Abstract
The whole-cell perforated patch clamp technique was used to study membrane currents in isolated rabbit corpus cavernosum smooth muscle cells. Depolarization from −80 mV to the range −40 to −10 mV evoked a nifedipine-sensitive Ca2+ current that was followed by a slower inward current that activated over several hundred milliseconds. The slow current reversed near the Cl− equilibrium potential (ECl) and was reduced by anthracene-9-carboxylic acid (A9C; 1 mm) and niflumic acid (100 μm), suggesting that it was a Ca2+-activated Cl− current. When held constantly at −60 mV, over 70% of cells fired spontaneous transient inward currents (STICs), the amplitudes of which were reduced by A9C and niflumic acid. STICs reversed near ECl in a symmetrical Cl− gradient and when [Cl−]o was substituted with glutamate or I−, the reversal potential shifted to more positive or more negative values, respectively, confirming that STICs were mediated by Cl− channels. STICS were also blocked by cyclopiazonic acid, 2-aminoethoxydiphenyl borate (2-APB) and 2-nitro-4-carboxyl-N,N-diphenylcarbamate (NCDC), suggesting that they depended on IP3-mediated Ca2+-release from the sarcoplasmic reticulum. Modulation by the NO–cGMP pathway was investigated by applying nitrosocysteine, 3-(5-hydroxymethyl-2-furyl)-1-benzyl indazole (YC-1), and 8-bromo cGMP, all three of which abolished STIC activity. YC-1 also reduced noradrenaline-evoked inward currents, but had no effect on similar currents evoked by caffeine, suggesting that cGMP selectively inhibited IP3-mediated Ca2+ release. We propose that Ca2+-activated Cl− currents underlie detumescent tone in the corpus cavernosum, and that modulation of this mechanism by the NO–cGMP pathway is important during penile erection.
Penile erection depends on decreased penile vascular resistance with resultant increased penile blood flow. It is generally agreed that these events depend not only on relaxation of the penile helicine arteries, but also on the corporeal smooth muscle that lines the cavernosal spaces (Andersson & Wagner, 1995). Just as erection depends on relaxation, detumescence depends on contraction of the corporal smooth muscle, which restricts blood flow to the erectile tissue. Although many factors have been shown to influence the contractile state of this smooth muscle, the physiological processes that regulate both the contraction and relaxation are incompletely understood (Andersson & Wagner, 1995; Andersson, 2001). Contraction is generally believed to depend on tonic activity in adrenergic nerves (Andersson, 2001), but isolated strips of corpus cavernosum develop dihydropyridine-sensitive spontaneous contractions, suggesting an underlying myogenic mechanism is also involved (Christ et al. 1990; Hoppner et al. 1996). Many inhibitory mechanisms affect the muscle, but since the discovery of a nitrergic innervation (Ignarro et al. 1990), the NO–cGMP pathway has been believed to be the most important (Andersson, 2001). Indeed, knowledge of this pathway has been successfully exploited in the use of sildenafil (Viagra) and other phosphodiesterae 5 inhibitors for treatment of erectile dysfunction. It may seem surprising therefore that the intracellular targets of cGMP in the corpus cavernosum have only been partly determined and are not well understood (Hedlund et al. 2000; Andersson, 2001).
Recently, a role for a Ca2+-activated Cl− current in generating spontaneous myogenic tone in the cavernosum was proposed (Karkanis et al. 2003). Under voltage clamp conditions, it was shown that rat and human corpus cavernosum cells fired spontaneous transient inward currents (STICs) that appeared to be mediated by the Cl− current. Under physiological (non-voltage clamped) conditions, the STICs would be expected to depolarize the cells into the range where voltage-dependent Ca2+ channels open, thus increasing Ca2+ influx. One possible mode of action of the NO–cGMP pathway may therefore be to reduce either the frequency or amplitude of the STICs. Despite this, at present there have been no reports of the effect of nitric oxide donors on STICs in the cavernosum, and an attempt to demonstrate effects of the cell-permeant cGMP anologue 8-bromo-cGMP proved equivocal, with little effect observed unless it was combined with 8-bromo-cAMP (Karkanis et al. 2003). The purpose of the present study was firstly to characterize the Cl− current in isolated rabbit corpus cavernosum cells, secondly to study the mechanism underlying STICs in these cells, and thirdly to determine the effects of an NO donor and cGMP on STICs. Although Cl− currents have not been previously demonstrated in the rabbit corpus cavernosum, we chose this preparation because the rabbit is a widely used animal model in contractile studies (Ignarro et al. 1990; Holmquist et al. 1992; Cellek & Moncada, 1997) and its cavernosum is large enough to yield a reasonable number of viable cells for study with the patch clamp technique.
Methods
Cell isolation
All procedures were carried out in accordance with current UK legislation. Male New Zealand white rabbits (>2.5 kg) were humanely killed with a lethal injection of pentobarbitone (i.v.) and the penis was removed. The tunica albugenia was opened bilaterally to expose both corpora cavernosa and these were carefully removed, placed in Hanks' Ca2+-free solution (see below) and cut in to 1 mm3 pieces. The pieces were stored in Hanks' Ca2+-free solution for 30 min before they were incubated in an enzyme medium containing (per 5 ml of Hanks' Ca2+-free solution): collagenase 15 mg (Sigma type 1a), protease 1 mg (Sigma type XXIV), BSA 10 mg (Sigma) and trypsin inhibitor 10 mg (Sigma) for 5–10 min at 37°C. They were then placed in Hanks' Ca2+-free solution and stirred for a further 5–10 min to release cells. Several drops of the cell suspension were added to Petri dishes containing Hanks' solution (100 μm Ca2+) and stored at 4°C for use within 8 h. To minimize use of animals, some experiments utilized cells that were dispersed from whole corpus cavernosum tissue that had been stored at 4°C overnight. The results from these experiments were indistinguishable from those obtained from fresh tissue.
Electrophysiological recording
Recordings were made using the amphotericin B perforated patch method (Rae et al. 1991). After gigaseals were obtained the series resistance fell over a 10–15-min period to 10–15 MΩ and remained stable for up to 1 h. Although amphotericin pores are cation selective, the permeability for Cl− is not negligible (Kleinberg & Finkelstein, 1984), so the reversal potential of Cl− current is determined by the bath and pipette Cl− concentrations (Horn & Marty, 1988). Where stated, reversal potential data were corrected for junction potentials of −3 mV in PSS and +2 mV in low-chloride solution according to the method described by Neher (1992). Voltage-clamp commands were delivered with an Axopatch 1D patch-clamp amplifier (Axon Instruments) and currents were recorded by means of a 12 bit AD/DA converter (Labmaster, Scientific Solutions) interfaced to an Intel computer running pCLAMP software (Axon Instruments).
Solutions and drugs
The following solutions were used (concentrations mm): Hanks' Ca2+-free solution (solution 1; for cell dispersal): 141 Na+, 5.8 K+, 130.3 Cl−, 15.5 HCO3−, 0.34 HPO42−, 0.44 H2PO4−, 10 dextrose, 2.9 sucrose, 10 Hepes (Melford) (pH adjusted to 7.4 with NaOH); PSS (solution 2): 130 Na+, 5.8 K+, 135 Cl−, 4.16 HCO3−, 0.34 HPO32−, 0.44 H2PO4−, 1.8 Ca2+, 0.9 Mg2+, 0.4 SO42−, 10 dextrose, 2.9 sucrose, 10 Hepes (pH adjusted to 7.4 with NaOH); Cs+ pipette solution (solution 3): 133 Cs+, 1 Mg2+, 135 Cl−, 0.5 EGTA (Sigma), 10 Hepes (pH adjusted to 7.2 with CsOH); low-chloride solution (solution 4): similar to solution 2 but with 86 glutamate and 49 Cl−, instead of 135 Cl−; iodide-substituted solution (solution 5): similar to solution 2 but with 135 I−, instead of 135 Cl−.
S-Nitroso-l-cysteine (nitrosocysteine) was synthesized by reacting l-cysteine with sodium nitrite under acidic conditions, as previously described (Field et al. 1978; Kowaluk & Fung, 1990). Reactant solution contained 1 n HCl (5 ml; Reidel-deHaen), MeOH (5 ml; Laboratory-Scan), 37 n H2SO4 (0.5 ml; BDH), sodium nitrite (10 mm; BDH) and l-cysteine (5 mm; BDH). In control experiments, dilutions of the reactants (containing all of the reactants except l-cysteine) equivalent to nitrosocysteine (16 μm) had no effect on the spontaneous Cl− currents.
Other drugs were used were as follows: caffeine (Sigma), anthracene-9-carboxylic acid (A9C; Sigma), niflumic acid (Sigma), 2-nitro-4-carboxyl-N,N-diphenylcarbamate (NCDC; Sigma), 2-aminoethoxydiphenyl borate (2APB; Acros), cyclopiazonic acid (CPA; Calbiochem) (3-(5-hydroxymethyl-2-furyl)-1-benzyl indazole (YC-1) and nifedipine (Bayer).
During experiments the dish containing the cells was superfused with PSS (solution 2). In addition, the cell under study was continuously superfused with PSS by means of a close delivery system consisting of a pipette (tip diameter 200 μm) placed approximately 300 μm away. This could be switched, with a dead space time of around 10 s, to a solution containing a drug. All experiments were carried out at 37°C. To deliver nitrosocysteine a variation in the normal procedure was necessary. This substance is stable in acidic stock solution, but breaks down with a half-life of ∼10 s (Kowaluk & Fung, 1990) in physiological saline. To deliver it to a cell firing STICs, 2 μl of 160 mm stock was rapidly mixed with 20 ml of PSS in a primed channel of the drug delivery system, thus providing a nominal final concentration of 16 μm. The actual concentration seen by the cell will be somewhat lower, depending on the time to mix the solution and the dead space time of the delivery system. We estimate that when the solution first reaches the cell the concentration will probably be less than 8 μm and this will continue to fall throughout the period of drug perfusion.
Statistics and analysis
Data are presented as the mean ±s.e.m., and statistical differences were compared using Students paired t test, taking the P < 0.05 level as significant. Throughout, n refers to the number of cells in each experimental series. In each case n was obtained from a minimum of 3 animals.
Results
Evoked Cl− currents
The majority of (80–90%) dispersed cells observed with bright field microscopy were spindle shaped, unbranched and contracted on application of caffeine or deliberate disruption of the cell membrane with a patch pipette. These were typical of smooth muscle cells described elsewhere in other tissues (Sanders, 1989). In addition to smooth muscle cells, a second less abundant (10–20%) cell type was observed. These were highly branched and failed to contract under the above conditions, resembling the interstitial cells previously observed in the rabbit urethra (Sergeant et al. 2000). However, for the purposes of the present study, we confined our recordings to typical smooth muscle cells.
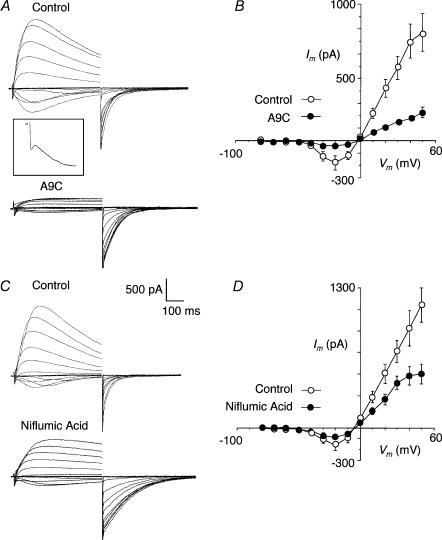
When cells were held at −80 mV and subjected to depolarizing potentials ranging from −70 to +50 mV, currents typical of those shown in Fig. 1A were evoked. These consisted of a transient inward current, similar in time course to the L-type Ca2+ current, followed by a large slowly developing inward current (Fig. 1A, inset) that reversed near 0 mV to give large, non-rectifying outward currents. In contrast to Cl− currents previously reported for rabbit and sheep urethra (Cotton et al. 1997; Sergeant et al. 2000), in most cells, these slow currents gradually decayed during the 500 ms depolarizing pulse to approximately 50% of their peak value. On repolarization to −80 mV inward tail currents were observed. A summary of the mean current–voltage (I–V) plot for the slow current in eight cells is shown in Fig. 1B. In these experiments the peak slow current was measured during the 500 ms sweep, excluding the first 30 ms to allow for inactivation of the L-type Ca2+ current. This produced a ‘U’ shaped I–V curve, similar to the Ca2+-activated Cl− current recorded in the urethra (Cotton et al. 1997; Sergeant et al. 2000). The mean inward current was maximal at −20 mV and reversed at near the chloride equilibrium potential (ECl = 0 mV).
Figure 1. Current–voltage relationships recorded using CsCl filled pipettes in corpus cavernosum myocytes.
A, family of currents recorded on stepping from −80 mV to potentials of −70 to +50 mV. Inward currents could be resolved in to fast and slow components. Inset shows the step to −10 mV at an expanded scale (× 3). Outward currents were observed on stepping positive to 0 mV and large inward tails were recorded on stepping back to −80 mV after the test step. The Cl− channel blocker A9C (1 mm) reduced the slow inward currents and the outward currents and prolonged the tails. B, current–voltage (I–V) relationship for the slow currents (measured as the peak) before and after A9C (1 mm) in 8 cells. C shows the effect of another Cl− channel blocker, niflumic acid (100 μm). This drug reduced the peak slow current and prolonged the tails. D, sumary effects of niflmic acid (100 μm) in 6 cells.
We next examined the effect of two Cl− channel blockers, A9C and niflumic acid. Figure 1A shows the effect of A9C (1 mm), which reduced the slow inward and outward currents and slightly prolonged the tail currents. A summary of the effect of A9C is shown in Fig. 1B where it reduced the current throughout the voltage range (P < 0.05; n = 8). These effects were very similar to those reported previously for the effects of A9C on Cl− currents in the sheep and rabbit urethra (Cotton et al. 1997; Sergeant et al. 2000). The effect of niflumic acid (100 μm) is shown in Fig. 1C where it reduced the peak outward currents by up to 50%, although it had little effect on the sustained components of outward current and only modestly reduced the inward component. Also, it increased the tail current amplitudes and slowed their rate of decay. Both of these effects have been observed in other smooth muscles (Hogg et al. 1994; Piper et al. 2002) and are explained further in the Discussion. A summary of its effects on the peak currents evoked during test steps is shown in Fig. 1D. Although less effective than A9C, niflumic acid significantly reduced the inward current at −20 mV, and the outward currents evoked by potentials of +10 through to +50 mV (P < 0.05; n = 6).
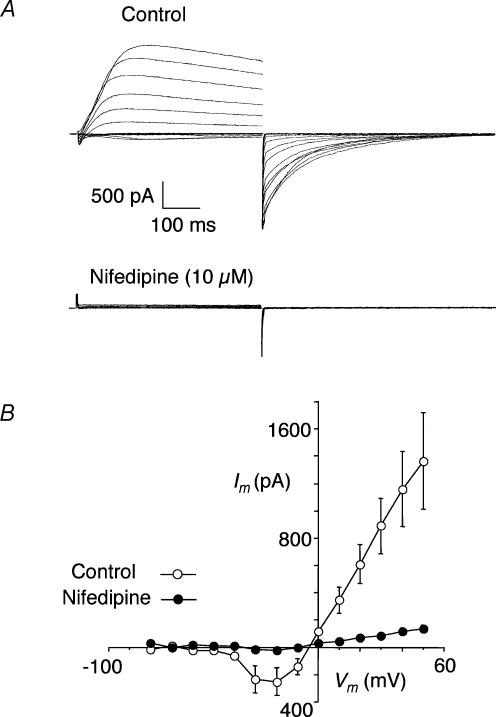
The Ca2+ dependence of the slow current was tested by using nifedipine to block Ca2+ influx during depolarization. An example is shown in Fig. 2A where 10 μm nifedipine completely blocked the initial Ca2+ current and all of the components of the slow current. A summary for four cells is presented in Fig. 2B where it is clear that nifedipine blocked the slow current at all potentials (P < 0.05).
Figure 2. Effect of nifedipine.
A, currents recorded using same protocol as in Fig. 1 before (Control) and after nifedipine (10 μm). B, summary I–V plot of the peak slow currents before and after nifedipine (10 μm) in 4 cells.
Spontaneous Cl− currents
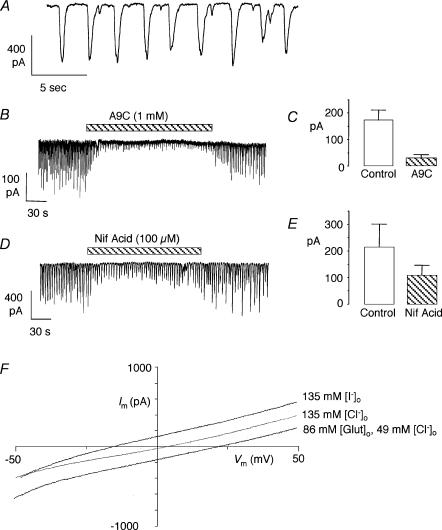
A characteristic of Ca2+-activated Cl− currents is that they can be activated by spontaneous Ca2+ release from stores to produce spontaneous transient inward currents (STICs). We found that the majority of myocytes (∼70%) in the corpus cavernosum exhibited this kind of activity. Sometimes (∼50%), the activity was irregular in amplitude and frequency, although more regular activity was also observed. An example is shown in Fig. 3A where both large (>400 pA) and small (<100 pA) STICs can be seen. Close inspection of this record reveals that the STICs were irregular in shape, often consisting of several phases. Usually, it was not possible to fit the decay phases of the STICs with simple exponential functions as described for the rabbit portal vein (Large & Wang, 1996). Figure 3B shows that STIC amplitudes were reduced with A9C (1 mm). For the purposes of summarizing these data, the average STIC amplitude in each cell was calculated for the 30 s period before application of the drug and again for a 30 s period after it had exerted its full effect. The average values were then used to calculate the means represented in Fig. 3C (n = 6). Overall, A9C reduced the amplitude of the STICs by >80% (P < 0.01), which is similar to its effects in the rabbit urethra (Sergeant et al. 2000). In contrast, niflumic acid was less effective than in the rabbit urethra. Figure 3D shows a typical example where 100 μm niflumic acid reduced STICs by approximately 50%, which is similar to its mean effect in five cells (Fig. 3E; P < 0.05). This contrasts with an 80% reduction achieved with only 10 μm of this blocker in the rabbit urethra (Sergeant et al. 2000).
Figure 3. Spontaneous currents (STICs).
A shows a record from cell firing STICs when held under voltage clamp at −60 mV. Note that the STICs were irregular in shape and amplitude. B, effect of A9C on STICs. C, summary of the effect of A9C (1 mm) on mean STIC amplitude in 6 cells. D, effect of niflumic acid on STICs. E, summary of the effect of niflumic acid (100 μm) on mean STIC amplitude in 5 cells. F, reversal potentials (Erev) of spontaneous currents in a cell subjected to ramp protocols (for details see text). Substitution of external Cl− with glutamate (Glut), an impermeant anion, shifted Erev in the positive direction, while substitution with I−, a more permeant anion than Cl−, shifted Erev in the negative direction. (Displayed data were uncorrected for junction potentials.)
To establish more clearly that the STICs were mediated by Cl− currents, their reversal potentials were examined using ramp protocols. In these experiments spontaneously active cells were repeatedly ramped from −50 to +50 mV over 300 ms, and held at 0 mV for 200 ms between ramps. When a ramp coincided with a STIC the reversal potential of the STIC could be measured. Contaminating currents (e.g. uncompensated capacitative current, leakage current, and possible voltage-activated currents) were minimized by subtracting a ‘null’ ramp from one that coincided with a STIC. Records obtained using this protocol from one cell are shown in Fig. 3F. In symmetrical Cl− concentrations ([Cl−]o = 135 mm; [Cl−]i = 135 mm) the current reversed at +3 mV (0 mV when corrected for a +3 mV junction potential) and hence corresponds to the calculated Cl− equilibrium potential (ECl = 0 mV). When [Cl−]o was reduced to 49 mm by substitution with the non-permeant anion glutamate, the reversal potential shifted to +22 (+24 mV when corrected for new junction potential), close to the new calculated value of ECl (+27 mV). When all the external Cl− was replaced with I−, an anion more permeant than Cl− through Ca2+-activated Cl− channels in smooth muscle (Large & Wang, 1996), the reversal potential shifted to −15 mV. The mean reversal potentials in five cells were −4 ± 2 mV in [Cl−]o = 135 mm, +20 ± 1 in [Cl−]o = 49 mm and −22 ± 3 in [I−]o = 135 mm. These results strongly support the idea that the STICs were mediated by Cl− channels.
Mechanisms underlying STICs
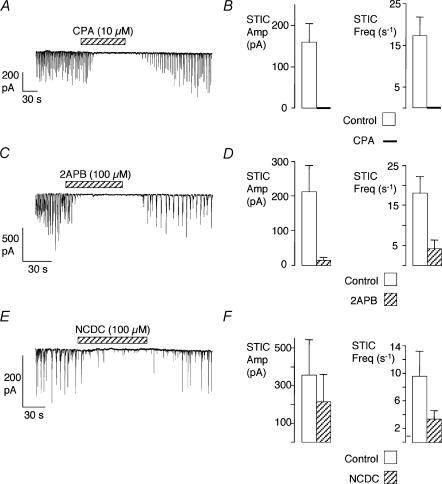
The effect of cyclopiazonic acid (CPA, 10 μm), a blocker of the sarcoplasmic Ca2+-ATPase, was examined to test the role of internal Ca2+ stores in generating STICs. Figure 4A shows a typical example where STICs were reduced in amplitude, and then abolished in the presence of this drug. Six such experiments are summarized in Fig. 4B, where it is clear that the STICs were completely inhibited (P < 0.01). Next we examined the effects of two drugs that interfere with IP3 mechanisms, namely 2APB, a blocker of IP3 receptors (IP3Rs), and NCDC, a blocker of phospholipase C (PLC). Figure 4C shows an example where 2APB (100 μm) reversibly inhibited STICs. Following wash-out the STICs returned at a reduced frequency, although their amplitude recovered completely. A summary of seven cells is presented in Fig. 4D, where both STIC amplitude and frequency were reduced by 93% (P < 0.05) and 86% (P < 0.01), respectively. NCDC had a similar effect to 2APB (Fig. 4E, F; reductions in frequency and amplitude significant, P < 0.05, n = 5). Taken together, these results suggest that Ca2+ release from IP3Rs was a requirement for STICS in these cells.
Figure 4. Effect on STICs of drugs which interfere with the SR.
A, effect of CPA on STICs in a cell held at −60 mV. B, summary of the effect of CPA (10 μm) in 6 cells. C, effect of 2APB on STICs in a cell held at −60 mV. D, summary of the effect of 2APB (100 μm) in 7 cells. E, effect of NCDC on STICs in a cell held at −60 mV. F, summary of the effect of NCDC (100 μm) in 5 cells.
Modulation of STICs by the NO–cGMP pathway
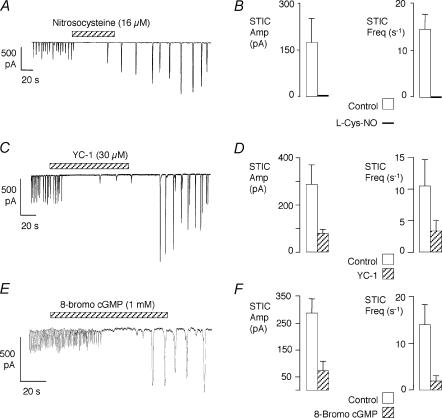
Since STICs are likely to cause cavernosum myocytes to contract (and therefore contribute to the detumescent state), it was of interest to find out if they were modulated by the NO–cGMP pathway. Firstly, the effect of the labile NO donor, nitrosocysteine, was examined. An example is shown in Fig. 5A, where nitrosocysteine completely inhibited STICs for ∼60 s, after which they returned. Interestingly, the STICs returned at a larger amplitude and lower frequency than control and this continued for about 10 min before control activity was restored. The effect of nitrosocysteine in five cells is shown in Fig. 5B. To summarize these data, a 30 s period of activity immediately before nitrosocysteine delivery was compared with the first 30 s after the onset of its action. During this period the activity was abolished in all of the cells. This suggests that NO can modulate the STIC activity of these cells.
Figure 5. Effect of drugs which stimulate the NO–cGMP pathway.
A, effect of the NO donor nitrosocysteine. B, summary of the effect of nitrosocysteine (16 μm, nominal) on STICs in 5 cells. C, effect of the soluble guanylate cyclase activator YC-1 on STICs. D, summary of the effect of YC-1 (30 μm) on STICs in 8 cells. E, effect of 8-bromo-cGMP on STICs. F, summary of 8-bromo-cGMP (1 mm) on STICs in 7 cells.
To assess the role of cGMP, the effect of YC-1, an activator of soluble guanylate cyclase, was tested (Friebe & Koesling, 2003). Preliminary experiments suggested that consistent effects could be obtained with 30 μm of this compound, while lower concentrations had more variable effects. An example is shown in Fig. 5C, where YC-1 (30 μm) initially abolished the activity followed by a period where a few small STICs seemed to break through. As with nitrosocysteine, the STICs returned with increased amplitude and at a slower rate than before. Figure 5D summarizes the effect of YC-1 in eight cells where it significantly reduced both the frequency (P < 0.05) and amplitude of the STICs (P < 0.01). A membrane-permeant analogue of cGMP, 8-bromo-cGMP, also inhibited the STICs with a similar pattern to that observed with nitrosocysteine and YC-1 (Fig. 5E). In seven cells both frequency (P < 0.05) and amplitude (P < 0.01) were significantly inhibited on exposure to 8-bromo-cGMP (1 mm; Fig. 5F). Taken together, these results suggest that NO, acting via cGMP, can inhibit STICs in corpus cavernosum myocytes.
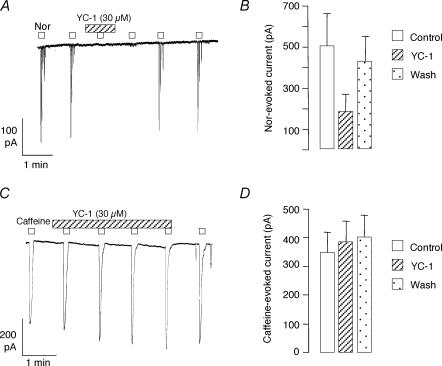
We also carried out experiments to examine the interaction of the cGMP pathway with the effect of noradrenaline on these cells. In general, noradrenaline (10 μm) caused a large inward Cl− current, followed by a burst of smaller currents at a higher frequency than the basal rate. Its effects were easiest to isolate in examples like that shown in Fig. 6A, where the cell was quiescent or had a low firing rate. In this experiment two applications of noradrenaline applied during control conditions evoked almost identical bursts of inward currents. YC-1 (30 μm) abolished these responses, although they could be repeated several minutes after wash-out. Summary data for six cells are presented in Fig. 6B, where the data were quantified by measuring the amplitude of the first large current evoked by noradrenaline. The amplitude of this response was reduced by > 60% in the presence of YC-1 (P < 0.05). To test whether the effect of YC-1 could be due to either direct blockade of Cl− channels, or a non-specific effect on Ca2+ stores, we also examined its effects on caffeine-evoked currents. As caffeine is known to evoke Ca2+ release by acting on ryanodine receptors (RyRs), rather than IP3Rs, its mode of action is different to that of noradrenaline. An example is shown in Fig. 6C, where it clear that YC-1 failed to reduce caffeine-evoked Cl− currents. Summary data in eight cells confirm that no reduction in caffeine-evoked currents occurred in the presence of YC-1 (indeed, a small but statistically significant increase in current was found, P < 0.01). These results suggest that the actions of cGMP are mediated by an interaction with IP3-dependent Ca2+ release.
Figure 6. Effect of YC-1 on responses to noradrenaline and caffeine.
A, noradrenaline (Nor, 10 μm) repeatedly evoked a burst of inward currents in a cell held at −60 mV. These responses were reversibly blocked in this cell by exposure to YC-1. B, summary of the effect of YC-1 (30 μm) on the amplitude of the largest noradrenaline-evoked current in each burst in 7 cells. YC-1 reversibly produced a mean 60% reduction in the noradrenaline-evoked current. C, caffeine (10 mm) repeatedly evoked inward currents in a cell held at −60 mV. These responses were unaffected by exposure to YC-1. D, summary of the effect of YC-1 (30 μm) on the amplitude of caffeine-evoked current in 8 cells. In contrast to noradrenaline-evoked currents, YC-1 failed to reduce the caffeine-evoked currents.
Discussion
In the initial part of the present study we have provided evidence that the smooth muscle cells of the rabbit corpus cavernosum possess a Ca2+-activated Cl− current. Our objectives were firstly to develop a preparation that would provide an adequate yield of cells to allow study of this current in a commonly used animal model of erectile function, and secondly to study possible modulation of the current by the NO–cGMP pathway. We believed that this was necessary as, although spontaneous Ca2+-activated Cl− currents (STICs) had previously been identified in rat and human corpus cavernousum, attempts to study modulation of the current by cGMP were inconclusive (Karkanis et al. 2003).
The current in the rabbit cavernosum was similar, although not identical, to the Cl− current previously described in rabbit and sheep urethra (Cotton et al. 1997; Sergeant et al. 2000). One difference was that in the urethra the current did not decay during depolarizing steps, while a decline of ∼50% was observed during 500 ms test pulses in the present study. Although we cannot exclude the possibility that this reflects a difference in Ca2+ handling between the two cell types, it may indicate that an inactivation mechanism is present in the cavernosum, but lacking in the urethra. Such a difference has also been found in other smooth muscle cell types. For example, a Ca2+–calmodulin-dependent kinase II (CaMKII)-mediated inactivation of Ca2+-activated Cl− currents has been demonstrated in trachea (Wang & Kotlikoff, 1997) and confirmed in aortic and pulmonary artery myocytes, but was less prominent in portal vein smooth muscle cells (Greenwood et al. 2001). At present we do not know why the current declines in the cavernosum, or what physiological significance, if any, this may have.
Another unusual feature of the Cl− current in the cavernosum was its insensitivity to blockade with niflumic acid. In the sheep and rabbit urethra as little as 10 μm of niflumic acid was sufficient to almost abolish the current (Cotton et al. 1997; Sergeant et al. 2000), while in the cavernosum up to 100 μm was necessary to produce only a modest block. Indeed, the sustained component of current was not reduced at all by this drug, while the amplitudes of the tail currents were even enhanced by it (Fig. 1C). Anomalous effects of niflumic acid on Ca2+-activated Cl− currents have been studied in more detail in rabbit pulmonary artery where the intracellular Ca2+ was clamped at 500 nm (Piper et al. 2002). Under these conditions niflumic acid was found to block the outward Cl− current elicited on stepping from −50 to +70 mV, but to enhance the inward current observed on stepping back to −50 mV. Ion substitution experiments performed to shift the reversal potential showed that these inhibitory and enhancing effects depended on the direction of current flow, rather than the potential at which the cell was held. Piper et al. (2002) therefore proposed that niflumic acid had a dual effect, acting as a blocker while simultaneously increasing the open probability of the channel. Since it was also a poorer blocker of outward Cl− current than inward Cl− current, the resultant effects were blockade of the outward current, but enhancement of the inward current. The enhancing effect had not previously been observed, and was only seen when the cells had been exposed to sustained increases in internal Ca2+. Thus the enhanced tail amplitudes during niflumic acid exposure in the present study might suggest that the corpus cavernosum cells have a higher resting Ca2+ level than many other smooth muscle cell types. Niflumic acid also markedly prolonged the tail currents in the present study, an effect also noted on the decay of STICs in the rabbit portal vein (Hogg et al. 1994). These authors suggested that this result could be explained if niflumic acid acted as an open channel blocker, which remained in the channel for a short time relative to the mean open duration of the channel. Their model assumed that a STIC was activated by a single Ca2+‘spark’ that decayed more rapidly than the STIC, so that the rate of STIC decay was determined by the kinetics of Cl− channel, rather than the rate of Ca2+ removal from the internal side of the cell membrane. The effect of the open channel blocker in these circumstances would be to prevent the channel from entering the normal closed state, causing prolonged ‘bursting’ activity between the open state and the blocked state. While this mechanism might contribute to prolongation of the tails under some circumstances, it is possible that the tails in the present study (especially longer ones, e.g. Fig. 2) reflect the rate of Ca2+ removal from the cell as well as simple channel kinetics. Also, the fact that the initial amplitude of the tails was enhanced, rather than reduced by niflumic acid, makes open channel block seem a less feasible explanation in this case. Therefore more study is required before the effects of niflumic acid in corpus cavernosum cells are fully understood.
We have also attempted to examine the mechanism underlying STICs in the corpus cavernosum. Blockade with CPA reversibly abolished them, suggesting that they were activated by cyclical Ca2+ release from the sarcoplasmic reticulum. STICs were also reduced by substances that interfered with IP3-mediated Ca2+ release, namely 2APB, an IP3 receptor blocker, and by NCDC, an inhibitor of PLC. These effects were similar to our findings in the rabbit urethra, where it was proposed that large STICs were evoked by large intracellular Ca2+ oscillations that required co-operative Ca2+ release from IP3 and ryanodine receptors (Sergeant et al. 2000, 2001). Strong evidence also exists for a central role for IP3-mediated Ca2+ release in generating slow waves in the gastrointestinal system (Suzuki et al. 2000; van Helden et al. 2000; Hirst & Edwards, 2001; Hirst et al. 2002).
To investigate modulation of STICs by the NO–cGMP pathway, we examined the effects of nitrosocysteine (an NO donor) and 8-bromo-cGMP (a cell-permeant analogue of cGMP). We also examined the effect of YC-1, which can activate soluble guanylate cyclase independently of NO by binding to a site different from the haem group, and also potentiates the stimulatory effect of NO on the enzyme (Friebe & Koesling, 2003). All three compounds had broadly similar effects, profoundly depressing both the frequency and amplitude of STICs. The effects with YC-1 were achieved with a similar concentration to that required to relax isolated strips of rabbit corpus cavernosum and in vitro (Hsieh et al. 2003). The inhibition by YC-1 was more long lasting than nitrosocysteine, possibly due its well-documented ability to inhibit the phosphodiesterases that break down cGMP (Galle et al. 1999). On wash-out the STIC frequency was slowed for up to 10 min and, sometimes, the amplitude was initially larger than in the control. We do not yet understand these wash-out effects, but speculate that the increased amplitude may be due to the reduced frequency, which would allow more time for store loading. Increased store loading may also have been due to increased sascoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) pump activity due to inhibition of phospholamban by cGMP (Raeymaekers et al. 1988).
In an attempt to understand how cGMP could inhibit STICs, we examined the effect of YC-1 on both caffeine- and noradrenaline-evoked Cl− currents. Noradrenaline is thought to stimulate IP3 production (and therefore stimulate IP3-dependent Ca2+ release) following binding to α1 adrenoceptors (Kobayashi et al. 1989), which are the predominant post-junctional subtype in the corpus cavernosum (Andersson, 2001). Caffeine, on the other hand, is thought to block IP3 receptors (Parker & Ivorra, 1991), but also to cause Ca2+ release from the SR by opening RyR (Zucchi & Ronca-Testoni, 1997). Caffeine therefore was utilized to examine the effect of YC-1 on IP3-independent Ca2+ release. The fact that the caffeine-induced responses were not blocked by YC-1 suggests that the modulation of STICs by cGMP occured at a site upstream from the activation of the Cl− channels by Ca2+. Also, this result makes it less likely that the YC-1 acted by causing direct Cl− channel blockade, or by non-specific interference with the SR Ca2+ store. In contrast to the caffeine responses, noradrenaline-induced Cl− currents were reduced by YC-1, suggesting that the NO–cGMP pathway could interfere with IP3-mediated Ca2+ release. These results also help to explain the observation by Cellek & Moncada (1997) that nitrergic inhibition appears to be specifically directed at the adrenergic-induced contraction in the cavernosum. They argued that this interaction took place predominantly at the post-junctional level and later suggested that this might occur at the level of Ca2+ signalling in the smooth muscle cells (Cellek, 2000). Although we have not measured intracellular Ca2+ directly, the fact that YC-1 blocked noradrenaline-induced Cl− currents suggests that a specific interaction did indeed occur at this level. Moreover, there is substantial evidence in the literature that cGMP modulates Ca2+ mobilization by inhibiting IP3 generation (Murthy et al. 1993; Ruth et al. 1993; Ding & Abdel-Latif, 1997) or by inhibiting Ca2+ release from IP3Rs (Komalavilas & Lincoln, 1996; Schlossmann et al. 2000; Feil et al. 2002; Murthy & Zhou, 2003). These effects appear to be mediated by cGMP-dependent protein kinase 1 (GK-1), absence of which in ‘knock out’ mice leads to low fecundity and reduced sensitivity to the relaxant action of NO in the corpus cavernosum (Hedlund et al. 2000).
Although the corpus cavernosum is often considered to be a tonic smooth muscle, it also readily develops spontaneous phasic contractions in vitro (Christ et al. 1990; Hoppner et al. 1996) and electromyographic (EMG) recordings suggest that it is a well synchronized muscle (Jiang et al. 2003). Implicit in our observations of STICs is the fact that the cytosolic Ca2+ levels spontaneously oscillate. Phasic contraction could therefore conceivably occur as a direct result of the oscillations, or indirectly due to activation of Cl− channels, followed by depolarization and Ca2+ influx via L-type Ca2+ channels. Although we know of no reports of intracellular microelectrode recordings in intact corpus cavernosum, spontaneous depolarizations similar to urethral slow waves have been reported in the corpus spongiosum of the guinea-pig (Hashitani et al. 2002). A physiological role for the Cl− current in the corpus cavernosum was suggested by Karkanis et al. (2003) when they showed that intracavernosal pressure in rats was increased by Cl− channel blockers, arguing that depolarization due to Cl− current activated voltage-dependent Ca2+ influx and caused contraction. This would be consistent with the findings that L-type Ca2+ channel antagonists reduce spontaneous phasic contractions, spontaneous tone and noradrenaline-induced tone in the cavernosum (Christ et al. 1990; Hoppner et al. 1996). The question, naturally, arises as to how an essentially oscillatory mechanism can often produce tone. The answer to this may lie in the recent discovery that the RhoA–Rho-kinase system is very highly expressed in the cavernosum (Wang et al. 2002). This system sensitizes the contractile proteins to Ca2+, enhancing and prolonging contraction, and would therefore increase the likelihood of temporal summation of the phasic contractions.
In conclusion, we have demonstrated that rabbit corpus cavernosum myocytes express a Ca2+-activated Cl− current and a high proportion of these cells also fire spontaneous Cl− currents. Our results suggest that oscillatory Ca2+ release from the SR underlies these events. Moreover, the currents are enhanced by noradrenaline and suppressed by NO–cGMP, suggesting that they are under the control of the normal physiological mechanisms that regulate tone in this tissue. We propose that detumescent tone is maintained by a combination of the underlying spontaneous mechanism and its enhancement by noradrenaline-induced Ca2+ release. To achieve penile erection, cGMP both suppresses the spontaneous mechanism and specifically inhibits the effect of noradrenaline, possibly at the level of either IP3 production or Ca2+ release from the IP3 receptor. These effects are likely to work in conjunction with additional cGMP-dependent mechanisms such as inhibition of RhoA-induced Ca2+ sensitization (Sauzeau et al. 2000; Mills et al. 2002), activation of myosin light chain phosphatase (Lee et al. 1997) and opening of K+ channels (Lee & Kang, 2001).
Acknowledgments
We wish to thank the Wellcome Trust (064212) and Diabetes UK (RD03/0002695) for financial support and the Department for Employment and Learning (N.I) for providing a postgraduate studentship for Michael Craven.
References
- Andersson KE. Pharmacology of penile erection. Pharmacol Rev. 2001;53:417–450. [PubMed] [Google Scholar]
- Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- Cellek S. Nitrergic–noradrenergic interaction in penile erection: a new insight into erectile dysfunction. Drugs Today (Barc) 2000;36:135–146. doi: 10.1358/dot.2000.36.2-3.568787. [DOI] [PubMed] [Google Scholar]
- Cellek S, Moncada S. Nitrergic control of peripheral sympathetic responses in the human corpus cavernosum: a comparison with other species. Proc Natl Acad Sci U S A. 1997;94:8226–8231. doi: 10.1073/pnas.94.15.8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ GJ, Maayani S, Valcic M, Melman A. Pharmacological studies of human erectile tissue: characteristics of spontaneous contractions and alterations in alpha-adrenoceptor responsiveness with age and disease in isolated tissues. Br J Pharmacol. 1990;101:375–381. doi: 10.1111/j.1476-5381.1990.tb12717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton KD, Hollywood MA, McHale NG, Thornbury KD. Ca2+ current and Ca2+-activated chloride current in isolated smooth muscle cells of the sheep urethra. J Physiol. 1997;505:121–131. doi: 10.1111/j.1469-7793.1997.121bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding KH, Abdel-Latif AA. Actions of C-type natriuretic peptide and sodium nitroprusside on carbachol-stimulated inositol phosphate formation and contraction in ciliary and iris sphincter smooth muscles. Invest Ophthalmol Vis Sci. 1997;38:2629–2638. [PubMed] [Google Scholar]
- Feil R, Gappa N, Rutz M, Schlossmann J, Rose CR, Konnerth A, Brummer S, Kuhbandner S, Hofmann F. Functional reconstitution of vascular smooth muscle cells with cGMP-dependent protein kinase I isoforms. Circ Res. 2002;90:1080–1086. doi: 10.1161/01.res.0000019586.95768.40. [DOI] [PubMed] [Google Scholar]
- Field L, Dilts RV, Ravichandran R, Lehhert PG, Carnahan GE. An unusually stable thionitrite from N-acetyl-D,1-penicillamine; X-ray crystal and molecular structure of 2-(acetylamino)-2-carboxy-1,1-dimethylethyl thionitrite. JCS Chem Comm. 1978:249–250. [Google Scholar]
- Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- Galle J, Zabel U, Hubner U, Hatzelmann A, Wagner B, Wanner C, Schmidt HH. Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br J Pharmacol. 1999;127:195–203. doi: 10.1038/sj.bjp.0702495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Ledoux J, Leblanc N. Differential regulation of Ca2+-activated Cl− currents in rabbit arterial and portal vein smooth muscle cells by Ca2+–calmodulin-dependent kinase. J Physiol. 2001;534:395–408. doi: 10.1111/j.1469-7793.2001.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Fukuta H, Dickens EJ, Suzuki H. Cellular mechanisms of nitric oxide-induced relaxation of corporeal smooth muscle in the guinea-pig. J Physiol. 2002;538:573–581. doi: 10.1113/jphysiol.2001.013049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund P, Aszodi A, Pfeifer A, Alm P, Hofmann F, Ahmad M, Fassler R, Andersson KE. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2349–2354. doi: 10.1073/pnas.030419997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Bramich NJ, Teramoto N, Suzuki H, Edwards FR. Regenerative component of slow waves in the guinea-pig gastric antrum involves a delayed increase in [Ca2+]i and Cl− channels. J Physiol. 2002;540:907–919. doi: 10.1113/jphysiol.2001.014803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Edwards FR. Generation of slow waves in the antral region of guinea-pig stomach-a stochastic process. J Physiol. 2001;535:165–180. doi: 10.1111/j.1469-7793.2001.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RC, Wang Q, Large WA. Action of niflumic acid on evoked and spontaneous calcium-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. Br J Pharmacol. 1994;112:977–984. doi: 10.1111/j.1476-5381.1994.tb13177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist F, Hedlund H, Andersson KE. Characterization of inhibitory neurotransmission in the isolated corpus cavernosum from rabbit and man. J Physiol. 1992;449:295–311. doi: 10.1113/jphysiol.1992.sp019087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppner CK, Stief CG, Jonas U, Mandrek K, Noack T, Golenhofen K. Electrical and chemical control of smooth muscle activity of rabbit corpus cavernosum in vitro. Urology. 1996;48:512–518. doi: 10.1016/S0090-4295(96)00218-X. [DOI] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J General Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh GC, O'Neill AB, Moreland RB, Sullivan JP, Brioni JD. YC-1 potentiates the nitric oxide/cyclic GMP pathway in corpus cavernosum and facilitates penile erection in rats. Eur J Pharmacol. 2003;458:183–189. doi: 10.1016/s0014-2999(02)02730-9. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- Jiang XG, Speel TG, Wagner G, Meuleman EJ, Wijkstra H. The value of corpus cavernosum electromyography in erectile dysfunction: current status and future prospect. Eur Urol. 2003;43:211–218. doi: 10.1016/s0302-2838(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Karkanis T, DeYoung L, Brock GB, Sims SM. Ca2+-activated Cl− channels in corpus cavernosum smooth muscle: a novel mechanism for control of penile erection. J Appl Physiol. 2003;94:301–313. doi: 10.1152/japplphysiol.00660.2002. [DOI] [PubMed] [Google Scholar]
- Kleinberg ME, Finkelstein A. Single-length and double-length channels formed by nystatin in lipid bilayer membranes. J Membr Biol. 1984;80:257–269. doi: 10.1007/BF01868444. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Kitazawa T, Somlyo AV, Somlyo AP. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989;264:17997–18004. [PubMed] [Google Scholar]
- Komalavilas P, Lincoln TM. Phosphorylation of the inositol 1,4,5-trisphosphate receptor. Cyclic GMP-dependent protein kinase mediates cAMP and cGMP dependent phosphorylation in the intact rat aorta. J Biol Chem. 1996;271:21933–21938. doi: 10.1074/jbc.271.36.21933. [DOI] [PubMed] [Google Scholar]
- Kowaluk EA, Fung HL. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J Pharmacol Exp Ther. 1990;255:1256–1264. [PubMed] [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physiol. 1996;271:C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kang TM. Effects of nitric oxide on the Ca2+-activated potassium channels in smooth muscle cells of the human corpus cavernosum. Urol Res. 2001;29:359–365. doi: 10.1007/s002400100211. [DOI] [PubMed] [Google Scholar]
- Lee MR, Li L, Kitazawa T. Cyclic fnm>GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J Biol Chem. 1997;272:5063–5068. doi: 10.1074/jbc.272.8.5063. [DOI] [PubMed] [Google Scholar]
- Mills TM, Chitaley K, Lewis RW, Webb RC. Nitric oxide inhibits RhoA/Rho-kinase signaling to cause penile erection. Eur J Pharmacol. 2002;439:173–174. doi: 10.1016/s0014-2999(02)01408-5. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Severi C, Grider JR, Makhlouf GM. Inhibition of IP3 and IP3-dependent Ca2+ mobilization by cyclic nucleotides in isolated gastric muscle cells. Am J Physiol. 1993;264:G967–G974. doi: 10.1152/ajpgi.1993.264.5.G967. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Zhou H. Selective phosphorylation of the IP3R-I in vivo by cGMP-dependent protein kinase in smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;284:G221–G230. doi: 10.1152/ajpgi.00401.2002. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Meth Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Parker I, Ivorra I. Caffeine inhibits inositol trisphosphate-mediated liberation of intracellular calcium in Xenopus oocytes. J Physiol. 1991;433:229–240. doi: 10.1113/jphysiol.1991.sp018423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper AS, Greenwood IA, Large WA. Dual effect of blocking agents on Ca2+-activated Cl− currents in rabbit pulmonary artery smooth muscle cells. J Physiol. 2002;539:119–131. doi: 10.1113/jphysiol.2001.013270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin-B. J Neurosc Met. 1991;37:5–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Raeymaekers L, Hofmann F, Casteels R. Cyclic GMP-dependent protein kinase phosphorylates phospholamban in isolated sarcoplasmic reticulum from cardiac and smooth muscle. Biochem J. 1988;252:269–273. doi: 10.1042/bj2520269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth P, Wang GX, Boekhoff I, May B, Pfeifer A, Penner R, Korth M, Breer H, Hofmann F. Transfected cGMP-dependent protein kinase suppresses calcium transients by inhibition of inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci U S A. 1993;90:2623–2627. doi: 10.1073/pnas.90.7.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM. Electrophysiology of dissociated gastrointestinal smooth muscle cells. In: Schultz SG, editor. Handbook of Physiology, section 6, The Gastrointestinal System. Vol. 1. Bethesda: The American Physiological Society; 1989. pp. 163–185. [Google Scholar]
- Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang GX, Allescher HD, Korth M, Wilm M, Hofmann F, Ruth P. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature. 2000;404:197–201. doi: 10.1038/35004606. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, McHale NG, Thornbury KD. Role of IP3 in modulation of spontaneous activity in pacemaker cells of rabbit urethra. Am J Physiol Cell Physiol. 2001;280:C1349–C1356. doi: 10.1152/ajpcell.2001.280.5.C1349. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000;525:105–111. doi: 10.1111/j.1469-7793.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helden DF, Imtiaz MS, Nurgaliyeva K, von der Weid P, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol. 2000;524:245–265. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Eto M, Steers WD, Somlyo AP, Somlyo AV. RhoA-mediated Ca2+ sensitization in erectile function. J Biol Chem. 2002;277:30614–30621. doi: 10.1074/jbc.M204262200. [DOI] [PubMed] [Google Scholar]
- Wang YX, Kotlikoff MI. Inactivation of calcium-activated chloride channels in smooth muscle by calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1997;94:14918–14923. doi: 10.1073/pnas.94.26.14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev. 1997;49:1–51. [PubMed] [Google Scholar]