Abstract
Juvenile king penguins develop adaptive thermogenesis after repeated immersion in cold water. However, the mechanisms of such metabolic adaptation in birds are unknown, as they lack brown adipose tissue and uncoupling protein-1 (UCP1), which mediate adaptive non-shivering thermogenesis in mammals. We used three different groups of juvenile king penguins to investigate the mitochondrial basis of avian adaptive thermogenesis in vitro. Skeletal muscle mitochondria isolated from penguins that had never been immersed in cold water showed no superoxide-stimulated proton conductance, indicating no functional avian UCP. Skeletal muscle mitochondria from penguins that had been either experimentally immersed or naturally adapted to cold water did possess functional avian UCP, demonstrated by a superoxide-stimulated, GDP-inhibitable proton conductance across their inner membrane. This was associated with a markedly greater abundance of avian UCP mRNA. In the presence (but not the absence) of fatty acids, these mitochondria also showed a greater adenine nucleotide translocase-catalysed proton conductance than those from never-immersed penguins. This was due to an increase in the amount of adenine nucleotide translocase. Therefore, adaptive thermogenesis in juvenile king penguins is linked to two separate mechanisms of uncoupling of oxidative phosphorylation in skeletal muscle mitochondria: increased proton transport activity of avian UCP (dependent on superoxide and inhibited by GDP) and increased proton transport activity of the adenine nucleotide translocase (dependent on fatty acids and inhibited by carboxyatractylate).
Mammals possess a mechanism of producing heat that does not require muscular contraction (Jansky, 1973). It is termed non-shivering thermogenesis and is defined as an increase in metabolic rate without any concomitant increase in skeletal muscle electrical activity upon exposure to cold. The mechanism of mammalian non-shivering thermogenesis is well understood and is mainly based on a regulated uncoupling of mitochondrial oxidative phosphorylation in brown adipose tissue. It is controlled by noradrenaline released from sympathetic nerves and is executed exclusively by uncoupling protein 1 (UCP1), which is present only in brown adipose tissue, and is stimulated by free fatty acids and inhibited by purine nucleoside di- and triphosphates (Cannon & Nedergaard, 2004).
Non-shivering thermogenesis also occurs in cold acclimated birds (ducklings, penguins and chickens) (El-Halawani et al. 1970; Barre et al. 1986; Duchamp et al. 1989). However, birds lack any detectable brown adipose tissue (Johnston, 1971; Saarela et al. 1991) so the mechanism that underlies non-shivering thermogenesis in birds must differ from that in mammals. At present this mechanism is unknown. In the absence of both brown adipose tissue and UCP1, skeletal muscle has been suggested to be the main site of non-shivering thermogenesis in birds (Duchamp et al. 1991; Duchamp & Barre, 1993). Compared to thermoneutral controls, skeletal muscle from cold acclimated penguins has a greater oxidative capacity, linked to increased mitochondrial inner membrane and cristae, and isolated mitochondria have lower respiratory control ratios (Duchamp et al. 1991).
Two separate models have been proposed to explain how skeletal muscle could control non-shivering thermogenesis in birds (Duchamp et al. 1999). The first is mitochondrial and involves a system analogous to the UCP1 mechanism in mammalian brown adipose tissue, whereby oxidative phosphorylation and ATP production are uncoupled. In this model the mitochondrial protonmotive force is partially dissipated by a regulated leak of protons, producing heat instead of ATP. Evidence for this in birds comes from the loose coupling of isolated muscle mitochondria from cold-adapted king penguins (Duchamp et al. 1991) or cold-acclimated ducklings (Roussel et al. 1998) and from the presence of an avian homologue of UCP1 (avUCP), whose distribution is restricted to skeletal muscle and whose mRNA expression is increased 3-fold during cold acclimation in ducklings (Raimbault et al. 2001). The penguin avUCP is 64%, 71% and 73% identical to UCP1, UCP2 and UCP3, respectively, and, like its mammalian and plant homologues, is activated by superoxide and inhibited by GDP, providing a diagnostic tool for investigating the presence of functional UCP (Talbot et al. 2003). However, it is unknown whether the physiological role of avUCP is thermogenic like UCP1, or more related to the as yet undefined functions of UCP2 and UCP3.
The second proposed mechanism for non-shivering thermogenesis in bird skeletal muscle is cytoplasmic and analogous to the thermogenic mechanism of the modified extraocular muscles of the heater organ found in endothermic fish (Block, 1994). This mechanism is less well defined than that of mammalian brown adipose tissue, but it is clear that Ca2+ release and re-uptake from the sarcoplasmic reticulum is involved. Generation of heat would be derived from the futile cycling of Ca2+, consuming ATP and stimulating mitochondrial respiration. Evidence for this cycle in birds comes from data showing an increase in both the sarcoplasmic reticulum calcium ATPase and Ca2+ release/ryanodine receptor densities in cold-acclimated ducklings (Dumonteil et al. 1993). The time course of the increase in sarcoplasmic reticulum calcium ATPase also correlates well with development of non-shivering thermogenesis (Dumonteil et al. 1995). These two mechanisms could coexist if the decrease in ATP production caused by partial uncoupling is not great enough to limit ATP-driven calcium cycling (Roussel et al. 1998).
Juvenile king penguins are terrestrial sea birds that maintain homeothermy after they emerge from the parental pouch by increasing thermal insulation by growth of down (Duchamp et al. 2002). Only after moulting at age 12–13 months do they face the thermogenic challenge of passage from shore to marine life in cold subantarctic seawater. During this period king penguins develop the capability for adaptive thermogenesis, acquired through several acclimation journeys into the sea (Barre & Roussel, 1986). We have exploited the unique ontogeny of king penguin thermoregulatory systems and examined the biochemical basis of penguin metabolic adaptation in response to thermal adaptation to marine life by investigating the functional characteristics of skeletal muscle mitochondria in vitro.
Methods
The study was conducted on the Crozet archipelago (Possession Island) at the French Alfred Faure Station (46° 25′ S, 52° 45′ E) from December to March, i.e. during the subantarctic summer. According to the Agreed Measures for the Preservation of Antarctic Fauna, the project received the agreement of the French Committee for Antarctic Research (Programme 131) and conformed to the Agreed Measures for the Conservation of Antarctic and Sub-Antarctic fauna.
Animals
Thirteen juvenile king penguins (Aptenodytes patagonicus) of both sexes, 11–12 months old, in the final stage of moulting and therefore not adapted to marine life, were captured on the nearby breeding colony at Baie du Marin (40 000 breeding pairs) and kept in an outside enclosure for up to 25 days. Care was taken to select birds that had not completed their moult and therefore had not undergone sea water immersion, which usually does not take place before complete moulting. The average air temperature during the experimental period was 7.7°C, which is within the thermoneutral zone of these birds (Duchamp et al. 1989). During summer, mean wind velocity was 3 m s−1, solar radiation was 190 W m−2, rainfall was 210 mm per month and humidity was 80%. Although harsh, these weather conditions during summer did not represent a marked cold stress for juvenile king penguins. The birds were split into two groups. The first, containing seven birds, acted as controls and formed the never immersed group. Simulation of passage to marine life was obtained in the second group by exposing the remaining six birds to 10 successive cold-water immersions at approximately 8°C for 5 h per immersion every second day as previously described (Barre & Roussel, 1986). Usual sea water temperature in the subantarctic area is around 5–6°C. This formed the immersed group. A further 11 birds of both sexes, 12–13 months old, were also captured. These birds had completed moulting and accomplished their passage to marine life, and formed the naturally adapted group. All birds were weighed every second day and force-fed ice fish (Champsocephalus gunnari) up to 500 g per day, to maintain a constant bodyweight. On completion of the study the penguins were released at the site of their capture.
Mitochondrial isolation
Penguins were anaesthetized by halothane inhalation, approximately 6 g of pectoralis muscle was removed, and skeletal muscle intermyofibrillar and subsarcolemmal mitochondria were isolated by homogenization, protease digestion and differential centrifugation in ice-cold isolation buffer (100 mm sucrose, 50 mm Tris base, 50 mm KCl, 5 mm EDTA, adjusted to pH 7.4 with HCl at 4°C) as previously described (Talbot et al. 2003) and suspended in medium containing 250 mm sucrose, 1 mm EGTA and 20 mm Tris base, pH 7.4. Protein concentration was determined using the biuret method with bovine serum albumin (BSA) as standard (Gornall et al. 1949). Penguin muscle mitochondria were well coupled, with respiratory control ratios of 2.6–3.7 with succinate as substrate. After surgery, birds were monitored for a few days and then released back into the colony. No adverse postsurgical outcomes were noted.
Measurement of proton conductance
The oxygen consumption of mitochondria, in the presence of oligomycin to inhibit ATP synthesis, is proportional to the rate at which protons leak across the mitochondrial inner membrane (assuming no slip in the proton pumps of the electron transport chain). The kinetic response of the proton conductance to its driving force (protonmotive force) can therefore be measured as the relationship between oxygen consumption and mitochondrial membrane potential when the potential is varied with titration with electron transport chain inhibitors (Nicholls, 1974; Brand, 1990; Cadenas et al. 2000) as previously described (Talbot et al. 2003). Because the yield of subsarcolemmal mitochondria was insufficient, proton conductance was measured only in intermyofibrillar mitochondria. Oxygen consumption was measured using a 2.5 ml Clark-type oxygen electrode (Hansatech, King's Lynn, UK) maintained at 38°C and calibrated with air-saturated assay medium (120 mm KCl, 5 mm KH2PO4, 3 mm Hepes, 1 mm EGTA, 1 mm MgCl2, 0.3% (w/v) BSA, pH 7.2), which was assumed to contain 402 nmol O ml−1 (Reynafarje et al. 1985). Electrode linearity was checked routinely by following the uncoupled respiration rate in the presence of 0.4 μm carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP) from 100 to 0% air saturation. Membrane potential was determined simultaneously using the potential-dependent probe triphenylmethyl phosphonium cation (TPMP+) (Brand, 1995). Mitochondria (0.35 mg protein ml−1) were incubated at 38°C in assay medium containing 5 μm rotenone, 1 μg ml−1 oligomycin and 65 ng ml−1 nigericin (to bring about the collapse of the difference in pH across the mitochondrial inner membrane). The TPMP+ electrode was calibrated with sequential additions of up to 2 μm TPMP+, and 4 mm succinate was added to start the reaction. Respiration and potential were inhibited progressively through successive steady states by additions of malonate up to 3 mm. At the end of each run, 0.4 μm FCCP was added to dissipate the membrane potential and release all TPMP+ back into the medium, allowing correction for any small electrode drift. The TPMP+ binding correction factor was taken as 0.45 per μl mg−1 of protein (Rolfe et al. 1994).
Where indicated, exogenous superoxide was generated using xanthine (50 μm) and xanthine oxidase (0.01 U per 2.5 ml). Stocks of xanthine at 0.35 mm and xanthine oxidase at 2 U ml−1 were prepared in assay medium. Xanthine and xanthine oxidase (XXO) were added before the TPMP+ calibration. Where XXO stimulated basal oxygen consumption before the addition of succinate, this was averaged over the period of the TPMP+ calibration and subtracted from all subsequent steady state respiration rates of that run. The maximum size of this correction was 10% of the state 4 rate. All experiments were conducted in the presence of 0.3% BSA and 150 μm palmitate unless stated otherwise. Where indicated, 12 U ml−1 superoxide dismutase, 1 mm guanosine diphosphate (GDP), or 1.2 μm carboxyatractylate (CAT) were added.
At the end of each set of experiments, the remaining mitochondria were frozen at −80°C and transported back to Europe for determination of avian adenine nucleotide translocase (avANT) content.
Plasma fatty acid analysis
The day prior to surgery on the penguins, blood samples of approximately 1 ml were taken from the marginal vein of the flipper either in the resting state at thermoneutrality (10°C in air) or immediately after 5 h of immersion in cold water. Blood samples were collected in heparin and immediately centrifuged at 5000 g for 5 min at 4°C to separate the plasma. Total non-esterified serum fatty acids were determined spectrophotometrically using the enzymatic NEFA C kit (WAKO Chemicals, Neuss, Germany) according to the manufacturer's guidelines.
Determination of avUCP mRNA relative abundance
Total RNA was isolated from frozen pectoralis muscle sampled on other batches of penguins submitted to the same protocol, using the TriReagent procedure (Sigma, St Quentin Fallavier, France) and the integrity of extracted RNA was assessed by electrophoresis on agarose gels. Reverse transcription (RT) of 1 μg of total RNA was performed using 200 U of M-MLV-RTase (Promega, France) according to the manufacturer's instructions and as previously described (Talbot et al. 2003). The resulting cDNAs were submitted to polymerase chain reaction (PCR) amplification in a total volume of 50 μl containing 1 μm of forward (5′-GTGGATGCCTACAGGACCAT-3′) and reverse (5′-ATGAACATCACCACGTTCCA-3′) primers (Invitrogen, Cergy Pontoise, France), 2.5 U of Taq DNA polymerase (Eurobio, Les Ullis, France), reaction buffer, 200 μm dNTPs and 1.5 mm MgCl2 as previously described (Talbot et al. 2003). After an initial denaturation step at 94°C for 2 min, 26 PCR cycles were run with the following parameters: denaturation at 94°C for 45 s, annealing at 65°C for 1 min and elongation at 72°C for 60 s using a Thermo Hybaid thermocycler (Ashford, UK). The amplified product (389 bp) was separated on agarose gel and the relative band intensity was quantified by Kodak Digital Science TM1D image analysis software. As an invariant gene, we used the β-actin gene with forward 5′-TGCGTGACATCAAGGAGAAG-3′ and reverse 5′-TGCCAGGGTACATTGTGGTA-3′ primers selected from the chicken β-actin sequence (GenBank accession no L08165). PCR parameters were the same as above except that annealing was at 58°C and 24 cycles of PCR were used, generating a 300 bp amplification product. All penguin avUCP and β-actin RT-PCR samples were in the linear range of logarithmic amplification without primer limitation.
Determination of mitochondrial ANT content
Mitochondrial adenine nucleotide translocase (ANT) content was determined using an assay based on the high-affinity binding of tritium-labelled atractylate ([3H]ATR) (Schonfeld et al. 1993). Frozen skeletal muscle mitochondria were thawed on ice and incubated at 1 mg protein ml−1 in 0.9 ml of assay buffer (without BSA or palmitate) containing 20 μm ADP and 5 μm[3H]ATR (custom synthesized, specific activity 1.16 GBq mol−1, a generous gift from Knoll Pharmaceuticals Ltd, Nottingham, UK). After 5 min at room temperature, the tubes were centrifuged at 16 000 g, the supernatant was discarded and the pellet was resuspended in 0.5 ml of assay buffer. Centrifugation and resuspension were repeated twice to wash off unbound [3H]ATR. Finally, the pellet was dissolved in 40 μl of 2% (v/v) sodium dodecyl sulphate. Radioactivity was assayed using liquid scintillation counting. Each incubation was performed in duplicate. To correct for radioactivity due to adherent [3H]ATR in the pellet not bound to ANT (blank radioactivity), each incubation was repeated in the presence of 5 μm CAT. The blank was subtracted from the total radioactivity measured in the absence of CAT and total nmol of ANT dimer per mg of mitochondrial protein calculated assuming one [3H]ATR molecule bound per ANT dimer (Riccio et al. 1975).
For Western blot analysis, mitochondrial preparations containing 40 μg mitochondrial protein were separated by SDS-PAGE on 12.8% acrylamide gels, and transferred to polyvinylidene fluoride membranes (PVDF membranes) (Immobilon-P, Millipore). Immunological detection was performed using a mouse anti-serum against bovine heart ANT1 (generous gift of B. Notario, T. Mampel and O. Vinas, Department of Biochemistry and Molecular Biology, University of Barcelona). The binding of antibody was detected with a horseradish peroxidase-coupled anti-mouse (Bio-Rad 170-6516; 1: 5000) secondary antibody and an enhanced chemiluminescence (ECL) detection kit (Amersham, UK). Protein size was determined using protein molecular-mass standards (Bio-Rad). Quantification of autoradiographs and ECL signals was performed by scanning densitometry. Specificity of immunoreactive detection of ANT was checked with a positive control (purified bovine heart ANT1).
Statistical analysis
Values are means ± s.e.m. Where indicated, unpaired Student's t tests and one-way ANOVA were performed with significance considered as P < 0.05.
Results
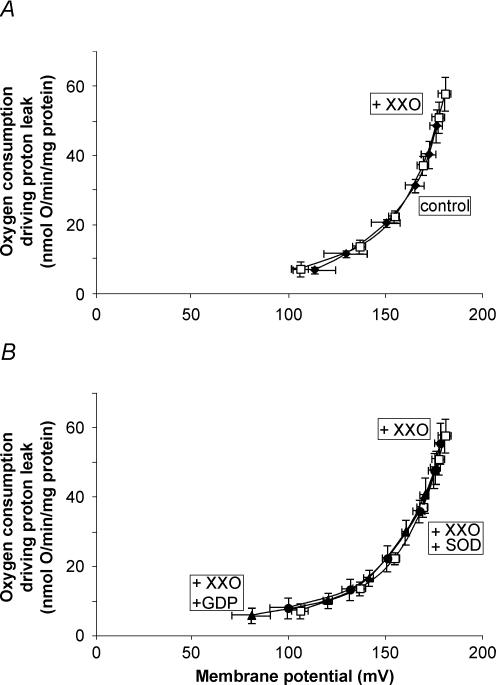
Figure 1 shows the kinetic response of the proton leak rate to its driving force, membrane potential, in skeletal muscle mitochondria from never-immersed penguins in the presence of BSA and 150 μm palmitate. Addition of the superoxide generating system, xanthine and xanthine oxidase (XXO), had no effect on the kinetics (Fig. 1A). The absence of a superoxide-induced proton conductance in these mitochondria led to the expected observation that neither GDP nor superoxide dismutase altered the proton conductance in the presence of superoxide (Fig. 1B). This result indicates there was no functional avUCP present in skeletal muscle mitochondria of juvenile penguins that had never been immersed in cold water.
Figure 1. No effect of superoxide on the proton conductance of skeletal muscle mitochondria from never-immersed penguins.
For details see Methods. Skeletal muscle mitochondria (0.35 mg ml−1) from never-immersed penguins were incubated in assay buffer and the mitochondrial proton leak kinetics were obtained by simultaneous measurement of membrane potential and oxygen consumption, using succinate as a substrate and varying the potential with sequential additions of malonate up to 3 mm. A, no difference in proton conductance in the presence or absence of superoxide. B, addition of GDP or superoxide dismutase in the presence of superoxide does not change the proton conductance. ♦, control; □, plus 50 μm xanthine and 0.01 U xanthine oxidase per 2.5 ml; ▴, plus xanthine, xanthine oxidase and 1 mm GDP; •, plus xanthine, xanthine oxidase and 12 U ml−1 superoxide dismutase. All data are means ± s.e.m. of five independent experiments each performed in duplicate.
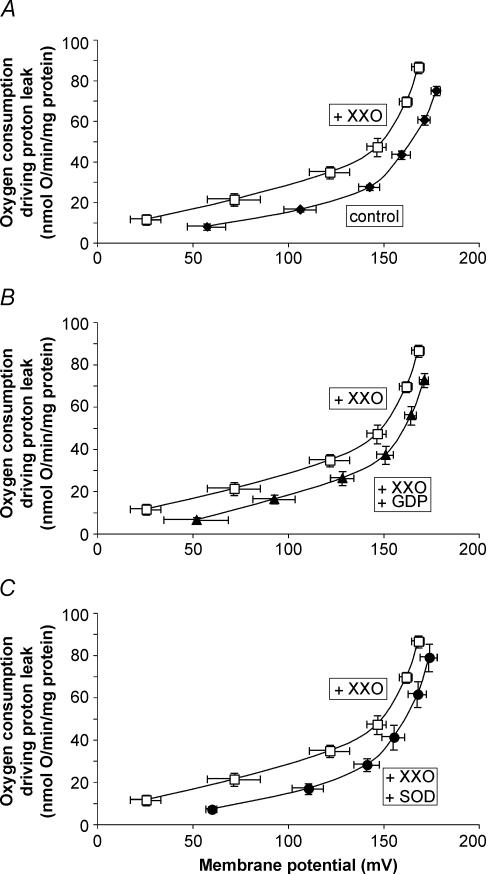
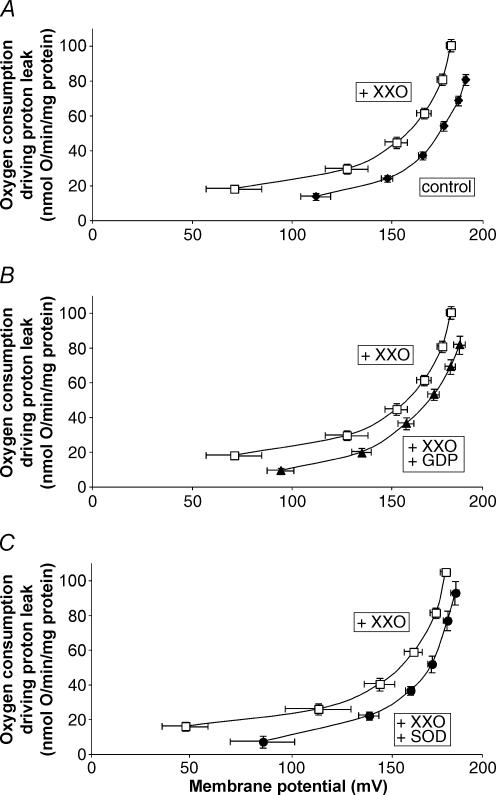
In stark contrast, Fig. 2A shows that mitochondria isolated from skeletal muscle of the experimentally immersed penguins did have a large superoxide-induced uncoupling: the respiration rate driving the proton leak at any given membrane potential was approximately 60% greater in the presence of XXO compared to its absence. The activation by superoxide was completely prevented by the addition of GDP (Fig. 2B). Since superoxide activation of proton conductance through UCP3 in mouse skeletal muscle mitochondria is prevented by GDP and absent in mitochondria from UCP3(−/−) mice (Echtay et al. 2002), this observation strongly suggests that the superoxide-activated, GDP-sensitive proton conductance in these penguin mitochondria was mediated by avUCP (Talbot et al. 2003). Furthermore, removal of superoxide by the addition of superoxide dismutase was also able to inhibit the XXO-stimulated proton conductance (Fig. 2C) in agreement with previous findings (Echtay et al. 2002; Talbot et al. 2003). Figure 3 shows that skeletal muscle mitochondria from the naturally adapted penguins possessed very similar properties to those of the experimentally immersed group, that is to say, a superoxide-induced proton conductance (Fig. 3A) that was inhibited by GDP (Fig. 3B) or superoxide dismutase (Fig. 3C). In summary, from Figs 1–3 it is clear that no functional avUCP is found in skeletal muscle mitochondria from never-immersed juvenile penguins, but either artificial or natural adaptation to cold water induces superoxide-activated, GDP-sensitive proton conductance by avUCP, in parallel to the appearance of adaptive thermogenesis in these birds.
Figure 2. Superoxide-induced, GDP-sensitive proton conductance of skeletal muscle mitochondria from experimentally immersed penguins.
For details see Methods. A, increased proton conductance in the presence of superoxide. B, inhibition of superoxide-induced proton conductance by addition of GDP. C, inhibition of superoxide-induced proton conductance by addition of superoxide dismutase. ♦, control; □, plus 50 μm xanthine and 0.01 U xanthine oxidase per 2.5 ml; ▴, plus xanthine, xanthine oxidase and 1 mm GDP; •, plus xanthine, xanthine oxidase and 12 U ml−1 superoxide dismutase. All data are means ± s.e.m. of six independent experiments each performed in duplicate.
Figure 3. Superoxide-induced, GDP-sensitive proton conductance of skeletal muscle mitochondria from naturally adapted penguins.
For details see Methods. A, increased proton conductance in the presence of superoxide. B, iInhibition of superoxide-induced proton conductance by addition of GDP. C, inhibition of superoxide-induced proton conductance by addition of superoxide dismutase. ♦, control; □ plus 50 μm xanthine and 0.01 U xanthine oxidase per 2.5 ml; ▴, plus xanthine, xanthine oxidase and 1 mm GDP; •, plus xanthine, xanthine oxidase and 12 U ml−1 superoxide dismutase. All data are means ± s.e.m. of eight independent experiments each performed in duplicate.
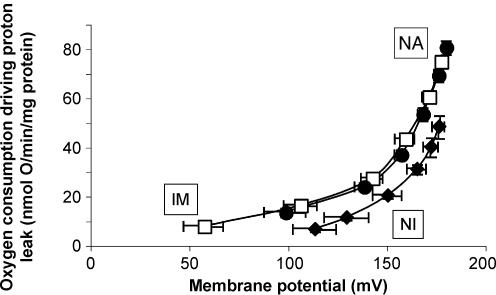
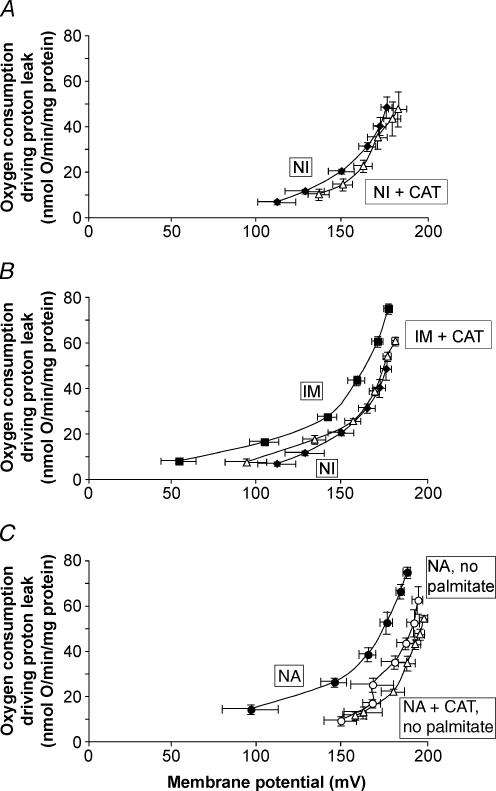
Next, we examined the proton conductance properties of penguin mitochondria in the absence of stimulation of avUCP by superoxide. Figure 4 presents a comparison of the kinetics of the unstimulated proton conductance in skeletal muscle mitochondria isolated from each of the three groups of penguins, measured under identical conditions of 0.3% BSA and 150 μm palmitic acid (to clamp the free fatty acid concentration) in the absence of other activators or inhibitors. Under these conditions, the kinetics of the proton conductance from immersed and naturally adapted penguin mitochondria were indistinguishable. However, mitochondria isolated from never-immersed penguins had a much lower proton conductance than mitochondria from the two groups that had been exposed to cold water. In this case, we have no evidence that the higher proton conductance of mitochondria from cold-adapted birds was caused by the presence of avUCP, since addition of GDP did not decrease it (Figs 2 and 3). The difference must have been due to some other constituent of the mitochondria.
Figure 4. Increased basal proton conductance of skeletal muscle mitochondria from immersed, never-immersed and naturally adapted penguins.
Data from Figs 1A, 2A and 3A. Skeletal muscle mitochondria (0.35 mg ml−1) from either never-immersed (NI) (♦) (n = 5), immersed (im) (♦) (n = 6), or naturally adapted (NA) (•) (n = 8) penguins were incubated in assay buffer containing 0.3% BSA and 150 μm palmitate and the kinetics of the proton conductance were obtained by varying the potential with malonate using 4 mm succinate as substrate. All data are means ± s.e.m.
In the presence of fatty acids, the adenine nucleotide translocase can catalyse carboxyatractylate-sensitive proton translocation (Andreyev et al. 1988; Brustovetsky & Klingenberg, 1994). Figure 5A demonstrates that in the presence of 0.3% BSA and 150 μm palmitic acid, addition of CAT to inhibit avANT in mitochondria from never-immersed penguins had little or no effect on the kinetics of proton conductance. However, addition of CAT to mitochondria from immersed penguins strongly decreased the proton conductance (Fig. 5B) and abolished the difference in proton conductance between the immersed and never-immersed groups demonstrated in Fig. 4. The same result was obtained with mitochondria from naturally adapted penguins (data not shown). In the presence of BSA only, with no added palmitate, the addition of CAT had no effect on the basal proton conductance of mitochondria isolated from the naturally adapted group (Fig. 5C), showing that CAT-sensitive uncoupling by avANT requires free fatty acids. In summary, Figs 4 and 5 demonstrate that cold adaptation in juvenile penguins leads to a second mechanism of increased proton conductance in skeletal muscle mitochondria: increased fatty acid-dependent, carboxyatractylate-sensitive proton transport by the adenine nucleotide translocase, in parallel to the appearance of adaptive thermogenesis in these birds.
Figure 5. ANT-mediated, fatty acid-dependent mechanism of increased basal proton conductance of skeletal muscle mitochondria from never-immersed and naturally adapted penguins.
For details see Methods. A, little carboxyatractylate (CAT)-sensitive proton conductance in skeletal muscle mitochondria from never-immersed (NI) penguins: ♦, control (n = 5); Δ, plus 1.2 μm CAT (n = 4). B, inhibition of ANT in skeletal muscle mitochondria from experimentally immersed (im) penguins strongly attenuates the difference in proton conductance between mitochondria from immersed and never-immersed penguins: ♦, ‘never-immersed’ proton conductance (n = 5); ▪, ‘immersed’ proton conductance (n = 6); ▵, ‘immersed’ plus 1.2 μm CAT (n = 6). C, omission of palmitate strongly decreases the ANT-mediated leak of skeletal muscle mitochondria from naturally adapted penguins: •, proton conductance of skeletal muscle mitochondria from naturally adapted (NA) penguins plus 0.3% BSA and 150 μm palmitate (n = 8); ○, ‘naturally adapted’ plus 0.3% BSA but omitting palmitate (n = 6); ▵, ‘naturally adapted’ plus 0.3% BSA and 1.2 μm CAT but omitting palmitate (n = 5). Data are means ± s.e.m.
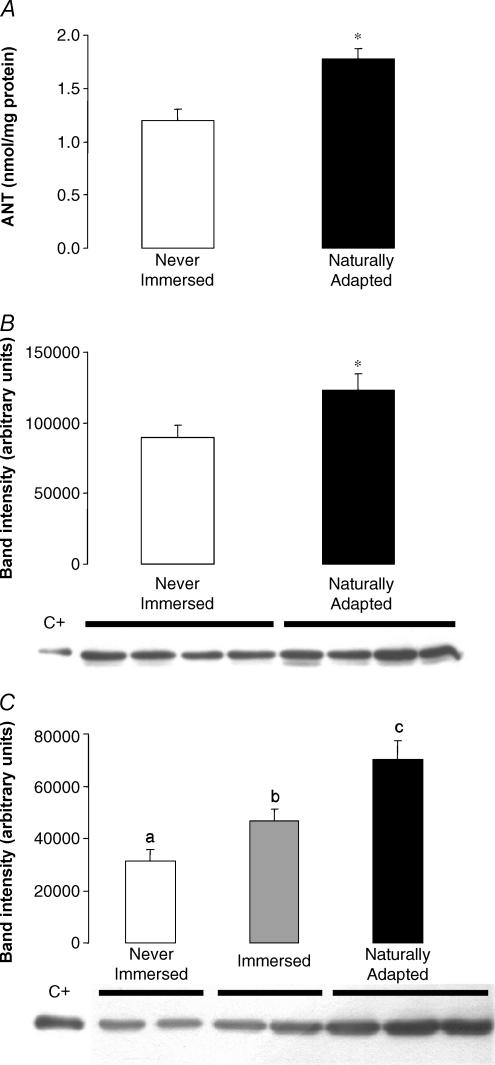
The greater CAT-sensitive avANT proton transport activity could have been caused by activation of ANT, or by an increase in its concentration. To distinguish between these possibilities, the ANT content of mitochondria was measured firstly using an assay based on the high affinity binding of tritium-labelled atractylate. Skeletal muscle mitochondria from naturally adapted penguins contained approximately 50% more ANT protein than mitochondria from never-immersed controls (Fig. 6A). Additional data could be obtained by Western blot using intermyofibrillar and subsarcolemmal muscle mitochondria from the same birds using an antibody against mammalian ANT1. As shown in Fig. 6B and C, an immunoreactive protein of the same size (around 31 kDa) as the positive control (purified bovine heart ANT1) was detected in penguin muscle mitochondria. Using intermyofibrillar mitochondria, the relative abundance of the protein was increased 1.5-fold after natural adaptation to marine life (Fig. 6B). Using subsarcolemmal mitochondria (Fig. 6C), the relative abundance of the protein was increased both after laboratory immersions (× 1.5) and after natural adaptation to marine life (× 2.2). Therefore, the causal factor for the greater CAT-sensitive proton conductance of mitochondria from cold-adapted penguins compared to mitochondria from never-immersed controls may be the greater amount of ANT protein per mg of total mitochondrial protein.
Figure 6. ANT content of penguin skeletal muscle mitochondria.
For details see Methods. A, frozen mitochondrial samples were thawed on ice and assayed for ANT protein content using an assay based on the high affinity binding of tritium-labelled atractylate. Data are means ± s.e.m. of measurements on 3 (never-immersed) or 4 (naturally adapted) independent preparations. *P < 0.005 using unpaired Student's t test. B, frozen intermyofibrillar mitochondrial samples (40 μg) from never-immersed or naturally adapted penguins were thawed on ice and assayed for ANT protein content by Western blot using antibodies against bovine ANT1. C+, positive control. Data are means ± s.e.m. of measurements on 4 independent preparations.*P < 0.005 using unpaired Student's t test. C, Western blot using subsarcolemmal mitochondrial samples (40 μg) from never-immersed, artificially immersed and naturally adapted penguins. Data are means ± s.e.m. of measurements on 4 or 6 (naturally adapted) independent preparations. Bars with different letters are significantly different (P < 0.05, Fisher's PLSD).
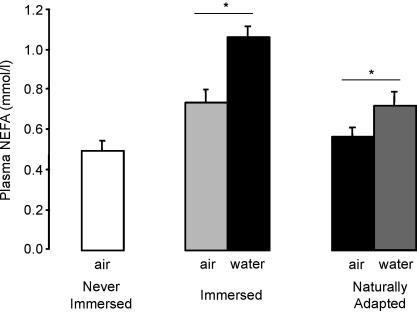
In principle, greater ANT-mediated proton conductance in penguins exposed to cold water could also be obtained by a greater stimulation caused by higher levels of non-esterified fatty acids (NEFA). Figure 7 shows that at thermoneutrality, concentrations of circulating NEFA in the serum of penguins that had previously been exposed to cold water were not significantly greater than those in never-immersed penguins (although there was a non-significant trend) so we have no evidence that increased NEFA activation of avANT plays a role in the greater mitochondrial proton conductance and in vivo thermogenesis during cold adaptation. Note, however, that acute immersion in cold water led to a rapid rise in plasma NEFA levels, presumably to fuel increased regulatory thermogenesis (Fig. 7).
Figure 7. Plasma levels of non-esterified fatty acids of penguins exposed to thermoneutral conditions in air (10°C) or after a 5 h immersion in cold water.
For details see Methods. Blood samples of approximately 1 ml were taken from the marginal vein of the flipper and centrifuged to separate the serum. Non-esterified fatty acids were determined using a commercial NEFA C kit. Data are means ± s.e.m. of measurements on 7 (never-immersed), 5 (immersed) or 7 (naturally adapted) independent preparations. In air, differences are non-significant (P > 0.10) using one-way ANOVA. Water immersion (5 h) led to a significant (*P < 0.05) rise in plasma levels using a paired t test.
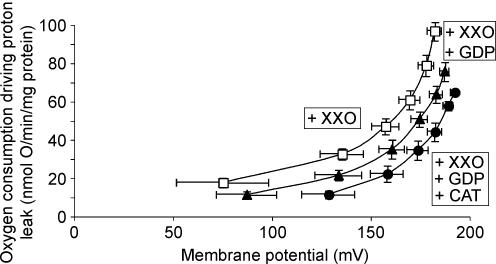
To establish whether the superoxide-activated, GDP-sensitive proton conductance mediated by avUCP shown in Figs 2A and 3A was independent of the fatty acid-activated, CAT-sensitive proton conductance mediated by avANT shown in Fig. 5B, further experiments were conducted using mitochondria from naturally adapted penguins. Figure 8 shows that the high proton conductance in the presence of superoxide was lowered by the addition of GDP, confirming the result in Fig. 3B. When CAT was also added, the conductance was lowered further, showing that avANT-mediated proton conductance was independent of superoxide activation of avUCP and therefore demonstrating the existence of two separate mechanisms operating in mitochondria from cold-adapted penguins to activate mitochondrial proton conductance.
Figure 8. Separate mechanisms of avUCP- and avANT-mediated proton conductance in skeletal muscle mitochondria from naturally adapted penguins.
For details see Methods. Skeletal muscle mitochondria (0.35 mg ml−1) from naturally adapted penguins were incubated in assay buffer containing 0.3% BSA and 150 μm palmitate with XXO (□), XXO plus GDP (▴) or XXO plus GDP and CAT (•). All data are means ± s.e.m. of five independent experiments performed in duplicate.
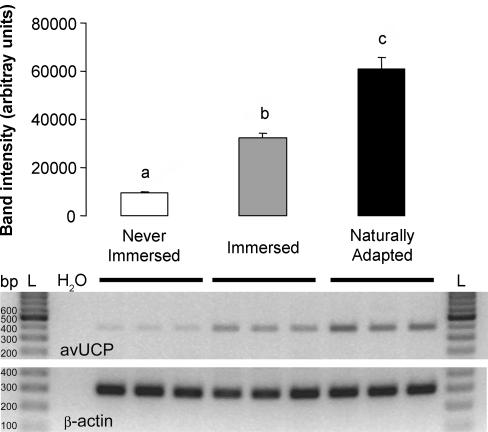
Finally, we investigated whether the induction of superoxide-activated, GDP-sensitive proton conductance by artificial or natural adaptation to cold water could be related to changes in the expression of avUCP. The relative abundance of avUCP mRNA, examined by RT-PCR, was low in pectoralis muscle from never-immersed penguins but was markedly increased in that of immersed (× 4.1) or naturally adapted (× 6.8) penguins (Fig. 9). These data show that an up-regulation of avUCP after artificial or natural adaptation to cold water occurs in parallel with the appearance of GDP-inhibitable proton conductance, supporting the proposal that they are linked.
Figure 9. RT-PCR analysis of avUCP mRNA levels in pectoralis muscle from never-immersed, immersed, or naturally adapted penguins.
For details see Methods. Values are means ± s.e.m. of five independent experiments. Bars with different letters are significantly different (P < 0.05 with Fisher's PLSD). β-Actin was used as an invariant gene. Gels showing RT-PCR from three birds of each group are presented for avUCP or β-actin. The size of the amplified product was 389 and 300 bp, respectively. L, 100 bp ladder; H2O, negative control of PCR.
Discussion
King penguins develop adaptive thermogenesis after prolonged immersion in cold water (Barre & Roussel, 1986). We show for the first time that in parallel with this, there is induction of functional avUCP in skeletal muscle mitochondria. Prior to first cold water immersion there is no superoxide-inducible, GDP-inhibitable mitochondrial proton conductance and hence no functional avUCP in skeletal muscle mitochondria (Fig. 1). After either experimental immersion (Fig. 2) or natural adaptation to marine life (Fig. 3) addition of superoxide to skeletal muscle mitochondria activates an avUCP-mediated increase in proton conductance. This occurs in parallel with a large increase in the relative abundance of avUCP mRNA.
The absence of functional avUCP in never-immersed penguins suggests interesting regulation of cold adaptation in birds. The absence of avUCP function before the massive cold challenge of immersion into cold water, despite the detection of its mRNA, might mean that the protein is either masked or lacking an endogenous activator that is released upon immersion. However, king penguins naturally require several journeys to sea before fully expressing adaptive thermogenesis, suggesting instead a slower mechanism, presumably protein synthesis. Present data showing that the abundance of avUCP mRNA markedly increased with repeated immersion would tend to favour this hypothesis. In agreement with the requirement of a time delay for cold adaptation to develop, induction of avUCP mRNA expression has been observed in cold-exposed ducklings after 4 weeks (Raimbault et al. 2001) and expression in chickens positively correlates with duration of cold exposure (Toyomizu et al. 2002; Collin et al. 2003). Accordingly, naturally adapted penguins showed a higher abundance of avUCP mRNA than artificially acclimated birds (Fig. 9). Interestingly, the fact that mitochondria from artificially or naturally immersed penguins did not show marked functional difference (at least with the parameters tested here) while the expression of avUCP was further increased by natural adaptation, suggests that other factors than just mRNA expression may control the concentration and/or activity of avUCP, as has been shown for mammalian UCP2 (Pecqueur et al. 2001).
The correlation between cold exposure and induction of avUCP activity does not necessarily reveal that avUCP in birds has the same thermogenic role as UCP1 in mammals. The extent of uncoupling mediated by avUCP in skeletal muscle mitochondria from birds is clearly much less than the maximum extent of uncoupling that can be achieved by UCP1 in brown adipose tissue mitochondria. This may relate to marked differences in the relative amounts of these proteins and to the fact that adaptive mechanisms in penguins occur in skeletal muscle, a tissue specialized in contractile function, as opposed to brown adipose tissue, the specialized thermogenic tissue of mammals. The level of avUCP-mediated uncoupling is likely to be limited by the demand for ATP production in muscle so that muscle contraction when swimming (or flying for other birds) is not compromised, which may, in turn, limit the potential thermogenic capacity of avUCP. However, there is a much greater mass of skeletal muscle in birds compared to brown adipose tissue in mammals, so perhaps only a small uncoupling of each mitochondrion may be sufficient to generate sufficient heat in birds.
Nonetheless, the primary function of avUCP may be unrelated to thermogenesis. Sequence comparison reveals that avUCP is more similar to mammalian UCP2 and UCP3 than it is to UCP1 (Raimbault et al. 2001; Talbot et al. 2003). UCP2 and UCP3 are unable to compensate for the loss of UCP1 and are therefore not significantly thermogenic (Nedergaard et al. 2001). Instead, there is a growing body of evidence that UCP2 and UCP3 function to limit mitochondrial superoxide production (Echtay et al. 2002; Talbot et al. 2004; Brand et al. 2004). Immersion in cold water might directly increase oxidative stress for penguins, or immersion might be a signal to express avUCP in preparation for a lifestyle of underwater hunting involving periods of muscle hypoperfusion and reperfusion, with concomitant oxidative stress. As superoxide is an activator of avUCP (Talbot et al. 2003), it is conceivable that repeated oxidative stress, together with the thermogenic challenge of cold water, may be the trigger for transcription and synthesis of the protein. Repeated increases in plasma NEFA levels induced by cold water immersion might also contribute to the induction of avUCP expression in muscle. Further studies are needed to clarify whether avUCP is switched on after cold-water immersion to protect against oxidative damage and/or as part of a thermogenic acclimation to cold.
Mitochondria from penguins either subjected to experimental cold-water immersion or naturally adapted to marine life showed a greater avUCP-independent proton conductance than those from never-immersed penguins (Fig. 4). All mitochondria possess a basal proton conductance across their inner membrane which contributes significantly to basal metabolic rate (Brand et al. 1999). Phylogeny, body mass (in both mammals and birds) and thyroid status all influence basal proton conductance (Hafner et al. 1988; Brand et al. 1991; Porter & Brand, 1993; Brand et al. 2003). However, this basal proton conductance is not catalysed by UCP3 in mouse skeletal muscle mitochondria (Cadenas et al. 2002) or by avUCP in penguin skeletal muscle mitochondria (Talbot et al. 2003), although both proteins can catalyse an inducible, superoxide-activatable proton leak. Similarly, other mitochondrial carriers, such as the adenine nucleotide translocase and the glutamate—aspartate antiporter, are able to mediate an inducible, fatty acid-dependent proton conductance across the mitochondrial inner membrane (Skulachev, 1999). Inhibition of ANT by carboxyatractylate had only a small effect on the proton conductance in mitochondria from never-immersed birds (Fig. 5A), whereas mitochondria from cold-adapted penguins had substantial CAT-sensitive, fatty acid-dependent (Fig. 5B and C) proton conductance. This was caused by an increase in ANT protein rather than by increased levels of circulating NEFA (Figs 6 and 7). Increased expression of ANT has already been reported in cold-acclimated birds at both the protein (Roussel et al. 2000) and the mRNA level (Toyomizu et al. 2002). Interestingly, the large increase in CAT-sensitive proton conductance (Fig. 5C) after immersion appears much greater than the modest increase in protein expression (Fig. 6). The reason for this is unclear, but may represent differential expression of ANT isoforms in skeletal muscle mitochondria, particularly in response to changes in oxidative metabolism (Stepien et al. 1992), or a change in the sensitivity of ANT to fatty acid-induced uncoupling. By analogy with the activation of UCP1-mediated uncoupling in mammalian brown adipose tissue, we can speculate that repeated immersion of penguins raises plasma NEFA levels, which stimulate avANT-mediated uncoupling, acutely increasing thermogenesis. All of these speculations clearly deserve further investigation.
The increase in fatty acid-dependent proton conductance mediated by the ANT may be thermogenic, but this may not be its primary purpose. Instead, the increase in ANT protein may be a response to extra demand for ATP in the cytosol, to fuel other potential heat-producing mechanisms. One possibility is that cold water immersion stimulates futile cycling of calcium ions across the sarcoplasmic reticulum membrane that consumes ATP and therefore increases mitochondrial respiration. This mechanism occurs in the modified extraocular muscles of the heater organ of endothermic fish (Block, 1994). There is some evidence for this mechanism in birds: cold-acclimated ducklings increase both sarcoplasmic reticulum calcium ATPase and Ca2+ release channels/ryanodine receptors (Dumonteil et al. 1993). Whether such mechanisms also take place in skeletal muscle from penguins during adaptation to sea life clearly deserves further investigation.
Previous studies have demonstrated increased mRNA for both avANT and avUCP in cold-acclimated birds (Roussel et al. 2000; Raimbault et al. 2001; Toyomizu et al. 2002; Collin et al. 2003). The data presented here extend these observations by demonstrating that repeated immersions of juvenile king penguins in cold water results in two separate changes to skeletal muscle mitochondria, both of which increase the proton conductance and potentially mediate adaptive thermogenesis. There are increases in the activities of avUCP (which can uncouple in response to activation by superoxide) and of ANT (which mediates a fatty acid-dependent proton conductance). However, the extent to which these mitochondrial modifications contribute to adaptive thermogenesis and the development of non-shivering thermogenesis in king penguins is unclear. While repeated exposure to cold water leads to changes in the expression and activity of avUCP and avANT, the changes could be for other reasons than thermogenesis, such as protection against oxidative stress, or to support increased ATP demand. Nevertheless, these changes could be an essential pre-requisite for long-term survival of penguins in cold water. Furthermore, whether these physiological changes are limited to penguins and their unique environment, or underlie the fundamental basis of heat production and non-shivering thermogenesis in all birds has yet to be determined.
Acknowledgments
This work was supported by grants from the Institut Polaire Français Paul Emile Victor (programme 131) and the Medical Research Council.
References
- Andreyev A, Bondareva TO, Dedukhova VI, Mokhova EN, Skulachev VP, Volkov NI. Carboxyatractylate inhibits the uncoupling effect of free fatty acids. FEBS Lett. 1988;226:265–269. doi: 10.1016/0014-5793(88)81436-4. [DOI] [PubMed] [Google Scholar]
- Barre H, Cohen-Adad F, Duchamp C, Rouanet JL. Multilocular adipocytes from muscovy ducklings differentiated in response to cold acclimation. J Physiol. 1986;375:27–38. doi: 10.1113/jphysiol.1986.sp016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre H, Roussel B. Thermal and metabolic adaptation to first cold-water immersion in juvenile penguins. Am J Physiol. 1986;251:R456–R462. doi: 10.1152/ajpregu.1986.251.3.R456. [DOI] [PubMed] [Google Scholar]
- Block BA. Thermogenesis in muscle. Ann Rev Physiol. 1994;56:535–577. doi: 10.1146/annurev.ph.56.030194.002535. [DOI] [PubMed] [Google Scholar]
- Brand MD. The proton leak across the mitochondrial inner membrane. Biochim Biophys Acta. 1990;1018:128–133. doi: 10.1016/0005-2728(90)90232-s. [DOI] [PubMed] [Google Scholar]
- Brand MD. Measurement of mitochondrial proton motive force. In: Cooper CE, editor. Bioenergetics — a Practical Approach. Oxford: IRL Press; 1995. pp. 39–62. [Google Scholar]
- Brand MD, Brindle KM, Buckingham JA, Harper JA, Rolfe DFS, Stuart JA. The significance and mechanism of mitochondrial proton conductance. Int J Obes. 1999;23(Suppl. 6):S4–S11. doi: 10.1038/sj.ijo.0800936. [DOI] [PubMed] [Google Scholar]
- Brand MD, Buckingham JA, Esteves TC, Green K, Lambert AJ, Miwa S, Murphy MP, Pakay JL, Talbot DA, Echtay KS. Mitochondrial superoxide and aging: uncoupling-protein activity and superoxide production. Biochem Soc Symp. 2004;71:203–213. doi: 10.1042/bss0710203. [DOI] [PubMed] [Google Scholar]
- Brand MD, Couture P, Else PL, Withers KW, Hulbert AJ. Evolution of energy metabolism. Proton permeability of the inner membrane of liver mitochondria is greater in a mammal than in a reptile. Biochem J. 1991;275:81–86. doi: 10.1042/bj2750081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Turner N, Ocloo A, Else PL, Hulbert AJ. Proton conductance and fatty acyl composition of liver mitochondria correlates with body mass in birds. Biochem J. 2003;376:741–748. doi: 10.1042/BJ20030984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustovetsky N, Klingenberg M. The reconstituted ADP/ATP carrier can mediate H+ transport by free fatty acids, which is further stimulated by mersalyl. J Biol Chem. 1994;269:27329–27336. [PubMed] [Google Scholar]
- Cadenas S, Buckingham JA, St-Pierre J, Dickinson K, Jones RB, Brand MD. AMP decreases the efficiency of skeletal-muscle mitochondria. Biochem J. 2000;351:307–311. [PMC free article] [PubMed] [Google Scholar]
- Cadenas S, Echtay KS, Harper JA, Jekabsons MB, Buckingham JA, Grau E, Abuin A, Chapman H, Clapham JC, Brand MD. The basal proton conductance of skeletal muscle mitochondria from transgenic mice overexpressing or lacking uncoupling protein-3. J Biol Chem. 2002;277:2773–2778. doi: 10.1074/jbc.M109736200. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Collin A, Buyse J, van As P, Darras VM, Malheiros RD, Moraes VM, Reyns GE, Taouis M, Decuypere E. Cold-induced enhancement of avian uncoupling protein expression, heat production, and triiodothyronine concentrations in broiler chicks. General Comp Endocrinol. 2003;130:70–77. doi: 10.1016/s0016-6480(02)00571-3. [DOI] [PubMed] [Google Scholar]
- Duchamp C, Barre H. Skeletal muscle as the major site of nonshivering thermogenesis in cold-acclimated ducklings. Am J Physiol. 1993;265:R1076–R1083. doi: 10.1152/ajpregu.1993.265.5.R1076. [DOI] [PubMed] [Google Scholar]
- Duchamp C, Barre H, Delage D, Rouanet JL, Cohen-Adad F, Minaire Y. Nonshivering thermogenesis and adaptation to fasting in king penguin chicks. Am J Physiol. 1989;257:R744–R751. doi: 10.1152/ajpregu.1989.257.4.R744. [DOI] [PubMed] [Google Scholar]
- Duchamp C, Barre H, Rouanet JL, Lanni A, Cohen-Adad F, Berne G, Brebion P. Nonshivering thermogenesis in king penguin chicks. I. Role of skeletal muscle. Am J Physiol. 1991;261:R1438–R1445. doi: 10.1152/ajpregu.1991.261.6.R1438. [DOI] [PubMed] [Google Scholar]
- Duchamp C, Marmonier F, Denjean F, Lachuer J, Eldershaw TP, Rouanet J-L, Morales A, Meister R, Benistant C, Roussel D, Barre H. Regulatory, cellular and molecular aspects of avian muscle nonshivering thermogenesis. Ornis Fennica. 1999;76:151–165. [Google Scholar]
- Duchamp C, Rouanet JL, Barre H. Ontogeny of thermoregulatory mechanisms in king penguin chicks (Aptenodytes patagonicus) Comp Biochem Physiol A. 2002;131:765–773. doi: 10.1016/s1095-6433(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Dumonteil E, Barre H, Meissner G. Sarcoplasmic reticulum Ca2+ ATPase and ryanodine receptor in cold-acclimated ducklings and thermogenesis. Am J Physiol. 1993;265:C507–C513. doi: 10.1152/ajpcell.1993.265.2.C507. [DOI] [PubMed] [Google Scholar]
- Dumonteil E, Barre H, Meissner G. Expression of sarcoplasmic reticulum Ca2+ transport proteins in cold-acclimating ducklings. Am J Physiol. 1995;269:C955–C960. doi: 10.1152/ajpcell.1995.269.4.C955. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- El-Halawani ME, Wilson WO, Burger RE. Cold-acclimation and the role of catecholamines in body temperature regulation in male Leghorns. Poultry Sci. 1970;49:621–632. doi: 10.3382/ps.0490621. [DOI] [PubMed] [Google Scholar]
- Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- Hafner RP, Nobes CD, McGown AD, Brand MD. Altered relationship between protonmotive force and respiration rate in non-phosphorylating liver mitochondria isolated from rats of different thyroid hormone status. Eur J Biochem. 1988;178:511–518. doi: 10.1111/j.1432-1033.1988.tb14477.x. [DOI] [PubMed] [Google Scholar]
- Jansky L. Non-shivering thermogenesis and its thermoregulatory significance. Biol Rev. 1973;48:85–132. doi: 10.1111/j.1469-185x.1973.tb01115.x. [DOI] [PubMed] [Google Scholar]
- Johnston DW. The absence of brown adipose tissue in birds. Comp Biochem Physiol A. 1971;40:1107–1108. doi: 10.1016/0300-9629(71)90298-2. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001;1504:82–106. doi: 10.1016/s0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974;50:305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Pecqueur C, Alves-Guerra MC, Gelly C, Levi-Meyrueis C, Couplan E, Collins S, Ricquier D, Bouillaud F, Miroux B. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J Biol Chem. 2001;276:8705–8712. doi: 10.1074/jbc.M006938200. [DOI] [PubMed] [Google Scholar]
- Porter RK, Brand MD. Body mass dependence of H+ leak in mitochondria and its relevance to metabolic rate. Nature. 1993;362:628–630. doi: 10.1038/362628a0. [DOI] [PubMed] [Google Scholar]
- Raimbault S, Dridi S, Denjean F, Lachuer J, Couplan E, Bouillaud F, Bordas A, Duchamp C, Taouis M, Ricquier D. An uncoupling protein homologue putatively involved in facultative muscle thermogenesis in birds. Biochem J. 2001;353:441–444. [Google Scholar]
- Reynafarje B, Costa LE, Lehninger AL. O2 solubility in aqueous media determined by a kinetic method. Anal Biochem. 1985;145:406–418. doi: 10.1016/0003-2697(85)90381-1. [DOI] [PubMed] [Google Scholar]
- Riccio P, Aquila H, Klingenberg M. Purification of the carboxy-atractylate binding protein from mitochondria. FEBS Lett. 1975;56:133–138. doi: 10.1016/0014-5793(75)80127-x. [DOI] [PubMed] [Google Scholar]
- Rolfe DFS, Hulbert AJ, Brand MD. Characteristics of mitochondrial proton leak and control of oxidative phosphorylation in the major oxygen-consuming tissues of the rat. Biochim Biophys Acta. 1994;1188:405–416. doi: 10.1016/0005-2728(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Roussel D, Chainier F, Rouanet J, Barre H. Increase in the adenine nucleotide translocase content of duckling subsarcolemmal mitochondria during cold acclimation. FEBS Lett. 2000;477:141–144. doi: 10.1016/s0014-5793(00)01790-7. [DOI] [PubMed] [Google Scholar]
- Roussel D, Rouanet JL, Duchamp C, Barre H. Effects of cold acclimation and palmitate on energy coupling in duckling skeletal muscle mitochondria. FEBS Lett. 1998;439:258–262. doi: 10.1016/s0014-5793(98)01382-9. [DOI] [PubMed] [Google Scholar]
- Saarela S, Keith JS, Hohtola E, Trayhurn P. Is the ‘mammalian’ brown fat-specific mitochondrial uncoupling protein present in adipose tissues of birds? Comp Biochem Physiol B. 1991;100:45–49. doi: 10.1016/0305-0491(91)90082-o. [DOI] [PubMed] [Google Scholar]
- Schonfeld P, Fritz S, Halangk W, Bohnensack R. Increase in the adenine nucleotide translocase protein contributes to the perinatal maturation of respiration in rat liver mitochondria. Biochim Biophys Acta. 1993;1144:353–358. doi: 10.1016/0005-2728(93)90120-5. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Anion carriers in fatty acid-mediated physiological uncoupling. J Bioenerg Biomembr. 1999;31:431–445. doi: 10.1023/a:1005492205984. [DOI] [PubMed] [Google Scholar]
- Stepien G, Torroni A, Chung AB, Hodge JA, Wallace DC. Differential expression of adenine nucleotide translocator isoforms in mammalian tissues and during muscle cell differentiation. J Biol Chem. 1992;267:14592–14597. [PubMed] [Google Scholar]
- Talbot DA, Hanuise N, Rey B, Rouanet J-L, Duchamp C, Brand MD. Superoxide activates a GDP-sensitive proton conductance in skeletal muscle mitochondria from king penguin (Aptenodytes patagonicus) Biochem Biophys Res Commun. 2003;312:983–988. doi: 10.1016/j.bbrc.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Talbot DA, Lambert AJ, Brand MD. Endogenous matrix superoxide produced from mitochondrial complex I activates uncoupling protein 3. FEBS Lett. 2004;556:111–115. doi: 10.1016/s0014-5793(03)01386-3. [DOI] [PubMed] [Google Scholar]
- Toyomizu M, Ueda M, Sato S, Seki Y, Sato K, Akiba Y. Cold-induced mitochondrial uncoupling and expression of chicken UCP and ANT mRNA in chicken skeletal muscle. FEBS Lett. 2002;529:313–318. doi: 10.1016/s0014-5793(02)03395-1. [DOI] [PubMed] [Google Scholar]