Abstract
The amiloride-insensitive salt taste receptor is the predominant transducer of salt taste in some mammalian species, including humans. The physiological, pharmacological and biochemical properties of the amiloride-insensitive salt taste receptor were investigated by RT-PCR, by the measurement of unilateral apical Na+ fluxes in polarized rat fungiform taste receptor cells and by chorda tympani taste nerve recordings. The chorda tympani responses to NaCl, KCl, NH4Cl and CaCl2 were recorded in Sprague-Dawley rats, and in wild-type and vanilloid receptor-1 (VR-1) knockout mice. The chorda tympani responses to mineral salts were monitored in the presence of vanilloids (resiniferatoxin and capsaicin), VR-1 antagonists (capsazepine and SB-366791), and at elevated temperatures. The results indicate that the amiloride-insensitive salt taste receptor is a constitutively active non-selective cation channel derived from the VR-1 gene. It accounts for all of the amiloride-insensitive chorda tympani taste nerve response to Na+ salts and part of the response to K+, NH4+ and Ca2+ salts. It is activated by vanilloids and temperature (> 38°C), and is inhibited by VR-1 antagonists. In the presence of vanilloids, external pH and ATP lower the temperature threshold of the channel. This allows for increased salt taste sensitivity without an increase in temperature. VR-1 knockout mice demonstrate no functional amiloride-insensitive salt taste receptor and no salt taste sensitivity to vanilloids and temperature. We conclude that the mammalian non-specific salt taste receptor is a VR-1 variant.
Mammals utilize two types of taste receptors to detect mineral salts: one that is Na+ specific, and a second that does not discriminate among Na+, K+ and NH4+ (Frank et al. 1983; Stewart et al. 1997; Lindemann, 2001). In the anterior tongue, the Na+-specific receptor in the fungiform taste receptor cells is the amiloride-sensitive epithelial Na+ channel (ENaC) (Frank et al. 1983; Kretz et al. 1999; Lin et al. 1999; Lindemann, 2001). However, in rat, mouse and hamster a significant part of the chorda tympani taste nerve response to NaCl (and to non-Na+ salts) is amiloride insensitive (Ninomiya et al. 1989; Hettinger & Frank, 1990; Ye et al. 1993). In some mouse strains, the entire NaCl chorda tympani response is amiloride insensitive (Ninomiya et al. 1989; Halpern, 1998). This suggests that amiloride-insensitive Na+ entry may also contribute to the net apical flux in fungiform taste receptor cells (DeSimone et al. 2001; Lyall et al. 2002). In the posterior tongue, rat glossopharyngeal taste nerve responses to NaCl are amiloride insensitive, suggesting the predominance of amiloride-insensitive pathways for Na+ influx across the apical membrane of circumvallate taste receptor cells. Paradoxically, all the ENaC subunits are detected immunocytochemically in circumvallate taste receptor cells, but the channel appears to be non-functional (Kretz et al. 1999; Lin et al. 1999). The differential distribution of the ENaC and the amiloride-insensitive Na+ transduction pathways vary widely across species. In humans, salty taste perception is predominantly amiloride insensitive (Halpern, 1998; Feldman et al. 2003; Smith & Ossebaard, 1995). While both amiloride-sensitive and amiloride-insensitive salt taste receptors are present in many species, including humans (Halpern, 1998; Feldman et al. 2003), the major mechanism mediating salt taste is amiloride insensitive. However, very little is known regarding these amiloride-insensitive pathways.
Recently, we identified an apical amiloride-insensitive cation pathway in rat fungiform taste receptor cells that is modulated by cetylpyridinium chloride (CPC) (DeSimone et al. 2001). Here, we used a rat model and a vanilloid receptor-1 (VR-1) knockout mouse model (Caterina et al. 2000) to demonstrate that the CPC-sensitive, amiloride-insensitive pathway is a non-selective cation channel that has functional similarities with cloned VR-1 (Caterina et al. 1997, 2000; Tominaga et al. 1998, 2001; Davis et al. 2002; Gunthorpe et al. 2002). The observation that this channel is non-functional in VR-1 knockout mice indicates that the amiloride-insensitive salt taste transducer is derived from the VR-1 gene.
Methods
Sprague-Dawley rats were housed in the Virginia Commonwealth University animal facility in accordance with institutional guidelines. All in vivo and in vitro animal protocols were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. At the end of the experiments the rats were humanely killed by an intraperitoneal overdose of pentobarbital (approx. 195 mg (kg body weight)−1).
Chorda tympani nerve recordings
Female Sprague-Dawley rats (150–200 g) were anaesthetized by intraperitoneal injection of pentobarbital (60 mg kg−1) and supplemental pentobarbital (20 mg kg−1) was administered as necessary to maintain surgical anaesthesia. The animal's corneal reflex and toe-pinch reflex were used to monitor the depth of surgical anaesthesia. Body temperatures were maintained at 36–37°C with a circulating water heating pad. The left chorda tympani nerve was exposed laterally as it exited the tympanic bulla and placed onto a 32G platinum–iridium wire electrode. An indifferent electrode was placed in nearby tissue. Neural responses were differentially amplified with a custom built, optically coupled isolation amplifier. For display, responses were filtered using a band-pass filter with cutoff frequencies 40 Hz to 3 kHz and fed to an oscilloscope. Responses were then full-wave rectified and integrated with a time constant of 1 s. Integrated neural responses and current and voltage records were recorded on a chart recorder and also captured on disk using LabVIEW software (National Instruments, Austin, TX, USA) and analysed off-line. Stimulus solutions were injected into a Lucite chamber (3 ml; 1 ml s−1) affixed by vacuum to a 28 mm2 patch of anterior dorsal lingual surface. The chamber was fitted with separate Ag–AgCl electrodes for measurement of current and potential. These electrodes served as inputs to a voltage–current clamp amplifier that permitted the recording of neural responses with the chemically stimulated receptive field under zero current clamp (0cc) or voltage clamp. The clamp voltages were referenced to the mucosal side of the tongue (Ye et al. 1993, 1994).
The anterior lingual surface was stimulated with a rinse solution containing 10 mm KCl and with a salt stimulus solution containing 10 mm KCl + 100 mm NaCl. Benzamil (Bz; 5 μm; Sigma, St Louis, MO, USA) was used to block Na+ ion entry via the apical epithelial Na+ channels (Lyall et al. 2002). In addition to NaCl, 100 mm KCl, 500 mm KCl, 100 mm NH4Cl, and 100 mm CaCl2 were also used as salt taste stimuli. Other taste stimuli included: 500 mm sucrose, 10 mm quinine, and 20 mm HCl. Typically stimulus solutions remained on the tongue for 2 min. Control stimuli consisting of 300 mm NaCl and 300 mm NH4Cl, applied at the beginning and at the end of the experiment, were used to assess preparation stability. The data were digitized and analysed off line. The numerical value of an integrated chorda tympani response was obtained in the quasi-steady-state part of the response as the area under the integrated chorda tympani response curve for a time interval of 1 min measured from the end of a typical 2 min stimulation period (Lyall et al. 2002). The change in area under the integrated chorda tympani response curves to various stimuli under different conditions was normalized to the response observed in each animal to 300 mm NH4Cl. This ratio of areas was averaged across the number of animals in each group (n) and expressed as the mean ± s.e.m. of n. Student's t test was employed to analyse the differences between sets of data.
The chorda tympani responses were also monitored in wild-type C57BL/6J and homozygous VR-1 knockout B6.129S4-Trpv1tm1jul mice (The Jackson Laboratory, Bar Harbor, ME, USA). Mice (30–40 g) were anaesthetized by intraperitoneal injection of pentobarbital (30 mg kg−1) and supplemental pentobarbital (10 mg kg−1) was administered as necessary to maintain surgical anaesthesia. The rest of the procedure was the same as described above for rats.
Measurement of the apical membrane cation conductance
If the amiloride-insensitive salt taste receptor has ion channel properties, one would expect an increase in conductance due to stimulation with salts plus agonist concentrations of vanilloids or CPC. The increase in apical membrane conductance can be detected in situ by measuring the sensitivity of chorda tympani responses to NaCl, KCl, and NH4Cl to applied lingual potential differences. The response–voltage data are well represented by the relationship:
| (1) |
where the amiloride-insensitive chorda tympani response, r, is assumed to be proportional to the cation flux through the ensemble of apical ion channels modulated by vanilloids and CPC. Here rmax is the maximum response (proportional to the maximum ion flux), c the salt concentration, Km is the concentration at which r is half-maximal, ϕ is the normalized potential, F Δ V/RT. Here ΔV is the applied potential difference relative to the zero current potential across the stimulated lingual epithelium (referenced to the oral cavity side), F, R and T having their usual thermodynamic meaning, and δ is the fraction of the voltage dropped across the apical membrane of the responding taste cells. In some applications the above equation can be linearized in both voltage and concentration (see below), yielding:
| (2) |
Here ro is the linearized response under zero current-clamp conditions, rmaxc/Km. Equation (2) shows that the variation of the chorda tympani response with applied voltage (ΔV) for given conditions of temperature, salt concentration and agonist concentration depends on the corresponding magnitude of the chorda tympani response in the absence of an applied voltage, ro. This dependence arises explicitly in the slope of the response curve which is proportional to ro. We may therefore expect that as RTX or CPC causes ro to either increase or decrease, the slope of the response will change proportionally with voltage. Because r is proportional to the cation flux through the apical conductance, the slope of the response–voltage curves will be proportional to the temperature-modulated or RTX- or CPC-modulated channel conductance. Accordingly, we designate the slope Fδro/RT as the response conductance (κ). Thus an increase in response is necessarily linked to an increase in response conductance.
Temperature studies
To investigate the effect of temperature on the chorda tympani responses to mineral salts, the lingual surface was superfused (8 ml min−1) with salt solutions using syringe pumps and heating coils maintained at temperatures between 23 and 55.5°C. The chorda tympani response or the product of absolute temperature (in kelvins, K), T, with response conductance, κ (i.e. Tκ), was plotted as a function of the temperature (°C) of the stimulus solution delivered to the tongue surface. The temperature dependence of the chorda tympani response and Tκ typically showed steep increases over a relatively narrow temperature interval followed by a slow decline from a maximum with further increases in temperature. The high degree of positive co-operativity in the data was modelled with a modified Hill equation of the form:
| (3) |
where r is the chorda tympani response and t is the temperature. The quantities: a, b, k, m, n and q are parameters used to fit the data according to least squares criteria.
[Na+]i measurement in polarized taste receptor cells
The rats were anaesthetized by exposing them to an inhalation anaesthetic, isoflurane (1.5 ml), in a desiccator. When the rats were fully unconscious, a midline incision was made in the chest wall and the aorta severed. The tongues were then rapidly removed and stored in ice-cold Ringer solution. The Ringer solution contained (mm): 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 sodium pyruvate, 10 glucose, 10 Hepes, pH 7.4. The lingual epithelium was isolated by collagenase treatment (Bèhèet al. 1990). A small piece of the anterior lingual epithelium containing a single fungiform papilla was mounted in a special microscopy chamber (Chu et al. 1995) as described earlier (Lyall et al. 2002). Relative changes in intracellular sodium activity ([Na+]i) were monitored in polarized taste receptor cells by loading the tissue with the sodium-sensitive fluoroprobe sodium green. Tissues were loaded with sodium green AM (30 μm; Molecular Probes, Eugene, OR, USA) at 4°C for 2 h. Before the experiment was started, the tissue was perfused on both sides with control solution for 15 min. The tissue was continuously perfused at the rate of 1 ml min−1 and the solution changes in the apical compartment were made using three-way miniature solenoid valves. The taste receptor cells in the taste bud were visualized from the basolateral side through a 40× objective (Zeiss; 0.9 NA) with a Zeiss Axioskop 2 plus upright fluorescence microscope and imaged with a set-up consisting of a cooled CCD camera attached to an image intensifier, an epifluorescent light source, a 515 nm dichroic beam splitter, and a 535 nm emission filter (20 nm band pass). In sodium green-loaded cells, the changes in [Na+]i were monitored by exciting the cells at 490 nm and the emitted light was imaged at 535 nm at 10 s intervals. Small regions of interest in the taste bud (diameter 2–3 μm) were chosen in which the changes in fluorescence intensity (F490) were analysed using imaging software TILL VisIon V3.3, TILL Photonics, Martinsried, Germany. Each region of interest contained two to three receptor cells. Thus the fluorescence intensity recorded for a region of interest represents the mean value from two to three receptor cells within the region of interest. In a typical experiment the fluorescence intensity measurements were made in an optical plane in the taste bud containing at least six regions of interest (approximately 18 cells). The background and autofluorescence at 490 nm were corrected from images of a taste bud without the dye. All experiments were done at room temperature (approximately 23°C).
Data analysis
In taste receptor cells loaded with sodium green the changes in [Na+]i were expressed relative to the fluorescence intensity (F490) under control conditions. The F490 under control conditions for each region of interest was taken as 100%. The data were also presented as the mean ± standard error of the mean from different tissue preparations. In this case n represented the number of taste buds. Student's t test was employed to analyse the differences between sets of data.
RT-PCR
Taste buds were harvested from rat fungiform papillae, aspirated with a micropipette and individually transferred onto coverslips, avoiding contaminating cells and debris (Vinnikova et al. 2004). Dorsal root ganglia were harvested as previously described (Depree & Bigbee, 1994). Total RNA was extracted using a RNeasy Mini kit (Qiagen, Valencia, CA, USA) with incorporation of the DNAse digestion step. The cDNA was generated and amplified using a Super SMART™ PCR cDNA Synthesis Kit (Clontech, Palo Alto, CA, USA) according to the manufacturer's protocol. PCR screening of the fungiform cDNA for the presence of VR-1 and its homologues was performed with HotStarTaq Poymerase (Qiagen) using primers and conditions described elsewhere (Liu & Simon, 2001). The following controls were performed with each PCR reaction: (i) a positive control using dorsal root ganglia cDNA and (ii) a negative control with omission of template. The PCR products were analysed by agarose gel electrophoresis. Bands of the predicted size were purified using the MinElute Gel Extraction kit (Qiagen) and directly sequenced (Davis Sequencing, Inc., Davis, CA, USA). Product sequences were analysed using BLAST search (National Center for Biotechnology Information, US National Library of Medicine, Bethesda, MD, USA).
Results
To investigate the physiological and pharmacological properties of the amiloride-insensitive salt taste receptor, we monitored rat and mouse chorda tympani responses to mineral salts in the presence of benzamil (Bz), a more specific ENaC inhibitor than amiloride. The chorda tympani responses were monitored under open circuit conditions, i.e. under zero current clamp (0cc) and under lingual voltage clamp (Ye et al. 1993; Kloub et al. 1997; Lyall et al. 2002).
Vanilloids, elevated temperature and VR-1 antagonists modulate rat CT responses to mineral salts
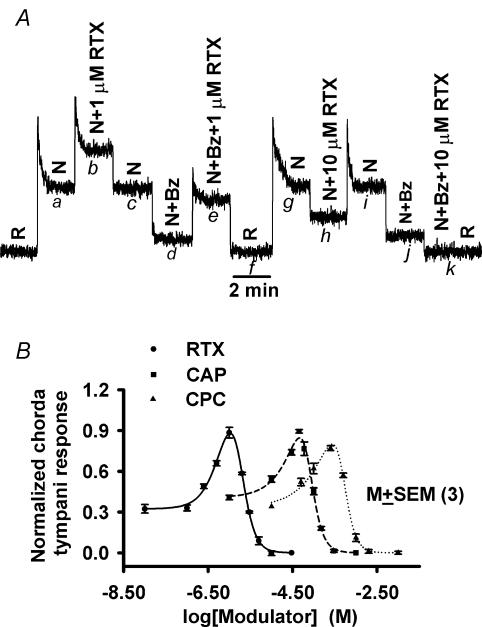
The effect of VR-1 agonists, resiniferatoxin (RTX) and capsaicin (CAP), was investigated on the NaCl chorda tympani responses. Stimulating the tongue with 100 mm NaCl + 1 μm RTX reversibly increased the chorda tympani response compared to 100 mm NaCl alone (Fig. 1A; a–b–c). The ENaC component of the chorda tympani response was blocked by 5 μm Bz (c–d). Superfusing the tongue with 100 mm NaCl + 5 μm Bz + 1 μm RTX gave the same magnitude of enhancement observed without Bz (Fig. 1A; d–e versus a–b). In contrast, stimulating with 100 mmNaCl + 10 μm RTX reversibly decreased the chorda tympani response (g–h–i) compared to 100 mm NaCl alone (f–g). In the next step, the ENaC component was again blocked with 5 μm Bz (i–j). Stimulating with NaCl + 5 μm Bz + 10 μm RTX reduced the response (j–k) to rinse level (R; 10 mm KCl). The data suggest that depending upon its concentration, RTX acts both as an agonist and antagonist of the Bz-insensitive NaCl chorda tympani response.
Figure 1. Effect of VR-1 agonists (RTX and CAP) and CPC on the rat chorda tympani response to NaCl.
The tongue was stimulated with a rinse solution (R; 10 mm KCl) and with 100 mm NaCl + 10 mm KCl (N) or with 100 mm NaCl + 10 mm KCl + 5 μm Bz (N + Bz). A, the Bz-insensitive NaCl chorda tympani response was enhanced (d–e) by 1 μm RTX and inhibited (j–k) by 10 μm RTX. B, increasing concentrations of RTX (•), CAP (▪) and CPC (▴) produced biphasic changes in the Bz-insensitive NaCl chorda tympani response. Each point represents the mean ± s.e.m. of the normalized chorda tympani response from 3 animals.
The rat Bz-insensitive NaCl chorda tympani response over a range of RTX, CAP and CPC concentrations gave bell-shaped concentration–response curves (Fig. 1B). RTX caused the chorda tympani response to NaCl (100 mm NaCl + 5 μm Bz) to increase monotonically between 0.1 μm and 1 μm; at higher RTX concentrations the NaCl response decreased reaching control levels around 3 μm. Above 3 μm RTX, the NaCl chorda tympani response was less than control, reaching rinse levels around 10 μm RTX concentration (Fig. 1B). CAP, also a VR-1 agonist, was similarly effective, although at relatively higher concentrations. Maximum activation of the NaCl chorda tympani response occurred around 40 μm CAP, and by 200 μm responses decreased to the rinse level (Fig. 1B). At the concentrations used in our experiments, we did not observe any effect of CAP or RTX on the Bz-sensitive NaCl chorda tympani response. It is likely that higher concentration of CAP (> 200 μm) may produce non-specific effects on the rat Na+-specific chorda tympani N-fibres (Osada et al. 1997). CPC, a compound previously shown to modulate the amiloride-insensitive chorda tympani response to mineral salts (DeSimone et al. 2001), demonstrated a similar bimodal effect on the NaCl chorda tympani response. Maximum activation of the NaCl chorda tympani response occurred around 250 μm CPC, and by 2 mm the response decreased to rinse level (Fig. 1B). In the presence of 5 μm Bz, addition of RTX (10 μm), CAP (200 μm), or CPC (2 mm) reduced the NaCl chorda tympani response to a baseline level that was indistinguishable from the rinse level. This suggests that the NaCl chorda tympani response is composed entirely of a Bz-sensitive component (ENaC) and a second Bz-insensitive component that is modulated by RTX, CAP and CPC with a rank order potency of RTX > CAP > CPC.
VR-1 is activated by heat (Caterina et al. 1997, 2000; Tominaga et al. 1998, 2001; Davis et al. 2002; Gunthorpe et al. 2002). Therefore, we tested the effect of temperature on the chorda tympani responses to NaCl. The tongue was superfused with a rinse solution (R; 10 mm KCl; pH 6) maintained at room temperature (23°C), and with NaCl stimulating solutions (100 mm NaCl + 5 μm Bz; pH 6) maintained at temperatures varying between 23 and 55.5°C. Elevating the temperature to 41°C (Fig. 2A) decreased the Bz-sensitive NaCl chorda tympani response (f–g) relative to 23°C (b–c). This indicates that at 41°C the ENaC activity is inhibited (Lundy & Contreras, 1997). In contrast, at 41°C the Bz-insensitive NaCl chorda tympani response (g–h) was enhanced relative to 23°C (c–d). Increasing the temperature to 38°C produced a sharp increase in the Bz-insensitive NaCl chorda tympani response; the maximum enhancement was achieved around 42°C, and above 42°C the responses decreased (Fig. 2B, 0 RTX).
Figure 2. Effect of elevated temperature on the rat chorda tympani response to NaCl.
The tongue was stimulated with a rinse solution (R; 10 mm KCl) and with 100 mm NaCl + 10 mm KCl (N) or with 100 mm NaCl + 10 mm KCl + 5 μm Bz (N + Bz). A, at 23°C superfusing with N + Bz inhibited 70% of the chorda tympani response (b–c) relative to a–b. At 41°C the NaCl chorda tympani response was smaller (e–f < a–b) and Bz produced a much smaller decrease in the NaCl chorda tympani response (f–g < b–c) relative to 23°C. B, variation of the chorda tympani response to N + Bz with temperature between 23 and 55.5°C in the presence of RTX (0–10 μm; pH 6). In a typical experiment the rat tongue was rinsed with 10 mm KCl (pH 6) at 23°C followed by stimulation with N + Bz + RTX at a fixed concentration of RTX varying between 0 and 10 μm (pH 6) over a range of temperatures between 23 and 55.5°C. Fitted curves in each case were drawn using eqn (3).
The amiloride-insensitive salt taste receptor integrates the effects of multiple stimuli. RTX increased the Bz-insensitive NaCl chorda tympani response at 23°C and shifted the temperature curve to the left in a dose-dependent manner (Fig. 2B). The mean temperature at which the Bz-insensitive NaCl chorda tympani response was enhanced by 50% (t0.5) at 0, 0.25, 0.50 and 1.0 μm RTX was 39.9 ± 0.1°C (n = 16), 37.9 ± 0.2°C (n = 9), 35.4 ± 0.3°C (n = 6) and 30.9 ± 0.4°C (n = 4), respectively. Increasing RTX to 10 μm completely inhibited the constitutively active Bz-insensitive NaCl chorda tympani response at 23°C and at elevated temperatures (Fig. 2B; n = 3).
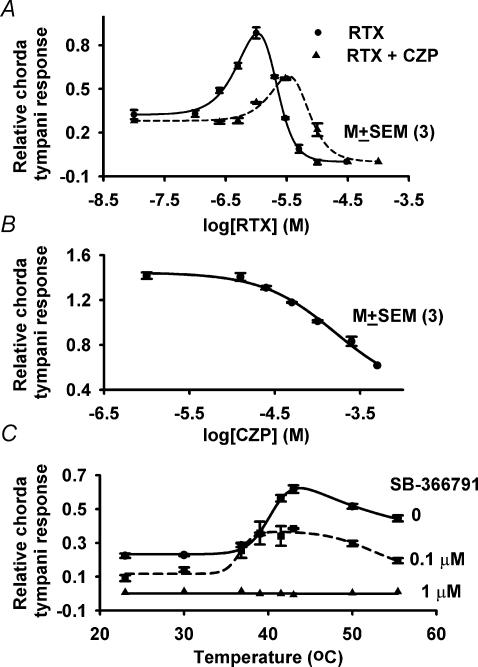
The VR-1 antagonists, capsazepine (CZP) and SB-366791, inhibited the effects of vanilloids, CPC and temperature on Bz-insensitive NaCl chorda tympani responses. CZP (10 μm) decreased the magnitude of the maximum Bz-insensitive NaCl chorda tympani response and shifted the RTX concentration–response curve to the right (Fig. 3A). Stimulating the tongue with NaCl solutions containing a fixed RTX concentration but increasing concentrations of CZP demonstrated a dose-dependent inhibition of the NaCl chorda tympani response (Fig. 3B). At a CZP concentration of 100 μm, chorda tympani responses decreased to the level of chorda tympani responses with NaCl alone. At CZP concentrations of 250 μm and above, the chorda tympani response was below the level of the chorda tympani response with NaCl alone. The Ki (the concentration of CZP that inhibits 50% of the RTX effect on the NaCl chorda tympani response) was 100 μm. The data suggest that at stimulating RTX concentrations, CZP acts as a competitive inhibitor of RTX.
Figure 3. Effect of VR-1 antagonists (CZP and SB-366791) on the rat chorda tympani response to NaCl.
A, chorda tympani responses were recorded during superfusion of the tongue with a rinse solution (10 mm KCl) and a stimulating solution (100 mm NaCl + 5 μm Bz + 10 μm CZP) containing RTX (0–10 μm). B, chorda tympani responses were recorded during superfusion of the tongue with a rinse solution (10 mm KCl) and stimulating solutions containing 10 mm KCl + 100 mm NaCl + 5 μm Bz + 0.75 μm RTX + CZP (0–500 μm). C, chorda tympani responses were recorded during superfusion of the tongue with a rinse solution (10 mm KCl) and a stimulating solution (100 mm NaCl + 5 μm Bz) containing 0 (•), 0.1 μm (▪) and 1 μm (▴) SB-366791. Each point represents the mean ± s.e.m. of the normalized chorda tympani response from 3 animals. Fitted curves in each case were drawn using eqn (3).
A more specific VR-1 antagonist, SB-366791, blocked the temperature-induced effects on the chorda tympani response to NaCl + Bz in a dose-dependent manner. At 1 μm, SB-366791 completely blocked the Bz-insensitive NaCl chorda tympani response at 23°C and at elevated temperatures (Fig. 3C).
The amiloride-insensitive salt taste receptor is non-functional in VR-1 knockout mice
The Bz-insensitive NaCl chorda tympani response is modulated by RTX, CAP and elevated temperature. The VR-1 antagonists, CZP and SB-366791, inhibit the effect of vanilloids, CPC and temperature on the Bz-insensitive NaCl chorda tympani response. Multiple stimuli produced an integrated effect on the Bz-insensitive NaCl chorda tympani response. The above results demonstrate that the amiloride-insensitive salt taste receptor has functional similarities with the VR-1 receptor. Consistent with this, a VR-1 mRNA transcript common to several channels in the transient receptor potential (TRP) receptor family was detected in rat fungiform taste receptor cells. We constructed a cDNA library from fungiform taste buds. Using primers sense 5′-TGAAAAACACCGTTGGGGAC-3′, and anti-sense 5′-GTAGACGAACATAAACCGGC-3′ (Liu & Simon, 2001), a single band of 338 bp was obtained (Fig. 4; lane 1), that yielded 100% homology with TRP channels: rVR-1, rVRL-1, rSIC and rVR5′sv. As a positive control, an identical PCR fragment was also amplified from a rat dorsal root ganglion cDNA library, known to contain the VR-1 transcript (Fig. 4; lane 2).
Figure 4. Rat fungiform taste receptor cells contain a VR-1 variant transducer.
A cDNA library from rat fungiform taste receptor cells was screened for VR-1 and its homologues (Liu & Simon, 2001) and yielded a single band of expected size (Lane 1; →). An identical PCR fragment was amplified from rat dorsal root ganglia cDNA (Lane 2). Lane 3: DNA ladder.
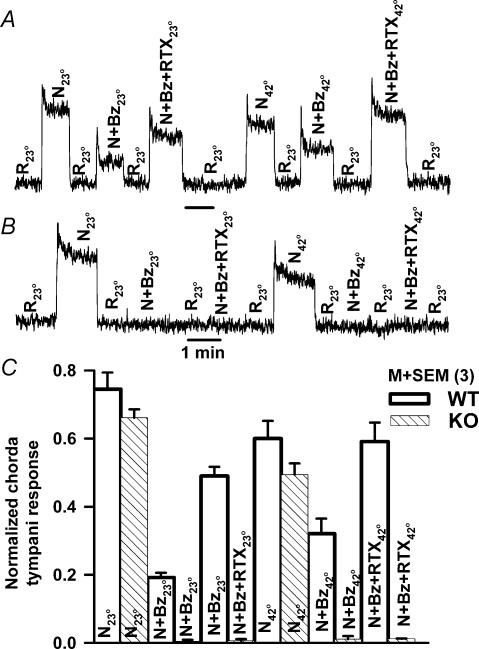
Homozygous VR-1 knockout mice, B6.129S4-Trpv1tm1jul (Caterina et al. 2000), demonstrated no Bz-insensitive NaCl chorda tympani response and no sensitivity to vanilloids and temperature. At 23°C, in wild-type C57BL/6J mice, about 25% of the NaCl chorda tympani response (N23°) was Bz insensitive (N + Bz23°) (Fig. 5A). Its magnitude was enhanced by RTX (N + Bz + RTX23°). At 42°C the Bz-insensitive NaCl chorda tympani response (N + Bz42°) and the response to RTX (N + Bz + RTX42°) were significantly enhanced relative to 23°C. The absence of a Bz-insensitive NaCl chorda tympani component indicates that in VR-1 null mice the entire NaCl response is composed of a Bz-sensitive ENaC component (N23°– (N + Bz23°); Fig. 5B). No effects of RTX and elevated temperature were observed in response to NaCl + Bz (N + Bz) and to NaCl + Bz + RTX (N + Bz + RTX) in VR-1 knockout mice (Fig. 5B). The data from three wild-type and three VR-1 knockout mice are summarized in Fig. 5C. In Fig. 5C, in VR-1 knockout mice, the magnitude of the chorda tympani responses with N + Bz23°, N + Bz + RTX23°, N + Bz42° and N + Bz + RTX42° were not significantly different from zero (P > 0.05; n = 3). The absence of a constitutively active Bz-insensitive NaCl chorda tympani response in VR-1 knockout mice demonstrates that the amiloride-insensitive salt taste receptor is derived from the VR-1 gene, and is most likely a homologue of VR-1.
Figure 5. Chorda tympani responses in wild-type mice (WT) (A) and VR-1 knockout mice (KO) (B).
Tongues were stimulated with 100 mm NaCl (N23°, N42°; subscripts refer to 23°C and 42°C temperatures, respectively), 100 mm NaCl + 5 μm Bz (N + Bz23°; N + Bz42°), and 100 mm NaCl + 5 μm Bz + 0.75 μm RTX (N + Bz + RTX23°; N + Bz + RTX42°) at either 23 or 42°C. Data from 3 wild-type mice and 3 VR-1 knockout mice are summarized in C. Each bar represents the mean ± s.e.m. of the normalized chorda tympani response from 3 animals.
The amiloride-insensitive salt taste receptor is a non-selective cation channel
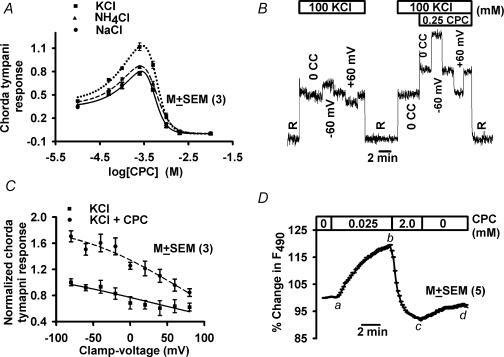
Stimulating the tongue with mixtures of CPC plus 100 mm KCl or NH4Cl (Fig. 6A) produced bell-shaped concentration–response relationships similar to those obtained with CPC + NaCl + Bz. RTX and CAP also produced similar bell-shaped responses with NaCl, KCl, NH4Cl and CaCl2 with the same rank order potency as obtained for NaCl + Bz (authors' unpublished observations). The chorda tympani responses to KCl and NH4Cl are amiloride insensitive (Hettinger & Frank, 1990; Kloub et al. 1997). Addition of 2 mm CPC (DeSimone et al. 2001), 0.01 mm RTX or 0.2 mm CAP (authors' unpublished observations) to KCl or NH4Cl stimulating solutions produced about 40% inhibition of the chorda tympani response compared to 100% in the case of NaCl + Bz. Therefore, non-Na+ salt taste is also mediated by more than one taste receptor. The results suggest that the amiloride-insensitive cation pathway accounts for all of the Bz-insensitive chorda tympani response to Na+ salts and part of the response to K+, NH4+ and Ca2+ salts.
Figure 6. Cation selectivity and voltage sensitivity of the amiloride-insensitive channel.
A, CPC induced biphasic changes in rat chorda tympani responses to 100 mm NaCl + 5 μm Bz (•), 100 mm NH4Cl (▴), and 100 mm KCl (▪). The CPC-sensitive chorda tympani responses to KCl and NH4Cl were obtained by subtracting the maximum suppression value at 10 mm CPC. Each point represents the mean ± s.e.m. of the normalized chorda tympani response from 3 animals. B, rat chorda tympani responses to 100 mm KCl at zero current clamp (0cc), −60 mV and +60 mV voltage clamp in the absence (left trace) and presence (right trace) of 0.25 mm CPC. C, rat chorda tympani responses to 500 mm KCl between −80 and +80 mV lingual voltage clamp in the absence (▪) and presence of 0.25 mm CPC (•). Each point represents the mean ± s.e.m. of the normalized chorda tympani response from 3 animals. D, relative changes in [Na+]i in polarized rat fungiform taste receptor cells loaded with sodium green. The changes in [Na+]i are expressed as the percentage change in fluorescence intensity (F490) of sodium green. Values are presented as mean ± s.e.m. from 6 regions of interest within the taste bud.
Agonist concentrations of vanilloids and CPC and elevated temperature increased the apical conductance to cations. The rat chorda tympani response to 100 mm KCl was slightly enhanced at −60 mV lingual voltage clamp (referenced to the oral cavity) and slightly suppressed at +60 mV (Fig. 6B). In the presence of 0.25 mm CPC, the same voltages exerted significantly larger effects on the response (Fig. 6B). Figure 6C shows the chorda tympani response to KCl under control conditions and after CPC treatment as a function of clamp voltages between −80 mV and +80 mV. In the presence of CPC, the mean value of the normalized chorda tympani response at −80 mV, 0 and +80 mV was significantly greater (P < 0.01; paired t test; n = 3; Fig. 6C) than its corresponding value in the absence of CPC. Thus the rat chorda tympani response to KCl across voltages shows that both the response and the slope of the response with voltage increased between −80 mV and +80 mV in the presence of CPC. The data were well represented by eqn (1). In Fig. 6C, the parameters for the response to KCl alone were: rmax= 1.34, Km= 0.37 m, and δ= 0.21. For KCl + CPC they were: rmax= 1.90, Km= 0.21 m, and δ= 0.37. Since the response is nearly linear with voltage, the relationship between the response and the slope of the response with voltage can be approximated by eqn (2). It is clear that the slope of the response curve (Fδro/RT; eqn (2)) is proportional to ro, so as CPC causes ro to increase, it also causes the slope of the response with voltage to increase. If the amiloride-insensitive salt taste receptor is an apical cation channel, r is expected to be proportional to the cation flux through apical conductances. Therefore, any agent that increases the response must do so by increasing the conductance of the cation channel transducers. This effect is observed macroscopically as an increase in the slope of the response–voltage curve, i.e. the response conductance, κ (Fδro/RT) which is proportional to the response, ro. This effect is observed in Fig. 6C where the agonist, CPC, is seen to increase both response and response conductance.
The presence of an apical CPC-modulated Na+ pathway was confirmed directly by the measurement of the apical Na+ flux (Lyall et al. 2002) in polarized fungiform TRCs. At an agonist concentration, CPC enhanced (Fig. 6D; a–b), and at an antagonist concentration, CPC inhibited (Fig. 6D; b–c) the unilateral apical Na+ flux.
We investigated the effect of RTX on the response conductance due to 100 mm NaCl + Bz. We observed that in the presence of RTX the response and consequently the slope of the response (κ) were each greater relative to their control values (Fig. 7A). In the presence of 0.75 μm RTX (Fig. 7A), κ increased from (1.29 ± 0.03) × 10−3 (n = 8) to (2.10 ± 0.32) × 10−3 response units mV−1 (n = 4; P < 0.05; unpaired t test). The fact that the size of the response and the response conductance are proportional is an indication that the taste receptor sensitive to RTX either is, or is tightly coupled to, an apical membrane conductance. If so, we can expect that any variable, such as a change in temperature or in RTX concentration, that modulates the response through this taste receptor will coincide with proportionate changes in response conductance. The effect of temperature on κ is best studied by first separating its dependence on temperature through the thermodynamic scale factor (RT) from its physiological temperature dependence as expressed through the temperature dependence of ro. This is accomplished by multiplying κ by T (in K) and treating Tκ as a new variable defined by:
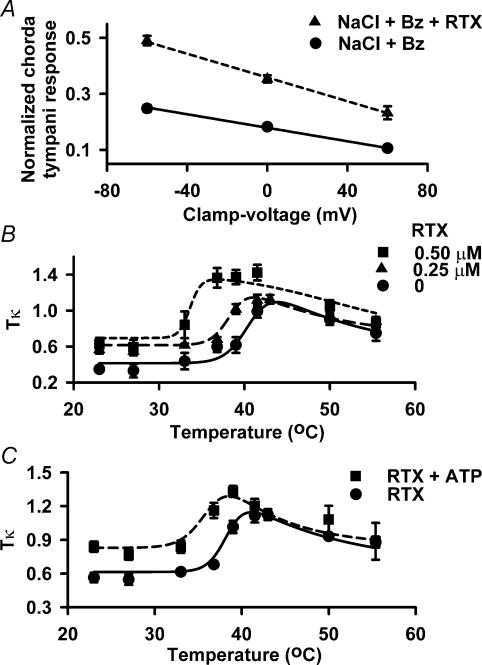
Figure 7. Effect of RTX, temperature and ATP on Tκ values of taste receptor cells in situ.
A, rat chorda tympani responses were recorded during superfusion of the tongue with a rinse solution (10 mm KCl) and with stimulation solutions containing 100 mm NaCl + 5 μm Bz (•; NaCl + Bz) or 100 mm NaCl + 5 μm Bz + 0.5 μm RTX (▴; NaCl + Bz + RTX) at 23°C. In the absence of RTX, n = 8 and in the presence of RTX, n = 4. B, chorda tympani responses were recorded during superfusion of the tongue with a rinse solution (10 mm KCl + 10 mm Hepes; pH 6.0, 23°) and stimulating solutions (10 mm KCl + 100 mm NaCl + 5 μm Bz + 10 mm Hepes; pH 6.0) containing 0 (•), 0.25 μm (▴), and 0.5 μm RTX (▪). For each RTX concentration tested the stimulus solution temperature was varied between 23 and 55.5°C. For 0, 0.25 and 0.5 μm RTX, n = 6, 4 and 3, respectively. C, chorda tympani responses were recorded during superfusion of the tongue with a rinse solution (10 mm KCl + 10 mm Hepes; pH 6.0, 23°C) and stimulating solutions (10 mm KCl + 100 mm NaCl + 5 μm Bz + 10 mm Hepes; pH 6.0) containing 0.25 μm RTX (•), and 0.25 μm RTX + 500 μm ATP (▪). For each RTX and RTX + ATP concentration tested the stimulus solution temperature was varied between 23 and 55.5°C. For 0.25 μm RTX and 0.25 μm RTX + 0.5 mm ATP, n = 4 and 3, respectively. Chorda tympani responses were recorded at zero current clamp (0cc) and at ± 60 mV lingual voltage clamp. In each case the NaCl chorda tympani responses were normalized to the corresponding chorda tympani responses obtained with 300 mm NH4Cl. In B and C the Tκ (product of absolute temperature (K), T, with response conductance (κ) data points were plotted as a function of temperature ranging from 23 to 55.5°C. The Tκ data were fitted toeqn (4) as described in the text. Values are presented as the mean ± s.e.m. of n, where n = number of animals.
| (4) |
where F and R are the usual thermodynamic constants, ro(t) is the chorda tympani response under zero current clamp conditions which depends on temperature, t, as shown in Fig. 2B, and δ has been defined in eqn (1). The value of Tκ corresponding to the κ-value for 100 mm NaCl + Bz at 23°C in Fig. 7A is accordingly 0.38 ± 0.01 response units K mV−1, and Tκ for 100 mm NaCl + Bz + 0.75 μm RTX at 23°C is 0.62 ± 0.09 response units K mV−1. Figure 7B shows the variation in Tκ with temperature in the presence of either 0, 0.25 μm or 0.5 μm RTX. In each case the fitted curve through the experimental points was drawn using Fδ/R as the only adjustable parameter. The remaining parameters were fixed at those values that were used to fit the corresponding dependence of ro on temperature in Fig. 2B. The single adjustable parameter yielded δ-values of 0.15, 0.15 and 0.17 for the 0, 0.25 μm and 0.5 μm RTX curves, respectively, suggesting that the apical channels sense from 15 to 17% of the voltage gradient applied across the stimulated anterior lingual taste region. As expected from eqn (4) Tκ shows the same temperature and RTX dependence as ro, displaying the same shift of t0.5 to lower temperatures with increasing RTX concentration. The strict proportionality observed here between response and response conductance in situ under various conditions of agonist concentration and temperature is consistent with the view that the proximate event in the amiloride-insensitive NaCl response is the passage of Na+ through apical membrane cation channels that are variants of VR-1.
Physiological and pharmacological properties not shared by the amiloride-insensitive salt taste receptor with VR-1
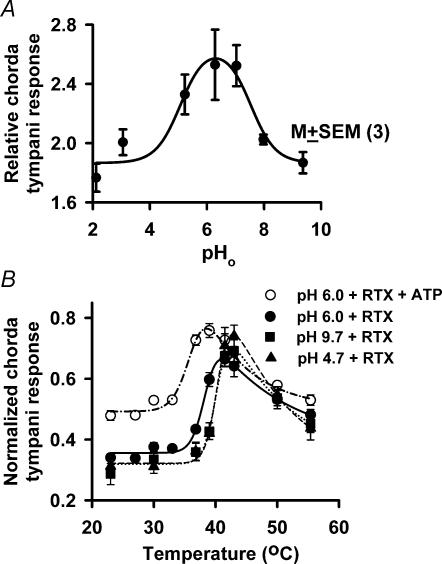
Besides some similarities, there are also significant physiological differences between cloned VR-1 and the amiloride-insensitive salt taste receptor. VR-1 is activated by a decrease in external pH (pHo) (Caterina et al. 1997; Davis et al. 2002; Gunthorpe et al. 2002). In the absence of a ligand, the Bz-insensitive NaCl chorda tympani response was not affected by changes in pHo (see Supplementary material; Lyall et al. 2002). However, the RTX-induced change in the Bz-insensitive NaCl chorda tympani response described a bell-shaped curve as a function of stimulus solution pHo (Fig. 8A). Similar to its effects on VR-1 (Caterina et al. 1997; Jordt et al. 2000; Cortright et al. 2001), RTX induced the greatest increase in the Bz-insensitive NaCl chorda tympani response under moderately acidic conditions (pH 6). Since at constant pHo, changes in taste receptor cell intracellular pH (pHi) do not affect the magnitude of the RTX-induced effects on the NaCl chorda tympani response (see Supplementary material), this suggests that the channel is modulated by the binding of protons to an external site on the amiloride-insensitive salt taste receptor.
Figure 8. Effect of external pH (pHo) on the NaCl chorda tympani response.
A, effect of pHo (2–10) on the rat chorda tympani response to 100 mm NaCl + 5 μm Bz + 0.5 μm RTX. Each point represents the mean ± s.e.m. of the normalized chorda tympani response from 3 animals. B, effect of pHo 4.7 (▴; n = 3), 6.0 (•; n = 9), 9.7 (▪; n = 6) and ATP (○; n = 4) on the temperature-induced chorda tympani response to 100 mm NaCl + 5 μm Bz + 0.25 μm RTX. Each point represents the mean ± s.e.m. of the normalized chorda tympani responses from n, number of animals. Fitted curves in each case were drawn using eqn (3).
Adenosine 5′-triphosphate (ATP) decreases the temperature threshold for VR-1 (Tominaga et al. 2001). ATP (500 μm) alone had no effect on the temperature threshold of the Bz-insensitive NaCl chorda tympani response (authors' unpublished observations). In the presence of 0.25 μm RTX (Fig. 8B), ATP (500 μm) decreased t0.5 from 37.9 ± 0.2°C (n = 9) to 35.3 ± 0.8°C (P < 0.05; n = 4). In the presence of RTX, the ATP-induced left shift in the temperature–response curve was accompanied by parallel changes in the Tκ (Fig. 7C). As in Fig. 7B, the RTX + ATP data were fitted according to eqn (4) using a single parameter. The corresponding δ-value was 0.15, which is consistent with the other response conductance studies (Fig. 7B).
Similarly, changes in pHo between 4.7 and 9.7 had no effect on the temperature threshold of the Bz-insensitive NaCl chorda tympani response in the absence of an agonist (authors' unpublished observations). However, in the presence of 0.25 μm RTX, changing pHo from 6.0 to pH 4.7 or to 9.7 (Fig. 8B) increased t0.5 from 37.9 ± 0.2°C (n = 9) to 40.0 ± 0.01°C (n = 3) or to 39.7 ± 0.02°C (n = 6; P < 0.05), respectively. Thus at a moderately acidic pH of 6.0, both pHo and RTX act synergistically to lower the temperature threshold of the Bz-insensitive NaCl chorda tympani response.
Vanilloids specifically affect chorda tympani responses to mineral salts
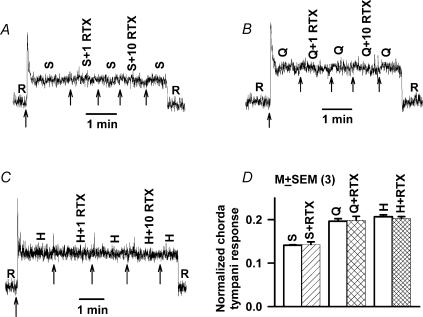
RTX at 1 μm or 10 μm did not affect the chorda tympani responses to 500 mm sucrose (Fig. 9A and D), 10 mm quinine (Fig. 9B and D), or 20 mm HCl (Fig. 9C and D). Thus RTX does not affect sucrose-best or quinine-best chorda tympani nerve fibres, and the ion pathway modulated by RTX does not transport H+ (Liu & Simon, 2001). Since amiloride and Bz eliminate neural activity in Na+-best single units (N-fibres) (Hettinger & Frank, 1990), RTX, CAP and CPC must therefore modulate the activity of the non-specific cation-sensitive salt units (E- or H-fibres) (Ninomiya & Funakoshi, 1988; Hettinger & Frank, 1990). The latter neural units presumably innervate taste receptor cells containing the VR-1 gene derived amiloride-insensitive salt taste receptor.
Figure 9. Effect of RTX on rat chorda tympani responses to sucrose, quinine and HCl.
Rat chorda tympani responses were recorded during superfusion of the tongue with a rinse solution (R; 10 mm KCl) and with stimulation solutions (arrows) containing 500 mm sucrose (S) (A), 10 mm quinine (Q) (B) and 20 mm HCl (H) (C) at 23°C with and without 1 μm and 10 μm RTX. Since RTX at 1 or 10 μm concentration induced no change in the chorda tympani responses to sucrose, quinine and HCl relative to control, the data for both concentrations of RTX were combined and are summarized in D. Each bar represents the mean ± s.e.m. of the normalized chorda tympani response in the presence of RTX relative to control from 3 animals. In the presence of RTX the chorda tympani responses to sucrose, quinine and HCl were not significantly different from control (P > 0.05).
Discussion
Our studies involving in vivo chorda tympani recordings and apical ion flux measurements in polarized fungiform taste receptor cells in vitro demonstrate that the amiloride-insensitive salt taste receptor is a non-selective cation channel that is permeable to Na+, K+, NH4+ and Ca2+ ions. The amiloride-insensitive cation channel is a member of the TRP channel family. It demonstrates functional similarities to the VR-1 receptor. It is modulated by vanilloids, temperature and VR-1 antagonists, and can integrate the effect of multiple stimuli.
However, there are also significant differences between VR-1 and the amiloride-insensitive salt taste receptor. In contrast to VR-1, the amiloride-insensitive cation channel is constitutively active in the absence of a ligand at 23°C (Figs 1, 2 and 3), and is not modulated by pHo (see Supplementary material) and ATP (authors' unpublished observations). The vanilloid receptors are expressed in rat dorsal root ganglion neurones (Caterina et al. 1997; Caterina & Julius, 2001). Isolated rat dorsal root ganglion neurones maintained in primary cultures on matrigel-coated glass coverslips and loaded with fura-2 (Depree & Bigbee, 1994), respond with an increase in [Ca2+]i when stimulated with RTX or CPC in the same rank order of potency as observed in taste receptor cells (see Supplementary material). This suggests that native vanilloid receptors expressed in dorsal root ganglion neurones are heterogeneous and respond to RTX and CPC in the same rank order of potency as in taste receptor cells. However, the recombinant VR-1 expressed in HEK 293 cells is not responsive to CPC (J. B. Davis, personal communication). These differences between the amiloride-insensitive cation channel and the VR-1 channel suggest that, while both are products of the same gene, the amiloride-insensitive salt taste receptor derives from an mRNA splice variant or is an alternatively transcribed protein of the gene. The difference may also arise if the taste receptor is a heteromultimer involving the VR-1 gene product.
The specificity of the channel as a transducer in salt taste is demonstrated by the observations that RTX has no effect on the chorda tympani responses to sucrose, quinine or H+ ions (Fig. 9). The channel is non-functional in VR-1 knockout mice (Fig. 5B and C). VR-1 null mice demonstrate no amiloride-insensitive NaCl chorda tympani response component and no salt taste sensitivity to RTX and temperature (Fig. 5B and C). In contrast, in TRPM5 (a taste TRP ion channel) knockout mice, sweet, amino acid, and bitter taste reception is abolished, without affecting sour or salty taste (Zhang et al. 2003). This suggests that the taste receptor cell VR-1 variant channel is specific for mineral salt detection. It accounts for the entire amiloride-insensitive chorda tympani response to NaCl (Fig. 1) and part of the response to K+, NH4+ (Fig. 6A) and Ca2+ salts (authors' unpublished observations). This suggests that the VR-1 variant channel in rat fungiform taste receptor cells is the amiloride-insensitive salt taste receptor.
The functional role of the amiloride-insensitive salt taste receptor is demonstrated by our studies with temperature. At 41°C about 70% of the Bz-sensitive NaCl chorda tympani response is inhibited relative to 23°C, and the Bz-insensitive component is elevated (Fig. 2A). Thus when ingesting hot food or beverages, the ENaC activity is inhibited (Lundy & Contreras, 1997), and salt taste transduction occurs predominantly through the amiloride-insensitive cation channel. The amiloride-insensitive cation channel activity increases in parallel with temperature (Figs 2B, 3C, 5A and 7B) and with the additive effects of RTX (Fig. 7A and B) and RTX + ATP (Fig. 7C). This indicates that the agonists and potentiators of the amiloride-insensitive cation channel interact to reduce the temperature threshold of the channel. This allows for increased salt taste sensitivity without an increase in temperature.
The amiloride-insensitive salt taste receptor may also play an important role in detecting Na+ while ingesting foods that are acidic. In mixtures containing NaCl and acidic stimuli, acid equivalents enter taste receptor cells and decrease pHi, inhibiting Na+ influx through the amiloride-sensitive ENaC and hence inhibiting the NaCl chorda tympani response (Lyall et al. 2002). Thus, in acid/salt mixtures, ENaC is inhibited and does not contribute to the overall salt taste. Unlike the amiloride-sensitive ENaC, the constitutively active amiloride-insensitive cation channel is insensitive to external pH. However, low pHo increases its sensitivity to vanilloids (Fig. 8A) and decreases the temperature threshold of the amiloride-insensitive cation channel (Fig. 8B). This suggests that in acid/salt mixtures salt taste is transduced predominantly by the amiloride-insensitive salt taste receptor.
The differential contribution of the amiloride-sensitive ENaC and the amiloride-insensitive cation channel to overall salt taste varies widely across species. In humans (Halpern, 1998; Feldman et al. 2003), the major mechanism mediating salt taste is amiloride insensitive. The modulation of the amiloride-insensitive salt taste receptor by vanilloid and non-vanilloid compounds suggests the possibility of developing specific salt taste suppressors and enhancers for humans. Such compounds could potentially have a significant role in the management of hypertension and cardiovascular disease.
In summary, Na+ transport across fungiform taste receptor cells occurs through both cellular and transcellular pathways. In the apical membranes of taste receptor cells salt taste transduction involves a Na+-specific receptor, the apical amiloride-sensitive ENaC and a VR-1 variant non-specific cation channel that is amiloride and Bz insensitive, resulting in the apical influx of Na+ into taste receptor cells (Lyall et al. 2002). The exit of Na+ from taste receptor cells occurs via the basolateral Na+–K+ ATPase. An additional Na+ transport mechanism involves the basolateral Na+–H+ exchanger isoform 1 (NHE-1) (Vinnikova et al. 2004). The apical Na+–H+ exchanger isoform 3 (NHE-3) seems to be quiescent (Vinnikova et al. 2004). The transcellular transport of Na+, K+, NH4+ and Ca2+ ions occurs via the paracellular shunt mechanism and is anion dependent (Ye et al. 1991; Kloub et al. 1997). The VR-1 variant channel is non-functional in VR-1 knockout mice. It is modulated by RTX, CAP, CPC and temperature, and is inhibited by VR-1 antagonists. In the presence of vanilloids, a decrease in pHo and the addition of ATP modulate the channel activity. In rat fungiform taste receptor cells, the VR-1 variant cation channel accounts for all of the Bz-insensitive chorda tympani response to Na+ salts and part of the chorda tympani response to K+, NH4+ and Ca2+ salts, and thus is the mammalian amiloride-insensitive salt taste receptor. Full molecular characterization of this channel and its modulation by vanilloid and non-vanilloid compounds should lead to the development of specific salt taste suppressors and enhancers.
Acknowledgments
This work was supported by the National Institute of Deafness and other Communication Disorders Grants DC-02422 and DC-00122. We thank Drs Shirley K. DeSimone, John R. Grider and Gabriel M. Makhlouf for helpful discussions and valuable criticisms.
Supplementary material
The online version of this paper can be found at:
DOI: 10.1113/jphysiol.2004.065656
http://jp.physoc.org/cgi/content/full/jphysiol.2004.065656v1/
DC1 and contains supplementary material entitled:
This material can also be found at:
http://blackwellpublishing.com/products/journals/suppmat/tjp/tjp332/tjp332sm.htm
References
- Bèhè P, DeSimone JA, Avenet P, Lindemann B. Membrane currents in taste cells of the rat fungiform papilla. Evidence for two types of Ca currents and inhibition of K currents by saccharin. J General Physiol. 1990;96:1061–1084. doi: 10.1085/jgp.96.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chu S, Brownell WE, Montrose MH. Quantitative confocal imaging along the crypt-to-surface axis of colonic crypts. Am J Physiol. 1995;269:C1557–C1564. doi: 10.1152/ajpcell.1995.269.6.C1557. [DOI] [PubMed] [Google Scholar]
- Cortright DN, Crandall M, Sanchez JF, Zou T, Krause JE, White G. The tissue distribution and functional characterization of human VR1. Biochem Biophys Res Commun. 2001;281:1183–1189. doi: 10.1006/bbrc.2001.4482. [DOI] [PubMed] [Google Scholar]
- Davis JB, Smart D, Gunthorpe MJ. The vanilloid receptor and vanilloid receptor-like genes: a hot topic getting hotter. Cell Transmissions. 2002;18:3–9. [Google Scholar]
- Depree JL, Bigbee JW. Retardation of neuritic outgrowth and cytoskeletal changes accompanying acetylcholine esterase inhibitor treatment in cultured rat dorsal root ganglion neurons. J Neurosci Res. 1994;39:567–575. doi: 10.1002/jnr.490390508. [DOI] [PubMed] [Google Scholar]
- DeSimone JA, Lyall V, Heck GL, Phan THT, Alam RI, Feldman GM, et al. A novel pharmacological probe links the amiloride-insensitive NaCl, KCl, and NH4Cl chorda tympani taste responses. J Neurophysiol. 2001;86:2638–2641. doi: 10.1152/jn.2001.86.5.2638. [DOI] [PubMed] [Google Scholar]
- Feldman GM, Mogyorosi A, Heck GL, DeSimone JA, Santos CR, Clary RA, et al. Salt-evoked lingual surface potential in humans. J Neurophysiol. 2003;90:2060–2064. doi: 10.1152/jn.00158.2003. [DOI] [PubMed] [Google Scholar]
- Frank ME, Contreras RJ, Hettinger TP. Nerve fibers sensitive to ionic taste stimuli in chorda tympani of the rat. J Neurophysiol. 1983;50:941–960. doi: 10.1152/jn.1983.50.4.941. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- Halpern BP. Amiloride and vertebrate gustatory responses to NaCl. Neurosci Biobehav Rev. 1998;23:5–47. doi: 10.1016/s0149-7634(97)00063-8. [DOI] [PubMed] [Google Scholar]
- Hettinger TP, Frank ME. Specificity of amiloride inhibition of hamster taste responses. Brain Res. 1990;513:24–34. doi: 10.1016/0006-8993(90)91085-u. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloub MA, Heck GL, DeSimone JA. Chorda tympani responses under lingual voltage clamp: implications for NH4 salt taste transduction. J Neurophysiol. 1997;77:1393–1406. doi: 10.1152/jn.1997.77.3.1393. [DOI] [PubMed] [Google Scholar]
- Kretz O, Barbry P, Bock R, Lindemann B. Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J Histochem Cytochem. 1999;47:51–64. doi: 10.1177/002215549904700106. [DOI] [PubMed] [Google Scholar]
- Lin W, Finger TE, Rossier BC, Kinnamon SC. Epithelial Na+ channel subunits in rat taste cells: localization and regulation by aldosterone. J Comp Neurol. 1999;405:406–420. doi: 10.1002/(sici)1096-9861(19990315)405:3<406::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lindemann B. Receptors and transduction in taste. Nature. 2001;413:219–225. doi: 10.1038/35093032. [DOI] [PubMed] [Google Scholar]
- Liu L, Simon SA. Acidic stimuli activates two distinct pathways in taste receptor cells from rat fungiform papillae. Brain Res. 2001;923:58–70. doi: 10.1016/s0006-8993(01)03190-0. [DOI] [PubMed] [Google Scholar]
- Lundy RF, Contreras RJ. Temperature and amiloride alter taste nerve responses to Na+, K+, and NH4+ salts in rats. Brain Res. 1997;744:309–317. doi: 10.1016/S0006-8993(96)01118-3. [DOI] [PubMed] [Google Scholar]
- Lyall V, Alam RI, Phan THT, Russell OF, Malik SA, Heck GL, et al. Modulation of rat chorda tympani NaCl responses and intracellular Na+ activity in polarized taste receptor cells by pH. J General Physiol. 2002;120:793–815. doi: 10.1085/jgp.20028656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Amiloride inhibition of responses of rat single chorda tympani fibers to chemical and electrical tongue stimulations. Brain Res. 1988;451:319–325. doi: 10.1016/0006-8993(88)90777-9. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Sako N, Funakoshi M. Strain differences in amiloride inhibition of NaCl responses in mice, Mus musculus. J Comp Physiol [A] 1989;166:1–5. doi: 10.1007/BF00190204. [DOI] [PubMed] [Google Scholar]
- Osada K, Komai M, Bryant BP, Suzuki H, Goto A, Tsunoda K, et al. Capsaicin modifies responses of rat chorda tympani nerve fibers to NaCl. Chem Senses. 1997;22:249–255. doi: 10.1093/chemse/22.3.249. [DOI] [PubMed] [Google Scholar]
- Smith DA, Ossebaard CA. Amiloride suppression of the taste intensity of NaCl: evidence from direct magnitude scaling. Physiol Behav. 1995;57:773–777. doi: 10.1016/0031-9384(94)00329-7. [DOI] [PubMed] [Google Scholar]
- Stewart RE, DeSimone JA, Hill DL. New perspectives in a gustatory physiology: transduction, development, and plasticity. Am J Physiol. 1997;272:C1–C26. doi: 10.1152/ajpcell.1997.272.1.C1. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinnikova AK, Alam RI, Malik SA, Ereso GL, Feldman GM, McCarty JM, et al. Na+-H+ exchange activity in taste receptor cells. J Neurophysiol. 2004;91:1297–1313. doi: 10.1152/jn.00809.2003. [DOI] [PubMed] [Google Scholar]
- Ye Q, Heck GL, DeSimone JA. The anion paradox in sodium taste reception: resolution by voltage-clamp studies. Science. 1991;254:724–726. doi: 10.1126/science.1948054. [DOI] [PubMed] [Google Scholar]
- Ye Q, Heck GL, DeSimone JA. Voltage dependence of the rat chorda tympani response to Na+ salts: implications for the functional organization of taste receptor cells. J Neurophysiol. 1993;70:167–178. doi: 10.1152/jn.1993.70.1.167. [DOI] [PubMed] [Google Scholar]
- Ye Q, Heck GL, DeSimone JA. Effects of voltage perturbation of the lingual receptive field on chorda tympani responses to Na+ and K+ salts in the rat: implications for gustatory transduction. J General Physiol. 1994;104:885–907. doi: 10.1085/jgp.104.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, et al. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online version of this paper can be found at:
DOI: 10.1113/jphysiol.2004.065656
http://jp.physoc.org/cgi/content/full/jphysiol.2004.065656v1/
DC1 and contains supplementary material entitled:
This material can also be found at:
http://blackwellpublishing.com/products/journals/suppmat/tjp/tjp332/tjp332sm.htm