Abstract
It has been claimed that transcranial magnetic stimulation (TMS) of the human motor cortex can produce a sense of movement of the contralateral hand, even when the hand is paralysed. The sense of movement was equated with a ‘corollary discharge’, a nulling mechanism originally posited for maintaining constancy of the visual field during eye movements. Our experiments were designed to test whether the sensation that accompanies TMS-evoked finger movements is generated centrally or whether it arises as a result of sensory feedback. Matched twitches of the left and right fingers were elicited either by bilateral electrical stimulation of forearm extensor muscles, or by a combination of TMS of left motor cortex (eliciting twitches of the right forefinger), and electrical stimulation of the left forearm muscles (eliciting twitches of the left forefinger). The time interval between stimuli activating left and right twitches was varied randomly (range ± 90 ms) from trial to trial. Subjects reported whether they sensed that the left or the right movement occurred first, or if they could detect no difference. The left and right movements evoked by bilateral electrical stimulation of muscles were sensed as near simultaneous when there was zero delay between them. When TMS was applied in conjunction with unilateral muscle stimulation, the TMS-evoked movement was felt, on average, 20 ms after the movement evoked by muscle stimulation. Similar results were obtained when the skin under the cathodal electrodes was anaesthetized. Since the TMS-evoked movements were felt later rather than earlier than the electrically evoked movements, the results do not support the idea that the sensation of movement was elicited centrally by TMS. Rather, they favour sensory feedback as the source of the sense of movement. The earlier perception of electrically evoked versus TMS-evoked movements was probably due to earlier sensory responses in the periphery rather than a suppression of the excitability of somatosensory cortex.
There have been several reports that TMS elicits sensations in limbs. When hand movements elicited by TMS were abolished by peripheral ischaemic block, some subjects reported a sense of movement in the paralysed hand (Amassian et al. 1989; Brazil-Neto et al. 1993) and these sensations were attributed to the generation of corollary discharge, otherwise known as ‘efference copy’. TMS also induced sensations of movement in the missing limbs of amputees, although the sensations were accompanied by movement of stump muscles (Cohen et al. 1991c). In spinal-cord-injured subjects with paralysed hands, TMS rarely elicited a sense of hand movement, but it was argued that the hand areas of the motor cortex might have been reorganized to become upper arm areas (Levy et al. 1990; Cohen et al. 1991c). Normal subjects did not experience a sense of movement during attempts voluntarily to move ischaemically paralysed fingers (Goodwin et al. 1972), but again it was argued that the command system for voluntary movement might inhibit perception of corollary discharge unless sensory feedback signalling movement is present.
An interesting recent approach to the problem exploited the fact that one cannot tickle oneself (Chronicle & Glover, 2003). Subjects' hands were attached to a lever which moved a blunt needle over their bare feet. Self-generated movements of the lever were not perceived as ticklish, but movements elicited involuntarily by TMS of motor cortex or electrical stimulation of arm muscles were. It was argued that in the voluntary movements, an efference copy generated in premotor areas provided a prediction of sensory input that somehow cancelled out the sense of tickle. TMS-evoked movements were ticklish, so it was concluded that TMS did not generate efference copy.
The idea that voluntary hand movements involve cortical efference copy received further support in another TMS study, albeit of a different type (MacDonald & Paus, 2003). In this case, repetitive TMS applied for 15 min to parietal or temporal cortex, impaired the subjective sense of timing of self-generated movements. Again it was suggested that the efference copy was generated in premotor rather than motor cortical regions. The parietal cortex was suggested as the area receiving and comparing the efference copy and sensory feedback.
The above reports concerning efference copy and sensations of movement induced by TMS of motor cortex thus appear to be inconsistent. It remains uncertain whether TMS can excite cortical elements that are instrumental in generating an efference copy of the movement elicited in the periphery.
Single-pulse TMS is effective in generating contraction in skeletal muscle through an action on neuronal elements in the motor cortex that elicits a brief volley in corticospinal neurones. Excitation of corticospinal axons is thought to occur either by stimulation of axons that are presynaptic and excitatory to corticospinal neurones or directly by depolarization of corticospinal neurones at the initial segment of the axon (Day et al. 1989a; Edgley et al. 1990; Burke et al. 1993). Stimulation thus occurs at or close to the output stage of the motor cortex through corticospinal neurones normally involved in the execution of voluntary movements.
In contrast, single-pulse TMS of somatosensory cortex at similar intensities does not elicit somatic sensations unless stimulus intensities are high enough to elicit actual movement (Andre-Obadia et al. 1999). This is in line with earlier work showing that single-pulse electrical stimuli applied to somatosensory cortex through subdural electrodes only elicited sensations of movement when intensities were high enough to cause actual movements (Libet et al. 1964). On the other hand, sensations were elicited by trains of electrical stimuli subthreshold for evoking movement if these lasted 100 ms or more. Interestingly, the subjective timing of the onset of sensation was referred by the subjects to the beginning rather than the end of the train of stimuli. Thus, the onset and duration of activity in somatosensory cortex is apparently not related to the subjective sensation of voluntary movement in a simple and direct way.
The subjective sense of timing of sensations (Libet et al. 1979), particularly in regard to those accompanying voluntary movements, has been debated extensively (Libet et al. 1983; Haggard & Eimer, 1999; Haggard et al. 2002; Pockett, 2002). The work presented here sets out to determine whether there is any immediate sense of movement elicited by a single pulse of TMS that would support the notion of an efference copy. That is, whether sensation is generated in advance of that expected by afferent feedback from peripheral sense organs excited by the peripheral movement. Given that a single pulse of TMS applied to motor cortex is sufficient to evoke a movement, it seems reasonable to posit that this may also elicit efference copy sufficient to evoke a sensation.
A preliminary report of this study has been published previously (Ellaway et al. 2003).
Methods
Experiments were carried out on right-handed, normal subjects (4 male, 5 female) aged 23–56 years. Ethical approval of the University of Alberta and written informed consent from the subjects, according to the Declaration of Helsinki, was obtained for the experimental procedures.
Experiment 1 – bilateral electrical stimulation of muscle
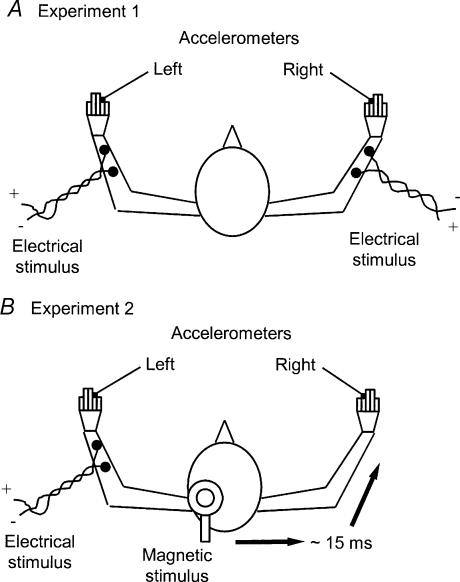
In one series of experiments, twitch contractions evoking left and right finger movements were elicited by surface electrical stimulation of muscles in both arms (Fig. 1A). Circular, cloth electrodes (wetted) with a surface area of approximately 15 cm2 were placed on the skin of the forearm, a cathode over finger extensor muscles and an anode just proximal to the wrist. The electrical stimuli consisted of two pulses, each of 0.16 ms duration, separated by 4 ms. A double pulse was used to elicit finger movements since it was found to minimize any associated skin sensation. It also produced finger movements that proved to be similar in duration to those produced by transcranial magnetic stimulation (TMS) of the motor cortex (see below). The position of the electrodes and strength of stimulation were adjusted so as to activate muscle that preferentially elicited finger extension limited to the forefinger. When this could not be achieved, the electrodes were placed so as to elicit closely similar finger movements in both hands. An accelerometer (weight 5 g) was attached to the appropriate digit of each hand and the signals monitored using a Digidata 1200 interface running Axoscope 8.1 software (Axon Instruments, CA, USA). The accelerometer recordings were used to help us match left and right finger movements in respect of duration and magnitude of acceleration. A custom-built, programmable stimulator was used to vary the time interval between stimuli delivered to the left and right arm. The unit could be set to select a number of different time intervals with either the left or the right stimulus occurring first, or the two could be set to occur simultaneously. Time intervals of up to 60–90 ms in increments of 15 ms were chosen. Subjects were presented with the following protocol. An audible bleep warned the subject that stimulation was about to commence. Two seconds after the bleep the first of three presentations of a pair (left and right) of stimuli separated by a particular time interval was made. This was followed at 3 s intervals by the two other pairs of stimuli. The subject was then allowed 5 s in which to voice an opinion as to whether the left movement was sensed ahead of the right, the right ahead of the left or that the order of the movements could not be distinguished. A new trial was then initiated. This protocol allowed the subject to experience a particular time interval between stimuli on three occasions before making a decision as to which occurred first. Each trial for a particular time interval between stimuli was repeated on six occasions, with the trials for those different intervals presented in random order.
Figure 1. Experimental arrangement for eliciting and recording finger movements.
A, Expt 1 in which movements of left and right fingers were elicited by percutaneous electrical stimulation of forearm muscles. B, Expt 2 in which movements of left fingers were elicited by percutaneous electrical stimulation of forearm muscles and of right fingers by transcranial magnetic stimulation of the contralateral motor cortex. The additional neural conduction time involved with cortical stimulation was, on average, 15 ms (indicated). The relative timing of left and right movements was varied randomly from trial to trial (see Methods). Movements were monitored in both experiments using lightweight accelerometers attached to the fingers.
Experiment 2 – unilateral electrical stimulation of muscle and TMS of motor cortex
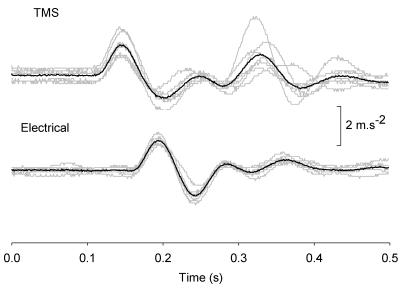
The same subjects participated in a second experiment in which the stimulus used to elicit finger movement in the right hand was replaced by TMS of the contralateral motor cortex (Fig. 1B). TMS was delivered by a MagStim 200 (The MagStim Company, Dyffed, Wales) using a 9 cm round coil. The position of the coil and the strength of stimulation were selected to obtain finger movement of the right hand that matched as closely as possible the finger movement of the left hand elicited by electrical stimulation. On occasions, the location of the cathodal surface electrode on the left arm and the strength of stimulation were adjusted so that the left finger movement more closely matched the right movement elicited by TMS. The time interval between left and right stimuli was again varied randomly (range ± 90 ms in 15 ms steps) from trial to trial. However, allowance was made for the extra conduction time from cortex to muscle incurred with TMS. This usually amounted to about 15 ms. Figure 2 shows representative accelerometer traces for a time interval between TMS and electrical stimuli selected so that the TMS-induced movement commenced 45 ms before the electrically induced movement. Experiment 2 was carried out on a different day to Expt 1.
Figure 2. Accelerometer records of finger movements elicited by TMS (above) and peripheral electrical stimuli (below).
The time interval between TMS and electrical stimuli was selected to produce TMS-induced finger movements in the right hand that occurred 45 ms in advance of the electrically induced movements in the left hand. Seven individual recordings (grey traces) and their averages (black traces) are shown.
Experiment 3 – anaesthetized skin
A group of four subjects participated in similar experiments but with the skin under the cathodal electrode of the left arm anaesthetized. A liberal coating of anaesthetic cream (lidocaine 2.5% and prilocaine 2.5%; Emla cream, AstraZeneca)) was applied to the skin before the start of the experiment and covered with a thin film of polythene to prevent drying. One hour after applying the local anaesthetic any excess cream was removed and the electrode placed within the anaesthetized patch of skin. Experiment 1 or 2 was then carried out with the skin over the muscle of the left arm anaesthetized. At the end of the experiment the sensitivity of the anaesthetized patch of skin was tested with a set of monofilament von-Frey hairs (Semmes-Weinstein aesthesiometer, Smith & Nephew, USA). Testing started with a thick (usually 4.56 ‘K’ gauge) filament. The subject's eyes were shut. Five consecutive indentations were performed by pushing the filament orthogonally into the skin until it bent and then allowing 2–3 s for the subject to report sensation. Successively smaller or larger gauge filaments were tested until the subject's detection rate was about 50%. This gauge was defined as our measure of perceptual threshold. A comparable area of skin on the forearm that had not been treated with the anaesthetic cream was also tested.
Experiment 4 – bilateral electrical stimulation of muscle and unilateral TMS of somatosensory cortex
Finally, three subjects participated in experiments similar to Expt 2, except that TMS was directed at the finger area of the somatosensory cortex, rather than motor cortex. These experiments were performed to test the idea that when TMS is applied over motor cortex, it may spread to and inhibit somatosensory cortex. This inhibition may block perception of TMS-elicited efference copy signals arriving from motor cortex. A figure-of-eight coil (9 cm diameter wings, 9 cm between centres, orientated 45 deg to the sagittal plane) was used to provide focal stimulation. First, we determined the region over motor cortex within which TMS elicited small, discrete extensions of the forefinger. The skin was marked at the centre of this region. The coil was then moved 2.5 cm posteriorly, which we assumed would position it over somatosensory cortex (Williams & Warwick, 1980). We confirmed that TMS pulses of the same intensity no longer elicited finger movement. Subjects also confirmed that they did not elicit a sense of movement. Experiment 1 was performed first to verify the subject's sense of timing without TMS. In modified Expt 2, in addition to bilateral electrical stimulation of muscles, TMS was applied to left somatosensory cortex, either at the same time as the electrical stimulus to the right forearm (case 1), or 15 ms after this stimulus (case 2). If the objection were valid, we would expect to see a significant disruption in the sense of timing of the electrically evoked movements.
Results
Experiment 1 – bilateral electrical stimulation of muscle
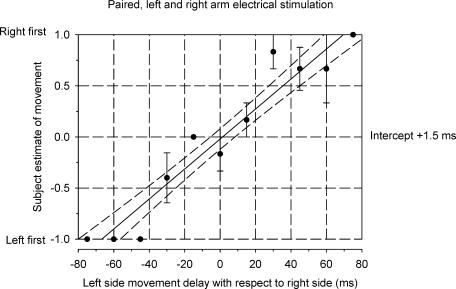
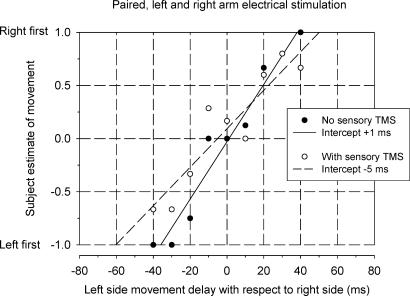
Sequence discrimination plots were constructed to display the subjective estimate of the order of left and right finger movements for different time intervals between left and right stimuli. Figure 3 shows such a psychometric function for one individual. Arbitrarily, a value of −1 was registered for the perception that the left preceded the right finger movement, +1 for the right movement preceding the left movement and a value of zero when the subject could not discriminate any order, i.e. the movements were perceived as occurring at the same time. Each point in Fig. 2 is the mean (±s.e.m.) of these values in six trials for a particular time interval. In this case, the left movement was correctly sensed as having occurred earlier than the right on all trials for intervals ≥45 ms. Consistent detection of the right preceding the left was not achieved until the separation reached 75 ms. A linear regression line has been fitted to the data points. The intercept of the linear regression line with the time axis represents the perceived time difference for movements that were actually simultaneous. The perceived time difference is very close to zero (+ 1.5 ms) for this subject. That is, the left and right movements were sensed as near simultaneous when there was zero delay between them. The perceived time difference for the six subjects ranged from −9.5 ms (right preceding left) to +3.5 ms (left preceding right) with an average value of −0.7 ms. The experiment was repeated for one subject. The perceived time difference had a value of −1 ms on both occasions.
Figure 3. Sequence discrimination plot for one subject using paired electrical stimulation of left and right forearm muscles to elicit finger movements.
The abscissa represents the delay between stimuli to left and right sides. Negative values indicate that the left side stimulation occurred before the right. The ordinate indicates the subject's perception of the order of left and right finger movements. A value of −1 was registered for the perception that the left preceded the right finger movement, +1 for the right movement preceding the left movement and a value of zero when the subject perceived the movements to have occurred at the same time. Each point is the mean (±s.e.m.) of six trials. A linear regression line (continuous line) with 95% confidence intervals (dashed lines) has been fitted to the data. The intercept of the regression line on the time axis (+ 1.5 ms) is regarded as the perceived time difference between two simultaneous movements.
Experiment 2 – unilateral electrical stimulation of muscle and TMS of motor cortex
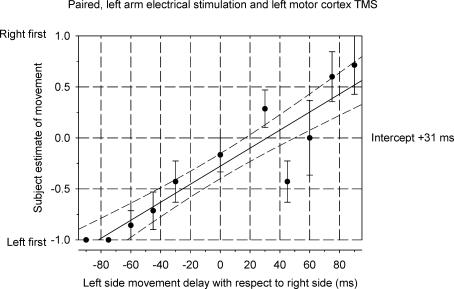
Figure 4 shows a sequence discrimination plot (same individual as illustrated in Fig. 3) when TMS of left motor cortex was combined with muscle stimulation of the left forearm. Movement traces for this subject are illustrated in Fig. 2. The slope of the regression line in Fig. 4 is less steep than with dual electrical stimulation. The perceived time difference is now +31 ms, indicating that on average the cortically evoked movement (right) was felt to have occurred after the movement (left) evoked by electrical muscle stimulation when, in fact, they were simultaneous. The perceived time difference for the six subjects ranged from −7 ms (right preceding left) to +53.5 ms (left preceding right) with an average value of +17.9 ms. The experiment was repeated for the one subject (see earlier) who had a perceived time difference value of −1.0 ms on the two occasions when tested with pairs of electrical stimuli. The values for perceived time difference when tested with paired electrical and TMS stimuli were +12 ms and +19.5 ms.
Figure 4. Sequence discrimination plot for the same subject as in Fig. 3, using electrical stimulation of the left forearm and TMS of the left motor cortex to elicit movements of the left and right fingers, respectively.
The plot is constructed as for Fig. 3. The intercept of the regression line on the time axis (+ 31 ms) indicates that movement of the right fingers elicited by TMS was perceived as having occurred later than a simultaneous movement of the left fingers elicited by electrical stimulation. Note that the extra conduction time involved with the TMS-induced movement (∼15 ms) has already been allowed for in the plot by advancing each cortical stimulus by an amount equal to that extra neural conduction time.
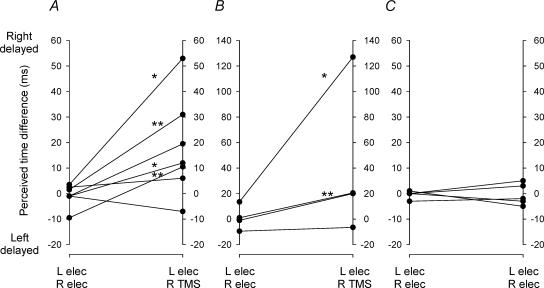
Figure 5A summarizes the results of the two experimental procedures in terms of the perceived time differences. Of the seven comparisons, four showed a statistically significant change (P < 0.05, t test) in the intercept of the regression line. In all four instances the perception was that the TMS movement was delayed with respect to the electrically induced movement. In no case was there a significant change in the opposite direction.
Figure 5. Perceived time differences in Expts 2, 3 and 4 (A, B and C).
In each panel the data on the left refer to paired finger movements evoked by bilateral electrical stimulation of muscles while the data on the right refer to: A, right finger movements evoked by TMS of left motor cortex and left finger movements evoked by muscle stimulation; B, as in A but with local skin anaesthesia under the cathodic muscle electrode on the left forearm; C, paired bilateral electrical stimulation of muscles plus TMS of somatosensory cortex synchronous with the electrical stimuli to the right muscle or 15 ms thereafter. Asterisks mark statistically significant changes in perceived time difference (*P < 0.05, **P < 0.01).
Experiment 3 – anaesthetized skin
All subjects reported a clear sensation of finger movement in response to both electrical stimulation and TMS. However, for some subjects the sensation elicited by electrical stimulation comprised both finger movement and a light tap to the skin felt under the cathode. The skin sensation arose presumably as a result of direct stimulation of cutaneous nerve fibres. This local skin sensation was reduced by employing a double shock (see Methods) that elicited the required movements at lower stimulus strengths. Nevertheless, in a further set of experiments, an attempt was made to block sensory input from the skin by applying local anaesthetic cream to the skin under the electrical cathode of the left arm for an hour before the stimulation trials (see Methods). Figure 5B shows the results of these trials from four subjects in whom the perceived time difference of movements was compared for dual electrical stimuli and electrical coupled with TMS. The perceived time differences were clustered close to zero (range −9.5 to +13.5 ms) for the movements elicited by electrical stimulation of both forearms. When movement on the right was elicited by TMS of the left motor cortex, the perceived time difference changed in all four subjects to a more positive value (range −6.5 to +127 ms). In two cases the change in the perceived time difference was statistically significant (P < 0.05, t test) and indicated that the TMS-induced movement was perceived as occurring later than the electrically induced movement. Perceptual thresholds of mechanical stimuli applied to the locally anaesthetized skin under the electrodes were tested with von Frey style monofilaments (see Methods) immediately after the stimulation trials. The perceptual thresholds were interpreted according to criteria established by Bell (1984). Three subjects lost the equivalent of protective sensation, retaining only rudimentary deep cutaneous sensation. In one subject there was only diminished light touch. However, none of the subjects reported any skin sensation during electrical stimulation under the stimulating electrode following application of the local anaesthetic.
Experiment 4 – bilateral electrical stimulation of muscle and unilateral TMS of left somatosensory cortex
TMS of somatosensory cortex has been shown to interfere with sensory inputs (McKay et al. 2003). It could be argued that in Expt 2, TMS spread to somatosensory cortex, subsequently inhibiting it. Thus although efference copy might indeed have issued forth from motor cortex, by the time it reached somatosensory cortex, the ability to perceive it was abolished. To test this idea, we repeated Expt 1 and a modified version of Expt 2, whereby in addition to bilateral electrical stimulation of muscles, TMS was applied to left somatosensory cortex, either at the same time as the electrical stimulus to the right forearm (case 1), or 15 ms after this stimulus (case 2). If the objection were valid, we would expect to see a significant disruption in the sense of timing of the electrically evoked movements. In case 1, the presumed inhibition of the left somatosensory cortex would have occurred about 15 ms before the arrival of the sensory input evoked by the electrical stimulus to the right forearm. Given that cortical inhibition is thought to have a time course of at least 100 ms, this should have reduced and possibly delayed the perception of the right finger movement, shifting the sequence discrimination plot to the right compared to the control trials of Expt 1. In case 2, TMS was timed to be coincident with the estimated arrival time of the sensory volley. We reasoned that this might have an even larger suppressive effect, and so should shift the sequence discrimination plots to the right even more.
Figure 6 shows typical sequence discrimination plots from one subject. Though the time axis intercepts were both within 5 ms of zero, the data from the TMS trial (case 1) were more scattered and the fitted line had a lower slope. Furthermore, all three subjects reported that in the TMS trials they felt less certain about their decisions. However, across subjects the differences in slopes were not statistically significant.
Figure 6. Time sequence plots for one subject in Expt 4, involving bilateral electrical stimulation of muscles with and without TMS of left somatosensory cortex.
○, TMS of left somatosensory cortex; •, without TMS. The perceived time differences (intercepts) are within 5 ms of zero for both procedures. The line of best fit was shallower in the TMS trial and the subject reported feeling less certain about the estimates; however, neither the time axis intercept nor the slope were significantly different in the two trials.
Figure 5C summarizes the results of these trials from all three subjects in whom the perceived time difference of movements was compared for dual electrical stimuli and electrical coupled with TMS of left somatosensory cortex. The perceived time differences were clustered close to zero (range −3 to +1 ms) for the movements elicited by electrical stimulation alone. The addition of TMS did not significantly change the perceived time differences, which remained clustered around zero (cases 1 and 2 lumped together, range −5 to +5 ms). Note that in one of these subjects, timing estimates were extremely variable in the first session, but in a second session in which she had been asked to attend more carefully, variability was greatly reduced so we discarded the data from the first session.
Discussion
The subjective impression of subjects in these experiments was that a TMS-induced finger movement in one hand was not perceived as occurring earlier than movement evoked in the opposite hand by direct muscle stimulation. Indeed, in some cases there was a significant delay in appreciation of the TMS-induced movement. The result was the same when skin under the stimulating cathode was anaesthetized, ruling out the possibility that electrically induced movements were sensed early due to immediate stimulation of cutaneous afferents. Since the perceived time difference for the TMS- and electrically induced movements ranged from close to zero up to tens of milliseconds delay for TMS, the results do not support the notion of an efference copy being elicited by TMS but favour sensory feedback as the source of the sense of movement.
How then should the claims of felt movements and other sensations that occur in response to TMS in subjects with paralysed or missing limbs be interpreted? First, only a third of subjects in a study of ischaemic nerve block (Brazil-Neto et al. 1993) reported sensation in the absence of movement. In similar experiments on three experienced subjects (the authors) movement was consistently felt but not always in the anticipated direction (Amassian et al. 1989). In spinal-cord-injured subjects, parasthesiae were felt below a complete spinal transection in response to TMS but as touch rather than movement (Levy et al. 1990; Cohen et al. 1991c). Finally, a sense of movement in response to TMS has been attributed to muscles removed by amputation but this was accompanied by contraction of stump muscles (Cohen et al. 1991a) that may have provided afferent feedback causing inappropriate interpretation of the peripheral events. It would appear therefore that a sense of movement is not an invariable consequence of TMS applied over the motor cortex. This makes it unlikely that some form of efference copy is a consistent corollary of the corticospinal output elicited by the TMS.
In those subjects who did experience a consistent sense of movement in response to TMS in absent or paralysed limbs, the experiments provided no detail about the timing of the sensation relative to the anticipated movement. A movement induced peripherally using direct electrical stimulation in normal subjects, as in the present experiments, is clearly felt by the subject. The question then arises as to whether the sensation evoked by a TMS- induced movement, or indeed by a voluntary movement, has an efference copy component or whether it is generated solely by afferent feedback. In the present experiments the perceived time difference for direct electrically induced and TMS-induced movements was negligible, or the TMS-induced movement was perceived as delayed. If there is an efference copy component generated by TMS then the present experiments indicate that it must be delayed by at least the loop time taken for neural transmission from the brain to muscle, muscle contraction and transmission of the resulting sensory activity back to the brain. Thus, in our experiments we cannot rule out the possibility that TMS evoked some form of cortical activity that contributed to the sense of movement. However, if it did so, then the experience of that sensation was that it occurred at or after the time expected due to sensory feedback from the peripheral receptors excited by the movement.
Another possibility is that TMS of motor cortex produced a lasting effect, either within motor or somatosensory cortex that conditioned the subsequent sensory experience generated by the afferent feedback related to the movement. Magnetic stimulation over the parietal lobe affects somatosensory evoked potentials recorded from the same side, enhancing the P25 wave and depressing later components (Kujirai et al. 1993; Seyal et al. 1995). This action of TMS is associated with attenuation or even block of the perception of peripheral stimulation. The sensation elicited by electrical stimulation of a finger was blocked by TMS delivered to the contralateral motor cortex simultaneously or up to 20 ms after the peripheral stimulus, but sensation was also attenuated to some degree over periods extending for ± 200 ms (Cohen et al. 1991b). During attenuation, no evidence was presented as to whether the diminished sensation generated by the peripheral stimulus was also perceived as being delayed. TMS can delay a cued voluntary motor response by an average 50–60 ms (Day et al. 1989b) without altering the expected sequence of muscle contractions. This suggests, at least on the motor side, that a magnetic stimulus to the cortex can delay events without necessarily degrading the pattern or program of neural events. Possibly it does so on the sensory side. There is further evidence relating to the influence of TMS suggesting that the cortical elements involved in suppressing or eliciting sensations are different to those leading to peripheral muscle contraction. By mapping optimal current directions induced by TMS, Pascual-Leone et al. (1994) concluded that the neuronal elements responsible for attenuation of sensation and for eliciting a sense of movement could not be distinguished but were different from those that elicited motor evoked potentials. However, the scalp positions for eliciting attenuation and MEPs were the same for all three, leading the authors to conclude that the different elements responsible for sensory and motor effects both lay in the motor cortex and, in terms of the sensory effect, were most likely to be cortico-cortical projections to the somatosensory cortex. What these authors did not address was the apparent conundrum that TMS at the one site and with the same induced current direction could either attenuate or elicit peripheral sensations.
In Expt 4 we directly tested the idea that TMS of somatosensory cortex might delay the sense of movement mediated by sensory signals from the arm. In none of the subjects was the sense of timing of the peripheral stimuli from right and left arms systematically altered by TMS. Though all three subjects reported that TMS seemed make their timing decisions harder, the intercepts in the time sequence plots did not shift to the right. Thus although TMS of somatosensory cortex may have suppressed the sensation of movement of the right forefinger, it did not significantly affect the perceived timing.
In Expt 2, there was no indication in any subject that a sense of movement occurred before actual movement. On the contrary, in several subjects the sense of movement in Expt 2 was delayed, typically by 20 ms or longer. Subjects were not asked to comment on the quality of the sensation associated with the TMS-induced movement or compare it to that produced by direct electrical stimulation. Certainly, there was no subject for whom the sensation of movement induced by TMS was absent. However, during the preliminary matching of finger movements produced by electrical stimulation of muscles and by TMS most subjects considered the TMS-induced movement to be less distinct when the transducer record actually showed the two movements to be well matched in form and amplitude. The use of a local anaesthetic to minimize direct stimulation of cutaneous afferents argues against the possibility that subjects were responding earlier to a volley of cutaneous afferent input elicited by the electrical, but not the TMS stimuli. One possibility is that the electrical stimuli directly elicited one or more synchronous volleys of action potentials in large muscle afferents and it was these volleys that were sensed ahead of the ensuing afferent responses to the muscle twitch. The volleys would have comprised responses to direct electrical stimulation of nerve axons and ‘early discharges’ in muscle spindle and tendon organ afferents that occur coincident with the onset of muscle contraction (Hunt & Kuffler, 1951). It has been argued that early discharges are elicited either by mechanical events at the onset of the twitch, or ephaptically by the synchronized action potentials in muscle fibres (Matthews, 1972). Either way, early discharges may also be elicited in large afferents in TMS-induced twitches, given the synchronicity of muscle activation involved. In conclusion, any initial afferent volleys directly evoked by electrical but not TMS stimuli might account for the slight time advance of perception of the movements induced by electrical stimuli.
In summary, an assessment has been made of the perceived time difference between finger movements elicited in one hand by peripheral electrical stimulation of muscles and in the opposite hand by TMS applied to the contralateral motor cortex. When the movements occurred simultaneously, subjects reported either that the two movements were indeed occurring at the same time or that the TMS-induced movement occurred late. The findings provide no evidence that TMS evokes an early efference copy.
Acknowledgments
This work was supported by the Alberta Heritage Foundation for Medical Research and the Wellcome Trust.
References
- Amassian VE, Cracco RQ, Maccabee PJ. A sense of movement elicited in paralyzed distal arm by focal magnetic coil stimulation of human motor cortex. Brain Res. 1989;479:355–360. doi: 10.1016/0006-8993(89)91640-5. [DOI] [PubMed] [Google Scholar]
- Andre-Obadia N, Garcia-Larrea L, Garassus P, Mauguiere F. Timing and characteristics of perceptual attenuation by transcranial stimulation: a study using magnetic cortical stimulation and somatosensory-evoked potentials. Psychophysiol. 1999;36:476–483. doi: 10.1017/s0048577299971676. [DOI] [PubMed] [Google Scholar]
- Bell JA. Light touch-deep pressure testing using Semmes-Weinstein monofilaments. In: Hunter JM, Schneider LH, Mackin EJ, Callahan AD, editors. Rehabilitation of the hand. Mosby, St Louis; 1984. pp. 399–406. [Google Scholar]
- Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, Cammarota A, Amassian VE, Cracco R, Maccabee P, Cracco J, Hallett M, Cohen LG. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116:511–525. doi: 10.1093/brain/116.3.511. [DOI] [PubMed] [Google Scholar]
- Burke D, Hicks R, Gandevia SC, Stephen J, Woodforth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J Physiol. 1993;470:383–393. doi: 10.1113/jphysiol.1993.sp019864. [published erratum appears in J Physiol (1994); 476, 553] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronicle EP, Glover J. A ticklish question: does magnetic stimulation of the primary motor cortex give rise to an ‘efference copy’? Cortex. 2003;39:105–110. doi: 10.1016/s0010-9452(08)70078-9. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Bandinelli S, Findley TW, Hallett M. Motor reorganization after upper limb amputation in man. A study with focal magnetic stimulation. Brain. 1991a;114:615–627. doi: 10.1093/brain/114.1.615. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Bandinelli S, Sato S, Kufta C, Hallett M. Attenuation in detection of somatosensory stimuli by transcranial magnetic stimulation. Electroenceph Clin Neurophysiol. 1991b;81:366–376. doi: 10.1016/0168-5597(91)90026-t. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Topka H, Cole RA, Hallett M. Leg paresthesias induced by magnetic brain stimulation in patients with thoracic spinal cord injury. Neurology. 1991c;41:1283–1288. doi: 10.1212/wnl.41.8.1283. [DOI] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens De Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989a;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [published erratum appears in J Physiol (Lond) 1990 November; 430: 617] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, Maertens De Noordhout A, Nakashima K, Shannon K, Marsden CD. Delay in the execution of voluntary movement by electrical or magnetic brain stimulation in intact man. Evidence for the storage of motor programs in the brain. Brain. 1989b;112:649–663. doi: 10.1093/brain/112.3.649. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. J Physiol. 1990;425:301–320. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Prochazka A, Chan M, Gauthier MJ. The sense of movement elicited by transcranial magnetic stimulation is due to sensory input, not efference copy. J Physiol. 2003;584.P:5–6P. doi: 10.1113/jphysiol.2003.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain. 1972;95:705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Haggard P, Clark S, Kalogeras J. Voluntary action and conscious awareness. Nat Neurosci. 2002;5:382–385. doi: 10.1038/nn827. [DOI] [PubMed] [Google Scholar]
- Haggard P, Eimer M. On the relation between brain potentials and the awareness of voluntary movements. Exp Brain Res. 1999;126:128–133. doi: 10.1007/s002210050722. [DOI] [PubMed] [Google Scholar]
- Hunt CC, Kuffler SW. Stretch receptor discharges during muscle contraction. J Physiol. 1951;113:298–315. doi: 10.1113/jphysiol.1951.sp004573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Sato M, Rothwell JC, Cohen LG. The effect of transcranial magnetic stimulation on median nerve somatosensory evoked potentials. Electroenceph Clin Neurophysiol. 1993;89:227–234. doi: 10.1016/0168-5597(93)90100-4. [DOI] [PubMed] [Google Scholar]
- Levy WJ, Jr, Amassian VE, Traad M, Cadwell J. Focal magnetic coil stimulation reveals motor cortical system reorganized in humans after traumatic quadriplegia. Brain Res. 1990;510:130–134. doi: 10.1016/0006-8993(90)90738-w. [DOI] [PubMed] [Google Scholar]
- Libet B, Alberts WW, Wright EW, Jr, Delattre LD, Levin G, Feinstein B. Production of threshold levels of conscious sensation by electrical stimulation of human somatosensory cortex. J Neurophysiol. 1964;27:546–578. doi: 10.1152/jn.1964.27.4.546. [DOI] [PubMed] [Google Scholar]
- Libet B, Gleason CA, Wright EW, Pearl DK. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983;106:623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- Libet B, Wright EW, Jr, Feinstein B, Pearl DK. Subjective referral of the timing for a conscious sensory experience: a functional role for the somatosensory specific projection system in man. Brain. 1979;102:193–224. doi: 10.1093/brain/102.1.193. [DOI] [PubMed] [Google Scholar]
- MacDonald PA, Paus T. The role of parietal cortex in awareness of self-generated movements: a transcranial magnetic stimulation study. Cereb Cortex. 2003;13:962–967. doi: 10.1093/cercor/13.9.962. [DOI] [PubMed] [Google Scholar]
- McKay DR, Ridding MC, Miles TS. Magnetic stimulation of motor and somatosensory cortices suppresses perception of ulnar nerve stimuli. Int J Psychophysiol. 2003;48:25–33. doi: 10.1016/s0167-8760(02)00159-9. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and Their Central Actions. London: Arnold; 1972. [Google Scholar]
- Pascual-Leone A, Cohen LG, Brasil-Neto JP, Valls-Sole J, Hallett M. Differentiation of sensorimotor neuronal structures responsible for induction of motor evoked potentials, attenuation in detection of somatosensory stimuli, and induction of sensation of movement by mapping of optimal current directions. Electroenceph Clin Neurophysiol. 1994;93:230–236. doi: 10.1016/0168-5597(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Pockett S. On subjective back-referral and how long it takes to become conscious of a stimulus: a reinterpretation of Libet's data. Conscious Cogn. 2002;11:144–161. doi: 10.1006/ccog.2002.0549. [DOI] [PubMed] [Google Scholar]
- Seyal M, Ro T, Rafal R. Increased sensitivity to ipsilateral cutaneous stimuli following transcranial magnetic stimulation of the parietal lobe. Ann Neurol. 1995;38:264–267. doi: 10.1002/ana.410380221. [DOI] [PubMed] [Google Scholar]
- Williams PL, Warwick R. Gray's Anatomy. 36. London: Churchill Livingstone; 1980. [Google Scholar]