Abstract
No studies to date have reported activation of satellite cells in vivo in human muscle after a single bout of high intensity exercise. In this investigation, eight individuals performed a single bout of high intensity exercise with one leg, the contralateral leg being the control. A significant increase in mononuclear cells staining for the neural cell adhesion molecule (N-CAM) and fetal antigen 1 (FA1) were observed within the exercised human vastus lateralis muscle on days 4 and 8 post exercise. In addition, a significant increase in the concentration of the FA1 protein was determined in intramuscular dialysate samples taken from the vastus lateralis muscle of the exercising leg (day 0: 1.89 ± 0.82 ng ml−1; day 2: 1.68 ± 0.37 ng ml−1; day 4: 3.26 ± 1.29 ng ml−1, P < 0.05 versus basal; day 8: 4.68 ± 2.06 ng ml−1, P < 0.05 versus basal and control). No change was noted in the control leg. Despite this increase in N-CAM- and FA1-positive mononuclear cells, an increased expression of myogenin and the neonatal isoform of the myosin heavy chain (MHCn) was not observed. Interestingly, myofibre lesions resulting from extensive damage to the proteins within the myofibre, particularly desmin or dystrophin, were not observed, and hence did not appear to induce the expression of either N-CAM or FA1. We therefore propose that satellite cells can be induced to re-enter the cell growth cycle after a single bout of unaccustomed high intensity exercise. However, a single bout of exercise is not sufficient for the satellite cell to undergo terminal differentiation.
Satellite cells are a population of muscle-derived stem cells responsible for myofibre development and renewal (Grounds, 1999; Asakura et al. 2001, 2002; Grounds et al. 2002). Activation, proliferation and fusion of this population of cells is required by a myofibre when undergoing fibrillar protein growth to maintain a constant nuclear/cytoplasmic ratio, or nuclear domain ratio. Satellite cell activation has been largely investigated in vitro (Allen et al. 1995; McGann et al. 2001) with limited studies being performed in animal models (Snow, 1990; Putman et al. 1998; Grounds et al. 2002) and fewer still using human subjects (Kadi et al. 1999a,b; Kadi & Thornell, 2000; Malm et al. 2000). To our knowledge, only one study has previously investigated satellite cell activation after a single bout of exercise (Malm et al. 2000). In this study no change, apart from that found with repeated muscle biopsy sampling, was observed after a single session of eccentric cycling (Malm et al. 2000).
The neural cell adhesion molecule (N-CAM, also known as Leu-19 or CD56) is a developmental molecule that is abundantly present on the surface of embryonic myotubes (Fidzianska & Kaminska, 1995). In non-pathological adult skeletal muscle, the N-CAM antigen is concentrated near the neuromuscular junctions and on satellite cells. It has been reported to be nearly undetectable in the non-synaptic portions of the myofibres and absent in non-myogenic cells (Fidzianska et al. 1995; Mesires & Doumit, 2002). N-CAM has become one of a number of useful markers of satellite cells in biopsy muscle tissue in humans (Kadi et al. 1999a,b; Malm et al. 2000; Thornell et al. 2003) as the electron microscopy method limits the number of cells that can be quantified. Other markers of satellite cell presence have been described, including m-cadherin, Pax-7, c-met, myf-5 and myoD. However, there is no consensus as to the exact state of proliferation or differentiation at which the markers are expressed and therefore, which is the optimal marker (Thornell et al. 2003).
The fetal antigen 1 (FA1) protein is a member of the epidermal growth factor superfamily (Floridon et al. 2000). Circulating FA1 is synthesized as a larger membrane-associated precursor defined by a delta-like mRNA (DLK1). Recently, DLK1 has been identified as one of six imprinted genes within the autosomal callipyge locus in sheep with a paternally inherited muscular hypertrophy (Charlier et al. 2001). FA1 is reported to be highly expressed in undifferentiated cells in a host of tissues including neonatal and fetal skeletal muscle fibres (Floridon et al. 2000), and is observed, histochemically, as densely stained mononuclear cells located beneath the basal lamina of myofibres during development. In normal adult human skeletal muscle, no FA1 staining is observed, although FA1-positive mononuclear cells have been shown in inflammatory myopathies in adult human muscle (C. H. Jensen, unpublished observation). Co-localization of staining of FA1 and Pax-7 in myogenic cells in sections of human neonatal skeletal muscle has been identified. In adult muscle, it is hypothesized that FA1 is expressed in a population of activated muscle-derived stem cells, distinct from the satellite cell population, and it disappears during the early stages of satellite cell commitment (C. H. Jensen, unpublished observation).
It has been proposed that quiescent satellite cells are activated by various growth factors including muscle growth factor, basic fibroblast growth factor, nitric oxide and hepatocyte growth factor (Musaròet al. 1999; Semsarian et al. 1999; Goldspink, 1999). The mechanism by which a bout of mechanical loading can induce the activation of these factors in vivo remains poorly understood, although muscle stretching has been proposed to play an important role (Goldspink, 1999). A current hypothesis suggests that repeated high mechanical force during an eccentric contraction causes disruption to proteins that maintain the cellular integrity of the myofibre. This disruption then triggers the release of certain growth factors from within the myofibre (Lieber & Fridén, 2002); however, this has not been confirmed in all studies (Yu & Thornell, 2002; Yu et al. 2002). It is currently not known, in human skeletal muscle, if an increase in satellite cells is possible without muscle fibre necrosis.
In this study we investigate the influence of acute high intensity voluntary muscle contractions on the number of satellite cells in human skeletal muscle by sampling both the muscle tissue directly and using intramuscular dialysate fluid.
Methods
Subjects
Eight healthy sedentary male subjects (age: 25 ± 3 years; height: 188 ± 9 cm; weight: 88 ± 12 kg) gave informed written consent to participate in this study. All subjects were unaccustomed to high intensity eccentric exercise and were not participating in any regular exercise regime. The Ethics Committees of the Municipalities of Copenhagen and Frederiksberg approved this study and all procedures conformed to the Declaration of Helsinki.
Exercise protocol
The exercise bout was performed with one leg, the contralateral leg serving as the control. The exercise protocol consisted of three exercise phases: (a) 50 one-leg ‘drop down’ jumps were performed from a stable platform of 45 cm; (b) eight sets of 10 maximal eccentric knee extensions at −30 deg s−1 using an isokinetic dynamometer (Kincom, USA); and (c) eight sets of 10 maximal eccentric knee extensions at −180 deg s−1 using an isokinetic dynamometer (Kincom). A 30 s rest phase between each set and a 5 min rest period between each exercise phase was scheduled.
Microdialysis
A microdialysis fibre was inserted, under ultrasound guidance, into the lower-to-mid vastus lateralis muscle of both legs on days 0, 2, 4 and 8. Each repeated insertion was placed 1 cm distal from the previous insertion site. The microdialysis tubing was inserted such that it ran parallel with the muscle fibres. The perfusate was infused at a flow rate of 5 µl min−1 and the dialysate was collected for 240 min. This procedure has been previously described in detail by Langberg et al. (1999, 2002). The intramuscular dialysate samples were immediately placed on dry ice and stored at −70°C pending analysis.
Fetal antigen 1 analysis
Fetal antigen 1 (FA1) concentrations from the microdialysate samples were determined using a sandwich ELISA technique which has been previously described (Jensen et al. 1997).
Muscle biopsy and sampling
Muscle biopsies were taken under local anaesthetic (1% lidocaine (lignocaine)) at a constant depth from the mid portion of the vastus lateralis in accordance with the needle biopsy technique of Bergstrom (1962). Muscle samples were taken from the control leg (n = 8) and the exercising leg (n = 4) on day 0. On days 2, 4 and 8 both legs were sampled (n = 8). Biopsy sites were at least 1 cm from previous biopsy sites and were similarly distant from regions subjected to microdialysis. Immediately after removal, the biopsy sample was frozen in isopentane that had been pre-cooled in liquid nitrogen and stored at −70°C.
Muscle histology
Transverse serial sections of the muscle biopsy samples were cut at 10 µm thickness using a cryostat microtome (Microm, Germany) at −22°C and mounted on slides. Serial sections were immunohistochemically stained for (a) markers of satellite cells: N-CAM (DAKO, M 0779, Denmark) and FA1 (developed at Odense University Hospital), (b) cell proliferation/differentiation markers: Ki-67 (DAKO M 7240, Denmark) and myogenin (DAKO M 3359, Denmark), (c) muscle regeneration marker: neonatal isoform of the myosin heavy chain (MHCn) (Novocastra NCL-MHCn, UK), (d) indicators of myofibre damage: desmin (Zymed 18-0016, USA), dystrophin (Novocastra NCL-DYS 2, UK) and fibronectin (Novocastra NCL-FIB, UK), and (e) inflammatory marker: CD68+ cells (DAKO 0718, Denmark). Negative and positive controls were included in each staining batch for all antibodies assessed. A routine haematoxylin and eosin stain was also performed to assess for morphological changes.
For quantification of N-CAM-positive cells, sample identity was concealed from the technician. Four hundred myofibres were counted from two individual sections per biopsy (200 muscle fibres per section) and the percentage of N-CAM-positive cells was calculated as the number of positive cells/(positive cells + myonuclear number) × 100 as previously described (Kadi et al. 1999a). An identical assessment was performed on the sections stained for FA1.
Statistics
The results for the histological quantification of N-CAM and FA1 are represented as mean ±s.d. The results for the quantification of FA1 from the microdialysate are represented as mean ±s.e.m. A repeated measures ANOVA with Dunn's post hoc test was performed. The level of significance was set at P < 0.05.
Results
N-CAM and FA1
To investigate the appearance of satellite cells in human skeletal muscle after a single bout of high intensity exercise, the histological expression of N-CAM and the FA1 protein was determined 5 h post exercise and again on days 2, 4 and 8 after the exercise bout (Fig. 1). A significant increase in cells positive for N-CAM was found in the histological sections taken from all subjects, on days 4 and 8 after the cessation of exercise (Table 1). Additionally, a significant increase in the percentage of mononuclear cells with positive staining for FA1 was found in the exercising leg, with no change from baseline occurring in the control leg (Table 2). The proliferation of active nuclei was identified with positive staining for Ki-67 in the exercised leg on days 4 and 8, indicating a small increase in the number of nuclei in the active phase of growth. Less than 0.2% of nuclei were positive in the control leg at any time point analysed (Fig. 2). Despite this, positive staining for myogenin and MHCn was only demonstrated in one subject, which commenced on day 2 post exercise and persisted 8 days after the exercise bout (data not shown). This increase in expression was associated with an individual who displayed muscle fibre necrosis (desmin-negative cells; dystrophin-negative cells; fibronectin-positive cells and centralized nuclei) (Fig. 3).
Figure 1. Detection of satellite cells.
Satellite cells were identified using both N-CAM (A, shown here on day 4 post exercise) and FA1 (B, displayed here on day 8 post exercise) antibodies. Only those cells that displayed a positive staining for N-CAM and included a nucleus were identified as a positive cell. The same methodology for counting was used to assess the expression of FA1-positive cells.
Table 1.
A significant increase in the percentage of N-CAM-positive cells was identified after a single bout of unaccustomed high intensity exercise
| Day 0 | Day 2 | Day 4 | Day 8 | |
|---|---|---|---|---|
| Control leg | 2.59 ± 1.05 | 3.24 ± 1.7 | 3.39 ± 0.08 | 3.07 ± 0.89 |
| Exercised leg | n.a. | 6.38 ± 1.23 | 7.57 ± 3.36* | 6.94 ± 8.27* |
P < 0.05 compared to both baseline and the control leg. n.a., due to small sample number (n = 4) on day 0 from the exercising leg, these values were not recorded.
Table 2.
A significant increase in the percentage of FA1-positive cells was identified after a single bout of unaccustomed high intensity exercise
| Day 0 | Day 2 | Day 4 | Day 8 | |
|---|---|---|---|---|
| Control leg | 0.29 ± 0.03 | 0.53 ± 0.08 | 0.39 ± 0.11 | 0.49 ± 0.04 |
| Exercised leg | n.a. | 2.12 ± 0.96 | 4.34 ± 2.72* | 3.34 ± 2.66* |
P < 0.05 compared to both baseline and the control leg. n.a., due to small sample number (n = 4) on day 0 from the exercising leg, these values were not recorded.
Figure 2. Detection of nuclei in the active phase of growth.
Nuclei in the active phase of growth were identified on day 4 (A) and day 8 (B) in the exercised leg only using a Ki-67 antibody. Areas positive for Ki-67 stained brown while the nuclei were counterstained blue using haematoxylin.
Figure 3. Detection of gross disturbance to the myofibre.
No gross morphological disruption to the myofibre was shown in 7 of the 8 subjects tested when histologically assessed with both haematoxylin and eosin (A1) and fibronectin (B1) (day 4 shown). However, a significant increase in staining for the N-CAM protein (C1) was observed (day 8 shown). In contrast, one subject displayed gross morphological disruption, shown here on day 4 (A2, haematoxylin and eosin; B2, fibronectin). On day 8 post exercise, a pattern consistent with alignment of satellite cells to form new myotubes was observed (C2, N-CAM).
Myofibre proteins
In seven of the eight subjects tested, no evidence of gross myofibre lesions leading to myofibre necrosis was observed during any of the testing days. In those subjects not showing myofibre lesions, individual fibres did show slight rounding in appearance. No positive staining of CD68+-reactive macrophages was noted within the myofibres. Additionally, no myofibres were observed which were negative for desmin, negative for dystrophin or positive for fibronectin (Fig. 3). Only one subject displayed myofibre necrosis with corresponding gross disturbances in the myofibre proteins (desmin-negative cells, dystrophin disruption and fibronectin-positive cells), which commenced on day 2 post exercise and was not fully recovered by day 8 post exercise (Fig. 3).
Intramuscular concentration of FA1
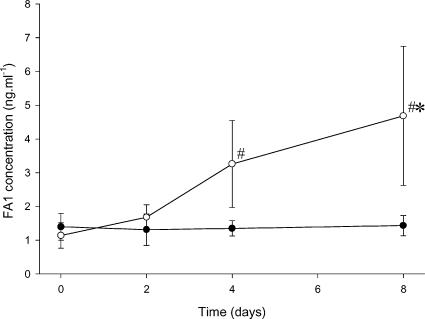
In the leg that was exercised, a significant increase in the concentration of FA1 within the intramuscular dialysate samples was shown on day 4 (3.26 ± 1.29 ng ml−1; P < 0.05) and day 8 (4.68 ± 2.06 ng ml−1; P < 0.05) when compared to baseline (1.89 ± 0.82 ng ml−1) (Fig. 4). No increase in the concentration of FA1 was seen in the samples taken from the control leg at any time sampled, with a significant difference between the exercised (4.68 ± 2.06 ng ml−1; P < 0.05) and control leg (1.43 ± 0.30 ng ml−1) being shown on day 8 post exercise.
Figure 4. Intramuscular concentration of FA1.
Concentration of FA1 in the intramuscular dialysate samples taken from the vastus lateralis muscle in the control (○) and exercising leg (•) is shown for days 0, 2, 4 and 8 post exercise. Values are presented as mean ±s.e.m. #Significantly different from baseline level (P < 0.05). *Significantly different from the control leg (P < 0.05).
Discussion
The mechanism by which an unaccustomed bout of high intensity exercise, particularly eccentric exercise, induces the activation of satellite cells is not well understood. It has been proposed that unaccustomed eccentric exercise induces damage to the myofibre proteins that maintain cellular integrity. Disruption of the desmin molecule has been reported to occur within 15 min of the onset of eccentric contractions (Fridén & Lieber, 2001; Barash et al. 2002; Lieber & Fridén, 2002) while fragmented staining of dystrophin and the presence of fibronectin have also been described shortly after the completion of a bout of high intensity eccentric exercise in rats (Biral et al. 2000; Komulainen et al. 2000). It is hypothesized that these disruptions to the myofibre proteins lead to myofibre necrosis, resulting in the onset of delayed muscle soreness, an increase in serum creatine kinase and a reduction in muscular strength in the days following the initial exercise bout (Fridén & Lieber, 2001). This has recently been challenged (Yu et al. 2002; Yu & Thornell, 2002; Peters et al. 2003). While disturbances in calcium metabolism have been implicated in the reported damage caused by unaccustomed eccentric exercise (Reid et al. 1994; Balnave & Allen, 1995), recent studies have suggested that higher tensile stresses produced during an eccentric contraction initiate these necrotic events, in turn activating the quiescent satellite cells. These findings have been established almost exclusively in animal models, with little muscle biopsy data being available on the lower limbs of human subjects. Despite 210 maximum voluntary contractions that were eccentric in nature being performed by the subjects in this study, seven of the eight subjects did not show myofibre lesions. Under light microscope examination, no disruption to the desmin or dystrophin proteins was found in the cellular membrane of the myofibres and, to support this, the fibronectin protein was not found within the myofibres. This indicated that the sarcolemma was undamaged. Disruption to the sarcolemma leading to membrane leakage has been previously shown in small animal models (Biral et al. 2000; Komulainen et al. 2000; Lieber & Fridén, 2002). This study confirms recent findings in human subjects that report no muscle fibre necrosis after downstairs running, eccentric bicycling and downhill treadmill running (Yu & Thornell, 2002). Therefore, it appears that in contrast to animal models (Fridén & Lieber, 2001), applying a maximal voluntary exercise stimulus in humans is not necessarily associated with disruption to the myofibre proteins leading to myofibre lesions.
Even with the lack of myofibre necrosis, an increase in the number of satellite cells was confirmed by positive staining for N-CAM, an abundant protein observed on the surface of early embryonic myotubes (Sanes et al. 1986). No change in N-CAM staining was observed in the control leg, removing the possibility that the increase in N-CAM was due to lesions induced by repeated biopsy sampling, as has been previously reported (Malm et al. 2000). In addition, an increase in the cell numbers staining positive for the membrane-bound protein, FA1, was found. In adult skeletal muscle the FA1 protein has been found to be absent except in regenerating muscle (data not shown). In a further characterization of the expression of this protein, FA1 has been shown to be absent in satellite cell cultures while explant cultures of the same muscle sample have shown intense staining (C. H. Jensen, unpublished observation). This data implies that FA1 is expressed in activated muscle-derived stem cells that can differentiate into myogenic cells via myocyte-mediated inductive interactions (Asakura et al. 2002). While further characterization is required, independent of the origin of the muscle stem cell, we have shown that two protein markers, that are proposed to reside within the muscle stem cell pathway, are increased after a single bout of high intensity exercise.
Using both histological staining and an ELISA technique we have been able to identify a quantitative increase in the expression of the FA1 protein occurring in the exercising leg only. While it is possible that the interstitial concentration of FA1 seen in the muscle could be attributed to other tissues, this is unlikely, as the control leg would also have been affected by an increase in systemic levels of FA1 derived from other tissues. The delay in the peak concentration between the interstitial concentration detected on day 8 and the histological analysis (peak day 4) is possibly due to differences in analysis techniques with the histological sections determining the bound form of the protein and the microdialysis determining the soluble form of the FA1 protein. The expression of both N-CAM and FA1 suggests that satellite cells independent of their origin can be activated to re-enter the growth cell cycle after a single bout of unaccustomed eccentric exercise. However, it appears that an additional exercise stress is required to induce terminal differentiation unless a muscle lesion occurs. In support of this hypothesis, it has been previously shown in rats that, after a single bout of exercise, the calculated satellite cell activation is far greater than required to repair the small number of necrotic fibres. Further, in those satellite cells that were activated, it was not shown if the proliferating myoblasts underwent fusion (Darr & Schultz, 1987). The inability to find myogenin-positive staining in seven of the eight subjects suggests that the number of cells expressing N-CAM and FA1 can upregulate in readiness for a second bout of exercise. Without this further exercise stimulus the satellite cells become quiescent again, and do not undergo terminal differentiation.
Atrophic processes due to ageing or as a consequence of disease or injury remains a contributing factor to morbidity. Identifying triggers for satellite cell activation in humans may expedite the development of treatments for sarcopenia and various muscular diseases. While in vitro models continue to elucidate signalling pathways, only in vivo investigations can examine the ability to activate these pathways with exercise. We show that an increase in the number of satellite cells is possible with a single bout of voluntary high intensity exercise. Terminal differentiation of these cells may not be possible without repeated bouts of exercise unless myofibre lesions occur.
Acknowledgments
We gratefully acknowledge the assistance Professor Miranda Grounds provided in the writing of this manuscript. We would also like to thank Ms Birgitte Lillethorup and Ms Anette Kliem for their assistance in performing the immunohistochemical analysis.
References
- Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balnave CD, Allen DG. Intracellular calcium and force in single mouse muscle fibres following repeated contractions with stretch. J Physiol. 1995;488:25–36. doi: 10.1113/jphysiol.1995.sp020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash IA, Peters D, Fridén J, Lutz GJ, Lieber RL. Desmin cytoskeletal modifications after a bout of eccentric exercise in the rat. Am J Physiol Regul Integr Comp Physiol. 2002;283:R958–R963. doi: 10.1152/ajpregu.00185.2002. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. Muscle electrolytes in man. Scand J Clin Lab Invest. 1962;14:511–513. [Google Scholar]
- Biral D, Jakubiec-Puka A, Ciechomska I, Sandri M, Rossini K, Carraro U, Betto R. Loss of dystrophin and some dystrophin-associated proteins with concomitant signs of apoptosis in rat leg muscle overworked in extension. Acta Neuropathol. 2000;100:618–626. doi: 10.1007/s004010000231. [DOI] [PubMed] [Google Scholar]
- Charlier C, Segers K, Wagenaar D, Karim L, Berghmans S, Jaillon O, Shay T, Weissenbach J, Cockett N, Gyapay G, Georges M. Human-ovine comparative sequencing of a 250-kb imprinted domain encompassing the callipyge (clpg) locus and identification of six imprinted transcript: DLK1, DAT, GTL2, PEG11, antiPEG11 and MEG8. Genome Res. 2001;11:850–862. doi: 10.1101/gr.172701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darr KC, Schultz E. Exercise-induced satellite cell activation in growing and mature skeletal muscle. J Appl Sci. 1987;63:1816–1821. doi: 10.1152/jappl.1987.63.5.1816. [DOI] [PubMed] [Google Scholar]
- Fidzianska A, Kaminska A. Neural cell adhesion molecule (N-CAM) as a marker of muscle tissue alternations. Review of the literature and own observations. Folia Neuropathol. 1995;33:125–128. [PubMed] [Google Scholar]
- Floridon C, Jensen CH, Thorsen P, Nielsen O, Sunde L, Westergaard JG, Thomsen SG, Teisner B. Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation. 2000;66:49–59. doi: 10.1046/j.1432-0436.2000.066001049.x. [DOI] [PubMed] [Google Scholar]
- Fridén J, Lieber RL. Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol Scand. 2001;171:321–326. doi: 10.1046/j.1365-201x.2001.00834.x. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat. 1999;194:323–334. doi: 10.1046/j.1469-7580.1999.19430323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds MD. Muscle regeneration: molecular aspects and therapeutic implications. Curr Opin Neurol. 1999;12:535–543. doi: 10.1097/00019052-199910000-00007. [DOI] [PubMed] [Google Scholar]
- Grounds MD, White JD, Rosenthal N, Bogoyevitch MA. The role of stem cells in skeletal and cardiac muscle repair. J Histochem Cytochem. 2002;50:589–610. doi: 10.1177/002215540205000501. [DOI] [PubMed] [Google Scholar]
- Jensen CH, Krogh TN, Stoving RK, Holmskov U, Teisner B. Fetal antigen 1 (FA1), a circulating member of the epidermal growth factor superfamily: ELISA development, physiology and metabolism in relation to renal function. Clin Chim Acta. 1997;268:1–20. doi: 10.1016/s0009-8981(97)00152-6. [DOI] [PubMed] [Google Scholar]
- Kadi F, Eriksson A, Holmer S, Butler-Browne GS, Thornell L-E. Cellular adaptation of the trapezius muscle in strength trained athletes. Histochem Cell Biol. 1999a;111:189–195. doi: 10.1007/s004180050348. [DOI] [PubMed] [Google Scholar]
- Kadi F, Eriksson A, Holmer S, Thornell L-E. Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med Sci Sports Exerc. 1999b;31:1528–1535. doi: 10.1097/00005768-199911000-00006. [DOI] [PubMed] [Google Scholar]
- Kadi F, Thornell L-E. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol. 2000;113:99–103. doi: 10.1007/s004180050012. [DOI] [PubMed] [Google Scholar]
- Komulainen J, Kalliokoski R, Koskinen SOA, Drost MR, Kuipers H, Hesselink MKC. Controlled lengthening or shortening contraction-induced damage is followed by fiber hypertrophy in rat skeletal muscle. Int J Sports Med. 2000;21:107–112. doi: 10.1055/s-2000-8869. [DOI] [PubMed] [Google Scholar]
- Langberg H, Bjørn C, Boushel R, Hellsten Y, Kjær M. Exercise-induced increase in interstitial bradykinin and adenosine concentrations in skeletal muscle and peritendinous tissue in humans. J Physiol. 2002;542:977–983. doi: 10.1113/jphysiol.2002.018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bülow J, Kjær M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999;521:299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber RL, Fridén J. Morphologic and mechanical basis of delayed-onset muscle soreness. J Am Acad Orthop Surg. 2002;10:67–73. [PubMed] [Google Scholar]
- McGann CJ, Odelberg SJ, Keating MT. Mammalian myotube dedifferentiation induced by newt regeneration extract. Proc Natl Acad Sci U S A. 2001;98:13699–13704. doi: 10.1073/pnas.221297398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm C, Nyberg P, Engström M, Sjödin B, Lenkei R, Ekblom B, Lundberg I. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol. 2000;529:243–262. doi: 10.1111/j.1469-7793.2000.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesires NT, Doumit ME. Satellite cell proliferation and differentiation during postnatal growth of porcine skeletal muscle. Am J Physiol Cell Physiol. 2002;282:C899–C906. doi: 10.1152/ajpcell.00341.2001. [DOI] [PubMed] [Google Scholar]
- Musarò A, McCullagh KJA, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- Peters D, Barash IA, Burdi M, Yuan PS, Mathew L, Friden J, Lieber RL. Asynchronous functional, cellular and transcriptional changes after a bout of eccentric exercise in the rat. J Physiol. 2003;553:947–957. doi: 10.1113/jphysiol.2003.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman C, Dusterhoft S, Pette D. Changes in satellite cell content and myosin isoforms in low frequency stimulated fast muscle of hypothyroid rat. J Appl Physiol. 1998;86:40–51. doi: 10.1152/jappl.1999.86.1.40. [DOI] [PubMed] [Google Scholar]
- Reid W, Huang J, Bryson S. Diaphragm injury and myofibrillar structure induced by resistive loading. J Appl Physiol. 1994;76:176–184. doi: 10.1152/jappl.1994.76.1.176. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Schachner M, Covault J. Expression of several adhesive macromolecules (N-CAM, L1, J1, NILE, uvomorulin, laminin, fibronectin and a heparan sulfate proteoglycan) in embryonic, adult and denervated adult skeletal muscle. Cell Biol. 1986;102:420–431. doi: 10.1083/jcb.102.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semsarian C, Wu MJ, Ju YK, Marciniec T, Yeoh T, Allen DG, Harvey RP, Graham RM. Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature. 1999;400:576–581. doi: 10.1038/23054. [DOI] [PubMed] [Google Scholar]
- Snow MH. Satellite cell response in rat soleus muscle undergoing hypertrophy due to surgical ablation of synergists. Anat Rec. 1990;227:437–446. doi: 10.1002/ar.1092270407. [DOI] [PubMed] [Google Scholar]
- Thornell L-E, Lindström M, Renault V, Mouly V, Butler-Browne GS. Satellite cells and training in the elderly. Scand J Med Sci Sports. 2003;13:48–55. doi: 10.1034/j.1600-0838.2003.20285.x. [DOI] [PubMed] [Google Scholar]
- Yu J-G, Malm C, Thornell L-E. Eccentric contractions leading to DOMS do not cause loss of desmin nor fibre necrosis in human muscle. Histochem Cell Biol. 2002;118:29–34. doi: 10.1007/s00418-002-0423-1. [DOI] [PubMed] [Google Scholar]
- Yu J-G, Thornell L-E. Desmin and actin alterations in human muscles affected by delayed onset of muscle soreness: a high resolution immunocytochemical study. Histochem Cell Biol. 2002;118:29–34. doi: 10.1007/s00418-002-0427-x. [DOI] [PubMed] [Google Scholar]