Abstract
Recent studies have proposed that release of calcium from the sarcoplasmic reticulum (SR) modulates the spontaneous activity of the sinoatrial node (SAN). Previously we have shown that several calcium regulatory proteins are expressed at a lower level in the centre of the SAN compared with the periphery. Such differences may produce heterogeneity of intracellular calcium handling and pacemaker activity across the SAN. Selective isolations showed that the centre of the SAN is composed of smaller cells than the periphery. Measurements of cytosolic calcium in spontaneously beating cells showed that diastolic calcium, systolic calcium, the calcium transient amplitude and spontaneous rate were greater in larger (likely to be peripheral) cells compared with smaller (likely to be central) SAN cells. The SR calcium content was greater in larger cells, although SR recruitment was more efficient in smaller cells. The sodium–calcium exchanger and sarcolemmal calcium ATPase had a lower activity and the exchanger was responsible for a larger proportion of sarcolemmal calcium extrusion in smaller cells compared with larger cells. Ryanodine had a greater effect on the spontaneous calcium transient in larger cells compared with smaller cells, and slowed pacemaker activity in larger cells but not smaller cells, thus abolishing the difference in cycle length. This study shows heterogeneity of intracellular calcium regulation within the SAN and this contributes to differences in pacemaker activity between cells from across the SAN. The smallest central cells of the leading pacemaker region of the SAN do not require SR calcium for spontaneous activity nor does disruption of the SR alter pacemaking in these primary pacemaker cells.
The mammalian sinoatrial node (SAN) is a heterogeneous structure (Boyett et al. 2000). During normal cardiac activation the spontaneous action potential originates at the centre of the SAN, propagates through the periphery to the crista terminalis and so conducts to the rest of the heart (Bleeker et al. 1980). Morphologically there are several differences across the SAN: cells in the centre, the ‘primary pacemaker cells', have been described as being apparently smaller than those found in the periphery (Bleeker et al. 1980; Boyett et al. 2000) and ‘empty’ in appearance, containing few distinguishable internal structures (Bleeker et al. 1980; Masson-Pevet et al. 1984). In addition there are differences in the expression of ion channels (Honjo et al. 1996,1999), the shape of the action potential (Honjo et al. 1996; Boyett et al. 1998) and intrinsic spontaneous rate (Boyett et al. 1998) between cells from the centre and periphery of the SAN.
Several recent studies have proposed that calcium release from the sarcoplasmic reticulum (SR) is critical for the spontaneous activity of the SAN (Rigg & Terrar, 1996; Hata et al. 1996; Li et al. 1997; Bogdanov et al. 2001; Vinogradova et al. 2002a;). The proposed mechanism is that calcium from spontaneous SR releases, termed ‘sparks’, occurring in late diastole is removed by the sarcolemmal sodium–calcium exchanger producing depolarizing current contributing to the initiation of the spontaneous action potential (Bogdanov et al. 2001). The importance of this mechanism in pacemaking remains controversial (DiFrancesco & Robinson, 2002; Vinogradova et al. 2002b; Honjo et al. 2003; Lakatta et al. 2003).
Recent work has identified regional differences in the expression of the L-type calcium channel, ryanodine receptor, and SERCA2a (the SR calcium pump) across the SAN (Musa et al. 2002), all of which are expressed at a lower level in the centre of the SAN compared with the periphery. Such differences may produce heterogeneity of calcium handling and potentially functional differences in pacemaking between cells from the centre and periphery of the SAN.
We have measured cytosolic calcium in cells from the rabbit SAN and found significant heterogeneity of intracellular calcium regulation. This heterogeneity can help explain differences in spontaneous activity between different regions of the SAN and may modulate the response of the SAN to physiological and pharmacological interventions. We conclude that calcium release from the SR is not a prerequisite for the spontaneous activity of the rabbit SAN, although it is a significant modulator of pacemaker activity and is a contributing factor to the previously noted differences in the spontaneous activity of the periphery and centre of the SAN.
Methods
Preparation of single SAN cells and experimental conditions
Single rabbit SAN and atrial cells were prepared as previously described (Lei et al. 2000). New Zealand White rabbits were purchased from Harlan Ltd (UK). All animal procedures were performed in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986. Animals were humanely killed by intravenous administration of an anaesthetic overdose (sodium pentobarbitone, 200 mg kg−1). During experiments, cells were continuously superfused with a Tyrode solution containing (mmol l−1): NaCl, 134; KCl, 4; MgCl2, 1; CaCl2, 2; glucose, 11; Hepes, 10; titrated with NaOH to a pH of 7.4. All experiments were conducted at 37°C. Ryanodine was used at 2 or 30 μmol l−1 and its effect measured under steady-state conditions after 10–15 min exposure. Caffeine (20 mmol l−1) was applied using a temperature-controlled rapid perfusion device (MPRE8, Cell MicroControls, VA, USA). Rapid perfusion was ensured by positioning the output of the perfusion device within 150 μm of the cell under study. Nickel (5 mmol l−1) was used to block the sodium–calcium exchanger.
Fluorescence measurements
Cells were loaded with the acetoxymethyl ester form of the Ca2+ indicator Fluo-3 (Fluo-3 AM, loaded at 5 μmol l−1 for 5 min at room temperature followed by 30 min for de-esterification). Fluo-3 fluorescence was excited using light with a wavelength of 488 nm and measured between 520 and 560 nm using a standard microscope fluorometry system (Cairn Research Ltd, UK). Briefly, Fluo-3-loaded cells were placed in a bath on the stage of a Nikon TMD inverted microscope and excitatory light focused on the sample; the resultant fluorescence was collected via a ×40 magnification oil-immersion objective. The fluorescence signal was recorded using a photomultiplier tube and the amplified signal saved to a PC for analysis. Movement artefacts were avoided by measuring fluorescence from the entire cell which was subject to uniform illumination intensity. Fluo-3 has negligible fluorescence in the absence of calcium and therefore may be calibrated according to the equation:
where F is the measured fluorescence, Fmax the fluorescence when the dye is saturated with calcium, and Kd the dissociation constant (864 nmol l−1; Merritt et al. 1990). Fmax was obtained by equilibrating the cytosolic calcium with extracellular calcium using either the ionophore 4-bromo-A-23187 or by damaging the cell with a patch pipette (Trafford et al. 1999). Approximately 20% of recordings were calibrated by using the 4-bromo-A23187 method. There were no significant differences between data calibrated using either method. A rectangular diaphragm was used to exclude fluorescence from neighbouring cells. Background fluorescence was subtracted from each trace as recorded with the cell removed from the field of observation.
Indo-1 was loaded using the acetoxymethyl ester form of the dye (2.5 μmol l−1 for 10 min at room temperature). Indo-1 signals were recorded and calibrated as previously described (Bassani et al. 1995). Not all recordings of intracellular calcium were calibrated.
Measurement of cell size
Images of cells were captured using a CCD camera and the projected area calculated using Scion Image (Scion Corporation, USA). The system was calibrated using 15 μm diameter FocalCheck beads (Molecular Probes, Oregon, USA).
Tissue samples from different regions of the SAN were taken using a circular punch-biopsy sampler (area 1 mm2) and used for cell isolation by the normal protocol. These samples were taken from immediately adjacent to the crista terminalis and centred on the normal location of the leading pacemaker site within the isolated rabbit sinoatrial node preparation. Of the five preparations used, in two the electrical activation pattern was assessed to determine the location of the leading pacemaker site using bi-polar extracellular electrodes prior to obtaining the tissue samples. In each case the leading pacemaker site was in a comparable anatomical location to that which has been previously well defined by other studies. This location thus appears to be the normal location of the leading pacemaker site in the rabbit sinoatrial node preparation under the conditions used and was used as the site for the other samples taken. Single cells obtained from the cell isolation protocol were loaded with calcein (5 μmol l−1 calcein-AM for 5 min). Cells were scanned using a laser scanning confocal microscope (LSM 510, Zeiss, Germany) to produce ultra-high resolution 3-D images of each cell. From these images, the projected area of the cell, the volume (using Bitplane Imaris Measurement Pro, Bitplane AG, Switzerland) and other morphometric parameters were measured.
Data analysis
Data are shown as means ±s.e.m. Statistical analysis was carried out using SigmaStat (SPSS Inc., Chicago, USA). A difference was taken as significant if P < 0.05. Comparisons between normally distributed data were analysed using ANOVA with Holm-Sidak comparisons. Various characteristics were plotted versus cell size. Comparisons of different linear and polynomial functions fitted to the data were made by F tests; the simplest model producing the best fit was used to describe the data. This in all cases proved to be a simple linear function. Therefore, data were fitted by linear regression. This yielded the probability P that there was no variation with cell size (i.e. the slope, m, of the fitted line was zero) and the correlation coefficient, r. Both P and r are reported. If P < 0.05, there was assumed to be a significant correlative variation with cell size. For some analyses, data were grouped according to cell size by dividing the total population into approximate thirds, permitting characteristics to be compared between the smallest (projected area <400 μm2), intermediate (400–700 μm2) and largest (>700 μm2) cells; the data were then analysed using ANOVA as described above.
Results
Cellular differences across the SAN
Throughout this study, we have used a simple measurement of cell size: the projected area of the cell image as observed during light microscopy. For the data shown in Fig. 1A, single myocytes were isolated from 1 mm2 pieces of tissue dissected from the centre of the SAN (centred on the leading pacemaker site), the periphery of the SAN adjacent to the crista-terminalis (CT), the CT and the right atrial appendage. Cells from the centre of the SAN had a significantly smaller projected area than cells from the periphery (410 ± 28 μm2versus 704 ± 36 μm2), and both peripheral and central cells were significantly smaller than cells from the CT (area, 1072 ± 71 μm2) and atrial tissue (1253 ± 138 μm2), although CT cells were not significantly smaller than atrial cells (n = 25 in each case). It is difficult to isolate cells separately from the periphery and centre of the SAN routinely, due to the small size of the tissue samples and associated small cell yields. It is more feasible to isolate cells from the whole of the SAN and use the projected area of the cell as an indicator of probable origin within the SAN. When isolating cells from the whole SAN, a continuum of cell sizes ranging from 205 to 1231 μm2 (projected area) were obtained. We hypothesize that these represent a continuous distribution of cells from the centre of the SAN, where the smallest cells are found, to the periphery, where the largest cells are found (Fig. 1A). Cellular properties correlating with cell size are therefore likely to vary regionally within the SAN.
Figure 1. SAN cell characteristics and spontaneous calcium transients.
A, mean ±s.e.m. projected cell area for single cells isolated from the right atrial appendage, crista terminalis, peripheral SAN and central SAN. * Significant difference (P < 0.05) by ANOVA with Holm-Sidak comparisons. B, spontaneous intracellular calcium transients recorded from large (area, 781 μm2; upper trace) and small (area 309 μm2; lower trace) cells.
Calcium transients in large and small cells from the SAN
Figure 1B shows recordings of spontaneous cytosolic calcium transients from a large cell (likely to be from the periphery of the SAN) and a small cell (likely to be from the centre of the SAN) illustrating significant differences in the amplitude and time course of the intracellular calcium transient. Figure 2A shows diastolic calcium and peak systolic calcium concentration versus cell size for all calibrated intracellular calcium records. Due to possible concerns regarding the calibration of the Fluo-3 signal, we confirmed the findings by performing some measurements using Indo-1 (Fig. 2A). Indo-1 is a ratiometric indicator, which is less prone to artefacts due to loss of dye and cell morphology changes, etc. However, Indo-1 is a stronger calcium buffer than Fluo-3 and Indo-1 loading had an adverse effect on cell viability and rendered many smaller cells quiescent. Fluo-3 loading did not significantly alter the spontaneous beating rate in SAN cells (mean cycle length in loaded cells was 348 ± 11 ms (n = 36) versus 354 ± 14 ms (n = 25) in unloaded cells; P > 0.3). This was true for all sizes of cells studied (mean cycle length in small cells was 397 ± 17 ms in loaded cells (n = 12) versus 387 ± 18 ms in unloaded cells (n = 9); mean cycle length in intermediate cells was 324 ± 14 ms in loaded cells (n = 14) versus 346 ± 17 ms in unloaded cells (n = 9); mean cycle length in large cells was 290 ± 14 ms in loaded cells (n = 10) versus 291 ± 9 ms in unloaded cells (n = 7)); further details are contained within the online data supplement. For this reason we considered that Fluo-3 was the most appropriate indicator.
Figure 2. Measurements of the spontaneous intracellular calcium transient.
A, diastolic (open symbols) and systolic (filled symbols) intracellular calcium plotted against projected cell area. Dotted symbols are data recorded using Indo-1 as the calcium indicator; all other recordings were obtained using Fluo-3. B, calcium transient amplitude plotted against projected cell area. In A and B, data have been fitted with a linear regression. C, mean ±s.e.m. diastolic calcium, systolic calcium and amplitude of the calcium transient for large (>700 μm2), intermediate (400–700 μm2) and small (<400 μm2) cells. * Significant difference (P < 0.05) by ANOVA with Holm-Sidak comparison from the small cell data.
For recordings using Fluo-3 and Indo-1 there were significant correlations between cell size and peak systolic calcium (Fluo-3, P < 0.0001, r = 0.79, n = 21; Indo-1, P = 0.03, r = 0.89, n = 7). For recordings using Fluo-3 there was a significant correlation between cell size and diastolic calcium (P = 0.0003, n = 21, r = 0.75). After combining the data obtained using the two dyes significant correlations were shown between cell size and peak systolic calcium and cell size and diastolic calcium (P < 0.0001, n= 28 in each case, r = 0.79 and 0.76, respectively). In Fig. 2A the data are shown fitted with a linear regression to highlight the correlation. Although the data on initial inspection appears to have a steeper correlation amongst smaller cells, multifactorial polynomial regressions did not produce a significantly improved fit to the data compared with a simple linear regression (assessed by F test of differing models).
Dividing the data according to cell size for comparative purposes showed there to be significant differences in diastolic calcium and peak systolic calcium between the largest (> 700 μm2, n = 8) and smallest (< 400 μm2, n = 10) cells and between the intermediate (400–700 μm2, n= 10) and smallest cells (Fig. 2C). There was no significant difference between the intermediate and largest cells. Diastolic calcium was 401 ± 19 nmol l−1 in the largest cells versus 386 ± 30 nmol l−1 in the intermediate cells and 279 ± 36 nmol l−1 in the smallest cells. Systolic calcium was 1143 ± 58 nmol l−1versus 996 ± 65 nmol l−1versus 534 ± 77 nmol l−1 for the largest, intermediate and smallest cells, respectively.
Figure 2B shows the amplitude of the calcium transient plotted against cell size for data obtained using Fluo-3 and Indo-1. For both dyes (individually and combined), the data are significantly correlated (P < 0.0001, r= 0.75, n= 28 for the combined data). In Fig. 2B again the data have been fitted with a linear regression to highlight the correlation. Dividing the data into categories according to cell size showed a significant difference in calcium transient amplitude between the largest and the smallest cells and the intermediate and the smallest cells but not between the largest and intermediate cells (Fig. 2C). The calcium transient amplitude was 741 ± 46 nmol l−1 in the largest cells versus 610 ± 50 nmol l−1 in the intermediate cells versus 254 ± 51 nmol l−1 in the smallest cells.
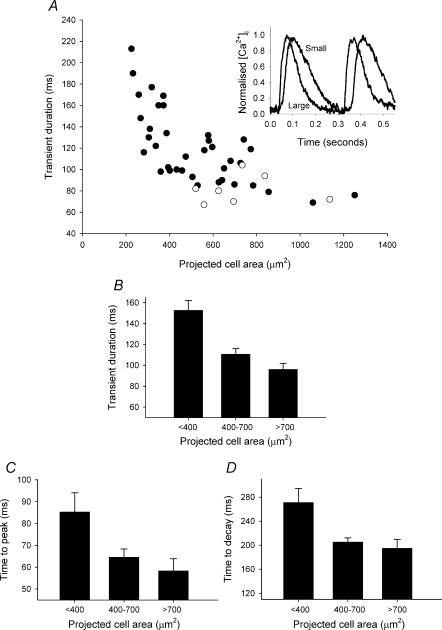
There is a significant negative correlation between calcium transient duration and cell size for SAN cells (P < 0.0001, r= 0.8, n = 37), but not atrial cells (electrically paced at 3 Hz; P = 0.95, n= 7) (Fig. 3A). The stimulation rate used for the atrial cells approximated the mean spontaneous rate of the SAN cells studied (2.9 Hz). Therefore the calcium transient kinetics of only SAN cells showed cell size dependence. As a measure of calcium transient duration, the time difference between a transient reaching half the peak systolic value and decaying halfway back to the diastolic level was used. The calcium transient in the smallest cells was longer (153 ± 10 ms; n= 12) than that in intermediate cells (110 ± 6 ms; n = 15) and in the largest cells (96 ± 6 ms; n = 10) (Fig. 3B). This is illustrated by the superimposed normalized representative recordings from small and large cells shown in the inset of Fig. 3A. The difference in the kinetics was apparent in both the rise and decay of the calcium transient, both of which were significantly slower in the smallest cells compared with the intermediate and largest cells. The time to peak was 58 ± 6 ms in large cells (n = 7), 65 ± 4 nmol l−1 in intermediate cells (n = 13) and 65 ± 4 ms in small cells (n = 12) (Fig. 3C) and the time for calcium to decay from its peak value to the diastolic level was 195 ± 15 ms in large cells, 205 ± 7 nmol l−1 in intermediate cells and 271 ± 24 ms in small cells (Fig. 3D). The difference in duration, time to peak and time to decay between the intermediate and largest cells was not significant.
Figure 3. Time course of the spontaneous calcium transient.
A, calcium transient duration plotted against cell area. Filled symbols are data from spontaneous SAN cells; open symbols are data from electrically paced left atrial cells. The inset shows representative normalized calcium transients for large (area, 781 μm2) and small (area, 309 μm2) cells. B, mean ±s.e.m. of calcium transient duration for large, intermediate and small cells. C and D, mean ±s.e.m. time to peak and time to decay, respectively, for large, intermediate and small cells.
Calcium buffering
A possible explanation for the differences observed in the calcium transient time course and amplitude is that there are differences in intracellular calcium buffering between cells from the periphery and centre of the SAN. Such a difference may be intrinsic or introduced by excessive loading of the calcium indicator Fluo-3, itself a calcium buffer, into smaller cells. However, control experiments (details available online as Supplementary Material) showed that calcium buffering by Fluo-3 is unlikely to be a significant factor.
SR calcium content and recruitment in spontaneous SAN cells
A smaller contribution of the SR to calcium handling in smaller cells compared with larger cells could also explain the smaller and slower calcium transient. We investigated calcium handling by the SR using caffeine to cause rapid release of the SR calcium content. Figure 4A shows the amplitude of the intracellular calcium transient evoked by rapid application of 20 mmol l−1 caffeine to spontaneously beating SAN cells. The caffeine application was timed to coincide with the diastolic phase of the spontaneous transients. The amplitude of the transient is expressed in terms of F/F0, where F is the peak fluorescence of the caffeine transient and F0 the lowest diastolic fluorescence. The amplitude of the caffeine transient significantly correlates with cell size (P < 0.0001, r = 0.76, n = 39). When data are divided according to cell size, the smallest cells were shown to have a significantly smaller caffeine transient (F/F0 = 2.2 ± 0.1, n = 12) than large cells (F/F0= 3.5 ± 0.2, n = 14) but not compared with intermediate cells (F/F0= 2.5 ± 0.2, n = 13); which also had a significantly smaller transient than the large cells.
Figure 4. Caffeine-evoked calcium transients in SAN cells.
A, amplitude of the caffeine-evoked transient plotted against projected cell area. The transient amplitude is expressed as F/F0, where F is the measured fluorescence and F0 the diastolic minimum fluorescence. B, fractional release of SR calcium during the spontaneous calcium transient plotted against projected cell area. In A and B, data have been fitted with a linear regression. C, an example of a caffeine-evoked transient in which resumption of spontaneous activity occurred in the presence of caffeine. Caffeine, 20 mmol l−1, was applied as indicated by the bar (cell area = 437 μm2).
The amplitude of the normal spontaneous transient as a fraction of the caffeine-evoked transient, termed fractional release, is an estimate of the proportion of the SR calcium content released during each spontaneous transient. Figure 4B shows the fractional release plotted against cell size. There is a significant negative correlation between fractional release and cell size (P = 0.017, r = 0.84, n = 39): small cells had spontaneous transients which were a larger fraction of the caffeine transient (0.74 ± 0.03, n = 12) than large cells (0.62 ± 0.03, n = 14) and therefore had a smaller reserve of calcium which remained unrecruited during normal spontaneous activity. Intermediate cells had a fractional release of 0.67 ± 0.04 (n = 13) which is not significantly different from that of small or large cells.
Some cells exposed to caffeine showed continued spontaneous activity in the presence of caffeine (Fig. 4C). Continued activity in the presence of caffeine occurred in 4 out of 12 small cells, 2 out of 13 intermediate cells and 1 out of 14 large cells (P = 0.4, χ2). This spontaneous activity persisted in the absence of any residual SR function at a reduced cycle length.
Calcium extrusion from SAN cells
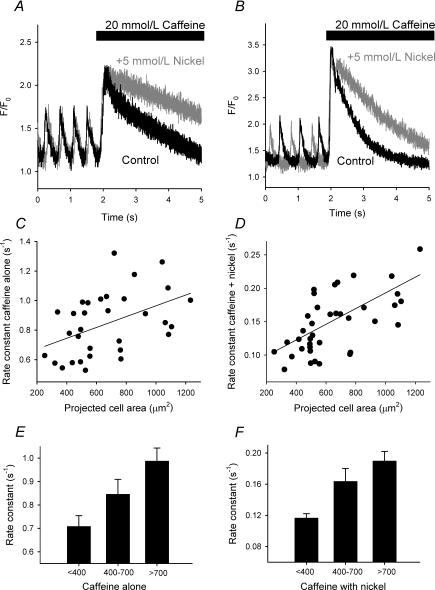
Figure 5 shows caffeine-evoked transients from spontaneously beating small (Fig. 5A) and large (Fig. 5B) SAN cells. The black trace shows the normal caffeine transient and the grey trace the transient produced when caffeine was applied in the presence of 5 mmol l−1 nickel (to block the sodium–calcium exchanger). In both instances, blockade of the exchanger caused a significant slowing of the decay of the caffeine transient. Figure 5C shows the rate constant for the decay of the caffeine transient with the exchanger active plotted against cell size and Fig. 5D the rate constant for the decay of the caffeine transient with the exchanger blocked plotted against cell size. There is a significant correlation between cell size and the rate of decay of the caffeine transient in both cases (P = 0.005, r = 0.79, n = 32 for the normal caffeine transient and P= 0.0002, r = 0.72, n = 39 for the caffeine transient in the presence of nickel). In some instances, it was not possible to obtain reliable data for the decay of the caffeine transient due to the rapid resumption of spontaneous activity in the presence of caffeine. Small cells have a slower rate of decay of the caffeine transient (in the absence of nickel) than large cells but not intermediate cells (Fig. 5E): 0.71 ± 0.05 s−1 for small cells (n = 8) versus 0.85 ± 0.06 s−1 for intermediate cells (n = 11) and 0.99 ± 0.06 s−1 for large cells (n = 13). This difference in rate constants persisted in the presence of nickel (Fig. 5F). With the sodium–calcium exchanger blocked, the rate constant for the decay of the caffeine transient was 0.12 ± 0.01 s−1 for small cells (n = 12), 0.16 ± 0.02 s−1 for intermediate cells (n = 13) and 0.19 ± 0.01 s−1 for large cells (n = 14). It is concluded that smaller cells have a lower rate of sarcolemmal calcium extrusion and this is due to a combination of a lower transport capability of the sarcolemmal sodium–calcium exchanger, calcium ATPase and other calcium transport pathways compared with large cells.
Figure 5. Calcium extrusion from SAN cells.
A and B, caffeine-evoked calcium transients in small (A) (cell area = 370 μm2) and large (B) (cell area = 1062 μm2) cells. The bar indicates when caffeine was applied. The black trace is when caffeine was applied alone. The grey trace is when caffeine was applied with 5 mmol l−1 nickel (to block the sodium–calcium exchanger). C and D, rate constants for the decay of the caffeine transient with (D) and without (C) the sodium–calcium exchanger blocked, plotted against projected cell area; the data have been fitted with a linear regression. E and F, comparisons between large, intermediate and small cells of the rate of decay of the caffeine transient in the absence (E) and presence (F) of nickel.
The contribution of the sodium–calcium exchanger and remaining (non-exchanger) calcium flux pathways to calcium removal from the cytoplasm can be calculated from the change in the rate constant for the decay of the caffeine transient when the exchanger is blocked. This assessment relies on the decay of the caffeine transients displaying simple first order kinetics, as was the case in the cells studied. The non-exchanger component is likely to be chiefly calcium extrusion from the cell on the sarcolemmal calcium ATPase, although flux of calcium into mitochondria via the calcium uniporter may contribute. Together these ‘non-exchanger’ calcium extrusion routes are termed the ‘slow’ calcium extrusion pathways.
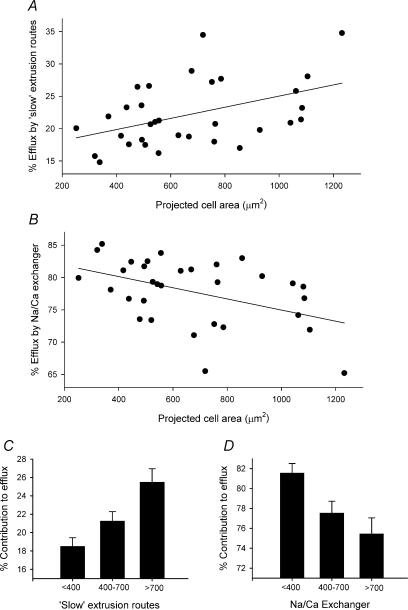
Figure 6A shows the percentage of cytosolic calcium that is removed from the cell on the slow calcium extrusion pathways versus cell size. These data are significantly correlated (P = 0.01, r = 0.8, n = 32). In the absence of SR calcium uptake the mean percentage removal by these routes (chiefly the sarcolemmal calcium ATPase) in all cells was 22.2 ± 0.9%. Figure 6B shows the percentage of the cytosolic calcium removed by the sodium–calcium exchanger. Again the data are significantly correlated with cell size (P = 0.007, r = 0.9, n = 32). The mean percentage removal by the exchanger in all cells was 77.8 ± 0.9%. The slow calcium removal pathways play a smaller role in extruding calcium in small cells than in large or intermediate cells (Fig. 6C). In small cells the slow calcium flux pathways removed 18.5 ± 1.0% of the released calcium (n = 8), compared to 21.2 ± 1.0% in intermediate cells (n = 11) and 25.5 ± 1.5% in large cells (n = 13). It follows that the sodium–calcium exchanger has a bigger contribution to calcium removal in smaller cells than in larger cells (Fig. 6D). In small cells, the exchanger was responsible for removing 81.6 ± 1.0% of cytoplasmic calcium versus 77.5 ± 1.2% in intermediate cells and 75.4 ± 1.6% in large cells.
Figure 6. Sarcolemmal calcium removal in SAN cells.
A and B, percentage contribution of the ‘slow’ calcium removal pathways (chiefly sarcolemmal calcium ATPase and the mitochondrial calcium uniporter) (A) and sodium–calcium exchanger (B) plotted against projected cell area. The data have been fitted with a linear regression. C and D, comparison between large, intermediate and small cells of the percentage contribution to sarcolemmal calcium extrusion by ‘slow’ calcium removal pathways (C) and sodium–calcium exchanger (D). Data are means ±s.e.m.
Effects of ryanodine on calcium transients
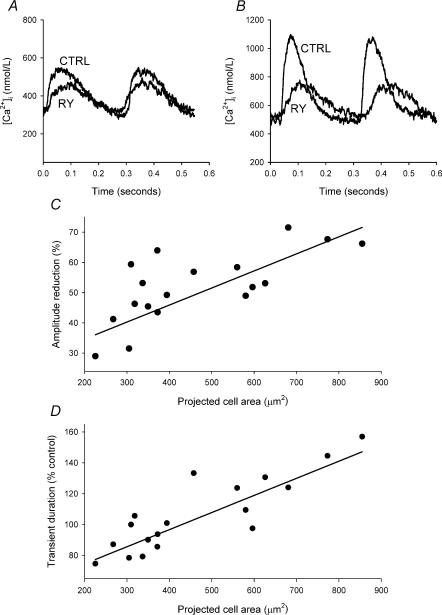
The role of the SR in SAN function can be investigated by depleting the SR of calcium using ryanodine. The effects of 2 μmol l−1 ryanodine on spontaneous intracellular calcium transients are illustrated in Fig. 7 for small (Fig. 7A) and large (Fig. 7B) cells – in both cases, ryanodine reduced the amplitude of the calcium transient. There is a significant correlation between the amount by which ryanodine reduced the calcium transient and cell size (P = 0.001, r = 0.85, n = 18) (Fig. 7C). A linear regression is shown fitted to the data to highlight the correlation. Ryanodine decreased the amplitude of the calcium transient to a significantly greater extent in large cells (65 ± 4%) compared with small cells (44 ± 4%) but not intermediate cells (53 ± 3%). Ryanodine did not alter diastolic calcium in large, intermediate or small cells.
Figure 7. Effect of ryanodine on the spontaneous calcium transient.
A and B, calcium transients recorded under control conditions (CTRL) and in the presence of 2 μmol l−1 ryanodine (RY) recorded from small (A) (cell area = 309 μm2) and large (B) (cell area = 781 μm2) cells. C, percentage reduction of the calcium transient by ryanodine plotted against projected cell area. D, calcium transient duration in the presence of ryanodine, expressed as a percentage of the control transient duration, plotted against projected cell area. In C and D, the data are fitted with a linear regression.
Ryanodine also had differential effects on the time course of the calcium transient; the duration was significantly prolonged in large cells (to 139 ± 7% of control), remained unaltered in intermediate cells (110 ± 8% of control) but was shortened in small cells (90 ± 4% of control). There is a significant correlation between the percentage change of the calcium transient duration and cell size (P < 0.0001, r = 0.9, n = 18) (Fig. 7D). Following the application of ryanodine, there was no longer a significant difference in the duration of the calcium transient between large and small cells.
Correlation of spontaneous activity with cell size – the SR influence
Under control conditions there was a significant correlation between cell size and spontaneous cycle length (time between spontaneous calcium transients) (P = 0.003, r = 0.8, n = 36) (Fig. 8A); large cells had a significantly shorter cycle length than small cells but not intermediate cells, although these in turn had a significantly shorter cycle length than small cells. The mean cycle length for large cells was 290 ± 14 ms (n = 10) versus 324 ± 14 ms for intermediate cells (n = 14) and 397 ± 17 ms for small cells (n = 12). A correlation between spontaneous cycle length and cell size was found in both unloaded cells and Fluo-3-loaded cells (see Supplementary Material available online). There was no significant alteration in spontaneous cycle length by Fluo-3 loading in small, intermediate or large cells.
Figure 8. Spontaneous activity and the effect of ryanodine.
A and B, cycle length (time between adjacent spontaneous calcium transients) plotted against cell area. In A, cells were in control solution; in B, cells had been treated with 2 μmol l−1 ryanodine. The data are fitted with linear regressions. C, percentage change in cycle length produced by ryanodine (2 and 30 μmol l−1 data combined) for small, intermediate and large cells. Data are means ±s.e.m.
Ryanodine abolished the correlation between cell size and spontaneous cycle length (Fig. 8B) by disparate effects on the spontaneous activity of large and small SAN cells (P = 0.3, n = 36). Ryanodine significantly slowed the spontaneous rate of large cells by 27 ± 8% (n = 9) (an amount comparable in magnitude to that reported by others for mammalian SAN cells; Li et al. 1997) but failed to significantly alter that of small (−5 ± 11%, n= 12) or intermediate (11 ± 10%, n= 14) cells.
To verify the ability of 2 μmol l−1 ryanodine to completely deplete the SR of calcium, the rapid application of 20 mmol l−1 caffeine was used to test for any remaining releasable calcium. No caffeine response was observed following the application of 2 μmol l−1 ryanodine (data not shown). Previously, we have shown that 30 μmol l−1 ryanodine causes a slowing of the spontaneous activity of isolated SAN cells by 22 ± 3% (Honjo et al. 2003). Analysis of the 30 μmol l−1 ryanodine data yielded similar conclusions to that of the 2 μmol l−1 ryanodine data. Ryanodine at 30 μmol l−1 increased spontaneous cycle length in large cells by 24 ± 6% and in intermediate cells by 16.4 ± 3% but failed to significantly alter the cycle length of small cells (change = 3.5 ± 2%). There were no significant differences between the effects of 2 or 30 μmol l−1 ryanodine, the lower concentration being apparently sufficient to cause full depletion of the SR and maximal effect. The combined data for the effects of 2 and 30 μmol l−1 ryanodine on the spontaneous cycle length of small, intermediate and large cells are shown in Fig. 8C.
Discussion
This study shows heterogeneity of intracellular calcium regulation and the spontaneous intracellular calcium transient in cells from the SAN. This heterogeneity correlates with differences in spontaneous activity. Spontaneous activity is greatest in the largest, postulated peripheral, cells of the SAN, which have the highest SR calcium content and largest spontaneous calcium transient. Removing the role of the SR calcium in pacemaking makes these cells become like the smallest, postulated central, SAN cells and removes differences in their pacemaker activity; however, differences in diastolic calcium and the intracellular calcium transient remain.
Cell size
Previous studies identified an apparent difference in terms of cell size between cells from the periphery and centre of the SAN (Bleeker et al. 1980). As part of this study, we have confirmed this in a quantitative manner. It follows that cell size can be used as a marker of location within the SAN (Fig. 1A), greatly simplifying the study of heterogeneity of the SAN. We extend this idea to hypothesize from this and previous data that there is a continuum of cell sizes within the SAN from the smallest cells in the centre to the largest cells in the periphery. This idea is supported by the discovery of apparent continuous trends in cellular properties and the lack of identified discontinuous subpopulations (Boyett et al. 2000). However, to confirm this will require further studies of the intact tissue.
Calcium handling
The smaller cells of the SAN, likely to be from the central leading pacemaker region, have a lower diastolic calcium, peak systolic calcium, spontaneous calcium transient amplitude and caffeine transient amplitude compared with the larger, putative peripheral, SAN cells. These findings correlate with data from immunohistochemical studies, showing a lower expression of the ryanodine receptor and SERCA2a in the central region of the SAN compared with the periphery, suggesting that SR is present at a lower density (Musa et al. 2002). In the presence of ryanodine, however, the calcium transient remained smaller in the small cells of the SAN compared with larger cells. Thus the SR is not responsible for all the differences in the calcium transient observed. The remaining differences are presumably due to previously identified differences in the density of the calcium current between large and small cells of the SAN (proposed to represent a centre–periphery difference; larger cells having a higher calcium current density; Musa et al. 2002). The identified differences in the contribution of the SR to the spontaneous calcium transient between large and small cells can, however, explain the slower kinetics of the calcium transient, smaller caffeine transient and smaller impact of ryanodine found in smaller cells compared with larger cells.
Calcium extrusion from the cells of the SAN by sarcolemmal calcium transport processes is slower in smaller cells compared with larger cells. This correlates well with previous work showing a lower expression of the sodium–calcium exchanger in cells from the centre of the SAN (Musa et al. 2002), although despite the reduced overall transport capacity the actual percentage contribution to calcium removal from the cytoplasm by the exchanger is greater in the smaller cells of the SAN compared with the larger cells. The principal remaining calcium transport process is likely to be efflux on the sarcolemmal calcium ATPase as has been shown in rabbit ventricular myocytes (Bassani et al. 1994). Another potentially important transport route is the mitochondrial calcium uniporter although in ventricular cells this route is slow with a limited capacity (Bassani et al. 1994) and is perhaps likely to be of even less significance in cells from the SAN which have been reported to have a low mitochondrial density (Masson-Pevet et al. 1984).
Intracellular calcium and pacemaking
In the present study, ryanodine had no significant effect on the pacemaking of the smallest, putative central SAN cells, but caused a modest slowing in the largest SAN cells likely to originate from the periphery (Fig. 8C). Previous work on single SAN cells (Bassani et al. 1997; Honjo et al. 2003), isolated intact SAN preparations (Honjo et al. 2003), and isolated right atria (Lipsius, 1989; Bassani et al. 1999) has shown that ryanodine causes a modest slowing of pacemaking. Ryanodine at low (2 μmol l−1) or high concentrations (30 μmol l−1) does not cause cessation of spontaneous activity (Honjo et al. 2003) nor does cyclopiazonic acid (Rigg & Terrar, 1996), thapsigargin (Bassani et al. 1997), or, in some cases, application of caffeine (Fig. 4C), despite the disruption of SR function by all these manoeuvres. The fact that the disruption of SR function can cause a slowing of spontaneous activity has been used to argue that SR calcium release contributes to the normal pacemaking of SAN cells (Vinogradova et al. 2002a; Lakatta et al. 2003). The conclusion of the current study is that SR calcium release is a significant modulator of pacemaking but its influence is greatest in peripheral SAN cells (latent pacemaker cells; Bassani et al. 1997; Huser et al. 2000), having an apparently limited influence on the pacemaking of the small cells of the SAN, which are likely to be from the region of the normal primary pacemaker. The present study shows SR differences confer on the larger (peripheral) cells faster pacemaking than smaller (central) cells (differences in the density of various ionic currents between large and small cells are also likely to contribute to the difference in pacemaking; Boyett et al. 2000). However, the peripheral cells are normally electrotonically suppressed by the surrounding atrial muscle, explaining the apparent paradoxical domination of the central cells of the SAN in normal pacemaking (Kirchhof et al. 1988; Watanabe et al. 1995). The modest effect of ryanodine on pacemaking in the intact SAN could result from its action on the peripheral SAN, which electrotonically influences the leading pacemaker site at the centre of the SAN rather than a direct effect on the central primary pacemaker cells.
The role of the SR in large peripheral cells may explain some cases of pacemaker shift, whereby in response to physiological and pharmacological interventions the initiation site of spontaneous activity may move from the centre of the SAN towards the periphery, such as has been observed in response to (-adrenergic stimulation (Opthof, 1988). It may prove under conditions of enhanced SR calcium loading, such as during β-adrenergic stimulation, peripheral cells show enhanced spontaneous activity and become the dominant pacemaker overcoming the normal electrotonic suppression.
The mechanism whereby intracellular calcium, and more specifically SR calcium, exerts its influence on pacemaker rate remains unclear. Intracellular calcium has a modulatory influence on numerous signalling pathways and ion channels making it difficult to specify which of its effects when combined may underlie the observed phenomena. The issue is complicated by the fact that cells from differing regions of the SAN are known to have different action potential forms, which are in turn due to differences in ion channel expression (Boyett et al. 2000). Consequently the effects on spontaneous activity of cells from the centre versus the periphery of the SAN due to changes in intracellular calcium may be influenced by the differing dominance of ionic currents across the SAN. To further understand the effects of intracellular calcium, further electrophysiological analysis is required to assess regional differences in the various ionic currents which underlie pacemaking and their sensitivity to intracellular calcium.
Conclusion
The finding that there is significant heterogeneity of intracellular calcium handling within the SAN, and that this in turn produces heterogeneity of pacemaker activity, has many implications for understanding the functioning of the SAN in normal physiology, disease and during pharmacological interventions. Intracellular calcium is an important modulator of pacemaker function, but calcium release from the SR does not drive or influence pacemaker activity equally in all pacemaker cells, particularly those apparently from the centre of the SAN, the normal origin of the cardiac action potential.
Acknowledgments
The authors would like to thank the British Heart Foundation who funded this work.
Supplementary material
The online version of this paper can be found at 10.1113/jphysiol.2003.057372 and contains supplementary material entitled ‘Fluo-3 loading does not significantly alter the properties of SAN cells', including three supporting figures.
References
- Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani JW, Bassani RA, Bers DM. Calibration of indo-1 and resting intracellular [Ca]i in intact rabbit cardiac myocytes. Biophys J. 1995;68:1453–1460. doi: 10.1016/S0006-3495(95)80318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani RA, Bassani JW, Lipsius SL, Bers DM. Diastolic SR Ca efflux in atrial pacemaker cells and Ca-overloaded myocytes. Am J Physiol. 1997;273:H886–H892. doi: 10.1152/ajpheart.1997.273.2.H886. [DOI] [PubMed] [Google Scholar]
- Bassani JW, Godoy CM, Bassani RA. Effect of ryanodine on sinus node recovery time determined in vitro. Braz J Med Biol Res. 1999;32:1039–1043. doi: 10.1590/s0100-879x1999000800015. [DOI] [PubMed] [Google Scholar]
- Bleeker WK, Mackaay AJ, Masson-Pevet M, Bouman LN, Becker AE. Functional and morphological organization of the rabbit sinus node. Circ Res. 1980;46:11–22. doi: 10.1161/01.res.46.1.11. [DOI] [PubMed] [Google Scholar]
- Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: molecular partners in pacemaker regulation. Circ Res. 2001;88:1254–1258. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000;47:658–687. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- Boyett MR, Honjo H, Yamamoto M, Nikmaram MR, Niwa R, Kodama I. Regional differences in effects of 4-aminopyridine within the sinoatrial node. Am J Physiol. 1998;275:H1158–H1168. doi: 10.1152/ajpheart.1998.275.4.H1158. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Robinson RB. Beta-modulation of pacemaker rate: novel mechanism or novel mechanics of an old one? Circ Res. 2002;90:E69. doi: 10.1161/01.res.0000014803.05780.e7. [DOI] [PubMed] [Google Scholar]
- Hata T, Noda T, Nishimura M, Watanabe Y. The role of Ca2+ release from sarcoplasmic reticulum in the regulation of sinoatrial node automaticity. Heart Vessels. 1996;11:234–241. doi: 10.1007/BF01746203. [DOI] [PubMed] [Google Scholar]
- Honjo H, Boyett MR, Kodama I, Toyama J. Correlation between electrical activity and the size of rabbit sino-atrial node cells. J Physiol. 1996;496:795–808. doi: 10.1113/jphysiol.1996.sp021728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo H, Inada S, Lancaster MK, Yamamoto M, Niwa R, Jones SA, Shibata N, Mitsui K, Horiuchi T, Kamiya K, Kodama I, Boyett MR. Sarcoplasmic reticulum Ca2+ release is not a dominating factor in sinoatrial node pacemaker activity. Circ Res. 2003;92:e41–e44. doi: 10.1161/01.res.0000055904.21974.be. [DOI] [PubMed] [Google Scholar]
- Honjo H, Lei M, Boyett MR, Kodama I. Heterogeneity of 4-aminopyridine-sensitive current in rabbit sinoatrial node cells. Am J Physiol. 1999;276:H1295–H1304. doi: 10.1152/ajpheart.1999.276.4.H1295. [DOI] [PubMed] [Google Scholar]
- Huser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol. 2000;524:415–422. doi: 10.1111/j.1469-7793.2000.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhof CJ, Bonke FI, Allessie MA. Evidence for the presence of electrotonic depression of pacemakers in the rabbit atrioventricular node. The effects of uncoupling from the surrounding myocardium. Basic Res Cardiol. 1988;83:190–201. doi: 10.1007/BF01907273. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Maltsev VA, Bogdanov KY, Stern MD, Vinogradova TM. Cyclic variation of intracellular calcium: a critical factor for cardiac pacemaker cell dominance. Circ Res. 2003;92:E45–E50. doi: 10.1161/01.res.0000055920.64384.fb. [DOI] [PubMed] [Google Scholar]
- Lei M, Honjo H, Kodama I, Boyett MR. Characterisation of the transient outward K+ current in rabbit sinoatrial node cells. Cardiovasc Res. 2000;46:433–441. doi: 10.1016/s0008-6363(00)00036-5. [DOI] [PubMed] [Google Scholar]
- Li J, Qu J, Nathan RD. Ionic basis of ryanodine's negative chronotropic effect on pacemaker cells isolated from the sinoatrial node. Am J Physiol. 1997;273:H2481–H2489. doi: 10.1152/ajpheart.1997.273.5.H2481. [DOI] [PubMed] [Google Scholar]
- Lipsius SL. Mechanisms of atrial subsidiary pacemaker function. In: Sayeed MM, editor. Cellular Pathophysiology. Boca Raton, Florida: CRC Press; 1989. pp. 1–39. [Google Scholar]
- Masson-Pevet MA, Bleeker WK, Besselsen E, Treytel BW, Jongsma HJ, Bouman LN. Pacemaker cell types in the rabbit sinus node: a correlative ultrastructural and electrophysiological study. J Mol Cell Cardiol. 1984;16:53–63. doi: 10.1016/s0022-2828(84)80714-2. [DOI] [PubMed] [Google Scholar]
- Merritt JE, McCarthy SA, Davies MP, Moores KE. Use of fluo-3 to measure cytosolic Ca2+ in platelets and neutrophils. Loading cells with the dye, calibration of traces, measurements in the presence of plasma, and buffering of cytosolic Ca2+ Biochem J. 1990;269:513–519. doi: 10.1042/bj2690513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa H, Lei M, Honjo H, Jones SA, Dobrzynski H, Lancaster MK, Takagishi Y, Henderson Z, Kodama I, Boyett MR. Heterogeneous expression of Ca2+ handling proteins in rabbit sinoatrial node. J Histochem Cytochem. 2002;50:311–324. doi: 10.1177/002215540205000303. [DOI] [PubMed] [Google Scholar]
- Opthof T. The mammalian sinoatrial node. Cardiovasc Drugs Ther. 1988;1:573–597. doi: 10.1007/BF02125744. [DOI] [PubMed] [Google Scholar]
- Rigg L, Terrar DA. Possible role of calcium release from the sarcoplasmic reticulum in pacemaking in guinea-pig sino-atrial node. Exp Physiol. 1996;81:877–880. doi: 10.1113/expphysiol.1996.sp003983. [DOI] [PubMed] [Google Scholar]
- Trafford AW, Diaz ME, Eisner DA. A novel, rapid and reversible method to measure Ca buffering and time-course of total sarcoplasmic reticulum Ca content in cardiac ventricular myocytes. Pflügers Arch. 1999;437:501–503. doi: 10.1007/s004240050808. [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Bogdanov KY, Lakatta EG. β-Adrenergic stimulation modulates ryanodine receptor Ca2+ release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002a;90:73–79. doi: 10.1161/hh0102.102271. [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Bogdanov KY, Lakatta EG. Novel perspectives on the beating rate of the heart. Circ Res. 2002b;91:e3. doi: 10.1161/01.res.0000031164.28289.55. [DOI] [PubMed] [Google Scholar]
- Watanabe EI, Honjo H, Anno T, Boyett MR, Kodama I, Toyama J. Modulation of pacemaker activity of sinoatrial node cells by electrical load imposed by an atrial cell model. Am J Physiol. 1995;269:H1735–H1742. doi: 10.1152/ajpheart.1995.269.5.H1735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online version of this paper can be found at 10.1113/jphysiol.2003.057372 and contains supplementary material entitled ‘Fluo-3 loading does not significantly alter the properties of SAN cells', including three supporting figures.