Abstract
The present study was undertaken to determine whether activity-dependent changes in axonal excitability are greater in motor axons than cutaneous afferents for the same impulse load. In nine healthy subjects, supramaximal stimulation at 8 Hz was delivered to the median nerve at the wrist. Changes in the threshold current required to generate compound motor and sensory potentials ∼50% of maximum and other indices of axonal excitability were tracked before and after repetitive stimulation for 10 min. The long-lasting stimulation produced a prolonged depression in the excitability of both cutaneous afferents and motor axons, with gradual recovery to control levels over 15–20 min. These changes in threshold were associated with a reduction in refractoriness, an increase in supernormality and a decrease in the strength–duration time constant, changes consistent with axonal hyperpolarization. Greater changes in threshold occurred in motor axons: threshold increased by 9.9% and 16.4% for test stimulus durations of 0.1 and 1 ms, respectively, for motor axons and by 5.4% and 8.3% for cutaneous afferents. With higher stimulus frequencies and thereby greater impulse loads, greater threshold changes could be induced in cutaneous afferents. It is argued that the hyperpolarization resulted from activity of the electrogenic Na+−K+ pump, that it requires > 125 ms to restore the resting state following an action potential, and that significant intracellular Na+ accumulation occurs during a steady 8-Hz train. These findings imply that physiological discharge rates will activate the pump and thereby produce axonal hyperpolarization, the extent of which will vary with impulse load. A plausible explanation is that greater activity-dependent hyperpolarization in motor axons is due to less inward rectification as a result of less activity of the hyperpolarization-activated cation conductance (IH) than in cutaneous afferents.
While neurological diseases commonly affect both sensory and motor axons, the resultant clinical features often imply more selective involvement of either cutaneous afferents or motor axons. The cause of this selectivity may be a targeted immune attack against axonal components specific to the type of axon. Often, however, there is little pathological evidence for selective nerve involvement, raising the possibility that the function of the axons has been affected more than their structure. A number of differences in the behaviour of cutaneous afferents and motor axons have been described, some reflecting differences in the function of specific ion channels (Bostock et al. 1994, 1998; Kiernan et al. 1996, 2001; Bostock & Rothwell, 1997; Burke et al. 1997; Mogyoros et al. 1997; Vagg et al. 1998; Kiernan & Bostock, 2000; Kiernan, 2002; Lin et al. 2002).
Conduction of impulses, either individually or in trains, can produce long-lasting effects on nerve excitability. In the case of prolonged impulse trains, the most prominent change is the development of axonal hyperpolarization, which presumably occurs due to activation of the electrogenic Na+−K+ pump (Gasser, 1935; Bergmans, 1970, 1982; Bostock & Grafe, 1985; Applegate & Burke, 1989; Morita et al. 1993; Bostock & Bergmans, 1994; Kiernan et al. 1997a,b; Burke et al. 2001; Kiernan, 2002). During activity Na+ ions accumulate inside the active axons, a process that drives the Na+−K+ pump, and its activity continues when the impulse train has ceased. The Na+−K+ pump exchanges three Na+ ions for two K+ ions, and the resulting ionic imbalance inevitably leads to axonal hyperpolarization. Using high-frequency electrical stimulation, evidence for activity-dependent hyperpolarization has been produced for human cutaneous afferents (Applegate & Burke, 1989; Kiernan et al. 1997a,b) and a single motor axon (Bostock & Bergmans, 1994). In addition, hyperpolarization can be produced in human motor axons due to the natural activity associated with a maximal voluntary contraction (Vagg et al. 1998). The extent and duration of hyperpolarization depend on both discharge rate and train length, i.e. on the impulse load on the axon (e.g. Morita et al. 1993; Bostock & Bergmans, 1994). Maximal voluntary contractions for as little as 15 s can reduce the excitability of motor axons for 5–10 min; contractions for 1 min produce greater threshold changes lasting 15–20 min or more (Vagg et al. 1998). This normal physiological response has been shown to be of sufficient magnitude to induce conduction failure in motor axons in demyelinating neuropathies (Cappelen-Smith et al. 2000; Kaji et al. 2000).
There is evidence for greater activity of the hyperpolarization-activated cation conductance (IH) in human cutaneous afferents than motor axons (Bostock et al. 1994; Lin et al. 2002), and this has been invoked to explain differences in their postischaemic behaviour. The physiological role of this accommodative conductance could be to limit the extent of hyperpolarization that occurs with activity, so limiting any tendency for conduction block at axonal branch points or sites where pathology has impaired the safety margin for impulse conduction (Pape, 1996). Given this difference in IH, one might expect that, for the same impulse load, the extent of hyperpolarization would be greater for motor axons than cutaneous afferents. The major aim of the present study was to compare the excitability changes produced in human cutaneous afferents and motor axons following prolonged repetitive stimulation that imposed an identical impulse load on the axons.
Methods
Recordings were undertaken on nine healthy subjects (aged 25–58 years; 7 male, 2 female), none of whom suffered from a peripheral nerve disorder or a systemic disease affecting peripheral nerve function. The volunteers gave informed written consent to the experimental procedures which had the approval of the Committee on Experimental Procedures Involving Human Subjects of the University of New South Wales. The study was in accordance with the Declaration of Helsinki.
In all studies, axonal excitability was measured for cutaneous afferents and motor axons by stimulating the median nerve at the wrist. The resultant antidromic compound sensory action potential (CSAP) was recorded from the index finger using ring electrodes. The orthodromic compound muscle action potential (CMAP) was recorded from abductor pollicis brevis using surface electrodes, with the active electrode at the motor point and the reference on the proximal phalanx.
The technique of ‘threshold tracking’ was used to measure the changes in excitability of motor and cutaneous afferent axons at the wrist following long stimulus trains, as previously described (see Bostock et al. 1998; Burke et al. 2001). The EMG and neurogram were amplified (gains 1000 and 50 000), filtered (bandwidths 1.6 Hz to 2 or 5 kHz) and digitized by computer (Pentium PC) with A/D board (DT2812, Data Translation Inc., MA, USA), using a sampling rate of 10 kHz. Stimulus waveforms generated by the computer were converted to current with a purpose-built isolated linear bipolar constant current source (maximal output ± 50 mA). Stimulus currents were applied via non-polarizable electrodes (Red Dot, 3M Health Care, Germany). Stimulation and recording were controlled by software written in BASIC (QTRAC version 4.3, © Professor H. Bostock, Institute of Neurology, University College London).
Different test stimuli were delivered at a rate of 2 Hz, either alone or preceded by a conditioning stimulus, rotating through a sequence of 10 different test stimuli, five for sensory and five for motor. A fixed supramaximal stimulus of 0.2 ms duration was delivered on channels 1 and 6 to produce a CSAP or CMAP of maximal amplitude. On channels 2–5 (for cutaneous afferents) and 7–10 (for motor axons), the threshold current required to elicit a CSAP/CMAP 50% of the maximal amplitude was tracked by the computer using ‘proportional’ tracking (Bostock et al. 1998). With proportional tracking, the change in stimulus current is proportional to the error between the recorded response and the target CSAP/CMAP (50% of maximal). The windows for the target potentials on channels 2–5 and 7–10 were referenced to the maximal potentials on channels 1 and 6, respectively. This ensured that the test potentials remained 50% of maximum despite changes in amplitude due to dispersion of the compound responses induced by the 10-min repetitive stimulus trains.
Unconditioned test stimuli of 0.1 ms and 1 ms duration tracking the 50% target were delivered on channels 2 and 3 (and channels 7 and 8), respectively. The strength—duration time constant (τSD) was calculated off-line from the thresholds recorded from channels 2 and 3 (and channels 7 and 8) according to Weiss's Law, which postulates a linear relationship between stimulus charge and stimulus duration (see Bostock & Bergmans, 1994; Mogyoros et al. 1996, 1997; Kiernan et al. 1997a,b; Bostock et al. 1998; Grosskreutz et al. 1999; Burke et al. 2001).
Channels 4 and 5 (or channels 9 and 10) were used to follow refractoriness and supernormality. Supramaximal conditioning stimuli of 0.2 ms duration, identical to those on channel 1 (or channel 6), preceded test stimuli of 0.1 ms duration tracking the 50% target potential. The conditioning—test intervals were 2 and 7 ms, respectively. For these recordings, the conditioned response was measured after on-line subtraction of the maximal CSAP (or CMAP) produced by the conditioning stimulus. Refractoriness is a measure of the decrease in excitability that occurs during the relatively refractory period following transmission of a nerve impulse, and was calculated off-line as the percentage increase in current required to produce the target CSAP/CMAP when a conditioning stimulus preceded the test stimulus by 2 ms. Supernormality was calculated as the percentage reduction in current required to produce the target potential when the conditioning—test interval was 7 ms, the interval at which supernormality is normally maximal (Kiernan et al. 1996). Both refractoriness and supernormality were normalized to their unconditioned controls.
The above excitability indices were followed for at least 5 min before repetitive stimulation for 10 min, and their recovery was followed for 35 min after the end of the 10-min stimulus train.
The amplitude of the CSAP was measured from peak to peak, and that of the CMAP from baseline to negative peak. Post-train changes in axonal excitability were initially compared in motor and sensory nerves following trains of stimuli delivered to the wrist at 8 Hz for 10 min. Each stimulus in the train was 1 ms in duration, and exceeded that required to produce the maximal CMAP or CSAP by 40%, whichever was greater. To minimize discomfort and inadvertent movement, stimulus intensity was first set using a stimulus rate of 1 Hz, and the rate was then increased over 60 s to 8 Hz. In separate studies, the digital nerves of the index finger were stimulated at frequencies of 20 Hz and 30 Hz for 10 min using supramaximal stimuli of duration 1 ms. These trains were delivered to the finger because that is less painful than similar trains at the wrist. In these experiments excitability was still measured at the wrist, as in other experiments, by reversing the stimulating and recording electrodes so that the antidromic CSAP was recorded from the digital nerves.
In all experiments skin temperature was monitored continuously near the stimulus site and was maintained at > 32°C by wrapping the limbs in blankets and applying radiant heat when necessary. All data are expressed as mean ± s.e.m.
Results
Excitability parameters were recorded for cutaneous afferents and motor axons at rest, prior to repetitive stimulation. The strength—duration time constant (τSD) was longer for cutaneous afferents of the median nerve (0.53 ± 0.03 ms) than for motor axons (0.44 ± 0.03 ms; mean ± s.e.m), consistent with previous studies (Mogyoros et al. 1996; Bostock & Rothwell, 1997). Refractoriness was similar for cutaneous afferents and motor axons (cutaneous afferents 35.8 ± 9.7%; motor 31 ± 6.8%; mean ± s.e.m), while supernormality was significantly greater for motor axons (cutaneous afferents 14.2 ± 1.6%; motor 20.7 ± 1.5%; mean ± s.e.m), the latter consistent with previous studies (Kiernan et al. 1996).
The basic experiment involved comparing changes in the above measures before and after repetitive stimulation of the median nerve at the wrist at 8 Hz for 10 min. This stimulus rate was chosen for three reasons. (i) With such rates there should be no failure of impulse conduction in the axon or its distal terminals, at axonal branch points, at the neuromuscular junction or in the muscle fibre. (ii) Any decrease in excitability would not be attributable to cumulative effects of mechanisms responsible for the ‘recovery cycle’ (particularly slow K+ conductances) because, for human nerves, the recovery of excitability following single impulses or brief trains is complete within ∼100 ms (Bergmans, 1970; Kiernan et al. 1996; Lin et al. 2000; Kiernan, 2002). (iii) In voluntary contractions, motor axons can maintain a tonic discharge at this frequency or even higher — i.e. the chosen rate is ‘physiological’.
Over the first minute after the stimulus train (8 Hz for 10 min), the latency of the maximal CMAP was increased by, on average, 0.02 ms (1.2%) and its amplitude was reduced to 92.9% of the pre-train value. For the maximal CSAP, latency was prolonged by 0.5 ms (1.6%) and the amplitude reduced to 92.3% of control. The latency prolongations reflect the hyperpolarization documented below. The amplitude changes reflect dispersion of the maximal potentials, an issue addressed in the Discussion.
Comparison of threshold changes induced by repetitive stimulation in cutaneous afferents and motor axons
Repetitive stimulation at 8 Hz for 10 min caused significant changes in nerve excitability for both cutaneous afferents and motor axons. To enable comparisons of the effects on cutaneous afferents and motor axons in different subjects, threshold changes were normalized to the control level established prior to commencement of repetitive stimulation.
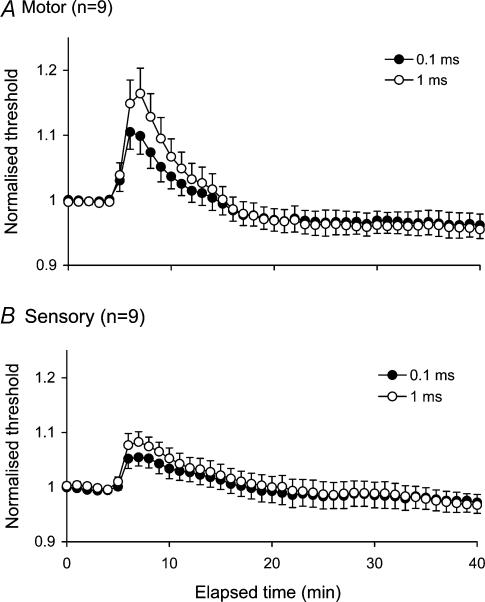
Following impulse trains delivered at 8 Hz for 10 min, axons became less excitable than before the train. As a result there was a prominent increase in the threshold current required to generate the target CSAP or CMAP (50% of maximum; Fig. 1). The delays inherent in threshold tracking have introduced a distortion in Figs 1 and 2 where the threshold changes appear to require a number of stimulus cycles to reach their maxima, whereas they were, in all probability, maximal immediately the train ended. There were clear quantitative differences in the extent of the threshold changes for cutaneous afferents and motor axons. The increases in threshold were significantly greater for motor axons than for cutaneous afferents (threshold increases of 9.9 ± 2.8% and 16.4 ± 3.9% for motor axons using unconditioned test stimuli of duration 0.1 and 1 ms, respectively; 5.4 ± 1.6% and 8.3 ± 1.9% for cutaneous afferents; P < 0.05 for both, Student's t test for paired data). Thresholds slowly returned to the control level over 15–20 min.
Figure 1. Activity-dependent changes in threshold for motor and sensory axons.
Mean changes in threshold (± s.e.m) recorded for 9 subjects following repetitive stimulation of the median nerve at the wrist at 8 Hz for 10 min. Changes are shown for motor (A) and sensory axons (B) using test stimuli of 0.1 and 1 ms duration. Immediately following cessation of impulse trains, axons became less excitable, with a prominent increase in threshold, significantly greater for motor axons when compared to sensory.
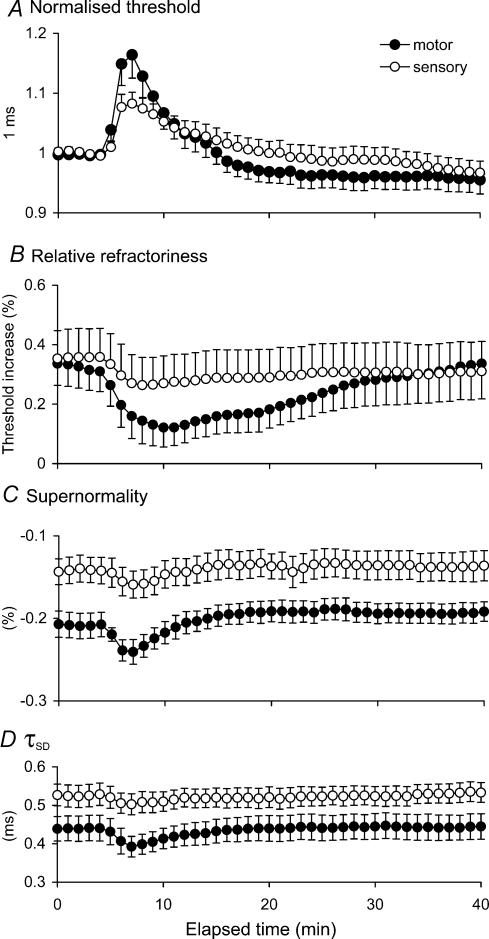
Figure 2. Comparison of changes in threshold and excitability indices for motor and sensory axons during and after repetitive stimulation.
A, mean data (± s.e.m) for 9 subjects recorded following repetitive stimulation of the median nerve at the wrist at 8 Hz for 10 min. Threshold change for a 1.0 ms test stimulus normalized to resting levels for motor (•) and sensory (○) axons following repetitive stimulation. B and C, relative refractoriness and supernormality expressed as the change in threshold for the test potential as a percentage of the unconditioned threshold. D, τSD calculated off-line from the thresholds measured using test stimuli of 0.1 and 1 ms duration.
Relationships between different indices of excitability and threshold following activity
The changes in threshold induced by repetitive stimulation were associated with appropriate changes in other indices of axonal excitability. Refractoriness decreased following activity, with a greater decrease for motor axons than cutaneous afferents (motor by 18.9%, cutaneous afferents by 9.4%; Fig. 2B); supernormality increased (motor by 3.3%, cutaneous afferents by 1.7%; Fig. 2C); and strength—duration time constant (τSD) decreased (motor by 4.1%, cutaneous afferents by 2.5%; Fig. 2D). The changes in these indices are qualitatively those expected for axonal hyperpolarization.
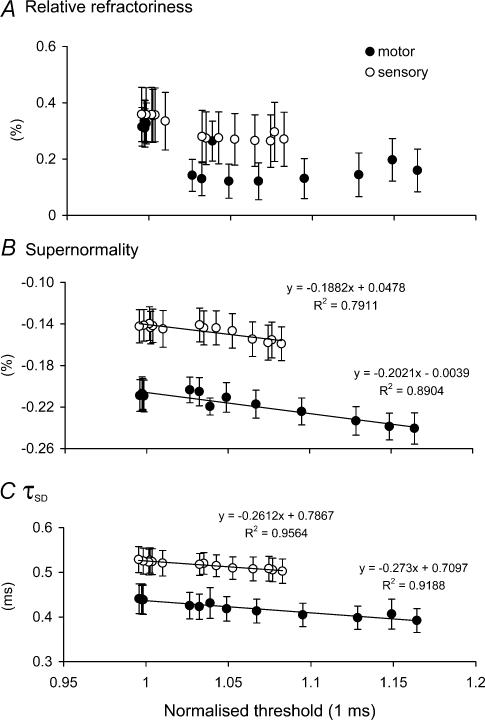
Threshold may be used as an indicator of membrane potential to establish whether the lesser changes in refractoriness, supernormality and τSD for cutaneous afferents were appropriate for the smaller threshold increase for those axons. Figure 3 illustrates the relationships between refractoriness, supernormality and τSD and threshold (as a surrogate for membrane potential). There was little difference in the relationships for motor axons and cutaneous afferents during recovery from activity, apart from the vertical shift due to differences in resting values. It is concluded (i) that the threshold changes are due to axonal hyperpolarization, and (ii) that the lesser changes in refractoriness, supernormality and τSD for cutaneous afferents than motor axons following activity can be attributed to the lesser activity-dependent hyperpolarization for the motor axons.
Figure 3. Relationships between normalized threshold and relative refractoriness, supernormality and τSD for motor and sensory axons following repetitive stimulation.
Mean data from Fig. 2 are plotted against normalized threshold (from Fig. 2A) for relative refractoriness (A), supernormality (B) and τSD (C) for motor (•) and sensory (○) axons following repetitive activity.
Effects of impulse load
To determine whether some property prevented a greater threshold change in cutaneous afferents (i.e. whether a higher impulse load would produce greater threshold increases than seen with motor axons), stimulus trains of higher frequency were delivered to cutaneous afferent axons in the digital nerves of the index finger. Excitability was tested at the wrist, as in the preceding experiments, to ensure that valid comparisons could be made with the preceding data.
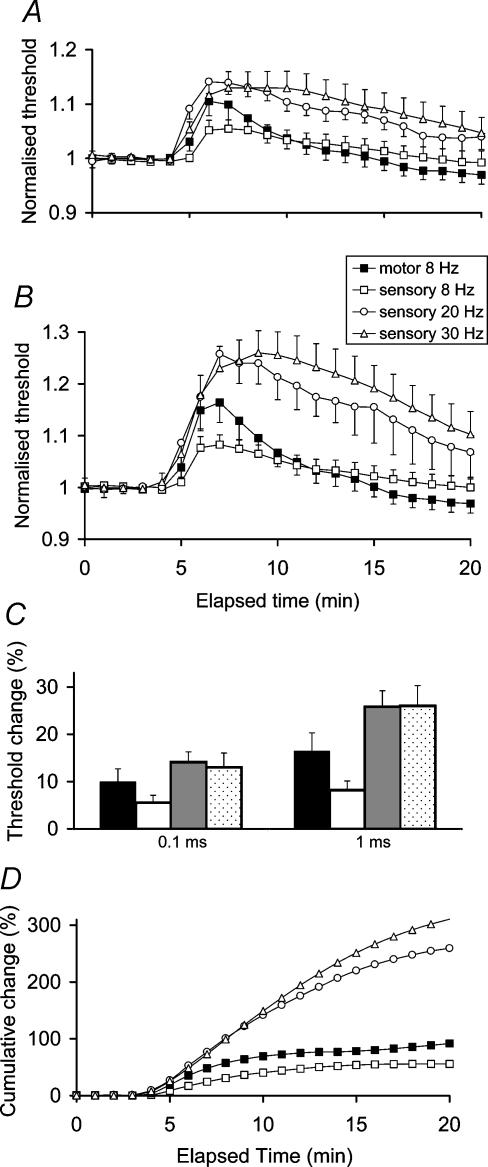
Stimulus trains at 30 Hz for 10 min produced a much larger increase in threshold of cutaneous afferents than had occurred with stimulus trains at 8 Hz for 10 min. The threshold changes were also of greater magnitude than produced by 8 Hz in motor axons (threshold increases of 13.0 ± 3.0% and 26.0 ± 4.3% for cutaneous afferents for stimulus durations of 0.1 ms and 1 ms, respectively, compared to the 9.9 ± 2.8% and 16.4 ± 3.9% for motor axons discharging at 8 Hz). In addition there was a longer time course for recovery (Fig. 4). The effects of a 20-Hz stimulus frequency were similar to those of 30 Hz for cutaneous afferents (threshold increase of 14.1 ± 2.2% and 25.8 ± 3.4% for test durations of 0.1 and 1 ms, respectively), again with a longer time course for recovery relative to stimulation at 8 Hz.
Figure 4. The effects of impulse load on axonal excitability.
Comparison of threshold changes for different rates of repetitive stimulation at 8 Hz, 20 Hz and 30 Hz in sensory axons with 8 Hz stimulation in motor axons for test stimulus durations of 0.1 ms (A) and 1.0 ms (B); peak threshold change again for test stimulus durations of 0.1 ms and 1.0 ms with data from Fig. 4A and B for motor at 8 Hz (black column) and sensory axons of progressive frequencies (C); and the cumulative threshold changes in area for 1 ms test stimuli from Fig. 4B (D).
These studies establish that the magnitude of the activity-dependent hyperpolarization induced by repetitive stimulation is related to impulse load but that the relationship may reach a plateau for cutaneous afferents at 20–30 Hz.
Discussion
The present study has demonstrated that cutaneous afferents and motor axons undergo qualitatively similar activity-dependent decreases in excitability following prolonged repetitive stimulation, but that for the same impulse load these changes are quantitatively greater in motor axons. The greater increase in threshold observed for motor axons resulted in appropriately greater changes in other indices of axonal excitability (refractoriness, supernormality and τSD). Greater threshold change could be induced in cutaneous afferents by increasing the frequency of the stimulus train, thereby increasing the impulse load imposed on the axons.
Choice of stimulus rate and train duration
As mentioned earlier, a number of reasons dictated the choice of the stimulus rate of 8 Hz. First, higher-frequency stimulation of the whole median nerve at the wrist is more painful, and this would make it more difficult for naive volunteers to relax. Secondly, the stimulus frequency should be one that does not jeopardise conduction in the distal axon, at branch points, at the neuromuscular junction or along the muscle fibre membrane. Impairment of conduction at these sites would affect conditioned potentials more than unconditioned. This would have increased the extent of refractoriness (see Kuwabara et al. 2002) and possibly have reduced the extent of supernormality, inappropriately so for the threshold change and in comparison with the neural volley. The data in Fig. 3 indicate that this was not a problem.
Inevitably, there were still some changes in the CMAP, presumably due to slowing of the propagation velocity of the muscle fibre action potential, and in the CSAP, presumably due to dispersion and phase cancellation associated with different degrees of slowing in cutaneous afferents. However, the effects of these changes were controlled by referencing the test potential every stimulus cycle to the current maximal CMAP (see Methods), so that the test potential always remained 50% of maximum. Had this not been done, a fixed target window based on the pre-train amplitude would have represented a greater percentage of maximum, and this of itself would have meant that the threshold current was erroneously too high.
Thirdly, the stimulus rate in the train should ideally be one that is ‘physiologically relevant’ (even if the train duration was not). It would then be easier to draw clinical implications from the findings. In this respect, 8 Hz is an appropriate frequency for motor axons which commonly maintain rates of 8–12 Hz during tonic contractions, particularly in postural muscles. However, this rate is less relevant for cutaneous afferents and, in any case, only slowly adapting mechanoreceptive afferents will maintain a tonic discharge.
Finally, the mechanisms underlying any threshold increase would be easier to interpret (see below) if the interstimulus interval was significantly greater than 100 ms.
It must be conceded that axons do not normally maintain a steady discharge for 10 min (except perhaps in some postural muscles, and even in them there will be fluctuations in discharge frequency). The train duration was chosen to avoid an effect that was too small to produce unequivocal changes in threshold. Lesser train durations would presumably have produced lesser threshold increases, but these might still have a significant effect on impulse conduction in peripheral nerve disorders if the safety margin for impulse conduction is sufficiently impaired by the pathology (see below, Clinical implications). Either way, the findings imply that ionic homeostasis cannot be restored within 125 ms, as discussed further below.
Mechanisms of the activity-dependent hyperpolarization
Two main mechanisms are believed to be responsible for the hyperpolarization that follows activity: activation of slow K+ conductances and activation of the electrogenic Na+−K+ pump. The former is mainly responsible for ‘H1’, the hyperpolarization that follows brief trains of impulses (Bergmans, 1970), analogous to the ‘P1’ afterpotential recorded by Gasser, (1935). The effects of this hyperpolarization have been documented for human nerve (e.g. Bergmans, 1970; Bostock & Bergmans, 1994; Miller et al. 1995; Lin et al. 2000). H1 is probably due to the cumulative effects of the late subnormal phase of the recovery of excitability following a single discharge; it is maximal with trains of 10–20 impulses, and like the late subnormal phase of the recovery cycle, it lasts only about 100 ms after the end of the stimulus train. The threshold increase produced by H1 has an exponential decay, consistent with its dependence on a single dominant mechanism (Lin et al. 2000).
With an 8-Hz train, it is unlikely that the mechanisms underlying the late subnormal phase and H1 contribute significantly to the activity-dependent hyperpolarization. Instead, it is likely that the hyperpolarization resulted from activation of the electrogenic Na+−K+ pump to correct the intracellular Na+ accumulation that occurs when an axon conducts trains of impulses (Bostock & Grafe, 1985; Morita et al. 1993). This does not mean that there are no other processes contributing to the change in membrane potential: e.g. at the peak of the action potential there may be reversed action of the Na+/Ca2+ exchanger (Tatsumi & Katayama, 1995; Kiernan et al. 1997b); Na+-gated K+ channels are co-located with Na+ channels and would have a hyperpolarizing action, driven by a high intracellular Na+ concentration (Koh et al. 1994; Vogel & Schwarz, 1995); and ATP-dependent K+ channels might be affected if the impulse train depleted ATP stores (Jonas et al. 1991; Vogel & Schwarz, 1995). Nevertheless, in animal experiments the hyperpolarization is best explained by pump activity, and this is probably the case in the present experiments.
Whether pump activity is the exclusive mechanism or not, the implication of the present findings is that 125 ms is insufficient to clear the post-spike Na+ influx. The Na+−K+ pump is active at rest, and contributes ∼10–15 mV to membrane potential (Grafe et al. 1994). Whether single impulses alter pump activity significantly is not known, but it has been suggested to play a role in the late subnormal phase of recovery of excitability following a single discharge (Stys & Waxman, 1994). Either way, the ion imbalance and the restorative mechanisms designed to correct it produce too small a change in excitability for effects to be demonstrated following single impulses or brief trains using currently available methods. The implication of the present study is that Na+ balance is not restored within 125 ms, and that an 8-Hz train will produce a cumulative imbalance that triggers restorative processes that result in hyperpolarization.
Reasons for the difference in activity-dependent hyperpolarization
There are a number of biophysical differences between human cutaneous afferents and motor axons (Bostock et al. 1998; Burke et al. 2001). The most relevant in hyperpolarized axons is the greater accommodation of cutaneous afferents to hyperpolarizing stimuli, probably due to a difference in the activity of IH (Bostock et al. 1994; Lin et al. 2002). There are appropriate differences in post-ischaemic hyperpolarization in cutaneous afferents and motor axons, in threshold electrotonus and in accommodation to long-lasting polarizing currents. In the modelling studies of Bostock et al. (1994), the differences in behaviour of cutaneous afferents and motor axons could be explained if the activity of IH in cutaneous afferents was twice that in motor axons. The present results might therefore have been expected if repetitive stimulation at 8 Hz could produce sufficient hyperpolarization.
There are alternative explanations for the differences in activity-dependent hyperpolarization. For example, greater activity of the Na+−K+ pump in motor axons would be expected to produce greater hyperpolarization. Pump activity can vary in different tissues dependent on cellular factors and physiological stimuli to which they are subjected (Therien & Blostein, 2000). Accordingly differences in pump activity would be expected if the discharge patterns and discharge rates of sensory and motor axons were different during normal function, as they probably are. The rates used in the present study may not have approached pump capacity for sensory axons. There are no definitive data for differences in Na+−K+ pump distribution or function in sensory and motor axons although indirect measures suggest if anything, that sensory axons may have a greater dependence on pump activity than motor axons to maintain resting membrane potential (Lin et al. 2002).
Clinical implications
The present study shows that modest discharge frequencies, well within those associated with normal function, can cause significant hyperpolarization of the active axons, and that the extent of hyperpolarization is greater for motor axons for the same impulse load. Bostock & Grafe (1985) found that rat nerve fibres with a reduced safety margin for conduction due to focal demyelination could be blocked by conduction of trains of impulses at rates within the physiological range. Natural activity can precipitate reversible conduction failure at sites of injury in single human axons studied using microneurography (Inglis et al. 1998). In patients with inflammatory demyelinating polyneuropathies, such as multifocal motor neuropathy and chronic inflammatory demyelinating polyneuropathy (CIDP), activity-dependent hyperpolarization can precipitate conduction failure at sites of impaired function (Cappelen-Smith et al. 2000; Kaji et al. 2000). In CIDP, it was estimated that significant conduction failure would occur if the axons hyperpolarized by ∼14% (Cappelen-Smith et al. 2000), the magnitude of change demonstrated for motor axons following tetanic stimulation at 8 Hz. It is of some interest that the clinical manifestations of acute and chronic inflammatory neuropathies are commonly predominantly motor, and this is consistent with the view that motor axons have a greater tendency to undergo conduction block in these diseases, perhaps because they accommodate less well to hyperpolarization.
Acknowledgments
Grant support from the NHMRC and Ramaciotti Foundation is gratefully acknowledged.
References
- Applegate C, Burke D. Changes in excitability of human cutaneous afferents following prolonged high-frequency stimulation. Brain. 1989;112:147–164. doi: 10.1093/brain/112.1.147. [DOI] [PubMed] [Google Scholar]
- Bergmans J. The Physiology of Single Human Nerve Fibres. Louvain Belgium: Vander; 1970. [Google Scholar]
- Bergmans J. Repetitive activity induced in single human motor axons: a model for pathological repetitive activity. In: Culp WJ, Ochoa J, editors. Abnormal Nerves and Muscles as Impulse Generators. Oxford: Oxford University Press; 1982. pp. 393–418. [Google Scholar]
- Bostock H, Bergmans J. Post-tetanic excitability changes and ectopic discharges in a human motor axon. Brain. 1994;117:913–928. doi: 10.1093/brain/117.5.913. [DOI] [PubMed] [Google Scholar]
- Bostock H, Burke D, Hales JP. Differences in behaviour of sensory and motor axons following release of ischaemia. Brain. 1994;117:225–234. doi: 10.1093/brain/117.2.225. [DOI] [PubMed] [Google Scholar]
- Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve. 1998;21:137–158. doi: 10.1002/(sici)1097-4598(199802)21:2<137::aid-mus1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bostock H, Grafe P. Activity-dependent excitability changes in normal and demyelinated rat spinal root axons. J Physiol. 1985;365:239–257. doi: 10.1113/jphysiol.1985.sp015769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Rothwell JC. Latent addition in motor and sensory fibres of human peripheral nerve. J Physiol. 1997;498:277–294. doi: 10.1113/jphysiol.1997.sp021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Kiernan MC, Bostock H. Excitability of human axons. Clin Neurophysiol. 2001;112:1575–1585. doi: 10.1016/s1388-2457(01)00595-8. [DOI] [PubMed] [Google Scholar]
- Burke D, Kiernan MC, Mogyoros I, Bostock H. Susceptibility to conduction block: differences in the biophysical properties of cutaneous afferents and motor axons. In: Kimura J, Kaji R, editors. Physiology of ALS and Related Disorders. Amsterdam: Elsevier; 1997. pp. 43–53. [Google Scholar]
- Cappelen-Smith C, Kuwabara S, Lin CS-Y, Mogyoros I, Burke D. Activity-dependent hyperpolarization and conduction block in chronic inflammatory demyelinating polyneuropathy. Ann Neurol. 2000;48:826–832. [PubMed] [Google Scholar]
- Gasser HS. Changes in nerve potentials produced by rapidly repeated stimuli and their relation to the responsiveness of nerve to stimulation. Am J Physiol. 1935;111:35–50. [Google Scholar]
- Grafe P, Bostock H, Schneider U. The effects of hyperglycaemic hypoxia on rectification in rat dorsal root axons. J Physiol. 1994;480:297–307. doi: 10.1113/jphysiol.1994.sp020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskreutz J, Lin C, Mogyoros I, Burke D. Changes in excitability indices of cutaneous afferents produced by ischaemia in human subjects. J Physiol. 1999;518:301–314. doi: 10.1111/j.1469-7793.1999.0301r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis JT, Leeper JB, Wilson LR, Gandevia SC, Burke D. The development of conduction block in single human axons following a focal nerve injury. J Physiol. 1998;513:127–133. doi: 10.1111/j.1469-7793.1998.127by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Koh D, Kampe K, Hermsteiner M, Vogel W. ATP-sensitive and Ca-activated K+ channels in vertebrate axons. novel links between metabolism and excitability. Pflugers Arch. 1991;418:68–73. doi: 10.1007/BF00370453. [DOI] [PubMed] [Google Scholar]
- Kaji R, Bostock H, Kohara N, Murase N, Kimura J, Shibasaki H. Activity-dependent conduction block in multifocal motor neuropathy. Brain. 2000;123:1602–1611. doi: 10.1093/brain/123.8.1602. [DOI] [PubMed] [Google Scholar]
- Kiernan MC. Pathophysiology of paraesthesiae. In: Reisen RC, Nuwer MR, Hallett M, Medina C, editors. Advances in Clinical Neurophysiology. Amsterdam: Elsevier Science; 2002. pp. 156–162. [Google Scholar]
- Kiernan MC, Bostock H. Effects of membrane polarization and ischaemia on the excitability properties of human motor axons. Brain. 2000;123:2542–2551. doi: 10.1093/brain/123.12.2542. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Hales JP, Gracies J-M, Mogyoros I, Burke D. Paraesthesiae induced by prolonged high frequency stimulation of human cutaneous afferents. J Physiol. 1997a;501:461–471. doi: 10.1111/j.1469-7793.1997.461bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Lin CS, Andersen KV, Bostock H. Clinical evaluation of excitability measures in sensory nerve. Muscle Nerve. 2001;24:883–893. doi: 10.1002/mus.1085. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Mogyoros I, Burke D. Differences in the recovery of excitability in sensory and motor axons of human median nerve. Brain. 1996;119:1099–1105. doi: 10.1093/brain/119.4.1099. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Mogyoros I, Hales JP, Gracies JM, Burke D. Excitability changes in human cutaneous afferents induced by prolonged repetitive axonal activity. J Physiol. 1997b;500:255–264. doi: 10.1113/jphysiol.1997.sp022015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DS, Jonas P, Vogel W. Na+-activated K+ channels localised in the nodal region of myelinated axons of Xenopus. J Physiol. 1994;479:183–197. doi: 10.1113/jphysiol.1994.sp020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara S, Ogawara K, Sung J-Y, Mori M, Kanai K, Hattori T, Yuki N, Lin CS-Y, Burke D, Bostock H. Differences in membrane properties of axonal and demyelinating Guillain—Barré syndromes. Ann Neurol. 2002;52:180–187. doi: 10.1002/ana.10275. [DOI] [PubMed] [Google Scholar]
- Lin CS-Y, Kuwabara S, Cappelen-Smith C, Burke D. Responses of human sensory and motor axons to the release of ischaemia and to hyperpolarizing currents. J Physiol. 2002;541:1025–1039. doi: 10.1113/jphysiol.2002.017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS-Y, Mogyoros I, Burke D. Recovery of excitability of cutaneous afferents in the median and sural nerves following activity. Muscle Nerve. 2000;23:763–770. doi: 10.1002/(sici)1097-4598(200005)23:5<763::aid-mus14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Miller TA, Kiernan MC, Mogyoros I, Burke D. Activity-dependent changes in impulse conduction in focal nerve lesion. Brain. 1995;118:1217–1224. doi: 10.1093/brain/118.5.1217. [DOI] [PubMed] [Google Scholar]
- Mogyoros I, Kiernan MC, Burke D. Strength-duration properties of human peripheral nerve. Brain. 1996;119:439–447. doi: 10.1093/brain/119.2.439. [DOI] [PubMed] [Google Scholar]
- Mogyoros I, Kiernan MC, Burke D, Bostock H. Excitability changes in human sensory and motor axons during hyperventilation and ischaemia. Brain. 1997;120:317–325. doi: 10.1093/brain/120.2.317. [DOI] [PubMed] [Google Scholar]
- Morita K, David G, Barrett JN, Barrett EF. Posttetanic hyperpolarization produced by electrogenic Na+/K+ pump in lizard axons impaled near their motor terminals. J Neurophysiol. 1993;70:1874–1884. doi: 10.1152/jn.1993.70.5.1874. [DOI] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Ann Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Stys PK, Waxman SG. Activity-dependent modulation of excitability: implications for axonal physiology and pathophysiology. Muscle Nerve. 1994;17:969–974. doi: 10.1002/mus.880170902. [DOI] [PubMed] [Google Scholar]
- Tatsumi H, Katayama Y. Na+ dependent Ca2+ influx induced by depolarisation in neurons dissociated from rat nucleus basalis. Neurosci Lett. 1995;196:9–12. doi: 10.1016/0304-3940(95)11823-f. [DOI] [PubMed] [Google Scholar]
- Therien AG, Blostein R. Mechanisms of sodium pump regulation. Am J Cell Physiol. 2000;279:C541–C566. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- Vagg R, Mogyoros I, Kiernan MC, Burke D. Activity-dependent hyperpolarization of human motor axons produced by natural activity. J Physiol. 1998;507:919–925. doi: 10.1111/j.1469-7793.1998.919bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W, Schwarz JR. Voltage-clamp studies on axons: macroscopic and single-channel currents. In: Waxman SG, Stys PK, Kocsis JD, editors. The Axon. Oxford: Oxford University Press; 1995. pp. 257–280. [Google Scholar]