected by the exercise mode (Fig. 4B). Accordingly, similar systemic vascular conductances were observed during all skiing techniques (Fig. 4B). However, the distribution of cardiac output was modified depending on the musculature engaged in the exercise. Thus, leg blood flow and vascular conductance were greater during leg skiing and lower during double poling (P < 0.05). In contrast, arm blood flow and conductance were maximal during double poling and the lowest during leg skiing (P < 0.05). The diagonal stride elicited an intermediate response. The lumped vascular conductance to the regional circulations apart from the limbs was 20% lower during double poling than during diagonal skiing (P < 0.05). Consequently, the blood flow to other territories than the limbs was reduced from 7.6 ± 1.4 l min−1 during the diagonal style to 4.9 ± 1.6 l min−1 during double poling (P < 0.05), a value similar to that observed during maximal exercise with the diagonal technique.
Limb O2 delivery and consumption
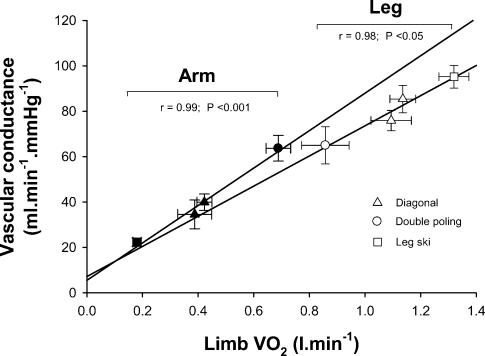
Leg O2 delivery was highest during leg skiing and lowest during double poling (Fig. 3A). Conversely, arm O2 delivery peaked during double poling and was lowest during leg skiing (Fig. 3A). The contribution of the legs to whole body V̇O2 was higher during leg skiing and lower during double poling, while the arm contributed maximally to whole V̇O2 during double poling and minimally during leg skiing. The regional vascular conductances were tightly regulated depending on the local V̇O2, as reflected by the close relationship obtained between vascular conductance and V̇O2 in arms (r = 0.99, P < 0.001) and legs (r = 0.98, P < 0.05) (Fig. 5). However, for a given V̇O2 arm vascular conductance was greater than leg vascular conductance during submaximal exercise, as shown by repeated measures analysis of variance using V̇O2 as a covariate (P < 0.05). In contrast, the percentage of O2 extraction was always higher for the legs (mean range from 83.3 ± 2.5 during double poling to 93.4 ± 1.3% during maximal exercise) than for the arms (mean range from 53.7 ± 4.3 during leg skiing to 85.2 ± 1.9% during maximal exercise; P < 0.05). The arm-to-leg observed difference in V̇O2 adjusted vascular conductance disappeared after accounting for the differences in O2 extraction.
Figure 5. Relationship between limb vascular conductance and V̇O2.
Vascular conductance values adjusted for V̇O2 were significantly higher for the upper than for the lower extremity. This difference disappeared after accounting for differences in O2 extraction between upper and lower extremities.
Arterial catecholamines
Both catecholamines increased during exercise, but the catecholamine response to exercise was not affected by the skiing technique during submaximal exercise. The arterial adrenaline concentration was 1–1.2 nmol l−1, and noradrenaline was 10–12 nmol l−1.
Discussion
This study reports for the first time combined measurements of cardiac output and leg and arm blood flow during upright submaximal and maximal exercise in healthy humans. The principal findings are as follows. (1) The combined maximal vascular conductance of arms and legs outweighs the maximal pumping capacity of the heart, implying that the muscular vasodilatory response during maximal exercise must be restrained to maintain perfusion pressure. (2) Limb vascular conductance is linearly related to limb V̇O2 during submaximal exercise. However, (3) for a given submaximal V̇O2 a greater level of vasodilatation is induced in the arms than in the legs, reflecting the lower extraction capacity of the arms compared with the leg muscles. (4) Exercise mean blood pressure is maintained at levels close to those observed in the pre-exercise resting conditions during submaximal and maximal exercise during upright whole body exercise.
Maximal arm blood flow in humans
This is the first investigation where the subclavian vein blood flow during arm exercise has been measured in humans. It is very likely that our subjects reached almost maximal arm blood flow and V̇O2 during double poling (5.2 l min−1). All skiers reported that they were performing close to their maximum for the arms while skiing with this technique. In support, very low PO2 and pH and high lactate values were observed in the subclavian vein during this type of exercise (Van Hall et al. 2003). As depicted in Table 1, the skiers reached arm flows that are 39–74% higher than previously observed during submaximal arm cranking at ∼80% of arm maximal power output (Jensen-Urstad & Ahlborg, 1992; Volianitis & Secher, 2002; Volianitis et al. 2003). Using the arm blood flow–power output relationship previously reported by Ahlborg & Jensen-Urstad (1991), we have calculated that our subjects will reach a similar blood flow when working in the arm crank ergometer at a power output of 234 W. Likewise, from the data of Ahlborg & Jensen-Urstad an arm V̇O2 of 678 ml min−1 can be estimated, which agree amazingly well with the 688 ml min−1 actually measured. This implies that the admixture of blood from tissues apart from the active arm and shoulder muscles in the subclavian vein is negligible or similar to that present in the study of Ahlborg & Jensen-Urstad, who placed the arm catheter in the axillary vein at the level of the coracoid process.
Table 1.
Summary of investigations where arm blood flow has been measured during dynamic exercise in humans
| Exercise | Subjects | Height Weight Age | Pulmonary V̇O2,max (ml kg−1 min−1) | Vessel | Flow assessment | Blood flow (l min−1) | MAP (mmHg) | Vascular conductance (ml min−1 mmHg−1) | O2extraction (%) | V̇O2 (l min−1) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm cranking 80% V̇O2,maxa 134 W | 5 men 2 women | 182 cm 79 kg 24 years | 51.7 | Axillary vein brachial artery | Constant infusion of cardiogreen | 3.77 | — | — | — | 0.52 | Jensen-Urstad & Ahlborg (1992) |
| Arm cranking 78% V̇O2,maxa 90 W | 8 men | 182 cm 72.3 kg 25 years | 48.4 | Axillary vein | Constant infusion of cardiogreen brachial artery | 2.43 | — | — | — | 0.32 | Ahlborg & Jensen-Urstad (1991) |
| Arm cranking 80% Ẇmaxb 122 W | 10 men | 183 cm 82 kg 21 years | 49.3 | Axillary vein | Thermodilution bolus injection | 3.00 | 112 | 27 (29) f | 57 | 0.45 | Volianitis & Secher (2002) |
| Arm cranking 80% Ẇmax (90 W)b + leg 60% Ẇmaxc | 10 men | 183 cm 82 kg 21 years | 49.3 | Axillary vein | Thermodilution bolus injection | 2.42 | 103 | 23 (25) f | 70 | 0.40 | Volianitis & Secher (2002) |
| Arm cranking 80% Ẇmaxb 136 W + leg cycling 204 Wd | 7 men | 183 cm 82 kg 21 years | ∼45 | Axillary vein | Thermodilution constant infusion | 2.97 | 103 | 29 (31) f | 62 | 0.37 | Volianitis et al.(2003) |
| Double poling 85% V̇O2,max during double poling | 6 men | 180 cm 72 kg 24 years | 71 | Subclavian vein under fluoroscopy control | Thermodilution constant infusion | 5.23 | 87 | 64 f | 68 | 0.69 | e |
Pulmonary V̇O2,max measured during arm cranking.
Ẇmax: maximal power output during arm cranking.
Ẇmax: maximal power output during leg cycling.
This condition elicited 95% of subjects V̇O2,max during an incremental exercise test to exhaustion in the cycle ergometer.
Present investigation.
Value in brackets calculated assuming a subclavian vein pressure of 7 mmHg, as in the present investigation.
Limb vascular conductance
In our experimental conditions the legs received a maximum of 60% of the maximal cardiac output, while the arms received a maximum of 35%. Yet, to perfuse at maximal level both territories, cardiac output should have been increased to 33–34 l min−1 (approximately 9.1 l min−1 to each leg and 5.2 l min−1 to each arm and 5–6 l min−1 to perfuse the rest of the body), i.e. 3–4 l min−1 more than actually observed. In Table 2 we summarize the data published on maximal leg vascular conductance in healthy humans. In agreement with our results a peak thigh blood flow close to 9 l min−1 has also been reported during adenosine-induced vasodilatation in humans with an estimated maximal cardiac output of 23–25 l min−1 (Radegran & Calbet, 2001). Likewise leg blood flow between 8 and 12.5 l min−1 has been reported during maximal exercise in the cycle ergometer (Knight et al. 1993; Schaffartzik et al. 1993; Richardson et al. 1995; Calbet et al. 2003; Gonzalez-Alonso & Calbet, 2003). In well trained subjects having a maximal cardiac output of 29 l min−1, i.e. just a bit lower than our skiers, Gonzalez-Alonso & Calbet (2003) reported a peak leg blood flow of 12.5 l min−1. The 3 l min−1 difference in peak leg blood flow between the present investigation and that reported by Gonzalez-Alonso & Calbet corresponds very well with the flow directed to the arms during maximal skiing exercise with the diagonal style. Assuming that the arm muscle mass is 4–5 kg and the leg muscle mass 10–11 kg (Jensen-Urstad & Ahlborg, 1992; Calbet et al. 2001) it can be estimated that the maximal blood flow per muscle mass of the legs and the arms is rather similar in trained subjects or is just a bit higher in the arms, but still far below the level of perfusion reported for the quadriceps muscle during knee extension exercise (Andersen & Saltin, 1985; Richardson et al. 1993). But when leg and arm blood flow are normalized as a function of V̇O2 (i.e. flow/V̇O2) it can be realized that for a given V̇O2 the arms receive about 27% more blood flow than the legs, reflecting the lower O2 extraction capacity of the arms (Clausen et al. 1973; Ahlborg & Jensen-Urstad, 1991; Jensen-Urstad & Ahlborg, 1992; Volianitis & Secher, 2002). In fact, a lower O2 extraction capacity is compensated for by increasing blood flow during submaximal knee extension exercise with carbon monoxide and hyperoxia, a condition where the O2 content of blood is maintained at normal levels but the affinity of haemoglobin for O2 is increased (Gonzalez-Alonso et al. 2001).
Table 2.
Summary of investigations where single-leg blood flow, intra-arterial blood pressure and vascular conductance have been measured in humans
| Exercise | Subjects | Height Weight Age | Pulmonary V̇O2,max (ml kg−1 min−1) | Vessel | Flow assessment | Blood flow (l min−1) | MAP (mmHg) | Leg Vascular conductance (ml min−1 mmHg−1) | O2 extraction (%) | V̇O2 (l min−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Resting seateda | 7 men Active | 184 cm 81 kg 24 years | — | Femoral artery | Ultrasound Doppler | 9.07 | 80 g | 113 (120)h | 1 | 0.18 | Radegran & Calbet (2001) |
| Two-legged knee extension Ẇmax: 143 W | 7 men Active | 183 cm 85 kg 24 years | 55 d | Femoral vein | Thermodilution constant infusion | 6.68 | 118 g | 57 (59)g (64)i | 73 | 0.98 | Roach et al. (1999) |
| Two-legged knee extension b | 5 men Cyclist | 178 cm 75 kg 23 years | 58 d | Femoral vein | Thermodilution constant infusion | 8.8 | 132 g | 67 (69)g (75)i | 84 | 1.34 | Richardson et al. (1995) |
| Cycle ergometer Ẇmax: 298 W | 5 men 4 women Active | 176 cm 74 kg 24 years | 56 | Femoral vein | Thermodilution constant infusion | 8.77 | 124 g | 71 (80)i | 85 | 1.42 | Calbet et al. (2003) |
| Cycle ergometer Constant intensity at 356 Wc | 8 men Cyclist | 181 cm 78 kg 24 years | 60 | Femoral vein | Thermodilution constant infusion | 12.52 | 131 g | 96 (107)i | 87 | 2.14 | Gonzalez-Alonso & Calbet (2003) |
| Diagonal at V̇O2,max | 3 men Elite skiers | 181 cm 75 kg 23 years | 74 e | Femoral vein fluoroscopy controlled | Thermodilution constant infusion | 9.10 | 96 | 95 (99)g (110)h | 93 | 1.61 | f |
Maximal vasodilatation achieved with increasing doses of adenosine. During the adenosine infusions a cuff placed just below the knee joint was inflated at ≥ 240 mmHg.
Reported values correspond to 90% of maximal power output, since values at V̇O2,max for blood pressure were not reported.
Load chosen to elicit V̇O2,max in 3–5 min and exhaustion in 5–10 min. The measurements obtained at V̇O2,max are reported.
V̇O2,max during leg cycling. Measured with the diagonal technique before the invasive experiments.
Present investigation.
At the height of the inguinal ligament.
Value in brackets, Assuming a 4 mmHg femoral vein pressure.
Value in brackets, Assuming a 14 mmHg femoral vein pressure.
The maximal value of arm vascular conductance observed in our skiers (64 ml min−1 mmHg−1) was attained during double poling at an intensity corresponding to 85% of the maximal V̇O2 that our skiers can achieve with this technique (H. C. Holmberg, unpublished observations). This vascular conductance is almost twice the arm vascular conductance reported during arm exercise at similar relative intensity in non-arm trained subjects of similar body size (Volianitis & Secher, 2002; Volianitis et al. 2003) (Table 1). This finding suggests that exercise maximal arm vascular conductance may be remarkably increased by training. The latter agrees with the findings of Snell et al. (1987), who measured resting post-ischaemic exercise blood flow in the calf muscles with plethysmography. It is likely that the arm muscles were almost fully vasodilated during double poling, since vascular conductance was similar to that observed during adenosine-induced vasodilatation of the leg muscles in resting humans (Radegran & Calbet, 2001). During maximal exercise with the diagonal technique arm O2 extraction, arm lactate release and subclavian vein temperature achieved peak values, while subclavian vein pH reached the lowest value. This implies that the arms were exercising at the same or even at a higher intensity than during double poling. However, arm blood flow was ∼40% and arm V̇O2 ∼ 20% lower during maximal exercise with the diagonal technique than during double poling. The great activation of anaerobic metabolism in the arms during maximal exercise with the diagonal technique reflects a mismatch between O2 demand and O2 delivery. The fact that arm vascular conductance was also lower during maximal exercise with the diagonal technique than during submaximal exercise with the double poling technique further supports our conclusion. That is, some level of vasoconstriction should be opposing the vasodilatory signals elicited by arm muscle contractions to preserve mean arterial pressure during whole body exercise. It is likely that the strong activation of the so-called central command combined with the activation of the metaboreflex may have led to a high sympathetic tone which could have blunted the metabolic elicited vasodilatation (Joyner et al. 1990) more efficiently in the arms than in the legs.
Systemic vascular conductance and pumping capacity of the heart
In the present investigation we have measured for the first time the maximal leg vascular conductance in elite athletes during maximal exercise while standing up. The value obtained, 110 ml min−1 mmHg−1, is a bit lower than that measured in the thigh during adenosine-induced vasodilatation in resting humans (Radegran & Calbet, 2001), since the muscle mass of the thigh is lower that that of the full lower extremity. When calculated in the same way, our skiers reached a maximal leg vascular conductance that was also below the value observed during maximal exercise in the cycle ergometer, in the upright position (Calbet et al. 2003; Gonzalez-Alonso & Calbet, 2003) (Table 2). Since elite endurance athletes should reach greater skeletal muscle maximal vascular conductances (Andersen & Saltin, 1985; Snell et al. 1987; Richardson et al. 1995), it is suggested that during maximal skiing with the diagonal technique some degree of vasoconstriction should also be restraining blood flow at the lower extremities.
In our experimental conditions the maximal combined vascular conductance of legs and arms was about 350 ml min−1 mmHg−1. With the observed vascular conductance for the rest of the vascular system of 50–60 ml min−1 mmHg−1 at maximal exercise, it can be calculated that without some vasoconstriction on the active muscles mean blood pressure would have dropped from the observed 95 to 75–77 mmHg in our experimental conditions. In fact, exercise mean arterial blood pressure drops to values between 60 and 65 mmHg in paraplegics who lack of a functional sympathetic system (Dela et al. 2003). For a systemic vascular conductance of 400–410 ml min−1 mmHg−1 and a maximal cardiac output of 20–25 l min−1, as usually observed in non-physically active young adults, the corresponding maximal exercise mean blood pressure would drop into a range between 49 and 63 mmHg, and will compromise cerebral blood flow (Lassen, 1959). Therefore, when combined maximal leg and arm exercise is performed a vasoconstrictor action on the active muscles is needed to maintain systemic blood pressure. Otherwise a cardiac output of 37–40 l min−1 would have been needed to maximally perfuse arms and legs, without compromising systemic blood pressure and perfusion in other vascular beds. In fact, a very high maximal cardiac output – more than twice the value observed in athletic humans after accounting for differences in body size – is a common feature in athletic quadrupeds with higher V̇O2,max than the best humans (Rose et al. 1994).
Mean arterial pressure during whole body exercise
The pumping capacity of the heart is limited by the maximum work that the heart can perform. It is well know that the heart V̇O2 and work increase depending on the cardiac output, but also depending on the mean arterial blood pressure. A higher maximal cardiac output can be attained if the mean arterial pressure is maintained at a low, but tolerable, level. Increasing the mean arterial pressure implies that the maximal cardiac output, and hence systemic O2 delivery, would be lower. It is not a surprise then that maximal cardiac output values are more elevated during running than during cycling, inasmuch as the mean arterial pressure during running is lower than during cycling (Hermansen et al. 1970; Calbet et al. 2003; Gonzalez-Alonso & Calbet, 2003). The muscle vascular conductance is also limited; once maximal vasodilatation has been achieved the only mechanism available to enhance muscular O2 delivery is by increasing mean arterial pressure. But mean arterial pressure will only contribute efficiently to elevate muscular perfusion during exercise models in which the maximal pumping capacity of the heart is not taxed, otherwise the benefit reached by increasing perfusion pressure will be discounted by the reduction of maximal cardiac output and its surrogate leg blood flow as has been shown during exercise in severe acute hypoxia (Calbet et al. 2003).
The present investigation shows that during dynamic exercise it is possible to achieve high muscular perfusion levels with just a mild or even without an elevation of mean blood pressure, at least during whole body exercise in the upright position. Similar levels of intra-arterial MAP have been reported during running uphill on the treadmill (Hermansen et al. 1970) and during combined near maximal arm and leg cranking seated upright (Volianitis & Secher, 2002; Volianitis et al. 2003). Actually, a discrete reduction of mean blood pressure was observed during submaximal exercise, when the average MAP of all skiing styles was compared with resting blood pressure. This has an important implication for devising healthy modes of exercise, particularly for people with hypertension or cardiac diseases.
V̇O2,max during whole body exercise
This study also shows unequivocally that V̇O2,max is limited by blood flow and its surrogate oxygen delivery in elite cross-country skiers. The arm V̇O2 was lower during maximal exercise with the diagonal technique than during submaximal double poling, implying that not all the potential V̇O2 is used during maximal exercise with the four extremities. In another group of elite cross-country skiers we determined the V̇O2,max using three different protocols: diagonal style, maximal double poling style, and running uphill (H. C. Holmberg, unpublished data). The greatest V̇O2,max value (6.2 l min−1, n = 7) was obtained with the diagonal style, while significantly 4 and 14% lower values were observed during running uphill and during incremental exercise to exhaustion with the double poling technique. If the arm V̇O2 represents the same percentage of the pulmonary V̇O2 during double poling at exercise intensities between 73 and 100% of V̇O2,max, then during maximal double poling exercise the arms should be able to reach an 18% higher V̇O2,max than observed in the present investigation. During running about 75–80% of the pulmonary V̇O2 is generated in the muscles of the lower extremities, 10–15% by the respiratory muscles and 10–15% by the heart, central nervous system, skeletal muscles of the trunk and other tissues (Harms et al. 1997). The latter implies that our skiers should have also been able to reach 15–22% greater leg V̇O2 than observed during maximal exercise with the diagonal style. Given the fact that O2 extraction cannot be increased further than the 93% observed already at maximal exercise with the diagonal style, a 15–22% greater blood flow or O2 content would be necessary to allow for a 15–22% higher leg V̇O2 during maximal skiing exercise with the diagonal technique.
In summary, this study indicates that oxygen delivery is the primary variable regulated; vascular conductance and blood pressure are adjusted to match O2 delivery with the local tissue O2 demand. Limb vascular conductance is linearly related to limb V̇O2 during submaximal exercise. This study shows that for a given submaximal V̇O2 a greater level of vasodilatation is required in the arms than in the legs due to the lower O2 extraction capacity of the arms. Finally, the data presented show that the muscular vasodilatory response during maximal whole body exercise should be restrained. If not, the systemic vascular conductance would overwhelm the maximal pumping capacity of the heart and hypotension would ensue.
Acknowledgments
The help from the staff and use of the facilities at the Department of Cardiology and Clinical Physiology of the Karolinska Hospital, Sweden were highly appreciated. We would also acknowledge the excellent technical assistance provided by Birgitte Jessen and Karen Juel. This study was supported by grants from the Swedish Olympic Committee, Team Danmark, and the Sport Research Council of the Ministry of Culture. The Copenhagen Muscle Research Centre is funded by a grant from the Danish National Research Foundation (Grant no. 504-14).
References
- Ahlborg G, Jensen-Urstad M. Arm blood flow at rest and during arm exercise. J Appl Physiol. 1991;70:928–933. doi: 10.1152/jappl.1991.70.2.928. [DOI] [PubMed] [Google Scholar]
- Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R291–R303. doi: 10.1152/ajpregu.00155.2002. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Dorado C, Diaz-Herrera P, Rodriguez-Rodriguez LP. High femoral bone mineral content and density in male football (soccer) players. Med Sci Sports Exerc. 2001;33:1682–1687. doi: 10.1097/00005768-200110000-00011. [DOI] [PubMed] [Google Scholar]
- Clausen JP, Klausen K, Rasmussen B, Trap-Jensen J. Central and peripheral circulatory changes after training of the arms or legs. Am J Physiol. 1973;225:675–682. doi: 10.1152/ajplegacy.1973.225.3.675. [DOI] [PubMed] [Google Scholar]
- Dela F, Mohr T, Jensen CM, Haahr HL, Secher NH, Biering-Sorensen F, Kjaer M. Cardiovascular control during exercise: insights from spinal cord-injured humans. Circulation. 2003;107:2127–2133. doi: 10.1161/01.CIR.0000065225.18093.E4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Calbet JA. Reductions in systemic and skeletal muscle blood flow and oxygen delivery limit maximal aerobic capacity in humans. Circulation. 2003;107:824–830. doi: 10.1161/01.cir.0000049746.29175.3f. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol. 2001;530:331–341. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallman H, Farnebo LO, Hamberger B, Johnsson G. A sensitive method for the determination of plasma catecholamines using liquid chromatography with electrochemical detection. Life Sci. 1978;23:1049–1052. doi: 10.1016/0024-3205(78)90665-3. [DOI] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Hermansen L, Ekblom B, Saltin B. Cardiac output during submaximal and maximal treadmill and bicycle exercise. J Appl Physiol. 1970;29:82–86. doi: 10.1152/jappl.1970.29.1.82. [DOI] [PubMed] [Google Scholar]
- Jensen-Urstad M, Ahlborg G. Is the high lactate release during arm exercise due to a low training status? Clin Physiol. 1992;12:487–496. doi: 10.1111/j.1475-097x.1992.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Lennon RL, Wedel DJ, Rose SH, Shepherd JT. Blood flow to contracting human muscles: influence of increased sympathetic activity. J Appl Physiol. 1990;68:1453–1457. doi: 10.1152/jappl.1990.68.4.1453. [DOI] [PubMed] [Google Scholar]
- Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol. 1993;75:2586–2594. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39:183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- Radegran G, Blomstrand E, Saltin B. Peak muscle perfusion and oxygen uptake in humans: importance of precise estimates of muscle mass. J Appl Physiol. 1999;87:2375–2380. doi: 10.1152/jappl.1999.87.6.2375. [DOI] [PubMed] [Google Scholar]
- Radegran G, Calbet JA. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand. 2001;171:177–185. doi: 10.1046/j.1365-201x.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Human femoral artery diameter in relation to knee extensor muscle mass, peak blood flow, and oxygen uptake. Am J Physiol Heart Circ Physiol. 2000;278:H162–H167. doi: 10.1152/ajpheart.2000.278.1.H162. [DOI] [PubMed] [Google Scholar]
- Ray CA, Dudley GA. Muscle use during dynamic knee extension: implication for perfusion and metabolism. J Appl Physiol. 1998;85:1194–1197. doi: 10.1152/jappl.1998.85.3.1194. [DOI] [PubMed] [Google Scholar]
- Reeves JT, Groves BM, Cymerman A, Sutton JR, Wagner PD, Turkevich D, Houston CS. Operation Everest II: cardiac filling pressures during cycle exercise at sea level. Respir Physiol. 1990;80:147–154. doi: 10.1016/0034-5687(90)90078-d. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Kennedy B, Knight DR, Wagner PD. High muscle blood flows are not attenuated by recruitment of additional muscle mass. Am J Physiol. 1995;269:H1545–H1552. doi: 10.1152/ajpheart.1995.269.5.H1545. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol. 1993;75:1911–1916. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol. 1999;276:H438–H445. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Cluer D, Saltin B. Some comparative aspects on the camel as a racing animal. Acta Physiol Scand Suppl. 1994;617:87–95. [PubMed] [Google Scholar]
- Schaffartzik W, Barton ED, Poole DC, Tsukimoto K, Hogan MC, Bebout DE, Wagner PD. Effect of reduced hemoglobin concentration on leg oxygen uptake during maximal exercise in humans [editorial] J Appl Physiol. 1993;75:491–498. doi: 10.1152/jappl.1993.75.2.491. [DOI] [PubMed] [Google Scholar]
- Snell PG, Martin WH, Buckey JC, Blomqvist CG. Maximal vascular leg conductance in trained and untrained men. J Appl Physiol. 1987;62:606–610. doi: 10.1152/jappl.1987.62.2.606. [DOI] [PubMed] [Google Scholar]
- Van Hall G, Jensen-Urstad M, Rosdahl H, Holmberg HC, Saltin B, Calbet JA. Leg and arm lactate and substrate kinetics during exercise. Am J Physiol Endocrinol Metab. 2003;284:E193–E205. doi: 10.1152/ajpendo.00273.2002. [DOI] [PubMed] [Google Scholar]
- Volianitis S, Krustrup P, Dawson E, Secher NH. Arm blood flow and oxygenation on the transition from arm to combined arm and leg exercise in humans. J Physiol. 2003;547:641–648. doi: 10.1113/jphysiol.2002.034496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volianitis S, Secher NH. Arm blood flow and metabolism during arm and combined arm and leg exercise in humans. J Physiol. 2002;544:977–984. doi: 10.1113/jphysiol.2002.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]