Abstract
The effects of neuroactive steroids on the function of GABAA receptors were studied using cell-attached records of single channel activity recorded from HEK293 cells transfected with α1 β2 γ2L subunits. Activity was elicited with a half-maximal (50 μm) concentration of GABA. Two steroids were studied in detail: ACN ((3α,5α,17β)-3-hydroxyandrostane-17-carbonitrile) and B285 ((3α,5β,17β)-3-hydroxy-18-norandrostane-17-carbonitrile). Four effects on channel activity were seen, two on open time distributions and two on closed times. When clusters of openings were elicited in the absence of steroid, the open time distribution contained three components. ACN produced concentration-dependent alterations in the open time distribution. The prevalence of the longest duration class of open times was increased from about 15% to about 40% (EC50 about 180 nm ACN), while the duration of the longest class increased from 7.4 ms to 27 ms (EC50 about 35 nm ACN). B285 also increased the prevalence of the longest duration open times (EC50 about 18 nm B285) but increased the duration only at concentrations close to 10 μm. The differences in the actions of these two steroids suggest that the effects on proportion and duration of the long duration open time component are produced by independent mechanisms and that there are separate recognition sites for the steroids which are associated with the two functional actions. The closed time distributions also showed three components in the absence of steroid. The rate of occurrence of the two brief duration closed time components decreased with increasing ACN, with an EC50 of about 50 nm ACN. In contrast, B285 did not reduce the rate of occurrence of the brief closings until high concentrations were applied. However, both B285 and ACN reduced the rate of occurrence of the activation-related closed state selectively, with comparable IC50 concentrations (about 40 nm ACN, 20 nm B285). As in the case for action on open times these data suggest that there are two recognition sites and two independent mechanisms, perhaps the sites and mechanisms associated with actions on open times. The presence of 1 μm ACN had no effect on the estimated channel opening rate or on the apparent affinity of the receptor for GABA. Mutation of the carboxy terminus of the γ2 subunit, but not the α1 or β2 subunits, abolished the ability of ACN to increase the duration of OT3 but had no effect on the reduction of the rate of occurrence of the activation-related closed state. These observations are also consistent with the idea that there is more than one distinguishable steroid recognition site on the GABAA receptor.
Neuroactive steroids are known to act on the γ-aminobutyric acid A (GABAA) receptor; many act to potentiate the response of the receptor to low concentrations of GABA, while some (particularly sulphated steroids) block the response at all concentrations of GABA (Macdonald & Olsen, 1993; Lambert et al. 2001a,b). Potentiation and inhibition by steroids appear to be distinct processes and probably involve distinct binding sites, rather than reflecting opposing effects on a single process (e.g. agonism and inverse agonism; (Zaman et al. 1992; Park-Chung et al. 1999; Akk et al. 2001; Wang et al. 2002). We have studied potentiation by neuroactive steroids, since potentiation has been associated with activity as hypnotics and anaesthetics (Macdonald & Olsen, 1993; Lambert et al. 1996, 2001a), and it has been suggested that some behavioural effects of endogenous neurosteroids may reflect actions at the GABAA receptor (Majewska et al. 1986). Steroids which potentiate GABAA receptor activation also prolong GABAergic inhibitory postsynaptic currents, although the amplitude is generally not increased (Harrison et al. 1987; Zorumski et al. 1998; Haage & Johansson, 1999). Although the phenomenon of potentiation has been known for many years (Harrison & Simmonds, 1984), the mechanism by which it occurs is still poorly understood. Macroscopic responses decay more slowly, an effect which has been attributed to a decrease in desensitization (Zhu & Vicini, 1997), while single channel recordings have found an increase in the mean open time and an increase in the rate of bursts of activity elicited by a low concentration of GABA (Twyman & Macdonald, 1992). Indeed, it is not known whether a potentiating steroid has a single mechanism by which it acts, or more than one (Srinivasan et al. 1999).
The site(s) for steroid recognition which mediate potentiation of the GABAA receptor are not known, although neuroactive steroids do not bind to the GABA-binding site, nor to sites characterized for other drugs active at the GABAA receptor.
We have begun a study of the actions of neuroactive steroids on single channel currents elicited by GABA, to determine the kinetic basis for steroid potentiation. We have taken advantage of the synthesis of a variety of steroids and steroid analogues to explore the structure–activity relationship of these steroids. We chose two steroids for particular study, based on their differential ability to decrease the binding of t-buty/bicyclophosphorothionate (TBPS) to GABAA receptors. (3α,5α,17β)-3-Hydroxyandrostane-17-carbonitrile (ACN) shows a monophasic inhibition curve, with a concentration for half-maximal inhibition (IC50) of 40 nm and a Hill coefficient of 0.87. In contrast, (3α,5β,17β)-3-hydroxy-18-norandrostane-17-carbonitrile (B285) shows a shallow concentration–effect curve, which can be described by the sum of two curves with Hill coefficients of 1 and IC50 values of 30 nm and 108 μm (A. Evers, in preparation). Our initial studies involve the analysis of channel activity within clusters of activity elicited by a close to half-maximal concentration of GABA (50 μm), because the analysis of this activity is more established (cf. Akk et al. 2001; Steinbach & Akk, 2001). A preliminary report of some of these observations has been included in a study of interactions between neuroactive steroids and ethanol at the GABAA receptor (Akk & Steinbach, 2003).
Methods
Expression systems and electrophysiology
Rat GABAA receptor α1, β2 and γ2L subunit cDNAs were subcloned into a cytomegalovirus (CMV) promoter-based vector pcDNAIII (Invitrogen Corp., San Diego, CA, USA), and transiently expressed in human embryonic kidney (HEK) 293 cells using a calcium phosphate precipitation-based transfection technique (Akk et al. 2001; Akk, 2002). Generation of mutated subunits was done by standard methods, using the QuikChange kit (Stratagene, La Jolla CA, USA). All constructs were sequenced over the entire coding region, and no extraneous mutations were present.
Most drugs and chemicals were purchased from Sigma Chemical Company (St Louis, MO, USA). ACN and B285 were synthesized in our laboratories. Steroids were initially dissolved in DMSO at 10 mm concentration, and diluted on the day of the experiment. Concentrations of steroid above 20 μm were not often used, as the steroid solubility is not known precisely but is likely to be about 20–50 μm.
The single channel currents were recorded using the patch clamp technique in the cell-attached configuration (Hamill et al. 1981). The bath solution contained (mm): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose and 10 Hepes; pH 7.4. The pipette solution contained (mm): 120 NaCl, 5 KCl, 10 MgCl2, 0.1 CaCl2, 20 tetraethylammonium chloride, 5 4-aminopyridine, 10 glucose, 10 Hepes; pH 7.4. In addition, the pipette solution contained the indicated concentrations of GABA and steroid. Agonist was added to the saline, and no correction for osmolarity was made. The pipette potential was held at +60 mV. We assume that the cell membrane potential was ∼−40 mV, and thus the total potential across the patch membrane was about −100 mV. The channel activity was recorded with an Axopatch 200B amplifier, low-pass filtered at 10 kHz, acquired with a Digidata 1200 series interface at 50 kHz using pCLAMP software (Axon Instruments, Union City, CA, USA) and stored on a PC hard drive.
Kinetic analysis
The kinetic analysis has been described earlier (see: Akk et al. 2001; Steinbach & Akk, 2001). The analysis was performed on single channel clusters. A cluster is defined as a series of openings and closings of a single ion channel which starts as the channel returns from the long-lived desensitized state(s) and ends when the channel enters the long-lived desensitized state(s). The duration of a typical cluster is 2–3 s while the average lifetime of the receptor in the desensitized state(s) can be > 10 s (cf. Jones & Westbrook, 1995). Due to a low number of receptors in the patch and a relatively short lifetime of clusters compared to the dwells in long-lived desensitized state(s), the activity in most patches consisted of episodes of intense activity of a single channel (a cluster) separated from other clusters by intervals lasting seconds.
To isolate single channel clusters we used a cutoff duration, τcrit. A cluster termination was called when the duration of the closed time interval between two neighbouring openings exceeded that of τcrit. In theory, the optimal duration of τcrit depends on the intracluster closed times and should be at least five times longer than the mean duration of the longest intracluster closed time. This assures that the misclassification of closed time events is insignificant. In the present case, clusters at all steroid concentrations contained relatively long-lived gaps. To include such gaps in the analysis, we used a τcrit of at least 500 ms. In some cases the activity occurred at a sufficiently high frequency that clusters contained rare overlapping openings from two channels. In some of these cases, segments of the data which did not contain overlapping events were analysed to obtain estimates of the mean open time and the open time distribution, but not closed times.
The isolated clusters were low-pass filtered at 3 kHz and idealized using the segmented-k-means algorithm (program SKM, Qin et al. 1996). The intracluster open and closed times were estimated using maximum likelihood methods which incorporate a correction for missed events (program MIL, Qin et al. 1997). Since we do not have a complete scheme for activation of the GABAA receptor, we analysed the closed and open time distributions separately (Akk et al. 2001; Steinbach & Akk, 2001). We note that this approach ignores the information which is contained in the association between kinetically identifiable states – for example, whether brief openings are preferentially associated with long closings. However, we do not have a complete kinetic model which incorporates all of the open and closed states which can be distinguished kinetically.
Concentration–effect relationships were fitted to the set of individual values across the concentration range of the steroid (rather than to mean values) using the non-linear curve fitting method in SigmaPlot (SPSS, Chicago, IL, USA). The best fitting parameter value is given in the text, with the estimated standard error derived from the fitting.
Results
Actions of steroids on open times in clusters of activity elicited by 50 μm GABA
Sample traces of clusters of activity elicited by 50 μm GABA are shown in Fig. 1, in the absence of steroids and in the presence of 1 μm ACN or 1 μm B285. Inspection of the traces suggests that the channel open time is increased by 1 μm ACN, but not by 1 μm B285. The mean open time was increased in a concentration-dependent fashion by ACN (Fig. 2A), from about 3.2 ms to about 15 ms, with a concentration producing a half-maximal increase (EC50) of 120 nm ACN (see Table 1). In contrast, B285 produced an increase in the mean open time only at concentrations near 10 μm (Fig. 2A).
Figure 1. Single channel currents elicited by 50 μm GABA in the absence or presence of steroids.
Sample clusters are shown in the left column, while the distributions of open times in clusters are shown on the right. Superimposed on the histograms are the fits for three exponential components (thin lines) and the sum of all three (heavy lines). ACN at 10 nm has little effect, while 1 μm ACN increases both the fraction and the mean duration of the long duration component (note the shift in the right-most component fitted to the data). In contrast, 1 μm B285 increases the proportion of long duration openings (note increased area under the right-most component) but not the mean duration. Finally, 30 μm B285 increases both the fraction and the mean duration of the long duration open time component. The structures of ACN and B285 are shown at the bottom of the figure. Note that ACN has the same structure as allopregnanolone, except for the presence of a carbonitrile at the 17-position instead of a methylketone. B285 differs in two places: the absence of a methyl group at the 18 position, and altered ring fusion at the 5 position.
Figure 2. Effects of steroids on mean durations.
The arithmetic mean open times (A), closed times (B) and calculated Popen (C) in clusters elicited by 50 μm GABA in the presence of various concentrations of ACN (•) or B285 (▵) are shown. The curves show the fit of the equation Y([steroid]) =Y0+ (Ymax–Y0)[steroid]/([steroid]+ EC50) for data obtained in the presence of ACN (continuous lines) and B285 (dashed lines). (The continuous line in B shows the mean closed time in the presence of ACN; no fit was performed to these data.) The dotted lines show the mean values for data obtained using 50 μm GABA in the absence of steroid. Values for the fit parameters are given in Table 1.
Table 1.
Parameters fit to concentration-effect curves for steroid actions
| ACN | B285 | Y0 | |
|---|---|---|---|
| Mean closed time (ms) | |||
| Ymax | N.D. | 2.7 ± 1.4 | |
| EC50 | N.D. | 17 ± 33 | 4.84 |
| Mean open time (ms) | |||
| Ymax | 14.6 ± 3.3 | 14.6** | |
| EC50 | 118 ± 152 | 15040 ± 20160 | 3.21 |
| Calculated Popen | |||
| Ymax | 0.71 ± 0.14 | 0.71 ± 0.06 | |
| EC50 | 136 ± 296 | 105 ± 83 | 0.40 |
| Fraction OT2 | |||
| Ymax | 0.25 ± 0.28 | 0.24 ± 0.35 | |
| EC50 | 60 ± 111 | 4.7 ± 10.4 | 0.63 |
| Fraction OT3 | |||
| Ymax | 0.42 ± 0.14 | 0.47 ± 0.19 | |
| EC50 | 178 ± 239 | 18 ± 25 | 0.15 |
| Mean duration OT3 (ms) | |||
| Ymax | 27 ± 16 | 27** | |
| EC50 | 35 ± 72 | 27980 ± 276700 | 7.4 |
| Rate to enter CT1 (s−1) | |||
| Ymax | 58 ± 128 | 58** | |
| EC50 | 38 ± 48 | 1904 ± 3187 | 300 |
| Rate to enter CT2 (s−1) | |||
| Ymax | 20 ± 46 | 20** | |
| EC50 | 49 ± 108 | 32110 ± 5327000 | 70 |
| Rate to enter CT3 (s−1) | |||
| Ymax | 0 ± 86 | 23 ± 24 | |
| EC50 | 38 ± 54 | 19 ± 9 | 150 |
| Sum closing rate (s−1) | |||
| Ymax | 84 ± 228 | 234 ± 164 | |
| EC50 | 44 ± 60 | 147 ± 253 | 500 |
The fitted values for the EC50 (nm) and value at a saturating concentration (Ymax) for various actions of steroids are shown, with the estimated standard error of the parameter. As indicated by the magnitude of the estimated error, these values are only approximations. However, they indicate that the EC50 values for actions of ACN are generally of the order of 100 nm. The EC50 values for actions of B285 fall in 2 groups, one near 100 nm and the other around 10 μm. In each case, the data were fitted with the equation Y([steroid]) =Y0+ (Ymax−Y0) [steroid]/([steroid]+ EC50). The data were sufficiently scattered that all fits were constrained by setting the value for Y0 to the mean value for GABA alone (shown in the column headed Y0), and in some cases the value for Ymax was set to the fitted value for data with ACN (indicated by
When clusters of activity are elicited by 50 μm GABA in the absence of steroids (Steinbach & Akk, 2001), the distribution of open times shows three exponential components (OT1 with mean duration ∼0.2 ms; OT2, 3.1 ms; OT3, 7.4 ms). The number of components was not altered in the presence of 1 μm ACN. However, both the proportion and the mean duration of the longest duration component were increased (Fig. 1). In the presence of 1 μm B285, the relative frequency of OT3 was increased, but the mean duration of OT3 was not (Fig. 1).
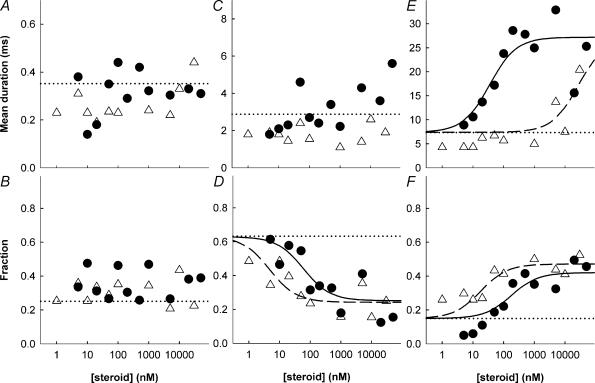
When open times were examined over a range of steroid concentrations both the proportion and mean duration of OT3 increased with the concentration of ACN, with an EC50 of about 180 and 35 nm, respectively (Fig. 3; Table 1). In contrast, the proportion increased with [B285] with an EC50 of about 18 nm, while the mean duration of OT3 showed a small increase at concentrations near 10 μm (Fig. 3). For both steroids, the increase in the proportion of openings in OT3 was accompanied by a decrease in the proportion in OT2. The mean durations of OT1 and OT2 did not change consistently with concentration (Fig. 3).
Figure 3. Effects of steroids on prevalence and duration of open times.
The fitted mean durations (top row) and fraction of total openings (bottom row) of the open time components are shown (ACN: • B285: ▵). Data are shown for the brief duration component (OT1, panels A and B), the intermediate duration component (OT2, panels C and D) and the long duration component (OT3, panels E and F). The lines are as described in the legend to Fig. 2; if no fitted curve is shown then no fit was attempted. Values for fit parameters are given in Table 1.
These data indicate that there are two distinguishable effects on open times which, together, result in the increase in the mean open time. ACN produces both effects with similar EC50 values near 100 nm. B285 has an EC50 for enhancing the prevalence of OT3 similar to that of ACN, while the increase in duration of OT3 occurs only at much higher concentrations. The increase in the overall mean open time is dominated by the increase in the duration of OT3.
The observation that two distinguishable effects on open times are seen, which have different concentration dependences on the concentrations of ACN and B285, suggests that there are at least two steroid binding sites on a single GABAA receptor.
In the absence of GABA, ACN is a weak activator of the GABAA receptor. The activity is characterized by brief openings which usually occur as isolated events. The characteristics of steroid-elicited activity will be described more fully when we analyse the actions of steroids on activity elicited by low concentrations of GABA (Akk et al. in preparation).
Actions of steroids on closed times in clusters of activity elicited by 50 μm GABA
ACN produced little change in the mean closed time in clusters, while B285 resulted in a reduction (Fig. 2B). The calculated probability of being open in a cluster (Popen) increased for both ACN and B285 (Fig. 2C).
When clusters of activity are elicited by 50 μm GABA in the absence of steroids (Steinbach & Akk, 2001), the distribution of closed times within clusters shows three components (CT1 with mean duration ∼0.2 ms; CT2, 1.5 ms; CT3, 18 ms). Previous work has indicated that the CT3 component seen at 50 μm GABA actually contains two components. One is a short duration desensitized state (which will be termed Csd, mean duration about 20 ms) which we have resolved at all GABA concentrations above 100 μm. The second is a component we have associated with channel activation (Cact) whose duration decreases with increasing GABA concentrations (Steinbach & Akk, 2001; Akk et al. 2004). When the distributions of closed times were examined over a range of steroid concentrations, neither ACN nor B285 changed the mean durations of any of the closed states. The mean duration of CT1 was about 0.2 ms (GABA: 0.16 ± 0.03, 5 patches; ACN: 0.19 ± 0.09, 14 patches; B285: 0.18 ± 0.05, 13 patches), for CT2 about 1.5 ms (GABA: 1.53 ± 0.43; ACN: 1.44 ± 0.48; B285: 1.40 ± 0.43) and for CT3 about 15 ms (GABA: 17.6 ± 5.6; ACN: 17.5 ± 6.6, 11 patches; B285: 14.9 ± 4.1). In some patches with ACN, the CT3 component was so rare that it was not distinguished (see below). At concentrations of ACN above 20 nm, a long duration closed time component appeared with duration about 100 ms (93 ± 51, 10 patches), while a residual component with mean duration about 16 ms remained. In contrast, B285 did not result in the appearance of this long duration component.
ACN decreased the rate of occurrence of CT1, CT2 and CT3 (Fig. 4), as would be expected since the mean open time increased. The new, very long duration component seen in the presence of ACN had a low rate of occurrence which was close to the rate of occurrence of Csd in control records (Fig. 4). Hence, it is possible that this particular component reflects a prolongation of a normally occurring short duration desensitized state by ACN. B285 decreased the rate of occurrence of CT3 (Fig. 4). At concentrations approaching 10 μm, B285 produced some reduction in the rate of entering CT1 and CT2.
Figure 4. Effects of steroids on the rates of entry into closed channel states.
The rates of occurrence for various closed time components (per second of open time) are shown. The left column shows data obtained in the presence of ACN, and the right column shows data in the presence of B285. In each panel, the point plotted at the left shows data obtained using 50 μm GABA in the absence of steroid, while the dotted lines show the mean values obtained over a range of GABA concentrations in the absence of steroid. The fitted curves were obtained as described in the legend to Table 1. The top panels (A and D) show the sum of all closing rates (▾) and the apparent short duration desensitized state (□). (Note that the short duration desensitized state was not resolved in the presence of B285, nor in the presence of 50 μm GABA alone.) The middle panels (B and E) show the rate of occurrence of the CT3 component. The bottom panels (C and F) show the two brief duration components, CT2 (○) and CT1 (♦). Note that ACN has more profound effects to decrease the rates of occurrence of CT1, CT2 and CT3, and, accordingly, a more profound effect on the overall closing rate. However, B285 does decrease the rate of occurrence of CT3 with a similar EC50, while only the highest concentrations of B285 affect the rates of occurrence of CT1 and CT2. Parameters for the fitted curves are given in Table 1.
The reduction by B285 in the mean closed time within clusters results from the decreased rate of occurrence of the CT3 component. In contrast, in the presence of ACN the rates of occurrence of both brief (CT1 and CT2) and long (CT3) closed times are reduced, so that the overall mean closed time is relatively unaffected.
ACN does not change the channel opening rate, while both B285 and ACN decrease the closing rate
The two shortest duration closed states, CT1 and CT2, are likely to reflect transient channel closures which do not lead back towards the unbinding of agonist (see Scheme 1 for a simplified kinetic model). This interpretation is based on the findings that the mean duration and the rate of occurrence of CT1 and CT2 do not depend on agonist concentration (Haas & Macdonald, 1999; Steinbach & Akk, 2001).
In Scheme 1, A indicates an agonist molecule, R a GABAA receptor with a closed channel, R* a receptor with an open channel and CT1 and CT2 the states underlying those two components in the closed time histogram. α is the channel closing rate constant, β is the channel opening rate constant, and k− is the dissociation rate for the first agonist molecule to leave the receptor.
As mentioned above, previous work has identified a component in the closed time distributions which becomes briefer as the GABA concentration is raised (Steinbach & Akk, 2001). The inverse of the mean duration of this component (the effective opening rate, β′) increases, then reaches a saturating value which we have interpreted as the channel opening rate (β). The concentration dependence of β′ provides an estimate of the affinity of the resting GABAA receptor for GABA. Accordingly, we examined the dependence of β′ on the GABA concentration in the presence of 1 μm ACN. As shown in Fig. 5, neither β nor the EC50 for β′ was affected by ACN, indicating that the channel opening rate and the affinity of the receptor were unaltered.
Figure 5. Lack of effect of 1 μm ACN on the channel effective opening rate.
The channel effective opening rate is plotted against the concentration of GABA, in the absence (⋄) and presence (•) of 1 μm ACN. The data were fitted with the equation β′([GABA]) =β([GABA]n)/{[GABA]n+ EC50n}. The values fitted were: GABA alone, β= 1883 ± 686 s−1, EC50= 359 ± 162 μm, n = 1.7 ± 0.2 and GABA + 1 μm ACN, β= 1475 ± 947 s−1, EC50= 374 ± 365 μm, n = 1.4 ± 0.3.
These data indicate that the residual CT3 component in the presence of ACN has the properties of an activation-related closed state on the return path between AnR* and R (see Scheme 1). In particular, the rate of occurrence of the state reflects the channel closing rate α. Accordingly, the selective reduction in the rate of occurrence in this component by both ACN and B285 indicates that both steroids decrease the channel closing rate. The estimated half-maximally effective concentrations are about 40 nm (ACN) and 20 nm (B285). ACN, in addition, reduces the rates for entering CT1 and CT2 with EC50 values of about 50 nm, while B285 has effects only at high concentrations. As in the case of open times, these observations are consistent with the idea that there are at least two steroid binding sites on the GABAA receptor.
The effects of a mixture of ACN and B285 on activity in clusters
To determine whether ACN and B285 were acting at the same sites, we examined the effects of an intermediate concentration of B285 on the actions of ACN. B285 was applied at a concentration of 1 μm, which is about 10-fold higher than the EC50 for the high affinity actions and about 10-fold lower than the EC50 for the low affinity actions, so that the expected occupancy of the high affinity site would be large (> 90%) and that of the low affinity site low (< 10%). Clusters of activity were elicited by 50 μm GABA in the presence of both 1 μm B285 and increasing concentrations of ACN. We examined whether the nature or concentration dependence of the effects of ACN was altered in the presence of B285.
The mean open time increased very little with increasing [ACN] in the presence of 1 μm B285. Over the range of 500 nm to 10 μm ACN, the average of the mean open times was 6.9 ± 3.4 ms (11 patches), which is significantly less (P = 0.001 from 2-tailed t test) than for ACN in the absence of B285 (13.8 ± 4.9 ms, 8 patches; see Fig. 2). The mean open time was longer than for GABA alone (3.2 ± 1.0 ms; 6 patches; P = 0.005). The mean closed time was reduced (3.1 ± 2.0 ms), as found with B285 alone, and did not change with [ACN]. These observations demonstrated that ACN and B285 interact, so the distributions of open and closed times were examined.
The distribution of open times in clusters showed three components in the mixture of steroids. The mean duration of OT3 increased at higher [ACN] to a lesser extent than in the absence of B285 (Fig. 6), while the proportion of openings in the long duration component was high for all mixtures and did not change with [ACN]. The finding that 1 μm B285 maximally enhanced the proportion of openings in the long duration component, and occluded the ability of ACN to enhance the proportion, is consistent with full occupancy of a high affinity site by B285 (although other, more complicated models would also be compatible with the data). However, the reduced ability of ACN to prolong the mean duration of the OT3 component cannot be explained by simple competition, since occupancy of this site by B285 would be expected to be very low from the data obtained using B285 alone (see above).
Figure 6. The presence of 1 μm B285 reduces effects of ACN on the long duration open time component.
The mean duration of the long duration open time component (OT3) is shown in A, while the fraction of the total openings which belonged to that component is shown in B, for data obtained in the presence of 1 μm B285 plus the indicated concentrations of ACN (▾). The continuous lines are the fits to data obtained with ACN alone (see Fig. 3), the dashed lines show mean values obtained in the presence of 1 μm B285 alone and the dotted lines show the mean values for data obtained using 50 μm GABA in the absence of steroid. No attempt was made to fit the data.
The durations of closed time components were not affected by the mixture of steroids (data not shown), as expected from the lack of effect of applications of single steroids (see above). The 100 ms closed time component was present at higher [ACN] in the presence of 1 μm B285. The rates of occurrence of the various components in the closed time distribution were reduced in the presence of a mixture of steroids, and did not show a noticeable dependence on [ACN] (Fig. 7). Overall, the rates of occurrence were similar to the rates seen in the presence of 1 μm B285 alone.
Figure 7. The presence of 1 μm B285 reduces effects of ACN on the rates of entering closed channel states.
The rates of occurrence of the various closed time components in the presence of 1 μm B285 plus the indicated concentration of ACN are shown. The curves show the fits to data obtained in the presence of ACN alone (see Fig. 4), the dashed lines show values in the presence of 1 μm B285 alone and the dotted lines show mean values obtained in the presence of GABA alone. No attempt was made to fit the data. Note that the presence of 1 μm B285 reduces the actions of ACN.
Overall, these data indicate that the presence of 1 μm B285 saturates the particular effects of steroid which show an EC50 for B285 of about 100 nm (increase in the fraction of openings in OT3, reduction in the rate of occurrence of CT3). This is consistent with the idea that B285 and ACN bind to a common site to produce these effects. However, 1 μm B285 also reduces the effects of ACN for the actions which show an EC50 for B285 of about 10 μm (the increase in mean open time, duration of OT3 and the rate of entry into CT1 and CT2). A possible interpretation of these observations is that there are two binding sites for steroids on a GABAA receptor, which interact allosterically. This idea will be discussed more fully below (see Discussion).
Mutation of the carboxy terminus of the γ2 subunit selectively alters neurosteroid actions
Previous studies of neuronal nicotinic receptors have identified the carboxy terminus of the α4 subunit as a region of the receptor which is involved in recognition of the steroid, 17β-oestradiol (Paradiso et al. 2001). We tested the possibility that this region may be involved in some of the potentiating actions of neurosteroids on the GABAA receptor. We replaced the final amino acids of the α1, β2 and γ2 subunits with sequence derived from the ρ1 subunit of the GABAC receptor, as this receptor is relatively insensitive to steroids (Morris et al. 1999). In these constructs the final residues of the α1 subunit (WATYLNREPQLKAPTPH), the β2 subunit (WLYYV) and the γ2 subunit (WVSYLY) were replaced with the residues WSIFS.
The behaviour of the receptors containing mutated subunits was qualitatively normal (Fig. 8), in that activity elicited by 50 μm GABA occurred in clusters. The calculated mean open times and closed times were within a factor of 2 of the wild-type receptors for receptors containing single mutated subunits (Table 2), although there were apparent effects on both mean open and mean closed times for some of the constructs. A full characterization of the effects of the mutations will be published in the future. At the moment, the focus is on the actions of steroids on the activity elicited by 50 μm GABA.
Figure 8. Single channel currents elicited by 50 μm GABA from GABAA receptors containing mutated subunits.
Clusters of activity are shown, from receptors containing (α1–ρ)β2γ2 subunits elicited by 50 μm GABA (top row) or GABA plus 1 μm ACN (2nd row). Note that the sojourns in open states are prolonged in the presence of ACN. Clusters from receptors containing α1β2(γ2–ρ) subunits elicited by 50 μm GABA (3rd row) or GABA plus 1 μm ACN (bottom row). Note that open sojourns are not obviously prolonged, although closed state sojourns appear to be reduced in duration.
Table 2.
Parameters for steroid actions on receptors containing mutated subunits
| Receptor | Condition | Mean open time (ms) | Mean closed time (ms) | Mean OT3 (ms) | Fraction OT3 |
|---|---|---|---|---|---|
| Wild-type | 50 GABA | 3.21 ± 0.89 (4) | 4.65 ± 1.40 (4) | 8.3 ± 2.7 (4) | 0.15 ± 0.08 (4) |
| α1β2(γ2–ρ) | 50 GABA | 4.34 ± 1.17 (3) | 6.10 ± 2.01 (3) | 8.2 ± 0.7 (3) | 0.40 ± 0.20 (3) |
| (versus wild-type) | (ratio) | 1.35 | 1.31 | 0.98 | 2.66 |
| (α1-ρ)(β2–ρ)(γ2–ρ) | 50 GABA | 7.2 ± 1.1 (4)* | 12.4 ± 8.2 (4) | 13.6 ± 4.2 (4) | 0.42 ± 0.12 (4)* |
| (versus wild-type) | (ratio) | 2.24* | 2.67 | 1.63 | 2.80* |
| (α1-ρ)β2γ2 | 50 GABA | 2.56 ± 0.71 (3) | 9.35 ± 4.56 (3) | 5.4 ± 1.7 (3) | 0.30 ± 0.08 (3) |
| (versus wild-type) | (ratio) | 0.80 | 2.01 | 0.65 | 2.01 |
| α1(β2–ρ)γ2 | 50 GABA | 2.39 ± 0.51 (5) | 6.41 ± 2.57 (5) | 4.8 ± 0.9 (5)* | 0.34 ± 0.19 (5) |
| (versus wild-type) | (ratio) | 0.75 | 1.38 | 0.58* | 2.27 |
| Wild-type | + ACN | 13.3 ± 5.8 (3)* | 4.59 ± 2.40 (3) | 37 ± 22 (3)* | 0.35 ± 0.06 (3)* |
| (versus 50 GABA) | (ratio) | 4.15* | 0.99 | 4.49* | 2.32* |
| α1β2(γ2–ρ) | + ACN | 3.49 ± 0.30 (6) | 1.77 ± 0.40 (6)* | 8.4 ± 1.5 (6) | 0.37 ± 0.06 (6) |
| (versus 50 GABA) | (ratio) | 0.80 | 0.29* | 1.02 | 0.92 |
| (α1-ρ)(β2–ρ)(γ2–ρ) | + ACN | 5.64 ± 2.53 (3) | 6.86 ± 5.92 (3) | 9.9 ± 4.3 (3) | 0.46 ± 0.07 (3) |
| (versus 50 GABA) | (ratio) | 0.78 | 0.55 | 0.72 | 1.09 |
| (α1-ρ)β2γ2 | + ACN | 10.4 ± 2.4 (5)* | 3.46 ± 1.68 (5)* | 19.6 ± 4.9 (5)* | 0.51 ± 0.10 (5)* |
| (versus 50 GABA) | (ratio) | 4.07* | 0.37* | 3.63* | 1.68* |
| α1(β2–ρ)γ2 | + ACN | 6.09 ± 1.84 (6)* | 2.3 ± 0.73 (6)* | 14.2 ± 4.0 (6)* | 0.39 ± 0.05 (6) |
| (versus 50 GABA) | (ratio) | 2.54* | 0.36* | 2.94* | 1.14 |
| Wild-type | + B285 | 4.31 ± 1.98 (3) | 2.55 ± 0.08 (3) | 8.7 ± 4.5 (3) | 0.45 ± 0.05 (3)* |
| (versus 50 GABA) | (ratio) | 1.34 | 0.55 | 1.05 | 2.97* |
| α1β2(γ2–ρ) | + B285 | 3.6 ± 0.35 (5) | 2.44 ± 1.26 (5)* | 9.4 ± 2.6 (5) | 0.35 ± 0.12 (5) |
| (versus 50 GABA) | (ratio) | 0.83 | 0.40* | 1.15 | 0.86 |
| Wild-type | + PB | 5.61 ± 1.25 (6)* | (5.0) | 14.3 ± 2.5 (6)* | 0.32 ± 0.07 (6)* |
| (versus 50 GABA) | (ratio) | 1.75* | — | 1.71* | 2.13* |
| α1β2(γ2–ρ) | + PB | 7.99 ± 2.09 (5)* | 2.98 ± 1.4 (5)* | 13.1 ± 2.2 (5)* | 0.50 ± 0.19 (5) |
| (versus 50 GABA) | (ratio) | 1.84* | 0.49* | 1.61* | 1.23 |
The mean values for various parameters describing data obtained from receptors containing the subunits given in the first column. The column headed condition indicates the drugs present: control data were obtained in the presence of 50 μm GABA alone. Data in the presence of 1 μm ACN (+ ACN), 1–5 μm B285 (+ B285) or 40 μm pentobarbital (+ PB) are shown. The data for wild-type receptors in the presence of pentobarbital are from Steinbach & Akk (2001), and were obtained using GABA concentrations of 50–1000 μm. All other data were obtained using 50 μm GABA. The next 2 columns show calculated mean open and closed times (mean ±s.d., with number of patches given in parentheses), with the ratio of the mean to specified control mean given below the means (for example, versus 50 GABA indicates that the control was data from the given construct elicited by GABA alone).
P < 0.05 (2 tailed t test). The final 2 columns give data for the long duration OT3 component.
As illustrated in Fig. 8, receptors containing the α1–ρ construct showed a similar response to ACN as did wild-type receptors: open times were prolonged and closed times appeared to be reduced in duration. In contrast, when receptors contained the γ2–ρ construct the open times were not prolonged. The calculated mean open and closed times are presented in Table 2. Receptors which contained the α1–ρ or β2–ρ constructs (with other subunits being wild-type) showed open times which were prolonged by ACN, while receptors containing the γ2–ρ construct (with either wild-type or mutated α1 and β2 subunits) did not. The distributions of open times were then analysed. In each case, the distributions showed three components (as in the wild-type receptor). However, the receptors containing any of the constructs showed, in general, a higher fraction of openings in the long duration component (Table 2), and there were some differences in the mean duration for the long duration component (OT3). The addition of ACN prolonged the duration of OT3 for receptors which contained a wild-type γ2 subunit, but failed to prolong it for receptors with the γ2–ρ construct (Table 2, Fig. 9). Since the fraction of OT3 was already high for the receptors containing the γ2–ρ construct, it is not clear how to interpret the observation that ACN failed to increase that fraction. The addition of B285, as expected, had no effect on the duration of OT3 for receptors containing the γ2–ρ construct.
Figure 9. The relative changes produced by ACN or pentobarbital on receptors containing wild-type or mutated subunits.
A, mean duration of the OT3 component in the presence of 1 μm ACN, normalized to the value in the presence of GABA alone, and similar data in the presence of 40 μm pentobarbital (PB). Data were obtained from receptors containing α1β2γ2 (•), (α1–ρ)β2γ2 (▴), α1(β2–ρ)γ2 (▀), α1β2(γ2–ρ) (□) and (α1–ρ)(β2–ρ)(γ2–ρ) (▵) subunits. B, data for the rates of entry to the CT1, CT2, CT3 states and the sums of those rates. Data are shown for receptors in the presence of 1 μm ACN (•, wild-type; ○, α1β2(γ2–ρ)) or 40 μm PB (▴, wild-type; ▵, α1β2(γ2–ρ)); normalized to the value in the presence of GABA alone. Note that the ordinate in panel A is linear, while that in panel B is logarithmic. Data are means ±s.d. Non-normalized data and results of statistical tests are shown in Tables 2 and 3.
It has already been reported that 40 μm pentobarbital increases the mean open time for activity elicited by 50 μm GABA from wild-type receptors (Steinbach & Akk, 2001). The increase results from an increase the fraction of openings in OT3 and an increase in the mean duration of OT3. In marked contrast to the results with ACN, pentobarbital prolonged the mean open time and the mean duration of OT3 for receptors containing the γ2–ρ construct, in a fashion indistinguishable from effects on wild-type receptors (Table 2, Fig. 9).
These observations demonstrate that the carboxy terminus of the γ2 subunit is required for the ability of ACN to prolong the duration of openings, but not for the ability of pentobarbital.
We then analysed the distributions of closed times for activity elicited by 50 μm GABA from receptors containing the γ2–ρ construct. Qualitatively, the data in Fig. 8 and Table 2 suggest that ACN does affect closed times for these receptors. The distributions showed three components, similar to those seen for wild-type receptors. The durations for the CT1, CT2 and CT3 components were all similar to those for wild-type receptors (data not shown). The rates of occurrence for the components were also similar in the absence of steroid (Table 3). In the presence of ACN there was no decrease in the rates of occurrence in the CT1 and CT2 components (Table 3, Fig. 9), as might be expected from the lack of an increase in the mean open time. However, there was still a significant decrease in the rate of occurrence of the CT3 component in the presence of either ACN or B285 (Table 3, Fig. 9). Pentobarbital produced a decrease in the rates of entering CT1 and CT3 which are comparable to those on wild-type receptors (Table 3), although they did not reach significance at the 0.05 level. Hence, the effect of the mutations to the γ2 subunit is to selectively remove the ability of ACN to reduce the rate of entry into the CT1 and CT2 components while sparing the reduction of entry into the activation-related closed time component. In contrast, an analysis of the closed time distributions for receptors containing mutated α1 or β2 subunits, but a wild-type γ2 subunit, showed that 1 μm ACN produced significant decreases in the rates of occurrence of all three closed time components, CT1, CT2 and CT3 (data not shown).
Table 3.
Effects of steroids on rates for entering closed channel states
| Receptor | Condition | k+1 (s−1) | k+2 (s−1) | k+3 (s−1) | sum (s−1) |
|---|---|---|---|---|---|
| Wild-type | 50 GABA | 248 ± 51 (4) | 49 ± 10 (4) | 82 ± 33 (4) | 379 ± 86 (4) |
| α1β2(γ2–ρ) | 50 GABA | 141 ± 66 (2) | 53 ± 15 (2) | 53 ± 6 (2) | 246 ± 75 (2) |
| P versus wild-type | P = 0.088 | P = 0.741 | P = 0.305 | P = 0.138 | |
| Ratio to wild-type | 0.57 | 1.07 | 0.64 | 0.65 | |
| Wild-type | + ACN | 56 ± 28 (3)* | 27 ± 8 (3)* | 13 ± 11 (2) | 92 ± 44 (3)* |
| P versus 50 GABA | P = 0.002 | P = 0.025 | P = 0.052 | P = 0.003 | |
| Ratio to 50 GABA | 0.23* | 0.55* | 0.16 | 0.24* | |
| α1β2(γ2–ρ) | + ACN | 194 ± 44 (5) | 99 ± 21 (5)* | 17 ± 6 (5)* | 310 ± 29 (5) |
| P versus 50 GABA | P = 0.244 | P = 0.038 | P = 0.001 | P = 0.131 | |
| Ratio to 50 GABA | 1.38 | 1.88* | 0.32* | 1.26 | |
| Wild-type | + B285 | 177 ± 38 (3) | 98 ± 42 (3) | 23 ± 9 (3)* | 298 ± 82 (3) |
| P versus 50 GABA | P = 0.102 | P = 0.070 | P = 0.031 | P = 0.262 | |
| Ratio to 50 GABA | 0.72 | 1.99 | 0.28* | 0.79 | |
| α1β2(γ2–ρ) | + B285 | 206 ± 15 (2) | 99 ± 33 (2) | 25 ± 4 (2)* | 329 ± 52 (2) |
| P versus 50 GABA | P = 0.306 | P = 0.216 | P = 0.030 | P = 0.328 | |
| Ratio to 50 GABA | 1.46 | 1.88 | 0.47* | 1.34 | |
| Wild-type | + PB | 142 ± 51 (5)* | 39 ± 14 (5) | 46 ± 18 (5) | 227 ± 64 (5)* |
| P versus 50 GABA | 0.017* | 0.265 | 0.071 | 0.018* | |
| Ratio to 50 GABA | 0.57 | 0.79 | 0.56 | 0.60* | |
| α1β2(γ2–ρ) | + PB | 81 ± 16 (5) | 51 ± 11 (5) | 20 ± 17 (5) | 152 ± 35 (5) |
| P versus 50 GABA | 0.082 | 0.870 | 0.054 | 0.057 | |
| Ratio to 50 GABA | 0.58 | 0.97 | 0.38 | 0.62 |
The estimated rates of entry into the CT1, CT2 and CT3 states and the sum of the rates, for receptors containing the subunits given in the first column. The data are shown as mean ±s.d. for data obtained from the numbers of patches given in parentheses. Conditions and format are the same as used in Table 2.
P < 0.05 (2 tailed t test).
Overall, the replacement of the carboxy terminal segment of the γ2 subunit with residues from the ρ1 subunit removed steroid effects which showed selectivity for ACN (prolongation of mean open times, prolongation of OT3 and reduction in rates on entering CT1 and CT2), while sparing one action of both ACN and B285 (reduction of entry into CT3). The mutation appeared to directly affect the prevalence of OT3, so the loss of that steroid effect is difficult to interpret. We have not yet identified the specific residues involved in the change in steroid action. Previous studies of the nicotinic α4 subunit demonstrated that both specific residues and the overall conformation of the carboxy terminal region were critical in conferring the ability of 17β-oestradiol to potentiate that receptor.
Discussion
These data demonstrate that neuroactive steroids have multiple effects on the function of GABAA receptors. A major effect is to increase the duration of channel openings by increasing both the duration and the prevalence of the longest component in the open time distributions. The closed times are also affected, by selectively decreasing the rate of occurrence of the activation related component and, in the case of ACN, by generally decreasing the rate for entering closed states. Finally, some steroids result in the appearance of a longer duration closed state, which might reflect the stabilization of a short-lived desensitized state. One important observation is that ACN had no significant effect on the dependence of the effective opening rate on the GABA concentration. The apparent affinity of the resting receptor for GABA and the channel opening rate were not affected.
Comparison to previous studies
Previous studies of the effects of steroids on channel properties of GABAA receptors have used low concentrations of GABA so that only the properties of bursts of openings can be examined. All studies have agreed that neuroactive steroids have no effect on single channel conductance. An analysis of current fluctuations recorded from rat spinal cord neurones in cell cultures found that alphaxalone decreased the cut-off frequency of a fit Lorentzian, corresponding to an increase in the mean burst duration from about 30 ms to 74 ms (Barker et al. 1987). However a study of receptors expressed in HEK cells after transfection of α1, β1 and γ2 subunits found no change in spectra recorded in the absence or presence of allopregnanolone, although macroscopic responses were potentiated (Puia et al. 1990). Bursts recorded from bovine chromaffin cells were significantly prolonged (Lambert et al. 1990). In the most extensive study, bursts of openings elicited by 2 μm GABA from cultured mouse spinal cord neurones were analysed (Twyman & Macdonald, 1992). In these data, pregnanolone or androsterone increased the mean open time by about 2-fold, with an EC50 less than 1 μm steroid. Three components were identified in the open time distribution. The relative frequency of the longest duration component was increased, but the mean duration was unchanged. The prevalence of the briefest duration component was decreased, while the intermediate duration component was largely unaffected.
Overall, then, several previous studies have found that neuroactive steroids increase the burst duration or channel open duration. However, there are differences among the reports, and to the observations we have made using 50 μm GABA to activate the receptor. In particular, no prolongation of the mean open time components was seen in the previous studies. In addition, there were no indications, at least with the steroids used, that there was more than a single site at which steroids acted in potentiating responses to GABA. Twyman & Macdonald (1992) also reported that the burst frequency elicited from outside-out patches was increased in the presence of steroid, and suggested that it might result from a change in GABA affinity. However, this observation can be difficult to interpret since even at low concentrations of GABA the receptor may enter relatively long-lived closed states (cf. Maconochie et al. 1994; Jones & Westbrook, 1995; Burkat et al. 2001), which can make it difficult to interpret burst frequencies. We have used a more direct approach to estimating the affinity of the resting receptor for GABA and the channel opening rate, and found that 1 μm ACN did not change either opening rate or affinity for GABA. Some of the divergence in observations might result from differences in preparations, in GABAA receptor subunit combinations, or in the particular steroids studied.
Sites for steroid interactions
The use of two steroids allowed us to demonstrate that the steroid structure differentially affected the functional effects (see Table 1). Qualitatively, the concentrations of ACN producing half-maximal effects were similar for all the actions, near 100 nm. On the other hand, the effects of B285 fell into two groups. For one group, comprising the increase in prevalence for the long duration open time and the decrease in the rate of occurrence of CT3, the EC50 was near 20 nm. For the other group, the increase in duration of OT3 and the decrease in the rates for entering CT1 and CT2, the EC50 was 10 μm or more. These observations indicate that at least two binding sites are involved, which recognize different features of the steroid molecule. For convenience in discussion, we will assume that there are only two sites. The sites will be called site A (underlying the increase in prevalence for OT3 and the decrease in entering CT3) and site B (underlying the increase in duration of OT3 and the decrease in the rates for entering CT1 and CT2).
We applied a mixture of steroids to examine this possible model in more detail. The results suggest that B285 and ACN both bind to site A. In the presence of a saturating concentration of B285 (1 μm or about 50 times the apparent EC50), the increase in the prevalence of OT3 and the decrease in the rate of occurrence of CT3 appear to be saturated. However, other observations cannot be interpreted in terms of simple competitive interactions. B285 apparently has a very low affinity for site B (EC50 > 10 μm) while the presence of 1 μm B285 blocks the ability of ACN to reduce the rates for entering brief closed states or prolong the duration of OT3. A simple hypothesis to explain this observation is that binding of a steroid to site A affects the affinity at site B. In this example, occupancy of site A by ACN would result in a higher affinity for steroid binding at site B, whereas occupancy of site A by B285 would result in a lower affinity at site B. The site involved in production of the 100 ms closed time component appears to be distinct from either A or B. Of course, there may be additional sites and additional interactions at the level of the mechanism by which potentiation is produced.
Mutation of the subunits indicated that the carboxy terminus of the γ2 subunit, but not the α1 or β2 subunits, contained residues which were required for the ability of ACN to prolong OT3 and to reduce the rates for entering CT1 and CT2 (both site B effects). In contrast, the ability of ACN or B285 to reduce the rate of entry into CT3 (site A effect) was not affected. The other site A effect, an increase in the prevalence of the OT3 component, was removed, but this observation is confounded by the fact that the mutation itself enhanced the prevalence of OT3 in the absence of steroids. It is important to note that pentobarbital was able to increase the mean open time and the duration of OT3 for receptors containing the mutated γ2 subunit. This observation indicates that the mutation has not removed all allosteric mechanisms which act to prolong OT3, but shows selectivity for the action of steroid potentiators. These observations are consistent with the idea that there is more than one distinct site for steroid interaction with a single GABAA receptor, and that occupancy of the sites is coupled with distinct functional effects.
Some previous studies have suggested the presence of multiple sites for steroid interactions with GABAA receptors. The sites for the inhibitory and potentiating actions of neurosteroids appear to be distinct (see Introduction). Based on physiological studies, the authors of one study have suggested that more than one site is involved in positive actions of steroids, perhaps one site for direct gating and another for potentiation (Puia et al. 1990). However, the present work significantly extends this idea by providing direct evidence for two sites which recognize specific features of the steroid molecule, which are coupled to distinct functional consequences and which are likely to be physically distinct. The data do not provide any information about whether one or both sites are involved in gating by steroids as well as potentiation.
Biochemical studies have also reported evidence that there is more than one steroid binding site on the GABAA receptor. The initial study (Hawkinson et al. 1994) examined the inhibition of TBPS binding by steroids, and found that the inhibition was biphasic with two apparent binding sites. However, this study used brain homogenates and could not distinguish between the possibilities that the apparent multiple sites were on separate populations of receptors, or that a single receptor had more than one site. A subsequent study made similar observations using recombinant GABAA receptors expressed in insect SF9 cells (Srinivasan et al. 1999), supporting the idea that two sites exist on a single receptor (although heterogeneity in expressed receptors is possible). These studies did not, however, distinguish these sites in terms of recognition of steroid structural features and did not associate the sites with any physiological actions.
Most recently, an extensive examination of a number of neurosteroid analogues has found that two sites are necessary to explain the ability of steroids to modulate TBPS binding (A. Evers, personal communication). As mentioned in the Introduction, early results from these studies were used to select ACN and B285 for physiological characterization, as they differed greatly in their ability to modulate TBPS binding. The biochemical data also indicate that the two steroid recognition sites are allosterically linked, as our present data obtained with mixtures of steroids suggest.
Mechanisms for steroid effects
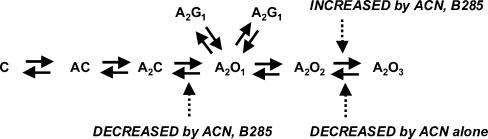
We identified four major effects of steroids. It seems likely that the mechanism which underlies the increased duration of OT3 is the reduction in the rates for entering CT1 and CT2. It is possible that the increased prevalence of OT3 and the reduced α have a common mechanism, based simply on the structure–effect relationship for ACN and B285. We have begun to explore possible kinetic mechanisms for these effects, but have not found a suitable scheme. One simple kinetic scheme which captures many of the features of our observations is shown in Fig. 10. In this scheme, the three open states are connected linearly with a single channel closing rate to A2C. The brief closed states, CT1 and CT2, arise from the first open state. In this scheme, both ACN and B285 reduce α (acting at site A). Both ACN and B285 increase the rate from A2O2 to A2O3, to increase the proportion of openings in the OT3 component (site A). Finally, ACN reduces the rate for leaving A2O3 (acting at site B) with high potency.
Figure 10. A hypothetical kinetic scheme which accounts for many of the actions of neuroactive steroids on GABAA receptor function.
A receptor with a closed channel is designated by C, an agonist molecule by A, a receptor with an open channel as O, and the brief duration closed states which underlie the CT1 and CT2 components as G1 and G1′. The actions of steroids are hypothesized to occur by actions to increase the rate constant for going from A2O2 to A2O3 (site A effect), to decrease the rate for going from A2O1 to A2C (site A effect), and to decrease the rate for going from A2O3 to A2O2 (site B effect).
There are similarities between the actions of ACN and pentobarbital on GABAA receptors. Both drugs affect the rates at which the channel enters non-conducting states, with no changes seen in channel opening or receptor affinity. Both drugs selectively reduce the intrinsic channel closing rate (α). Finally, both drugs increase the prevalence and duration of OT3. However, there are some differences in effects. An analysis of adjacent dwells (data not shown) indicates that in the presence of ACN long sojourns in an open state are associated with brief sojourns in a closed state whereas the converse is true in the presence of pentobarbital. In addition, the effects of the mutations in the γ2 subunit suggest that at least some of the binding sites for pentobarbital and ACN are different.
Recently, Bianchi & Macdonald (2003) have provided evidence that neurosteroids potentiate GABAA receptor activation when low efficacy agonists are used, by altering the kinetic mode to a higher efficacy mode, as was suggested earlier for barbiturates (Steinbach & Akk, 2001). Our data are compatible with this suggestion. However, there are several aspects of this idea which remain to be clarified. One is the question of how modes are connected, and which states are common to different modes. What are the rates for mode switching? A second aspect is to determine how the various actions of either barbiturates or steroids are accommodated in the framework of mode switching. For example, is the mode-controlling site distinct from the sites underlying the actions examined in the present study?
Mechanisms for potentiation
Potentiation of macroscopic responses to a relatively high concentration of GABA would be expected to follow the increase in Popen. There are two effects of steroids which result in an increase in the Popen in a cluster – an increase in open time and a decrease in closed time. ACN increases Popen by increasing the open time, while B285 has more action to reduce the closed time. In preliminary experiments, we examined the abilities of ACN and B285 to potentiate the responses of Xenopus oocytes expressing GABAA receptors when responses were elicited with a GABA concentration which produces a response equivalent to about 30% to 50% of maximal (20 μm). As expected, both steroids potentiated responses in the oocytes. ACN potentiated responses 1.5-fold with an EC50 of about 120 nm (fitted by a Hill equation with the Hill coefficient set to 1) while B285 potentiated responses 2.3-fold, with an EC50 of 3 μm (data not shown). The Popen curves predicted from our single channel data for ACN and B285 are very similar to each other (Fig. 2, Table 1), with EC50 values of 136 nm for ACN and 105 nm for B285 and maximal potentiation of 1.8-fold for both. There is very good agreement for the results obtained with ACN, but only qualitative agreement for data with B285.
Very few studies of potentiation use a half-maximal concentration of GABA. Instead, potentiation of responses to low concentrations of GABA or prolongation of postsynaptic currents are examined. In both of these cases, the properties of bursts of single channel activity would underlie potentiation, rather than Popen in a cluster. The burst duration depends on the number of openings in a burst and the mean duration of the open time. Our data would predict that the number of openings should increase, as a result of the reduction in α, and that the open duration should increase in the case of ACN. Accordingly, we would expect that ACN should be more effective at potentiating responses to low concentrations of GABA and at prolonging the decay of postsynaptic currents. The actions of these steroids on responses to a low concentration of GABA will be examined in a subsequent publication (Akk et al. in preparation).
Conclusions
Our data demonstrate that neuroactive steroids have several actions on GABAA receptors, requiring at least two binding sites and operating through at least two mechanisms. This multiplicity of actions may help explain why previous attempts to identify a site for neurosteroid binding to GABAA receptors have not succeeded. The differences between B285 and ACN are likely to reflect two features of the steroid structure; both the difference in the stereochemistry at the 5-position and the presence or absence of the 18-methyl group probably contribute to the differential actions of ACN and B285. Further studies of additional steroids will be required to determine whether this idea has validity.
Acknowledgments
This work was supported by a grant from the NIH (GM P01-47969 to D.C., A.E. and J.H.S.) and the Alcoholic Beverage Medical Research Foundation (to G.A.). T.D. was supported in part by a WU/HHMI Summer Undergraduate Research Fellowship funded by an Undergraduate Biological Sciences Education Program grant from the Howard Hughes Medical Institute to Washington University. J.H.S. is the Russell and Mary Shelden Professor of Anaesthesiology.
References
- Akk G. Contributions of the non-α subunit residues (loop D) to agonist binding and channel gating in the muscle nicotinic acetylcholine receptor. J Physiol. 2002;544:695–705. doi: 10.1113/jphysiol.2002.029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes J, Steinbach JH. Pregnenolone sulfate block of GABAA receptors: mechanism and involvement of a residue in the M2 region of the α subunit. J Physiol. 2001;532:673–684. doi: 10.1111/j.1469-7793.2001.0673e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes JR, Steinbach JH. Activation of GABAA receptors containing the α4 subunit by GABA and pentobarbital. J Physiol. 2004;556:387–399. doi: 10.1113/jphysiol.2003.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Steinbach JH. Low doses of ethanol and a neuroactive steroid positively interact to modulate rat GABAA receptor function. J Physiol. 2003;546:641–646. doi: 10.1113/jphysiol.2002.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JL, Harrison NL, Lange GD, Owen DG. Potentiation of γ-aminobutyric-acid-activated chloride conductance by a steroid anaesthetic in cultured rat spinal neurones. J Physiol. 1987;386:485–501. doi: 10.1113/jphysiol.1987.sp016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkat PM, Yang J, Gingrich KJ. Dominant gating governing transient GABAA receptor activity: a first latency and Po/o analysis. J Neurosci. 2001;21:7026–7036. doi: 10.1523/JNEUROSCI.21-18-07026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haage D, Johansson S. Neurosteroid modulation of synaptic and GABA-evoked currents in neurons from the rat medial preoptic nucleus. J Neurophysiol. 1999;82:143–151. doi: 10.1152/jn.1999.82.1.143. [DOI] [PubMed] [Google Scholar]
- Haas KF, Macdonald RL. GABAA receptor subunit γ2 and δ subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol. 1999;514:27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Simmonds MA. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323:287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Vicini S, Barker JL. A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci. 1987;7:604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkinson JE, Kimbrough CL, McCauley LD, Bolger MB, Lan NC, Gee KW. The neuroactive steroid 3 α-hydroxy-5 β-pregnan-20-one is a two-component modulator of ligand binding to the GABAA receptor. Eur J Pharmacol. 1994;269:157–163. doi: 10.1016/0922-4106(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Harney SC, Peters JA, Frenguelli BG. Modulation of native and recombinant GABAA receptors by endogenous and synthetic neuroactive steroids. Brain Res Brain Res Rev. 2001a;37:68–80. doi: 10.1016/s0165-0173(01)00124-2. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Callachan H, Peters JA. Neurosteroid modulation of native and recombinant GABAA receptors. Cell Mol Neurobiol. 1996;16:155–174. doi: 10.1007/BF02088174. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Peters JA, Harney SC, Belelli D. Steroid modulation of GABAA receptors. In: Mohler H, editor. Pharmacology of GABA and Glycine Neurotransmission. Berlin: Springer Verlag; 2001b. pp. 117–140. [Google Scholar]
- Lambert JJ, Peters JA, Sturgess NC, Hales TG. Steroid modulation of the GABAA receptor complex: electrophysiological studies. Ciba Found Symp. 1990;153:56–71. doi: 10.1002/9780470513989.ch4. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1993;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Maconochie DJ, Zempel JM, Steinbach JH. How quickly can GABAA receptors open. Neuron. 1994;12:61–71. doi: 10.1016/0896-6273(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Morris K, Moorefield CN, Amin J. Differential modulation of the γ-aminobutyric acid type C receptor by neuroactive steroids. Mol Pharmacol. 1999;56:752–759. [PubMed] [Google Scholar]
- Paradiso K, Zhang J, Steinbach JH. The C terminus of the human nicotinic α4β2 receptor forms a binding site required for potentiation by an estrogenic steroid. J Neurosci. 2001;21:6561–6568. doi: 10.1523/JNEUROSCI.21-17-06561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate γ-aminobutyric acid receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J. 1996;70:264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. Maximum likelihood estimation of aggregated Markov processes. Proc R Soc Lond B Biol Sci. 1997;264:375–383. doi: 10.1098/rspb.1997.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Sapp DW, Tobin AJ, Olsen RW. Biphasic modulation of GABA receptor binding by steroids suggests functional correlates. Neurochem Res. 1999;24:1363–1372. doi: 10.1023/a:1022524421464. [DOI] [PubMed] [Google Scholar]
- Steinbach JH, Akk G. Modulation of GABAA receptor gating by pentobarbital. J Physiol. 2001;537:715–733. doi: 10.1111/j.1469-7793.2001.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, He Y, Eisenman LN, Fields C, Zeng CM, Mathews J, Benz A, Fu T, Zorumski E, Steinbach JH, Covey DF, Zorumski CF, Mennerick S. 3β-hydroxypregnane steroids are pregnenolone sulfate-like GABAA receptor antagonists. J Neurosci. 2002;22:3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman SH, Shingai R, Harvey RJ, Darlison MG, Barnard EA. Effects of subunit types of the recombinant GABAA receptor on the response to a neurosteroid. Eur J Pharmacol. 1992;225:321–330. doi: 10.1016/0922-4106(92)90106-6. [DOI] [PubMed] [Google Scholar]
- Zhu WJ, Vicini S. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J Neurosci. 1997;17:4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick SJ, Covey DF. Enantioselective modulation of GABAergic synaptic transmission by steroids and benz[e]indenes in hippocampal microcultures. Synapse. 1998;29:162–171. doi: 10.1002/(SICI)1098-2396(199806)29:2<162::AID-SYN7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]