Abstract
It is known that brain noradrenaline (norepinephrine) mediates fever, but the neuronal group involved is unknown. We studied the role of the major noradrenergic nucleus, the locus coeruleus (LC), in lipopolysaccharide (LPS)-induced fever. Male Wistar rats had their LC completely ablated electrolytically or their catecholaminergic LC neurones selectively lesioned by microinjection of 6-hydroxydopamine; the controls were sham-operated. Both lesions resulted in a marked attenuation of LPS (1 or 10 μg kg−1, i.v.) fever at a subneutral (23°C) ambient temperature (Ta). Because electrolytic and chemical lesions produced similar effects, the role of the LC in fever was further investigated using electrolytic lesions only. The levels of prostaglandin (PG) E2, the terminal mediator of fever, were equally raised in the anteroventral third ventricular region of LC-lesioned and sham-operated rats during the course of LPS fever, indicating that LC neurones are not involved in febrigenic signalling to the brain. To investigate the potential involvement of the LC in an efferent thermoregulatory neuronal pathway, the thermoregulatory response to PGE2 (25 ng, i.c.v.) was studied at a subneutral (23°C, when fever is brought about by thermogenesis) or neutral (28°C, when fever is brought about by tail skin vasoconstriction) Ta. The PGE2-induced increases in metabolic rate (an index of thermogenesis) and fever were attenuated in LC-lesioned rats at 23°C, whereas PGE2-induced skin vasoconstriction and fever normally developed in LC-lesioned rats at 28°C. The LC-lesioned rats had attenuated PGE2 thermogenesis despite the fact that they were fully capable of activating thermogenesis in response to noradrenaline and cold exposure. It is concluded that LC neurones are part of a neuronal network that is specifically activated by PGE2 to increase thermogenesis and produce fever.
The brain plays a central role in coordinating several responses to systemic inflammation, including fever. It transduces the febrigenic signals from the periphery into appropriate adjustments of thermoeffector activity to ultimately increase body core temperature (Tc). Signals from the periphery may gain access to the brain by three routes: (1) via afferent fibres that travel mostly through the vagus nerve and make their first synapse in the nucleus of the solitary tract (Blatteis et al. 2000; Romanovsky et al. 2000); (2) via circumventricular organs, such as the organum vasculosum laminae terminalis and the subfornical organ, which lack a blood—brain barrier (Takahashi et al. 1997; Blatteis et al. 2000); and (3) via interaction with cells located in the blood—brain interface, i.e. endothelial (Tilders et al. 1994; Cao et al. 1996) and perivascular (Elmquist et al. 1997; Schiltz & Sawchenko, 2003) cells. Activation of these afferent pathways ultimately increases the level of PGE2 in the brain (Blatteis & Sehic, 1997b). By interacting with EP3 receptors (Ushikubi et al. 1998; Oka et al. 2003) and consequently decreasing the intracellular level of cyclic AMP in preoptic neurones (Steiner et al. 2002; Steiner & Branco, 2003), PGE2 triggers an appropriate thermoeffector response to increase Tc. The preoptic region sends both inhibitory and excitatory efferent projections to nuclei located in the hypothalamus, midbrain, and brainstem that are involved in the control of thermoeffector activity (Nagashima et al. 2000).
There is strong evidence supporting an involvement of brain noradrenaline in the genesis of fever. First, systemic administration of exogenous and endogenous pyrogens causes a rapid activation of noradrenergic terminals in the preoptic region (Dunn & Wang, 1995; Linthorst et al. 1995). Second, both in vitro (Malik & Sehic, 1990) and in vivo (Sehic et al. 1996) studies have shown that noradrenaline triggers the release of the terminal mediator of fever, PGE2 (Blatteis & Sehic, 1997a). Third, and most important, depletion of brain catecholamines using the neurotoxin 6-hydroxydopamine abolishes the fever induced by interleukin-1 in rats (Ovadia et al. 1989). However, the noradrenergic neuronal group involved in fever remains unknown.
The locus coeruleus (LC), a well-delineated cluster of noradrenaline-containing neurones located adjacent to the fourth ventricle in the pontine brainstem (Berridge & Waterhouse, 2003), is the major noradrenergic nucleus in the brain. It is estimated that ∼50% of all of the noradrenergic projections in the central nervous system originate in the LC (Aston-Jones et al. 1995; Berridge & Waterhouse, 2003). Hence, it is reasonable to hypothesize that LC neurones play a major role in the development of fever. Because of its strategic location, the LC may convey febrigenic messages to the preoptic region and/or control the activity of thermoeffector neuronal pathways. For example, by receiving inputs from the nucleus of the solitary tract (Van Bockstaele et al. 1999) and projecting to the preoptic region (Aston-Jones et al. 1995), LC neurones may be involved in febrigenic signalling via the vagus nerve. Yet, the LC neurones may be involved in the control of thermoeffector (thermogenesis and/or heat loss) activity because they receive inputs from the preoptic region (Steininger et al. 2001) and project to brain regions involved in the control of thermoeffector activity (Jones & Yang, 1985; Grzanna & Fritschy, 1991; Morrison et al. 1999; Uno & Shibata, 2001). To date, however, the involvement of the LC in the febrile response has not been addressed. In the present study, we addressed the role of the LC in the fever induced by bacterial lipopolysaccharide (LPS) in rats.
Methods
Animals
Experiments were performed on adult male Wistar rats weighing 290–320 g at the time of the experiments. The rats were kept in a room with a temperature of 24–26°C and a 12: 12 h light–dark cycle (lights on at 06.00 h). They had free access to water and food. Animals assigned to experiments involving biotelemetry and brain harvesting for PGE2 determination were handled daily during 5 days and habituated to their experimental setup. Animals designated to an experiment involving measurement of tail skin temperature (Tsk) were handled regularly and habituated (seven daily training sessions, 3–4 h each) to spend time inside cylindrical confiners made of stainless steel wire, which were used later in the experiment. The confiners limited the animals' back and forth movements and prevented them from turning around. As previously described (Romanovsky et al. 1998), well-adapted rats exhibit neither stress fever nor any other sign of stress when they are confined. Each rat was used in only one experiment and subjected to only one treatment. The experiments were started at 09.00 h. The protocols were approved by the Animal Care and Use Committee of the University of Sao Paulo.
Surgery
All surgical procedures were performed under anaesthesia with 2,2,2-tribromoethanol (250 mg kg−1, i.p.) and antibiotic protection (160 000 U kg−1 benzylpenicillin, 33.3 mg kg−1 streptomycin, and 33.3 mg kg−1 di-hydrostreptomycin, i.m.). The rats were subjected to electrolytic lesions of the LC or to selective chemical lesion of LC catecholaminergic neurones; all lesions were bilateral. The lesions were made in rats fixed to a stereotaxic frame; the LC was located at the following stereotaxic coordinates: −3.4 mm from lambda; ± 1.2 mm from midline; −6.8 mm from the skull surface; incisive bar at 0 mm; inclination of vertical stereotaxic bar at 15 deg. For the electrolytic lesions, rats had the uncoated tip of a stainless steel electrode inserted into the LC, and an anodic current (2 mA, 10 s) was passed. Sham-operated rats had the tip of the same electrode inserted 2 mm above the LC, and no current was passed. For the chemical lesions, the tip of a glass micropipette was inserted into the LC, and 6-hydroxydopamine (6-OHDA; Sigma, St Louis, MO, USA) was injected (4 μg in 1 μl of vehicle over 5 min). Sham-operated rats were injected with vehicle (1 μg ascorbic acid in 1 μl of saline over 5 min).
Rats designated to receive intracerebroventricular (i.c.v.) injections were chronically implanted with a stainless steel guide cannula (0.7 mm o.d.), whose tip was located into the third cerebral ventricle (coordinates: −0.4 mm from bregma; 0 mm from midline; −7.8 to −8.5 mm from the skull surface; incisive bar at −3.3 mm; inclination of vertical stereotaxic bar at 0 deg). The displacement of the meniscus in a water manometer ensured correct positioning of the cannula. Stainless steel microscrews were fixed to the bone, and the guide cannula was attached to the screws with the use of acrylic cement. A tight-fitting stylet was kept inside the guide cannula.
Rats designated to have their Tc monitored were implanted intraperitoneally with a biotelemetry transmitter (model: ER-4000, Mini-Mitter Co. Inc., Sunriver, OR, USA) through a medial laparotomy.
Rats designated to receive intravenous (i.v.) injections were chronically implanted with a silicon catheter though the external jugular vein into the right atrium according to Harms & Ojeda (1974). The distal end of the catheter was knotted, tunnelled under the skin, and exteriorized at the nape. The venous catheters were flushed daily with heparinized (50 U ml−1) saline.
Instrumentation
The experiments were performed on day 3 (electrolytic lesion) or day 5 (chemical lesion) post-surgery. On these days, the body weights of the rats that had their LC lesioned electrolytically (288 ± 7 g) or chemically (301 ± 11 g) did not significantly differ from those of the corresponding sham-operated controls (300 ± 4 g and 310 ± 5 g, respectively). In all experiments, except for the experiment involving measurement of Tsk, the rats were freely moving and housed singly. The abdominal temperature (an index of Tc) of freely moving rats was measured by biotelemetry (Mini-Mitter). Rats designated for an experiment involving measurement of oxygen consumption were housed in a sealed chamber. At the time of measurement, the air flow of the chamber was interrupted, and five samples (30 ml each) of the air inside the chamber were taken at 1 min intervals and passed through an oxygen analyser (model OA 272; Saylor Servomex, UK). During the period that the chamber was sealed, the oxygen fraction did not drop to less than 18%. The temporal evolution of the oxygen fraction decay was plotted, and the slope of the resulting curve corresponded to the rate of oxygen consumption.
For the experiment involving measurement of Tsk, previously adapted rats were placed in a confiner and instrumented with two thermoprobes (type YSI 402, Cole-Parmer Instrument Co., CO, USA), one for recording colonic temperature (an index of Tc) and another for recording Tsk (an index of sensible heat loss). The colonic thermocouple was inserted ∼8 cm beyond the anal sphincter and fixed to the base of the tail with tape. The skin thermoprobe was positioned on the lateral surface of the tail at the boundary of its proximal and middle thirds and fixed in place with tape.
Experimental protocols
Five experiments were conducted.
Expt 1
This experiment was performed to evaluate the effect of LC lesions on LPS-induced fever. At a Ta of 23°C, rats that had their LC lesioned electrolytically or chemically, or the corresponding sham-operated controls, were i.v. bolus-injected with two doses (1 or 10 μg kg−1) of LPS (serotype 0111:B4; Sigma) or with pyrogen-free saline. It is known that the lower dose (1 μg kg−1) of LPS evokes a monophasic fever, which is blocked by subdiaphragmatic vagotomy, whereas the higher dose (10 μg kg−1) induces a fever with a greater magnitude and duration, which is not altered by subdiaphragmatic vagotomy (Romanovsky et al. 1997b). Because electrolytic and chemical lesions similarly attenuated LPS fever (see Results), the role of the LC in fever was further investigated in the subsequent experiments by using electrolytic lesions only.
Expt 2
This experiment was aimed at evaluating whether the LC is involved in febrigenic signalling to the brain. The level of PGE2 (the terminal mediator of fever) in its presumed site of action (the preoptic/anteroventral third ventricular region; Scammel et al. 1996; Blatteis & Sehic, 1997b) was used as an index of febrigenic signalling. LC-lesioned (electrolytically) or sham-operated animals kept at 23°C were injected with LPS at a dose that evokes either a vagus-dependent (1 μg kg−1) or -independent (10 μg kg−1) fever, or with saline. The rats were decapitated 2 h postinjection, their brains immediately excised, and the anteroventral third ventricular (AV3V) region, which contains the preoptic region, was dissected on the basis of anatomic landmarks previously described (Knuepfer et al. 1984; Johnson, 1985). The AV3V region was cut at its borders with the vertical limb of the diagonal band of Broca (anterior), the posterior end of the optic chiasm (posterior), the ventral thalamus (dorsal), and the lateral hypothalamus (lateral); the ventral limit of the AV3V region was the optic chiasm. The samples were frozen under liquid nitrogen and stored at −70°C until assayed. They were homogenized on ice in 150 μl of methanol containing indomethacin (1 m) using a digital 600 W microprocessor cell disrupter (Vir Tris, NY, USA). The homogenates were centrifuged at 10 000 g for 10 min at 2°C. The resulting supernatants and pellets were used for PGE2 and protein determination, respectively. The supernatants were purified using C18 minicolumns (Part No. WAT023590; Sep-Pak) according to the manufacturer's instructions, and the samples were dried in a Savant Speed Vac Concentrator System (model SC 210A; GMI, Albertville, MN, USA). The samples were reconstituted in 500 μl of the assay buffer provided in the kit (part no. RPN 222; Amersham, UK), and PGE2 levels were measured using enzyme immunoassay according to the manufacturer's instructions. The pellets were diluted in 1 ml of a sodium hydroxide (0.1 m) solution, and protein content was determined by the Bio-Rad protein assay (code no. 500-0006; Bio-Rad, CA, USA).
Expt 3
This experiment was performed to address the possible involvement of the LC in an efferent neuronal pathway for fever generation. The effect of bilateral electrolytic lesions of the LC on the fever induced by PGE2 was determined. PGE2 acts directly on the preoptic region to activate thermoeffector neuronal pathways (Scammell et al. 1996; Blatteis & Sehic, 1997b). To distinguish a possible involvement of the LC in the control of thermogenesis from an involvement in heat loss, this experiment was performed at a Ta that is below or within the thermoneutral zone of the rats. This strategy was successfully used in a previous study (Steiner et al. 2004) and is based on the fact that the set of thermoeffectors recruited to produce fever strongly depends on Ta. At a subneutral Ta, PGE2 fever is brought about mainly by an increase in metabolic heat production; at a neutral Ta, PGE2 fever is brought about mainly by tail skin vasoconstriction — a heat conservation mechanism (Stitt, 1973; Crawshaw & Stitt, 1975). Using the method of Romanovsky et al. (2002), which is based on the fact that rats have moderate tail skin vasodilatation in a neutral environment, we found that a Ta of 28°C was neutral and a Ta of 23°C was subneutral for the rats in our experimental setup. These Ta values were selected for the experiment.
LC-lesioned or sham-operated rats were injected i.c.v. with PGE2 (25 ng; Sigma) or its vehicle (20% ethanol in saline) at 23°C or 28°C. Tc and Tsk were measured at both Ta levels, and metabolic rate (assessed by the rate of oxygen consumption) was determined at 23°C. A 705-LT 10 μl Hamilton syringe connected to an injection needle (200 μm o.d.) by a PE-10 catheter was used for the injections. At the time of drug administration, the stylet was replaced with the injection needle and 2 μl was infused over a period of 1 min. To avoid reflux, the needle was kept inside the guide cannula for 1 min after the end of the infusion.
Expt 4
This experiment was designed to address whether thermogenic tissues were fully functional in LC-lesioned rats. Because i.v. noradrenaline increases metabolic heat production by acting directly on the brown adipose tissue of rats (Cannon & Nedergaard, 1998), it was used to determine whether LC-lesioned rats are fully capable of producing heat in the presence of appropriate stimulation. LC-lesioned (electrolytically) and sham-operated rats were infused i.v. with noradrenaline (4 μg kg−1 min−1, 16.7 μl kg−1 min−1, over 60 min; Sigma) or saline (16.7 μl kg−1 min−1, over 60 min), and their Tc and rate of oxygen consumption were monitored. The total dose infused (240 μg kg−1) was chosen on the basis of a previous study (Hayashi & Nagasaka, 1983). To prevent noradrenaline-induced skin vasocontriction from affecting Tc, this experiment was performed at a subneutral Ta (23°C), when the vessels of the tail would already be maximally vasoconstricted (Romanovsky et al. 2002).
Expt 5
This experiment was aimed at testing whether lesions of the LC alter the capacity of the rats to sustain their Tc during acute exposure to severe cold. Such a capacity is directly related to the rats' ability to activate metabolic heat production, and has been used by many as an index of cold-activated thermogenesis (Horn et al. 1994; Gordon, 2000). LC-lesioned (electrolytically) and sham-operated rats previously kept at 23°C were transferred to a climatic chamber set to 4°C, and their Tc was monitored.
Assessment of lesion effectiveness and placement
At the end of the experiments, the animals were anaesthetized with 2,2,2-tribromoethanol (250 mg kg−1, i.p.) and perfused transcardially (right atrium cut) with phosphate-buffered saline (PBS, 0.1 m, pH 7.4) followed by 4% paraformaldehyde in PBS. The brains were removed, postfixed with 4% paraformaldehyde for 4 h, and then immersed in a 30% sucrose solution for 48 h. Serial sections (20 μm) of the brainstem were made using a cryostat microtome (Leica, Model CM1850, Germany) and mounted on gelatin-coated glass slides. To verify the correct placement of the electrolytic lesions, the sections were stained by the Nissl method. The electrolytic lesions were visualized as a cluster of necrotic tissue. To verify the correct placement and effectiveness of the chemical lesions, tyrosine hydroxylase (TH) immunoreactivity, a marker of catecholaminergic neurones (Xu et al. 2003), was assessed. To this end, the sections were incubated overnight with a rabbit polyclonal anti-TH antibody (1 : 200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by a 2 h incubation with a biotinylated goat polyclonal anti-rabbit IgG antibody (1 : 400; Vector Laboratories, Burlingame, CA, USA). The biotinylated antibody was complexed with avidin DH—biotinylated horseradish peroxidase (Vector, code PK-4001), and the complex was developed by addition of the peroxidase substrate 3,3′-diaminobenzidine tetrahydrochloride (DAB) according to manufacturer's instructions (Sigma). The sections from LC-lesioned rats were counterstained with Harris' haematoxylin to reveal fibres of passage.
Data processing and analysis
The absolute values of Tc and rate of oxygen consumption were used to evaluate deep body temperature responses and metabolic heat production, respectively. The heat loss index (HLI) was used to evaluate thermoeffector responses of the tail skin vasculature. As justified elsewhere (Romanovsky et al. 2002), the HLI was calculated according to the formula: HLI = (Tsk − Ta)/(Tc − Ta). The theoretical limits of the HLI are 0 (maximal skin vasoconstriction) and 1 (maximal vasodilatation). In practice, however, the upper limit depends on the position of the tail skin thermocouple. When Tsk is measured at the boundary of the proximal and middle thirds of the tail, as in the present study, the HLI rarely exceeds 0.6 (Romanovsky et al. 2002). The Tc, HLI, and oxygen consumption responses were analysed by two-way ANOVA, with time (0–360 min for Expts 1 and 5, and 0–60 min for Expts 3 and 4) as the within-subject and treatment as the between-subject factors. The PGE2 responses (Expt 2) were analysed by one-way ANOVA followed by the Tukey's multiple comparison test. Values were considered significantly different when P < 0.05. All values are reported as means ± s.e.m.
Results
Lesion effectiveness and placement
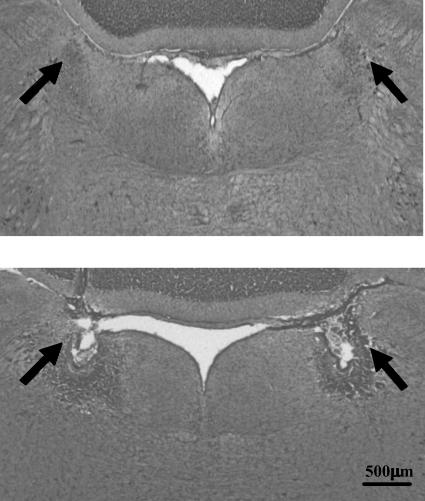
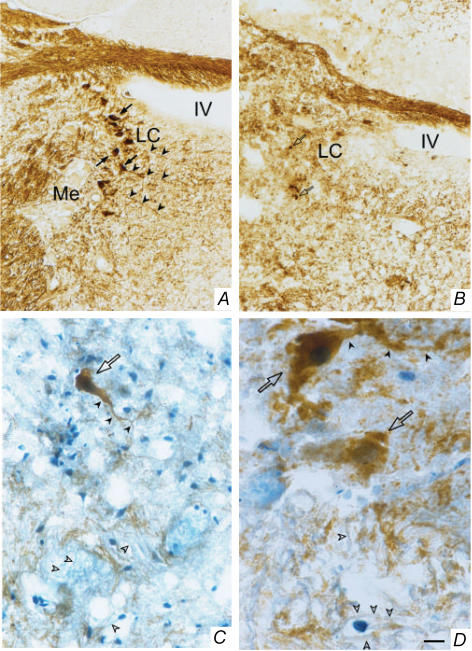
The LC of sham-operated rats was intact and typically appeared as a bilateral compact cluster of intensely stained, catecholaminergic (TH-containing) neurones adjacent to the fourth ventricle in the pontine brainstem (Figs 1 and 2). Successful electrolytic lesions of the LC were visualized as a necrotic core surrounded by a zone of gliosis in the region where the LC is usually found in intact rats (Fig. 1). Successful chemical (6-OHDA) lesions of the LC were revealed mostly by the disappearance of TH-positive perikarya; a few pycnotic perikarya, presumably non-functional, were also seen in some chemically lesioned rats; fibres of passage were intact in 6-OHDA-lesioned rats (Fig. 2). Only rats in which more than 70% of the LC was bilaterally lesioned were included in the study.
Figure 1. Representative photomicrograph of an electrolytic lesion of the locus coeruleus (LC).
The figure shows coronal sections at the pons level illustrating the location of the LC (upper panel) and a typical LC lesion (lower panel). The arrows indicate either the intact LC or the site of lesion.
Figure 2. Representative photomicrographs of a chemical (6-hydroxydopamine) lesion of the LC.
The catecholaminergic cell bodies were identified by tyrosine hydroxylase immunohistochemical staining (dark brown) of the brains of sham-operated (A) or LC-lesioned rats (B–D). The number of neurones (black arrows) and fibres (black arrowheads) positive to tyrosine hydroxylase were dramatically reduced in LC-lesioned rats (B). Note also the presence of a few catecholaminergic neurones with pycnotic, presumably non-functional, perikarya (open arrows) in LC-lesioned rats (B); a detailed (higher magnification) morphology of these neurones is shown in C and D. Tyrosine hydroxylase-negative fibres (blue staining; open arrowheads), i.e. fibres of passage, were found in abundance in the epicentre of the lesion (C and D). LC, locus coeruleus; Me, mesencephalic trigeminal nucleus; IV, fourth ventricle. Scale bars: 48 μm in A and B, 24 μm in C, and 9.6 μm in D.
Expt 1
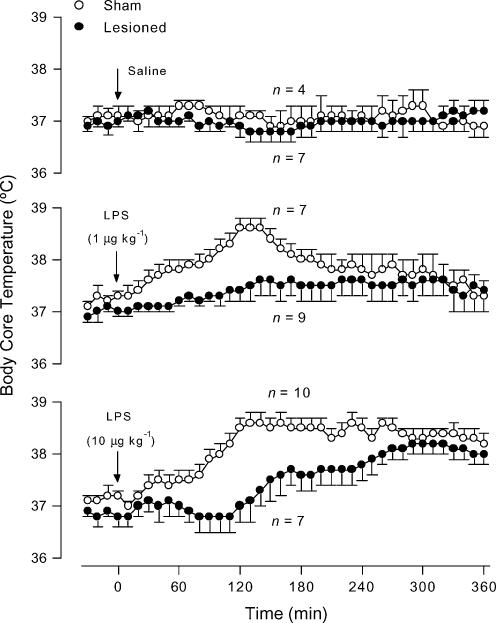
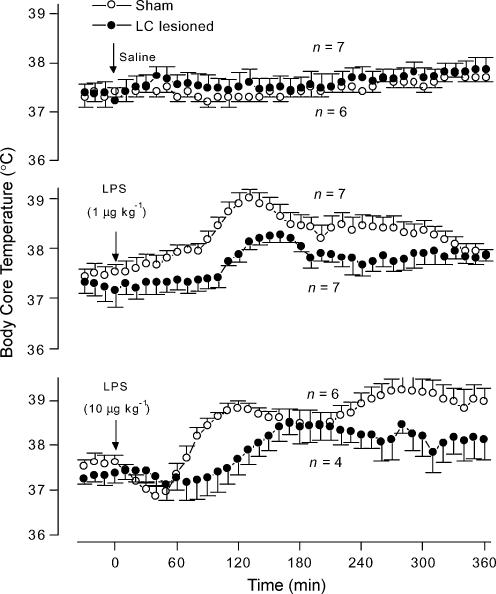
In the sham-operated rats, the low dose (1 μg kg−1, i.v.) of LPS elicited a slowly developing, monophasic fever (F1,36 = 81.15, P < 0.01; compared to saline-treated controls), whereas the moderate dose (10 μg kg−1, i.v.) of LPS caused a long-lasting fever (F1,36 = 188.7, P < 0.01; compared to saline-treated controls) (Figs 3 and 4). In relation to the corresponding sham-operated controls, the rats with electrolytic lesion of the LC responded to the lower dose of LPS with no fever (F1,36 = 49.93, P < 0.01; Fig. 3) and to the moderate dose of LPS with a markedly delayed and attenuated fever (F1,36 = 134.1, P < 0.01; Fig. 3). Selective chemical lesion of catecholaminergic cell bodies of the LC produced similar results. The chemically lesioned rats responded to the lower and higher doses of LPS with significantly (F1,36 = 121.1, P < 0.01 and F1,36 = 61.52, P < 0.01, respectively) attenuated fevers in relation to the responses of the corresponding sham-operated controls (Fig. 4). Saline (i.v.) produced no thermal effect in either sham-operated or LC-lesioned (electrolytically or chemically) rats (Figs 3 and 4).
Figure 3. LPS-induced fever in LC-lesioned and sham-operated rats.
The figure shows body core temperature responses to saline (upper panel) and intravenous LPS doses of 1 μg kg−1 (middle panel) and 10 μg kg−1 (bottom panel) of LC-lesioned and sham-operated rats. The experiment was conducted at an ambient temperature of 23°C. The arrows indicate the times of injection. n, number of animals per group.
Figure 4. LPS-induced fever in chemically LC-lesioned and sham-operated rats.
The figure shows body core temperature responses to saline (upper panel) and intravenous LPS doses of 1 μg kg−1 (middle panel) and 10 μg kg−1 (bottom panel) of chemically LC-lesioned and sham-operated rats. The experiment was conducted at an ambient temperature of 23°C. The arrows indicate the times of injection. n, number of animals per group.
Expt 2
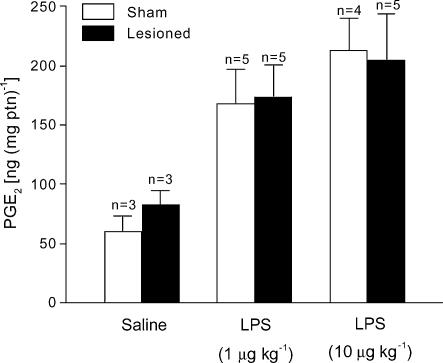
To address whether the impaired LPS-induced fever in LC-lesioned rats resulted from interrupted febrigenic signalling to the brain, we measured the levels of the terminal fever mediator PGE2 in the AV3V region of LC-lesioned (electrolytically) and sham-operated rats challenged with LPS or saline. Immediately before the peak of LPS fever (120 min postinjection), the PGE2 levels in the sham-operated rats treated with LPS were significantly (P < 0.05) higher than the levels of the corresponding saline-treated controls (Fig. 5). The levels of PGE2 in the AV3V of LC-lesioned rats were similarly increased in response to both the lower and higher doses of LPS (P < 0.05; Fig. 5).
Figure 5. Changes in PGE2 levels induced by LPS in LC-lesioned and sham-operated rats.
The levels of PGE2 were measured in the anteroventral third ventricular (AV3V) region of LC-lesioned and sham-operated rats 120 min after challenge with intravenous LPS (1 or 10 μg kg−1) or saline. The experiment was conducted at 23°C. n, number of animals per group.
Expt 3
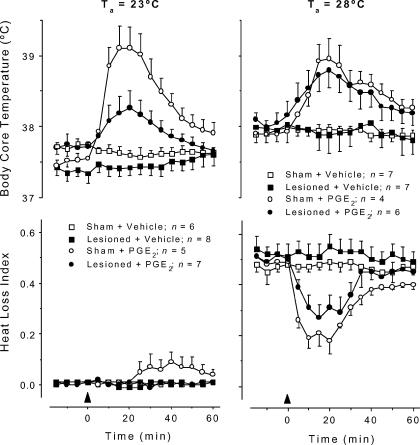
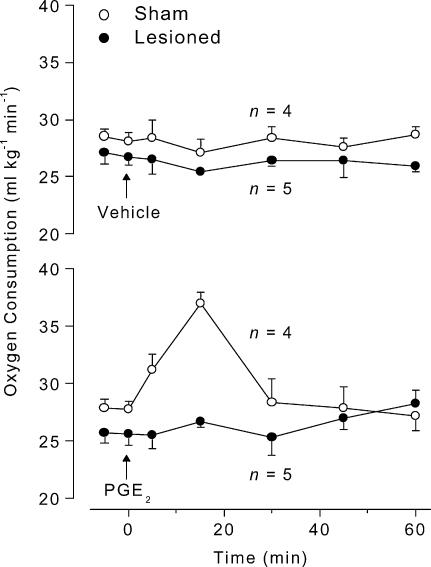
To determine whether lesion of the LC attenuates fever by impairing the normal operation of a thermoeffector neuronal pathway, the fever induced by PGE2 (which activates thermoeffector febrigenic pathways by acting directly on the preoptic/AV3V region) was studied in LC-lesioned (electrolytically) and sham-operated rats. This experiment was performed at Ta of 23°C and 28°C, which are subneutral and neutral values, respectively. At both Ta levels, PGE2 (25 ng, i.c.v.) injection of sham-operated rats evoked a typical fever (Fig. 6); this response was significantly different from that of vehicle-treated rats (at 23°C: F1,12 = 124.7, P < 0.01; at 28°C: F1,12 = 119.5, P < 0.01). At 23°C, the HLI was nearly zero (maximal tail skin vasoconstriction) and did not change in the course of PGE2 fever (Fig. 6). At this Ta, PGE2-induced fever was associated with an activation of metabolic heat production, as evident from the increased rate of oxygen consumption (Fig. 7); the oxygen consumption response of PGE2-treated rats significantly differed from that of vehicle-treated rats (F1,5 = 7.538, P < 0.01). In relation to the sham-operated controls, the LC-lesioned rats responded to PGE2 with an attenuated fever (F1,12 = 32.35, P < 0.01; Fig. 6). The attenuated fever was associated with almost no increase — although a tendency to an increase was noticed — in the metabolic rate (Fig. 7); the HLI remained unaffected (Fig. 6). At 28°C, the HLI was ∼0.5 (tail skin vasodilatation) before any injection, and a pronounced decrease in HLI (vasoconstriction) preceded the PGE2-induced Tc rise (F1,12 = 96.58, P < 0.01, compared to the sham-operated rats treated with vehicle) (Fig. 6). Under this condition, both the febrile increase in Tc and the associated decrease in HLI in response to PGE2 were unaffected in LC-lesioned rats (Fig. 6). In no case did i.c.v. administration of vehicle affect Tc, HLI, or metabolic rate (Figs 6 and 7).
Figure 6. PGE2-induced fever in LC-lesioned and sham-operated rats.
The figure shows body core temperature and heat loss index responses to PGE2 (25 ng, i.c.v.) or its vehicle (20% ethanol in saline) of LC-lesioned and sham-operated rats. The experiment was conducted at an ambient temperature (Ta) of either 23°C (left panels) or 28°C (right panels). The arrowheads indicate the times of injection. n, number of animals per group.
Figure 7. PGE2-induced changes in oxygen consumption in LC-lesioned and sham-operated rats.
The figure shows oxygen consumption responses to PGE2 (25 ng, i.c.v.) or its vehicle (20% ethanol in saline) of LC-lesioned and sham-operated rats. The experiment was conducted at an ambient temperature (Ta) of 23°C. The arrows indicate the times of injection. n, number of animals per group.
Expt 4
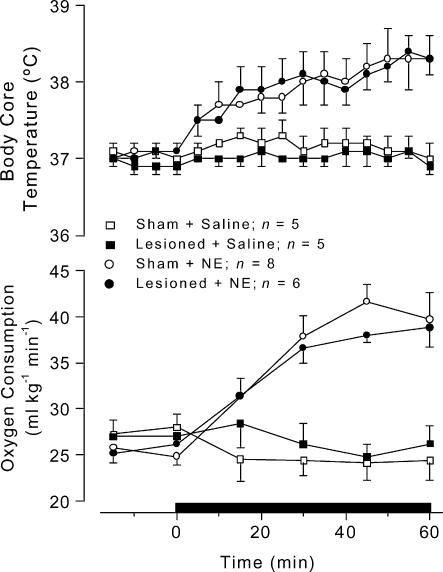
To determine whether thermogenic organs, especially the brown adipose tissue, were fully functional in LC-lesioned (electrolytically) rats, the effects of i.v. noradrenaline on Tc and metabolic rate of sham-operated rats were compared to those of the LC-lesioned rats at a Ta value of 23°C. Infusion of noradrenaline (240 μg kg−1 over 60 min) produced comparable increases in Tc and metabolic rate (assessed by the rate of oxygen consumption) of both LC-lesioned and sham-operated rats (Fig. 8). The Tc responses to noradrenaline of the LC-lesioned and sham-operated rats were significantly (F1,12 = 85.71, P < 0.01, and F1,12 = 47.32, P < 0.01, respectively) different from the responses to saline. The oxygen consumption responses of the same rats to noradrenaline were also significantly (F1,4 = 40.53, P < 0.01, LC-lesioned rats; F1,4 = 51.46, P < 0.01, sham-lesioned rats) different from the responses to saline. Infusion of saline produced no change in the parameters studied (Fig. 8).
Figure 8. Intravenous noradrenaline-induced changes in body core temperature and oxygen consumption of LC-lesioned and sham-operated rats.
Noradrenaline was intravenously infused at a total dose of 240 μg kg−1 over 60 min. Control rats received intravenous saline at the same rate. The horizontal bar indicates the time of infusion. n, number of animals per group.
Expt 5
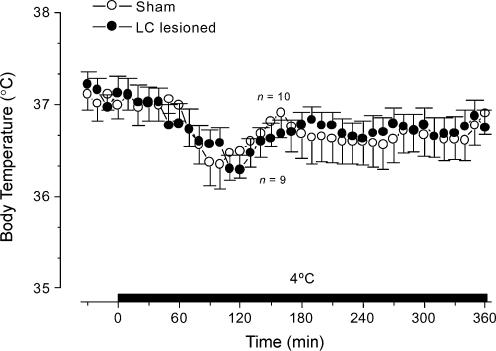
To determine whether lesion of the LC disrupts the rats' ability to activate thermogenesis and sustain Tc in response to severe cold exposure, LC-lesioned (electrolytically) and sham-operated rats were acutely exposed to 4°C, and their Tc was monitored. Both LC-lesioned and sham-operated rats were fully competent to sustain their Tc at 4°C (Fig. 9). Cold exposure resulted in an initial tendency for a decrease in Tc (Fig. 9). The rats rapidly recovered from this initial effect within less than 150 min and were able to sustain their Tc at baseline level until the end of the 360 min exposure (Fig. 9).
Figure 9. Changes in body core temperature of LC-lesioned and sham-operated rats exposed to a 4°C environment.
The figure shows Tc responses to cold exposure (4°C) of LC-lesioned and sham-operated rats. The horizontal bar indicates the time of cold exposure. n, number of animals per group.
Discussion
In the present study, we have used both electrolytic ablation of the LC and selective chemical lesion of LC catecholaminergic neurones to verify the possible involvement of this nucleus in the fever induced by LPS. Our data show that lesions of the LC resulted in an attenuation of LPS-induced fever at a subneutral Ta, even when catecholaminergic neurones, but not fibres of passage, were selectively lesioned by microinjection of 6-OHDA. This finding strongly supports a role of catecholaminergic LC neurones in the genesis of fever. Next, we investigated such a role in more detail. To this end, we used the practical and reproducible electrolytic lesioning approach, since both electrolytic and chemical lesions produced very similar — if not identical — effects on LPS fever.
Neuroanatomical evidence suggests that febrigenic signals from vagal afferents arriving in the nucleus of the tractus solitarius (NTS) may reach the preoptic region through a relay in the LC (Aston Jones et al. 1995; Van Bockstaele et al. 1999). Hence, it is possible that the LC is involved in febrigenic signalling via the vagus nerve. This possibility is, however, disproved in the present study by the following observations. First, lesions of the LC attenuated not only the monophasic (vagus-dependent; Romanovsky et al. 1997b) fever induced by a low dose of LPS, but also the long-lasting (vagus-independent; Romanovsky et al. 1997b) fever induced by a moderate dose of LPS. Second, lesion of the LC did not affect the increased production of PGE2 (the terminal mediator of fever) in the AV3V region during the course of LPS-induced fever, irrespectively of the LPS dose.
Another possibility is that the LC is part of a thermoeffector neuronal pathway activated by pyrogens. To address this possibility, we verified whether PGE2 (which acts directly on the preoptic region to activate febrigenic thermoeffector pathways; Blatteis & Sehic, 1997b) was able to produce fever in LC-lesioned rats. Remarkably, we found that PGE2 fever is substantially attenuated in LC-lesioned rats at a subneutral but not at a neutral Ta. In agreement with previous studies (Stitt, 1973; Crawshaw & Stitt, 1975), we observed that PGE2 fever was brought about by enhanced metabolic heat production (increase in oxygen consumption) at a subneutral Ta and by skin vasoconstriction (decrease in HLI) at a neutral Ta. At a subneutral Ta, the attenuated fevers of LC-lesioned rats were associated with a strong blockade of the PGE2-induced activation of metabolic heat production. At thermoneutrality, on the other hand, LC-lesioned rats showed marked tail skin vasoconstriction and were able to mount a normal febrile response to PGE2.
These results suggest the involvement of the LC in a thermogenic neuronal pathway, but an alternative explanation is also possible. The impaired capacity of LC-lesioned rats to develop increased thermogenesis and fever in response to LPS and PGE2 may be due to an atrophy of thermogenic tissues. Indeed, an impaired thermogenesis induced by malnutrition has been quoted as an important ‘side-effect’ of lesion (Shido et al. 1989) and denervation (Romanovsky et al. 1997a) experiments. To assess the thermogenic capacity of the rats, we verified the effects of i.v. noradrenaline on the metabolic rate (assessed by the rate of oxygen consumption) and Tc. This method is based on the fact that noradrenaline directly activates thermogenesis in the brown adipose tissue, the main thermogenic organ in rats (Cannon & Nedergaard, 1998). It was successfully employed in previous studies (Hayashi & Nagasaka, 1983; Gordon, 2000). That LC-lesioned rats responded to noradrenaline with increases in metabolic rate and Tc identical to those of sham-operated rats indicates that the thermogenic reserves of LC-lesioned rats were intact. Consequently, impaired thermogenic and febrile responses of LC-lesioned rats to LPS and PGE2 in a subthermoneutral environment can be ascribed to the interruption of a specific fever-inducing thermogenic neuronal pathway rather than atrophy of thermogenic tissues. Consistent with such a role of the LC are the studies showing that the number of neurones expressing c-fos (a marker of neuronal activation) increase in the LC after LPS administration (Hare et al. 1995; Xu et al. 2003). Moreover, neurochemical and electrical activation of LC neurones occur when febrigenic substances, i.e. LPS (Molina-Holgado & Guaza, 1996) and corticotropin-releasing factor (Valentino et al. 1983; Emoto et al. 1993), are administered at regular laboratory temperatures, which are subneutral for rats.
Although lesion of the LC markedly attenuated LPS- and PGE2-induced thermogenesis and fever, it had no effect on cold-induced thermogenesis. In agreement, studies by Cano et al. (2003) and Bachtell et al. (2003) revealed no activation of the LC (assessed by c-fos expression) during exposure to cold. Only one study (Kiyohara et al. 1995) showed cold-induced c-fos expression in the LC, but this effect may be unrelated to the activation of thermogenesis; it may be related to the activation of the stress response to a new environment. Indeed, a recent study showed that cold-induced c-fos expression in LC neurones disappears in rats systematically adapted to transfer to a cold environment, despite the fact that adapted rats still respond to cold with enhanced thermogenesis (Bachtell et al. 2003). It thus seems that catecholaminergic LC neurones are specifically involved in the activation of thermogenesis by pyrogens.
Further investigation is needed to understand the specific functional connections of the LC with the neuronal circuitry involved in the control of thermogenesis, but speculative scenarios can be proposed. Based on the results of their recent study using pseudorabies virus (a retrograde transynaptic tracer), Cano et al. (2003) have proposed that the LC is multisynaptically connected to the brown adipose tissue. This finding agrees with previous studies showing that there is no direct projection from the LC to the intermediolateral cell column of the spinal cord (Grzanna & Fritschy, 1991; Proudfit & Clark, 1991), where preganglionic sympathetic neurones involved in brown adipose tissue activation are located (Zhang et al. 2000; Cano et al. 2003). It also agrees with the facts that LC neurones receive projections from the preoptic region, either directly (Steininger et al. 2001) or via relays in the paraventricular hypothalamus (Aston-Jones et al. 1991), and project to several brainstem structures involved in the control of brown adipose tissue thermogenesis, e.g. the inferior olive (Grzanna & Fritschy, 1991; Uno & Shibata, 2001) and the raphe pallidus (Jones & Yang, 1985; Morrison et al. 1999).
In summary, the present study shows that catecholaminergic LC neurones are part of a thermoeffector neuronal pathway that is specifically activated by pyrogens (e.g. PGE2) to activate thermogenesis and produce fever in a subthermoneutral environment.
Acknowledgments
We thank Mauro F. Silva, Daniela Lima de Oliveira, Daoud Hidbrahim Elias Filho, Gustavo Michel Batista de Souza and Lidiane de Cássia Anastácio for excellent technical assistance. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Daoud Hidbrain Elias Filho and Lidiane de Cássia Anastácia are the recipients of FAPESP technician scholarships. M. C. Almeida is a recipient of a CNPq graduate scholarship.
References
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, Charléty P, Valentino RJ, Williams JT. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Grzanna R. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The Rat Nervous System. Sydney: Academic Press, Inc.; 1995. pp. 183–213. [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin E. Identification of temperature-sensitive neural circuits in mice using c-fos expression mapping. Brain Res. 2003;960:157–164. doi: 10.1016/s0006-8993(02)03807-6. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus—noradrenergic system: modulation of behavioral state and state-dependent cognitive processess. Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Sehic E. Fever: how may circulating pyrogens signal the brain? News Physiol Sci. 1997a;12:1–9. [Google Scholar]
- Blatteis CM, Sehic E. Prostaglandin E2: a putative fever mediator. In: Mackowiak PA, editor. Fever: Basic Mechanisms and Management. Philadelphia, USA: Lippincott-Raven Publishers; 1997b. pp. 117–145. [Google Scholar]
- Blatteis CM, Sehic E, Li S. Pyrogen sensing and signaling. Old views and new concepts. Clin Infect Dis. 2000;31:168–177. doi: 10.1086/317522. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Nonshivering thermogenesis and brown adipose tissue. In: Blatteis CM, editor. Physiology and Pathophysiology of Temperature Regulation. New Jersey: World Scientific Publishing Co, Pty Ltd; 1998. pp. 63–77. [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Cao C, Matsumura K, Yamagata K, Watanabe Y. Endothelial cells of the rat brain vasculature express cyclooxygenase-2 mRNA in response to systemic interleukin-1 beta: a possible site of prostaglandin synthesis responsible for fever. Brain Res. 1996;733:263–272. doi: 10.1016/0006-8993(96)00575-6. [DOI] [PubMed] [Google Scholar]
- Crawshaw LI, Stitt JT. Behavioural and autonomic induction of prostaglandin E-1 fever in squirrel monkeys. J Physiol. 1975;244:197–206. doi: 10.1113/jphysiol.1975.sp010791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Wang J. Cytokine effects on CNS biogenic amines. Neuroimmunomodulation. 1995;2:319–328. doi: 10.1159/000097211. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Breder CD, Sherin JE, Scammell TE, Hickey WF, Dewitt D, Saper CB. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J Comp Neurol. 1997;381:119–129. doi: 10.1002/(sici)1096-9861(19970505)381:2<119::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Emoto H, Tanaka M, Koga C, Yokoo H, Tsuda A, Yoshida M. Corticotropin-releasing factor activates the noradrenergic neuron system in the rat brain. Pharmacol Biochem Behav. 1993;45:419–422. doi: 10.1016/0091-3057(93)90259-v. [DOI] [PubMed] [Google Scholar]
- Gordon FJ. Effect of nucleus tractus solitarius lesions on fever produced by interleukin-1beta. Auton Neurosci. 2000;85:102–110. doi: 10.1016/s1566-0702(00)00228-9. [DOI] [PubMed] [Google Scholar]
- Grzanna R, Fritschy JM. Efferent projections of different subpopulations of central noradrenaline neurons. Prog Brain Res. 1991;88:89–101. doi: 10.1016/s0079-6123(08)63801-7. [DOI] [PubMed] [Google Scholar]
- Hare AS, Clarke G, Tolchard S. Bacterial lipopolysaccharide-induced changes in Fos protein expression in the rat brain: correlation with thermoregulatory changes and plasma corticosterone. Neuroendocrinol. 1995;7:791–799. doi: 10.1111/j.1365-2826.1995.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Harms PG, Ojeda SR. A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol. 1974;36:391–392. doi: 10.1152/jappl.1974.36.3.391. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nagasaka T. Suppression of norepinephrine-induced thermogenesis in brown adipose tissue by fasting. Am J Physiol. 1983;245:E582–E586. doi: 10.1152/ajpendo.1983.245.6.E582. [DOI] [PubMed] [Google Scholar]
- Horn T, Wilkinson MF, Landgraf R, Pittman QJ. Reduced febrile responses to pyrogens after lesions of the hypothalamic paraventricular nucleus. Am J Physiol. 1994;267:R323–R328. doi: 10.1152/ajpregu.1994.267.1.R323. [DOI] [PubMed] [Google Scholar]
- Johnson AK. The periventricular anteroventral third ventricle (AV3V): its relationship with the subfornical organ and neural systems involved in maintaining body fluid homeostasis. Brain Res Bull. 1985;15:595–601. doi: 10.1016/0361-9230(85)90209-6. [DOI] [PubMed] [Google Scholar]
- Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Kiyohara T, Miyata S, Nakamura T, Shido O, Nakashima T, Shibata M. Differences in Fos expression in rat brain between cold and warm ambient exposures. Brain Res Bull. 1995;38:193–201. doi: 10.1016/0361-9230(95)00093-t. [DOI] [PubMed] [Google Scholar]
- Knuepfer MM, Johnson AK, Brody MJ. Identification of brainstem projections mediating hemodynamic responses to stimulation of the anteroventral third ventricle (AV3V) region. Brain Res. 1984;294:305–314. doi: 10.1016/0006-8993(84)91042-4. [DOI] [PubMed] [Google Scholar]
- Linthorst ACE, Flachskamm C, Holsboer F, Reul JMHM. Intraperitoneal administration of bacterial endotoxin enhances noradrenergic neurotransmission in the rat preoptic area: Relationship with body temperature and hypothalamic-pituitary-adrenocortical activity. Eur J Neurosci. 1995;7:2418–2430. doi: 10.1111/j.1460-9568.1995.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Malik KU, Sehic E. Prostaglandins and the release of adrenergic transmitter. Ann N Y Acad Sci. 1990;604:222–236. doi: 10.1111/j.1749-6632.1990.tb31996.x. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F, Guaza C. Endotoxin administration induced differential neurochemical activation of the rat brain stem nuclei. Brain Res Bull. 1996;40:151–156. doi: 10.1016/0361-9230(96)00043-3. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol. 1999;276:R290–R297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci. 2000;85:18–25. doi: 10.1016/S1566-0702(00)00216-2. [DOI] [PubMed] [Google Scholar]
- Oka T, Oka K, Kobayashi T, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S, Saper SB. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J Physiol. 2003;551:945–954. doi: 10.1113/jphysiol.2003.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadia H, Abramsky O, Weidenfeld J. Evidence for the involvement of the central adrenergic system in the febrile response induced by interleukin-1 in rats. J Neuroimmunol. 1989;25:109–116. doi: 10.1016/0165-5728(89)90128-8. [DOI] [PubMed] [Google Scholar]
- Proudfit HK, Clark FM. The projection of the locus coeruleus to the spinal cord. Prog Brain Res. 1991;538:231–245. doi: 10.1016/0006-8993(91)90435-x. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Szekely M. Neural route of pyrogen signaling to the brain. Clin Infect Dis. 2000;31:S162–S167. doi: 10.1086/317515. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Kulchitsky VA, Simons CT, Sugimoto N, Szekely M. Febrile responsiveness of vagotomized rats is suppressed even in the absence of malnutrition. Am J Physiol. 1997a;273:R777–R783. doi: 10.1152/ajpregu.1997.273.2.R777. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Simons CT, Kulchitsky VA. ‘Biphasic’ fevers often consist of more than two phases. Am J Physiol. 1998;275:R323–R331. doi: 10.1152/ajpregu.1998.275.1.R323. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Simons CT, Szekely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am J Physiol. 1997b;273:R407–R413. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Sugimoto N, Simons CT, Hunter WS. The organum vasculosum laminae terminalis in immune-to-brain febrigenic signaling: a reappraisal of lesion experiments. Am J Physiol Regul Integr Comp Physiol. 2003;285:R420–R428. doi: 10.1152/ajpregu.00757.2002. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Elmquist JK, Griffin JD, Saper CB. Ventromedial preoptic prostaglandin E2 activates fever-producing autonomic pathways. J Neurosci. 1996;16:6246–6254. doi: 10.1523/JNEUROSCI.16-19-06246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE. Signaling the brain in systemic inflammation: the role of perivascular cells. Frontiers Biosci. 2003;8:s1321–s1329. doi: 10.2741/1211. [DOI] [PubMed] [Google Scholar]
- Sehic E, Ungar AL, Blatteis CM. Interaction between norepinephrine and prostaglandin E2 in the preoptic area of guinea pigs. Am J Physiol. 1996;271:R528–R536. doi: 10.1152/ajpregu.1996.271.3.R528. [DOI] [PubMed] [Google Scholar]
- Shido O, Nagasaka T, Watanabe T. Blunted febrile response to intravenous endotoxin in starved rats. J Appl Physiol. 1989;67:963–969. doi: 10.1152/jappl.1989.67.3.963. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Antunes-Rodrigues J, Branco LGS. Role of preoptic second messenger systems (cAMP and cGMP) in the febrile response. Brain Res. 2002;944:135–145. doi: 10.1016/s0006-8993(02)02738-5. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Branco LGS. Fever and anapyrexia in systemic inflammation: intracellular signaling by cyclic nucleotides. Front Biosci. 2003;8:s1398–s1408. doi: 10.2741/1188. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Rudaya AY, Ivanov AI, Romanovsky AA. Febrigenic signaling to the brain does not involve nitric oxide. Br J Pharmacol. 2004;141:1204–1213. doi: 10.1038/sj.bjp.0705713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger TL, Gong H, McGinty D, Szymusiak R. Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. J Comp Neurol. 2001;429:638–653. [PubMed] [Google Scholar]
- Stitt JT. Prosaglandin E1 fever induced in rabbits. J Physiol. 1973;232:163–179. doi: 10.1113/jphysiol.1973.sp010262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Smith P, Ferguson A, Pittman QJ. Circumventricular organs and fever. Am J Physiol. 1997;273:R1690–R1695. doi: 10.1152/ajpregu.1997.273.5.R1690. [DOI] [PubMed] [Google Scholar]
- Tilders FJ, DeRijk RH, Van Dam AM, Vincent VA, Schotanus K, Persoons JH. Activation of the hypothalamus-pituitary-adrenal axis by bacterial endotoxins: routes and intermediate signals. Psychoneuroendocrinology. 1994;19:209–232. doi: 10.1016/0306-4530(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Uno T, Shibata M. Role of inferior olive and thoracic IML neurons in nonshivering thermogenesis in rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R536–R546. doi: 10.1152/ajpregu.2001.280.2.R536. [DOI] [PubMed] [Google Scholar]
- Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tuboi K, Katsuyama M, Ichikawa A, Tanaka T, Yoshida N, Narumiya S. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270:363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Telegan P. Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J Comp Neurol. 1999;412:410–428. doi: 10.1002/(sici)1096-9861(19990927)412:3<410::aid-cne3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Xu S, Guo S, Jiang X, Yin Q, Umezawa T, Hisamitsu T. Effect of indomethacin on the c-fos expression in AVP and TH neurons in rat brain induced by lipopolysaccharide. Brain Res. 2003;966:13–18. doi: 10.1016/s0006-8993(02)04000-3. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Lu J, Elmquist JK, Saper CB. Lipopolysaccharide activates specific populations of hypothalamic and brainstem neurons that project to the spinal cord. J Neurosci. 2000;20:6578–6586. doi: 10.1523/JNEUROSCI.20-17-06578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]