Abstract
Small intestinal movements depend on the composition of the chyme with mixing predominating at high nutrient levels and propulsion being prevalent at low nutrient levels. The mechanisms coupling nutrients to motility are unknown. We used computer analysis of video recordings of isolated guinea-pig duodenum, jejunum and ileum to examine movements induced by a fatty acid, decanoic acid. Increasing intraluminal pressure past a threshold using control saline consistently evoked propulsive reflexes: lumen-occluding constrictions appeared at the oral end propagating at 20.4 ± 2.4 mm s−1 (mean ±s.d., jejunum) to the anal end before being repeated until the intraluminal pressure was returned to control. Subthreshold pressure increases sometimes evoked a transient series of constrictions appearing at the oral end and propagating anally at 18.4 ± 4.7 mm s−1 (jejunum). At basal pressures, decanoic acid dose-dependently induced motor activity consisting of 40–60 s episodes of constrictions separated by 40–200 s periods of quiescence and lasting up to 2 h. Five contraction patterns were identified within episodes including localized stationary constrictions; constrictions that propagated slowly (5–8 mm s−1) for short distances orally or anally; and constrictions that propagated orally or anally for the length of the preparation at 14–20 mm s−1. Decanoic acid induced motor activity was reversibly abolished by tetrodotoxin (3 μm), hyoscine (1 μm) and hexamethonium (100 μm), but was insensitive to blockade of P2 purinoceptors by PPADS (60 μm). Thus, decanoic acid induces motor activity equivalent to segmentation in guinea-pig small intestine in vitro and this depends on intrinsic neural pathways.
The movements of the gastrointestinal tract aid the digestion and absorption of nutrients by mixing food with digestive juices and exposing digested material to the absorptive epithelium. Mixing depends on a specific motor pattern, segmentation. In seminal studies of intestinal movements, in vivo, Cannon (1912) identified several motor patterns in the small intestine after a barium meal. The best known is ‘peristalsis’, a propulsive motor pattern, in which contraction of the circular muscle migrates, or ‘propagates’, anally along the intestine pushing the intestinal content ahead of it. However, the predominant motor pattern after a meal is the suite of movements that Cannon defined as segmentation. This consists of circular muscle contractions that remain stationary within the intestine and alternate with periods of relaxation, thereby dividing and redividing the intestinal content, i.e. mixing it. The relative incidence of segmenting versus propulsive contractions depends critically on the nutrient level within the intestinal contents (Borgstrom & Arborelius, 1975; Schemann & Ehrlein, 1986; Siegle & Ehrlein, 1988; Defilippi & Gomez, 1995). When nutrients (proteins, starches, lipids) are present segmentation predominates and intestinal transit is slow; when the intestine contains non-nutritive bulk, propulsive motor patterns (propagating contractions) predominate and intestinal transit is faster (Schemann & Ehrlein, 1986). The relative proportions of segmenting and propulsive contractions also differ in various pathological conditions, e.g. segmentation is reduced and propulsive contractions are increased in Crohn's disease, even when the disease is inactive (Annese et al. 1997).
The mechanisms coupling nutrient levels to segmentation are unknown. Indeed the effects of nutrients are often treated as inhibition of propulsive movements with segmentation as a default motor pattern (Keinke & Ehrlein, 1983; Schemann & Ehrlein, 1986; Schmid & Ehrlein, 1993). In most studies in vivo, segmentation is defined as contractions that do not propagate past two or more recording points along the small intestine. This negative definition makes analysis of the underlying mechanism difficult, if not impossible, and it is not clear whether the segmentation motor pattern is predominantly neurogenic or myogenic. Cannon (1912) believed that segmentation was due to a local reflex mediated by the enteric nervous system (ENS). By contrast, a recent paper speculates that the timing of segmentation contractions is largely, if not entirely, the result of slow wave activity in the intestinal smooth muscle coat (Thuneberg & Peters, 2001), i.e. that the ENS is not involved in determining this timing. However, a recent study has shown that spontaneous, temporally uncoordinated contractions, which may represent segmentation, still occur in the small intestine of W/Wv mutant mice that completely lack both slow waves and pacemaker interstitial cells of Cajal (Spencer et al. 2003). Resolution of this key question, and the issues arising from it, depends on knowing the precise sites of action of pharmacological agents affecting neurones and muscle, which has been hampered by the lack of a robust in vitro model of segmentation or the fed state.
Over the last few years, computer analysis of video images of the contracting intestine has provided a new tool for the investigation of motor patterns within both the small and large intestines (Benard et al. 1997; Hennig et al. 1999; Bercik et al. 2000; D'Antona et al. 2001). This method allows measurements of muscular activity with very high resolution in both time and space along the length of an isolated intestinal segment. Converting the diameter of the intestine to a greyscale allows the diameter to be plotted against distance along the segment and elapsed time to create spatiotemporal maps of the intestinal motility. This reveals motor patterns that are very difficult to detect with any other technique. The present study used computer analysis of video images to investigate motor patterns induced by a simple nutrient (decanoic acid) infused into the lumen of three regions of the guinea-pig small intestine in vitro: the duodenum, the upper jejunum and the ileum. The aim was to develop an in vitro model of the fed state, which would allow detailed analysis of the underlying mechanisms.
Methods
Tissue preparation
Guinea-pigs (250–380 g), of either sex, were killed by being stunned and having their carotid arteries severed. This procedure was in accordance with the guidelines of the National Health and Medical Research Council of Australia and was approved by the University of Melbourne Animal Experimentation Ethics Committee. The abdominal cavity was then opened and segments of duodenum, jejunum and/or ileum removed with their oral ends marked so that orientation could be preserved. The duodenum (5–8 cm in length) was dissected 5–10 mm anal to the gastro-duodenal junction. Proximal jejunum (8–10 cm in length) was taken just anal to the duodenum and distal ileum (8–10 cm in length) was taken 10–20 cm oral to the ileo-caecal junction. The segments were immediately flushed clean with physiological saline (composition, mm: NaCl 118, KCl 4.6, CaCl2 2.5, MgSO4 1.2, NaH2PO4 1, NaHCO3 25, d-glucose 11, bubbled with 95% O2 and 5% CO2) and placed in an organ bath containing 25 ml of this saline at 37°C. The saline was continuously superfused through the organ bath at a flow rate of 6 ml min−1. Cannulae were inserted into each end of the intestinal segments and secured with nylon thread. The orally placed cannula was connected via a two-way stopcock to a reservoir containing physiological saline. The height of the reservoir could be adjusted to change the pressure in the lumen. The anally placed cannula was connected via a three-way stopcock to a vertical outflow tube, which allowed the backpressure to be adjusted, to a pressure transducer (PVB 6003, Surgicare, Victoria, Australia) to measure changes in luminal pressure and to a draining line that allowed the lumen to be flushed. Pressure signals were processed via a BIOPAC Systems Inc. MP100 recording unit and stored on a personal computer using AcqKnowledge.2.4 software (SDR Clinical Technology, New South Wales, Australia).
Image acquisition and spatio-temporal maps
A Logitech Quickcam Pro camera was positioned 6–7 cm vertically above the organ bath to record video images of a 5–6 cm length of intestine. Images were captured at a frame rate of 6 per second with a resolution of 640 × 480 using Logitech Quickcam software (Version 5.0) and acquired directly to computer in avi format. The recordings were processed off-line with edge-detection software developed in-house using the Matlab system (version 12). The software converts the image of the intestine into a silhouette and then counts the number of vertical pixels for each horizontal pixel in the silhouette. This effectively measures the intestinal diameter at 640 points along the segment with a resolution in the order of 80 μm. The diameters were then converted to a grey scale, where white was the smallest diameter and black the largest. This allowed the diameter of the intestine (grey scale) to be plotted as a function of space (distance along the segment) and time (each video frame corresponds to one time point giving a resolution of 167 ms) on a two-dimensional image (a spatiotemporal map; see Fig. 1). Circular muscle contractions appeared as white regions in the spatiotemporal maps, while relaxations were seen as dilatations and appeared black; intermediate levels of activity are shown as shades of grey. These methods were also used to plot the intestinal diameter at any chosen point as a function of time (Fig. 2B).
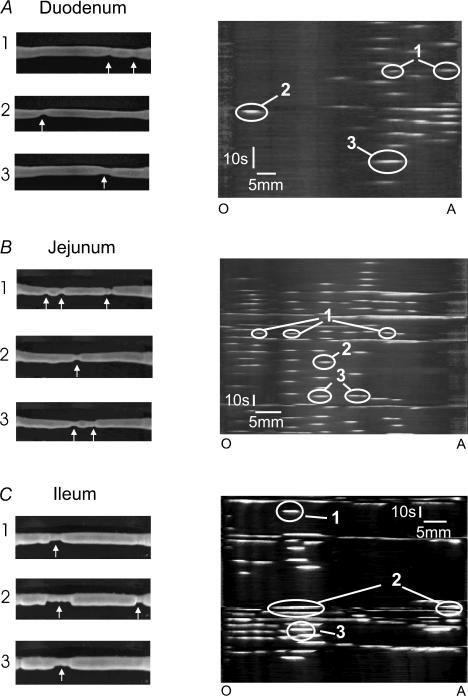
Figure 1. Video images and spatiotemporal maps of motor activity induced in each region of the small intestine by decanoic acid.
The left column shows individual video images taken at three distinct time points for individual segments of duodenum (A), jejunum (B) and ileum (C) in the presence of intraluminal decanoic acid. The arrows indicate constrictions, which were only seen in the presence of the fatty acid. The right column shows the corresponding spatiotemporal maps with the constrictions (white) indicated by arrows in the left column circled. The numbers indicate which video images correspond to particular contractions in the spatiotemporal maps. In this figure. and all subsequent figures the maps are orientated so that the record begins at the top and the oral end (O) of the segment is on the left and the anal end (A) is to the right.
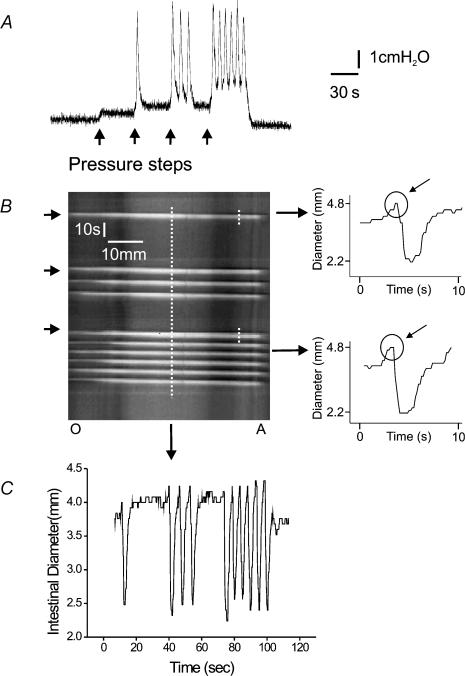
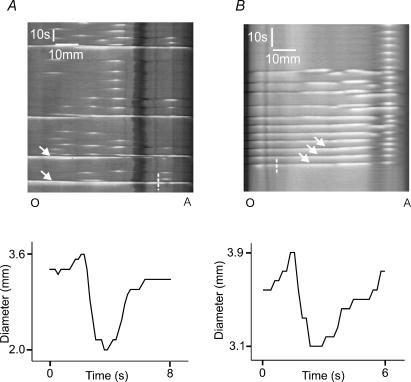
Figure 2. Response of an isolated segment of duodenum to increasing intraluminal pressure induced by saline.
A shows a pressure recording taken as the intraluminal pressure was increased in 1 cmH2O steps (arrows) from 2 cmH2O (the basal level) to 6 cmH2O. The second step triggered a single phasic contraction, while the third triggered a burst of contractions that did not continue though the stimulus was sustained. The last step triggered a series of contractions that continued until the infusion pressure was returned to baseline. The left panel of B shows the spatiotemporal map derived from video images corresponding to the pressure trace in A. The two right hand panels show the diameters at the same point tracked along the short dashed lines, with the circles indicating dilatations that preceded the contractions. C tracks the diameter of the duodenum at another point (long dashed line) showing both the phasic and tonic contractions.
Experimental protocols
After the tissues were set up, 10 ml of physiological saline was flushed through the lumen and the preparation left to equilibrate for 1 h. During this period, the height of the saline in the reservoir was kept at 2 cm above the level of the segment providing an intraluminal pressure of 2 centimetres of water (cmH2O). This is below the threshold for initiation of propulsive reflexes in guinea-pigs of this size. (Monro et al. 2002) The back pressure in the outflow tube was held at 2 cmH2O throughout the experiment.
Distension stimuli
The pressure threshold for initiation of persistent propulsive contractions (for definition see Results) was determined by raising the inflow pressure in steps of 1 cmH2O at intervals of approximately 30 s, until this motor pattern was initiated. The inflow pressure was then returned to control levels. The pressure, which triggered the persistent propulsive contractions, was consistent within one preparation for several hours and was taken as the threshold for propulsive motor activity (or peristalsis).
Nutrient stimulation
Decanoic acid (Sigma Aldrich, NSW, Australia) was first dissolved in 100% ethanol and then diluted with distilled water (1 : 1 ratio by volume) to form a 100 mm stock solution. This stock solution was then added to the physiological saline to give a final concentration of decanoic acid in the luminal perfusion solution of 100 μm to 1 mm. The maximum concentration of ethanol in the luminal solution was 0.4%. Control experiments using luminal solutions containing 0.7% ethanol indicated that this concentration of ethanol had no effect on motor behaviour of the intestinal segments (6 preparations).
In each experiment, the tissue was allowed to equilibrate with a control saline solution in the lumen at a basal pressure of 2 cmH2O for 1 h and then the threshold pressure for initiation of peristaltic contractions was determined as described above. The luminal saline then was flushed out with a solution containing decanoic acid and either the basal motor activity or the threshold for peristaltic motor activity was monitored over the next 1–2 h. The decanoic acid was then replaced with the control saline and threshold measurements were repeated after a 1-h period monitoring basal motor activity.
It is possible that the effects of decanoic acid described below resulted from damage to the mucosa caused by isolation of the intestinal segments and maintenance in vitro. The following experiment was undertaken to examine this possibility. Decanoic acid was flushed into the lumen of three preparations of jejunum without the normal equilibration period; this induced motor patterns indistinguishable from those seen after the 1 h incubation period (see Results). After 30 min, the preparations were fixed, dehydrated, embedded in paraffin, sectioned, mounted on slides and stained with haematoxylin–eosin. These sections were compared with control sections from preparations fixed without being placed in the organ bath and with sections from preparations fixed 2 or 4 h after mounting in the organ baths. The state of the mucosa in 20 sections from each preparation was evaluated using ×100 magnification by an experimenter blinded to the duration of incubation of the preparations. It was found that the mucosa deteriorated to the point that almost all villi were gone after 4 h, although the crypts remained intact at this time and there were no gaps in the mucosal layer separating the lumen from the submucosa. Thus, although there was a loss of epithelium, enteroendocrine and enterochromaffin cells would have remained. In contrast, there was little, or no, damage to the mucosa after 30 min, at which time decanoic acid already produced the full suite of motor activity seen at later times.
The effects of drugs on motor patterns induced by decanoic acid were tested as follows. Decanoic acid was infused into the lumen and the resulting motor pattern was imaged in 2–3 control video recordings (duration 1–4 min). A drug (e.g. hexamethonium) was then added to the bathing solution (i.e. serosal application) and the motor activity was monitored for up to 30 min with video recordings taken at 1–2 min intervals. After 30 min, the drug was washed out of the bath and a further set of video recordings were taken to monitor any recovery. Decanoic acid was kept within the lumen throughout this procedure.
Drugs
Drugs used in these experiments included hyoscine, hexamethonium and pyridoxal phosphate-6-axophenyl-2′-4′-disulphonic acid (PPADS) (all from Sigma Aldrich, NSW, Australia) and tetrodotoxin (TTX) (Alomone Laboratories Ltd, Jerusalem, Israel). All drugs were initially made up in distilled water to form stock solutions. Final concentrations of drug were achieved by adding appropriate aliquots of the stock to the saline used to superfuse the tissue.
Statistics
Data in the text are reported as means ± standard deviation, except where otherwise stated. Statistical comparisons were made using Student's paired and unpaired t tests and one-way analysis of variance (ANOVA) where appropriate. P values < 0.05 were taken as indicating statistical significance. In the text below, n is the number of preparations used for the analysis in all cases.
Results
Motor patterns induced by increasing intraluminal pressure with saline
After 1 h incubation at the resting intraluminal pressure of 2 cmH2O, each region of guinea-pig small intestine was quiescent. Increasing intraluminal pressure (distension) ultimately evoked a stereotyped motor pattern consisting of a series (= 5) of transient contractions that appeared at the oral end of the preparation and propagated to the anal end. The contractions were propulsive as they led to a net outflow of the intraluminal saline via the 2 cm resistance tube. They were repeated throughout a distension of up to 60 s and disappeared only when the pressure was reduced; accordingly, we have termed them ‘persistent propulsive contractions’. Each contraction was preceded at any given site by a brief increase (dilatation) in intestinal diameter (Fig. 2B).
Step increases in intraluminal pressure to levels below the threshold for persistent propulsive contractions often evoked one or more propagating contractions of the circular muscle (Fig. 2). These appeared at the oral end and propagated to the anal end producing a net outflow of intraluminal saline, so they were propulsive. They were preceded by a brief dilatation (Fig. 2B). This contractile pattern was transient: only 1–4 contractions were initiated during a maintained pressure step; we have termed them ‘transient propulsive contractions’.
The threshold pressure required to evoke persistent propulsive contractions in the duodenum (8.0 ± 0.2 cmH2O; mean ±s.d., n = 10) was significantly greater than that in the ileum (6.8 ± 0.3 cmH2O, n = 10), while the threshold in the jejunum was significantly lower still (5.6 ± 1.5 cmH2O, n = 10; P < 0.05 in each case).
The probability of a step increase in pressure, to 1 cmH2O below the threshold for initiation of persistent propulsive contractions, evoking transient propulsive contractions was lower in the duodenum (0.32), than in the jejunum (0.49) or ileum (0.46). Transient propagating contractions were seen in all preparations of jejunum (n = 10) and ileum (n = 10) and in 8 of 10 preparations of duodenum.
The speeds at which the propulsive contractions, whether in persistent or transient patterns, propagated along the intestine were measured from spatiotemporal maps. There were no significant differences in propagation speeds between the persistent propulsive contractions and the transient propulsive contractions in any one region. Propagation speeds in the duodenum and ileum were virtually identical (duodenum persistent 15.2 ± 2.4 mm s−1, n = 6, transient 16.8 ± 2.0 mm s−1, n = 6; ileum persistent 14.5 ± 2.9 mm s−1, n = 6, transient 15.0 ± 3.0 mm s−1, n = 6), but were significantly faster in the jejunum (persistent 20.4 ± 2.4 mm s−1n = 6, P < 0.01; transient 18.4 ± 4.7 n = 6, P < 0.05).
The magnitudes of the contractions were measured as the maximum pressure produced and the minimum diameter achieved as the contraction wave passed a specific site. The minimum diameter achieved during a propulsive contraction is presumably the diameter when the lumen is occluded and was used to compare such contractions with the other motor patterns described below.
The maximum pressure generated by the persistent propulsive contractions was much larger in the ileum (5.7 ± 2.4 cmH2O, n = 6, P < 0.025) than in the duodenum (3.9 ± 1.6 cmH2O, n = 6) or the jejunum (3.8 ± 0.9 cmH2O, n = 6). Pressures generated by transient propulsive contractions were significantly less than those produced by persistent propulsive contractions in the same preparation (P < 0.025 in each case), but the same regional distribution of amplitudes was observed (duodenum 2.9 ± 1.8 cmH2O; jejunum 2.5 ± 0.7 cmH2O; ileum 4.9 ± 1.8 cmH2O; P < 0.01). There were no significant regional differences in the minimum diameter achieved by either type of contraction evoked by increased intraluminal pressure (duodenum persistent 2.4 ± 1.1 mm, transient 2.6 ± 1.1 mm, n = 6; jejunum 2.5 ± 0.9 mm, n = 10; ileum 1.9 ± 0.9, n = 10; P > 0.1).
Motor patterns induced by intraluminal decanoic acid
Infusing decanoic acid (100 μm to 1 mm) into the lumen of isolated segments of small intestine without changing the intraluminal pressure induced episodes of contractions separated by periods of quiescence in both the duodenum and jejunum (Figs 1 and 3) after 20–30 min of exposure. Prior to this the tissue was largely quiescent. These contraction episodes were never seen in the absence of the decanoic acid. Detailed examination revealed that they could be resolved into five distinct motor patterns (Figs 4, 5 and 6), which overlapped in both space and time.
Figure 3. The time course of contraction patterns seen in duodenum (Duo) and jejunum (Jej) during a prolonged exposure to decanoic acid.
A shows a spatiotemporal map of the duodenum starting 25 min after infusing decanoic acid (1 mm) into the lumen. Note, episodes of contractions (white areas) are separated by similar duration periods of quiesence. A similar pattern is seen in B, which shows a map of the jejunum under identical conditions.
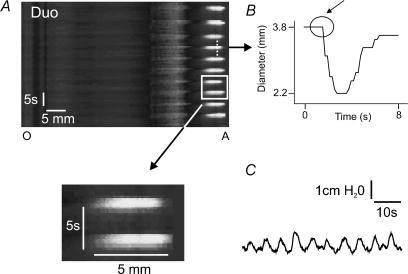
Figure 4. Stationary contractions induced by decanoic acid in a segment of ileum.
The upper panel of A shows a spatiotemporal map of isolated duodenum during infusion of decanoic acid (1 mm). The square highlights a region containing two constrictions that are shown with greater magnification in the lower panel. Note, the constriction appeared simultaneously at all points and did not extend to other regions at later times, i.e. it did not propagate. B tracks the diameter at a single point (dashed line) through one constriction. C shows a pressure recording corresponding to the entire time period shown in A.
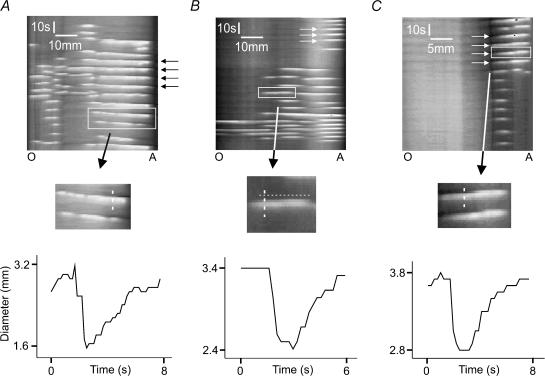
Figure 5. Short length (SL) contractions propagate both anally and orally in duodenum and jejunum.
The upper panel of A shows anally propagating SL contractions (black arrows) induced by decanoic acid (1 mm) in a segment of jejunum. The middle panel shows an expanded image of the region within the box in the upper panel. The bottom panel tracks the diameter at a single point shown by the dashed line. In this case, the constriction begins in the middle of the segment, is clearly preceded by a dilatation and propagates anally, although it does not reach the anal end. In B, orally propagating SL contractions (box) and stationary contractions (small arrows) can be seen in the jejunum and in this case the SL contractions are not accompanied by a dilatation. The horizontal dashed line in the middle panel provides a reference to allow the propagation of the constriction to more oral points to be clearly seen. In C, a band of orally propagating SL contractions in the duodenum can be seen (small arrows) and these are preceded by dilatations.
Figure 6. Whole length (WL) propagating contractions in the jejunum.
A shows a spatiotemporal map of a segment of jejunum in the presence of intraluminal decanoic acid (1 mm). Anally propagating constrictions that begin at the oral end of the preparation and continue to the anal end can be readily seen (arrows). When the diameter at a single point is tracked (lower panel), the constriction is clearly preceded by a dilatation. B shows constrictions that appear first close to the anal end of a similar segment and then propagate to the oral end (arrows). These too are preceded by a localized dilatation.
One type of contraction was transient, highly localized and did not propagate within the resolution of the spatiotemporal maps (Fig. 4) or when the frame rate was increased to 10 Hz. These contractions were associated with small intraluminal pressure changes, but could only be reliably distinguished from other types of contractions using the video-analysis method. These ‘stationary contractions’ were seen in all preparations that responded to decanoic acid.
Decanoic acid also induced contractions that sometimes appeared towards the centre of the preparation and then propagated either orally or anally, with the amplitude decreasing over 1–2 cm so they disappeared before reaching the end of the segment (Fig. 5). We refer to these as short length propagating contractions (SL contractions) and treat the orally and anally propagating forms as separate.
Two other motor patterns were evoked by luminal decanoic acid, these appeared at either the oral or the anal end of the preparation and propagated to the other end (Fig. 6). These have been termed whole length propagating contractions (WL contractions) with orally propagating and anally propagating forms being distinguishable.
The action of decanoic acid was concentration-dependent, as the number of preparations of duodenum and jejunum that responded with contractions increased as the luminal concentration of decanoic acid increased from 100 μm to 1 mm, the maximum effective concentration. For example, 0.1 mm decanoic acid induced motor activity in 4 of 13 preparations of duodenum, but 1 mm decanoic acid induced activity in 23 of 26 duodenal preparations. It was also partially region specific; 1 mm decanoic acid evoked activity in 88% (23/26) of duodenal and 92% (24/26) of jejunal preparations, but in only 55% (12/22) of ileal preparations (P < 0.01 comparing duodenum and ileum, χ2 test 1 d.f).
Time course of motor activity induced by decanoic acid
In duodenum and jejunum, motor activity appeared after 20–30 min of exposure to decanoic acid and then occurred in 14–120 s episodes separated by 30–380 s of quiescence (Fig. 3). The mean duration of contraction episodes was similar in the duodenum (48 ± 4 s, n = 6) and jejunum (51 ± 7 s, n = 6, P > 0.1). The intervals between episodes were shorter in the duodenum (79 ± 21 s, n = 6) than in the jejunum (119 ± 49 s, n = 6, P < 0.05). The pattern of alternating motor activity and quiescence continued throughout a 2 h exposure to decanoic acid.
The motor activity of the ileum took longer to manifest (up to 60 min) and died out within 60 min. In this region, the duration of contraction episodes was 48 ± 11 s and the interval between episodes was 93 ± 31 s (n = 4).
Properties of the stationary contractions
Comparisons of the properties of the stationary contractions were made with 1 mm decanoic acid. Stationary contractions (Fig. 4B) were observed in all preparations that responded. The mean length of intestine that contracted was identical in the jejunum (4.2 ± 2.2 mm, n = 16) and ileum (4.2 ± 1.5 mm, n = 6), but significantly longer in the duodenum (5.6 ± 1.9 mm, n = 20, P < 0.025). Individual contractions lasted 3–7 s in all regions and durations did not differ between regions. The minimum diameters seen during stationary contractions were similar in the jejunum (1.9 ± 0.4 mm, n = 16) and ileum (2.0 ± 0.2, n = 6, P < 0.2) and in these regions also did not differ significantly from those seen during persistent propulsive contractions (jejunum 1.8 ± 0.2 mm, n = 16, P > 0.1; ileum 1.7 ± 0.2 mm, n = 6, P > 0.05). The minimum diameter seen during stationary contractions of the duodenum (2.4 ± 0.6 mm, n = 20) was significantly greater than in other intestinal regions (P < 0.05) and than that seen during persistent propulsive contractions in the same preparations (1.8 ± 0.4 mm, n = 20, P < 0.02).
In most cases, stationary contractions were repeated at the same site several times during individual contraction episodes, so they appeared as vertical bands in the spatiotemporal maps (Fig. 4A). Such bands occurred in 87% (20 of 23) of duodenal preparations, 67% (16 of 24) of jejunal preparations and 50% of ileal preparations (6 of 12). Bands of stationary contractions were often seen at more than one site in a segment (Fig. 1) with the duodenum and ileum having fewer contraction sites (2.0 ± 0.3, n = 20; and 2.5 ± 1.2, n = 6, respectively) than the jejunum (3.4 ± 1.2, n = 16, P < 0.01). The frequency of contractions within bands did not differ between regions (duodenum 10.8 ± 4.2 min−1, jejunum 9.6 ± 6.0 min−1, ileum 9.6 ± 5.4 min−1).
Properties of SL (short length propagating) contractions
SL contractions were seen in all preparations of duodenum (n = 23) and jejunum (n = 24) that responded to 1 mm decanoic acid. Most exhibited both anally (duodenum 21, jejunum 24) and orally (duodenum 23, jejunum 21) propagating contractions. Eleven of the 12 responsive ileal preparations had both anally and orally propagating SL contractions. In some cases, SL contractions propagating both orally and anally from a single point were observed (duodenum 10, jejunum 12, ileum 3). The minimum diameters seen in SL contractions did not differ between the orally propagating and anally propagating forms or between regions (duodenum 2.1 ± 0.5 mm, jejunum 1.8 ± 0.4 mm, ileum 1.8 ± 0.2 mm, n = 6 for each, P > 0.4) and were the same as those seen during the persistent propagating contractions in these regions.
Anally directed SL contractions were always preceded by a dilatation, but such dilatations were less common with the orally directed SL contractions (Fig. 5) (4 of 15 preparations of duodenum, 5 of 15 preparations of jejunum analysed).
SL contractions were often initiated repeatedly at a single site so they appeared as bands in the spatiotemporal maps (Fig. 5). Bands of anally propagating SL contractions were more common than bands of orally propagating SL contractions (P < 0.01, χ2 test 1 d.f). Bands of anally propagating SL contractions were seen in 15 of 23 preparations of duodenum, but only 10 had bands of propagating SL contractions (3 had no bands of propagating SL contractions). These proportions were similar in the jejunum (13 anal, 7 oral, n = 24). Only anally propagating SL contractions formed bands in the ileum (5 of 12 preparations).
The frequency of contractions within bands of orally propagating SL contractions was very similar in the duodenum (14.5 ± 5.4 min−1) and jejunum (14.8 ± 5.8 min−1). The frequency was lower within bands of anally propagating SL contractions (duodenum 8.9 ± 4.4 min−1, jejunum 10.6 ± 7.7 min−1), but the difference was only statistically significant in the duodenum (P < 0.05).
Propagation speeds of orally and anally propagating SL contractions did not differ significantly within, or between, regions (duodenum oral 7.2 ± 0.6 mm s−1, anal 7.1 ± 1.6 mm s−1; jejunum oral 6.4 ± 1.5 mm s−1, anal 5.8 ± 5.3 mm s−1; ileum oral 8.5 ± 2.3 mm s−1, anal 6.3 ± 0.7 mm s−1; n = 6 in each case, P > 0.05). However, they were substantially lower than the propagation speeds of either persistent or transient propulsive contractions evoked by distension (see above) or the WL contractions evoked by decanoic acid (Table 1).
Table 1.
A comparison of the propagation speeds of SL, WL, transient and persistent propulsive contractions in duodenum, jejunum and ileum (mean ±s.e.m., mm s−1)
| SL sustained | WL | Transient | ||||
|---|---|---|---|---|---|---|
| Anal | Oral | Anal | Oral | Anal | Anal | |
| Duodenum | 7.0 ± 0.4† | 7.0 ± 0.3† | 17.9 ± 1.0 | 20.8 ± 1.8 | 15.9 ± 0.7 | 16.1 ± 1.0 |
| Jejunum | 5.8 ± 0.5† | 7.4 ± 0.5† | 15.9 ± 1.1 | 20.8 ± 0.8 | 18.7 ± 1.2 | 18.7 ± 0.7 |
| Ileum | 6.5 ± 0.3*† | 8.4 ± 0.6† | 15.3 ± 1.0 | 14.9 ± 1.1 | 15.2 ± 1.0 | |
Significance at P < 0.01 for comparison between SL and WL, transient or sustained contractions;
significance at P < 0.01 for comparison between anal and oral SL contractions.
Properties of WL (whole length propagating) contractions
Anally directed WL contractions were seen in all preparations with anally directed SL contractions, but differed markedly from the latter in their rates of propagation (Table 1). Orally propagating WL contractions were significantly rarer in each intestinal region (13 of 23 (56%) duodenum, 7 of 24 (29%) jejunum, 1 of 12 (8%) ileum). Both types of WL contractions were always preceded by a dilatation (Fig. 6).
Both types of WL contractions were grouped together in bands similar to those seen with SL and stationary contractions. The frequency of contractions within these bands was substantially lower than within bands of SL contractions and did not differ between regions or between orally and anally propagating WL contractions (duodenum oral 9.8 ± 3.2 min−1, anal 7.1 ± 1.2 min−1; jejunum oral 6.0 ± 2.4 min−1, anal 7.5 ± 0.1 min−1; n = 5 in each case, P > 0.08).
The propagation speeds of anally directed WL contractions (duodenum 17.9 ± 2.4 mm s−1, jejunum 15.9 ± 2.7 mm s−1, ileum 15.3 ± 2.3 mm s−1) did not differ (P > 0.05 in each case) from those of transient and persistent propulsive contractions evoked by distension of the same regions (Table 1). The propagation speed of orally directed WL contractions in the duodenum (20.8 ± 3.1 mm s−1) and jejunum (20.8 ± 1.4 mm s−1) was higher, but this was only significant for the jejunum (P < 0.05).
Pharmacology of motor patterns induced by decanoic acid
Because the effects of decanoic acid were less robust in the ileum, pharmacological studies were only undertaken in the duodenum and jejunum.
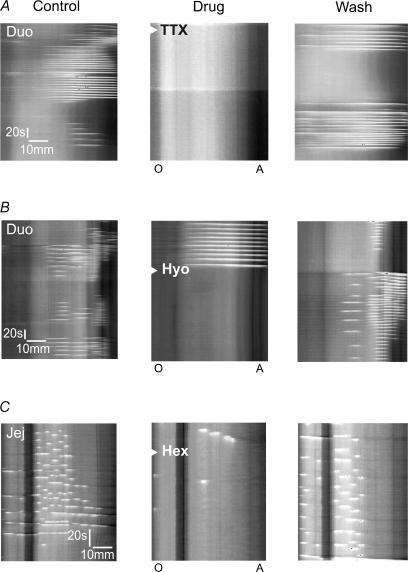
TTX (3 μm) in the superfusate completely, and reversibly, abolished the contraction bursts evoked in either duodenum (n = 6) or jejunum (n = 7) by luminal decanoic acid (1 mm) (Fig. 7A). These motility patterns were also abolished by the muscarinic antagonist hyoscine (1 μm) or the nicotinic antagonist hexamethonium (100 μm) when either was added to the superfusate (Fig. 7B and C). By contrast, the P2 antagonist PPADS (60 μm) had no significant effect on any of the measured parameters of the motor patterns evoked by decanoic acid.
Figure 7. Effects of blockade of neural activity, muscarinic transmission and nicotinic transmission on decanoic acid induced motor activity in the duodenum and jejunum.
The left hand panel of A shows decanoic acid induced contractions in the duodenum, these were abolished by tetrodotoxin (3 μm applied at the arrow head in the central panel) and the effects of tetrodotoxin reversed after 30 min washout (right panel). B shows a similar experiment in which muscarinic receptors were blocked by bath application of hyoscine (1 μm, arrowhead in middle panel), which then reversed after 1 h of washout (right panel). In C, contractions induced by decanoic acid in the jejunum (left panel) are blocked by hexamethonium (100 μm, arrowhead central panel), which then reversed on washout (right panel).
Discussion
The results described here indicate that luminal decanoic acid can trigger a suite of motor patterns in vitro that resemble the fed state seen in the same intestinal regions in vivo. The stationary contractions and orally and anally propagating SL contractions appear to be analogues of segmentation and are only seen in these preparations in the presence of decanoic acid. The anally propagating WL contractions are analogous to peristalsis, while orally propagating WL contractions may be equivalent to retropulsion. These motor patterns are episodic and, in the duodenum and jejunum, contraction episodes are repeated regularly as long as decanoic acid is in the lumen. They depend on neural activity in cholinergic pathways. The results also highlight significant regional differences in the responses of the small intestine to different stimuli, with the ileum responding preferentially to distension and the duodenum to the fatty acid.
Decanoic acid induces segmentation
Stationary contractions were seen in every response to decanoic acid, but never in response to distension. These contractions appear simultaneously in a confined region, typically 4–5 mm in length, and effectively occlude the lumen for a short period. Thus, like the stationary contractions underlying segmentation in vivo, stationary contractions induced by decanoic acid mix the intestinal contents.
The SL contractions, whether orally or anally propagating, probably serve a similar function. They occlude the lumen and these occlusions propagate slowly for 1–2 cm along the intestine and then dissipate. This matches fed state motor patterns seen in fluoroscopy studies. Neither type of SL contraction would be detected in manometry studies in vivo, because the spacing of recording sites is rarely less than 1 cm (Andrews et al. 2001) so SL contractions would never be seen at three adjacent sites sequentially and only rarely at two adjacent sites. Thus, they would be classed as stationary contractions in such studies.
Orally propagating WL contractions probably correspond to the retrograde pressure complexes seen during intralipid infusions in vivo (Andrews et al. 2001). The relatively low incidence of these contractions compared to anally directed WL contractions is consistent with this as retrograde pressure complexes (retropulsion) are less common than anterograde complexes (peristalsis) in vivo.
The anally propagating WL contractions evoked by decanoic acid were indistinguishable from the transient propulsive contractions evoked by step increases in intraluminal pressure. Thus, decanoic acid and distension induce a common motor pattern, but the fatty acid also induces several extra patterns.
Mechanisms responsible for decanoic acid-induced motor patterns
The segmentation induced by luminal decanoic acid is probably regulated by one or more motor pattern generators. However, the question remains as to whether these are located within the enteric nervous system or depend on the intrinsic pacemakers of the intestinal smooth muscle, the interstitial cells of Cajal. Several pieces of evidence in this and previous studies shed some light on this.
The abolition of the motor activity evoked by luminal decanoic acid by TTX indicates that these contractions depend on neural activity. Muscarinic blockade also abolishes this motor activity, which suggests that cholinergic motor neurones are a common element. As hexamethonium has a similar effect, nicotinic synapses within the intestinal wall are also critical. On the other hand, PPADS, at a concentration (60 μm) that blocks P2X-mediated transmission to inhibitory motor neurones and inhibitory junction potentials in the circular muscle of the ileum (Bian et al. 2000), has no effect suggesting that P2-mediated transmission is not involved. Thus, activity in at least two populations of enteric neurones is needed for the response to decanoic acid. However, it is possible that neural activity merely exerts a permissive effect and the intrinsic pacemakers of the muscle set the timing within the bands of stationary and SL contractions that make up the segmentation response to the fatty acid.
Bercik et al. (2000) used video-imaging to analyse contractile patterns in the arterially perfused rat ileum in vitro and detected one to three localized pacemaker sites in each segment. These produced slowly propagating contractions in the presence of TTX. Their interpretation was that the segments contained a few dominant pacemaker sites and that the propagating contractions were due to propagating slow waves. Similar findings have been obtained using video-mapping in guinea-pig proximal colon (D'Antona et al. 2001). This mechanism might account for the present finding that stationary contractions and SL contractions appear confined to one or more specific locations within a segment. Further, the slowly propagating contractions arising from myogenic pacemakers are superficially similar to the SL contractions evoked by decanoic acid. However, there are key differences. These include the observation that SL contractions are lumen-occluding over roughly 5 mm at a time, they are blocked by TTX, hyoscine or hexamethonium and they are only seen when decanoic acid is in the lumen.
The critical issue, however, is the timing of contractions within the bands of stationary and SL contractions. Slow waves are difficult to study in the guinea-pig small intestine, but there have been some reports, the most detailed of which is that by Smith (1989), which found that the slow wave frequency in the ileum was 16.4 per minute. This is distinctly different from the frequency within bands of stationary contractions or bands of SL contractions in any region examined above. Donnelly et al. (2001) reported that the frequency of slow waves in guinea-pig duodenum was 19 min−1 and 11 min−1 in the jejunum. The latter matches the frequencies of stationary contractions (10 min−1) and anally propagating SL contractions (11 min−1) in the jejunum, but the former clearly differs from what is seen in the duodenum (stationary 11 min−1, anally propagating SL 9 min−1). Thus, pacemakers intrinsic to the muscle may not determine the timing of segmentation induced by nutrient, at least in guinea-pig. This needs to be confirmed by simultaneous recording of slow waves and segmentation in the same preparations.
If slow waves do not underlie the motor patterns induced by decanoic acid, then this raises many questions about the enteric neural circuitry that the current literature is ill-equipped to answer. The anally propagating WL contractions are the only component of these motor patterns about which there is much evidence to identify an underlying neural mechanism. Because they are indistinguishable from the transient and persistent propulsive contractions evoked by distension, the same neural circuit may mediate all three types of behaviour, although the initiating mechanism clearly differs. The enteric circuitry is able to sustain anally propagating contraction waves (descending excitation) independent of the movement of intestinal content (Brookes et al. 1999,2001; Spencer et al. 1999,2000,2001; Monro et al. 2002) However, Monro et al. (2002) found that descending excitation is not sensitive to hexamethonium, but is depressed by PPADS, and Spencer et al. (2000) found an intermittent sensitivity to either antagonist. Thus, the pharmacology of the anally propagating WL contractions, which are blocked by hexamethonium and insensitive to PPADS, is distinctively different from that of descending excitation. This may reflect the feedback from the muscle which underpins descending excitation (Spencer et al. 2001) and would be enhanced when the intestinal tube is intact and contains a liquid.
Role of longitudinal muscle
Length changes in the longitudinal muscle can be estimated from the spatiotemporal maps using features that increase the intestinal diameter: Peyer's patches and/or attached mesentery. These appear in the maps as vertical dark lines (e.g. Figs 3A and 6A) and local movements of the longitudinal muscle during the decanoic acid induced motor patterns can be estimated from the horizontal deflections of these lines during motor activity. These movements in the presence of decanoic acid were typically no more than 1–2 mm, and were associated with constrictions of the circular muscle. They had little effect on either the speed of propagation or distance covered by SL or WL contractions.
Regional specialization
This study reveals clear regional differences in the responses of the guinea-pig small intestine to luminal stimuli, extending the study of Schulze-Delrieu (1991) who found that propulsive contractions in the jejunum were smaller than those in the ileum. The duodenum and upper jejunum respond robustly to luminal decanoic acid, but the ileum responded more slowly, less reliably and for shorter intervals. By contrast, both transient and persistent propulsive contractions evoked by increasing luminal pressure (distension) were substantially larger and were evoked by smaller pressure increases in the ileum than in the duodenum and jejunum. These differences fit with function in that the upper small intestine is largely involved in digestion and nutrient absorption, which depends on segmentation, and the lower small intestine is more involved in storage, water reabsorption and propulsion.
Conclusions
Computer analysis of video images of the intestine in vitro reveals that infusion of a fatty acid, decanoic acid, into the lumen initiates motor patterns similar in form to the fed state in vivo. These include contractions that are stationary or propagate slowly over very short distances, patterns that are analogous to peristalsis (anally propagating WL contractions) and patterns analogous to retropulsion (orally propagating WL contractions). Thus, the preparations described here represent the first quantifiable in vitro models of the fed state, and in particular nutrient-induced segmentation, which have been described. Generation of these patterns depends on neural activity. Further analysis of the mechanisms underlying decanoic acid induced motor activity of the upper small intestine may cast light on that most enigmatic of intestinal motor behaviours, segmentation.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia (114103), the Melbourne Research Grant Scheme and the Swedish Medical Research Council (8288).
References
- Andrews JM, Doran SM, Hebbard GS, Malbert CH, Horowitz M, Dent J. Nutrient-induced spatial patterning of human duodenal motor function. Am J Physiol Gastrointest Liver Physiol. 2001;280:G501–G509. doi: 10.1152/ajpgi.2001.280.3.G501. [DOI] [PubMed] [Google Scholar]
- Annese V, Bassotti G, Napolitano G, Usai P, Andriulli A, Vantrappen G. Gastrointestinal motility disorders in patients with inactive Crohn's disease. Scand J Gastroenterol. 1997;32:1107–1117. doi: 10.3109/00365529709002989. [DOI] [PubMed] [Google Scholar]
- Benard T, Bouchoucha M, Dupres M, Cugnenc P-H. In vitro analysis of rat intestinal wall movements at rest and during propagated contraction: a new method. Am J Physiol. 1997;273:G776–G784. doi: 10.1152/ajpgi.1997.273.4.G776. [DOI] [PubMed] [Google Scholar]
- Bercik P, Bouley L, Dutoit P, Blum A-L, Kucera P. Quantitative analysis of intestinal motor patterns: spatiotemporal organization of nonneural pacemaker sites in the rat ileum. Gastroenterology. 2000;119:386–394. doi: 10.1053/gast.2000.9306. [DOI] [PubMed] [Google Scholar]
- Bian X-C, Bertrand PP, Bornstein JC. Descending inhibitory reflexes involve P2X receptor-mediated transmission from interneurons to motor neurons in guinea-pig ileum. J Physiol. 2000;528:551–560. doi: 10.1111/j.1469-7793.2000.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgstrom S, Arborelius M., Jr Influence of a fatty acid on duodenal motility. Scand J Gastroenterol. 1975;10:599–601. [PubMed] [Google Scholar]
- Brookes SJH, Chen BN, Costa M, Humphreys CMS. Initiation of peristalsis by circumferential stretch of flat sheets of guinea-pig ileum. J Physiol. 1999;516:525–538. doi: 10.1111/j.1469-7793.1999.0525v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJH, D'Antona G, Zagorodnyuk VP, Humphreys CMS, Costa M. Propagating contractions of the circular muscle evoked by slow stretch in flat sheets of guinea-pig ileum. Neurogastroenterol Mot. 2001;13:519–531. doi: 10.1046/j.1365-2982.2001.00290.x. [DOI] [PubMed] [Google Scholar]
- Cannon WB. Peristalsis, segmentation and the myenteric reflex. Am J Physiol. 1912;30:114–128. [Google Scholar]
- D'Antona, Hennig GW, Costa M, Humphreys CMS, Brookes SJH. Analysis of motor patterns in the isolated guinea-pig large intestine by spatio-temporal maps. Neurogastroenterol Mot. 2001;13:483–492. doi: 10.1046/j.1365-2982.2001.00282.x. [DOI] [PubMed] [Google Scholar]
- Defilippi C, Gomez E. Effect of casein and casein hydrolysate on small bowel motility and d-xylose absorption in dogs. Neurogastroenterol Mot. 1995;7:229–234. doi: 10.1111/j.1365-2982.1995.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Donnelly G, Jackson TD, Ambrous K, Ye J, Safdar A, Farraway L, Huizinga JD. The myogenic component in distention-induced peristalsis in the guinea pig small intestine. Am J Physiol. 2001;280:G491–G500. doi: 10.1152/ajpgi.2001.280.3.G491. [DOI] [PubMed] [Google Scholar]
- Hennig GW, Costa M, Chen BG, Brookes SJH. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol. 1999;517:575–590. doi: 10.1111/j.1469-7793.1999.0575t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinke O, Ehrlein HJ. Effect of oleic acid on canine gastroduodenal motility, pyloric diameter and gastric emptying. Q J Exp Physiol. 1983;68:675–686. doi: 10.1113/expphysiol.1983.sp002757. [DOI] [PubMed] [Google Scholar]
- Monro RL, Bertrand PP, Bornstein JC. ATP and 5-HT are the principal neurotransmitters in the descending excitatory reflex pathway of the guinea-pig ileum. Neurogastroenterol Mot. 2002;14:255–264. doi: 10.1046/j.1365-2982.2002.00325.x. [DOI] [PubMed] [Google Scholar]
- Schemann M, Ehrlein HJ. Postprandial patterns of canine jejunal motility and transit of luminal content. Gastroenterology. 1986;90:991–1000. doi: 10.1016/0016-5085(86)90878-4. [DOI] [PubMed] [Google Scholar]
- Schmid HR, Ehrlein HJ. Effects of enteral infusion of hypertonic saline and nutrients on canine jejunal motor patterns. Dig Dis Sci. 1993;38:1062–1072. doi: 10.1007/BF01295722. [DOI] [PubMed] [Google Scholar]
- Schulze-Delrieu K. Intrinsic differences in the filling response of the guinea pig duodenum and ileum. J Laboratory Clin Med. 1991;117:44–50. [PubMed] [Google Scholar]
- Siegle ML, Ehrlein HJ. Digestive motor patterns and transit of luminal contents in canine ileum. Am J Physiol. 1988;254:G552–G559. doi: 10.1152/ajpgi.1988.254.4.G552. [DOI] [PubMed] [Google Scholar]
- Smith TK. Spontaneous junction potentials and slow waves in the circular muscle of isolated segments of guinea-pig ileum. J Auton Nerv Syst. 1989;27:147–154. doi: 10.1016/0165-1838(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Sanders KM, Smith TK. Migrating motor complexes do not require electrical slow waves in the mouse small intestine. J Physiol. 2003;533:881–893. doi: 10.1113/jphysiol.2003.049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Smith CB, Smith TK. Role of muscle tone in peristalsis in guinea-pig small intestine. J Physiol. 2001;530:295–306. doi: 10.1111/j.1469-7793.2001.0295l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N, Walsh M, Smith TK. Does the guinea-pig ileum obey the ‘law of the intestine’? J Physiol. 1999;517:889–898. doi: 10.1111/j.1469-7793.1999.0889s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Walsh M, Smith TK. Purinergic and cholinergic neuro-neuronal transmission underlying reflexes activated by mucosal stimulation in the isolated guinea-pig ileum. J Physiol. 2000;522:321–331. doi: 10.1111/j.1469-7793.2000.t01-1-00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuneberg L, Peters S. Toward a concept of stretch-coupling in smooth muscle. I. Anatomy of intestinal segmentation and sleeve contractions. Anat Rec. 2001;262:110–124. doi: 10.1002/1097-0185(20010101)262:1<110::AID-AR1016>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]