Abstract
We have previously shown that the splenorenal reflex controls renin release through splenic afferent and renal sympathetic nerves. We proposed that this reflex would also affect renal blood flow (RBF). RBF was measured in male Long Evans rats using transit-time flow probes. There were no significant differences between any of the experimental groups with respect to baseline values of RBF (8.9 ± 0.4 ml min−1, n = 25) or mean arterial pressure (MAP, 98.7 ± 2.5 mmHg, n = 25). Splenic venous pressure was selectively raised (from 7.9 ± 0.6 to 21.6 ± 0.3 mmHg, n = 25) in anaesthetized rats by partial ligation of the splenic vein. This caused an immediate fall in RBF (−2.1 ± 0.2 ml min−1, n = 7) and in MAP (−12.4 ± 2.8 mmHg, n = 7). The fall in RBF, but not the fall in blood pressure, was attenuated by renal denervation (ΔRBF: − 0.7 ± 0.1 ml min−1, n = 6), splenic denervation (ΔRBF: −0.8 ± 0.1 ml min−1, n = 6) and close renal arterial injection of the α1-adrenergic blocker phenoxybenzamine (12.5 μg; ΔRBF: −0.8 ± 0.1 ml min−1, n = 6). Renal conductance fell only in the intact control group, i.e. the residual fall in RBF in the denervated and phenoxybenzamine-treated animals could be attributed to the fall in MAP. We also showed that splenic vein occlusion increased both splenic afferent (from 3.0 ± 0.3 to 6.6 ± 0.6 spikes s−1, n = 5) and renal efferent (from 24.8 ± 2.0 to 50.2 ± 4.9 spikes s−1, n = 9) nerve activity. We conclude that obstruction to splenic venous outflow, such as would occur in portal hypertension, initiates increased splenic afferent nerve activity and renal vasoconstriction through the splenorenal reflex, as well as a fall in blood pressure. We propose that this contributes to the renal and cardiovascular dysfunction observed in portal hypertension.
Portal hypertension is defined as a pathological increase in portal venous pressure to above 10 mmHg (Gupta et al. 1997; Henderson et al. 1998). Cirrhotic/portal hypertensive patients have elevated levels of noradrenaline in the renal venous blood (Henriksen et al. 1984), as well as significantly lower baseline mean renal blood flow levels compared to control subjects; this is indicative of increased renal sympathetic tone (Gatta et al. 1982). These changes in renal haemodynamics in cirrhotic patients appear to be functional, i.e. there is no intrinsic renal disease (Koppel et al. 1969). Several studies have investigated the hepatorenal reflex whereby elevation of portal venous pressure causes increased hepatic afferent nerve activity, reflex increases in renal and cardiopulmonary sympathetic efferent nerve activity (DiBona & Sawin, 1995; Kostreva et al. 1980) and decreased renal blood flow (Jalan et al. 1997). In addition, acute superior mesenteric vein occlusion has also been shown to reduce renal blood flow, suggesting possible involvement of an intestinal–renal neural reflex (Miller et al. 1983). Thus, it has been proposed that altered neural reflexes mediate the abnormal renal haemodynamics observed in portal hypertension.
Blood from the splenic vein drains into the portal vein. Thus an increase in portal venous pressure causes a concomitant rise in splenic venous pressure. We wished to investigate whether changes in intrasplenic haemodynamics also alter renal function. There is both structural and functional evidence for a neural reflex pathway between the spleen and the kidneys (Herman et al. 1982; Calaresu et al. 1984; Meckler & Weaver, 1988; Deng & Kaufman, 2001). We proposed, therefore, that derangement of renal function in portal hypertension may be mediated by a splenorenal neurogenic reflex, whereby elevated portal and splenic venous pressure would increase splenic afferent nerve activity, which would induce an increase in efferent renal sympathetic nerve activity and decrease renal blood flow. In order to isolate the spleen as the initiator of the splenorenal reflex, we partly occluded the splenic vein to elevate splenic venous pressure to the degree observed in portal hypertension; this did not cause any obstruction to portal venous flow. The effects of renal denervation, splenic denervation, and pharmacological blockade of intrarenal α1-adrenergic receptors were studied. In addition, we measured the changes in splenic afferent and renal efferent nerve activity elicited by splenic venous occlusion.
Methods
The experimental procedures were approved by the local Animal Welfare Committee in accordance with the guidelines issued by the Canada Council on Animal Care. All animals were killed with an anaesthetic overdose of pentobarbitone sodium (96 mg, i.v., MTC Pharmaceuticals, Cambridge, Ontario, Canada) at the completion of each experiment. All data were recorded online (DATAQ Instruments, Akron, Ohio, USA) and analysed using WINDAQ software (DATAQ Instruments).
Animals
Male Long–Evans rats (350–600 g, Charles River, Montreal, Quebec, Canada) were housed in the University of Alberta Animal Facility for at least 1 week before experiments started. They were exposed to a 12 h–12 h light–dark cycle in a temperature- and humidity-controlled room. All rats were fed standard 0.3% sodium rat chow and water ad libitum. There were four experimental groups: intact control rats (n = 7), renal denervated rats (n = 6), splenic denervated rats (n = 6) and phenoxybenzamine-treated rats (n = 6).
Surgery
Anaesthesia was induced with pentobarbitone sodium (60 mg (kg body weight)−1, i.p.), followed 1 h later by Inactin (Sigma, Canada; ethyl-(methyl-propyl)-malonyl-thio-urea, 80 mg (kg body weight)−1, s.c.). Body temperature was maintained at 37°C with a homeothermic blanket (Harvard Apparatus).
The femoral vein and artery were cannulated with Silastic™ (Dow Corning, 0.51 mm i.d., 0.94 mm o.d.) and polyethylene tubing (VWR International, Mississauga, Ontario, Canada, PE 50, 0.58 mm i.d., 0.97 mm o.d.) for administration of isotonic saline (3 ml h−1) and monitoring of systemic blood pressure, respectively. Through a mid-line laparotomy, the gastric and renal vessels were exposed. A Silastic™ cannula (Dow Corning, 0.30 mm i.d., 0.64 mm o.d.) was inserted occlusively into the gastric vein, advanced until the tip lay at the junction with the splenic vein, and secured with a drop of Vetbond tissue glue (3M Animal Care Products, St. Paul, MN, USA). This cannula was connected to a pressure transducer for online monitoring of splenic venous pressure.
A Prolene 0.7 loop (Ethicon, USA) was placed loosely around the splenic vein at its junction with the portal vein; this allowed for controlled partial occlusion of the splenic vein. Similarly, a loose ligature (Prolene 1.5) was placed around the hepatic portal vein (rostral to the junction with the splenic vein), to induce portal hypertension; in this case, a cannula (polyethylene, 0.58 mm i.d., 0.97 mm o.d.) was inserted non-occlusively into the portal vein, and secured with tissue adhesive (3M Vetbond, Animal Care Products) to monitor portal venous pressure.
In renal denervation experiments, the renal nerves were stripped from the left renal artery and vein as previously described (Kaufman & Stelfox, 1987). Similarly, in splenic denervation experiments, the splenic nerves were stripped from the splenic artery and vein, distal to branching of the vessels toward the spleen (Andrew et al. 2001).
Renal blood flow (RBF)
A factory-calibrated flow probe (1RB series, Transonic Systems, Ithaca, NY, USA) was placed around the left renal artery and covered in conducting jelly. A 35–40 min stabilization time was allowed, during which time body temperature, mean arterial pressure (MAP) and RBF were monitored. Baseline values of RBF, MAP and splenic venous pressure were then recorded for 20 min, after which tension was applied to the splenic venous ligature to raise splenic venous pressure to 20–24 mmHg. Splenic venous pressure, MAP and RBF were recorded for a further 20 min.
Phenoxybenzamine (12.5 μg in 150 μl heparinized isotonic saline, Sigma, Canada) was administered over 30 s into the renal artery with application of vibration to ensure even distribution throughout the kidney (Hamza & Kaufman, 2004). After a stabilization period of 20–25 min, the effect of splenic venous occlusion on RBF was measured as described above. At the end of each experiment, it was confirmed that the phenoxybenzamine had completely blocked intrarenal α1-adrenergic receptors; the α1-adrenergic agonist phenylephrine (0.15 μg in 150 μl heparinized isotonic saline, Sabex, Boucherville, Quebec, Canada) did not cause any change in RBF.
Extracellular nerve recording
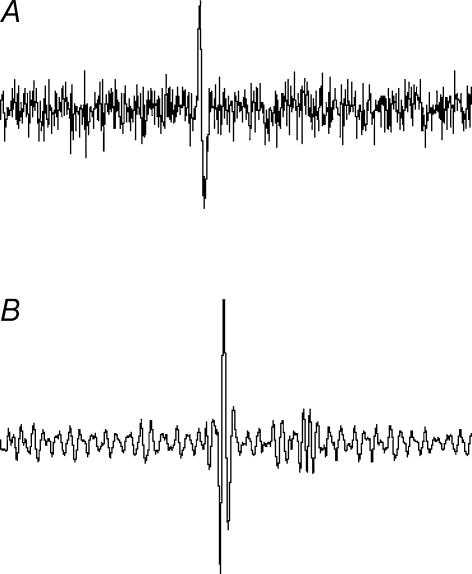
Separate groups of rats were used for these experiments. Nerve recordings were performed as previously described (Kaufman & Levasseur, 2003). The abdominal cavity was filled with mineral oil, and the splenic nerve isolated and cut. Splenic sensory afferent nerve activity was measured by placing the distal end of a small nerve fibre (closer to the spleen) onto bipolar platinum recording electrodes, and covering them with Kwik-Cast (WPI, Sarasota, FL, USA). The nerve signal was amplified and filtered between 100 and 1000 Hz (Leaf Electronics Ltd QT-B; WPI LPF-30, Sarasota, FL, USA). Output from the amplifier was fed to a loudspeaker and displayed on a PC (sampling rate between 3 and 10 kHz, WINDAQ, DATAQ Instruments, Akron, OH, USA). After stabilization (20 min), afferent nerve activity was recorded online. Twenty minutes later, the splenic venous ligature was tightened until splenic venous pressure measured between 20 and 24 mmHg. Nerve recording continued for a further 20 min. A similar procedure was used to measure renal efferent nerve activity except that, in this case, the proximal end (closer to the spinal cord) of a small severed nerve fibre was placed on the recording electrodes. The analysis of nerve discharge was based on average discharge rate (spikes s−1) of visually identified action potentials in the raw filtered recordings (Fig. 1).
Figure 1. Raw recordings of action potentials.
A, recordings from splenic afferent nerves (sampling rate, 10 kHz). B, recordings from renal efferent nerves (sampling rate, 3.3 kHz).
Drugs
Phenoxybenzamine (Sigma, Canada) was dissolved in heparinized isotonic saline (heparin 10 000 IU l−1) at a concentration of 12.5 μg in 150 μl. This dose of phenoxybenzamine has previously been shown to block intrarenal α-adrenergic receptors (Smyth et al. 1984). Phenylephrine hydrochloride (Sabex, Canada; 10 mg ml−1) was diluted with heparinized isotonic saline (heparin 10 000 IU l−1) to a final concentration of 0.15 μg in a 150 μl volume.
Data analysis
Results are based on the last 10 min of each 20 min recording period described above. The data were analysed using Student's paired t test, or ANOVA followed by Student–Newman–Keuls post hoc test. Significance was accepted at P < 0.05.
Results
Splenic venous pressure
Preliminary experiments revealed that, when portal venous pressure was increased from 3.9 ± 0.4 to 13.6 ± 0.3 mmHg, there was an accompanying increase in splenic venous pressure from 10.1 ± 0.8 to 23.0 ± 0.7 mmHg (n = 5). In the subsequent series of experiments, mean baseline splenic venous pressure for all the animals was 7.9 ± 0.6 mmHg (n = 25). This was increased to 20–24 mmHg (mean, 21.6 ± 0.3 mmHg, n = 25) by partial occlusion of the splenic vein, i.e. to that pressure which would be associated with a portal venous pressure of 12–15 mmHg. There were no significant differences between the four experimental groups with regard to the baseline or experimental splenic venous pressures.
Renal blood flow
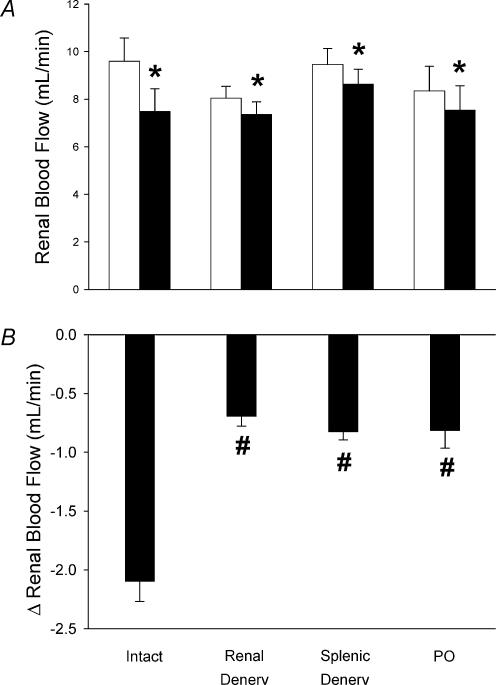
Mean baseline RBF for all the animals was 8.9 ± 0.4 ml min−1 (n = 25). There were no significant differences between the baseline renal blood flows for any of the four experimental groups (Fig. 2). When splenic venous pressure was increased, there was an immediate decrease in RBF in the intact animals (−2.1 ± 0.2 ml min−1, n = 7; Fig. 2). This response was greatly attenuated after renal denervation (−0.7 ± 0.1 ml min−1, n = 6), after splenic denervation (−0.8 ± 0.1 ml min−1, n = 6) and after intrarenal administration of phenoxybenzamine (−0.8 ± 0.1 ml min−1, n = 6; Fig. 2).
Figure 2. Effect of partial splenic vein ligation on RBF of intact (n = 7), renal denervated (Renal Denerv, n = 6), splenic denervated (Splenic Denerv, n = 6) and intrarenal phenoxybenzamine-treated (PO, 12.5 μg, n = 6) rats.
A, RBF before (▪) and after (□) partial splenic venous occlusion. B, change in RBF during partial splenic venous occlusion. Data are presented as means ± s.e.m. *Significant change in renal blood flow, P < 0.05. #Significant difference compared with intact group, P < 0.05.
Mean arterial pressure
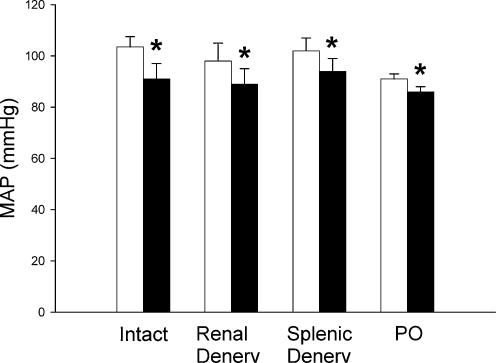
Mean baseline MAP for all the animals was 98.7 ± 2.5 mmHg (n = 25). There were no significant differences between the baseline values for any of the four experimental groups (Fig. 3). Moreover, the fall in MAP during splenic vein occlusion did not differ significantly between the intact (−12.4 ± 2.8 mmHg, n = 7), renal denervated (−8.3 ± 1.9 mmHg, n = 6), splenic denervated (−8.2 ± 1.5 mmHg, n = 6) and phenoxybenzamine-treated animals (−5.0 ± 0.33 mmHg, n = 6; Fig. 3). Although the baseline MAP and the fall in pressure tended to be lower in the phenoxybenzamine-treated animals, this did not reach significance (baseline MAP, P = 0.306; change in MAP, P = 0.133).
Figure 3. Effect of partial splenic vein ligation on MAP of intact (n = 7), renal denervated (Renal Denerv, n = 6), splenic denervated (Splenic Denerv, n = 6) and intrarenal phenoxybenzamine-treated (PO, 12.5 μg, n = 6) rats.
□, baseline values; ▪, values during partial splenic venous occlusion. The data are presented as means ±s.e.m., *Significant difference between baseline MAP and MAP during partial splenic vein ligation, P < 0.05.
Renal conductance
Renal conductance (K) was calculated as the ratio of flow (Q̇) to renal perfusion pressure (P): K = Q̇/P. There was a significant fall in renal conductance (from 0.088 ± 0.011 to 0.079 ± 0.014 ml min−1 mmHg−1, n = 7) in intact animals during splenic vein occlusion. However, there was no such change in the renal denervated (from 0.086 ± 0.011 to 0.086 ± 0.012 ml min−1 mmHg−1, n = 6), splenic denervated (from 0.094 ± 0.008 to 0.094 ± 0.009 mmHg ml−1 min−1 n = 6), or pheno-xybenzamine-treated animals (from 0.092 ± 0.012 to 0.088 ± 0.013 ml min−1 mmHg−1 n = 6).
Nerve activity
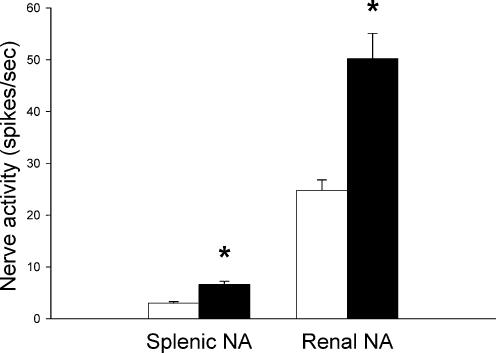
Increased splenic venous pressure (partial splenic vein occlusion) caused an immediate and significant increase both in splenic afferent (from 3.0 ± 0.3 to 6.6 ± 0.6 spikes s−1, n = 5) and renal efferent nerve activity (from 24.8 ± 2.0 to 50.2 ± 4.9 spikes s−1, n = 9; Fig. 4).
Figure 4. Effect of partial splenic vein ligation on splenic afferent (Splenic NA, n = 5) and renal efferent (Renal NA, n = 9) nerve activity.
□, baseline values; ▪, values during partial splenic venous occlusion. Data are presented as means ± s.e.m. *Significant difference between baseline nerve activity and nerve activity during partial splenic vein ligation, P < 0.05.
Discussion
Splenic venous occlusion caused an immediate fall in RBF, which was attenuated by renal denervation, splenic denervation and close renal arterial injection of the α1-adrenergic blocker phenoxybenzamine. This was accompanied, in all groups, by a fall in MAP. Renal conductance fell only in the intact group. We also showed that splenic vein occlusion increased both splenic sensory afferent and renal efferent nerve activity. We conclude that obstruction to splenic venous outflow, such as would occur in portal hypertension, increases splenic sensory afferent nerve activity and renal sympathetic nerve activity through the splenorenal reflex. This causes renal vasoconstriction and a fall in RBF. There is also a fall in systemic blood pressure.
Portal hypertension is associated with perturbations of renal function which are mediated, at least in part, through the renal sympathetic nerves (Anderson et al. 1976). There is evidence that several neurogenic pathways are involved. The hepatorenal reflex has been shown to regulate RBF (Jalan et al. 1997; Kew et al. 1972); portal vein occlusion increases hepatic sensory afferent nerve activity and induces reflex increases in renal sympathetic nerve activity (Kostreva et al. 1980). It has also been shown that, in dogs, acute occlusion of the superior mesenteric vein causes a reduction in renal arterial blood flow (Miller et al. 1983). The authors concluded that increased mesenteric outflow pressure, as would be associated with portal hypertension, initiates an intestinal–renal neural reflex. The model used in the present study, ligation of the splenic vein, does not cause any changes in portal venous pressure or mesenteric vascular pressure. The reflex changes we observed in RBF were thus initiated by the spleen, there being no direct contribution from the liver or intestines.
α1-Adrenergic receptors mediate renal vasoconstriction (Schmitz et al. 1981). The dose of phenoxybenzamine (12.5 μg), an irreversibly binding receptor antagonist, was chosen to completely block renal α1-adrenergic receptors, but to have minimal systemic effects (Harvey & Nickerson, 1954; Smyth et al. 1984). We verified that the intrarenal α1-adrenergic receptors were indeed blocked, by administering intrarenal phenylephrine at a dose (0.15 μg) known normally to cause significant renal vasoconstriction (Hamza & Kaufman, 2004). However, there was probably some phenoxybenzamine spillover into the systemic circulation. Although baseline MAP tended to be lower in the phenoxybenzamine-treated animals, this did not reach significance (which may reflect the limited sample size rather than lack of any systemic effect). This does not, however, detract from our finding that, in the phenoxybenzamine-treated animals, there was still a significant fall in blood pressure in response to splenic vein ligation.
We had hypothesized that phenoxybenzamine would block the effects of the renal sympathetic nerves on the renal arterioles, and prevent the drop in RBF seen after splenic venous occlusion. While it has previously been shown that phenoxybenzamine administration completely inhibits renal nerve-stimulated vasoconstriction (Chapman et al. 1982), administration of phenoxybenzamine in our study attenuated, but did not abolish, the decrease in RBF during splenic venous occlusion. However, given that MAP fell during splenic venous occlusion, we reasoned that the residual drop in RBF seen in the denervated and phenoxybenzamine-treated animals might have been secondary to the fall in blood pressure. Calculation of renal arterial conductance confirmed that, while conductance fell in the intact animals, there was no such change in the denervated and phenoxybenzamine-treated animals, i.e. the residual decrease in RBF after interruption of the splenorenal reflex could indeed be accounted for by the fall in renal perfusion pressure. One might question why autoregulation did not prevent the fall in renal perfusion pressure from inducing a fall in RBF, given that MAP was within the normal autoregulatory range of the kidney. However, it has been shown that moderate sympathetic activation, such as would have been induced by splenic vein occlusion, raises the lower limit of autoregulatory control of RBF (Persson et al. 1990). As such, the fall in MAP could indeed have reduced renal blood flow, without changing renal vascular conductance.
Portal hypertension, induced either by cirrhosis or by portal vein ligation, is associated with a fall in MAP (Albillos et al. 1992; Bomzon et al. 1993; Kaufman & Levasseur, 2003), which is not blocked by renal denervation (Anderson et al. 1976). This is consistent with our observation that the fall in MAP induced by partial splenic vein ligation was likewise not blocked by splenic or renal denervation.
In conclusion, we have demonstrated that obstruction to splenic venous outflow, such as would be associated with portal hypertension, causes a reduction in RBF and a fall in MAP. While the change in RBF is neurally mediated through the splenorenal reflex, the fall in systemic blood pressure is unaltered by either splenic or renal denervation. It has previously been established that, in portal hypertension, renal function is influenced by both hepatorenal and intestinal–renal reflex pathways (Jalan et al. 1997; Miller et al. 1983). We now propose that, in addition, concomitant changes in intrasplenic haemodynamics also initiate splenorenal reflex pathways which contribute to the perturbations in renal and cardiovascular function associated with the condition.
Acknowledgments
This research was supported by grants from the Heart and Stroke Foundation of Alberta, NWT and Nunavut. We gratefully acknowledge the technical assistance of Jody Levasseur and Siu Lin Tam for measuring splenic and renal nerve activity.
References
- Albillos A, Colombato LA, Groszmann RJ. Vasodilatation and sodium retention in prehepatic portal hypertension. Gastroenterology. 1992;102:931–935. doi: 10.1016/0016-5085(92)90179-3. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Cronin RE, McDonald KM, Schrier RW. Mechanisms of portal hypertension-induced alterations in renal hemodynamics, renal water excretion, and renin secretion. J Clin Invest. 1976;58:964–970. doi: 10.1172/JCI108550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew PS, Deng Y, Kaufman S. Splenic denervation worsens lipopolysaccharide-induced hypotension, hemoconcentration and hypovolemia. Am J Physiol. 2001;280:R1564–R1572. doi: 10.1152/ajpregu.2001.280.5.R1564. [DOI] [PubMed] [Google Scholar]
- Bomzon A, Binah O, Blendis LM. Hypotension in experimental cirrhosis – is loss of vascular responsiveness to norepinephrine the cause of hypotension in chronic bile-duct-ligated dogs. J Hepatol. 1993;17:116–123. doi: 10.1016/s0168-8278(05)80531-8. [DOI] [PubMed] [Google Scholar]
- Calaresu FR, Tobey JC, Heidemann SR, Weaver LC. Splenic and renal sympathetic responses to stimulation of splenic receptors in cats. Am J Physiol. 1984;247:R856–R865. doi: 10.1152/ajpregu.1984.247.5.R856. [DOI] [PubMed] [Google Scholar]
- Chapman BJ, Horn NM, Robertson MJ. Renal blood-flow changes during renal nerve stimulation in rats treated with α-adrenergic and dopaminergic blockers. J Physiol. 1982;325:67–77. doi: 10.1113/jphysiol.1982.sp014136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Kaufman S. Splenorenal reflex regulation of arterial pressure. Hypertension. 2001;38:348–352. doi: 10.1161/01.hyp.38.3.348. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Sawin LL. Hepatorenal baroreflex in cirrhotic rats. Am J Physiol. 1995;269:G29–G33. doi: 10.1152/ajpgi.1995.269.1.G29. [DOI] [PubMed] [Google Scholar]
- Gatta A, Merkel C, Grassetto M, Milani L, Zuin R, Ruol A. Enhanced renal sympathetic tone in liver cirrhosis: evaluation by intrarenal administration of dihydroergocristine. Nephron. 1982;30:364–367. doi: 10.1159/000182519. [DOI] [PubMed] [Google Scholar]
- Gupta TK, Chen L, Groszmann RJ. Pathophysiology of portal hypertension. Baillière's Clin Gastroenterol. 1997;11:203–219. doi: 10.1016/s0950-3528(97)90036-1. [DOI] [PubMed] [Google Scholar]
- Hamza SM, Kaufman S. Vibrator prevents streaming during close-arterial infusion into kidney. Am J Physiol. 2004;286:F643–F646. doi: 10.1152/ajprenal.00290.2003. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Nickerson M. Reactions of dibenamine and some congeners with substances of biological interest in relation to the mechanism of adrenergic blockade. J Pharmacol Exp Ther. 1954;112:274–290. [PubMed] [Google Scholar]
- Henderson JM, Barnes DS, Geisinger MA. Portal hypertension. Curr Probl Surg. 1998;35:379–452. [PubMed] [Google Scholar]
- Henriksen JH, Ring-Larson H, Kanstrup IL, Christensen NJ. Splanchnic and renal elimination and release of catecholamines in cirrhosis. Evidence of enhanced sympathetic nervous activity in patients with decompensated cirrhosis. Gut. 1984;25:1034–1043. doi: 10.1136/gut.25.10.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman NL, Kostreva DR, Kampine JP. Splenic afferents and some of their reflex responses. Am J Physiol. 1982;242:R247–R254. doi: 10.1152/ajpregu.1982.242.3.R247. [DOI] [PubMed] [Google Scholar]
- Jalan R, Forrest EH, Redhead DN, Dillon JF, Hayes PC. Reduction in renal blood flow following acute increase in the portal pressure: evidence of the existance of a hepatorenal reflex in man? Gut. 1997;40:664–670. doi: 10.1136/gut.40.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman S, Levasseur J. Effect of portal hypertension on splenic blood flow, intrasplenic extravasation and systemic blood pressure. Am J Physiol. 2003;284:R1580–R1585. doi: 10.1152/ajpregu.00516.2002. [DOI] [PubMed] [Google Scholar]
- Kaufman S, Stelfox J. Atrial stretch induced diuresis in Brattleboro rats. Am J Physiol. 1987;252:R503–R506. doi: 10.1152/ajpregu.1987.252.3.R503. [DOI] [PubMed] [Google Scholar]
- Kew MC, Limbrick C, Varma RR, Sherlock S. Renal and intrarenal blood flow in non-cirrhotic portal hypertension. Gut. 1972;13:763–767. doi: 10.1136/gut.13.10.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel MH, Coburn JW, Mims MM, Goldstein H, Boyle JD, Rubini ME. Transplantation of cadaveric kidneys from patients with hepatorenal syndrome. Evidence for the functional nature of renal failure in advanced liver disease. N Engl J Med. 1969;280:1367–1371. doi: 10.1056/NEJM196906192802501. [DOI] [PubMed] [Google Scholar]
- Kostreva DR, Castaner A, Kampine JP. Reflex effects of hepatic baroreceptors on renal and cardiac sympathetic nerve activity. Am J Physiol. 1980;238:R390–R394. doi: 10.1152/ajpregu.1980.238.5.R390. [DOI] [PubMed] [Google Scholar]
- Meckler RL, Weaver LC. Persistent firing of splenic and renal afferent nerves after acute decentralization but failure to produce ganglionic reflexes. Neurosci Lett. 1988;88:167–172. doi: 10.1016/0304-3940(88)90120-6. [DOI] [PubMed] [Google Scholar]
- Miller RL, Sarfeh IJ, Sanderfer T, Malki A, Tidwell R, Mason GR. Acute portal hypertension and reduced renal blood flow: an intestinal-renal neurogenic reflex. J Surg Res. 1983;34:319–324. doi: 10.1016/0022-4804(83)90078-1. [DOI] [PubMed] [Google Scholar]
- Persson PB, Ehmke H, Nafz B, Kirchheim HR. Sympathetic modulation of renal autoregulation by carotid occlusion in conscious dogs. Am J Physiol. 1990;258:F364–F370. doi: 10.1152/ajprenal.1990.258.2.F364. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Graham RM, Sagalowsky A, Pettinger WA. Renal alpha-1 and alpha-2 adrenergic receptors: biochemical and pharmacological correlations. J Pharmacol Exp Ther. 1981;219:400–406. [PubMed] [Google Scholar]
- Smyth DD, Umemura S, Pettinger WA. Alpha-1 adrenoceptor selectivity of phenoxybenzamine in the rat kidney. J Pharmacol Exp Ther. 1984;230:387–392. [PubMed] [Google Scholar]