Abstract
The role of tropomyosin (Tm) in the elementary steps of the cross-bridge cycle in bovine myocardium was investigated. The thin filament was selectively removed using gelsolin (thin filament severing protein), and the actin filament was reconstituted from G-actin. Tm was further reconstituted without troponin (Tn), and the kinetic constants of the elementary steps of the cross-bridge cycle were deduced using sinusoidal analysis at pCa ≤ 4.66, pH 7.00, and 25°C. The association constant of MgATP to cross-bridges (K1) after reconstitution of Tm was 20.7 ± 2.3 mm−1, which was about 2 × the control (untreated) myocardium (9.1 ± 1.3 mm−1). Following reconstitution of Tm, the equilibrium constant of the cross-bridge detachment step (K2), the phosphate (Pi) association constant (K5) and the equilibrium constant of the force-generation step (K4), which significantly changed in the actin filament-reconstituted myocardium, recovered to those of the control myocardium. Active tension after reconstitution of Tm was 0.69 × the control myocardium, a value between the control (1.00 ×) and the actin filament-reconstituted myocardium (0.59 ×). Tm-reconstituted myocardium was further reconstituted with Tn, and the effect of MgATP on the rate constants (K1, K2) was studied. Following reconstitution with Tn, the myocardium regained the Ca2+-sensitivity and the active tension became 0.83 × the control myocardium. In addition, K1 recovered to the value of the control myocardium with Tn reconstitution. These results indicate that both Tm and Tn enhance the force generated by each cross-bridge, and that Tm is primarily responsible for the change in the kinetic constants of the elementary steps of the cross-bridge cycle.
It has been known for some time that force generation in muscle is accomplished by a cyclic interaction between the myosin molecule, the main component of the thick filament, and the actin molecule, the main component of the thin filament. Myosin and actin convert the chemical energy stored in ATP to mechanical work, resulting in a sliding motion between the thick and thin filaments (Huxley & Niedergerke, 1954; Huxley & Hanson, 1954). In skeletal and cardiac muscles, the Ca2+-dependent regulatory switch, the tropomyosin (Tm)–troponin (Tn) system, is located on the thin filament (Ebashi & Endo, 1968). Tm is a rod-like dimeric protein which spans seven actin monomers along the thin filament with one Tn complex attached to the Tm dimer. In the steric blocking mechanism, Tm blocks the myosin binding site on the actin filament when Ca2+ is absent, resulting in an inhibition of the actomyosin interaction (Haselgrove, 1973; Huxley, 1973). The binding of Ca2+ to Tn removes this inhibition by azimuthal movement of Tm, allowing myosin to interact freely with actin (Lehman et al. 1994,1995).
Although the steric blocking mechanism is supported by many lines of evidence, the regulation of actomyosin interaction by a simple movement of Tm from a blocked position to an open position cannot explain some of the biochemical observations (McKillop & Geeves, 1993; Tobacman & Butters, 2000). While it has been shown in regulated actin that the ATP hydrolysis rate is inhibited by 96% in the absence of Ca2+, the association constant of myosin subfragment 1 (S1) to actin is almost unchanged regardless of the presence of Ca2+ (Chalovich et al. 1981; Chalovich & Eisenberg, 1982). Covalently cross-linked acto-S1 still possesses Ca2+ regulation of the ATP hydrolysis rate when Tm and Tn are present (King & Greene, 1985). These findings indicate that the Tm–Tn system does not act just as a shield between actin and myosin, but serves a more complex role that alters actomyosin interaction and concomitant cross-bridge kinetics. Furthermore, a partial extraction of TnC is known to alter the kinetics of the cross-bridge cycle by promoting ATP binding and the rate of cross-bridge detachment and suppressing the rate of force generation (Zhao et al. 1996). Actomyosin ATPase activity is known to increase when Tm is added (Bremel et al. 1972; Murray et al. 1980). These observations also indicate that the Tm–Tn system modulates muscle contraction via a more complex mechanism.
Recently, we have demonstrated, using the thin filament-reconstitution method, that the Tm–Tn system alters the kinetic constants of the elementary steps of the cross-bridge cycle and increases the force supported by each cross-bridge in bovine myocardium (Fujita et al. 2002). In our experiments, the thin filament in bovine myocardium was selectively removed by plasma protein gelsolin, and the actin filament was reconstituted by adding purified G-actin in a polymerizing condition (Fujita et al. 1996). The actin filament-reconstituted myocardium generates active tension in a Ca2+-insensitive manner because of the lack of regulatory proteins. This tension was about 2/3 of the control myocardium. The thin filament with full Ca2+ sensitivity can be reconstituted by adding native tropomyosin (nTm: a complex of Tm and Tn) to the actin filament-reconstituted myocardium. This reconstitution resulted in a larger tension similar to that of control myocardium (Fujita et al. 2002). Because in our previous studies, the regulatory system was reconstituted as nTm, it remained to be seen whether the effect was caused by Tm, Tn, or both. In this study, we reconstituted Tm and Tn sequentially after reconstitution of the actin filament. We found that Tm is primarily responsible for the change in the kinetic constants that we observed using nTm (except for the MgATP association constant), and that both Tm and Tn contribute to an increase of force supported by each cross-bridge.
Methods
Solutions
The relaxing solution (Rx) contained (mm): 6 EGTA, 2.2 MgATP, 5 ATP, 8 Pi, 41 NaProp (Prop = propionate), 75 KProp, 10 Mops (3-(N-morpholino) propane sulphonic acid), and 40 BDM (2,3-butanedione 2-monoxime). The rigor solution (Rg) contained 8 Pi, 55 NaProp, 122 KProp, and 10 Mops. Experimental solutions are indicated by mSnP, where m represents the millimolar concentration of MgATP2−, and n represents the millimolar concentration of phosphate (Pi). The 5S0P solution contained (mm): 6 CaEGTA, 5.83 MgATP, 1.36 ATP, 15 phosphocreatine (CP), 1 NaProp, 92 KProp, 10 NaN3, and 10 Mops. The 5S32P solution contained (mm): 6 CaEGTA, 5.7 MgATP, 1.36 ATP, 15 CP, 32 Pi, 1 NaProp, 17 KProp, 10 NaN3, and 10 Mops. The 0S8P solution contained (mm): 6 CaEGTA, 0.85 MgProp2, 15 CP, 8 Pi, 15 NaProp, 88 KProp, 10 NaN3, and 10 Mops. The 5S8P solution contained (mm): 6 CaEGTA, 5.8 MgATP, 1.36 ATP, 15 CP, 8 Pi, 1 NaProp, 73 KProp, 10 NaN3, and 10 Mops. The –Ca solution (5S8P solution without Ca) contained (mm): 6 EGTA, 5.9 MgATP, 1.25 ATP, 15 CP, 8 Pi, 1 NaProp, 73 KProp, 10 NaN3, and 10 Mops. The 5S8P solution is also called the standard activating solution. The pCa of all activating solutions was ≤ 4.66, pH was adjusted to 7.00 by KOH, the Mg2+ concentration was 0.5 mm, the total sodium concentration was 55 mm, ionic strength was adjusted to 200 mm by NaProp and KProp, and all activating solutions contained 320 units ml−1 (=0.64 mg ml−1) creatine kinase (CK). All solution components were added from neutral stock solutions: EGTA as K2H2EGTA, CaEGTA as K2CaEGTA, Pi as K1.5H1.5PO4, MgATP as Na2MgATP, ATP as Na2K1.7H0.3ATP, and CP as Na2CP. Multiple equilibria were assumed, and individual concentrations of multivalent ionic species were calculated using our computer program with the following apparent association constants at pH 7.0 (log values): CaEGTA, 6.28; MgEGTA, 1.61; CaATP, 3.70; MgATP, 4.00; CaCP, 1.15; MgCP, 1.30. The experiments were performed at 25°C.
Na2CP, Na2H2ATP, Mops and H4EGTA were purchased from Sigma Chemical Co. (St Louis, MO, USA); CaCO3, MgO, NaOH, KOH, KH2PO4, K2HPO4, NaN3 and propionic acid were purchased from Fisher Scientific Co. (Itasca, IL, USA); creatine kinase was purchased from Boehringer Mannheim (Indianapolis, IN, USA).
Muscle fibres and proteins
Bovine hearts were obtained from a slaughterhouse within 15 min of death, and immediately cooled with ice. The muscle bundles (∼2 mm in diameter, ∼10 mm in length) were excised from a straight portion of the ventricular papillary muscles and incubated in the sodium-skinning solution (mm): 2 DTT (dithiothreitol), 30 BDM, 10 EGTA, 5 ATP, 2 MgATP, 122 NaProp and 10 Mops (pH 7.0) for 3 h at 0°C. The sodium-skinning solution was used to minimize contraction. For further skinning, the solution was replaced with potassium-skinning solution containing (mm): 2 DTT, 30 BDM, 10 EGTA, 5 ATP, 2 MgATP, 122 KProp and 10 Mops (pH 7.0), and stored overnight at 0°C. BDM and EGTA were used to minimize force generation. The solution was further replaced with one containing 50% (v/v) glycerol and (mm): 2 DTT, 30 BDM, 10 EGTA, 5 ATP, 2 MgATP, 122 KProp and 10 Mops (pH 7.0) and stored at 0°C. The solution was replaced once again the next morning, and the muscle bundles were stored at a low temperature (−20°C).
G-actin was extracted and purified from acetone powder (Kondo & Ishiwata, 1976) of rabbit white skeletal muscles as described by Spudich & Watt (1971). G-actin was stored at 0°C and used within 2 weeks of extraction. Tm and Tn were prepared from bovine cardiac muscle as described by Ebashi et al. (1968) and purified using DEAE Sephadex A-25 (Pharmacia, Sweden). Tm was further purified as described by Yamaguchi et al. (1974). Bovine plasma gelsolin was prepared as described by Kurokawa et al. (1990).
Selective removal and reconstitution of actin filament were performed as reported by Fujita et al. (1996),2002. Tm and Tn were reconstituted as reported by Fujita & Ishiwata, (1999).
Experimental procedure and deduction of kinetic constants
A strip of myocardium (90–160 μm in width and 2 mm in length) was dissected from a skinned muscle bundle. One end of the myocardium was connected to a tension transducer via a stainless steel wire (210 μm in diameter), and the other end was connected to a length driver via another stainless steel wire. Nail polish was used to glue the ends of myocardium to the wires. The myocardium was stretched until a small passive tension was observed, when the length (L0) of the myocardium was determined. The width was measured under a dissecting microscope (20 ×) and the cross-sectional area was estimated assuming a circular cross-section. At this stage, the myocardium was chemically skinned further in the relaxing solution containing 1% (v/v) Triton X-100 for 20 min.
Two muscle models (Tm-reconstituted and Tm–Tn-reconstituted myocardium) were maximally activated in the presence of saturating Ca2+ in a bath in which the temperature was controlled at 25°C. A 0.25%L0 peak-to-peak sinusoidal waveform at 18 discrete frequencies between 0.13 and 100 Hz was digitally synthesized in a 386 CPU PC (Industrial Computer Source, San Diego, CA, USA) that controlled the length driver via a 14-bit DAC. Tension and length signals were simultaneously sampled by two 16-bit A/D converters and complex modulus data Y(f) were calculated as the ratio of the force change to the length change in the frequency domain. The complex modulus data observed during relaxation was subtracted. The complex modulus data were resolved into two exponential processes (B and C) by fitting the data to eqn (1) by minimizing the sum of modulus squares (Kawai & Brandt, 1980; Wannenburg et al. 2000):
| (1) |
where i=√−1. Uppercase letters B and C represent their respective magnitudes (amplitudes) and lower case letters b and c represent the characteristic frequencies of the respective processes. 2πb and 2πc are the apparent rate constants of the respective processes. Process B corresponds to phase 3, process C corresponds to phase 2, and Y(∞) corresponds to phase 1 of tension transients of step analysis (Huxley & Simmons, 1971). In relaxed and rigor muscle fibres, these exponential processes were absent, indicating that these processes are signatures of cycling cross-bridges. Y(∞) is referred to ‘stiffness’ in this paper. The complex modulus data were corrected by those of the rigor condition.
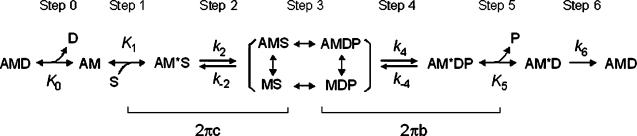
The results were analysed based on scheme 1, where A = actin, M = myosin, S = MgATP, D = MgADP, and P = Pi= phosphate. The kinetic constants of elementary step 1 and 2 of the cross-bridge cycle were determined by fitting the MgATP dependence of 2πc to eqn (2) by minimizing the sum of squares (Kawai & Halvorson, 1989).
| (2) |
The kinetic constants of elementary steps 4 and 5 of the cross-bridge cycle were determined by fitting the Pi dependence of 2πb to eqn (3) by minimizing the sum of squares (Kawai & Halvorson, 1991).
| (3) |
where
| (4) |
In these equations, S and P indicate their respective concentrations: S =[MgATP] and P =[Pi]. K1 and K2 obtained from the MgATP study and S= 5 mm were used for calculation of σ in eqn (4). Details of the sinusoidal analysis technique have been published (Kawai & Brandt, 1980).
Scheme 1.
Elementary steps of the cross-bridge cycle.
SDS-gel electrophoresis
Approximately 10 muscle fibres, each similar in size to those for mechanical measurements, were placed in the corner (500 μl) of a small, tilted petri dish and followed through the extraction and reconstitution protocol. Five petri dishes (50 fibres) corresponding to 5 different kinds of treatment were used. They were then solubilized and electrophoresed at 20 mA for about 90 min at 22°C using acrylamide gradient (8–16%) ready gels (Tris-HCl, 15 wells, Cat. No. 161-1223, Bio-Rad), as described by Laemmli (1970). Gels were stained with Coomassie Brilliant Blue R250.
Confocal fluorescence microscopy
Tetramethyl rhodamine-5-iodoacetamide (Rh-IA) and fluorescein phalloidin (Fl-Ph) were purchased from Molecular Probes (Eugene, OR, USA). Rh-IA-labelled Tm was prepared according to the method of Ishii & Lehrer (1990). The thin filament was first removed by treatment with gelsolin for 80 min, and then the actin filament was reconstituted for a total of 28 min (7 min × 4). Then, the myocardium was incubated in relaxing solution containing Rh-IA-labelled Tm for 12 h. Next, the myocardium was fixed with relaxing solution containing 1% formaldehyde for 30 min and stained with 6.6 μm Fl-Ph in relaxing solution for 5 h. For observation, the myocardium was mounted on a cover slip and washed with relaxing solution containing 4.5 mg ml−1 glucose, 0.22 mg ml−1 glucose oxidase, 0.036 mg ml−1 catalase, and 10 mm DTT. This preparation was observed under a laser scanning confocal microscope equipped with 25 mW Ar laser at 488 nm (Fluoview-IX/AR; Olympus Co., Tokyo). No crossover between the fluorescence images of rhodamine (> 610 nm) and fluorescein (510–540 nm) was detected.
Results
Removal and reconstitution of the thin filament
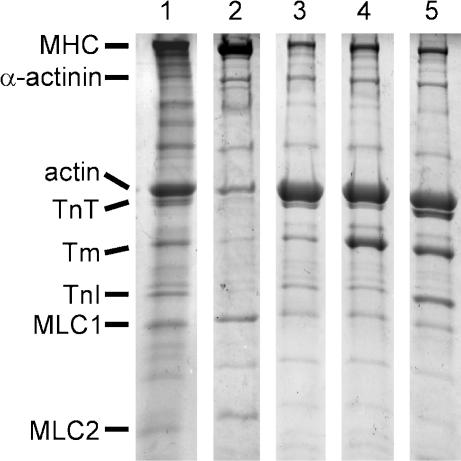
Figure 1 shows SDS-PAGE of myocardium at each step of reconstitution. In control myocardium (lane 1), thick filament proteins, myosin heavy chain and myosin light chains 1 and 2, can be seen. Also seen are thin filament proteins, actin, TnT, Tm and TnI. TnC could not be stained with the method we used. In gelsolin-treated myocardium (lane 2), the amount of thin filament proteins decreased significantly, but the amount of MLC1 and MLC2 remained about the same. In actin filament-reconstituted myocardium (lane 3), the amount of actin increased significantly. In Tm-reconstituted myocardium (lane 4), the amount of Tm increased significantly. In Tm–Tn-reconstituted myocardium (lane 5), the amount of TnT and TnI increased significantly, hence all thin filament proteins were observed here. In lane 2, some residual actin (∼20% of lane 1) can be seen, although a similarly treated preparation did not develop tension (Fig. 3B). This residual actin is presumably located within and/or near to the Z-line, but does not overlap with the thick filament. This amount of actin is essential for a successful reconstitution of the actin filament (Fujita et al. 2002).
Figure 1. SDS-PAGE of myocardium at each step of reconstitution.
Lane 1, control myocardium; lane 2, gelsolin-treated myocardium; lane 3, actin filament-reconstituted myocardium; lane 4, Tm-reconstituted myocardium; lane 5, Tm–Tn-reconstituted myocardium. The Tm-reconstituted myocardium (lane 4) was first treated with gelsolin, then actin filament was reconstituted before Tm was reconstituted. The Tm–Tn-reconstituted myocardium (lane 5) was first treated with gelsolin, then actin filament was reconstituted, followed by sequential reconstitution of Tm and Tn. In lane 2, gelsolin is visible just below the α-actinin band. Lane 1 is from one gel, and lanes 2–5 are from another gel with a different order. Abbreviations: MHC, myosin heavy chain; Tm, tropomyosin; TnT, troponin T; TnI, troponin I; MLC1, myosin light chain 1; MLC2, myosin light chain 2.
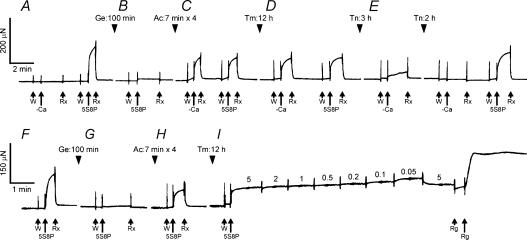
Figure 3. Slow pen traces of isometric tension at each step of reconstitution.
A–E, a slow pen trace of isometric tension at each step of removal and reconstitution of the thin filament in one myocardium. In A, a control (untreated) myocardium was first tested with a solution without Ca2+ (−Ca) to demonstrate that active tension did not develop without Ca2+. Then the myocardium was treated with the standard activating solution (5S8P, pCa 4.66) at 25°C. Before activation, the myocardium was immersed in the same 5S8P solution at 0°C (labelled W) to wash out BDM, which was present in the Rx solution. The myocardium did not develop active tension in W because of the low temperature. In B, the myocardium was treated by gelsolin (Ge) for 100 min, and then immersed in the 5S8P solution at 25°C with the result that no tension developed, thus demonstrating that the thin filament was sufficiently removed so that there is no overlap between the thick and the thin filaments. In C, the actin filament was reconstituted for 28 min over four sessions (Ac), then the myocardium was immersed in the solution without Ca2+ (−Ca) followed by the solution with Ca2+ (5S8P) at 25°C. Both treatments resulted in the same active tension development, demonstrating the absence of the regulatory system. In D, Tm was reconstituted for 12 h (Tm). Once again, the active tension developed irrespective of Ca2+. In E, reconstitution with Tn was first performed for 3 h; this turned out to be insufficient, as can be seen by the Ca2+-insensitive tension. An additional reconstitution for 2 h was necessary to fully restore the Ca2+ sensitivity, as represented by no tension in the absence of Ca2+, and tension levels close to the original tension (A) in the presence of Ca2+. F–I, another myocardium was similarly treated as in A–D, resulting in reconstitution of the actin filament with Tm. In this myocardium, the complex modulus data were collected at 7 different MgATP concentrations (0.05–5 mm), as indicated in I. The standard activation at 5 mm MgATP (5S8P) was repeated to detect any decrease in active tension; the preparation was discarded if more than 20% of tension was lost. This activation was followed by an induction of the rigor state with the Rg solution. In all experiments, relaxation was obtained in the solution containing 40 mm BDM (Rx) at 0°C. All activations including rigor (Rg) were performed at 25°C.
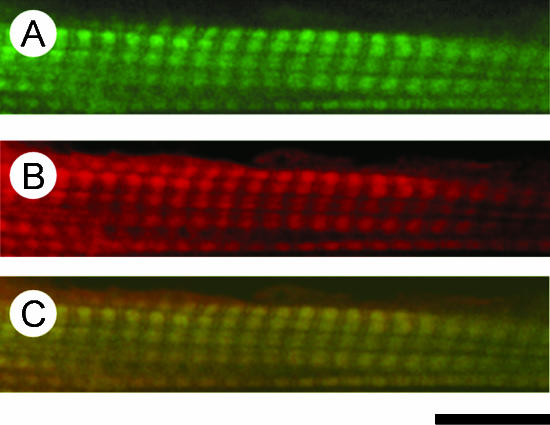
Figure 2 shows confocal fluorescent microscope images of myocardium reconstituted with Rh-IA-labelled Tm and stained with Fl-Ph. The distribution of Fl fluorescence (green), indicating the actin filament, shows a repetitive pattern of bright and dark bands (Fig. 2A). The centre of the bright band corresponds to the Z-line. The distribution of Rh fluorescence (red), indicating Tm, similarly shows a repetitive pattern (Fig. 2B), and the banding pattern appears to be identical to that of the actin filament. To demonstrate colocalization of Tm and the actin filament, both images were superimposed in Fig. 2C, which shows that Tm was homogeneously incorporated onto the actin filament.
Figure 2. Confocal fluorescence micrographs of bovine myocardium reconstituted with Rh-IA-labelled Tm and labelled with Fl-Ph.
The thin filament was first removed by gelsolin, and sequentially reconstituted with actin, followed by Rh-IA-labelled Tm. The preparation was then treated with Fl-conjugated phalloidin to label the actin filament. A, Fl fluorescence showing the distribution of the actin filament; B, Rh fluorescence showing the distribution of Tm; C, colocalization of the actin filament and Tm. Calibration bar, 10 μm.
Figure 3A–E shows slow pen traces of isometric tension at each step of the thin filament extraction and reconstitution. The myocardium was initially activated with the 5S8P solution and the standard active tension was measured at 25°C (Fig. 3A). Then the myocardium was treated with gelsolin for 100 min (Fig. 3B, Ge) at 2°C in the solution that contained (mm): 117 KCl, 4.25 MgCl2 (2.2 mm free Mg2+), 2.2 ATP (2.0 MgATP2−), 2.0 EGTA, 20 Mops (pH 7.0), 2 CaCl2, 40 BDM, and 0.3 mg ml−1 gelsolin. BDM was used to suppress tension development during the gelsolin treatment, because Ca2+ is a cofactor for this protein and needed for the extraction. After the gelsolin treatment, active tension did not develop in the standard activating solution (Fig. 3B), demonstrating a removal of the thin filament.
The myocardium was then immersed in the actin-polymerizing solution containing (mm): 80 KI, 4 MgCl2, 4 ATP, 4 EGTA, 40 BDM, 20 KPi (pH 7.0), and 1.0 mg ml−1 G-actin at 2°C to reconstitute the actin filament (Fig. 3C, Ac). The actin-polymerizing solution was replaced every 7 min to avoid spontaneous nucleation in the muscle chamber. Similarly, KI instead of KCl was used to deter the nucleation (Funatsu et al. 1994). Actin polymerization was performed for a total of 28 min (=7 min × 4). Subsequently, the myocardium was tested with the –Ca solution (5S8P solution without Ca2+), and then with the Ca2+ solution (5S8P) at 25°C (Fig. 3C). Because of the lack of regulatory proteins, this myocardium developed the same tension regardless of the presence of Ca2+. Active tension after reconstitution of actin filament was 59 ± 2% (±s.e.m., n= 17) of the control myocardium when tested by the standard activating solution (5S8P) that had 8 mm Pi. Relaxation was achieved by including 40 mm BDM in the relaxing solution.
To reconstitute Tm, actin filament-reconstituted myocardium was immersed in the relaxing solution containing 1.2 mg ml−1 Tm for 12 h at 2°C (Fig. 3D, Tm). Tm-reconstituted myocardium developed active tension regardless of the presence of Ca2+ (Fig. 3D). At 25°C, this tension was generally greater than that of actin filament-reconstituted myocardium: active tension after Tm reconstitution was 69 ± 6% (n= 17) of control myocardium, an increase of 10% (=69%-59%) over the actin filament-reconstituted myocardium.
The myocardium was further reconstituted with Tn by immersing it in the relaxing solution containing 1.0 mg ml−1 Tn at 2°C (Fig. 3E, Tn). As shown in this figure, 3 h reconstitution with Tn was not adequate, because ∼1/3 of Ca2+-insensitive tension developed, and an additional 2 h was needed to fully restore the Ca2+ sensitivity; the Tm–Tn-reconstituted myocardium did not develop active tension when Ca2+ was absent, but it did develop active tension when Ca2+ was present (Fig. 3E). This observation indicates that Tm and Tn were fully reconstituted. With the reconstitution of Tn, active tension increased to 83 ± 9% (n= 7) of control myocardium, an increase of 14% (=83%− 69%) over the Tm-reconstituted myocardium.
Effect of MgATP on the exponential process C
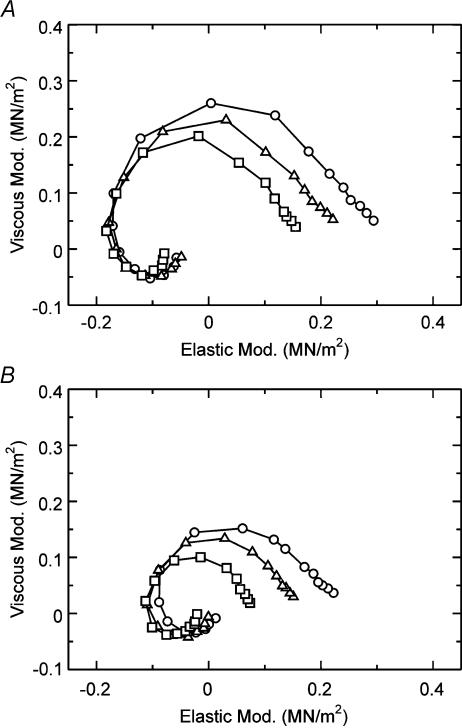
Figure 3F–I shows isometric tension of another myocardium as the thin filament was extracted and reconstituted. In this series of experiments, the reconstitution was only through Tm, and the MgATP and the Pi studies were performed. The purpose of these experiments was to investigate whether the larger force observed in the presence of Tm than in its absence is related to a larger force per cross-bridge or to a larger number of force-generating cross-bridges. The effect of MgATP on exponential process C on Tm-reconstituted and Tm–Tn-reconstituted myocardium was studied in the range of 0.05–5 mm in the presence of 8 mm Pi under the maximal Ca2+-activating condition (pCa ≤ 4.66) and as shown in Fig. 3I. This study characterizes the elementary steps of the MgATP binding step 1 and subsequent cross-bridge detachment step 2 in Scheme 1. Figure 4A shows the Nyquist plot of the complex modulus Y(f) in the Tm-reconstituted myocardium activated at three different MgATP concentrations (0.1 mm, 0.5 mm, 5 mm). These plots and the MgATP effect are similar to other myocardial systems reported earlier (Kawai et al. 1993; Zhao & Kawai, 1996; Wannenburg et al. 2000; Fujita et al. 2002).
Figure 4. Nyquist plots of the complex modulus of Tm-reconstituted myocardium.
A, the effect of MgATP on the complex modulus Y(f) in Tm-reconstituted myocardium (average of 9 data). ○, 0.1 mm MgATP; ▵, 0.5 mm MgATP; □, 5 mm MgATP. The phosphate concentration was fixed at 8 mm.B, the effect of Pi on the complex modulus Y(f) in Tm-reconstituted myocardium (average of 11 data). ○, 2 mm Pi; ▵, 8 mm Pi; □, 32 mm Pi. The MgATP concentration was fixed at 5 mm. Peak-to-peak amplitude was 0.25%L0. Data are shown in the Nyquist plot, which is a plot of elastic modulus in the abscissa versus viscous modulus in the ordinate. Frequencies used are (clockwise): 0.13, 0.25, 0.35, 0.5, 0.7, 1, 1.4, 2, 3.1, 5, 7.5, 11, 17, 25, 35, 50, 70 and 100 Hz.
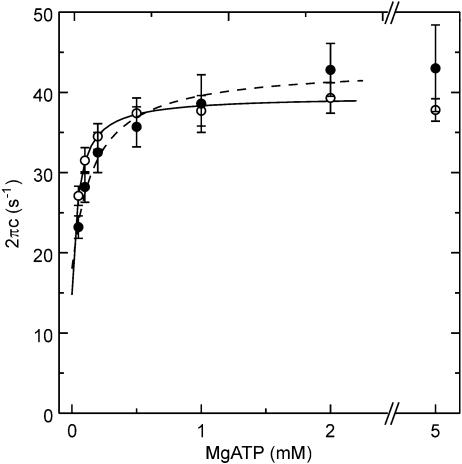
The MgATP dependence on 2πc of Tm-reconstituted (○), and Tm–Tn-reconstituted (•) myocardium is shown in Fig. 5 with s.e.m. error bars. The kinetic constants of elementary steps 1 and 2 of the cross-bridge cycle were determined by fitting the MgATP dependence of 2πc to eqn (2), and the results are summarized in Table 1. In Tm-reconstituted myocardium, the association constant of MgATP to cross-bridges (K1) was 20.7 ± 2.3 mm−1 (±s.e.m., n= 9). This value is about twice of that of control bovine myocardium (9.1 mm−1) reported previously (Fujita et al. 2002). The forward rate constant of cross-bridge detachment step 2 (k2) was 25.7 ± 2.7 s−1, the backward rate constant (k−2) was 13.8 ± 1.8 s−1, and the equilibrium constant K2(=k2/k−2) was 2.3 ± 0.5. These values are almost the same as those of control bovine myocardium reported previously (k2= 26.6, k−2= 12.1, and K2= 2.6; Fujita et al. 2002). In Tm–Tn-reconstituted myocardium, K1 decreased significantly to 13.3 ± 1.9 mm−1 (n= 7), which is comparable to the control myocardium. The k2, k−2, and K2 values did not change significantly by the reconstitution of Tn.
Figure 5. The rate constant 2πc is plotted as a function of the MgATP concentration in Tm- and Tm-Tn-reconstituted myocardium.
○, Tm-reconstituted myocardium (n= 9); •, Tm-Tn-reconstituted myocardium (n= 7). Error bars represent s.e.m. Continuous curves are based on eqn (2) with best fit parameters.
Table 1.
The kinetic constants of Tm-reconstituted and Tm-Tn-reconstituted bovine myocardium
| Kinetic constants | Units | Tm-reconstituted | Tm–Tn-reconstituted |
|---|---|---|---|
| K1 | mm−1 | 20.7 ± 2.3 (9) | 13.3 ± 1.9 (7) |
| k2 | s−1 | 25.7 ± 2.7 (9) | 27.6 ± 3.6 (7) |
| k−2 | s−1 | 13.8 ± 1.8 (9) | 12.4 ± 1.6 (7) |
| K2 | — | 2.32 ± 0.48 (9) | 2.70 ± 0.61 (7) |
| k4 | s−1 | 6.80 ± 0.60 (11) | — |
| k−4 | s−1 | 13.5 ± 1.70 (11) | — |
| K4 | — | 0.57 ± 0.08 (11) | — |
| K5 | mm−1 | 0.13 ± 0.05 (11) | — |
Values are means ±s.e.m. The number of observations is shown in parentheses.
Effects of Pi on the exponential process B
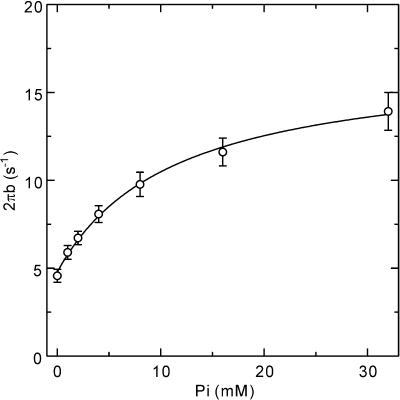
To determine the kinetic constants associated with elementary steps 4 and 5 of Scheme 1, we studied the effect of Pi in the range of 0–32 mm on exponential process B in Tm-reconstituted myocardium. The study was carried out in the presence of the saturating MgATP concentration (5 mm) under maximal Ca2+-activating conditions (pCa 4.66). Figure 4B shows the Nyquist plot of the complex modulus Y(f) in the Tm-reconstituted myocardium activated at three different Pi concentrations (2 mm, 8 mm, 32 mm). The complex modulus data were fitted to eqn (1) to obtain the apparent rate constant 2πb. 2πb data were then plotted against Pi concentration (Fig. 6) and fitted to eqn (3) (Kawai & Halvorson, 1991) to deduce the kinetic constants of elementary steps 4 and 5. The results are summarized in Table 1. In the Tm-reconstituted myocardium, the rate constant of the force generation step (k4) was 6.8 ± 0.6 s−1 (n= 11) and its reversal step (k−4) was 13.5 ± 1.7 s−1. Its equilibrium constant K4 (=k4/k−4) was 0.57 ± 0.08, and the Pi association constant (K5) was 0.13 ± 0.05 mm−1. These values are qualitatively the same compared to the control myocardium reported earlier (k4= 7.1, k−4= 12.6, K4= 0.59, and K5= 0.14; Fujita et al. 2002).
Figure 6. The rate constant 2πb is plotted as a function of the Pi concentration in Tm-reconstituted myocardium.
Error bars represent s.e.m. (n= 11). A continuous curve is based on eqn (3) with best fit parameters.
Isometric tension and stiffness
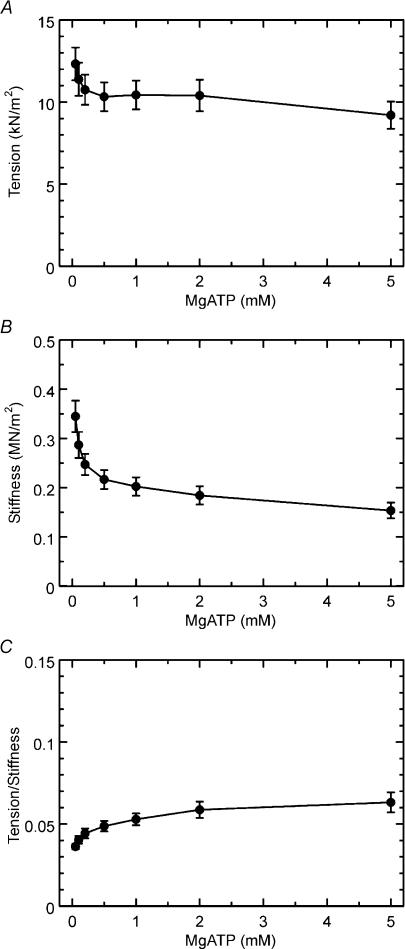
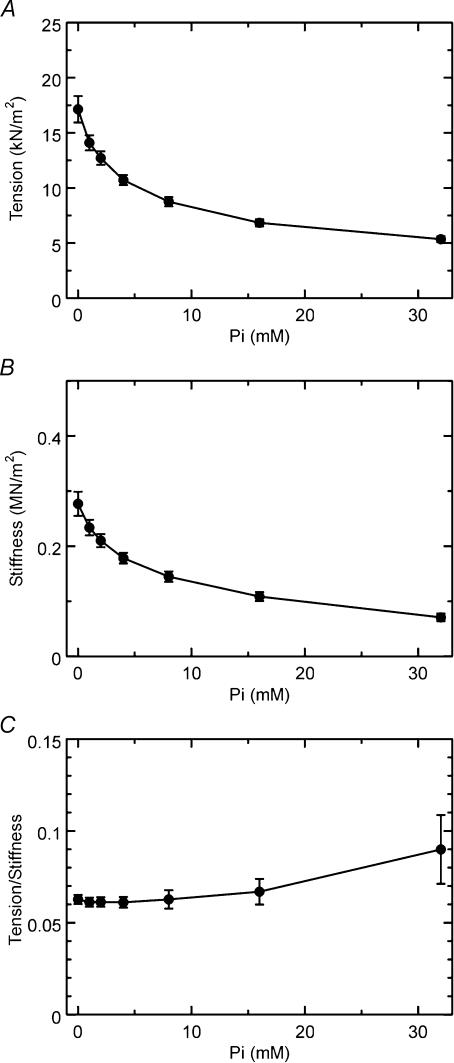
Figure 7 shows isometric tension, stiffness, and the tension/stiffness ratio plotted against MgATP concentration in the Tm-reconstituted myocardium. These results are based on the same experiments as shown in Fig. 5. Both isometric tension and stiffness decreased whereas the ratio increased by the increase in the MgATP concentration. This result is in agreement with previous results in myocardium (Kawai et al. 1993; Fujita et al. 2002) as well as in skeletal muscle fibres (Kawai & Zhao, 1993). Figure 8 shows isometric tension, stiffness, and the tension/stiffness ratio plotted against the Pi concentration for the same experiments as shown in Fig. 6 in Tm-reconstituted myocardium. Both isometric tension and stiffness decreased with the increase in the Pi concentration, which is in agreement with previous results in myocardium (Nosek et al. 1990; Kawai et al. 1993; Fujita et al. 2002) as well as in skeletal muscle fibres (Dantzig et al. 1992; Kawai & Zhao, 1993).
Figure 7. Isometric tension (A), stiffness (B), and the ratio (tension/stiffness) (C) are plotted against MgATP concentration in Tm-reconstituted myocardium.
Error bars represent s.e.m. (n= 9). Experiments were performed in the presence of 8 mm Pi.
Figure 8. Isometric tension (A), stiffness (B), and the ratio (tension/stiffness) (C) are plotted against the Pi concentration in Tm-reconstituted myocardium.
Error bars represent s.e.m. (n= 11). Experiments were performed in the presence of 5 mm MgATP.
In the presence of 8 mm Pi (standard activating condition with 5S8P), isometric tension in the control myocardium was 15.4 ± 1.1 kN m−2 (n= 17). In the absence of added Pi (5S0P), isometric tension in the control myocardium was 27.6 ± 3.2 kN m−2 (n= 11). Isometric tension after reconstitution of the actin filament decreased to 61 ± 5% (n= 11) of the control myocardium when tested with the 5S0P solution. After reconstitution of Tm, the active tension increased to 69 ± 7% (n= 11) of the control myocardium with the 5S0P solution.
Cross-bridge distribution
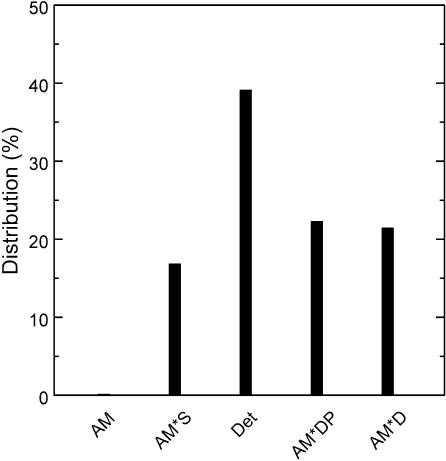
Cross-bridge distribution at the standard activation condition (5S8P) of Tm-reconstituted myocardium was calculated based on eqn (18) of Kawai & Halvorson (1991) using the equilibrium constants in Table 1, and shown in Fig. 9. The probability of cross-bridges in the AM state was 0.16% and not significantly populated. This is because K1 is large and a saturating concentration of MgATP was used. The probability of force-generating states AM*DP and AM*D was about 22% each, and the probability of the AM*S state was about 17%. Cross-bridges were mostly populated in the ‘Det’ state, which was about 39%. The Det state is a combination of detached states (MS and MDP) and weakly attached states (AMS and AMDP) (see Scheme 1). These states cannot be distinguished with our analysis, which depends on strongly attached states. This cross-bridge distribution was not significantly different from the control bovine myocardium or the nTm-reconstituted myocardium reported previously (cf. Fig. 6 of Fujita et al. 2002).
Figure 9. Calculated cross-bridge distribution in Tm-reconstituted myocardium.
Calculated cross-bridge distribution based on the equilibrium constants shown in Table 1 and under standard activating conditions (5S8P). Det, detached state, which is a combination of weakly attached states (AMS and AMDP) and truly detached states (MS and MDP). Other abbreviations are the same as those in Scheme 1.
Discussion
Reconstitution of Tm and Tn
We have succeeded in selectively removing the thin filament from bovine myocardium, and sequentially reconstituting the myocardium with actin, Tm, and then with Tn. The degree of reconstitution was quantified by SDS-PAGE (Fig. 1), confocal images (Fig. 2), and isometric tension (Fig. 3). The SDS-PAGE demonstrates removal of the thin filament (Fig. 1, lane 2), reconstitution of the actin filament (lane 3), reconstitution of Tm (lane 4), and reconstitution of Tn (lane 5). In lane 5, TnT and TnI can be identified. As expected, the confocal images demonstrate that actin (Fig. 2A) and Tm (Fig. 2B) can be seen colocalized (Fig. 2C), indicating that Tm was incorporated into the actin filament homogeneously. The functional reconstitution was assessed by isometric tension (Fig. 3). Both actin filament-reconstituted myocardium and Tm-reconstituted myocardium developed tension irrespective of the presence of Ca2+ (Figs 3C and D). These results demonstrate that the Ca2+ regulatory system was absent in these preparations as expected. In Tm–Tn-reconstituted myocardium, the Ca2+ sensitivity returned: the myocardium developed tension when Ca2+ was added to the activating solution, and the myocardium relaxed when Ca2+ was removed from the solution (Fig. 3E). These results imply that the Ca2+ regulatory system was restored by the Tm and Tn reconstitution. Thus, we conclude that the reconstitution of the thin filament-extracted myocardium with actin, Tm and Tn was both structurally and functionally complete.
Tension augmentation by reconstitution of Tm
Our previous study (Fujita et al. 2002) was focused on the effect of native tropomyosin (nTm), which is a complex of Tm and Tn. We noticed enhancement of active tension by 40% (relative to initial control tension) with nTm, from which we concluded that the enhancement was based on an increase in the force supported by each cross-bridge. This conclusion was also supported by our observation that the number of force-generating cross-bridges decreased by about 20% with the nTm reconstitution (Fujita et al. 2002). However, at that time we were not able to tell whether this enhancement was based on Tm, Tn or both. To determine which one of these proteins enhances isometric tension, we reconstituted Tm and Tn sequentially in this report. We found that Tm in the absence of Tn enhanced isometric tension by about 10%. This result demonstrates that Tm partially contributes to the enhancement of isometric tension. Previous studies using in vitro motility assays on actin and heavy mero myosin (HMM) demonstrated a similar contribution of Tm to force (VanBuren et al. 1999; Bing et al. 2000). This coincidence of results from two very different methods is remarkable, because fibre experiments are typically carried out at or near physiological ionic strength (∼200 mm), whereas in vitro motility assays are typically carried out at low ionic strength (50–70 mm). It has been known for some time that the mechanisms of force generation depend on ionic interaction (Sutoh, 1993) as well as hydrophobic interaction (Zhao & Kawai, 1994). The ionic interaction is diminished by an increase in the ionic strength, whereas the hydrophobic interaction is unchanged by ionic strength.
Tension augmentation by reconstitution of Tn
We then added Tn to the Tm-reconstituted myocardium. We found that the isometric tension increased further by 14% when Tn was added to the Tm-reconstituted myocardium in the presence of Ca2+. This fact implies that Tn has a further activating role in the actomyosin interaction. This activating role must be mediated by Tm, actin, or both. Such an enhancement of tension by Tn and Ca2+ was observed previously in single molecule experiments on tension and gliding speed (Gordon et al. 1998; Bing et al. 2000; Homsher et al. 2000) using in vitro motility assays. The combined recovery of isometric tension was 24%, which is consistent with our earlier results (Fujita et al. 2002) using nTm to reconstitute the regulatory system.
The effect of Tm on the elementary steps of the cross-bridge cycle
We studied the cross-bridge kinetics of Tm-reconstituted myocardium by sinusoidal analysis, and found that most kinetic constants of the elementary steps resumed those of the control myocardium as soon as Tm was reconstituted. An exception was K1 (MgATP binding constant) which was about 2 × the control myocardium. These results contrast with our previous study of actin-filament-reconstituted myocardium, in which K2, K4 and K5 became 0.23×, 4.4× and 2.8× of the control myocardium, respectivley (Fujita et al. 2002); there was little change in K1. Thus, it can be concluded that Tm enhances ATP binding, but otherwise Tm reconstitution recovers the equilibrium constants to the control level. The distribution of cross-bridge states is not very different between Tm-reconstituted (Fig. 9), nTm-reconstituted, and control myocardium (Fig. 6 of Fujita et al. 2002). These facts imply that Tm partially modifies actin configuration so as to enhance stereospecific and hydrophobic interaction between actin and myosin molecules. This modification increases the force generated by each cross-bridge. A similar line of evidence was presented by using Δ23Tm, a Tm mutant (Lu et al. 2003). A model that accounts for the regulatory proteins–actin interaction was proposed by Tobacman & Butters (2000).
The effect of Tn on the elementary steps of the cross-bridge cycle
We found that the kinetic constants simulated those of the control myocardium as soon as Tn was reconstituted and Ca2+ was included in the activating saline. This fact implies that the reconstitution was functionally complete. What is important is that isometric tension increased further to 1.2 × with the Tn reconstitution. The kinetic constants of Tm-reconstituted myocardium and Tm–Tn-reconstituted myocardium were almost the same, except that K1 decreased following the Tn reconstitution (see above). This decrease, however, does not seriously affect the overall distribution of cross-bridges among the six states; hence the number of force-generating cross-bridges is approximately the same before and after the Tn reconstitution (cf. Fig. 9 and Fig. 6 of Fujita et al. 2002). The reason for the tension increase must therefore be based on an increase in cross-bridge force, i.e. each cross-bridge must generate a larger force as Tn is reconstituted. The fact that the addition of Tn enhanced isometric tension by 14% over the Tm-reconstituted myocardium implies that the tension on each cross-bridge increased by ∼14%.
It may be interesting to discuss why K1 was altered at each step of reconstitution. This observation is consistent with the idea that Tm and Tn affect the microenvironment of the nucleotide-binding site on myosin. This effect must be mediated through chain reactions that include Tm, actin, actomyosin interface and the myosin head. How could this be possible? One possibility is that isometric tension itself can mediate such chain reactions: we have known from previous studies that K1 becomes larger with smaller tensions in rabbit psoas fibres (Zhao et al. 1996). Furthermore, our earlier studies have shown that different muscle types have different K1 values when the amino acid sequence of the nucleotide-binding site is nearly identical (Wang & Kawai, 2001). This difference may depend on the charge distribution of loop 1 of MHC: if it is more positive, the MgATP2− molecule may bind more strongly. It is likely that the positively charged loop 1 readily attracts the negatively charged MgATP molecule. Therefore, the possibility arises that the charge distribution and the position of loop 1 may contribute to the ATP binding affinity (Kelley et al. 1993; Rovner et al. 1997; Sweeney et al. 1998; Wang & Kawai, 2001). In fact, it is known that loop 1 comes close to the nucleotide-binding pocket (Rayment et al. 1993); hence loop 1 could serve as a local ATP carrier. This loop exists between the N-terminus 25K and 50K domains of the myosin head, and its position may change depending on the state of the thin filament and/or tension imposed on the myosin head. An interesting hypothesis is that the position of the loop 1 is altered by each step of reconstitution of Tm and Tn on the actin filament, so as to change the binding affinity of the MgATP2− molecule.
Acknowledgments
The authors would like to thank to Dr Lary Tobacman for his gift of tropomyosin and troponin at the final stage of this work, and to Miss Mary Bryant for her excellent technical assistance. This work was supported in part by grants from NSF IBN 98-14441 and NIH HL70041 to M.K. This research was also supported in part by Grants-in-Aid for Specially Promoted Research, for the Bioventure Project, and for the 21st century COE program (Physics of Self-organization Systems) at Waseda University from the Ministry of Education, Sports, Culture, Science and Technology of Japan to S.I. Any opinions, findings, and conclusions or the contents of this work are solely the responsibility of the authors and do not necessarily reflect the views of the National Science Foundation. Similarly, the contents of this work are solely the responsibility of the authors and do not necessarily represent the official view of National Institute of Health.
References
- Bing W, Knott A, Marston SB. A simple method for measuring the relative force exerted by myosin on actin filaments in the in vitro motility assay: evidence that tropomyosin and troponin increase force in single thin filaments. Biochem J. 2000;350:693–699. [PMC free article] [PubMed] [Google Scholar]
- Bremel RD, Murray JM, Weber A. Manifestations of cooperative behavior in the regulated actin filament during actin-activated ATP hydrolysis in the presence of calcium. Cold Spring Hbr Symp Quant Biol. 1972;37:267–275. [Google Scholar]
- Chalovich JM, Chock PB, Eisenberg E. Mechanism of action of troponin·tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin. J Biol Chem. 1981;256:575–578. [PMC free article] [PubMed] [Google Scholar]
- Chalovich JM, Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982;257:2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Dantzig JA, Goldman YE, Millar NC, Lacktis J, Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol. 1992;451:247–278. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S, Endo M. Calcium ions and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ebashi S, Kodama A, Ebashi F. Troponin. I. Preparation and physiological function. J Biochem (Tokyo) 1968;64:465–477. doi: 10.1093/oxfordjournals.jbchem.a128918. [DOI] [PubMed] [Google Scholar]
- Fujita H, Ishiwata S. Tropomyosin modulates pH dependence of isometric tension. Biophys J. 1999;77:1540–1546. doi: 10.1016/S0006-3495(99)77001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Sasaki D, Ishiwata S, Kawai M. Elementary steps of the cross-bridge cycle in bovine myocardium with and without regulatory proteins. Biophys J. 2002;82:915–928. doi: 10.1016/S0006-3495(02)75453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Yasuda K, Niitsu S, Funatsu T, Ishiwata S. Structural and functional reconstitution of thin filaments in the contractile apparatus of cardiac muscle. Biophys J. 1996;71:2307–2318. doi: 10.1016/S0006-3495(96)79465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatsu T, Anazawa T, Ishiwata S. Structural and functional reconstitution of thin filaments in skeletal muscle. J Muscle Res Cell Motil. 1994;15:158–171. doi: 10.1007/BF00130426. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Chen Y, Liang B, Lamadrid M, Luo Z, Chase PB. Skeletal muscle regulatory proteins enhance F-actin in vitro motility. Adv Exp Med Biol. 1998;453:187–196. doi: 10.1007/978-1-4684-6039-1_22. [DOI] [PubMed] [Google Scholar]
- Haselgrove JC. X-ray evidence for a conformational change in the actin-containing filaments of vertebrate striated muscle. Cold Spg Hbr Symp Quant Biol. 1973;37:341–352. [Google Scholar]
- Homsher E, Lee DM, Morris C, Pavlov D, Tobacman LS. Regulation of force and unloaded sliding speed in single thin filaments: effects of regulatory proteins and calcium. J Physiol. 2000;524:233–243. doi: 10.1111/j.1469-7793.2000.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF, Niedergerke R. Interference microscopy of living muscle fibres. Nature. 1954;173:971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Simmons RM. Proposal mechanisms of force generation in striated muscle. Nature. 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley HE. Structural changes in the actin- and myosin-containing filaments during contraction. Cold Spg Hbr Symp Quant Biol. 1973;37:361–376. [Google Scholar]
- Huxley HE, Hanson J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954;173:973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Lehrer SS. Excimer fluorescence of pyrenyliodoacetamide-labeled tropomyosin: a probe of the state of tropomyosin in reconstituted muscle thin filaments. Biochem. 1990;29:1160–1166. doi: 10.1021/bi00457a010. [DOI] [PubMed] [Google Scholar]
- Kawai M, Brandt PW. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscle of rabbit, frog and crayfish. J Muscle Res Cell Mot. 1980;1:279–303. doi: 10.1007/BF00711932. [DOI] [PubMed] [Google Scholar]
- Kawai M, Halvorson H. Role of MgATP and MgADP in the cross-bridge kinetics in chemically skinned rabbit psoas fibers. Study of a fast exponential process (C) Biophys J. 1989;55:595–603. doi: 10.1016/S0006-3495(89)82857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Halvorson HR. Two step mechanism of phosphate release and the mechanism of force generation in chemically skinned fibers of rabbit psoas muscle. Biophys J. 1991;59:329–342. doi: 10.1016/S0006-3495(91)82227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Saeki Y, Zhao Y. Crossbridge scheme and the kinetic constants of elementary steps deduced from chemically skinned papillary and trabecular muscles of ferret. Circ Res. 1993;73:35–50. doi: 10.1161/01.res.73.1.35. [DOI] [PubMed] [Google Scholar]
- Kawai M, Zhao Y. Cross-bridge scheme and force per cross-bridge state in skinned rabbit psoas muscle fibers. Biophys J. 1993;5:638–651. doi: 10.1016/S0006-3495(93)81109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley CA, Takahashi M, Yu JH, Adelstein RS. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J Biol Chem. 1993;268:12828–12848. [PubMed] [Google Scholar]
- King RT, Greene LE. Regulation of the adenosinetriphosphatase activity of cross-linked actin-myosin subfragment 1 by troponin-tropomyosin. Biochem. 1985;24:7009–7014. doi: 10.1021/bi00345a039. [DOI] [PubMed] [Google Scholar]
- Kondo H, Ishiwata S. Uni-directional growth of F-actin. J Biochem (Tokyo) 1976;79:159–171. doi: 10.1093/oxfordjournals.jbchem.a131043. [DOI] [PubMed] [Google Scholar]
- Kurokawa H, Fujii W, Ohmi K, Sakurai T, Nonomura Y. Simple and rapid purification of brevin. Biochem Biophys Res Commu. 1990;168:451–457. doi: 10.1016/0006-291x(90)92342-w. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehman W, Craig R, Vibert P. Ca2+-induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstitution. Nature. 1994;368:65–67. doi: 10.1038/368065a0. [DOI] [PubMed] [Google Scholar]
- Lehman W, Vibert P, Uman P, Craig R. Steric-blocking by tropomyosin visualized in relaxed vertebrate muscle thin filaments. J Mol Biol. 1995;251:191–196. doi: 10.1006/jmbi.1995.0425. [DOI] [PubMed] [Google Scholar]
- Lu X, Tobacman LS, Kawai M. Effects of tropomyosin internal deletion Δ23Tm on isometric tension and the cross-bridge kinetics in bovine myocardium. J Physiol. 2003;553:457–471. doi: 10.1113/jphysiol.2003.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JM, Knox MK, Trueblood CE, Weber A. Do tropomyosin and myosin compete for actin sites in the presence of calcium? FEBS Lett. 1980;114:169–173. doi: 10.1016/0014-5793(80)80886-6. [DOI] [PubMed] [Google Scholar]
- Nosek TM, Leal-Cardoso JH, Mclaughlin M, Godt RE. Inhibitory influence of phosphate and arsenate on contraction of skinned skeletal and cardiac muscle. Am J Physiol. 1990;259:C933–C939. doi: 10.1152/ajpcell.1990.259.6.C933. [DOI] [PubMed] [Google Scholar]
- Rayment I, Rypniewski WR, Schmidt-Bäse K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993a;261:50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Rovner AS, Freyzon Y, Trybus KM. An insert in the motor domain determines the functional properties of expressed smooth muscle myosin isoforms. J Muscle Res Cell Mot. 1997;18:103–110. doi: 10.1023/a:1018689102122. [DOI] [PubMed] [Google Scholar]
- Spudich JA, Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- Sutoh K. Identification of actin surface interacting with myosin during the actin-myosin sliding. Adv Exp Med Bio. 1993;332:241–245. doi: 10.1007/978-1-4615-2872-2_23. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Rosenfeld SS, Brown F, Faust L, Smith J, Xin J, Stein LA, Sellers JR. Kinetic tuning of myosin via a flexible loop adjacent to the nucleotide binding pocket. J Biol Chem. 1998;273:6262–6270. doi: 10.1074/jbc.273.11.6262. [DOI] [PubMed] [Google Scholar]
- Tobacman LS, Butters CA. A new model of cooperative myosin-thin filament binding. J Biol Chem. 2000;275:27587–27593. doi: 10.1074/jbc.M003648200. [DOI] [PubMed] [Google Scholar]
- VanBuren P, Palmiter KA, Warshaw DM. Tropomyosin directly modulates actomyosin mechanical performance at the level of a single actin filament. Proc Natl Acad Sci U S A. 1999;96:12488–12493. doi: 10.1073/pnas.96.22.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Kawai M. Effect of temperature on elementary steps of the cross-bridge cycle in rabbit soleus slow-twitch muscle fibres. J Physiol (Lond) 531. 2001;1:219–234. doi: 10.1111/j.1469-7793.2001.0219j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannenburg T, Heijne GH, Geerdink JH, Van den Dool HW, Janssen PML, de Tombe PP. Cross-bridge kinetics in rat myocardium: Effect of sarcomere length and calcium activation. Am J Physiol. 2000;279:H779–H790. doi: 10.1152/ajpheart.2000.279.2.H779. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Greaser ML, Cassens RG. Interactions of troponin subunits with different forms of tropomyosin. J Ultrastruct Res. 1974;48:33–58. doi: 10.1016/s0022-5320(74)80043-2. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Kawai M. Kinetic and thermodynamic studies of the cross-bridge cycle in rabbit psoas muscle fibers. Biophys J. 1994;67:1655–1668. doi: 10.1016/S0006-3495(94)80638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Kawai M. Inotropic agent EMD-53998 weakens nucleotide and phosphate binding to cross bridges in porcine myocardium. Am J Physiol. 1996;271:H1394–H1406. doi: 10.1152/ajpheart.1996.271.4.H1394. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Swamy PMG, Humphries KA, Kawai M. The effect of partial extraction of troponin C on the elementary steps of the cross-bridge cycle in rabbit psoas fibers. Biophys J. 1996;71:2759–2773. doi: 10.1016/S0006-3495(96)79469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]