Abstract
Synaptic transmission between neurones intrinsic to the wall of the intestine involves multiple neurotransmitters. This study aimed to identify neurotransmitters responsible for non-cholinergic excitatory synaptic transmission in the submucous plexus of the guinea pig ileum. Intracellular recordings were made from secretomotor and vasodilator neurones. A single electrical stimulus to a fibre tract evoked excitatory postsynaptic potentials (EPSPs) with three different time courses – fast, slow and an EPSP with an intermediate time course (latency 96 ms, duration 1.2 s). In all neurones, blocking nicotinic receptors reduced fast EPSPs, but they were abolished in only 57 of 78 neurones. Fast EPSPs were also reduced by P2 purinoceptor blockade (5 of 27 neurones) or 5-HT3 receptor blockade (3 of 20 neurones). The intermediate EPSP was abolished by P2 receptor blockade (13 of 13 neurones) or by the specific P2Y1 receptor antagonist MRS 2179 (5 of 5 neurones) and was always preceded by a nicotinic or mixed nicotinic/purinergic fast EPSP. Intermediate EPSPs were observed in over half of all neurones including most non-cholinergic secretomotor neurones identified by immunoreactivity for vasoactive intestinal peptide. The slow EPSP evoked by a single pulse stimulus was also abolished by P2 receptor blockade (5 of 5 neurones) or by MRS 2179 (3 of 3 neurones). We conclude that fast EPSPs in submucous neurones are mediated by acetylcholine acting at nicotinic receptors, ATP acting at P2X receptors and 5-HT acting at 5-HT3 receptors. Both the intermediate EPSP and the single stimulus slow EPSP are mediated by ATP acting at P2Y1 receptors.
ATP acting via P2X receptors has been established as a neurotransmitter in several parts of the central nervous system (Robertson et al. 2001). However, it is only in the enteric nervous system (ENS) that a function for fast excitatory postsynaptic potentials (EPSPs) mediated via P2X receptors has been shown. They mediate transmission between interneurones and motor neurones in descending inhibitory reflexes of guinea-pig ileum (Bian et al. 2000). Their specific role elsewhere within the ENS (Spencer et al. 2000; Monro et al. 2002) has not been established although ATP may play a role in regulating secretomotor neurones via slow EPSPs mediated by P2Y1 receptors (Hu et al. 2003) (for reviews see Galligan, 2002; Bertrand, 2003).
The enteric nervous system is composed of two ganglionated plexuses: the myenteric plexus that controls motility and the submucous plexus that controls secretion/absorption. Submucous neurones in the guinea pig ileum display up to six distinct synaptic potentials in response to electrical stimulation of fibre tracts. These are fast EPSPs, two types of slow EPSP, two types of inhibitory postsynaptic potential (IPSP) and an EPSP with a time course longer than that of a fast EPSP, but shorter than a slow EPSP (Hirst & McKirdy, 1975; Surprenant, 1984; Bornstein et al. 1986,1988; Mihara et al. 1987). This last potential has been termed an intermediate EPSP and is not as well characterized as the other synaptic potentials (Bornstein et al. 1986). Functionally there are four classes of neurone in the submucous plexus: cholinergic secretomotor neurones (containing neuropeptide Y), non-cholinergic secretomotor neurones (containing vasoactive intestinal peptide, VIP), cholinergic vasodilator neurones, and intrinsic sensory neurones. Fast EPSPs are common in secretomotor and vasodilator neurones, which together comprise around 85% of the total number of neurones (Bornstein et al. 1986). The remaining 15% are intrinsic sensory neurones and rarely display fast EPSPs (Bornstein et al. 1989). Slow EPSPs evoked by a train of stimuli are seen in all classes of neurone, but those evoked by a single stimulus are confined to the non-cholinergic secretomotor neurones (Bornstein et al. 1986). IPSPs are also confined to the non-cholinergic secretomotor class (Bornstein et al. 1986; Evans et al. 1994).
Fast EPSPs in the myenteric plexus are mediated by acetylcholine (ACh), ATP and, to a lesser extent, 5-HT (Galligan & Bertrand, 1994; LePard et al. 1997; LePard & Galligan, 1999; Zhou & Galligan, 1999). Studies of submucosal fast EPSPs have, however, concluded that they are mediated exclusively by ACh acting at nicotinic receptors (Hirst & McKirdy, 1975; Moore & Vanner, 1998,2000). Nonetheless, submucous neurones express P2X2 (Castelucci et al. 2002) and P2X3 (Poole et al. 2002; Van Nassauw et al. 2002) receptors and there is evidence that ATP is released from several classes of presynaptic neurone (Hu et al. 2003). Myenteric neurones immunoreactive for 5-HT project to the submucous plexus (Costa et al. 1982) and submucous neurones respond to both 5-HT and ATP with fast ligand-gated depolarizations that are blocked by 5-HT3 (Surprenant & Crist, 1988) or P2 (Barajas-Lopez et al. 2000) receptor antagonists, respectively.
We used intracellular recordings and receptor antagonists to determine the contribution of ATP and 5-HT to EPSPs in submucous neurones. We found that ATP can participate in three different types of EPSP, sometimes all in a single neurone: fast EPSPs via P2X receptors, slow EPSPs via P2Y1 receptors, and intermediate EPSPs, also via P2Y1 receptors. An increase in chloride ion conductance underlies the intermediate EPSP, which occurred in most non-cholinergic and some cholinergic secretomotor neurones. Fast EPSPs mediated by 5-HT were also identified, though most fast EPSPs were mediated by ACh.
Methods
Tissue preparation
Guinea pigs of either sex in the weight range 180–350 g were stunned and killed by a blow to the back of the head followed by severing of the carotid arteries and spinal cord, in accordance with the guidelines of the University of Melbourne Animal Experimentation Ethics Committee. The abdominal cavity was opened and a 3 cm segment of ileum was removed 10–20 cm from the ileocaecal junction. This was placed into physiological salt solution (composition (mM): NaCl 118, NaHCO3 25, d-glucose 11, KCl 4.8, CaCl2 2.5, MgSO4 1.2, NaH2PO4 1.0) at room temperature and bubbled with 95% O2, 5% CO2. The lumen was flushed clear of contents with the physiological salt solution and the segment of ileum trimmed of mesentery and cut open along the mesenteric border. It was then tightly pinned flat with mucosa uppermost in a Petri dish lined with Sylgard (Compound 184, Dow Corning Corp, Midland, MI, USA) and the mucosa stripped off with fine forceps. A section of submucous plexus approximately 0.75–1.5 cm wide and 0.5–0.8 cm long was then removed from the underlying circular muscle and pinned tightly with serosal side up in an organ bath (volume 0.3–1.3 ml) lined with Sylgard. Oxygenated physiological salt solution was prewarmed to 34–36°C and superfused at a rate of 2–4 ml min−1. The preparation was allowed to equilibrate for 1 h before intracellular recordings began.
Electrophysiology
Submucosal ganglia were visualized at ×300 using an inverted microscope (IMT-2 or IX-70, Olympus, Tokyo, Japan) with either Nomarski or Hoffman differential interference contrast optics. Neurones were impaled with glass microelectrodes containing 1 m KCl and 2% biocytin (w/v) (tip resistance 100–230 MΩ) using conventional techniques and characterized electrophysiologically as either S or AH type (Hirst et al. 1974). Neurones that could be classified as AH neurones based on their action potential shape or long after-hyperpolarizing potential were excluded from this study, even though submucosal AH neurones do exhibit some fast EPSPs (Bornstein et al. 1989). Voltage recordings were made using an Axoprobe 1A or Axoclamp 2A (bridge mode) amplifier, digitized at 5–20 kHz (Digidata 1200B) and recorded on a personal computer using Axoscope 9 (all from Axon Instruments, Foster City, CA, USA). Impalement times ranged from 30 min to over 4 h with neuronal characterization starting at least 10 min after impalement.
Synaptic potentials were evoked by electrical stimulation of interganglionic fibre tracts located further than one neighbouring ganglion away from the impaled neurone (up to 1.2 mm away) using a 25 μm insulated tungsten monopolar stimulating electrode. Fast EPSPs, intermediate EPSPs and single stimulus slow EPSPs were evoked using a single pulse (Bornstein et al. 1986), typically 0.8 mA (0.6–1.2 mA) and 0.5 ms (up to 2.0 ms) duration (Master-8 stimulator, ISO-Flex stimulus isolation unit; both from AMPI, Jerusalem, Israel). A train of 5 pulses at 20 Hz or 3 pulses at 30 Hz was used to evoke IPSPs and/or multistimuli slow EPSPs (Surprenant, 1984; Bornstein et al. 1986). Fast and intermediate EPSPs were recorded at membrane potentials of −70 to −90 mV to maximize their amplitude and to reduce the likelihood of action potential initiation. Slow EPSPs and IPSPs were recorded at, or close to, resting membrane potential (about −50 mV) as they are predominately due to changes in potassium conductance (Surprenant, 1984).
For each neurone, the current that evoked the smallest distinct fast EPSP was determined (threshold stimulus) and this was increased until a fast EPSP of maximal amplitude was evoked (maximal stimulus). The current was then increased to 20–30% beyond maximal (supramaximal stimulus) and used for the remainder of the experiment. One of the consequences of using a supramaximal stimulus was that fast EPSPs were likely to be the product of several presynaptic fibres (Bornstein et al. 1987), but this stimulus regime was required to ensure that variations in the amplitude of fast EPSPs were not simply due to changes in the number of presynaptic fibres activated. As the stimulating electrode was typically around 1 mm from the impaled neurone, the arrival of action potentials in the different terminals would be nearly simultaneous (within 1–2 ms of each other). Thus, these fast EPSPs should still show relatively simple onset and decay kinetics.
Once the electrophysiological and pharmacological characterizations were completed cells were filled with biocytin and the position of the neurone within the ganglion, and the ganglion within the preparation, was noted for later identification following immunohistochemical processing.
Immunohistochemistry
Preparations were processed immunohistochemically to reveal the presence of VIP and biocytin. Briefly, preparations were fixed in Zamboni's fixative (2% formaldehyde plus 0.2% picric acid in 0.1 m phosphate buffer, pH 7.0) at 4°C for between 1 and 24 h, cleared of fixative and permeabilized using dimethyl sulfoxide (3 × 10 min washes), and then washed in phosphate-buffered saline (3 × 10 min washes). The preparations were incubated in a humidifier at room temperature with rabbit anti-VIP antibody (code 7913, a gift from Professor J. B. Furness, Department of Anatomy and Cell Biology, University of Melbourne, Australia) at 1: 400 for 48 h. Preparations were then washed and incubated with donkey anti-rabbit FITC (fluorescein isothiocynate) antibody (1: 50; Amersham Biosciences Pty Ltd, Castle Hill, NSW, Australia) and streptavidin–Texas Red (1: 200 or 1: 400; Amersham Biosciences) for 2–2.5 h to reveal the VIP- and/or biocytin-containing neurones. After washing, the preparations were mounted in buffered glycerol (pH 8.7) for viewing under a fluorescence microscope (Axioskop, Carl Zeiss, Oberkochen, Germany) using appropriate filter cubes for discriminating between FITC and Texas Red fluorescence. Images were acquired at 12 bits using a CCD camera and saved as taken as 16 bit greyscale TIFFs using Spot Advanced software (both from Diagnostic Instruments Incorporated, Sterling Heights, MI, USA).
Drugs and solutions
Receptor antagonists were prepared as stock solutions (generally 1000-fold concentration) in distilled water and kept at 4°C. They included hexamethonium, idazoxan, mecamylamine, pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), suramin (all from Sigma-Aldrich Fine Chemicals, Sydney, Australia), dihydro-β-erythroidine (RBI, Natick, MA, USA), granisetron (a kind gift from SmithKline Beecham, Australia) and MRS 2179 (Tocris, Australian Laboratory Services, Sydney, Australia). The voltage-gated sodium channel blocker, tetrodotoxin (TTX; Alomone Laboratories, Jerusalem, Israel), was also used. Drugs were diluted to the final concentration in physiological salt solution before addition to the organ bath. They were allowed to equilibrate for 5 min before synaptic potentials were evoked in their presence and were washed out for 20–30 min before recovery of responses were sought.
The physiological salt solution was used as the basis for the low Ca2+ (0.25 mm) high Mg2+ (12 mm) solution used to prevent calcium-dependent transmitter release, and for the isethionate (118 mm) and choline (50 mm) substitutions for chloride (118 mm) and sodium (50 mm), respectively (sodium isethionate and choline chloride, both from Sigma-Aldrich Fine Chemicals). These solutions were superfused for approximately 3 min before testing for synaptic potentials and approximately 10 min was allowed afterwards for the physiological salt solution to establish normal ion concentrations again.
Choline chloride (1 mm) was prepared in HEPES (10 mm)-buffered saline (120 mm NaCl, pH 7.2) on the day of use. It was applied to the cell body by pressure ejection (10–20 p.s.i., 100 ms) from a micropipette (tip diameter approximately 10 μm) positioned approximately 200 μm away (Picospritzer III, Parker Instrumentation, Hadland Photonics Pty Ltd, Glen Waverly, Victoria, Australia).
Statistics
Data were analysed using Axoscope 9 and Origin 6 (MicroCal Software, Northampton, MA, USA). Parameters measured were: amplitude (for fast, intermediate and slow EPSPs), latency (fast and intermediate EPSPs), duration at 90% return to baseline (intermediate – some durations were extrapolated, and slow EPSPs), full width at half-amplitude (fast EPSPs), and time to peak (fast EPSPs). The average of between three and six repetitions obtained were used for comparisons. n refers to the number of neurones recorded. Unless otherwise stated numbers given are mean ±s.e.m. and the range and/or median, where relevant, is given in parentheses. Student's t test was used to compare data for significant differences with a P value of < 0.05 taken as the cut-off for significance. All t tests were one-tailed and paired unless noted.
Results
A total of 134 S-type neurones from 129 preparations were characterized electrophysiologically for basic cellular and synaptic properties. The average initial resting membrane potential measured was −54 ± 0.7 mV (from −40 to −70 mV; n = 118) and input resistance 237 ± 13 MΩ (36–898 MΩ). Action potential (AP) shape was observed by applying a 5 ms depolarizing pulse through the recording electrode to evoke a single AP; the duration of the falling phase of the AP at half-amplitude (the second half-width) was 0.81 ± 0.03 (0.45–2 ms; n = 83). Failure to fire an AP occurred in 16 neurones despite the presence of a fast EPSP. A prominent shoulder was observed on repolarization from a hyperpolarizing current pulse (due to an A-type potassium-like current, IA-like) in 92 neurones (69%) and a sag during the hyperpolarizing pulse (due to a hyperpolarization-activated cation-like current, IH-like) was seen in 29 neurones (22%; for correlation with cell types see Table 1).
Table 1.
Basic electrophysiological properties of neurones
| RMP (mV) | Rin (MΩ) | IA | IH | Maximum APs fired | Duration of firing (ms) | Frequency of firing (Hz) | AP 1st half-width (ms) | AP 2nd half-width (ms) | |
|---|---|---|---|---|---|---|---|---|---|
| VIP +ve | −57 ± 1 | 193 ± 21 | 14 | 4 | 7 ± 2 | 137 ± 27 | 52 ± 6 | 0.65 ± 0.06 | 0.79 ± 0.05 |
| (n = 22) | (n = 19) | (n = 18) | (n = 16) | (n = 16) | (n = 10) | (n = 14) | |||
| VIP −ve | −53 ±2* | 272 ± 43 | 11 | 5 | 14 ± 4 | 223 ± 53 | 61 ± 8 | 0.58 ± 0.05 | 0.69 ± 0.04 |
| (n = 17) | (n = 13) | (n = 14) | (n = 14) | (n = 14) | (n = 9) | (n = 14) | |||
| Not tested | −53 ± 1 | 235 ± 13 | 67 | 20 | 9 ± 1 | 187 ± 19 | 56 ± 3 | 0.66 ± 0.03 | 0.86 ± 0.04 |
| (n = 95) | (n = 85) | (n = 74) | (n = 74) | (n = 29) | (n = 53) | ||||
| All | −54 ± 0.6 | 232 ± 13 | 92 | 29 | 10 ± 1 | 184 ± 16 | 56 ± 3 | 0.64 ± 0.03 | 0.82 ± 0.03 |
| (n = 134) | (n = 117) | (n = 116) | (n = 104) | (n = 104) | (n = 48) | (n = 81) |
Rows 1 and 2 show the neurones processed immunohistochemically for VIP while row 3 shows those that were not processed. Row 4 shows the values for all neurones. A 500 ms hyperpolarizing pulse was passed through the recording electrode to reveal the presence of an A-type K+-like current or hyperpolarization-activated cation-like current (number represents the number of neurones in which it was found). A 500 ms depolarizing pulse was passed to determine the AP firing patterns and a 5 ms pulse was passed to examine a single AP in detail.
P < 0.05, considered a significant difference between VIP +ve and VIP −ve neurones (Student's unpaired two-tailed t test). AP, action potential; half-width, duration of the AP at half-amplitude (divided into rising phase (1st half) and falling phase (2nd half)); IA, A-type K+-like current; IH, hyperpolarization-activated cation-like current; n, number of neurones examined; RMP, resting membrane potential; Rin= input resistance; VIP, vasoactive intestinal peptide: −ve = negative, +ve = positive.
A single pulse stimulus (0.5–2 ms, 0.6–1.2 mA) applied to an interganglionic fibre tract evoked up to four kinds of synaptic potential: fast and slow EPSPs, IPSPs, and an excitatory potential with an intermediate time course that could occur between a fast EPSP and a slow EPSP, the intermediate EPSP. Fast EPSPs could be evoked in all 134 neurones (Fig. 1A), slow EPSPs in 13 neurones (10%; Fig. 1D), IPSPs in 26 neurones (20%; see Fig. 6A), and intermediate EPSPs in 71 neurones (53%; Fig. 1B). Spontaneously occurring fast EPSPs were seen in 113 neurones (84%). Trains of stimuli (3 pulses at 30 Hz; 5 pulses at 20 Hz) evoked slow EPSPs and/or an IPSP in 43 (32%) and 41 (32%) neurones, respectively (Fig. 1E and C).
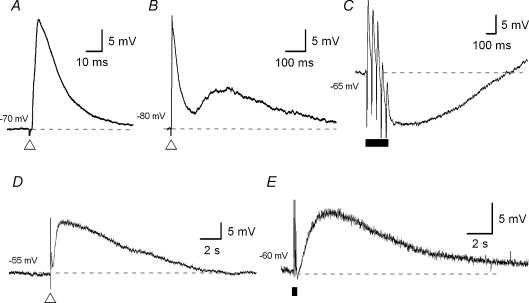
Figure 1. Five types of synaptic potential evoked by electrical stimulation in submucous plexus neurones.
Voltage traces are taken from five different submucous neurones (A–E); each trace is the average of 3–6 records. The dashed lines represent the membrane potential at which the neurones were held (noted to the left of each trace). A single pulse electrical stimulus (duration 0.5 ms, 0.8 mA) was applied, at the open triangle, to an interganglionic fibre tract to evoke excitatory postsynaptic potentials (EPSPs) with three distinct time courses. A, a fast EPSP was evoked at a membrane potential of −70 mV and lasted approximately 30 ms. B, an intermediate EPSP (shown with a preceding fast EPSP) was evoked at a membrane potential of −80 mV and lasted for about 1 s. D, a slow EPSP was evoked at resting membrane potential (RMP, −55 mV) and lasted approximately 10 s. Trains of presynaptic stimuli (5 pulses, 20 Hz) were applied, during the period represented by the filled bar, to the fibre tracts to evoke inhibitory postsynaptic potentials (IPSPs) (C, RMP = −65 mV) and slow EPSPs (E, RMP = −60 mV). Note, IPSPs can sometimes also be evoked by a single electrical stimulus (see Fig. 6A).
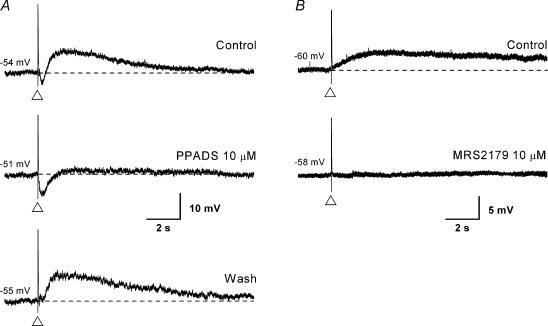
Figure 6. Effect of P2 receptor antagonists on the single pulse slow EPSP.
Voltage traces taken from two submucosal neurones (A and B); the traces in A are single records while those in B are the average of 3 records. The scale shown on the bottom trace applies to upper and lower traces as well. The dashed lines represent the membrane potential (noted to the left of each trace). A, a single pulse electrical stimulus (at the open triangle) evoked, in the following order: a fast EPSP, an intermediate EPSP (bottom) or a small IPSP (top, middle), and a slow EPSP. Application of the P2 receptor antagonist PPADS (30 μm, middle trace) abolished the slow EPSP. Note, the IPSP amplitude is enhanced in the middle trace by the blockade of the intermediate EPSP and the slow EPSP. B, in a different neurone (in the presence of the α1 antagonist idazoxan (1 μm) to block the IPSP), the selective P2Y1 receptor antagonist MRS 2179 (10 μm) was applied instead of PPADS. As in the case with the intermediate EPSP, the slow EPSP was abolished while the fast EPSP was spared.
Fast EPSPs
Fast EPSPs from 78 neurones were examined pharmacologically. The mean amplitude of the fast EPSPs was 23 ± 1 mV (median 23 mV) and duration at half-amplitude was 24 ± 1 ms (range 9–47 ms, median 24 ms, n = 60). Many fast EPSPs also had distinct peaks or bumps occurring, some with consistent latencies, after the initial peak on the falling phase of the potential. One interpretation is that some of the individual fast EPSPs that make up the response were arriving through multiple synapses.
Fast EPSPs could be blocked by the addition of tetrodotoxin (TTX; 300 nm; n = 2) to block voltage-gated sodium channels and, thus, prevent axonal propagation of action potentials, or by substituting the physiological salt solution for one containing a lower concentration of Ca2+ (0.25 mm) and a higher concentration of Mg2+ (12 mm) to block calcium-dependent transmitter release (n = 14).
Effect of nicotinic receptor antagonists
Fast EPSPs were abolished in 57 of 73 neurones (78%) by a nicotinic receptor antagonist (1 mV remaining from 21 mV; Fig. 2A). In the remaining 16 neurones (22%), the fast EPSPs were reduced from 32 mV to 11 mV (34% of control), thus, revealing a non-nicotinic component.
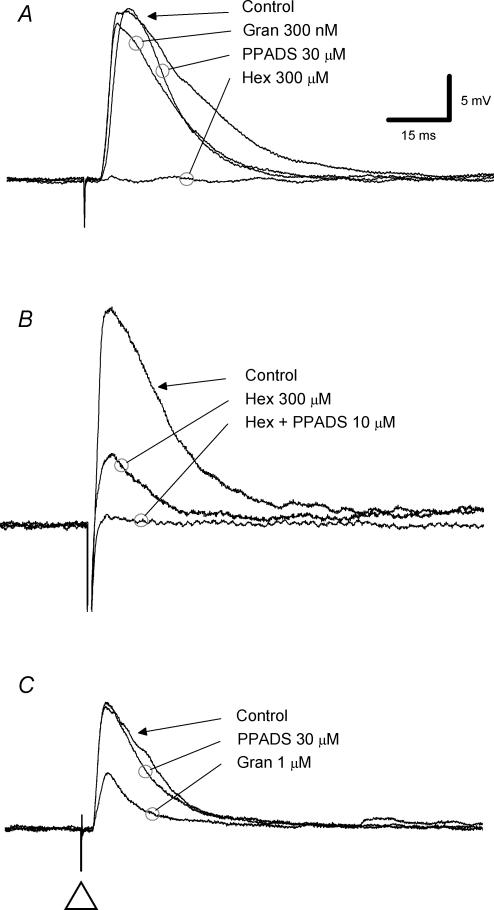
Figure 2. Effects of antagonists for nicotinic, P2 and 5-HT3 receptors on fast EPSPs.
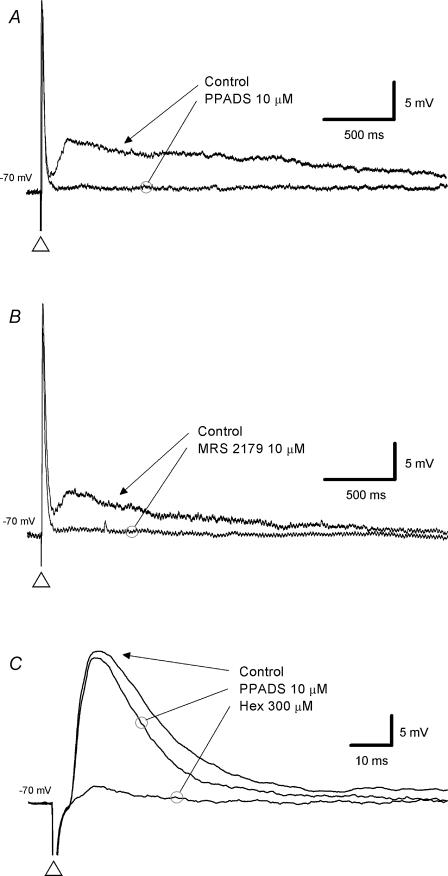
Voltage traces from three submucosal neurones (A–C) are shown; each trace is the average of 6 records. A, a single electrical stimulus (duration 0.5–2 ms, 0.8 mA) was applied to a presynaptic fibre tract to evoke a fast EPSP (membrane potential −70 mV). Neither the P2 receptor antagonist PPADS nor the 5-HT3 receptor antagonist granisetron (Gran) reduced the fast EPSP. Application of the nicotinic receptor antagonist hexamethonium (300 μm, Hex) abolished the fast EPSP. B, in this neurone (membrane potential −80 mV), hexamethonium (300 μm) depressed the fast EPSP and PPADS (10 μm) abolished the hexamethonium-resistant component. C, in this neurone (membrane potential −70 mV), PPADS (10 μm) had no effect on the fast EPSP, but granisetron depressed it by approximately 50%.
The effects of the main nicotinic receptor antagonist used, hexamethonium, were concentration dependent, as in some experiments 100 μm hexamethonium left a residual fast EPSP that was blocked by increasing the concentration to 300 μm. The blockade was specific because two other nicotinic receptor antagonists, mecamylamine (30 μm, n = 5) and dihydro-β-erythroidine (30 μm, n = 2), were as effective as the higher concentration of hexamethonium.
Effect of P2X receptor antagonists
P2 receptor antagonists were tested on 27 neurones, 7 of which had fast EPSPs with a non-nicotinic component. In 5 of the 27 neurones, P2 receptor blockade (either with or without additional nicotinic receptor blockade) reduced the fast EPSP amplitude. In two neurones, addition of PPADS (pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid, 10 or 30 μm) to the bath reduced the fast EPSP amplitude from 21 to 14 mV (67% of control); suramin (100 μm) was tested in one of these neurones and also attenuated the fast EPSP. In three neurones, hexamethonium left a 15 mV residual fast EPSP (39% of control) and addition of PPADS and hexamethonium together abolished this (Fig. 2B).
In 7 of the 27 neurones, PPADS caused an increase in the amplitude of the fast EPSP (from 31 to 38 mV; 123% of control); this increase was not reversed following washout. In 5 of the 7 neurones, hexamethonium abolished the fast EPSP. In another neurone, the fast EPSP was reduced by hexamethonium, increased by PPADS, but was abolished by the combination of PPADS and hexamethonium. In two neurones both PPADS and suramin were tested; PPADS acted to increase the fast EPSP amplitude, whereas suramin had no effect or decreased it.
In 15 of the 27 neurones, P2 receptor antagonists had no effect on fast EPSP amplitude. It was, however, noted that PPADS narrowed the width (at half-amplitude) of the compound fast EPSP from 21 to 18 ms (86%, n = 10, P < 0.05). This reduction in width was associated with a disappearance of the later distinct peaks on the falling phase of the potential. We interpret this to mean that PPADS had blocked multisynaptic fast EPSPs and was thus having an effect at an upstream synapse.
Effect of 5-HT3 receptor antagonists
The 5-HT3 receptor antagonist granisetron was tested in 20 neurones. In 3 of the 20 neurones, granisetron (300 nm or 1 μm) caused a clear decrease in the fast EPSP amplitude from 23 to 12 mV (52% of control; Fig. 2C); 2 neurones with a hexamethonium-resistant component and 1 neurone that was untested with nicotinic blockade.
In 17 of 20 neurones, granisetron had no effect. In 13 neurones, there was a nicotinic-only fast EPSP while in 4 neurones there was a hexamethonium-resistant component to the fast EPSP. In 5 neurones where granisetron did not alter the amplitude of the fast EPSP it did cause a non-significant decrease in the duration of the average fast EPSP response. This suggests that again, multisynaptic fast EPSPs may have been blocked by granisetron at an upstream synapse.
Intermediate EPSPs
Characteristics of the intermediate EPSP
An EPSP with a duration of 500–1500 ms has previously been described in the submucous plexus by Bornstein et al. (1986). As it had a time course longer than a fast EPSP (tens of milliseconds), but shorter than a slow EPSP (tens of seconds), it was termed an intermediate EPSP. In the present study, of the 134 neurones, 71 (53%) responded to a single electrical stimulus with a depolarization resembling the intermediate EPSP. Of these, 40 neurones were analysed further. At membrane potentials between −70 and −90 mV, this intermediate EPSP had a mean amplitude of 4 ± 0.3 mV (1−23 mV, n = 40), duration of 1250 ± 80 ms (450–2450 ms, n = 40) and latency of 94 ± 4 ms (30–189 ms, n = 40). The amplitude was highly dependent on the input resistance of the neurone, with a lower input resistance, such as occurs at potentials more negative than −90 mV, giving rise to a smaller amplitude (see Fig. 5A). When the intermediate EPSP was evoked at, or close to, the resting membrane potential it could initiate an AP (not illustrated. Bornstein et al. 1986).
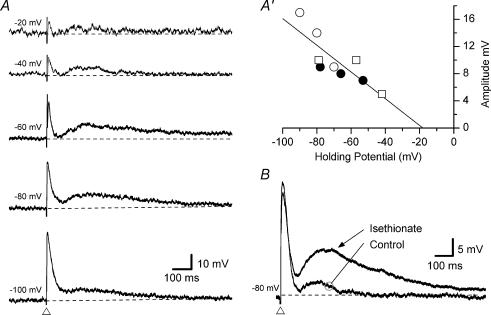
Figure 5. Effect of holding potential and reduced extracellular chloride concentrations on the intermediate EPSP.
Voltage traces taken from two submucosal neurones (A and B). Traces from A are single records while traces for B are an average of 6 records. The dashed lines represent the holding membrane potential (noted to the left of each trace). A, an intermediate EPSP (with a preceding fast EPSP) was evoked, at the open triangle, at different membrane potentials (the scale shown on the bottom trace applies to all). The resistance of the neurone was reduced at holding potentials more negative than about −70 mV (not shown). A′, the amplitude of the intermediate EPSP is plotted against the holding potential for three neurones (•, ○ and □) with three data points used from each to plot a linear regression (shown as the line). Extrapolation of the y-axis intercept found the average reversal potential to be −18 mV (n = 3). B, substituting isethionate for chloride in the extracellular solution resulted in an increased amplitude of the intermediate EPSP (evoked at the open triangle). Note, the amplitude of the preceding fast EPSP was unchanged during superfusion with isethionate.
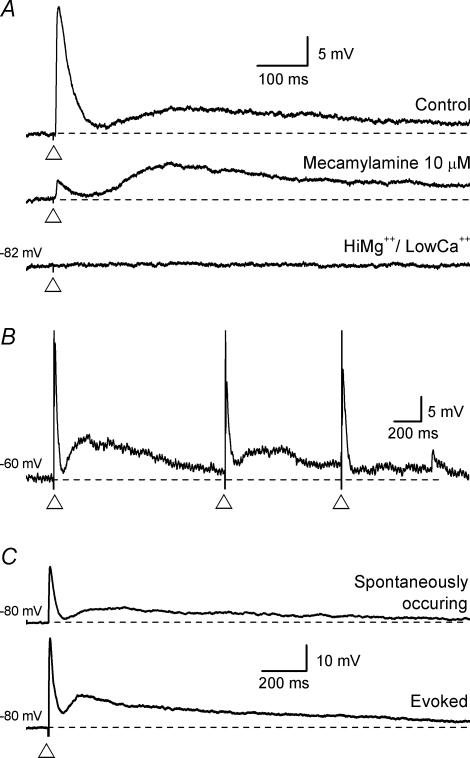
The evoked intermediate EPSP was unaffected when the preceding fast EPSP was abolished by nicotinic receptor blockade (for details see below and Fig. 3A) and was blocked by TTX (300 nm, n = 2; not illustrated) or by a low Ca2+ (0.25 mm), high Mg2+ (12 mm) solution (n = 12; Fig. 3A). It was observed that multiple intermediate EPSPs could result from the one stimulus with new intermediate EPSPs, presumably arriving through a multisynaptic pathway, occurring well after the peak of the previous intermediate EPSP (not illustrated). When a short train of stimuli (5 pulses, 20 Hz) was used it resulted in a larger amplitude of the intermediate EPSP. Single stimuli delivered approximately once a second (1 Hz) resulted in a lowering of the amplitude, i.e. rundown (Fig. 3B). Spontaneously occurring intermediate EPSPs were seen with (Fig. 3C) or without a preceding fast EPSP. The time course of the spontaneously occurring intermediate EPSPs did not appear to differ greatly from an evoked intermediate EPSP from the same neurone (Fig. 3C), though this was not analysed in detail.
Figure 3. Characteristics of the intermediate EPSP.
Voltage traces from three submucosal neurones (A–C); each trace is the average of 6 records except for B which is a single record. The dashed lines represent the holding membrane potential (noted to the left of each trace). A, an intermediate EPSP (with a preceding fast EPSP) was evoked, at the open triangle, in control conditions and following superfusion with the nicotinic receptor antagonist mecamylamine (10 μm). Mecamylamine reduced the fast EPSP to 90% of control, but did not affect the intermediate EPSP. Subsequent superfusion with a high Mg2+, low Ca2+ solution, sufficient to block calcium-dependent transmitter release, abolished both the fast EPSP and the intermediate EPSP (the scale shown on the top trace applies to all). B, repetitive stimulation at around 1 Hz (at the open triangle) caused a reduction in amplitude of both the fast EPSP (shown with an evoked AP) and the intermediate EPSP. The intermediate EPSP recovered in less than 10 s. C, some neurones that had evoked intermediate EPSPs (at the open triangle) also had spontaneously occurring fast EPSP/intermediate EPSP combinations. (Both traces are the average of 6 records, the scale shown on the bottom trace applies to both traces.)
Pharmacology of the intermediate EPSP
The nicotinic receptor antagonist hexamethonium had no effect on the intermediate EPSP (control, 4 mV; hexamethonium, 4 mV; n = 32). This demonstrates that nicotinic receptors are not involved in the generation of the intermediate EPSPs and suggests the intermediate EPSP is not secondary to the depolarization caused by the fast EPSP. In contrast, addition of PPADS (10 or 30 μm; Fig. 4A), suramin (100 μm) or MRS 2179 (10 μm; Fig. 4B) attenuated or abolished (control 6 mV to 1 mV) the intermediate EPSP in all neurones tested (n = 12, 4 and 5, respectively). Addition of granisetron (300 nm or 1 μm) to the bath had no effect on the intermediate EPSP (control 2 mV, granisetron 2 mV, n = 9, not shown).
Figure 4. Effect of P2 receptor antagonists on the intermediate EPSP.
Voltage traces taken from a single submucosal neurone (A–C); each trace is the average of 6 records. The holding potential is noted to the left of each trace. Intermediate EPSPs (evoked at the open triangle) were abolished by the P2 receptor antagonists PPADS (10 μm;A) and the specific P2Y1 receptor antagonist MRS 2179 (10 μm;B). The preceding fast EPSP from A has been expanded in C to show that PPADS caused a slight reduction in the amplitude and duration of the fast EPSP while the nicotinic receptor antagonist, hexamethonium (300 μm), greatly reduced it.
Pharmacology of the preceding fast EPSP
The electrically evoked intermediate EPSP was always preceded by a fast EPSP (e.g. Fig. 3). This fast EPSP was blocked by hexamethonium in 24 neurones (Fig. 4C), or was sensitive to both hexamethonium and PPADS in 4 neurones (P2 receptor antagonists were not tested in 6 neurones where there was a non-nicotinic component; nicotinic receptor antagonists were not tested in 4 neurones); there was no effect of granisetron (13 tested). All neurones with a purinergic component to the fast EPSP had an intermediate EPSP.
Ionic basis of the intermediate EPSP
When the holding potential was varied between −20 and −90 mV, we found the amplitude of the intermediate EPSP was smaller at membrane potentials approaching −20 mV, but did not continue to increase in size when the membrane potential was hyperpolarized past −75 mV (Fig. 5A). The reversal potential, extrapolated from the linear part of the current–voltage plot, was −18 mV (n = 3). A small decrease in input resistance also occurred during the intermediate EPSP (8 ± 3% mean decrease, minimum 0%, maximum 40%), but this was difficult to measure.
To determine what conductance was responsible for this depolarization, we carried out an ion substitution in the extracellular solution. As the resistance during the intermediate EPSP decreased and the amplitude decreased at depolarized membrane potentials, this suggested either a chloride or mixed cation conductance. A sodium substitution would probably compromise the sodium-dependent AP that is required to evoke the intermediate EPSP, thus we first tried substituting sodium isethionate (118 mm) for sodium chloride (118 mm). Chloride substitution resulted in an increase in intermediate EPSP amplitude from 4 to 9 mV (225% of control, n = 7; Fig. 5).
We substituted 50 mm choline chloride for 50 mm of the sodium chloride in the bathing solution, and under these conditions somatic depolarization could still evoke an AP suggesting synaptic transmission should be intact. Despite this, synaptic transmission still appeared to be depressed. It was noted that as the choline chloride entered the bath the neurones were strongly depolarized. To assess the effects of choline more directly, choline chloride at 1 mm (in HEPES-buffered saline) was applied by pressure ejection onto the cell bodies. In 4 of 4 neurones tested choline caused a fast depolarization that was reduced or abolished by nicotinic receptor blockade (300 μm hexamethonium) suggesting choline is a potent agonist at the subtype of nicotinic receptor present on submucosal neurones (Seddik et al. 2003).
Slow EPSPs
A single pulse stimulus evoked a slow EPSP, as previously reported by Surprenant (1984) and Bornstein et al. (1986), in 13 of 134 neurones (10%). This slow EPSP had an average amplitude of 7 ± 1 mV and duration of 14 ± 1 s. Addition of PPADS (10 or 30 μm) to the bath resulted in a large reduction in the amplitude of the single pulse slow EPSP from 7 to 2 mV in three neurones (Fig. 6A). The selective P2Y1 antagonist MRS 2179 (10 μm) also greatly reduced the amplitude of the single pulse slow EPSP from 5 to 1 mV in the three neurones tested (Fig. 6B).
Correlation between the three excitatory synaptic potentials (fast, intermediate and slow) was tested in seven neurones with a single shock slow EPSP: six neurones had an intermediate EPSP, five had a fast EPSP that was blocked by hexamethonium, while two had a fast EPSP sensitive to PPADS.
Immunohistochemical correlation
Of the 134 neurones characterized electrophysiologically, 39 were also characterized immunohistochemically. Table 1 summarizes the basic electrophysiological properties of these neurones. Of the 39 neurones, 22 were immunoreactive for VIP. Single pulse slow EPSPs were seen in 3 of these neurones and 19 had intermediate EPSPs (Table 2). Pharmacological characterization of synaptic potentials was carried out in 33 of the 39 neurones (Table 3), 17 of which were immunoreactive for VIP. Fast EPSPs were completely blocked by hexamethonium in 13 neurones and 4 neurones were partially resistant to hexamethonium – 2 being sensitive to PPADS while 2 were not tested with PPADS.
Table 2.
Synaptic potentials observed, evoked by a single pulse stimulus or a train, in neurones processed immunohistochemically for vasoactive intestinal peptide
| Fast EPSPs | Intermediate EPSPs | Single pulse slow EPSPs | Multiple pulse slow EPSPs | Single pulse IPSPs | Multiple pulse IPSPs | Spontaneous fast EPSPs | |
|---|---|---|---|---|---|---|---|
| VIP +ve (n = 22) | 22 | 19 | 3 | 10 | 4 | 8 | 19 |
| VIP −ve (n = 17) | 17 | 10 | 2 | 3 | 4 | 3 | 14 |
Values represent the number of neurones in which synaptic potentials were observed. EPSP, excitatory postsynaptic potential; IPSP, inhibitory postsynaptic potential; n= number of neurones examined; VIP, vasoactive intestinal peptide: −ve = negative, +ve = positive.
Table 3.
Fast EPSP pharmacology, and presence of intermediate EPSPs, in neurones processed immunohistochemically for vasoactive intestinal peptide
| Intermediate EPSP | Abolished by nicotinic blockade | Resistance to nicotinic blockade | Nicotinic blockade not tested | Sensitive to P2 blockade | P2 blockade not tested | |
|---|---|---|---|---|---|---|
| VIP +ve | Yes, n = 17 | 13 | 4 | 2 | 2 | |
| (n = 17) | ||||||
| VIP −ve | Yes, n = 9 | 5 | 2 | 2† | 2 | |
| (n = 16) | No, n = 7 | 6 | 1‡ |
Suramin increased fast EPSP amplitude in one neurone
PPADS increased fast EPSP amplitude. n= number of neurones examined VIP, vasoactive intestinal peptide: −ve = negative, +ve = positive.
Of the 17 neurones not immunoreactive for VIP, 2 had a single pulse slow EPSP and 10 had an intermediate EPSP (Table 2). Fast EPSPs were completely blocked by hexamethonium in 5 neurones, 2 neurones were partially resistant to hexamethonium but PPADS was not tested, and 2 neurones were not tested with nicotinic receptor blockade but suramin increased the amplitude in 1 neurone (Table 3).
Of the 7 neurones not immunoreactive for VIP and lacking an intermediate EPSP, 6 had fast EPSPs blocked by hexamethonium and 1 was sensitive to hexamethonium but PPADS increased the amplitude (Table 3). The cells with a fast EPSP sensitive to granisetron were not processed immunohistochemically.
Discussion
This study has shown that ATP has a considerable role in excitatory synaptic transmission in the submucous plexus of the guinea-pig ileum as it mediates three distinct classes of EPSP: fast, intermediate and slow. We confirm that the fast EPSP in most neurones is exclusively nicotinic but have shown that approximately 20% have a mixed nicotinic/purinergic fast EPSP while a smaller proportion have a mixed nicotinic/serotonergic fast EPSP.
Cholinergic fast EPSPs
Approximately half the neurones in the submucous plexus are immunoreactive for VIP, a marker for non-cholinergic secretomotor neurones. Submucosal neurones without VIP immunoreactivity contain the ACh-synthesizing enzyme choline acetyltransferase (ChAT; Furness et al. 1984) and are therefore cholinergic. These cholinergic neurones can be functionally subdivided into secretomotor (Bornstein & Furness, 1988) and vasodilator neurones (Brookes et al. 1991). The AH/intrinsic sensory neurones excluded from the present study are also cholinergic (Bornstein et al. 1989; Furness et al. 1998). In the present study, all fast EPSPs were depressed by nicotinic receptor antagonists. Thus, the three classes of neurone studied, non-cholinergic and cholinergic secretomotor neurones and vasodilator neurones, all exhibit fast EPSPs mediated at least partly by ACh acting at nicotinic receptors. We found that in some secretomotor neurones of each class, the fast EPSP was abolished by nicotinic receptor blockade indicating that ACh is the only transmitter involved. These data are consistent with previous electrophysiological studies of synaptic inputs to submucous neurones that found that the predominant excitatory input is via nicotinic receptors (Hirst & McKirdy, 1975; Surprenant, 1984; Moore & Vanner, 2000; Reed & Vanner, 2001). It is also consistent with studies of mucosal chloride secretion and vasodilatation, which have shown that all secretomotor neurones respond to nicotinic receptor agonists (Keast et al. 1985; Hendriks et al. 1989) and that nicotinic transmission is essential for reflex activation of vasodilator neurones (Vanner, 2000).
Non-cholinergic fast EPSPs
In approximately 20% of neurones, nicotinic receptor blockade left a residual non-cholinergic fast EPSP. This could be blocked by a P2 receptor antagonist or a 5-HT3 receptor antagonist and hence was due to the actions of synaptically released ATP or 5-HT. This is similar to transmission in the myenteric plexus, where both ATP and 5-HT mediate some fast EPSPs (Galligan & Bertrand, 1994; Zhou & Galligan, 1999).
P2X receptors have been identified on submucous neurones electrophysiologically (Barajas-Lopez et al. 1998) and immunohistochemically with P2X2 receptors confined to non-cholinergic secretomotor neurones (Castelucci et al. 2002) and P2X3 receptors on cholinergic vasodilator neurones and some cholinergic secretomotor neurones (Poole et al. 2002; Van Nassauw et al. 2002). The chloride secretion evoked by ATP in colonic mucosa (Cuthbert & Hickman, 1985) may be through these P2X receptors (see below).
As in the myenteric plexus (Zhou & Galligan, 1999), fast EPSPs mediated by 5-HT were confined to a subset of submucous neurones. They were not seen in neurones with purinergic EPSPs, whether fast, intermediate or slow. Studies of neurally mediated secretion show that cholinergic secretomotor neurones are preferentially activated via 5-HT3 receptors, while non-cholinergic secretomotor neurones are preferentially activated via higher affinity 5-HT receptors (Hendriks et al. 1989; Johnson et al. 1994). This suggests that 5-HT-mediated fast EPSPs occur in the cholinergic secretomotor neurones. This is also consistent with the observation that these cholinergic neurones lack P2X2 receptors (Castelucci et al. 2002) and that P2X3 receptors are rare (Poole et al. 2002).
The 5-HT-containing terminals in submucous ganglia originate from descending interneurones in the myenteric plexus (Furness & Costa, 1982) and so the fast EPSPs mediated by 5-HT must be evoked from these myenteric neurones. These interneurones have a role in descending excitatory motor pathways (Monro et al. 2002) suggesting that the 5-HT3-mediated fast EPSPs may play a role in coordinating secretion with motility.
Intermediate EPSPs
Most submucous neurones, including virtually all non-cholinergic secretomotor neurones, exhibit an intermediate EPSP. They can be evoked by a single electrical pulse stimulus or can occur spontaneously and can initiate action potentials. Intermediate EPSPs had a latency of around 90 ms and a duration ranging from 450 ms to 2.5 s, placing their time course between that of the fast EPSP and slow EPSP (Fig. 6C, bottom panel). They were abolished by tetrodotoxin and by a low Ca2+, high Mg2+ solution confirming that they result from action potential-evoked, calcium-dependent, transmitter release. Intermediate EPSPs have previously been identified in the cholinergic secretomotor neurones (those containing neuropeptide Y (NPY); Bornstein et al. 1986) but this is the first description in non-cholinergic secretomotor (VIP-containing) neurones. Intermediate EPSPs were seen in virtually all (86%) VIP-immunoreactive neurones and in some VIP-negative neurones. These latter neurones are likely to be the cholinergic secretomotor neurones. Nearly half of submucous neurones are VIP-immunoreactive and another 30% are NPY-immunoreactive (Furness et al. 1984), so approximately 60% of all submucous neurones have an intermediate EPSP, suggesting a more prominent role for the intermediate EPSP in submucosal circuitry than previously thought.
Bornstein et al. (1986) reported a decrease in input resistance during the intermediate EPSP and a reversal potential close to zero. The present study supports these findings showing that there is a small decrease in input resistance and an extrapolated reversal potential of −18 mV. Substitution of isethionate for Cl− in the bathing solution enhanced the intermediate EPSP amplitude. Together, these data suggest that an increase in chloride conductance is responsible for the intermediate EPSP. A contribution of a cation conductance cannot, however, be excluded because substitution of choline for extracellular Na+ did not produce definitive results. In fact, we found that choline was a potent agonist at nicotinic receptors on submucosal neurones, suggesting functional α7 subunits are likely to be on submucous neurones (Kirchgessner & Liu, 1998). Choline as an agonist at nicotinic receptors has been reported in the central nervous system (for review see Pereira et al. 2002) and peripheral nervous system (Seddik et al. 2003) but not to our knowledge in the enteric nervous system.
The intermediate EPSP is abolished by P2 receptor antagonists and their time course argues that they are mediated by G-protein-coupled receptors. A specific P2Y1 receptor antagonist, MRS 2179 (Nandanan et al. 2000), abolished the intermediate EPSP in all neurones tested. Together, these data show that ATP is acting at P2Y receptors and suggests they are of the P2Y1 subtype.
Intermediate EPSPs were only seen in neurones with purely nicotinic fast EPSPs or mixed nicotinic/purinergic fast EPSPs. Purinergic fast EPSPs were also confined to neurones with an intermediate EPSP, suggesting that cholinergic secretomotor neurones activated by 5-HT3 receptors are not the ones that express P2X3 receptors. This implies that different subclasses of cholinergic secretomotor neurones receive input from distinct neural pathways, one using ATP and the other 5-HT.
Spontaneously occurring intermediate EPSPs were mostly preceded by a fast EPSP that was phase-locked (Fig. 3C). This suggests that both EPSPs resulted from the activation of the same presynaptic nerve terminal and, thus, that ACh and ATP may be coreleased. Some spontaneously occurring intermediate EPSPs were seen without a preceding fast EPSP indicating that non-cholinergic neurones also release ATP that can mediate these synaptic potentials. It is not clear which neurones release ATP, but a study by Hu et al. (2003) has shown that the ATP mediating slow EPSPs in submucous neurones (see below) may come from submucous neurones, myenteric neurones and even extrinsic sympathetic nerves.
Slow EPSPs
Two types of slow EPSP are seen in submucous neurones (Bornstein et al. 1986). One is similar to that seen in myenteric neurones that is evoked by a train of stimuli and involves a decrease in potassium conductance. The other is usually evoked by a single stimulus in non-cholinergic secretomotor neurones and has been reported to involve a conductance increase (Hu et al. 2003) or decrease (Surprenant, 1984; Bornstein et al. 1986). In the present study we found this second type of slow EPSP had very little change in conductance and was blocked by a selective P2Y1 receptor antagonist, confirming a recent study by Hu et al. (2003). Therefore, this synaptic potential is also mediated by ATP, and we can conclude that ATP mediates three types of EPSP in the submucous plexus. Indeed, all three types were evoked by a single stimulus in some non-cholinergic secretomotor neurones in this study.
Potentiation of fast EPSPs
P2 receptor blockade increased the fast EPSP amplitude in several neurones that had purely cholinergic fast EPSPs. Postsynaptic interactions between nicotinic and P2X receptors have been reported in enteric neurones (Barajas-Lopez et al. 1998; Khakh et al. 2000), but it seems more likely this is a presynaptic effect. Kamiji et al. (1994) reported that presynaptic P2 receptors inhibit synaptic transmission in the myenteric plexus. Alternatively, inhibitory adenosine receptors might be involved (Barajas-Lopez et al. 1996) as PPADS blocks the breakdown of ATP to adenosine (Lambrecht, 1996). Regardless, this observation suggests that synaptically released ATP has inhibitory effects in addition to its postsynaptic excitatory effects.
Functional significance
Non-cholinergic secretomotor neurones are the major sites of integration for neural regulation of mucosal electrolyte secretion (Bornstein & Furness, 1988). They receive cholinergic fast EPSPs and slow EPSPs from myenteric and submucous neurones, and IPSPs from sympathetic neurones and myenteric neurones (Bornstein et al. 1987, 1988; Moore & Vanner, 1998, 2000; Reed & Vanner, 2001; Hu et al. 2003). ATP evokes chloride secretion via activation of secretomotor neurones in rat colonic mucosa (Cuthbert & Hickman, 1985), but whether P2X or the P2Y receptors mediate this is unknown. In guinea pig distal colon, P2Y1 receptors appear to play a major role in mechanically evoked reflex secretion (Cooke et al. 2004). We have shown that purinergic fast and intermediate EPSPs, some of which come from submucous neurones, are likely to be important in the control of secretion. Intermediate EPSPs are well suited to bridge the gap in time domains left between the short time course fast EPSPs and long time course slow EPSPs in the submucosal circuitry. They would also interact with fast EPSPs evoked by low frequency excitation occurring immediately after a burst of activity.
Acknowledgments
This work was supported by the National Health and Medical Research Council, Australia, grants 114103 and 251504, fellowship 007703 (P.P.B), scholarship 209181 (R.L.M.). We would like to thank Professor John Furness for his kind donation of the VIP antibody, and Heather Robbins and Melanie Coffey for their expert teaching of the immunohistochemical methods. We are also grateful to Phil Davies and Evan Thomas for valuable comments on the manuscript.
References
- Barajas-Lopez C, Espinosa-Luna R, Christofi FL. Changes in intracellular Ca2+ by activation of P2 receptors in submucosal neurons in short-term cultures. Eur J Pharmacol. 2000;409:243–257. doi: 10.1016/s0014-2999(00)00848-7. [DOI] [PubMed] [Google Scholar]
- Barajas-Lopez C, Espinosa-Luna R, Zhu Y. Functional interactions between nicotinic and P2X channels in short-term cultures of guinea-pig submucosal neurons. J Physiol. 1998;513:671–683. doi: 10.1111/j.1469-7793.1998.671ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas-Lopez C, Peres AL, Espinosa-Luna R. Cellular mechanisms underlying adenosine actions on cholinergic transmission in enteric neurons. Am J Physiol. 1996;271:C264–C275. doi: 10.1152/ajpcell.1996.271.1.C264. [DOI] [PubMed] [Google Scholar]
- Bertrand PP. ATP and sensory transduction in the enteric nervous system. Neuroscientist. 2003;9:243–260. doi: 10.1177/1073858403253768. [DOI] [PubMed] [Google Scholar]
- Bian X, Bertrand PP, Bornstein JC. Descending inhibitory reflexes involve P2X receptor-mediated transmission from interneurons to motor neurons in guinea-pig ileum. J Physiol. 2000;528:551–560. doi: 10.1111/j.1469-7793.2000.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Costa M, Furness JB. Synaptic inputs to immunohistochemically identified neurones in the submucous plexus of the guinea-pig small intestine. J Physiol. 1986;381:465–482. doi: 10.1113/jphysiol.1986.sp016339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Costa M, Furness JB. Intrinsic and extrinsic inhibitory synaptic inputs to submucous neurones of the guinea-pig small intestine. J Physiol. 1988;398:371–390. doi: 10.1113/jphysiol.1988.sp017048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB. Correlated electrophysiological and histochemical studies of submucous neurons and their contribution to understanding enteric neural circuits. J Auton Nerv Syst. 1988;25:1–13. doi: 10.1016/0165-1838(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Costa M. Sources of excitatory synaptic inputs to neurochemically identified submucous neurons of guinea-pig small intestine. J Auton Nerv Syst. 1987;18:83–91. doi: 10.1016/0165-1838(87)90137-8. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Costa M. An electrophysiological comparison of substance P-immunoreactive neurons with other neurons in the guinea-pig submucous plexus. J Auton Nerv Syst. 1989;26:113–120. doi: 10.1016/0165-1838(89)90159-8. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Steele PA, Costa M. Calretinin immunoreactivity in cholinergic motor neurones, interneurones and vasomotor neurones in the guinea-pig small intestine. Cell Tissue Res. 1991;263:471–481. doi: 10.1007/BF00327280. [DOI] [PubMed] [Google Scholar]
- Castelucci P, Robbins HL, Poole DP, Furness JB. The distribution of purine P2X2 receptors in the guinea-pig enteric nervous system. Histochem Cell Biol. 2002;117:415–422. doi: 10.1007/s00418-002-0404-4. [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Xue J, Yu JG, Wunderlich J, Wang YZ, Guzman J, Javed N, Christofi FL. Mechanical stimulation releases nucleotides that activate P2Y1 receptors to trigger neural reflex chloride secretion in guinea pig distal colon. J Comp Neurol. 2004;469:1–15. doi: 10.1002/cne.10960. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB, Cuello ACU, Verhofstad AA, Steinbusch HW, Elde RP. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their visualization and reactions to drug treatment. Neuroscience. 1982;7:351–363. doi: 10.1016/0306-4522(82)90272-x. [DOI] [PubMed] [Google Scholar]
- Cuthbert AW, Hickman ME. Indirect effects of adenosine triphosphate on chloride secretion in mammalian colon. J Membr Biol. 1985;86:157–166. doi: 10.1007/BF01870782. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Jiang MM, Surprenant A. Morphological properties and projections of electrophysiologically characterized neurons in the guinea-pig submucosal plexus. Neuroscience. 1994;59:1093–1110. doi: 10.1016/0306-4522(94)90308-5. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their projections in the guinea-pig small intestine. Neuroscience. 1982;7:341–349. doi: 10.1016/0306-4522(82)90271-8. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M, Keast JR. Choline acetyltransferase- and peptide immunoreactivity of submucous neurons in the small intestine of the guinea-pig. Cell Tissue Res. 1984;237:329–336. doi: 10.1007/BF00217152. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Galligan JJ. Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol. 2002;2:623–629. doi: 10.1016/s1471-4892(02)00212-6. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. J Neurosci. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks R, Bornstein JC, Furness JB. Evidence for two types of 5-hydroxytryptamine receptor on secretomotor neurons of the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1989;339:409–414. doi: 10.1007/BF00736055. [DOI] [PubMed] [Google Scholar]
- Hirst GDS, Holman ME, Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974;236:303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, McKirdy HC. Synaptic potentials recorded from neurones of the submucous plexus of guinea-pig small intestine. J Physiol. 1975;249:369–385. doi: 10.1113/jphysiol.1975.sp011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HZ, Gao N, Zhu MX, Liu S, Ren J, Gao C, Xia Y, Wood JD. Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol. 2003;550:493–504. doi: 10.1113/jphysiol.2003.041731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PJ, Bornstein JC, Furness JB, Woollard DJ, Orrman-Rossiter SL. Characterization of 5-hydroxytryptamine receptors mediating mucosal secretion in guinea-pig ileum. Br J Pharmacol. 1994;111:1240–1244. doi: 10.1111/j.1476-5381.1994.tb14878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiji T, Morita K, Katayama Y. ATP regulates synaptic transmission by pre- and postsynaptic mechanisms in guinea-pig myenteric neurons. Neuroscience. 1994;59:165–174. doi: 10.1016/0306-4522(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Keast JR, Furness JB, Costa M. Investigations of nerve populations influencing ion transport that can be stimulated electrically, by serotonin and by a nicotinic agonist. Naunyn Schmiedebergs Arch Pharmacol. 1985;331:260–266. doi: 10.1007/BF00634247. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Zhou X, Sydes J, Galligan JJ, Lester HA. State-dependent cross-inhibition between transmitter-gated cation channels. Nature. 2000;406:405–410. doi: 10.1038/35019066. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu MT. Immunohistochemical localization of nicotinic acetylcholine receptors in the guinea pig bowel and pancreas. J Comp Neurol. 1998;390:497–514. [PubMed] [Google Scholar]
- Lambrecht G. Design and pharmacology of selective P2-purinoceptor antagonists. J Auton Pharmacol. 1996;16:341–344. doi: 10.1111/j.1474-8673.1996.tb00049.x. [DOI] [PubMed] [Google Scholar]
- LePard KJ, Galligan JJ. Analysis of fast synaptic pathways in myenteric plexus of guinea pig ileum. Am J Physiol. 1999;276:G529–G538. doi: 10.1152/ajpgi.1999.276.2.G529. [DOI] [PubMed] [Google Scholar]
- LePard KJ, Messori E, Galligan JJ. Purinergic fast excitatory postsynaptic potentials in myenteric neurons of guinea pig: distribution and pharmacology. Gastroenterology. 1997;113:1522–1534. doi: 10.1053/gast.1997.v113.pm9352854. [DOI] [PubMed] [Google Scholar]
- Mihara S, Nishi S, North RA, Surprenant A. A non-adrenergic, non-cholinergic slow inhibitory post-synaptic potential in neurones of the guinea-pig submucous plexus. J Physiol. 1987;390:357–365. doi: 10.1113/jphysiol.1987.sp016705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monro RL, Bertrand PP, Bornstein JC. ATP and 5-HT are the principal neurotransmitters in the descending excitatory reflex pathway of the guinea-pig ileum. Neurogastroenterol Motil. 2002;14:255–264. doi: 10.1046/j.1365-2982.2002.00325.x. [DOI] [PubMed] [Google Scholar]
- Moore BA, Vanner S. Organization of intrinsic cholinergic neurons projecting within submucosal plexus of guinea pig ileum. Am J Physiol. 1998;275:G490–G497. doi: 10.1152/ajpgi.1998.275.3.G490. [DOI] [PubMed] [Google Scholar]
- Moore BA, Vanner S. Properties of synaptic inputs from myenteric neurons innervating submucosal S neurons in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol. 2000;278:G273–G280. doi: 10.1152/ajpgi.2000.278.2.G273. [DOI] [PubMed] [Google Scholar]
- Nandanan E, Jang SY, Moro S, Kim HO, Siddiqui MA, Russ P, Marquez VE, Busson R, Herdewijn P, Harden TK, Boyer JL, Jacobson KA. Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y1 receptor ligands. J Med Chem. 2000;43:829–842. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB. The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci. 2002;101:39–47. doi: 10.1016/s1566-0702(02)00179-0. [DOI] [PubMed] [Google Scholar]
- Pereira EF, Hilmas C, Santos MD, Alkondon M, Maelicke A, Albuquerque EX. Unconventional ligands and modulators of nicotinic receptors. J Neurobiol. 2002;53:479–500. doi: 10.1002/neu.10146. [DOI] [PubMed] [Google Scholar]
- Reed DE, Vanner SJ. Converging and diverging cholinergic inputs from submucosal neurons amplify activity of secretomotor neurons in guinea-pig ileal submucosa. Neuroscience. 2001;107:685–696. doi: 10.1016/s0306-4522(01)00392-x. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Ennion SJ, Evans RJ, Edwards FA. Synaptic P2X receptors. Curr Opin Neurobiol. 2001;11:378–386. doi: 10.1016/s0959-4388(00)00222-1. [DOI] [PubMed] [Google Scholar]
- Seddik R, Bradaia A, Trouslard J. Choline induces Ca2+ entry in cultured sympathetic neurones isolated from rat superior cervical ganglion. Eur J Pharmacol. 2003;471:165–176. doi: 10.1016/s0014-2999(03)01860-0. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Walsh M, Smith TK. Purinergic and cholinergic neuro-neuronal transmission underlying reflexes activated by mucosal stimulation in the isolated guinea-pig ileum. J Physiol. 2000;522:321–331. doi: 10.1111/j.1469-7793.2000.t01-1-00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A. Slow excitatory synaptic potentials recorded from neurones of guinea-pig submucous plexus. J Physiol. 1984;351:343–361. doi: 10.1113/jphysiol.1984.sp015249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Crist J. Electrophysiological characterization of functionally distinct 5-hydroxytryptamine receptors on guinea-pig submucous plexus. Neuroscience. 1988;24:283–295. doi: 10.1016/0306-4522(88)90331-4. [DOI] [PubMed] [Google Scholar]
- Van Nassauw L, Brouns I, Adriaensen D, Burnstock G, Timmermans JP. Neurochemical identification of enteric neurons expressing P2X3 receptors in the guinea-pig ileum. Histochem Cell Biol. 2002;118:193–203. doi: 10.1007/s00418-002-0447-6. [DOI] [PubMed] [Google Scholar]
- Vanner S. Myenteric neurons activate submucosal vasodilator neurons in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol. 2000;279:G380–G387. doi: 10.1152/ajpgi.2000.279.2.G380. [DOI] [PubMed] [Google Scholar]
- Zhou X, Galligan JJ. Synaptic activation and properties of 5-hydroxytryptamine(3) receptors in myenteric neurons of guinea pig intestine. J Pharmacol Exp Ther. 1999;290:803–810. [PubMed] [Google Scholar]