Abstract
In the present study, we investigated the effects of the exogenous application of tetrahydrobiopterin on the endothelium-dependent vasorelaxation and superoxide anion generation in the mesenteric microvessels of intrauterine undernourished rats. In addition, we investigated the presence of peroxynitrite in these rats by evaluation of nitrotyrosine-containing proteins, a stable end-product of peroxynitrite oxidation. For this, female pregnant Wistar rats were fed either normal or 50% of the normal intake diets during the whole gestational period. Male offspring (16 weeks of age) were studied to assess microvascular reactivity, superoxide production using a hydroethidine staining assay, nitric oxide synthase (NOS) activity and nitric oxide (NO) production. Western blot analysis was used to quantify nitrotyrosine-containing proteins and relative multiplex RT-PCR analysis for endothelial NOS (eNOS) mRNA expression. Superfusion with tetrahydrobiopterin significantly decreased superoxide generation and improved vascular function. Intrauterine malnutrition induced a decrement of NOS activity and NO production without affecting the gene expression of eNOS. However, incubation with tetrahydrobiopterin significantly improved NO production after stimulation with acetylcholine or bradykinin in intrauterine undernourished rats. The fact that the nitrotyrosine-containing proteins were increased could, at first sight, suggest that the peroxynitrite is the mediator responsible for the excessive oxidation and depletion of tetrahydrobiopterin. Our study shows that exogenous application of tetrahydrobiopterin leads to a significant improvement of endothelium-dependent vasodilatation, enhanced NO production and decreased superoxide generation in microvessels of intrauterine undernourished rats. Since we found a decrease in NOS activity without an alteration in the gene expression of eNOS, we suggest that impaired NOS-dependent responses of mesenteric arterioles are related to the impairment of tetrahydrobiopterin pathways.
It is well known that intrauterine malnutrition contributes to the development of hypertension in adulthood (Barker et al. 1993; Krishnaswamy et al. 2002; Yliharsila et al. 2003). The association between malnutrition during fetal life and subsequent hypertension is associated with alterations in endothelium-dependent vasodilatation. In fact, recent human studies have associated the intrauterine grown restriction with endothelium dysfunction in children (Leeson et al. 1997; Martin et al. 2000) and young adult (Goodfellow et al. 1998; Leeson et al. 2001), suggesting that the endothelium-derived nitric oxide (NO) system is impaired after chronic exposure to fetal malnutrition.
The mechanisms by which intrauterine malnutrition exerts its effects on the endothelium are not completely elucidated. We recently demonstrated that endothelial dysfunction in intrauterine undernourished rats is partially due to an increased oxidative stress, characterized by decreased superoxide dismutase (SOD) activity (Franco et al. 2002a) as well as by an excess generation of superoxide anion by a NADPH-dependent mechanism via activation of angiotensin II (Franco et al. 2003). On the other hand, reduced NO synthesis might also contribute to the endothelium dysfunction observed in intrauterine undernourished rats. Alves et al. (2002) reported that rats submitted to intrauterine malnutrition have diminished urinary excretion of NO end-products. In addition, we previously found that intrauterine malnutrition induced a decrement of NOS activity in the isolated aortic rings from adult offspring, and this may be due to the reduction in eNOS gene expression (Franco et al. 2002b). However, the possible role of other factors in the decreased NO production is undefined.
Tetrahydrobiopterin, a critical cofactor for eNOS, may be deficient in various conditions associated with altered endothelial function. In fact, treatment with tetrahydrobiopterin has been shown to augment endothelium-dependent vasodilatation in patients with hypercholesterolaemia and atherosclerosis, and in smokers (Stroes et al. 1997; Maier et al. 2000; Ueda et al. 2000; Heitzer et al. 2000a). In addition, biochemical studies demonstrated that in the presence of suboptimal concentrations of tetrahydrobiopterin, activation of eNOS leads to ‘uncoupling of eNOS’ with subsequent increased formation of superoxide anions (Vasquez-Vivar et al. 1998; Wever et al. 1998). Thus the optimal concentration of this cofactor is of fundamental importance for normal function of eNOS and vascular endothelial cells.
In the present study, we investigated the effects of the exogenous application of tetrahydrobiopterin on the endothelium-dependent vasorelaxation and superoxide anion generation in rats submitted to intrauterine malnutrition. In addition, we investigated the presence of peroxynitrite in intrauterine undernourished rats by evaluation of nitrotyrosine-containing proteins, a stable end-product of peroxynitrite oxidation.
Methods
All procedures used in this study were approved and performed in accordance with guidelines of the Ethics Committee of the Institute of Biomedical Sciences, University of São Paulo, and conformed with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). Wistar rats from our colony (Laboratory of Hypertension, Institute of Biomedical Sciences, University of São Paulo) were maintained in a room at 22 ± 1°C with a 12 h light cycle and 60% humidity.
Dietary protocol
Timed mating was performed in female Wistar rats (age range 9–11 weeks). A total of 18 adult virgin female Wistar rats were used to generate the 90 males used in the study. Day 1 of pregnancy was determined by the presence of spermatozoa in the vaginal smear. Following confirmation that mating had occurred, the females were housed individually in standard rat cages and randomly divided into two groups: control (n = 8), fed standard chow ad libitum with an approximate composition of (g kg−1): 220 protein, 435 carbohydrates, 40 fat, 80 cellulose, 100 minerals, 125 water, plus salt and vitamin mixtures; and restricted group (n = 10), fed 50% of the ad libitum intake, determined by the amount of food consumed by the control group from day 1 of pregnancy until parturition. Following parturition, both groups of offspring received food ad libitum. In order to prevent any variation in neonatal growth through availability of milk intake during suckling, litter size was standardized to 8 pups at day 1. The litter size of each dam was noted and each neonate was individually weighed. At 16 weeks of age males from both control and restricted groups were used in a randomized manner for the studies detailed below.
Characterization of adult offspring
At 16 weeks of age, the rats from both groups were placed in a metabolic cage during 24 h to evaluate the food and water consumption and the urine volume. Glycosuria was qualitatively assessed in urine with the aid of reagent strips. The systolic blood pressure was determined in conscious rats from both groups by an indirect tail-cuff method according to Franco et al. (2002b) (pneumatic transducer, PowerLab 4/S, ADInstruments Pty Ltd). In another series of experiments, rats from control (n = 7) and restricted (n = 7) groups were submitted to an oral glucose tolerance test (OGTT). After 13 h of food deprivation, glucose (200 g l−1) was administered at a dose of 2 g kg−1 by gastric gavage. The samples were obtained from the cut tip of the tail 0, 5, 30 and 60 min later and blood glucose concentrations were determined with a glucose monitor. The glucose responses during the OGTT were calculated by estimation of the total area under the glucose curve (ΔG) using Prism GraphPad 4.04 software.
Vascular reactivity ‘in vivo’ in mesenteric microvessels
Rats were anaesthetized with chloral hydrate (450 mg kg−1, s.c.), and the mesentery was exteriorized and arranged for microscopic observation in situ according to Zweifach (1948) with slight modifications (Fortes et al. 1984). Changes in vessel diameter were estimated after superfusion (1.0 ml min−1) with a buffer solution containing tetrahydrobiopterin (10−7m) for 2 min before topical application of acetylcholine (ACh, 2 × 10−3m) or bradykinin (BK, 3 × 10−6m) in a standard volume of 0.01 ml and were removed by washing with warmed Ringer-Locke solution. The dose and the time delay necessary for the effect of tetrahydrobiopterin were chosen in preliminary experiments. For each animal, at least three different microscopic fields were observed and the arteriole diameter measured. The rats were killed by an overdose of anaesthetic.
Hydroethidine staining assay for superoxide formation
Superoxide anion was estimated by imaging microfluorometry of the oxidation of hydroethidine into bromide ethidium as previously described (Suzuki et al. 1995; Dantas et al. 2002). Rats were anaesthetized with chloral hydrate (450 mg kg−1, s.c.), and the mesentery was set for microscopic observation in situ (Fortes et al. 1984). After an initial 30 min stabilization period, the mesentery preparation was then superfused with a buffer solution containing hydroethidine (HE, 10.0 μmol l−1; Polysciences) for 60 min. The number of nuclei labelled with ethidium bromide (EB-positive nuclei) along the arteriolar wall (NEB) (extension corresponding to 200 μm) was determined 60 min after the onset of HE superfusion. At the end of the experiments, the preparation was superfused with absolute ethanol for 5 min followed by a superfusion with 10% EB solution (v/v) to establish the total number of nuclei along the arteriolar wall under observation (NT). The EB-positive number was counted (double blind) and expressed as a percentage of EB-positive nuclei = (NEB/NT) × 100. To evaluate the role of tetrahydrobiopterin in superoxide generation, the mesenteries from control and restricted groups were superfused with a buffer solution containing hydroethidine plus tetrahydrobiopterin (10−7m) during a 30 min stabilization period and maintained throughout the experiments. This dose was chosen in preliminary experiments. The rats were killed by an overdose of anaesthetic.
Determination of NOS activity
NOS activity was measured by the biochemical conversion of l-[3H]arginine to l-[3H]citrulline according to the method described by Rees et al. (1996). Briefly, arterioles from the mesenteric arteriolar bed were dissected and immediately homogenized in ice-cold buffer containing (mm): 20 Hepes, 0.32 sucrose, 1.0 DTT, 0.1 EDTA, 1.0 pepstatin, 1.0 phenylmethylsulfonyl fluo-ride (PMFS) in the presence of 10 g ml−1 leupeptin. The incubation (37°C for 60 min) was performed in buffer containing: 4 μm FAD/FMN, 10 μg ml−1 calmodulin, 1.25 mm Ca2+; 1 mm NADPH, 120 nml-arginine, 50 nm[3H]arginine (specific activity: 45.2 Ci mmol−1; NEN Life Science Products, Inc., Boston, MA, USA) in the presence of 10 μm BH4. Cation-exchange resin (Dowex 50WX8-400 equilibrated with 50 mm Hepes, pH 5.5) was added to the reaction mixture to remove the excess l-[3H]arginine. The supernatants were collected in vials with scintillation liquid and the radioactivity was quantified.
Measurement of NO production in the transverse arteriolar sections
NO was measured by the use of 4,5-diaminofluorescein diacetate (DAF-2), an NO-sensitive fluorescent dye (Kojima et al. 1998). Mesenteric arterioles were quickly dissected, frozen, and embedded in a freezing medium. Transverse arteriolar sections (20 μm) were obtained on a cryostat, collected on glass slides, and incubated at 37°C with 12.5 μm DAF-2 in 0.1 m phosphate buffer (pH 7.4) containing 0.45 μm CaCl2. After 1.5 h, the sections were stimulated with ACh (10−4m) or BK (10−6m) in the presence or absence of tetrahydrobiopterin (10−6m). The selection of these concentrations was based on previous reports (Shinozaki et al. 1999). Control sections received the same volume of phosphate buffer. After an additional 1 h, digital images were collected on a Nikon E1000 microscope equipped for epifluorescence (excitation at 485 nm; emission 538 nm). The images were analysed using Image software (NIH, USA) by measuring the mean optical density of the fluorescence observed in the endothelium in relation to the background staining. This fluorescence ratio was evaluated in at least three locations in each image and in at least four arterioles from different animals.
Multiplex relative RT-PCR
Arterioles from the mesenteric bed were dissected and immediately frozen in liquid nitrogen. Storage temperature was −70°C. Total RNA was isolated from the mesenteries using TRizol Reagent (Invitrogen) according to the manufacturer's instructions. cDNA was generated from 2 μg of total RNA using SuperScript II (Invitrogen). Products of the RT reaction were subjected to PCR amplification using TaqDNA polymerase (Invitrogen) and specific oligonucleotide primers for eNOS (size, 165 base pairs; 30 cycles; annealing temperature, 65°C; forward: GCCAGGAGGGACTGCAGTAC; reverse: GCGGGGAAGTGATGTCCAGG) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (size, 406 base pairs; 24 cycles; annealing temperature, 65°C; forward: GGTGCTGAGTATGTCGTGGA; reverse: TTCAGCTCTGGGATGACCTT). The conditions for PCR were as follows: initial denaturation at 94°C for 5 min was carried out, followed by 30 cycles of 94°C for 1 min; annealing temperature of 65°C for 1 min, and 72°C for 1 min. The PCR was terminated with a final extension step at 72°C for 10 min. PCR products were electrophoretically resolved by 1% agarose gel and visualized with ethidium bromide. The bands intensities were measured using a software package (Kodak Digital Science).
SDS-PAGE and Western blot analysis for nitrotyrosine
Arterioles from the mesenteric bed were dissected and immediately homogenized in ice-cold buffer containing (mm): 50.0 Tris-HCl, 0.2 DTT; 0.5 PMFS. Equal quantities of protein from each sample were resolved by SDS-PAGE (10% polyacrilamide) electroblotted onto nitrocellulose membrane. After blocking non-specific sites with 5% non-fat dry milk, the membranes were incubated overnight at 4°C with the primary mouse monoclonal antibody raised against nitrotyrosine-modified keyhole limpet hemocyanin (KLH) (500 ng ml−1, Upstate). Membranes were washed with Tris-buffered saline containing 0.1% Tween 20, and incubated with an alkaline phosphatase-conjugated rabbit anti-mouse secondary antibody. A chemiluminescent assay kit (Immun-Star; Bio-Rad, USA) was used to detect immunoreactive nitrotyrosine-containing proteins, and the intensity of all bands was estimated by densitometric analysis with a ChemImager 5500 system (Alpha Innotech).
Statistical analysis
Data are presented as mean ± s.e.m. Statistical analysis was performed using one-way ANOVA (post hoc: Tukey-Kramer multiple comparisons test) or unpaired t test when appropriate (SigmaStat, version 2.0, Jandel Scientific Software). Values were considered statistically significant when P < 0.05.
Results
Characteristics of the pregnant rats and their offspring
During pregnancy, maternal weight gain was monitored weekly. The nutritional restriction during pregnancy resulted in a marked reduction in maternal body weight from conception until day 15 of gestation. From day 15 of gestation until parturition at day 23, the undernourished dams gained weight, returning to their pre-mating weights (Table 1). At birth, the body weight in offspring exposed to intrauterine malnutrition was lower (5.2 ± 0.8 g; n = 53; P < 0.05) than in the control group (8.1 ± 0.6 g; n = 37). The litter size at birth did not differ between the control (10.14 ± 0.14 pups per litter, n = 8) and restricted (10.50 ± 0.68 pups per litter, n = 10) groups. At 16 weeks of age, the systolic blood pressure was significantly increase in the restricted (148 ± 7.8 mmHg; n = 53; P < 0.05) compared with the control group (123 ± 3.4 mmHg; n = 37). However, no differences were observed in food and water intake, urine volume, glycosuria, adult body weight and ΔG (OGTT test) when comparing control and restricted rats (Table 2).
Table 1.
Body weight (g) of the pregnant rats
| Gestation (days) | Control dams (n = 8) | Restricted dams (n = 10) |
|---|---|---|
| 1 | 220 ± 3 | 219 ± 5 |
| 5 | 242 ± 3 | 211 ± 3* |
| 10 | 254 ± 5 | 195 ± 5* |
| 15 | 285 ± 4 | 209 ± 4* |
| 22 | 309 ± 4 | 218 ± 5* |
Values are means ±s.e.m. n represents the number of animals in the group.
P < 0.05 versus control dams.
Table 2.
Characteristics of the adult offspring
| Group (n = 7) | Body weight (g) | Food intake (g (24 h)−1) | Water intake (ml (24 h)−1) | Urine volume (ml (24 h)−1) | Glycosuria (mg dl−1) | ΔGa (mm) |
|---|---|---|---|---|---|---|
| Control | 286.9 ± 14.3 | 22.6 ± 2.3 | 37.6 ± 2.2 | 4.4 ± 1.4 | Undetectable | 95.54 ± 14.0 |
| Restricted | 298.8 ± 19.1 | 21.4 ± 0.8 | 38.4 ± 1.7 | 4.5 ± 1.7 | Undetectable | 134.35 ± 19.8 |
Values are means ±s.e.m. n represents the number of animals in the group.
The glucose responses during the oral glucose tolerance test were calculated by estimation of the total area under the glucose curve (ΔG).
Role of tetrahydrobiopterin in vascular reactivity
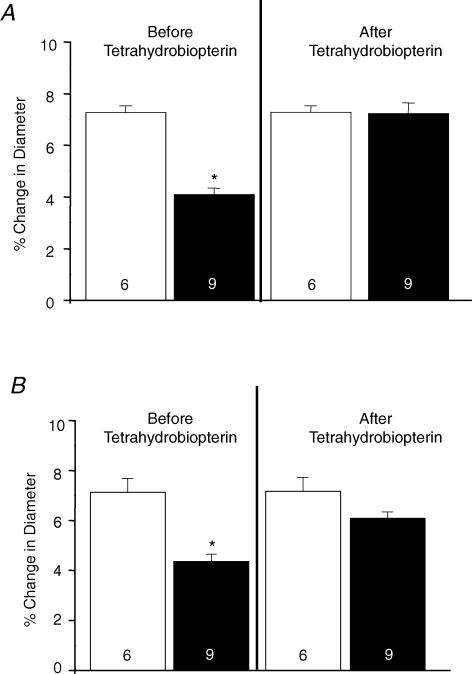
There were no differences in the baseline diameters between the control and restricted group (19.60 ± 0.65 versus 19.63 ± 0.45 μm). The magnitude of acetylcholine (Fig. 1A) and bradykinin (Fig. 1B) responses was significantly less in the restricted compared to the control group (ACh: 4.10 ± 0.24 versus 7.28 ± 0.25%; BK: 4.37 ± 0.30 versus 7.15 ± 0.55%; P < 0.001). The superfusion with tetrahydrobiopterin did not alter the responses to acetylcholine and bradykinin in control rats (data not shown). In contrast, superfusion with tetrahydrobiopterin significantly improved the response to both agents in the restricted group (ACh: 7.24 ± 0.41 versus 7.28 ± 0.25%; BK: 6.10 ± 0.26 versus 7.15 ± 0.55%; P < 0.01) (Fig. 1A and B).
Figure 1. Response of the mesenteric arterioles to acetylcholine and bradykinin in control and restricted groups, before and after superfusion with tetrahydrobiopterin.
A, response to acetylcholine; B, response to bradykinin. Control, open columns; restricted group, filled columns. Values are expressed as mean ± s.e.m. Numbers inside the columns represent the number of animals used. *P < 0.05 compared with control group.
Role of tetrahydrobiopterin in superoxide generation
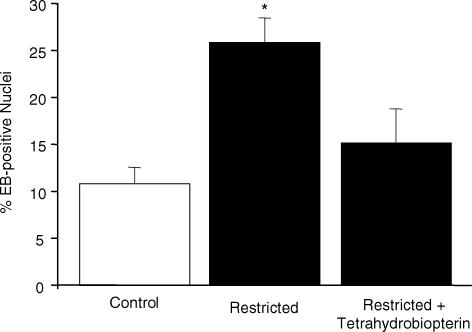
Superoxide anion generation was higher in mesenteries from the restricted group (25.84 ± 2.60%; n = 9; P < 0.01) than from the control (10.83 ± 1.72%; n = 7) group (Fig. 2). The superfusion with tetrahydrobiopterin did not alter the superoxide generation in the control rats (data not shown). However, the enhanced number of EB-positive nuclei observed in restricted rats was significantly reduced by tetrahydrobiopterin (15.17 ± 3.59; n = 8; P < 0.05) (Fig. 2), suggesting that this cofactor is involved in the overproduction of superoxide in restricted rats.
Figure 2. Effect of tetrahydrobiopterin on number of ethidium bromide (EB)-positive nuclei along mesenteric arterioles.
Graph shows percentage of EB-positive nuclei along mesenteric arterioles of control (n = 7), restricted (n = 9) and restricted + tetrahydrobiopterin (n = 8) groups. Values are expressed as mean ±s.e.m. *P < 0.05 compared with control group.
NOS activity and NO measurements
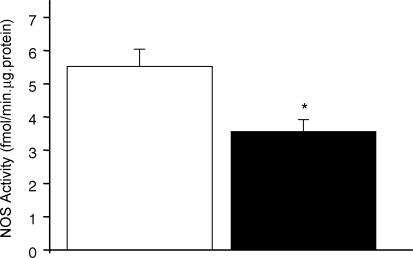
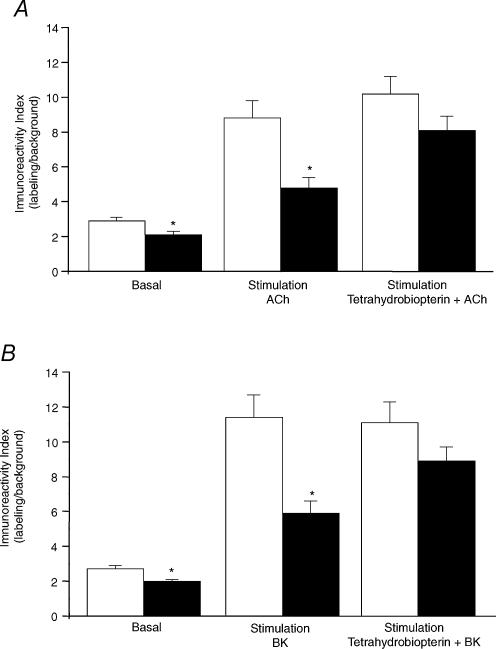
As illustrated in Fig. 3 the NOS activity was markedly reduced in mesenteric arteriolar beds from the restricted (3.56 ± 0.35 fmol min−1 (μg protein)−1; n = 8; P < 0.01) in comparison to the control (5.51 ± 0.52 fmol min−1 (μg protein)−1; n = 7) group. In addition, basal NO production (measured as the fluorescence ratio) was significantly depressed in restricted (2.1 ± 0.2; n = 4; P < 0.05) in comparison to the control (2.9 ± 0.2; n = 4) rats (Fig. 4A and B). The NO production was significantly increased after stimulation with acetylcholine (Fig. 4A) or bradykinin (Fig. 4B) in all groups, the increase being greater in control than in restricted rats (Fig. 4A and B). Incubation with tetrahydrobiopterin significantly improved NO production after stimulation with acetylcholine or bradykinin in the restricted group (Fig. 4A and B).
Figure 3. NOS activity in mesenteric arterioles of control versus restricted intake groups.
Control group, open column (n = 7); restricted group, filled column (n = 8). Values are expressed as mean ±s.e.m. *P < 0.05 compared with control group.
Figure 4. NO production in mesenteric arterioles of control versus restricted intake groups.
A, production of NO in basal conditions, after stimulation with acetylcholine, and after acetylcholine plus tetrahydrobiopterin; B, production of NO in basal conditions, after stimulation with bradykinin, and after bradykinin plus tetrahydrobiopterin. Control group, open columns; restricted group, filled columns. Values are expressed as mean ± s.e.m. and are representative of 4 animals. *P < 0.05 compared with control group.
Western Blot analysis for nitrotyrosine
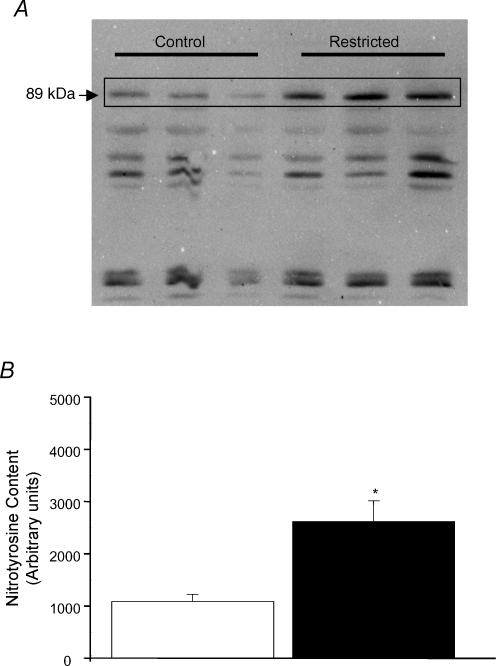
Western blot analysis showed increased nitrotyrosine-containing proteins (densitometric analysis, arbitrary units) in mesenteric vessels from the restricted (2614.7 ± 499.8; n = 7; P < 0.01) compared with the control (1084.0 ± 140.5; n = 5) group (Fig. 5).
Figure 5. Nitrotyrosine expression in mesenteric arterioles from control and restricted intake groups.
A, representative Western blot (SDS-PAGE) showing nitrotyrosine expression in mesenteric arterioles from control (left 3 lanes) and restricted (right 3 lanes) groups. B, densitometric analysis of nitrotyrosine expression in control (open column, n = 5) and restricted (filled column, n = 7) groups. Values are expressed as mean ± s.e.m. *P < 0.05 compared with control group.
eNOS mRNA expression
Relative multiplex RT-PCR analysis of mesenteric vessels for eNOS in restricted and control groups was performed. However, no changes in mRNA expression for this gene could be found in the restricted (0.96 ± 0.02; n = 5) compared with the control (0.95 ± 0.01; n = 5) group.
Discussion
Tetrahydrobiopterin is an essential cofactor for eNOS and has antioxidant activity for scavenging reactive oxygen species (ROS) (Cosentino & Luscher, 1999; Thony et al. 2000) therefore decreased availability of tetrahydrobiopterin could lead to reduced NO production and/or increased superoxide formation resulting in endothelial dysfunction (Katusic, 2001). In the present study, we demonstrated that exogenous application of tetrahydrobiopterin to mesenteric microvessels of intrauterine undernourished rats lead to a significant improvement of endothelium-dependent vasodilatation and caused a reduction in superoxide anion generation. Furthermore, preincubation with this cofactor caused an enhanced NO production. These results suggest that tetrahydrobiopterin affects endothelium-dependent pathways in intrauterine undernourished rat vessels.
Biopterin metabolism is critical for the regulation of NOS activity. It has been suggested that depletion of tetrahydrobiopterin is important for the regulation of endothelial production of superoxide as well as of NO (Wemer et al. 1995; Shinozaki et al. 1999). In fact, a number of studies have shown that the treatment of diabetic human subjects (Heitzer et al. 2000b) or diabetic animals (Pieper, 1997; Meininger et al. 2000) with tetrahydrobiopterin ameliorates impaired NOS-dependent vasoreactivity of peripheral blood vessels. Recent studies have shown that tetrahydrobiopterin can restore impaired vascular function in human patients (Maier et al. 2000) and pigs (Tiefenbacher et al. 2000) with atherosclerosis. In the present study, the impaired responses to acetylcholine and bradykinin were corrected after exogenous application of tetrahydrobiopterin. Consistent with these data, the NO production after stimulation with acetylcholine or bradykinin was corrected by tetrahydrobiopterin. Therefore, decreased NO bioactivity in intrauterine undernourished rats is at least in part due to an alteration in tetrahydrobiopterin pathways.
Contrasting with our previous study in aorta (Franco et al. 2002b), in the present study we showed that the intrauterine malnutrition induced a decrement of NOS activity without affecting the gene expression of eNOS in the microvessels of Wistar rats. Therefore, impaired endothelium-dependent relaxation might be associated with a reduction in NOS activity rather than with a reduction in the expression of the protein. The discrepancy between our previous and the present study could be due to differences in the type and size of the vessel, as well as to the particular experimental conditions of the study. In addition, metabolic and structural differences between endothelial cells from different vascular beds might also be involved.
Intrauterine malnutrition causes enhanced oxidative stress at the microcirculation level mainly due to the release of superoxide anion from endothelial cells (Franco et al. 2002a). We recently reported that the superoxide generation was significantly attenuated by treatment with NADPH oxidase-inhibitor apocynin (Franco et al. 2003). On the other hand, the findings of the present study support a role for NOS in superoxide production, since reduction of vascular superoxide production after exogenous application of tetrahydrobiopterin was observed. In fact, the effects of tetrahydrobiopterin on vascular oxidative stress have been extensively reported. Cosentino et al. (1998) have shown that tetrahydrobiopterin alters superoxide release in pre-hypertensive rats. In addition, Shinozaki et al. (2000) demonstrated that oral administration of tetrahydrobiopterin prevents vascular oxidative stress in the aortas of insulin-resistant rats. Although the exact mechanism underlying the beneficial effect of tetrahydrobiopterin is still unknown, the most likely explanation is the prevention of eNOS uncoupling leading to increased NO production (Vasquez-Vivar et al. 1998; Andrew & Mayer, 1999; Milstien & Katusic, 1999). On the other hand, restoring the tetrahydrobiopterin levels may be beneficial since pterins (including tetrahydrobiopterin) possess antioxidant properties by virtue of their scavenging activity for reactive oxygen species (Thony et al. 2000; Tarpey, 2002). However, our present data demonstrated that the reduction of superoxide anion generation induced by tetrahydrobiopterin could be due to the prevention of eNOS uncoupling, since an increased NO production after preincubation with tetrahydrobiopterin was observed. These data suggest that tetrahydrobiopterin-induced improvements in endothelial function in intrauterine undernourished rats reflect a specific effect on eNOS rather than being the consequence of a non-specific antioxidant action.
A critical question that remains to be answered is how the intrauterine malnutrition affects tetrahydrobiopterin-dependent pathways. The biosynthesis of this cofactor depends on a balanced cellular redox state, it is therefore reasonable to hypothesize that oxidative stress may lead to excessive oxidation and depletion of tetrahydrobiopterin (Komori et al. 1995). In fact, it has been demonstrated that peroxynitrite can oxidize tetrahydrobiopterin under in vitro conditions (Milstien & Katusic, 1999). Laursen et al. (2001) provided the first evidence that, in intact arteries, the oxidation of tetrahydrobiopterin by peroxynitrite may have important implications in the pathogenesis of endothelial dysfunction. Somers et al. (1998) demonstrated that peroxynitrite, and not superoxide or hydrogen peroxide, avidly oxidizes tetrahydrobiopterin to dihydrobiopterin in atherosclerosis. Peroxynitrite has been associated with increased oxidative reaction, DNA damage, and reaction with lipids, and aromatic amino acids such as tryptophan and tyrosine (Salgo et al. 1995; Zhuang & Simon, 2000). Nitration of tyrosine residues leads to the production of 3-nitrotyrosine that may be considered as a marker of peroxynitrite-dependent oxidative damage (El-Remessy et al. 2003). The present findings of increased nitrotyrosine-containing proteins in the mesenteric arteriolar bed isolated from intrauterine undernourished rats could, at first sight, suggest that the peroxynitrite is the mediator responsible for the tetrahydrobiopterin oxidation. Consistent with these results, Franco et al. (2002a) reported that the treatment with MnTMPyP, a SOD mimetic and scavenger of peroxynitrite (Pfeiffer et al. 1998; Perez & Cerbaum, 2002), improves the impairment of endothelium-dependent vasodilatation in intrauterine undernourished rats, reinforcing the role of peroxynitrite in endothelium dysfunction induced by intrauterine malnutrition. However, additional study is needed to evaluate the precise role of peroxynitrite in tetrahydrobiopterin oxidation in these animals.
Taken together, our findings provide data to propose a new mechanism whereby intrauterine malnutrition can affect endothelial production of NO. Exogenous application of tetrahydrobiopterin leads to a significant improvement of endothelium-dependent vasodilatation, enhanced NO production and decreased superoxide generation in microvessels of intrauterine undernourished rats. Since we found a decrease in NOS activity without alteration in gene expression of eNOS, we suggest that impaired NOS-dependent responses of mesenteric arterioles are related to the impairment of tetrahydrobiopterin pathways.
Acknowledgments
The present study was supported by grants from FAPESP. The authors thank Marta Rodrigues da Silva and Tiyeko Anna Eliza Vieira de Moraes Urakawa for excellent technical support.
References
- Alves MG, Barão MA, Odo LN, Nascimento G, Franco MCP, Nigro D, Lucas SSR, Laurindo FRM, Brandizzi LIV, Gil ZF. L-Arginine effects on blood pressure and renal function of intrauterine restricted rats. Ped Nephrol. 2002;17:856–862. doi: 10.1007/s00467-002-0941-z. [DOI] [PubMed] [Google Scholar]
- Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43:521–531. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- Barker DJP, Gluckman PD, Godfrey KM, Hardind JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;391:939–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Luscher TF. Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc Res. 1999;43:274–278. doi: 10.1016/s0008-6363(99)00134-0. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Patton S, Werner ER, Werner-Felmayer G, Moreau P, Malinski T, Luscher TF. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J Clin Invest. 1998;101:1530–1537. doi: 10.1172/JCI650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas APV, Tostes RCA, Fortes ZB, Nigro D, Costa SG, Carvalho MHC. In vivo evidence for antioxidant potential of estrogen in microvessels of female spontaneously hypertensive rats. Hypertension. 2002;39:405–411. doi: 10.1161/hy0202.102993. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Abou-Mohamed G, Cadwell RW, Cadwell RB. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci. 2003;44:3135–3143. doi: 10.1167/iovs.02-1022. [DOI] [PubMed] [Google Scholar]
- Fortes ZB, Garcia-Leme J, Scivoletto R. Vascular reactivity in diabetes mellitus: possible role of insulin on endothelial cell. Br J Pharmacol. 1984;83:635–643. doi: 10.1111/j.1476-5381.1984.tb16217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M do CP, Akamine EH, Di Marco GS, Casarini DE, Fortes ZB, Tostes RCA, Carvalho MHC, Nigro D. NADPH oxidase and enhanced superoxide generation in intrauterine undernourished rats: involvement of the renin-angiotensin system. Cardiovasc Res. 2003;59:767–775. doi: 10.1016/s0008-6363(03)00461-9. [DOI] [PubMed] [Google Scholar]
- Franco MCP, Dantas APV, Akamine EH, Kawamoto EM, Fortes ZB, Scavone C, Carvalho MHC, Tostes RCA, Nigro D. Enhanced oxidative stress as a potential mechanism underlying the programming of hypertension in utero. J Cardiovasc Pharmacol. 2002a;40:501–509. doi: 10.1097/00005344-200210000-00002. [DOI] [PubMed] [Google Scholar]
- Franco MCP, Arruda RMP, Dantas APV, Kawamoto EM, Fortes ZB, Scavone C, Carvalho MHC, Tostes RCA, Nigro D. Intrauterine undernutrition: expression and activity of the endothelial nitric oxide synthase in male and female adult offspring. Cardiovasc Res. 2002b;56:145–153. doi: 10.1016/s0008-6363(02)00508-4. [DOI] [PubMed] [Google Scholar]
- Goodfellow J, Bellamy MF, Gorman ST, Browlee M, Ramsey MW, Lewis MJ, Davies DP, Henderson AH. Endothelial function is impaired in fit young adults of low birth weight. Cardiovasc Res. 1998;40:600–606. doi: 10.1016/s0008-6363(98)00197-7. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Munzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: Evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000a;86:E36–E41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000b;43:143–148. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- Katusic ZS. Vascular endothelial dysfunction: does tetrahydrobiopterin play a role? Am J Physiol Heart Circ Physiol. 2001;281:H981–H986. doi: 10.1152/ajpheart.2001.281.3.H981. [DOI] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Urano Y, Higuchi T, Tanaka J, Kudo Y, Nagano T. Direct evidence of NO production in rat hippocampus and cortex using a new fluorescent indicator: DAF-2DA. Neuroreport. 1998;15:3345–3348. doi: 10.1097/00001756-199810260-00001. [DOI] [PubMed] [Google Scholar]
- Komori Y, Hyun J, Chiang K, Fukuto JM. The role of thiols in the apparent activation of rat brain nitric oxide synthase. J Biochem. 1995;117:923–927. doi: 10.1093/oxfordjournals.jbchem.a124797. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy K, Naidu AN, Prasad MP, Reddy GA. Fetal malnutrition and adult chronic disease. Nutr Rev. 2002;60:S35–S39. doi: 10.1301/00296640260130713. [DOI] [PubMed] [Google Scholar]
- Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in ApoE-deficient mice. Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- Leeson CPM, Kattenhorn M, Morley R, Lucas A, Deanfield JE. Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation. 2001;103:1264–1268. doi: 10.1161/01.cir.103.9.1264. [DOI] [PubMed] [Google Scholar]
- Leeson CPM, Whincup PH, Cook DG, Donald AE, Papacosta O, Lucas A, Deanfield JE. Flow-mediated dilatation in 9–11 year old children: the influence of intrauterine and childhood factors. Circulation. 1997;96:2233–2238. doi: 10.1161/01.cir.96.7.2233. [DOI] [PubMed] [Google Scholar]
- Maier W, Cosentino F, Lutolf RB, Fleisch M, Seiler C, Hess OM, Meier B, Luscher TF. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol. 2000;35:173–178. doi: 10.1097/00005344-200002000-00001. [DOI] [PubMed] [Google Scholar]
- Martin H, Hu J, Gennser G, Norman M. Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birthweight. Circulation. 2000;102:2739–2744. doi: 10.1161/01.cir.102.22.2739. [DOI] [PubMed] [Google Scholar]
- Meininger CJ, Marinos RS, Hatakeyama K, Martinez-Zaguilan R, Rojas JD, Kelly KA, Wu G. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem J. 2000;349:353–356. doi: 10.1042/0264-6021:3490353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstien S, Katusic ZS. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- Perez MJ, Cerbaum A. Antioxidant and pro-oxidant effects of a manganese porphyrin complex against CYP2E1-dependent toxicity. Free Rad Biol Med. 2002;33:111–127. doi: 10.1016/s0891-5849(02)00865-1. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Schrammel A, Koesling D, Schmidt K, Mayer B. Molecular actions of a Mn (III) pophyrin superoxide dismutase mimetic and peroxynitrite scavenger: reaction with nitric oxide and direct inhibition of NOS and soluble guanylyl cyclase. Mol Pharmacol. 1998;53:795–800. doi: 10.1124/mol.53.4.795. [DOI] [PubMed] [Google Scholar]
- Pieper GM. Acute amelioration of diabetic endothelial dysfunction with a derivative of the nitric oxide synthase cofactor, tetrahydrobiopterin. J Cardiovasc Pharmacol. 1997;29:8–15. doi: 10.1097/00005344-199701000-00002. [DOI] [PubMed] [Google Scholar]
- Rees D, Ben-Ishay D, Moncada S. Nitric oxide and the regulation of blood pressure in the hypertension-prone and hypertension-resistant Sabra rat. Hypertension. 1996;97:1916–1923. doi: 10.1161/01.hyp.28.3.367. [DOI] [PubMed] [Google Scholar]
- Salgo MG, Bermudez E, Squadrito GL, Pryor WA. Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytes. Arch Biochem Biophys. 1995;322:500–505. doi: 10.1006/abbi.1995.1493. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, Toda N, Kikkawa R. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/superoxide imbalance in insulin-resistant rat aorta. Diabetes. 1999;48:2437–2445. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Nishio Y, Okamura T, Yoshida Y, Maegawa H, Kojima H, Masada M, Toda N, Kikkawa R, Kashiwagi A. Oral administration of tetrahydrobiopterin prevents endothelial dysfunction and vascular oxidative stress in the aortas of insulin-resistant rats. Circ Res. 2000;87:566–573. doi: 10.1161/01.res.87.7.566. [DOI] [PubMed] [Google Scholar]
- Somers M, Falgui BT, Laursen BJ, Harrison DG. Interactions between peroxynitrite and tetrahydrobiopterin as a source of altered endothelium-dependent vascular relaxation in atherosclerosis. Circulation. 1998;98:I-735. [Google Scholar]
- Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher TF, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Swei A, Zweifach BW, Schmid-Schonbein GW. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hypertension. 1995;25:1083–1089. doi: 10.1161/01.hyp.25.5.1083. [DOI] [PubMed] [Google Scholar]
- Tarpey NM. Sepiapterin treatment in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1519–1521. doi: 10.1161/01.atv.0000038144.37823.bf. [DOI] [PubMed] [Google Scholar]
- Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347:1–16. [PMC free article] [PubMed] [Google Scholar]
- Tiefenbacher CP, Bleeke T, Vahl C, Amann K, Vogt A, Kubler W. Endothelial dysfunction of coronary resistance arteries is improved by tetrahydrobiopterin in atherosclerosis. Circulation. 2000;102:2172–2179. doi: 10.1161/01.cir.102.18.2172. [DOI] [PubMed] [Google Scholar]
- Ueda S, Matsuoka H, Miyazaki H, Usui M, Okuda S, Imaizumi T. Tetrahydrobiopterin restores endothelial function in long-term smokers. J Am Coll Cardiol. 2000;35:71–75. doi: 10.1016/s0735-1097(99)00523-9. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Mastres BS, Karoui H, Tordo P, Pritchard KA. Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemer ER, Werner-Felmayer G, Wachter H, Mayer B. Biosynthesis of nitric oxide: dependence on pterine metabolism. Rev Physiol Biochem Pharmacol. 1995;127:97–135. doi: 10.1007/BFb0048266. [DOI] [PubMed] [Google Scholar]
- Wever RMF, Van Dam T, Van Rijn HJM, de Groot PF, Rabelink TJ. Tetrahydrobiopterin regulated superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. J Biol Chem. 1998;273:25804–25808. doi: 10.1006/bbrc.1997.7069. [DOI] [PubMed] [Google Scholar]
- Yliharsila H, Eriksson JG, Forsén T, Kajantie E, Osmond C, Barker DJP. Self-perpetuating effects of birth size on blood pressure levels in elderly people. Hypertension. 2003;41:446–450. doi: 10.1161/01.HYP.0000055780.21222.96. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Simon G. Peroxynitrite-induced apoptosis involves activation of multiple caspases in HL-60 cells. Am J Physiol Cell Physiol. 2000;279:C341–C351. doi: 10.1152/ajpcell.2000.279.2.C341. [DOI] [PubMed] [Google Scholar]
- Zweifach BW. Indirect methods for regional blood flow: microscopic observation of circulation in rat mesoappendix and dogomentum, use in study of vasotropic substances. Meth Med Res. 1948;1:131–138. [PubMed] [Google Scholar]