Abstract
Pregnant guinea pigs were treated with dexamethasone (1 mg kg−1) or vehicle on days 40–41, 50–51 and 60–61 of gestation, after which animals delivered normally. Adult male offspring were catheterized at 145 days of age and subjected to tests of hypothalamic–pituitary–adrenal (HPA) axis function in basal and activated states. Animals exposed to dexamethasone in utero (mat-dex) exhibited increased hippocampus-to-brain weight ratio, increased adrenal-to-body weight ratio and increased mean arterial pressure. There were no effects on gestation length, birth weight and postnatal growth. There were no overall differences in diurnal plasma adrenocorticotropic hormone (ACTH) and cortisol profiles, though there were subtle differences during the subjective afternoon between control and mat-dex offspring. A significant decrease in initial ACTH suppression was observed following dexamethasone injection in mat-dex offspring compared to control offspring. Molecular analysis revealed significantly increased MR mRNA expression in the limbic system and particularly in the dentate gyrus in mat-dex offspring. In the anterior pituitary, both pro-opiomelanocortin (POMC) and glucocorticoid receptor (GR) mRNA levels were significantly elevated in mat-dex offspring. In conclusion, (1) repeated prenatal treatment with synthetic glucocorticoid (sGC) permanently programmes organ growth, blood pressure and HPA regulation in mature male offspring and these changes involve modification of corticosteroid receptor expression in the brain and pituitary; (2) the effects of prenatal sGC exposure on HPA function appear to change as a function of age, indicating the importance of investigating HPA and cardiovascular outcome at multiple time points throughout life.
Preterm delivery occurs in approximately 7% of all pregnancies and is the most serious cause of neonatal mortality and morbidity. Synthetic glucocorticoids (sGC) are routinely administered to pregnant women at risk of preterm delivery (NIH, 1995). Such treatment is highly effective in decreasing the incidence of respiratory distress syndrome and other complications in preterm infants (Liggins & Howie, 1972). Until recently, multiple course glucocorticoid therapy had become common practice in Australia, Europe and North America, if the risk of preterm delivery persisted (Brocklehurst et al. 1999; Smith et al. 2000). However, a recent NIH consensus update conference recommended that such practice be confined to ongoing clinical trials in which the efficacy of multiple course therapy is being investigated. Notwithstanding, practice over the last 5–10 years has led to a very significant number of infants/children that had received multiple exposures to sGC. Recent experimental data in guinea pigs, rats and sheep indicate that excess antenatal glucocorticoid exposure can lead to alteration or programming of the fetal hypothalamic—pituitary—adrenal (HPA) system, resulting in altered regulation of the axis throughout life (Liu et al. 2001; Weinstock, 2001; Welberg & Seckl, 2001; Welberg et al. 2001; Matthews, 2002; Sloboda et al. 2002). Long-term alterations in HPA function have been linked to the premature development of adult onset pathologies in rats, sheep and humans; these include insulin resistance and elevated blood pressure (Nyirenda et al. 2001; Reynolds et al. 2001a,b; Welberg et al. 2001; Phillips, 2002).
It is emerging from animal studies that perinatal programming of HPA function involves a permanent resetting of glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) levels in the hippocampus, hypothalamus and pituitary (Meaney et al. 2000; Liu et al. 2001; Welberg & Seckl, 2001; Matthews, 2002). In the guinea pig, we have previously reported that repeated fetal exposure to dexamethasone (1 mg kg−1) in late gestation results in young (postnatal day (PND) 70) adult male offspring with reduced basal HPA function (Liu et al. 2001). This was associated with a large increase in plasma testosterone, but no significant change in blood pressure. The guinea pig GR exhibits 4-fold lower binding affinity for dexamethasone than the human GR (Keightley et al. 1998). To accommodate this, we used a dose of dexamethasone (1 mg kg−1) which is comparable to that received by pregnant women who are at risk of preterm labour (∼0.25 mg kg−1 day−1; NIH, 1995). Unlike rats and more similar to primates, guinea pigs give birth to neuroanatomically mature young (Dobbing & Sands, 1979), with dynamic changes in development of GRs and MRs occurring throughout gestation (Matthews, 1998; Owen & Matthews, 2003). In the present study, we hypothesized that the effects of repeated prenatal treatment with sGC exposure on HPA function and blood pressure become more pronounced as animals age (PND150).
Methods
Animals and treatment
Female guinea pigs were mated in our animal facility as previously described (Dean & Matthews, 1999). Food and water were available ad libitum. The animals were kept on a 12 : 12 h light—dark (LD) cycle. Studies were performed according to protocols approved by the Animal Care Committee at the University of Toronto, in accordance with the Canadian Council for Animal Care. Pregnant guinea pigs were treated with subcutaneous (s.c.) injections of dexamethasone sodium phosphate (mat-dex, n = 22, 1 mg kg−1; Dexamethasone 2, Austin Vetoquinol, Lavaltrie, Canada) or saline vehicle (control, n = 22, 0.3 ml) on gestational days (GD) 40/41, 50/51 and 60/61, after which animals delivered normally. These times correspond to rapid phases of neurogenesis (GD40), brain growth and initiation of electrical activity (GD50) and myelination (GD60) in the fetal guinea pig brain (Dobbing & Sands, 1979). We have shown that the doses of dexamethasone used in this study cause down-regulation of maternal and fetal HPA activity (McCabe et al. 2001). At birth, gestation length, birth weight and sex of all neonates were recorded. Male offspring were weaned at 30 days of age and then housed separately, but within visual, auditory and olfactory contact of other guinea pigs. Offspring were left undisturbed apart from daily animal care and weight measurement on PND60, PND120 and before surgery (PND145). On PND145, polyvinyl catheters (PE90) were surgically implanted into the carotid artery of a subset of non-sibling male offspring (control n = 16, mat-dex n = 17). Animals were anaesthetized intramuscularly (i.m.) using ketamine (40 mg kg−1, MTC Pharmaceuticals, Cambridge, Canada) and xylazine (4 mg kg−1, Bayer Inc., Toronto, Canada) and surgery was carried out under standard aseptic conditions. Local anaesthetic (lidocaine hydrochloride, Astra Zeneca, Toronto, Canada) was administered as necessary. Immediately after surgery, the animals were treated with yohimbine (0.1 mg kg−1, i.v., Lloyd Laboratories, Shenandoah, USA) to reverse the actions of xylazine. A small jacket with a spring was then fitted to the guinea pig, and the catheters passed up through the spring and attached to a Teflon swivel (Lomir Biomedicals, Montreal, Canada). This allowed full rotation of the catheter and unrestricted movement of the guinea pig. Analgesic (buprenorphine, Schering-Plough Ltd, Hertfordshire, UK) was given before the animal was taken off the anaesthetic and 24 h following surgery. Repeated sampling of animals catheterized in this way does not result in activation of the HPA axis (Liu & Matthews, 1999; Liu et al. 2001). Catheters were filled with heparinized saline and flushed daily. Animals were left to recover for a minimum of 3 days following surgery.
Experiments in adult offspring
At PND150, HPA axis function was assessed in the catheterized animals using four tests, carried in the same order in each animal. (1) Hourly blood sampling during the subjective day (guinea pig active phase; 07.00–18.00 h) to assess basal pituitary—adrenal activity. (2) Dexamethasone—corticotropin-releasing hormone (CRH) challenge to assess glucocorticoid feedback sensitivity and responsiveness to CRH. Dexamethasone (1 mg kg−1, i.v.) was given at 09.00 h and blood samples were taken prior to dexamethasone administration (0) and at 30, 60, 120 and 240 min following injection. This was followed by an injection of human CRH (0.5 μg kg−1, i.v.) at 13.00 h and continued blood sample collection at 245, 255, 270, 300 and 360 min. (3) ACTH1-24 challenge (0.5 μg kg−1, i.v.) at 13.00 h to assess adrenal sensitivity. Blood samples were collected at −30, 0, 15, 30, 60 and 120 min. (4) Restraint (30 min) to assess responsiveness of the HPA axis to mild stress with blood sample collection at 0, 15, 30, 60 and 120. ACTH1-24 and CRH were purchased from Peninsula Laboratories (Belmont, CA, USA). The doses of dexamethasone, CRH and ACTH used in the current study were derived from pilot studies and previous reports (Liu & Matthews, 1999; Liu et al. 2001). At least 2 days of recovery were allowed between the tests. Plasma samples were stored at −20°C.
Blood pressure and heart rate were measured on the second rest day between tests, via the carotid artery cannula. A small displacement pressure transducer was connected to a MacLab/4e data acquisition system and a PowerMac Macintosh computer driven by MacLab Chart 3.5.6 software (AD Instruments, Castle Hill, Australia). Blood pressure was measured (5 min) between 14.00 and 15.00 h. Chart analysis was performed using the same software to calculate systolic, diastolic and mean arterial pressure (MAP) as well as heart rate (HR).
Animals were given at least 48 h recovery time following restraint and were killed using a solution of embutramide with mebozonium iodide and tetracaine hydrochloride in dimethylformamide as preservative (2.5 ml, i.v.; T61, Hoechst Roussel Vet, Regina, Canada). Brains, pituitaries and adrenal glands were rapidly dissected and frozen (−80°C). Prior to freezing, brains were sagitally hemisected at an angle and slightly off midline, such that the left block contained the left hippocampus and the entire hypothalamus. The right hippocampus was dissected from the remaining block and weighed. Other peripheral organs (heart, lung, left kidney and testes) were removed and weighed.
Endocrine analysis
Double-antibody (DA) and coated tube (CT) RIA kits (ICN Biomedical Inc., Costa Mesa, CA, USA) were used to determine levels of immunoreactive (ir) plasma ACTH, cortisol and testosterone. These assays have been previously used in the guinea pig (Liu et al. 2001; McCabe et al. 2001; Owen & Matthews, 2003). All samples from within each test were run in the same assay to negate interassay bias.
In situ hybridization (ISH)
The method of ISH has been described in detail previously (Matthews & Challis, 1995a,b; Matthews, 1998). Coronal cryosections of brain, pituitary and adrenals (10 μm; control n = 8; mat-dex n = 8) were mounted onto poly-l-lysine-coated slides, dried and fixed in paraformaldehyde (4%). Oligonucleotide probes were synthesized by Sigma Genosys (Oakville, Canada). The use of the antisense GR, MR, POMC and CRH oligonucleotide probes have been previously described (Dean & Matthews, 1999; Go et al. 2001; McCabe et al. 2001; Owen & Matthews, 2003). The antisense probes for melanocortin 2 receptor (MC2-R) and cytochrome P450C17 (CYP17) mRNA were complementary to bases 514–559 and 1094–1138 of the guinea pig MC2-R and CYP17 (GenBank ref AF104058 and S75277), respectively. The steroidogenic factor-1 (SF-1) mRNA antisense probe was designed complimentary to bases 1449–1493 of human nuclear receptor subfamily 5, group A, member 1 (NR5A1) (GenBank ref NM_004959) which shows 100% homology with the mouse. The antisense probe for steroidogenic acute regulatory protein (StAR) mRNA was complementary to bases 469–513 of rat StAR (GenBank ref NM_031558), a region 100% homologous to mouse StAR (GenBank ref AY032730). The probes were labelled with [35S]-deoxyadenosine-5′-α-thiophosphate (1300 Ci mmol−1, Perkin-Elmer, Woodbridge, Canada) using terminal deoxynucleotidyl transferase (TdT; 15 U μl−1; Invitrogen Canada Inc., Burlington, Canada) to a specific activity of 1.0 × 109 counts min−1μg−1. Labelled probes in hybridization buffer were applied to slides which were incubated overnight (42°C). After washing in 1 × standard sodium citrate (SSC; 30 min) at 22°C and then in 1 × SSC (30 min) at 55°C, the slides were dehydrated and exposed to autoradiographic film (Biomax, Kodak) for variable time periods (hippocampal GR 21 days; hippocampal MR 14 days; pituitary GR 7 days; anterior lobe POMC 72 h; intermediate lobe POMC 17 h; paraventricular nucleus (PVN) CRH and GR 42 days; adrenal MC2-R 48 h; CYP17 6 days; SF-1 96 h; StAR 72 h). All sections to be compared for a given mRNA species were processed in the same ISH experiment and exposed simultaneously to allow direct comparisons to be made. 14C standards (American Radiochemical, St Louis, MO, USA) were used to ensure that analysis was undertaken in the linear range of the autoradiographic film. The relative optical density (ROD) of the signal on autoradiographic film was quantified, after subtraction of background values, with a computerized image analysis system (Imaging Research, St Catharines, Canada). GR mRNA levels were measured in four regions of anterior hippocampus (CA1/2, CA3 and CA4), dentate gyrus (DG), hypothalamic paraventricular nucleus (PVN) and anterior pituitary gland. MR mRNA levels were measured in hippocampus (as for GR) and DG. POMC mRNA was measured in the intermediate lobe and anterior lobe (superior and inferior regions) of the pituitary gland. CRH mRNA was measured in hypothalamic PVN. MC2-R, CYP17, SF-1 and StAR mRNA expression was measured throughout the entire adrenal cortex followed by separated analysis of the glomerulosa region and fasciculata + reticularis regions. ROD was measured in at least six sections per region per animal, and averaged for statistical analysis. All analyses were undertaken by an operator unaware of the treatment. Incubation of sense probes with tissue sections known to contain target mRNA revealed no hybridization signal.
Statistical analysis
All data were expressed as mean ± s.e.m. For all tests, significance was set at P < 0.05. Statistical analysis was performed using GraphPad Prism version 3.0cx for Mac OS X (GraphPad Software, San Diego, CA, USA) and Statistica version 5.0 for Windows (Statsoft Inc., Tulsa, OK, USA). Data were statistically analysed using multivariate analysis of variance (ANOVA), followed by Duncan's method of post hoc comparison or a Bonferroni post hoc test to compare replicate means. Gestation length, litter size, birth weight, blood pressure, heart rate, organ weights, body weights, organ-to-body weight ratios, organ-to-brain weight ratios, GR and CRH mRNA in PVN and POMC mRNA in intermediate pituitary were analysed by one-way (prenatal treatment) ANOVA. Growth and basal plasma testosterone concentration were analysed by two-way (prenatal treatment × time) repeated-measures (RM) ANOVA. Hippocampal GR and MR mRNA, GR and POMC mRNA in anterior pituitary gland and adrenal StAR, SF-1, CYP17 and MC2-R mRNA were analysed by two-way (prenatal treatment × region) ANOVA. Diurnal changes in basal ACTH and cortisol concentrations were analysed in the control and dexamethasone-treated males separately using one-way (time) RM ANOVA. Effects of prenatal treatment on basal cortisol and ACTH were measured across the entire day by two-way (prenatal treatment × time) RM ANOVA. Effects of dexamethasone (0–240 min) and CRH (240–360) on plasma ACTH and cortisol were analysed independently using one-way (time) RM ANOVA in each of the prenatal treatment groups. For analysis of prenatal treatment effects, ACTH and cortisol responses to dexamethasone suppression (0–240 min) and CRH stimulation (240–360 min) were analysed by two-way (prenatal treatment × time) RM ANOVA and by one-way (prenatal treatment) ANOVA on the area above the curve (dexamethasone suppression, AAC) and area under the curve (CRH stimulation, AUC). Effects of ACTH challenge and restraint on plasma cortisol concentrations were analysed by performing one-way (time) ANOVA in each of the prenatal treatment groups. Two-way (prenatal treatment × time) RM-ANOVA was used for analysis of differences between prenatal treatment groups. This was followed by one-way (prenatal treatment) ANOVA on the total AUC.
Results
Prenatal glucocorticoid treatment had no effect on gestation length (control 69.1 ± 0.3 days, mat-dex 69.2 ± 0.3 days), litter size (control 3.2 ± 0.8 pups, mat-dex 3.1 ± 0.8 pups), birth weight or growth (Table 1). Organ-to-body weight ratios, hippocampus-to-brain weight ratios and pituitary-to-brain weight ratios in adult offspring are presented in Table 2. Analysis revealed that there was a significant increase in adrenal-to-body weight (P < 0.05) and hippocampal-to-brain weight (P < 0.01) ratios in mat-dex offspring. The increase in adrenal-to-body weight ratio corresponded to a significant increase in adrenal weight (P < 0.05; control 0.252 ± 0.008 g, mat-dex 0.281 ± 0.009 g), while the increase in hippocampal-to-brain weight was associated with a strong tendency towards an increase in hippocampal weight (control 0.107 ± 0.007 g, mat-dex 0.119 ± 0.004 g) and a tendency towards a decrease in brain weight (control 3.145 ± 0.037 g, mat-dex 3.063 ± 0.056 g) in the mat-dex offspring. There were no differences between prenatal treatment groups in any of the other organs measured.
Table 1.
Birth weights (PND0) and body weights (PND60 and PND120) in adult male offspring whose mothers had been injected with vehicle (control) or dexamethasone (mat-dex; 1 mg kg−1) on days 40–41, 50–51, and 60–61 of gestation
| PND0 | PND60 | PND120 | |
|---|---|---|---|
| Control | 95 ± 2 (32) | 554 ± 9 (29) | 810 ± 11 (32) |
| Mat-dex | 101 ± 3 (29) | 561 ± 15 (20) | 828 ± 16 (27) |
Results are expressed as mean ± s.e.m. in grams; numbers in parentheses indicate number of animals in each group.
Table 2.
Organ weights expressed as organ-to-body weight (brain, adrenal and peripheral) or organ-to-brain weight (hippocampus and pituitary) ratios in adult male offspring whose mothers had been injected with vehicle (control) or dexamethasone (mat-dex; 1 mg kg−1) on days 40–41, 50–51, and 60–61 of gestation
| HPA axis | Peripheral | |||||||
|---|---|---|---|---|---|---|---|---|
| Hippocampus | Pituitary | Brain | Adrenal | Heart | Left lung | Left kidney | Gonads | |
| Control | 3.3 ± 0.2 (15) | 5.9 ± 0.2 (16) | 4.2 ± 0.1 (16) | 3.4 ± 0.1 (16) | 4.9 ± 0.3 (16) | 2.5 ± 0.2 (16) | 4.3 ± 0.2 (16) | 3.1 ± 0.1 (16) |
| Mat-dex | 3.9 ± 0.1 (17)** | 6.1 ± 0.2 (17) | 4.1 ± 0.1 (17) | 3.8 ± 0.1 (16)* | 4.5 ± 0.4 (17) | 2.4 ± 0.1 (17) | 4.6 ± 0.2 (17) | 3.2 ± 0.1 (16) |
Results are expressed as mean ± s.e.m.; numbers in parentheses indicate numbers of animals in each group. Ratios were multiplied by 102 (hippocampus), 103 (pituitary, brain and peripheral) and 104 (adrenal).
P < 0.05
P < 0.01, significant difference between mat-dex and controls.
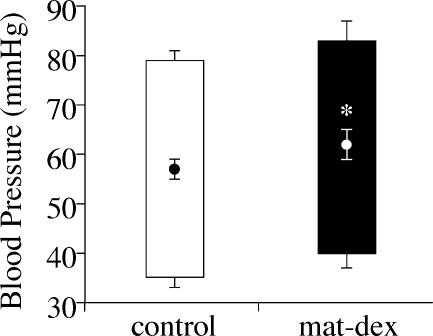
Mean arterial, diastolic and systolic blood pressures are presented in Fig. 1. Mean arterial pressure was significantly (P < 0.05) elevated in mat-dex offspring compared to controls. Though there was a trend towards an increase in systolic blood pressure, there were no significant differences in any of the other cardiovascular parameters measured (heart rate: control 325 ± 6 beats min−1, n = 10; mat-dex 324 ± 9 beats min−1, n = 8).
Figure 1. Mean arterial, systolic and diastolic blood pressure (mean ± s.e.m.) in adult male offspring whose mothers had been injected with vehicle (control) or dexamethasone (mat-dex).
Open column, control (n = 10); Filled column, mat-dex (1 mg kg−1; n = 8). Circles and bars inside the columns represent mean arterial pressure: systolic pressure is represented by the top bars and diastolic pressure is represented by the bottom bars. *P < 0.05, significant difference between prenatal treatments.
Endocrine function
Basal
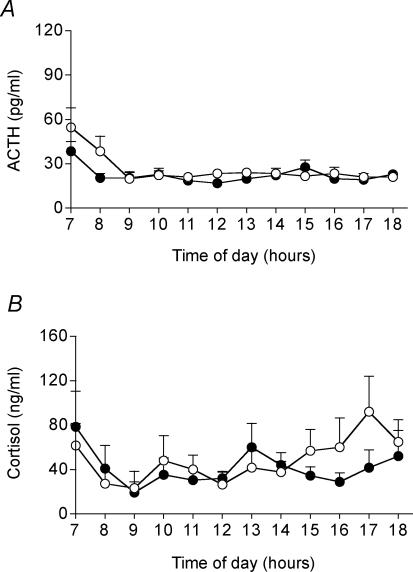
There was no significant difference in plasma testosterone concentrations (Table 3) throughout the subjective day between the two prenatal treatment groups. There was a significant effect of time on plasma ACTH concentrations (control P < 0.02, mat-dex P < 0.001), with higher levels in the early subjective morning compared to the rest of the subjective day (Fig. 2A). Plasma ACTH concentrations were not significantly different between the prenatal treatment groups, though there was a tendency towards lower early morning concentrations in the mat-dex offspring. There was no significant effect of time on cortisol concentrations during the subjective day, although there was a trend towards an increase in cortisol concentration in the subjective afternoon in the control group (Fig. 2B). No similar trend was observed in the mat-dex group. There was no overall difference in cortisol concentration throughout the subjective day between the two prenatal treatment groups, and no significant difference between individual time points following post hoc analysis.
Table 3.
Plasma testosterone concentrations (ng ml−1) in adult male offspring whose mothers had been injected with vehicle (control) or dexamethasone (mat-dex; 1 mg kg−1) on days 40–41, 50–51, and 60–61 of gestation
| n | 08.00 h | 09.00 h | 10.00 h | 13.00 h | 14.00 h | 15.00 h | 17.00 h | 18.00 h | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 8 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.5 ± 0.1 | 0.8 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.3 |
| Mat-dex | 11 | 0.7 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.1 | 1.2 ± 0.2 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
Results are expressed as mean ± s.e.m.; n indicates numbers of animals in each group.
Figure 2. Resting (basal) plasma ACTH (A) and cortisol (B) concentrations during the subjective day (07.00–18.00 h) in adult male offspring whose mothers had been injected with vehicle or dexamethasone on days 40–41, 50–51, and 60–61 of gestation.
Data are means ± s.e.m. ○ control (n = 9); •, dexamethasone (1 mg kg−1; n = 10).
Dexamethasone—CRH challenge
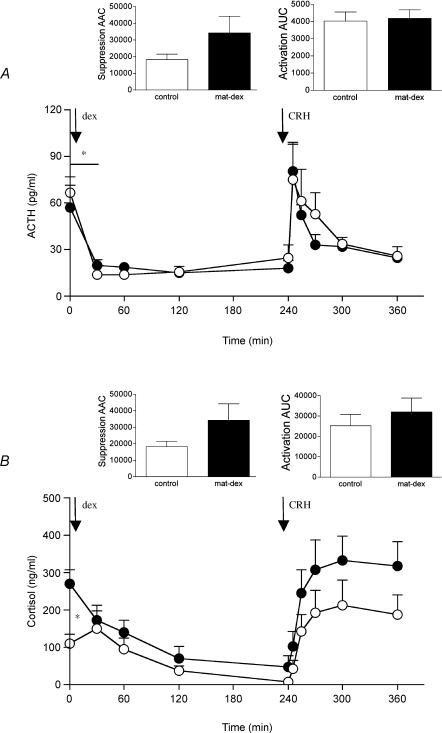
Administration of dexamethasone at 09.00 h caused a significant suppression of both plasma ACTH (control P < 0.001, mat-dex P < 0.005) and cortisol (control P < 0.001, mat-dex P < 0.001) concentrations (Fig. 3A and B). The magnitude of the initial suppression (0–30 min) of plasma ACTH concentrations was significantly lower (P < 0.05, AAC one-way (prenatal treatment) ANOVA) in mat-dex offspring compared to controls (Fig. 3A). While basal plasma cortisol concentrations (09.00 h) were significantly elevated (P < 0.05, one-way (prenatal treatment) ANOVA) in mat-dex offspring compared to controls, there was no overall difference in the level of plasma cortisol suppression between prenatal treatments (Fig. 3B). Injection of CRH significantly increased plasma ACTH (control P < 0.002, mat-dex P < 0.0001) and cortisol (control P < 0.0001, mat-dex P < 0.0001) in both prenatal treatment groups (Fig. 3A and B). The initial plasma ACTH response to CRH injection was similar between control offspring and mat-dex offspring. However, the activation appeared to be terminated faster in offspring born to sGC-treated mothers (Fig. 3A), but this failed to attain significance. Plasma cortisol levels in mat-dex offspring showed what appeared to be an increased response to CRH, though again it failed to reach significance (Fig. 3B).
Figure 3. Plasma ACTH (A) and cortisol (B) concentrations before and during dexamethasone and corticotropin-releasing hormone challenge in adult male offspring whose mothers had been injected with vehicle or dexamethasone on days 40–41, 50–51, and 60–61 of gestation.
Data are means ± s.e.m. Dexamethasone (dex), 1 mg kg−1; corticotropin-releasing hormone (CRH), 0.5 μg kg−1. ○, control, (ACTH n = 8, cortisol n = 7); •, dexamethasone (ACTH n = 6, cortisol n = 6) *P < 0.05, significant difference between prenatal treatments.
ACTH challenge
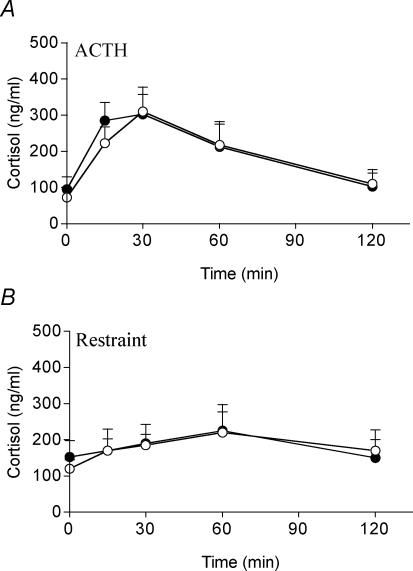
Injection of ACTH significantly increased plasma cortisol concentrations (control P < 0.01, mat-dex P < 0.01, one-way (time) ANOVA), which peaked at 30 min (Fig. 4A). There was no significant difference in the response between control and mat-dex offspring.
Figure 4. Plasma cortisol concentrations before and during ACTH challenge (0.5 μg kg−1; A) and restraint (30 min; B) in adult male offspring whose mothers had been injected with vehicle or dexamethasone on days 40–41, 50–51 and 60–61 of gestation.
Data are means ± s.e.m. ○, control (ACTH n = 8, restraint n = 8); •, dexamethasone (1 mg kg−1; ACTH n = 7, restraint n = 8).
Restraint
Restraint represented a very mild stress resulting in only a tendency toward increased cortisol levels. There was no significant difference in the cortisol responses between the two prenatal treatment groups.
Molecular regulation of the HPA axis
Hippocampus
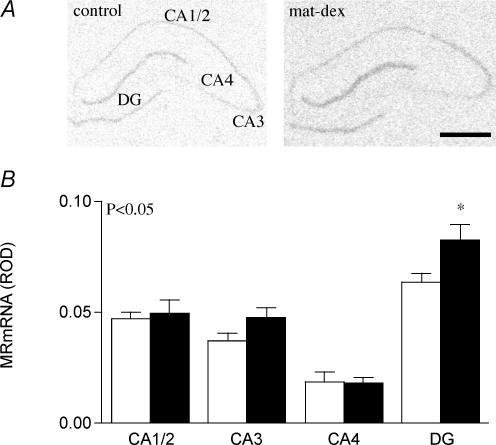
The expression pattern for MR mRNA in hippocampal cornu ammonis (CA) and dentate gyrus (DG) of adult guinea pigs was DG > CA1/2 > CA3 > CA4 (Fig. 5A). Analysis using two-way (prenatal treatment × region) ANOVA revealed significantly higher (P < 0.05) levels of MR mRNA in the limbic structures in the mat-dex offspring compared to controls. This effect was most prominent in the DG, as determined through post hoc analysis (P < 0.05, Fig. 5A and B). The glucocorticoid receptor mRNA expression pattern differed slightly from MR mRNA, being CA1/2 > DG > CA3 > CA4 (Table 4). There were no significant differences in limbic GR mRNA between control offspring and those born to sGC-treated mothers (Table 4).
Figure 5. Hippocampal MR mRNA.
A, representative expression of MR mRNA in the hippocampus (CA1/2, CA3, CA4) and dentate gyrus (DG) in adult male offspring whose mothers had been injected with vehicle (control) or dexamethasone (1 mg kg−1; mat-dex) on days 40–41, 50–51 and 60–61 of gestation. Bar = 1 mm. B, relative optical density (ROD) of MR mRNA expression (mean ± s.e.m.) in the hippocampus (CA1/2, CA3, CA4) and dentate gyrus (DG) in adult male offspring whose mothers had been injected with vehicle (control, open columns, n = 8) or dexamethasone (1 mg kg−1; filled columns, n = 8) on days 40–41, 50–51 and 60–61 of gestation. *P < 0.05, significant difference between prenatal treatments.
Table 4.
Relative optical density (ROD) of GR mRNA expression in hippocampus (CA1/2, CA3 and CA4) and dentate gyrus (DG) in adult male offspring whose mothers had been injected with vehicle (control) or dexamethasone (mat-dex; 1 mg kg−1) on days 40–41, 50–51, and 60–61 of gestation
| n | CA1/2 | CA3 | CA4 | DG | |
|---|---|---|---|---|---|
| Control | 8 | 0.52 ± 0.02 | 0.25 ± 0.01 | 0.21 ± 0.02 | 0.44 ± 0.03 |
| Mat-dex | 8 | 0.51 ± 0.04 | 0.25 ± 0.02 | 0.21 ± 0.02 | 0.45 ± 0.02 |
Results are expressed as mean ± s.e.m.; n indicates numbers of animals in each group.
Hypothalamus
GR and CRH mRNA were expressed at high levels in the hypothalamic PVN (not illustrated). However, there were no significant differences in GR or CRH between the prenatal treatment groups (GR: control 0.21 ± 0.02, mat-dex 0.20 ± 0.02; CRH: control 0.83 ± 0.02, mat-dex 0.80 ± 0.01 ROD).
Pituitary gland
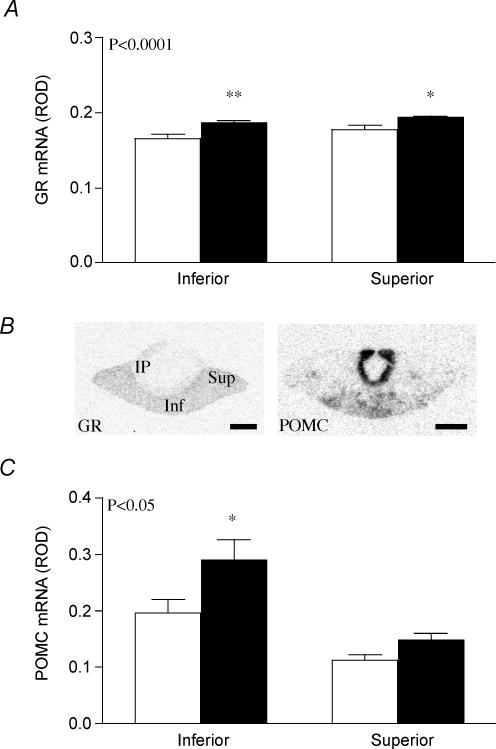
Two-way ANOVA (prenatal treatment × region) analysis of GR mRNA and POMC mRNA levels in the anterior lobe of the pituitary gland revealed significant differences in regional expression between prenatal treatment groups. There was a significant increase (P < 0.0001) in GR mRNA expression in the anterior pituitary in mat-dex offspring compared to controls (Fig. 6A). Post hoc analysis revealed this increase to be significant in both the inferior region (P < 0.01) and the superior region (P < 0.05) of the anterior pituitary gland. The pattern of POMC mRNA expression in the anterior pituitary gland was inferior region > superior region (Fig. 6B and C). Comparison between prenatal treatments revealed a significant overall increase (P < 0.05; Fig. 6B and C) in POMC mRNA expression in the anterior pituitary gland of mat-dex offspring. This effect was most prominent in the inferior region (P < 0.05). POMC mRNA was present at high level in the intermediate lobe (Fig. 6B). However, there was no effect of prenatal treatment on POMC mRNA levels in this region (control 1.2 ± 0.1, mat-dex 1.5 ± 0.2 ROD).
Figure 6. GR mRNA and POMC mRNA in the pituitary gland.
A, relative optical density (ROD) of GR mRNA expression (mean ± s.e.m.) in the anterior pituitary (inferior and superior) in adult male offspring whose mothers had been injected with vehicle (control, open columns, n = 7) or dexamethasone (1 mg kg−1; filled columns, n = 8) on days 40–41, 50–51 and 60–61 of gestation. *P < 0.05, **P < 0.01, significant difference between prenatal treatments. B, representative expression of GR mRNA (left panel, GR) and POMC mRNA (right panel, POMC) in the inferior (inf) and superior (sup) regions of the anterior pituitary and in the intermediate pituitary (ip). Bar = 1 mm. C, relative optical density (ROD) of POMC mRNA expression (mean ± s.e.m.) in the anterior pituitary (inferior and superior) in adult male offspring whose mothers had been treated as described above.
Adrenal cortex
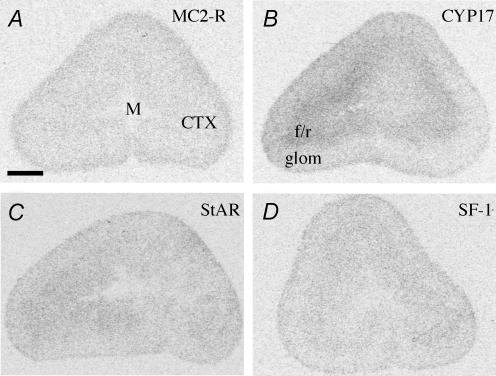
Analysis using two-way (prenatal treatment × region) ANOVA of mRNA expression of MC2-R, the key steroidogenic enzyme cytochrome P450C17 (CYP17), steroidogenic acute regulatory protein (StAR) and steroidogenic factor 1 (SF-1) expression revealed specific regional distribution (Fig. 7), but no difference between prenatal treatments (Table 5). The expression pattern of MC2-R in the adrenal cortex was glomerulosa > fasciculata/reticularis (Fig. 7A). The CYP17 mRNA expression pattern exhibited a tendency towards fasciculata/reticularis > glomerulosa, but the difference failed to attain significance (Fig. 7B). Analysis of StAR and SF-1 mRNA revealed uniform expression across all of the regions of the adrenal cortex (Fig. 7C and D).
Figure 7. Representative expression of MC2-R (A), CYP 17 (B), StAR (C) and SF-1 (D) mRNA in the glomerulosa (glom) and fasciculata/reticularis (f/r) regions of the adrenal cortex (CTX).
Adrenal medulla is represented by M. Bar = 1 mm.
Table 5.
Relative optical density (ROD) of MC2-R, CYP17, StAR and SF-1 mRNA expression in glomerulosa (glom) and fasciculata/reticularis (f/r) regions of adrenal cortex in adult male offspring whose mothers had been injected with vehicle (control) or dexamethasone (mat-dex; 1 mg kg−1) on days 40–41, 50–51, and 60–61 of gestation
| MC2-R | CYP17 | StAR | SF-1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | glom | f/r | glom | f/r | glom | f/r | glom | f/r | |
| Control | 8 | 0.14 ± 0.02 | 0.10 ± 0.01 | 0.32 ± 0.04 | 0.41 ± 0.04 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Mat-dex | 8 | 0.12 ± 0.01 | 0.09 ± 0.00 | 0.31 ± 0.04 | 0.37 ± 0.06 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 |
Results are expressed as mean ± s.e.m.; n indicates numbers of animals in each group.
Discussion
In the present study, we have demonstrated that repeated prenatal exposure to sGC has long-term effects on specific aspects of HPA regulation, hippocampal and adrenal size and blood pressure in mature adult male offspring at 150 days of age. Interestingly, at the level of pituitary—adrenal activity, these effects are less pronounced than those observed in younger adult male offspring at 80 days of age (Liu et al. 2001).
The dose of sGC administered to pregnant animals in this study, and those undertaken previously in our laboratory (1 mg kg−1) is comparable to that prescribed to pregnant women (0.2–0.3 mg kg−1) (Liggins & Howie, 1972), since the guinea pig GR has a 4-fold lower affinity for glucocorticoid than the human GR (Keightley et al. 1998). One of the principal concerns following repeated prenatal sGC exposure is the effect on fetal and postnatal growth. In the rat, daily maternal dexamethasone injections over the last week of pregnancy (term = 21 days) significantly reduces birth weight (Levitt et al. 1996). However, as was previously reported (Liu et al. 2001), there was no significant effect of prenatal sGC exposure on fetal or postnatal growth in the guinea pig. In this regard, both fetal rat and sheep growth, but not postnatal growth, are highly sensitive to sGC exposure in utero (Newnham & Moss, 2001). Relative duration and timing of sGC exposure and species sensitivity are the most likely explanations for this divergence. Three courses (2 injections per course) of sGC during a guinea pig pregnancy (term = 70 days) are quite different from daily injections in the last 7 days of a 21-day pregnancy in the rat. Though there were no significant differences in body weight and brain weight, there were significant increases in hippocampal weight relative to brain and in adrenal-to-body weight ratio.
In previous studies, we had shown that prenatal exposure to sGC led to reduced basal and activated HPA function in young adult male offspring (70 days of age). This was associated with a significant increase in MR expression in the limbic system and a strong tendency for reduced CRH mRNA levels in the hypothalamic PVN (Liu et al. 2001). There was also a very significant increase in plasma testosterone concentrations in the young adult males that had been exposed to sGC in utero. We suggested that the increase in hippocampal MR expression, combined with the increase in plasma testosterone, which is known to inhibit HPA function in the rat (Viau et al. 1999), resulted in reduced HPA function. Male guinea pigs are sexually mature by approximately 8 weeks of age (Hennessy et al. 2003). Castration of male guinea pigs, rats and hamsters has been shown to increase ACTH and cortisol production as well as CRH and arginine vasopressin (AVP), while androgen replacement reversed this effect (El Hani et al. 1980; Viau & Meaney, 1991; Handa et al. 1994; McCormick et al. 1998). In the present study, we undertook more sophisticated testing of HPA function than in our previous study (Liu et al. 2001) by incorporating an additional dexamethasone suppression—CRH challenge test and hourly monitoring of plasma ACTH, cortisol and testosterone concentrations throughout the subjective day.
In the mature males, there was no overall reduction in basal or activated pituitary—adrenal function, as we had noted in earlier studies (Liu et al. 2001). A longitudinal study in the sheep has also demonstrated that prenatal sGC exposure is associated with dynamic changes in HPA activity which vary as a function of age (Sloboda et al. 2002). In the present study, the powerful effect of prenatal exposure to sGC on plasma testosterone concentrations was no longer apparent. However, the elevation in hippocampal MR expression remained. The fact that basal pituitary—adrenal function was normalized, at least in the early part of the subjective day, along with normalization of the plasma testosterone concentrations in mature adult males, would suggest that elevated testosterone was a primary mediator of the suppression of HPA function in the young adult males (Liu et al. 2001). Further, the finding that hippocampal MR remains elevated, particularly in the dentate gyrus, brings into question the role of the elevated MR in modulating changes in HPA function following prenatal exposure to sGC. In the present study, we did not measure MR binding or protein levels in individual regions of the hippocampus or dentate gyrus. However, previous studies have linked changes in MR mRNA with changes in MR binding and modification of basal HPA activity (Carey et al. 1995; de Kloet et al. 1998). Variations (25%) in MR mRNA associated with stages of the reproductive and diurnal cycles have been shown to be mirrored by changes in receptor binding in adult rats (Carey et al. 1995). It is likely that a similar relationship between mRNA and active receptors exists in the adult guinea pig.
Further detailed studies are required to probe the interaction of altered MR regulation with HPA function in this model. The hippocampal MR has other roles including cardiovascular regulation (van den Berg et al. 1990, 1994a,b) and behaviour (de Kloet et al. 1998).
In the current study, there were additional significant but subtle changes in HPA function associated with prenatal exposure to sGC. Plasma ACTH concentrations were high during the early subjective morning (lights on) in both prenatal treatment groups, levels then decreased. While ACTH concentrations remained unchanged for the remainder of the day in both groups, plasma cortisol levels showed a tendency towards an increase in the late afternoon, but only in the control offspring. The temporal difference observed might be a result of diurnal fluctuations in adrenal sensitivity (Kaneko et al. 1981; Sage et al. 2001, 2002). It is also possible that prenatal sGC treatment affects circadian rhythmicity of the HPA axis in adulthood. In the rat, it has been shown that prenatal stress affects sleep—wake patterns and wheel-running rhythms, and this is associated with increased corticosterone levels (Koehl et al. 1997, 1999; Dugovic et al. 1999). To assess possible changes in adrenal sensitivity in the present study, animals were challenged with ACTH at 13.00 h. At this time of day, there were no differences in the plasma cortisol response to ACTH injection, suggesting no difference in adrenal sensitivity between the prenatal treatment groups. However, this does not preclude the possibility that differences in sensitivity emerge later in the afternoon or that the dose of ACTH that we used did not allow differences in functional sensitivity to be established. The latter is unlikely as we have previously shown that the dose of ACTH that we used (0.5 μg kg−1) leads to submaximal stimulation of plasma cortisol concentrations in the adult guinea pig (Lingas & Matthews, 2001; Liu et al. 2001). Though assessed in tissue samples derived from animals at 09.00 h, the lack of difference in MC2-R between the prenatal treatment groups corroborates the observations following ACTH challenge. Further, the fact that adrenocortical CYP17, StAR and SF-1 mRNA levels were not different between control and mat-dex offspring would suggest that there are no fundamental differences in adrenal sensitivity to ACTH. However, it is possible that there were differences in enzyme activity, MC2-R binding and intracellular shuttling of StAR that occur independently of changes in expression in animals that were prenatally exposed to sGC. Further studies are required to address these possibilities.
Dexamethasone suppression followed by CRH challenge has been used extensively to assess HPA function in human subjects (Evans et al. 1983; Newport et al. 2004). Indeed, a reduced ability to suppress plasma cortisol concentrations following dexamethasone exposure has become a sensitive test for human depression. In the present study, though there was no overall difference in ACTH or cortisol suppression following dexamethasone treatment, the initial fall in plasma ACTH concentrations following dexamethasone treatment was of a lower magnitude in mat-dex males. CRH challenge following dexamethasone suppression activated pituitary—adrenocortical function. There was a strong tendency for an increased plasma cortisol response to CRH following dexamethasone suppression in mat-dex offspring. Also, on the day of the dexamethasone suppression, basal cortisol concentrations were significantly elevated in the 09.00 h pre-dexamethasone sample. This difference was highly significant but is difficult to reconcile given that there was no similar elevation in plasma ACTH concentrations and that animals from the different prenatal treatment groups were handled identically.
The tendency for an amplified cortisol response to CRH injection observed in offspring exposed to sGC in utero correlates with the increased pro-opiomelanocortin (POMC) mRNA levels observed in the anterior pituitary gland. However, a concurrent increase in both GR mRNA and POMC mRNA is somewhat counterintuitive. Increased glucocorticoid sensitivity at the level of the pituitary gland might be expected to decrease HPA activity, and hence, lower POMC mRNA would be expected. Nevertheless, overall POMC production in the anterior pituitary is not solely regulated by negative feedback through GRs. It is determined by a balance of the stimulatory influence of CRH and arginine vasopressin (AVP) secretion from the hypothalamus and pituitary sensitivity to CRH and AVP, in addition to POMC sensitivity to the negative glucocorticoid feedback. In this context, the different response observed between CRH and ACTH challenges in the current study, may support altered pituitary sensitivity to CRH in animals exposed to sGC in utero. Furthermore, increased levels of GR mRNA could also be partly responsible for the shortened ACTH response to CRH injection that we observed in the offspring whose mothers were treated with sGC. It should also be noted that we did not colocalize changes in GR mRNA to corticotrophs. In this regard, it is known that GRs exist in a number of different endocrine cell types in the anterior pituitary (Matthews et al. 1995). It is important to note that we currently have no information on potential differences in AVP expression in the parvocellular region of the hypothalamic PVN. In the guinea pig, a single study has shown that AVP is without effect on ACTH secretion, though it does synergize with the actions of CRH at the pituitary corticotroph (Pradier et al. 1990). Further studies are required to identify whether hypothalamic AVP (parvocellular) and its pituitary receptor, or the relationship between AVP and CRH are altered in animals prenatally exposed to sGC.
It is of interest that the levels of MR mRNA in the limbic system remain high, despite the normalization of HPA function. Given the role of the hippocampal MR in basal HPA regulation, one would predict a reduction in HPA activity. However, the functional significance of up-regulation of MR in the limbic systems and particularly the dentate gyrus (DG) remains unclear. In this connection, the limbic MR has been implicated in the regulation of cardiovascular function, including blood pressure. Intracerebroventricular injections of low concentrations of aldosterone increase, while the MR antagonist (RU28318) decreases blood pressure (van den Berg et al. 1990, 1994a,b).
An effect of increased antenatal GC exposure on adult blood pressure has been documented in a number of species (Dodic & Wintour, 1994; Levitt et al. 1996; Celsi et al. 1998; Langley-Evans & Nwagwu, 1998; Dodic et al. 1999). In the current study, there was a significant increase in MAP in offspring exposed to sGC in utero. The underlying mechanism of fetal programming of hypertension has been shown to include changes in the brain and kidney renin—angiotensin system (Dodic et al. 1998; Rosmond & Bjorntorp, 1998), altered activity and expression of GC inducible genes and enzymes (Gardner et al. 1998) and changes in vascular wall constituents and structural properties of vascular smooth muscle (Berry, 1978; Keeley & Johnson, 1987; Bendeck et al. 1994). We would suggest that the up-regulation of the hippocampal MR might also be involved in the increased blood pressure following prenatal exposure to sGC. It would be interesting to determine whether blood pressure continues to increase as the guinea pigs age.
The ability of DG to replace granule neurones in adult life and its anatomical and functional connections to entorhinal cortex and the amygdala suggest its importance for the memory of aversive and fear-producing experiences (LeDoux, 1995) and cognition (Schmidt & Meyer, 1994). In addition, acute stressful experiences have been shown to suppress the ongoing neurogenesis through regulation by adrenal steroids (Gould et al. 1997; Tanapat et al. 1998, 2001; Sapolsky, 2000; Sapolsky et al. 2000). In the rhesus monkey, fetal glucocorticoid exposure was shown to cause considerable hippocampal damage (Uno et al. 1994). However, the overall decrease in endogenous glucocorticoid exposure during early adult life in the male guinea pig following prenatal sGC exposure (Liu et al. 2001) may have influenced neurogenesis and could, at least in part, be responsible for the increased hippocampal weight that we observe in the male mat-dex offspring at 150 days of age.
In conclusion, we have shown that there are long-term effects of prenatal exposure to sGC on HPA and cardiovascular function, but these effects are different from those identified at earlier stages of adult life. Indeed, effects of prenatal sGC treatment on pituitary—adrenal activity appear to become less pronounced with age whereas those on blood pressure become more pronounced. These studies suggest that programming of these parameters does not remain constant throughout the life course and highlights the importance of identifying the impact of prenatal sGC exposure at several time points after birth. The present study also further reinforces the necessity to exercise caution in the use of repeat course prenatal sGC therapy in humans.
Acknowledgments
We would like to thank Dawn Owen, Alice Kostaki, Elaine Setiawan and Marcus Andrews for their assistance with the experiments and manuscript preparation. This work was supported by the Canadian Institutes of Health Research (MOP-49511) and the Premier's Research Excellence Award (PREA) awarded to S.G.M.
References
- Bendeck MP, Keeley FW, Langille BL. Perinatal accumulation of arterial wall constituents: relation to hemodynamic changes at birth. Am J Physiol. 1994;267:H2268–H2279. doi: 10.1152/ajpheart.1994.267.6.H2268. [DOI] [PubMed] [Google Scholar]
- Berry CL. Hypertension and arterial development. Long-term considerations. Br Heart J. 1978;40:709–717. doi: 10.1136/hrt.40.7.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst P, Gates S, McKenzie-McHarg K, Alfirevic Z, Chamberlain G. Are we prescribing multiple courses of antenatal corticosteroids? A survey of practice in the UK. Br J Obstet Gynaecol. 1999;106:977–979. doi: 10.1111/j.1471-0528.1999.tb08440.x. [DOI] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, De Koning J, Helmerhorst F, De Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Celsi G, Kistner A, Aizman R, Eklof AC, Ceccatelli S, De Santiago A, Jacobson SH. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res. 1998;44:317–322. doi: 10.1203/00006450-199809000-00009. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Dean F, Matthews SG. Maternal dexamethasone treatment in late gestation alters glucocorticoid and mineralocorticoid receptor mRNA in the fetal guinea pig brain. Brain Res. 1999;846:253–259. doi: 10.1016/s0006-8993(99)02064-8. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 1998;94:149–155. doi: 10.1042/cs0940149. [DOI] [PubMed] [Google Scholar]
- Dodic M, Peers A, Coghlan JP, May CN, Lumbers E, Yu Z, Wintour EM. Altered cardiovascular haemodynamics and baroreceptor-heart rate reflex in adult sheep after prenatal exposure to dexamethasone. Clin Sci (Lond) 1999;97:103–109. [PubMed] [Google Scholar]
- Dodic M, Wintour EM. Effects of prolonged (48 h) infusion of cortisol on blood pressure, renal function and fetal fluids in the immature ovine foetus. Clin Exp Pharmacol Physiol. 1994;21:971–980. doi: 10.1111/j.1440-1681.1994.tb02659.x. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Maccari S, Weibel L, Turek FW, Van Reeth O. High corticosterone levels in prenatally stressed rats predict persistent paradoxical sleep alterations. J Neurosci. 1999;19:8656–8664. doi: 10.1523/JNEUROSCI.19-19-08656.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hani A, Dalle M, Delost P. Role of testosterone in the sexual dimorphism of adrenal activity at puberty in the guinea-pig. J Endocrinol. 1980;87:455–461. doi: 10.1677/joe.0.0870455. [DOI] [PubMed] [Google Scholar]
- Evans DL, Burnett GB, Nemeroff CB. The dexamethasone suppression test in the clinical setting. Am J Psychiatry. 1983;140:586–589. doi: 10.1176/ajp.140.5.586. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Jackson AA, Langley-Evans SC. The effect of prenatal diet and glucocorticoids on growth and systolic blood pressure in the rat. Proc Nutr Soc. 1998;57:235–240. doi: 10.1079/pns19980037. [DOI] [PubMed] [Google Scholar]
- Go KS, Lingas R, Wheeler MB, Irwin DM, Matthews SG. Decreased CRH mRNA expression in the fetal guinea pig hypothalamus following maternal nutrient restriction. Brain Res. 2001;896:179–182. doi: 10.1016/s0006-8993(01)02089-3. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Reed J, Wilson SE, Pitstick L. Sexual interactions of maturing male guinea pigs with their mothers, sisters, and unfamiliar adult females in the home cage. Dev Psychobiol. 2003;42:91–96. doi: 10.1002/dev.10083. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Kaneko K, Shinsako J, Dallman MF. Adrenal sensitivity to adrenocorticotropin varies diurnally. Endocrinology. 1981;109:70–75. doi: 10.1210/endo-109-1-70. [DOI] [PubMed] [Google Scholar]
- Keeley FW, Johnson DJ. Age differences in the effect of hydrocortisone on the synthesis of insoluble elastin in aortic tissue of growing chicks. Connect Tissue Res. 1987;16:259–268. doi: 10.3109/03008208709006980. [DOI] [PubMed] [Google Scholar]
- Keightley MC, Curtis AJ, Chu S, Fuller PJ. Structural determinants of cortisol resistance in the guinea pig glucocorticoid receptor. Endocrinology. 1998;139:2479–2485. doi: 10.1210/endo.139.5.5982. [DOI] [PubMed] [Google Scholar]
- Koehl M, Barbazanges A, Le Moal M, Maccari S. Prenatal stress induces a phase advance of circadian corticosterone rhythm in adult rats which is prevented by postnatal stress. Brain Res. 1997;759:317–320. doi: 10.1016/s0006-8993(97)00394-6. [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40:302–315. [PubMed] [Google Scholar]
- Langley-Evans SC, Nwagwu M. Impaired growth and increased glucocorticoid-sensitive enzyme activities in tissues of rat fetuses exposed to maternal low protein diets. Life Sci. 1998;63:605–615. doi: 10.1016/s0024-3205(98)00311-7. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. In search of emotional system in the brain: leaping from fear to emotion and consciousness. In: Gazzaniga M, editor. The Cognitive Neurosciences. Cambridge, MA, USA: MIT Press; 1995. pp. 1049–1061. [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525. [PubMed] [Google Scholar]
- Lingas RI, Matthews SG. A short period of maternal nutrient restriction in late gestation modifies pituitary-adrenal function in adult guinea pig offspring. Neuroendocrinology. 2001;73:302–311. doi: 10.1159/000054647. [DOI] [PubMed] [Google Scholar]
- Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J Physiol Endocrinol Metab. 2001;280:E729–E739. doi: 10.1152/ajpendo.2001.280.5.E729. [DOI] [PubMed] [Google Scholar]
- Liu L, Matthews SG. Adrenocortical response profiles to corticotrophin-releasing hormone and adrenocorticotrophin challenge in the chronically catheterized adult guinea-pig. Exp Physiol. 1999;84:971–977. [PubMed] [Google Scholar]
- McCabe L, Marash D, Li A, Matthews SG. Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotropin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea-pig brain. J Neuroendocrinol. 2001;13:425–431. doi: 10.1046/j.1365-2826.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have ‘organizational’ effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Res Dev Brain Res. 1998;105:295–307. doi: 10.1016/s0165-3806(97)00155-7. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Dynamic changes in glucocorticoid and mineralocorticoid receptor mRNA in the developing guinea pig brain. Brain Res Dev Brain Res. 1998;107:123–132. doi: 10.1016/s0165-3806(98)00008-x. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- Matthews SG, Challis JR. Levels of pro-opiomelanocortin and prolactin mRNA in the fetal sheep pituitary following hypoxaemia and glucocorticoid treatment in late gestation. J Endocrinol. 1995a;147:139–146. doi: 10.1677/joe.0.1470139. [DOI] [PubMed] [Google Scholar]
- Matthews SG, Challis JR. Regulation of CRH and AVP mRNA in the developing ovine hypothalamus: effects of stress and glucocorticoids. Am J Physiol. 1995b;268:E1096–E1107. doi: 10.1152/ajpendo.1995.268.6.E1096. [DOI] [PubMed] [Google Scholar]
- Matthews SG, Yang K, Challis JR. Changes in glucocorticoid receptor mRNA in the developing ovine pituitary and the effects of exogenous cortisol. J Endocrinol. 1995;144:483–490. doi: 10.1677/joe.0.1440483. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Weaver S, Yau J, Chapman K, Seckl JR. Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: the effects of thyroid hormones and serotonin. J Neurosci. 2000;20:3926–3935. doi: 10.1523/JNEUROSCI.20-10-03926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnham JP, Moss TJ. Antenatal glucocorticoids and growth: single versus multiple doses in animal and human studies. Semin Neonatol. 2001;6:285–292. doi: 10.1053/siny.2001.0064. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Heim C, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biol Psychiatry. 2004;55:10–20. doi: 10.1016/s0006-3223(03)00692-9. [DOI] [PubMed] [Google Scholar]
- NIH. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. Jama. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- Nyirenda MJ, Welberg LA, Seckl JR. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids-fetal effect or maternal influence? J Endocrinol. 2001;170:653–660. doi: 10.1677/joe.0.1700653. [DOI] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144:2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Phillips D. Endocrine programming and fetal origins of adult disease. Trends Endocrinol Metab. 2002;13:363. doi: 10.1016/s1043-2760(02)00696-3. [DOI] [PubMed] [Google Scholar]
- Pradier P, Tournaire C, Dalle M. Pituitary and adrenal responses to ovine corticotropin releasing factor and vasopressin injected into young and adult guinea-pigs. J Dev Physiol. 1990;14:163–169. [PubMed] [Google Scholar]
- Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Whorwood CB, Phillips DI. Altered control of cortisol secretion in adult men with low birth weight and cardiovascular risk factors. J Clin Endocrinol Metab. 2001a;86:245–250. doi: 10.1210/jcem.86.1.7145. [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Walker BR, Syddall HE, Whorwood CB, Wood PJ, Phillips DI. Elevated plasma cortisol in glucose-intolerant men: differences in responses to glucose and habituation to venepuncture. J Clin Endocrinol Metab. 2001b;86:1149–1153. doi: 10.1210/jcem.86.3.7300. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Bjorntorp P. The interactions between hypothalamic-pituitary-adrenal axis activity, testosterone, insulin-like growth factor I and abdominal obesity with metabolism and blood pressure in men. Int J Obes Relat Metab Disord. 1998;22:1184–1196. doi: 10.1038/sj.ijo.0800745. [DOI] [PubMed] [Google Scholar]
- Sage D, Maurel D, Bosler O. Involvement of the suprachiasmatic nucleus in diurnal ACTH and corticosterone responsiveness to stress. Am J Physiol Endocrinol Metab. 2001;280:E260–E269. doi: 10.1152/ajpendo.2001.280.2.E260. [DOI] [PubMed] [Google Scholar]
- Sage D, Maurel D, Bosler O. Corticosterone-dependent driving influence of the suprachiasmatic nucleus on adrenal sensitivity to ACTH. Am J Physiol Endocrinol Metab. 2002;282:E458–E465. doi: 10.1152/ajpendo.00287.2001. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schmidt TJ, Meyer AS. Autoregulation of corticosteroid receptors. How, when, where, and why? Receptor. 1994;4:229–257. [PubMed] [Google Scholar]
- Sloboda DM, Moss TJ, Gurrin LC, Newnham JP, Challis JR. The effect of prenatal betamethasone administration on postnatal ovine hypothalamic-pituitary-adrenal function. J Endocrinol. 2002;172:71–81. doi: 10.1677/joe.0.1720071. [DOI] [PubMed] [Google Scholar]
- Smith GN, Kingdom JC, Penning DH, Matthews SG. Antenatal corticosteroids: is more better? Lancet. 2000;355:251–252. doi: 10.1016/s0140-6736(99)00448-1. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Galea LA, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int J Dev Neurosci. 1998;16:235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- van den Berg DT, de Jong W, de Kloet ER. Mineralocorticoid antagonist inhibits stress-induced blood pressure response after repeated daily warming. Am J Physiol. 1994a;267:E921–E926. doi: 10.1152/ajpendo.1994.267.6.E921. [DOI] [PubMed] [Google Scholar]
- van den Berg DT, de Kloet ER, de Jong W. Central effects of mineralocorticoid antagonist RU-28318 on blood pressure of DOCA-salt hypertensive rats. Am J Physiol. 1994b;267:E927–E933. doi: 10.1152/ajpendo.1994.267.6.E927. [DOI] [PubMed] [Google Scholar]
- van den Berg DT, de Kloet ER, van Dijken HH, de Jong W. Differential central effects of mineralocorticoid and glucocorticoid agonists and antagonists on blood pressure. Endocrinology. 1990;126:118–124. doi: 10.1210/endo-126-1-118. [DOI] [PubMed] [Google Scholar]
- Viau V, Chu A, Soriano L, Dallman MF. Independent and overlapping effects of corticosterone and testosterone on corticotropin-releasing hormone and arginine vasopressin mRNA expression in the paraventricular nucleus of the hypothalamus and stress-induced adrenocorticotropic hormone release. J Neurosci. 1999;19:6684–6693. doi: 10.1523/JNEUROSCI.19-15-06684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]