Abstract
Indigenous microbiota have several beneficial effects on host physiological functions; however, little is known about whether or not postnatal microbial colonization can affect the development of brain plasticity and a subsequent physiological system response. To test the idea that such microbes may affect the development of neural systems that govern the endocrine response to stress, we investigated hypothalamic–pituitary–adrenal (HPA) reaction to stress by comparing germfree (GF), specific pathogen free (SPF) and gnotobiotic mice. Plasma ACTH and corticosterone elevation in response to restraint stress was substantially higher in GF mice than in SPF mice, but not in response to stimulation with ether. Moreover, GF mice also exhibited reduced brain-derived neurotrophic factor expression levels in the cortex and hippocampus relative to SPF mice. The exaggerated HPA stress response by GF mice was reversed by reconstitution with Bifidobacterium infantis. In contrast, monoassociation with enteropathogenic Escherichia coli, but not with its mutant strain devoid of the translocated intimin receptor gene, enhanced the response to stress. Importantly, the enhanced HPA response of GF mice was partly corrected by reconstitution with SPF faeces at an early stage, but not by any reconstitution exerted at a later stage, which therefore indicates that exposure to microbes at an early developmental stage is required for the HPA system to become fully susceptible to inhibitory neural regulation. These results suggest that commensal microbiota can affect the postnatal development of the HPA stress response in mice.
Early postnatal life represents a period of bacterial colonization, a time when a previously sterile milieu is inhabited by microorganisms that are likely to remain as residents throughout the life of the animal. The human intestine is more densely populated with microbes than any other organ, and 1014 bacteria inhabit the gastrointestinal tract of adult humans, which exceeds the number of eukaryotic cells (1013) of which the human body is constituted (Tannock, 1999; Borrielo, 2002). Therefore, it seems natural that such colonizing bacteria would play a principal role in the postnatal maturation of the mammalian immune system (Sudo et al. 1997; Hooper & Gordon, 2001). In addition, these bacteria aid in the digestion and absorption of macromolecules and act as a barrier to gut pathogens by blocking attachment to gut binding sites, which is the first step of bacterial pathogenicity (Finlay & Falkow, 1990). Thus, there is no doubt that most of our bacterial symbionts have several beneficial effects on host physiological functions; however, little is known about whether or not such microbes can affect the development of brain plasticity and a subsequent physiological system response.
The hypothalamic—pituitary—adrenal (HPA) axis is a neuroendocrine system that is subjected to programming by early life events. For example, as adults, neonatally handled animals exhibit dampened HPA responses to stress compared with non-handled animals (Meaney et al. 1988). In contrast, adult animals exposed to repeated periods of prolonged maternal deprivation as neonates display increased HPA response to stress (Schmidt et al. 2002). These effects persist throughout the life of the animal and the resulting differences in HPA activity are associated with the incidence of age-related neuropathology (Meaney et al. 1988). Because of the close, bidirectional communication between the neural and immune systems (Turnbull & Rivier, 1999) early in life, a time when the central nervous system (CNS) is especially susceptible to environmental influences, we speculated that such microbial colonization and subsequent immune reaction during early life might alter the development of HPA responsiveness.
To test this hypothesis, we investigated the HPA response to stress by comparing genetically identical mice that had no exposure to microorganisms (germfree; GF), mice raised with a normal functional microbiota but not with specific pathogens (specific pathogen free; SPF) and mice raised with a selected group of organisms (gnotobiotic).
Methods
Animals
GF and SPF BALB/c mice (male, 9 weeks old) were maintained in Trexler-type flexible-film plastic isolators with sterile food and water (Sudo et al. 1997). Surveillance for bacterial contamination was done by a periodic bacteriological examination of faeces. To obtain Bifidobacterium infantis-, rabbit-derived enteropathogenic Escherichia coli (EPEC)-, or EPEC mutant strain (ΔTir)-monoassociated mice whose flora were composed of a single strain of bacterium, the parent GF mice were administered a bacterium orally, and their offspring thus became infected with this bacterium at the neonate stage. These mice were used for the experiment at 9 weeks of age. To produce SPF flora-reconstituted mice, the GF mice were inoculated with 0.5 ml of a 1 × 10−2 dilution of fresh SPF mouse faeces at either 1 or 3 weeks before the commencement of the stress protocol. Such reconstituted mice were subjected to the stress regimen at 9 or 17 weeks of age. All experiments were approved by the Ethics Committee for Animal Experiments of Kyushu University.
Stress protocol
Acute restraint stress was applied by placing the animals in a 50 ml conical tube (Nukina et al. 1998, 2001). Mice were killed by cervical dislocation before (basal), and immediately, 30, 60, or 120 min after being subjected to 1 h of restraint stress. This procedure was performed according to our Institutional Guidelines for Animal Experiments. For ether stress (Diorio et al. 1993), animals were maintained for 2.5 min in a glass container lined with absorbent paper soaked with ether, then killed by cervical dislocation before (basal), and immediately, 30, 60, or 120 min after ether exposure. Blood samples for ACTH and corticosterone measurements were obtained by cardiac puncture and stored at −80°C before assay. To avoid fluctuations in the biological measurements resulting from differences in circadian rhythm, all samples were collected at the same time of day (between 9.00 and 11.00 h).
Maternal behaviour
As described previously (Anisman et al. 1998; Francis et al. 1999), maternal behaviour was scored for three 60 min observation periods daily (starting at 09.00, 13.00 and 17.00 h) on the second and third days postpartum. The behaviour of each mother (n = 8) was scored every 4 min (total of 360 samplings per group). The data were analysed as the percentage of observations in which animals engaged in the target behaviour. Individuals unaware of the origin of the animals checked the following behaviours: mother off pups, mother licking/grooming any pup, mother nursing pups in an arched-back posture, nursing in a ‘blanket’ posture in which the mother lays over the pups, or nursing in a passive posture in which the mother is lying either on her back or side while the pups nurse. Mean (± s.d.) frequency of the behaviours is expressed as a percentage of the total observation.
Determination of ACTH and corticosterone levels
The plasma level of ACTH was measured by an immunoradiometric assay using the Allégro HS-ACTH kit (Nihon Medi-Physics Co., Ltd, Nishinomiya Japan; Zahradnik et al. 1989). In this assay, the concentration of ACTH is linearly related to the amount of radioactivity bound to the beads over a wide analytical range (1–1500 pg ml−1). This assay system has been demonstrated to have a reliable sensitivity of 1 pg ml−1.
The plasma level of corticosterone was measured using a commercially available radioimmunoassay kit (ICN Biomedicals, Costa Mesa, CA, USA). The concentration of corticosterone in the serum samples was calculated from a standard curve and expressed in nanograms per millilitre. The detection limit of the assay was about 1 ng ml−1.
Determination of faecal bacterial flora
The faecal bacterial flora was determined according to methods previously described (Mitsuoka et al. 1965; Benno & Mitsuoka, 1992; Sudo et al. 2000). Briefly, approximately 1 g (wet weight) was suspended in an anaerobic diluent, and then serial tenfold dilutions from 10−1 to 10−8 were prepared. From the appropriate dilution, a 0.05 ml aliquot was then spread on three non-selective agar plates (trypticase soy blood agar, glucose blood liver agar, Eggerth-Gagnon agar) and selective agar plates to allow the incubation of Streptococci, Enterobacteria, Staphylococci, yeasts, Pseudomonas, Bacteroides, Bifidobacteria, Eubacteria, Veillonella, Lactobacilli and Clostridia (Eiken, Tokyo, Japan; OXOID, Basingstoke, UK). Staphylococcus medium no. 110 supplemented with 2.5% egg yolk and 5 μg ml−1 methicillin (Nissui, Tokyo, Japan) was used for methicillin-resistant Staphylococcus aureus and methicillin-sensitive Staphylococcus aureus. After incubation for 2 days (aerobes) or 3 days (anaerobes), 13 bacterial groups and yeasts were identified by colonial and cellular morphology, Gram staining, spore formation, and aerobic and anaerobic growth.
Semi-quantitative RT-PCR methods
Gene expression levels of corticotropin-releasing factor (CRH), glucocorticoid receptor (GR) and NMDA receptor subunits (NR-1 and NR-2a) were analysed by a semiquantitative RT-PCR method. The animals were killed by cervical dislocation and each brain was removed immediately. Total RNA was extracted from the cortex, hippocampus and hypothalamus of the GF and SPF mice using a commercially available kit (Sepasol-RNA II, Nacalai Tesque, Kyoto, Japan), according to the manufacturer's instructions. Each brain section was identified according to a stereotaxic atlas (Paxinos & Franklin, 2001). To normalize the signals from different RNA samples, glyceraldehydes-3-phosphate dehydrogenase (GAPDH) mRNA was co-amplified as an internal standard and then the relative values of each transcript to GAPDH mRNA were calculated. In preliminary experiments, optimal reaction parameters were adjusted to obtain a linear relationship between the number of PCR cycles and RT-PCR products and between the initial amount of RNA and RT-PCR products. Additionally, to verify the linearity of GAPDH signals in each experiment, RT-PCR products were collected after 26, 28, 30, 32 and 34 cycles in each sample and then checked to see if the GAPDH signals would be within the linear range of product accumulation. The following primers were designed according to the method described in the indicated literature, and their validity was checked in our laboratory: GAPDH (Nukina et al. 2001), sense primer 5′-TCCTGCACCACCAACTGCTTAG-3′, antisense primer 5′-TCTTACTCCTTGGAGGCCATGT-3′; c-Fos (Arrieta et al. 2000), sense primer 5′-CCCCTGTCAACACACAGGAC-3′, antisense primer 5′-CCGATGCTCTGCGCTCTGC-3′; CRH (Glasgow et al. 1999), sense primer 5′-AACTCAGAGCCCAAGTACGTTGAG-3′, antisense primer 5′-TCACCCATGCGGATCAGAATC-3′; GR (Kizaki et al. 1996), sense primer 5′-GCATGGAGAATTATGACCAC-3′, antisense primer 5′-ATCAGATCAGGAGCAAAGCA-3′; NR-1 (Cai & Rhodes, 2001), sense primer 5′-CTCCCACCAGTCCAGCGTCT-3′, antisense primer 5′-GTCATGTTCAGCATTGCGGC-3′; NR-2a (Cai & Rhodes, 2001), sense primer 5′-GGCTGTCAGCACTGAATCCAAAGG-3′, antisense primer 5′-CGAAAGGCAGCTTCTGCAATGTGTG-3′. PCR products were separated by electrophoresis on 1.5% agarose gel and visualized by ethidium bromide staining and UV light. Densitometric analysis was then done to quantify the mRNA levels using public domain NIH image software.
Measurement of neurotrophin and CRF protein levels
Sections of cortex, hippocampus and hypothalamus were quickly removed after the animals were killed and samples homogenized in a lysis buffer (137 mm NaCl, 20 mm Tris, 1% NP-40, 10% glycerol, 1 mm PMSF, 10 μg ml−1 aprotinin, 1 μg ml−1 leupeptin and 0.5 mm sodium vanadate). Homogenates were centrifuged and the supernatants used as enzyme-linked immunosorbent assay (ELISA) samples. Neurotrophin protein concentrations were determined by the method of Bradford, using bovine serum albumin as a standard. ELISAs were performed using the brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), or nerve growth factor (NGF) Emax ImmunoAssay System kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. CRF protein levels in the hypothalamus were measured using a commercially available radioimmunoassay kit (Phoenix Pharmaceuticals Inc., Belmont, CA, USA).
Assays for cytokine levels in plasma
The plasma bioactivity of interleukin (IL)-6 was determined by measuring the proliferation of the B9 cell line, an IL-6-dependent B cell hybridoma, as previously described (Nukina et al. 1998, 2001). The B9 cell line was kindly provided by Dr L. Aarden, the Netherlands Red Cross Blood Transfusion Service, Amsterdam, the Netherlands (Aarden, 1987). Briefly, B9 cells (5 × 103 per 100 μl) were cultured in 96-well microtiter plates with serial dilutions of the plasma samples. After 72 h of incubation at 37°C with 5% CO2, 20 μl of MTT tetrazolium (5 mg ml−1; Research Organics Inc., Cleveland, OH, USA) was added to determine the proliferation. After an additional 4 h of incubation the supernatant was removed and 100 μl of 10% SDS with 0.01 n HCl was added to dissolve the crystals. The level of cell proliferation was determined using a microplate ELISA reader at 570 nm. The IL-6 activity in the plasma samples was calculated based on a purified recombinant mouse IL-6 standard (Boehringer Mannheim Corp., Mannheim, Germany) run in the same assay. The sensitivity of the assay was about 1 pg ml−1 and the specificity of the assay was confirmed by using IL-6 neutralizing antibody, which can antagonize 95% of the B9 cell proliferation induced by the mouse plasma.
The IL-1β level of the plasma samples was assayed using a commercially available ELISA kit (BioSource International, Camarillo, CA, USA).
Anti-IL-6 treatment of mice
In some experiments, pre-treatment with anti-IL-6 antibody was done to neutralize the plasma IL-6 activity upon exposure to Bifidobacterium infantis. Hybridoma producing rat monoclonal antibody to mouse rIL-6, MP5-20F3 clone (Starnes et al. 1990) was obtained from the American Type Culture Collection (Rockville, MD, USA) by courtesy of the DNAX Research Institute of Molecular and Cellular Biology (Palo Alto, CA, USA). Purified monoclonal antibodies from the ascitic fluid from athymic nude mice were then used in the experiment. The proper dosage of anti-IL-6 antibody, that which sufficiently neutralizes plasma IL-6 response, was determined according to previous reports (Neta et al. 1992; Nukina et al. 1998). Chromatographically purified rat IgG (Seikagaku Corp., Tokyo, Japan) was also used as a control antibody.
Statistical analysis
All data are expressed as the means ±s.d. The data were analysed by Dunnett's post hoc test after the factorial analysis of variance. In some experiments, statistical analysis was done using the Mann—Whitney U test. A value of P < 0.05 was considered to indicate significant difference.
Results
Plasma ACTH and corticosterone responses of GF mice were more susceptible to restraint stress than those of SPF mice
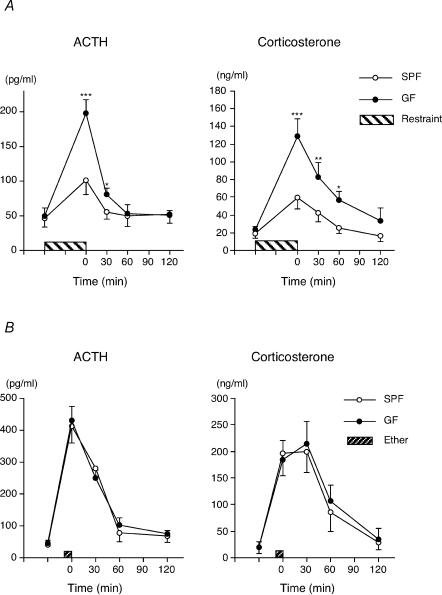
To investigate the difference in HPA response to stress stimuli between the GF and SPF mice, both groups of mice were subjected to either 1 h of restraint stress or ether exposure. Plasma ACTH and corticosterone elevation in response to restraint stress was substantially higher in GF mice than in SPF mice (Fig. 1A). When the mice were exposed to ether stimulus, no significant difference in plasma ACTH or corticosterone response was found between the groups of animals (Fig. 1B).
Figure 1. Increased plasma ACTH and corticosterone response to restraint stress, but not to ether exposure in GF mice.
A, mice were subjected to a 1 h period of restraint stress (GF, n = 6–11 for each time point, total of 52 animals; SPF, n = 6–11 for each time point, total of 50 animals). The baseline data were obtained by cardiac puncture from mice that were killed by cervical dislocation before stress exposure. The baseline ACTH and corticosterone levels in the GF and SPF mice were 49 ± 12 pg ml−1 and 23 ± 4.2 ng ml−1, in the GF mice, 46 ± 13 pg ml−1 and 19 ± 5.6 ng ml−1, respectively. P < 0.05, **P < 0.01, ***P < 0.001 in Dunnett's post hoc test between GF and SPF. B, GF and SPF mice failed to show any difference in HPA response to ether exposure (n = 6 for each time point, total of 30 animals per group).
GF mice revealed reduced expression levels of cortical GR transcript
Morphological examination of the adrenal and pituitary glands by HE staining failed to reveal any obvious difference of structure or cell type between the GF and SPF mice. The average adrenal cortical thicknesses were 310 ± 35.4 μm in GF mice (n = 5) and 306.6 ± 28.8 μm in SPF mice (n = 6). Furthermore, when pituitary sections were stained with anti-ACTH antibodies, no quantitative difference was noted in the corticotrophs between the groups of animals. However, in the hypothalamus, the mRNA expression level of CRF transcript, a main stimulator of ACTH secretion from the pituitary gland, was significantly higher in GF mice than in SPF mice (relative values of CRF to GAPDH mRNA: GF 1.36 ± 0.10 versus SPF 0.85 ± 0.13, n = 5 per group, P < 0.05 by Mann—Whitney U test). Such elevated CRF mRNA levels in the hypothalamus of GF mice were also confirmed by CRF protein concentrations (GF 9.8 ± 3.4 ng mg−1 protein versus SPF 5.3 ± 2.7 ng mg−1 protein, n = 6 per group, P < 0.05). The mRNA expression level of GR, which negatively regulates HPA axis activation by inhibiting hypothalamic CRF gene expression, was significantly lower in the cortex, but not the hypothalamus or hippocampus, of GF mice than in SPF mice (relative values of GR to GAPDH mRNA: cortex, GF 0.94 ± 0.69 versus SPF 1.82 ± 0.62; hypothalamus, GF 0.49 ± 0.16 versus SPF 0.48 ± 0.34; hippocampus, GF 3.21 ± 0.72 versus SPF 3.92 ± 0.77; n = 4–7 per group, P < 0.05).
The GF condition failed to affect maternal behaviour
In view of previous data indicating that interaction of the dam with her litter can program HPA development (Liu et al. 1997, 2000), maternal behaviour was scored for three 60 min observation periods daily on the second and third days postpartum. No difference in maternal behaviour was observed, with GF and SPF dams equally arch-backed-nursing or grooming/licking their pups (arch-backed nursing: GF 13.0 ± 2.9% versus SPF 11.8 ± 3.9%; grooming licking: GF 5.7 ± 1.3% versus SPF 5.2 ± 1.7%), suggesting that the enhanced HPA stress response of the GF mice was unlikely to have been due to reduced maternal contact.
The GF mice showed reduced expression levels of cortical and hippocampal BDNF
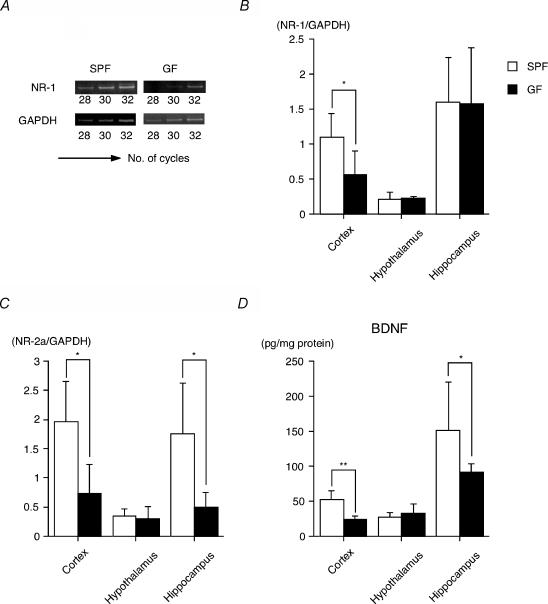
Previous reports demonstrated that early life events that take place during brain maturation can modulate the expression of neurotrophins of cellular plasticity within selected brain regions (Liu et al. 2000; Roceri et al. 2002). We therefore compared the expression levels of neurotrophins and their related receptors in the various brain areas of the GF mice with those of the SPF mice. A semiquantitative RT-PCR analysis of NR subunits, neurotransmitters that regulate the expression of BDNF, showed decreased gene expression of cortical NR-1 and NR-2a and hippocampal NR-2a subunits in GF mice compared with SPF mice (Fig. 2A–C). Consistent with these results, the BDNF protein level in the cortex and hippocampus was significantly lower in GF mice than in SPF mice (Fig. 2D), whereas other neurotrophins, NT-3 and NGF, in these areas of GF mice were identical to those of SPF mice (NT-3: cortex, GF 66.8 ± 13.2 versus SPF 65.8 ± 10.0; hippocampus, GF 126.5 ± 37.8 versus SPF 124.0 ± 14.1; NGF: cortex, GF 169.0 ± 87.0 versus SPF 188.7 ± 82.8; hippocampus, GF 136.4 ± 58.1 versus SPF 159.0 ± 87.5 pg (mg protein) −1).
Figure 2. NR subunit gene expression and protein BDNF concentration.
A, a typical RT-PCR product with NR-1 specific primers revealed PCR products with the predicted 333 bp length of NR-1 mRNA. Total RNA was extracted from a single cerebral cortex of GF and SPF mice. NR-1 (B) and NR-2a transcripts (C) were detected by RT-PCR in the cortex, hypothalamus or hippocampus of GF and SPF mice (9 weeks old). Histograms show the relative band intensities on densitometric analysis as ratios of NR subunit and GAPDH mRNA after 30 cycles of amplification (n = 4–7 per group). D, BDNF protein concentration was measured by ELISA (n = 6–10 per group). *P < 0.05, **P < 0.01 by Mann—Whitney U test.
HPA response to stress in the gnotobiotic mice
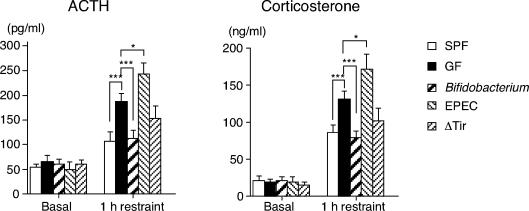
To further elucidate the involvement of gut microbiota in the HPA stress response, gnotobiotic mice whose flora were composed of a single strain of bacterium at the neonate stage were tested for their susceptibility to restraint stress at 9 weeks of age. Monoassociation with Bifidobacterium infantis, which is a representative inhabitant of the neonate gut, dampened the HPA stress response to the SPF (Fig. 3). In accordance with previous reports that neonatal endotoxin treatment increases the HPA stress response later in life (Shanks et al. 1995, 2000), the hormonal stress response in rabbit-derived enteropathogenic Escherichia coli (EPEC)-monoassociated mice was substantially higher than that in GF mice, although no such exaggerated response was found in the mice reconstituted with an EPEC mutant strain, ΔTir (Kenny et al. 1997), which is not internalized owing to defects in the translocated intimin receptor. Since there was no difference in the number of intestinal bacteria between the EPEC- and ΔTir-associated mice, these results indicate that bacterial internalization to the intestinal epithelial layer is an indispensable condition that enables the EPEC strain to influence the regulatory system of the HPA response.
Figure 3. Effects of restraint stress on plasma ACTH and corticosterone levels in gnotobiotic mice.
Plasma ACTH and corticosterone levels were measured before or immediately after 1 h restraint in GF (n = 20), SPF (n = 18) and monoassociated mice (n = 18–24 per group) at 9 weeks of age. *P < 0.05, ***P < 0.001 by Dunnett's test.
Colonization by bacterium induced increases in the c-Fos mRNA levels in the paraventricular nucleus and in the corticosterone and cytokine concentrations in the plasma
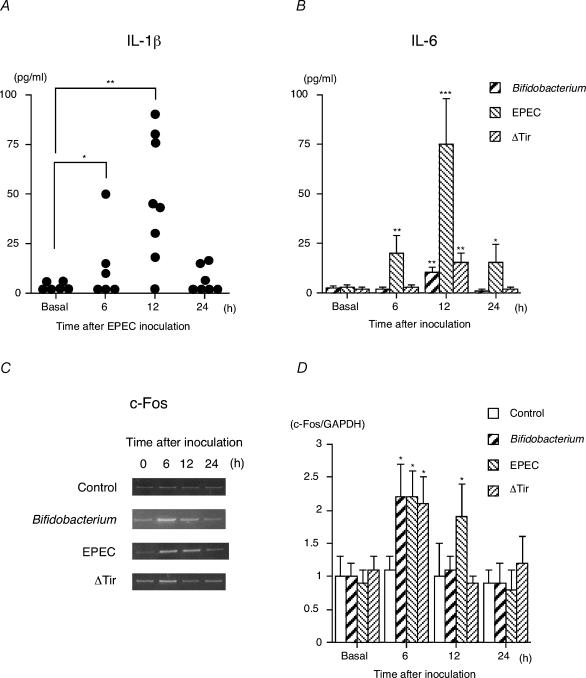
To further clarify the mechanism involved in the different sensitivity of each gnotobiotic mouse to stress, GF mice were orally inoculated with EPEC, ΔTir or Bifidobacterium infantis. Then either the IL-1β and IL-6 levels in the plasma or the c-Fos expression level in the paraventricular nucleus, a marker for neuronal activity, was measured before and 6, 12 and 24 h after the inoculation with each bacterium. IL-1β and IL-6 levels in the plasma substantially increased and reached a peak at 12 h after the inoculation with EPEC (Fig. 4A and B). Administration of ΔTir or Bifidobacterium infantis also triggered a small but significant increase in plasma IL-6 levels at 12 h after the inoculation without elevating the plasma IL-1β levels. Interestingly, the c-Fos mRNA level in the paraventricular nucleus was already elevated at 6 h after the inoculation regardless of which of the bacterial strains was used (Fig. 4C and D). This c-Fos response was accompanied by a concomitant increase in plasma corticosterone levels on exposure to microbes (control: basal 19 ± 10 ng ml−1, 6 h 25 ± 12 ng ml−1, 12 h 26 ± 16 ng ml−1, 24 h 17 ± 9 ng ml−1; Bifidobacterium: basal 20 ± 11 ng ml−1, 6 h 142 ± 36*** ng ml−1, 12 h 35 ± 26 ng ml−1, 24 h 30 ± 19 ng ml−1; EPEC: basal 19 ± 8 ng ml−1, 6 h 175 ± 41*** ng ml−1, 12 h 120 ± 29*** ng ml−1, 24 h 86 ± 24** ng ml−1; ΔTir: basal 22 ± 14 ng ml−1, 6 h 116 ± 32*** ng ml−1, 12 h 60 ± 41 ng ml−1, 24 h 51 ± 32 ng ml−1; n = 5–7 for each time point; *P < 0.05, **P < 0.01, ***P < 0.001 versus each corresponding basal value). Moreover, pretreatment with anti-IL-6 antibody failed to affect the elevated c-Fos response in the paraventricular nucleus (Fig. 5A and B) and corticosterone response in the plasma (Fig. 5C) on exposure to Bifidobacterium infantis. These results taken together indicate that visceral information derived from bacterial colonization can be transmitted to the brain at least partly through a humoral cytokine-independent pathway, probably via a neural route.
Figure 4. Kinetics of cytokine concentration in the plasma and c-Fos gene expression in the paraventricular nucleus upon exposure to Bifidobacterium infantis, EPEC or ΔTir.
GF mice at 5 weeks of age received a gavage of either 0.5 ml skimmed milk containing one of the bacterial strains (1 × 109 CFU; Bifidobacterium infantis, EPEC or ΔTir) or skimmed milk alone (control), after which plasma and brain samples were collected before (basal) and 6, 12 or 24 h after inoculation with each bacterium. A, the plasma IL-1β level of the mice exposed to EPEC was measured by ELISA (n = 6–8 for each time point). No significant IL-1β elevation in the plasma was found after inoculation with Bifidobacterium infantis, ΔTir or skimmed milk alone (control). B, plasma IL-6 levels were analysed by the B9 cell bioassay as described in the Methods (n = 6–8 for each time point). C, a typical RT-PCR product with c-Fos specific primers revealed PCR products with the predicted 247 bp length of c-Fos mRNA. D, histogram shows the relative band intensities on densitometric analysis as ratios of c-Fos and GAPDH mRNA after 30 cycles of amplification (n = 4 for each time point). *P < 0.05, **P < 0.01 and ***P < 0.001 were considered to be significantly different from the corresponding basal values.
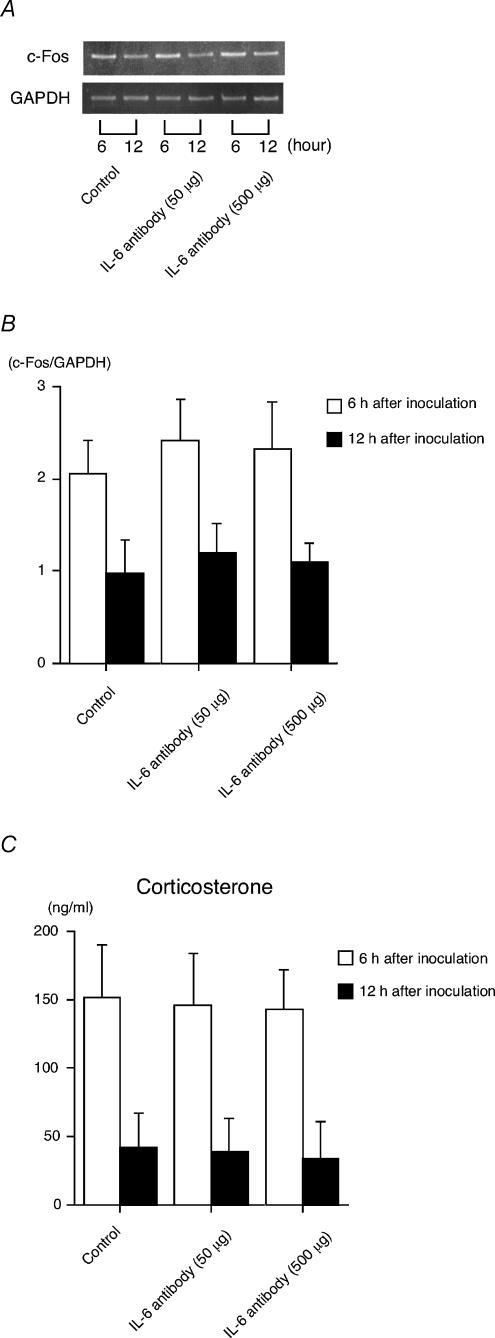
Figure 5. Effects of anti-IL-6 treatment on the c-Fos expression in the paraventricular nucleus and the corticosterone response in the plasma.
GF mice at 5 weeks of age were injected intraperitoneally with either anti-IL-6 antibody (MP5-20F3; 50 or 500 μg) or control rat IgG antibody (control) 1 h before being inoculated with Bifidobacterium infantis. The analysis of c-Fos mRNA expression levels in the paraventricular nucleus was done at 6 or 12 h after the inoculation. A, the results shown are representative of 4 independent experiments. B, histogram shows the relative band intensities on densitometric analysis as ratios of c-Fos and GAPDH mRNA after 30 cycles of amplification (n = 4 for each time point). C, determination of the plasma corticosterone levels was carried out according to the protocol described in the Methods (n = 6 for each time point).
A complete SPF flora partly reversed the HPA response to stress only when the flora was introduced at an early stage of development
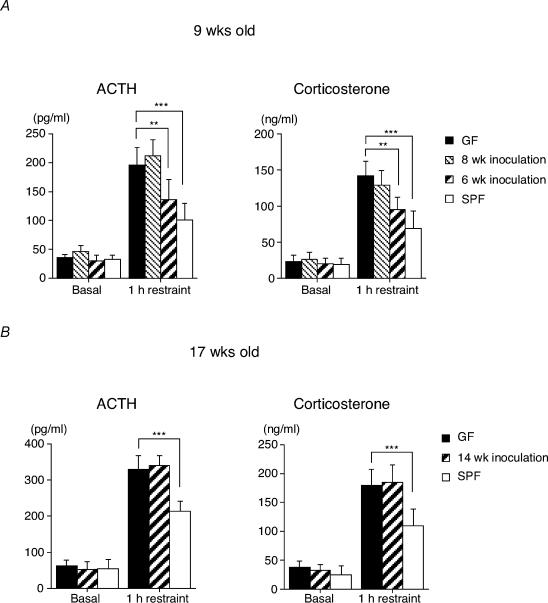
Finally, the effects of a complete SPF flora on the hormonal response to stress were examined. The enhanced HPA stress response of GF mice was partly corrected 3 weeks after reconstitution of SPF faeces at an early stage of development (Fig. 6A), while such correction was not found following any reconstitution exerted at a later stage (Fig. 6B). Bacterial examination of faecal samples revealed that colonization was almost completely accomplished 3 weeks after the administration of the SPF faeces, whereas aerobes, such as Escherichia coli, were more prominent than anaerobes 1 week after the administration of the SPF faeces. Collectively, these findings suggest that exposure to indigenous microbiota at an early developmental stage, when brain plasticity may still be preserved, is required for the HPA system to become fully susceptible to inhibitory neural regulation.
Figure 6. Effects of restraint stress on plasma ACTH and corticosterone levels in mice reconstituted with SPF faeces.
SPF flora-reconstituted mice were established by orally introducing fresh SPF mouse faeces into the GF mice at either 1 or 3 weeks before they were subjected to the stress protocol. Restraint stress was applied to these reconstituted mice at 9 (A) and 17 weeks of age (B) (n = 18–24 per group). **P < 0.01, ***P < 0.001 by Dunnett's post hoc test.
Discussion
Accumulating evidence has demonstrated a bidirectional communication between the brain and the gut. Researchers in this field preferentially call this cross-talk the ‘brain—gut axis’ (Aziz & Thompson, 1998). Indeed, it has been shown that a stressful experience can lead to altered gastrointestinal motility, secretions and blood flow; while, in turn, such alteration in gastrointestinal function is transmitted to the brain and can ultimately bring about the perception of visceral events such as nausea, satiety and pain (Drossman, 1998). Interestingly, a number of research papers reported that physical and psychological stress can affect the composition of intestinal microbiota in rodents (Porter & Rettger, 1940; Tannock & Savage, 1974; Suzuki et al. 1983) and primates (Holdeman et al. 1976; Bailey & Coe, 1999). The present results, in which colonizing microbes altered the HPA response to restraint stress, indicate that the interaction of gut bacteria with the brain is also bidirectional, just like the brain—gut axis. To our knowledge, this is the first report that shows commensal microbes affecting the neural network responsible for controlling stress responsiveness.
In this study, the HPA response of the GF mice was more sensitive to restraint stress than that of the SPF mice, while both groups of mice failed to show any difference in the sensitivity to ether stress. Additionally, GF mice also exhibited reduced BDNF expression levels in the cortex and hippocampus relative to SPF mice. Because the HPA response to restraint stress is affected by the limbic system, including the prefrontal cortex, hippocampus and amygdala, and requires assembly and processing of signals from multiple sensory modalities before initiation of a stress response, whereas ether stress does not (Herman & Cullian, 1997), these results indicate that cognitive processing in the limbic system may be involved in an exaggerated HPA response by GF mice. Nonetheless, it should be noted that we cannot rule out the possibility that the sensitivity of our methods is too low to detect a minimal change in the GR and NR subunit mRNA levels in the hypothalamus. More quantitative methods, such as real-time PCR and in situ hybridization, are needed to definitely conclude region-specific changes in the GR or NR subunit gene expression.
To date, we cannot clearly explain the exact mechanism by which the visceral signals originating from bacterial colonization are transmitted to the brain; however, the following pathways have been suggested to be involved: one is through a cytokine-mediated humoral route and the other is via a neural pathway. As summarized in a recent review by Turnbull & Rivier (1999), there is now overwhelming evidence that several cytokine families, especially interleukin-1, increase the secretory activity of the HPA axis; hence, it is reasonable to suppose that endotoxin and/or peptidoglycan, components of the bacterial cell wall, stimulate immune cells within the gut or elsewhere to release these cytokines, which consequently influence the parts of the CNS involved in the regulation of the HPA axis response. In fact, a previous report by Dahlgren et al. (1995) and our results showing that plasma IL-1 and IL-6 levels substantially increase upon exposure to EPEC also support the significant role of this humoral route. In this respect, since it was demonstrated that Gram-negative bacteria, especially bacteria such as Escherichia coli, translocated in large numbers to the mesenteric lymph node, whereas obligately anaerobic bacteria did so at only very low levels (Steffen et al. 1988), such pronounced cytokine response after exposure to EPEC may be due to the enhanced translocation rates of this strain.
Alternatively, c-Fos activation in the paraventricular nucleus was rapidly induced at 6 h after the inoculation of Bifidobacterium infantis or ΔTir, when plasma cytokine levels had not yet elevated. Together with results showing the inability of anti-IL-6 antibody pretreatment to block such an increase in the brain c-Fos expression and plasma corticosterone elevation upon exposure to Bifidobacterium infantis, these results thus suggest the importance of another pathway, probably via a neural-mediated pathway. In accord with this speculation, Wang et al. (2002) reported that subdiaphragmatic vagotomy attenuates c-Fos expression in the paraventricular nucleus and supraoptic nucleus in rats inoculated with Salmonella Typhimurium. Clearly, further studies, especially using molecular techniques, will be necessary to clarify how and to what extent neural- and cytokine-mediated pathways can contribute to the flora-mediated modulation of the HPA response. However, our experimental system using gnotobiotic animals proved to be a useful animal model for this clarification.
Recently, Rescigno and colleagues (Rescigno et al. 2001) have reported a new mechanism for bacterial uptake in the mucosal tissues that is mediated by dendritic cells, which open the tight junctions between epithelial cells, send dendrites outside the epithelium and directly sample both pathogenic and non-pathogenic bacteria that are unable to induce their own phagocytosis through the M cells. In addition, a recent in vitro study (Dunzendorfer et al. 2001) showed a variety of neuropeptides, including calcitonin gene-related peptide, vasoactive intestinal polypeptide and the neurotransmitter secretin, to induce chemotaxis of immature dendritic cells. Since there is a close anatomical association between the neuropeptide-containing nerves and dendritic cells (Bellinger et al. 2001), these findings taken together indicate the potentially important role of dendritic cells in signal transmission from the gut lumen to the CNS. A new project in our laboratory is now in progress, in which a DNA microarray technique is being applied to the gnotobiotic animal model to further clarify the precise molecular mechanism whereby dendritic cells interact with intestinal bacteria.
The series of experiments performed by Husebye et al. (1994, 2001) has shown different bacterial strains to differentially affect the motility of the gastrointestinal tract. Therefore, it should be noted that such a change in the gut motility after bacterial colonization is perceived as new visceral information and may then indirectly modulate the microbiota-induced alteration in the endocrine stress response.
Although the glucocorticoid response to stress is essential for survival, prolonged glucocorticoid elevation can present serious health risks, including diabetes, hypertension, hyperlipidaemia, hypercholesterolaemia, arterial disease, amenorrhoea, impairment of growth and tissue repair and immunosuppression (McEwen, 1998). In an elegant study by Shanks et al. (2000), it was clearly demonstrated that exposure of neonatal rats to a low dose of endotoxin (0.05 mg kg−1) resulted in long-term changes in HPA axis activity, with elevated mean plasma corticosterone concentrations that resulted from increased corticosterone pulse frequency and pulse amplitude. In addition, they also showed neonatal endotoxin exposure to have long-lasting effects on immune regulation, including increased sensitivity of lymphocytes to stress-induced suppression of proliferation and a remarkable protection from adjuvant-induced arthritis. Such a protective effect of pretreatment with endotoxin in adults on the development of arthritis was also confirmed in a later study by Harbuz et al. (2002). Together with the present results that the EPEC strain enhanced the HPA response to stress, these findings lead to the clinically important possibility that neonatal infection with pathological bacteria may alter the development of neural systems that govern the endocrine response to stress and may thereby predispose the individuals to stress-related pathology later in life. Alternatively, the enhanced HPA response to stress in GF mice was partly reversed by the addition of a complete SPF faeces at an early stage of development, indicating that either a complete indigenous microbiota containing aerobic and anaerobic bacteria or some strains of anaerobic bacteria including Bifidobacterium may play a protective role against the deleterious effects of elevated plasma glucocorticoid levels.
In summary, the present study shows that commensal microbiota are an environmental determinant that regulates the development of the HPA stress response. These findings indicate that the series of events in the gastrointestinal tract following postnatal microbial colonization can have a long-lasting impact on the neural processing of sensory information regarding the endocrine stress axis. Our concept, based on in vivo findings, provides evidence of a novel link between indigenous microorganisms and the nervous system and shows a new aspect of the brain—gut axis.
Acknowledgments
This work was supported in part by a Grant-in-Aid for General Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (no. 13470014-0) and by a Grant-in-Aid from the Yakult Bioscience Research Foundation.
References
- Aarden LA, DeGroot ER, Schaap OL, Lansdorf PM. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17:1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors. Int J Dev Neurosci. 1998;16:149–164. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Arrieta I, Camacho-Arroyo I, Mendoza-Rodríguez CA, Cerbón MA. c-Fos gene expression pattern in the hypothalamus and the preoptic area of defeminized rats. Brain Res. 2000;867:100–106. doi: 10.1016/s0006-8993(00)02244-7. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology. 1998;114:559–578. doi: 10.1016/s0016-5085(98)70540-2. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol. 1999;35:146–155. [PubMed] [Google Scholar]
- Bellinger DL, Lorton D, Lubahn C, Felten DL. Innervation of lymphoid organs: association of nerves with cells of the immune system and their implications in disease. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. 3. San Diego, CA, USA: Academic Press; 2001. pp. 55–111. [Google Scholar]
- Benno Y, Mitsuoka T. Impact of Bifidobacterium longum on human fecal microflora. Microbiol Immunol. 1992;36:683–694. doi: 10.1111/j.1348-0421.1992.tb02071.x. [DOI] [PubMed] [Google Scholar]
- Borrielo SP. The normal flora of the gastrointestinal tract. In: Hart AL, Stagg AJ, Graffner H, Glise H, Falk P, Kamm NA, editors. Gut Ecology. Trowbridge, UK: Cromwell Press; 2002. pp. 1–24. [Google Scholar]
- Cai Z, Rhodes PG. Intrauterine hypoxia-ischemia alters expression of the NMDA receptor in the young rat brain. Neurochem Res. 2001;26:487–495. doi: 10.1023/a:1010904727225. [DOI] [PubMed] [Google Scholar]
- Dahlgren UI, Midtvedt T, Tarkowski A. Transient appearance of circulating interleukin-6 and tumor necrosis factor in germ-free C3H/HeJ and C3H/HeN mice upon intestinal exposure to E. coli. In: Mestecky J, Russell MW, editors. Advances in Mucosal Immunology. New York, NY, USA: Plenum Press; 1995. pp. 459–462. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the median prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman DA. Presidential address: gastrointestinal illness and the biopsychosocial model. Psychosom Med. 1998;60:258–267. doi: 10.1097/00006842-199805000-00007. [DOI] [PubMed] [Google Scholar]
- Dunzendorfer S, Kaser A, Meierhofer C, Tilg H, Wiedermann CJ. Cutting edge: peripheral neuropeptides attract immature and arrest mature blood-derived dendritic cells. J Immunol. 2001;166:2167–2172. doi: 10.4049/jimmunol.166.4.2167. [DOI] [PubMed] [Google Scholar]
- Finlay BB, Falkow S. Cell adhesion and invasion mechanisms in microbial pathogenesis. Curr Opin Cell Biol. 1990;2:815–820. doi: 10.1016/0955-0674(90)90078-s. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Glasgow E, Kusano K, Chin H, Mezey É, Young WS, III, Gainer H. Single cell reverse transcription-polymerase chain reaction analysis of rat supraoptic magnocellular neurons: neuropeptide phenotypes and high voltage-gated calcium channel subtypes. Endocrinology. 1999;140:5391–5401. doi: 10.1210/endo.140.11.7136. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Chover-Gonzalez A, Gibert-Rahola J, Jessop DS. Protective effect of prior acute immune challenge but not footshock, on inflammation in the rat. Brain Behav Immun. 2002;16:439–449. doi: 10.1006/brbi.2001.0658. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullian WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Holdeman LV, Good IJ, Moore WEC. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol. 1976;31:359–375. doi: 10.1128/aem.31.3.359-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- Husebye E, Hellstrom PM, Midtvedt T. Intestinal microflora stimulates myoelectric activity of rat small intestine by promoting cyclic initiation and aboral propagation of migrating myoelectric complex. Dig Dis Sci. 1994;39:946–956. doi: 10.1007/BF02087542. [DOI] [PubMed] [Google Scholar]
- Husebye E, Hellström PM, Sundler F, Chen J, Midtwedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol. 2001;280:G368–G380. doi: 10.1152/ajpgi.2001.280.3.G368. [DOI] [PubMed] [Google Scholar]
- Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. Enteropathogenic E-coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- Kizaki T, Oh-ishi S, Ookawara T, Yamamoto M, Izawa T, Ohno H. Glucocorticoid-mediated generation of suppressor macrophages with high density FcγRII during acute cold stress. Endocrinology. 1996;137:4260–4267. doi: 10.1210/endo.137.10.8828485. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis D, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Bhatnagar S, Van Berkel C, Sapolsky RM. Postnatal handling attenuates neuroendocrine, anatomical, and cognitive impairments related to the aged hippocampus. Science. 1988;238:1766–1768. [Google Scholar]
- Mitsuoka T, Sega T, Yamamoto S. Eine verbesserte Methodik der qualitativen und quantitaven Analyse der Darmflora von Menschen und Tieren. Zbl Bakt Hyg I Abt Orig. 1965;195:445–469. [PubMed] [Google Scholar]
- Neta R, Perlstein R, Vogel SN, Ledney D, Abrams J. Role of interleukin 6 in protection from lethal irradiation and in endocrine responses to IL-1 and tumor necrosis factor. J Exp Med. 1992;175:689–694. doi: 10.1084/jem.175.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukina H, Sudo N, Aiba Y, Oyama N, Koga Y, Kubo C. Restraint stress elevates the plasma interleukin-6 levels in germ-free mice. J Neuroimmunol. 2001;115:46–52. doi: 10.1016/s0165-5728(01)00260-0. [DOI] [PubMed] [Google Scholar]
- Nukina H, Sudo N, Komaki G, Yu XN, Mine K, Kubo C. The restraint stress-induced elevation in plasma interleukin-6 negatively regulates the plasma TNF-α level. Neuroimmunomodulation. 1998;5:323–327. doi: 10.1159/000026352. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. 2. London: Academic Press; 2001. [Google Scholar]
- Porter JR, Rettger LF. Influence of diet on the distribution of bacteria in the stomach, small intestine and cecum of the white rat. J Infect Dis. 1940;66:104–110. [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry. 2002;7:609–616. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Oitzl MS, Levine S, de Kloet ER. The HPA system during the postnatal development of CD1 mice and the effects of maternal deprivation. Dev Brain Res. 2002;139:39–49. doi: 10.1016/s0165-3806(02)00519-9. [DOI] [PubMed] [Google Scholar]
- Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity. J Neurosci. 1995;15:376–384. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman S. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci U S A. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnes HF, Pearce MK, Tewari A, Yim HH, Zou JC, Abrams JS. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-α challenge in mice. J Immunol. 1990;145:4185–4191. [PubMed] [Google Scholar]
- Steffen EK, Berg RD, Deitch EA. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis. 1988;157:1032–1038. doi: 10.1093/infdis/157.5.1032. [DOI] [PubMed] [Google Scholar]
- Sudo N, Aiba Y, Takaki A, Yu XN, Oyama N, Koga Y, Kubo C. Dietary nucleic acids promote a shift in Th1/Th2 balance toward Th1-dominant immunity. Clin Exp Allergy. 2000;30:979–987. doi: 10.1046/j.1365-2222.2000.00811.x. [DOI] [PubMed] [Google Scholar]
- Sudo N, Sawamura SA, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–1745. [PubMed] [Google Scholar]
- Suzuki K, Harasawa Y, Yoshitake Y, Mitsuoka T. Effects of crowding and heat stress on intestinal flora, body weight gain, and feed efficiency of growing rats and chickens. Jpn J Vet Sci. 1983;45:331–338. doi: 10.1292/jvms1939.45.331. [DOI] [PubMed] [Google Scholar]
- Tannock GW. The normal microflora: an introduction. In: Tannock GW, editor. Medical Importance of the Normal Micro Flora. Dordrecht, The Netherlands: Kluwer; 1999. pp. 1–23. [Google Scholar]
- Tannock GW, Savage DC. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun. 1974;9:591–598. doi: 10.1128/iai.9.3.591-598.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang BR, Zhang XJ, Xu Z, Ding YQ, Ju G. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J Gastroenterol. 2002;8:540–545. doi: 10.3748/wjg.v8.i3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradnik R, Brennam G, Hutchison JS, Odell WD. Immunoradiometric assay of corticotropin with use of avidin-biotin separation. Clin Chem. 1989;35:804–807. [PubMed] [Google Scholar]