Abstract
Tibetan highlanders develop at altitude peak aerobic power levels (V̇O2peak) close to those of Caucasians at sea level. In order to establish whether this feature is genetic and, as a consequence, retained by Tibetan lowlanders, altitude-induced changes of peak aerobic performance (ΔV̇O2peak) were assessed in four groups of volunteers with different ethnic, altitude exposure and fitness characteristics, i.e. eight untrained second-generation Tibetans (Tib 2) born and living at 1300 m; seven altitude Sherpas living at ∼2800–3500 m; and 10 untrained and five trained Caucasians. Measurements were carried out at sea level or at Kathmandu (1300 m, Nepal) (PRE), and after 2–4 (ALT1), 14–16 (ALT2), and 26–28 (ALT3) days at 5050 m. At ALT3, ΔV̇O2peak of untrained and trained Caucasians was –31% and –46%, respectively. By contrast, ΔV̇O2peak of Tib 2 and Sherpas was –8% and –15%, respectively. At ALT3, peak heart rate (HRpeak) of untrained and trained Caucasians was 148 ± 11 and 149 ± 7 beats min−1, respectively; blood oxygen saturation at peak exercise (SaO2peak) was 76 ± 6% and 73 ± 6%, and haemoglobin concentration ([Hb]) was 19.4 ± 1.0 and 18.6 ± 1.2 g dl−1, respectively. Compared to Caucasians, Tib 2 and Sherpas exhibited at ALT3 higher HRpeak (179 ± 9 and 171 ± 4 beats min−1, P < 0.001), lower [Hb] (16.6 ± 0.6 and 17.4 ± 0.9 g dl−1, respectively, P < 0.001), and slightly but non-significantly greater average SaO2peak values (82 ± 6 and 80 ± 7%). The above findings and the time course of adjustment of the investigated variables suggest that Tibetan lowlanders acclimatize to chronic hypoxia more quickly than Caucasians, independent of the degree of fitness of the latter.
As is well known, with increasing altitude, peak aerobic power (V̇O2peak) of acclimatized lowlanders undergoes a progressive decrease as a consequence of the decline of barometric pressure and of oxygen partial pressure in inspired air. At any given altitude, however, the percentage reduction of V̇O2peak compared to sea level control values (ΔV̇O2peak,%) varies widely among individuals (Cerretelli & Hoppeler, 1996). Such variability appears to be mainly related to the extent of acclimatization and to the degree of fitness. A prolonged sojourn at very high altitude (>5500 m) may affect V̇O2peak of lowlanders both positively, by the increase of blood O2 carrying capacity (Grassi et al. 1996) and negatively, as a consequence of a progressive reduction of muscle mass (Cerretelli, 1976) and, possibly, of muscle deterioration (Martinelli et al. 1990). If the duration of the exposure is long enough (years) and altitude does not exceed ∼4000 m, developmental adaptation may occur (Moore, 2001) allowing a progressive recovery of the V̇O2peak towards levels only slightly lower than those found at sea level in age-, fitness- or training-matched individuals (Frisancho et al. 1973; Greska et al. 1985; Sun et al. 1990; Niu et al. 1995; Chen et al. 1997). With regard to fitness, in acute hypoxia, the peak aerobic power reduction was found to be greater in physically active than in inactive Caucasians (Lawler et al. 1988; Shephard et al. 1988; Martin & O'Kroy, 1993; Koistinen et al. 1995) as a consequence, among others, of a greater lung diffusion limitation (Dempsey et al. 1984; Dempsey & Wagner, 1999). Also in subchronic (Young et al. 1985) and chronic (Marzorati et al. 1995) hypoxia, there are hints that trained individuals may be more penalized in terms of ΔV̇O2peak than untrained subjects.
Compared to acclimatized lowlanders and even Andean populations, altitude Tibetans exhibit at peak exercise peculiar adaptive features, such as higher arterial O2 saturation (Zhuang et al. 1996) and heart rate values (Niu et al. 1995), and absolute V̇O2peak levels (Sun et al. 1990; Niu et al. 1995; Chen et al. 1997) close to those found in Caucasians at sea level. These findings, along with a less pronounced polycythemic response (Beall et al. 1998), a reduced hypoxic pulmonary vasoconstriction (Groves et al. 1993), and a lower prevalence of chronic mountain sickness (Moore et al. 1998), suggest that in altitude Tibetans the pattern of adaptation to chronic hypoxia is different compared to that of any other population.
The present study was designed primarily to establish whether resistance to hypoxia and, particularly the greater aerobic working capacity found in altitude Tibetans has a genetic basis. Should this be the case, Tibetan lowlanders born with the genetic adaptations of their ancestors, i.e. long-term processes occurring over generations (Moore, 2001), could be expected to acclimatize to high altitude more quickly than Caucasians. As an additional aim, we investigated the role of aerobic fitness, independent of ethnicity, on the preservation of peak aerobic performance at altitude. To achieve these aims, the respiratory and cardiovascular responses to peak exercise, particularly the altitude-induced decrease of V̇O2peak (ΔV̇O2peak) were assessed in Tibetans with different altitude exposure history and in Caucasian lowlanders with different levels of aerobic fitness, following an identical (26–28 days) altitude (5050 m) exposure protocol.
Methods
Subjects
The study was conducted, over several years, on a total of 30 male subjects with different characteristics, as follows.
Eight second-generation Tibetan lowlanders (Tib 2), born and living in Kathmandu (Nepal, 1300 m), offspring of migrants from the Tibetan plateau (3000–4500 m). None of them had ever been at altitudes above 2000 m for longer than 1 day. They frequently rode bicycles for transportation but were not engaged in any specific endurance training programme.
Seven Sherpas (Sh). Based on their genetic background, Sherpas are Tibetans born in, and lifelong residents of, the Solu Khumbu region (2800–3500 m). Our subjects, recruited among the porters of an expedition, were involved for a few weeks in house-keeping tasks at the Pyramid-laboratory at 5050 m. Assuming adaptation and full acclimatization to this altitude they were chosen as the reference group for comparison with Tibetan lowlanders.
Ten untrained Caucasian lowlanders (UT).
Five trained Caucasian lowlanders (T), running or cycling, on average, 4–8 h per week.
None of the Caucasian subjects were exposed to altitudes above 3000 m in the preceding year, and above 1200 m for 3 months before control test.
Age, anthropometrical characteristics and blood haemoglobin concentration of the subjects are given in Table 1. They underwent a preliminary clinical screening which included history taking, physical examination and resting ECG. All were highly motivated and quite cooperative. They were informed about the experimental procedure and gave consent to participate in the study, which was carried out in accordance with the principles outlined in the Declaration of Helsinki (2000) of the World Medical Association. The study was approved by the ethical committees and research review boards of the National Research Council (Milan, Italy), the Royal Nepal Academy of Science and Technologies (RONAST) and the Royal Nepal Ministry of Health (Kathmandu, Nepal).
Table 1.
Age, height, body mass, body mass index (BMI) and blood haemoglobin concentration ([Hb]) of 2nd generation Tibetan lowlanders (Tib 2), altitude Sherpas (Sh), untrained (UT) and trained (T) Caucasians in control conditions (PRE) and after 26–28 days at 5050 m (ALT3)
| n | Tibetans 8 | Sherpas 7 | Caucasians 10 | Caucasians 5 | |
|---|---|---|---|---|---|
| Age (year) | 20 ± 2 | 25 ± 2 | 34 ± 7*§ | 32 ± 4*§ | |
| Heigth (cm) | 170 ± 8 | 169 ± 6 | 179 ± 9 | 173 ± 1 | |
| Body mass (kg) | PRE | 57.3 ± 8.5 | 54.5 ± 4.4 | 82.8 ± 12.5*§ | 65.9 ± 5.6† |
| ALT3 | 57.7 ± 7.7 | 54.7 ± 3.9 | 76.6 ± 14.2*§a | 63.7 ± 5.2 | |
| BMI (kg m−2) | PRE | 19.8 ± 1.7 | 19.1 ± 0.9 | 25.9 ± 3.7*§ | 21.9 ± 1.7† |
| ALT3 | 20.0 ± 1.6 | 19.1 ± 0.8 | 23.9 ± 3.9 | 21.2 ± 1.6 | |
| [Hb] (g dl−1) | PRE | 13.5 ± 1.3 | 16.8 ± 0.8* | 15.3 ± 1.0* | 14.9 ± 0.9§ |
| ALT3 | 16.6 ± 0.6a | 17.4 ± 0.9 | 19.4 ± 1.0*§a | 18.6 ± 1.2*a |
Values are means ± s.d. Significantly different from Tib 2
Values are means ± s.d. Significantly different from Sh
Values are means ± s.d. Significantly different from UT.
P < 0.05 when comparing PRE and ALT3.
Testing sequence
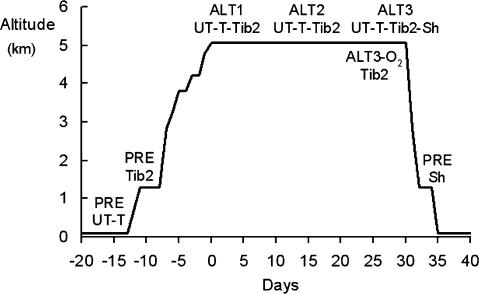
Figure 1 indicates the altitude profile and summarizes the sequence of the testing sessions. Control metabolic measurements (PRE) on Caucasians were carried out in Milan (Italy, 122 m) 10–15 days before departure to Nepal. The same data were adopted also at Kathmandu (1300 m), based on the established consensus that V̇O2peak of non-athletic subjects is not affected by altitudes below 1500 m (Terrados et al. 1985; Gore et al. 1996). Control measurements on Tib 2 were performed in Kathmandu. All subjects flew from Kathmandu to Lukla (2850 m) and reached Lobuche (5050 m) in the Khumbu Valley after a 7-day trek. Subjects walked 3–5 h daily at a moderate pace carrying light loads. Two days were allowed at 3800 and 4200 m, respectively, for rest and acclimatization. Sherpas reached Lukla from their native villages and thereafter followed the same walking schedule to Lobuche as did Caucasians and Tib 2. All groups, including Sherpas, stayed 26–28 days at 5050 m. Altitude measurements were carried out in a permanent research station (the Ev-K2-CNR Pyramid-Laboratory located at 5050 m, at ∼425 mmHg barometric pressure), equipped with stabilized electrical supply powered by a water turbine. Temperature inside the laboratory ranged from 17 to 22°C. Drinks and a wide variety of palatable food were freely available.
Figure 1. Altitude profile and the sequence of testing sessions for untrained (UT) and trained (T) Caucasians, and for Tibetan lowlanders (Tib 2) and Sherpas (Sh).
Untrained and trained Caucasians underwent control measurements (PRE) at sea level. The same data were adopted also at 1300 m, based on the observation that V̇O2peak of sedentary subjects is not affected by altitudes below 1500 m (Terrados et al. 1985; Gore et al. 1996). Control measurements (PRE) in Tibetan lowlanders were performed at 1300 m. Experiments were carried out 2–4 (ALT1), 14–16 (ALT2), and 26–28 (ALT3) days after arrival at the Pyramid-Laboratory. At ALT3, Tibetan lowlanders repeated the test also in acute hypobaric normoxia (ALT3-O2). At altitude, Sherpas underwent experiments only on one occasion (ALT3) and thereafter they were brought and tested at 1300 m (PRE).
Caucasians (UT and T) and Tib 2 underwent measurements 2–4 (ALT1), 14–16 (ALT2), and 26–28 days (ALT3) after arrival at the Pyramid-Laboratory. During this period T kept active walking in the neighbourhood or exercising on a bicycle ergometer daily for about 1 h. Being lifelong altitude residents, Sherpas were tested only at the end of the sojourn (ALT3). After completion of the ALT3 session, Tib 2 repeated the test in acute hypobaric normoxia (ALT3-O2) breathing a humidified O2-enriched mixture (∼40% O2 in N2). Sherpas were transferred by helicopter to Kathmandu where control measurements (PRE, see Fig. 1) were performed within 1–2 days. This procedure is necessarily different from that adopted for the other investigated groups, but it represents the only possible basis for differential metabolic measurements as a function of altitude.
Experimental procedure
Before performing the test, enough time was allowed for each subject to become familiar with the equipment, the testing procedure, and, particularly for Sherpas, with the use of the bicycle ergometer. A graded incremental exercise on an electrically braked, carefully calibrated bicycle ergometer (Cardioline STS-3, Remco, Italy) was adopted to determine peak aerobic power (V̇O2peak). After a 5-min period at rest, subjects performed a 4-min constant-load warming-up (30–90 watts, W, depending on altitude, age, body mass and fitness). Subsequently, the exercise workload was increased stepwise by 30 W every 3 min up to voluntary exhaustion. During the test, the subjects kept a constant pedalling rate (∼60 r.p.m.) with the aid of a digital display. Exhaustion was defined as the inability to keep the imposed rate for longer than 30 s.
Gas exchange, heart rate and haemoglobin
A computerized O2–CO2 analyser–flowmeter combination (Vmax 2900, SensorMedics, Yorba Linda, CA, USA) was used for breath-by-breath assessment of tidal volume (VT), pulmonary ventilation (V̇E) and gas exchange (V̇O2, V̇CO2). VT and (V̇E) were calculated by integration of the flow tracings recorded at the mouth of the subject by means of a pair of heated stainless steel wires (Mass Flow Sensor). Volume and gas analyser calibrations were performed prior to each measurement using a 3-litre syringe (Hewlett Packard 14278B), at three different flow rates, and by means of gas mixtures of known composition, respectively. Heart rate (HR) from ECG, and the arterialized blood oxygen saturation (SaO2) by earlobe pulse oximetry (Biox 3740, Pulse Oximeter, Ohmeda, Denver, CO, USA) were monitored throughout the tests.
Blood haemoglobin concentration ([Hb]) was measured at rest on venous blood samples by a photometric method (Compur M1000, Germany).
Data analysis and statistics
Steady-state values of gas exchange, HR and SaO2 were obtained by averaging the breath-by-breath or the beat-by-beat data over 30–45 s time periods, at rest, as well as at the end of each workload. Data are expressed as means ±s.d. To determine the statistical significance of differences between two means, a paired two-tailed Student's t test was performed. To check the statistical significance of differences among more than two means, a one-way or a repeated-measures analysis of variance (ANOVA) was performed, when applicable. If a significant F-value was identified, the Tukey-Kramer multiple comparison test was used. Linear least squares regression analyses were performed when applicable. The level of significance was set at P < 0.05. For statistical analyses a commercially available software package (InStat, Graph Pad Software, San Diego, CA, USA) was used.
Results
During altitude acclimatization, [Hb] of Tibetan lowlanders and Sherpas increased slightly, attaining at ALT3 similar levels (16.6 ± 0.6 and 17.4 ± 0.9 g dl−1, respectively). By contrast, at ALT3, [Hb] of untrained and trained Caucasians was 19.4 ± 1.0 and 18.6 ± 1.2 g dl−1, respectively, i.e. values significantly greater (P < 0.05) than those of Tibetan lowlanders and Sherpas (see Table 1).
Mean resting V̇O2, respiratory gas exchange ratio (R), V̇E HR and SaO2 values of the four investigated groups of subjects before (PRE) and throughout altitude exposure (ALT1, ALT2, ALT3 and ALT3-O2) are shown in Table 2. V̇O2 levels were significantly (P < 0.05) higher in Sherpas than in the other groups only at PRE. At PRE and at ALT3 resting gas exchange ratio (R) of Tibetan lowlanders and Sherpas was close to 1, and greater than that of Caucasians. Resting PRE V̇E was higher in Tibetans and Sherpas than in Caucasians (P < 0.05). Resting HR values did not differ among ethnic groups. Only trained Caucasians had lower HR (P < 0.05), particularly at PRE. Resting haemoglobin O2 saturation values were similar among groups at PRE, ALT1 and ALT2. At ALT3, SaO2 was significantly (P < 0.05) lower in untrained and trained Caucasians than in Tibetans.
Table 2.
Resting oxygen consumption (V̇O2), gas exchange ratio (R), pulmonary ventilation (V̇E), heart rate (HR) and arterial oxygen saturation (SaO2) of 2nd generation Tibetan lowlanders (Tib 2), altitude Sherpas (Sh) untrained (UT) and trained (T) Caucasians in control conditions (PRE) and throughout altitude exposure (ALT1, ALT2, ALT3 and ALT3-O2)
| Tibetans (Tib 2) | Sherpas (Sh) | Caucasians (UT) | Caucasians (T) | ||
|---|---|---|---|---|---|
| V̇O2 (ml kg−1 min−1) | PRE | 4.3 ± 0.7 | 5.2 ± 0.9 | 3.7 ± 0.4§ | 4.3 ± 0.4 |
| ALT1 | 4.6 ± 0.5 | — | 4.2 ± 0.9 | 4.5 ± 1.3 | |
| ALT2 | 4.5 ± 0.5 | — | 3.9 ± 0.5 | 4.1 ± 1.1 | |
| ALT3 | 5.4 ± 0.6a | 5.1 ± 1.0 | 4.4 ± 0.8 | 4.5 ± 0.6 | |
| ALT3-O2 | 5.3 ± 1.0a | — | — | — | |
| R | PRE | 0.99 ± 0.07 | 0.94 ± 0.05 | 0.84 ± 0.12* | 0.79 ± 0.10*§ |
| ALT1 | 0.91 ± 0.15 | — | 0.83 ± 0.13 | 0.86 ± 0.16 | |
| ALT2 | 0.98 ± 0.08 | — | 0.91 ± 0.12 | 0.98 ± 0.19 | |
| ALT3 | 1.02 ± 0.09 | 0.98 ± 0.06 | 0.84 ± 0.12*§ | 0.88 ± 0.08 | |
| ALT3-O2 | 0.75 ± 0.12abcd | — | — | — | |
| V̇E (l min−1) | PRE | 12.8 ± 1.4 | 13.5 ± 2.0 | 9.2 ± 1.2*§ | 8.2 ± 1.8*§ |
| ALT1 | 15.5 ± 2.8a | — | 17.3 ± 7.6a | 14.4 ± 3.7a | |
| ALT2 | 15.7 ± 2.5a | — | 16.7 ± 3.0a | 15.2 ± 2.9a | |
| ALT3 | 18.3 ± 1.2abc | 15.3 ± 3.2 | 16.4 ± 3.7a | 14.2 ± 3.1a | |
| ALT3-O2 | 16.0 ± 3.0a | — | — | — | |
| HR (beats min−1) | PRE | 81 ± 12 | 70 ± 12 | 78 ± 12 | 58 ± 3*† |
| ALT1 | 95 ± 16 | — | 92 ± 10a | 80 ± 19 | |
| ALT2 | 105 ± 9a | — | 87 ± 6* | 78 ± 8* | |
| ALT3 | 93 ± 8 | 87 ± 9a | 96 ± 13a | 79 ± 12 | |
| ALT3-O2 | 92 ± 10 | — | — | — | |
| SaO2 (%) | PRE | 97.5 ± 1.1 | 97.9 ± 0.9 | 97.3 ± 1.5 | 96.5 ± 0.7 |
| ALT1 | 89.1 ± 2.5a | — | 82.5 ± 7.5a | 84.1 ± 5.0a | |
| ALT2 | 91.0 ± 2.2a | — | 84.4 ± 6.2a | 85.9 ± 4.7 | |
| ALT3 | 91.6 ± 3.5a | 89.0 ± 3.0a | 84.6 ± 5.0*a | 84.0 ± 3.6*a | |
| ALT3-O2 | 97.6 ± 1.2bcd | — | — | — |
Values are means ±s.d. Significantly different from Tib 2,
Values are means ±s.d. Significantly different from Sh
Values are means ±s.d. Significantly different from UT.
Significantly different from PRE
Significantly different from ALT1
Significantly different from ALT2
Significantly different from ALT3.
Peak exercise data at PRE and during the sojourn at altitude are shown in Table 3.
Table 3.
Power output (Ẇ), oxygen consumption (V̇O2) gas exchange ratio (R), pulmonary ventilation (V̇E), heart rate (HR) and arterial oxygen saturation (SaO2) of 2nd generation Tibetan lowlanders (Tib 2), altitude Sherpas (Sh), untrained (UT) and trained (T) Caucasians) at peak exercise carried out in control conditions (PRE) and throughout altitude exposure (ALT1, ALT2 ALT3 and ALT3-O2)
| Tibetans | Sherpas | Caucasians | Caucasians | ||
|---|---|---|---|---|---|
| Ẇ (W) | PRE | 156 ± 25 | 167 ± 24 | 210 ± 37* | 276 ± 39*§† |
| ALT1 | 131 ± 16a | — | 144 ± 37a | 174 ± 25†a | |
| ALT2 | 130 ± 19a | — | 158 ± 14*a | 180 ± 24*a | |
| ALT3 | 146 ± 25bc | 154 ± 21 | 159 ± 20a | 173 ± 29a | |
| ALT3-O2 | 176 ± 19abcd | — | — | — | |
| Ẇ (W kg−1) | PRE | 2.7 ± 0.3 | 3.1 ± 0.4 | 2.6 ± 0.4§ | 4.2 ± 0.4*§† |
| ALT1 | 2.3 ± 0.1a | — | 1.8 ± 0.4*a | 2.7 ± 0.3*†a | |
| ALT2 | 2.3 ± 0.2a | — | 2.0 ± 0.4a | 2.9 ± 0.2*†a | |
| ALT3 | 2.5 ± 0.3bc | 2.8 ± 0.3 | 2.1 ± 0.4§ | 2.8 ± 0.4*† | |
| ALT3-O2 | 3.1 ± 0.2abcd | — | — | — | |
| V̇O2 (l min−1) | PRE | 2.16 ± 0.31 | 2.62 ± 0.40 | 3.21 ± 0.54*§ | 3.99 ± 0.32*§† |
| ALT1 | 1.65 ± 0.15a | — | 1.85 ± 0.42a | 2.08 ± 0.44a | |
| ALT2 | 1.71 ± 0.21a | — | 2.00 ± 0.32a | 2.09 ± 0.50a | |
| ALT3 | 2.01 ± 0.25bc | 2.25 ± 0.21a | 2.03 ± 0.38a | 2.07 ± 0.24a | |
| ALT3-O2 | 2.29 ± 0.29bcd | — | — | — | |
| V̇O2 (ml kg−1min−1) | PRE | 38.0 ± 4.6 | 48.9 ± 6.8* | 39.0 ± 6.0§ | 60.7 ± 3.2*§† |
| ALT1 | 28.7 ± 3.3a | — | 23.0 ± 5.7a | 33.0 ± 4.4†a | |
| ALT2 | 30.0 ± 3.8a | — | 25.0 ± 2.6a* | 32.8 ± 5.2†a | |
| ALT3 | 35.0 ± 3.7bc | 41.1 ± 2.9*a | 26.9 ± 4.5*§a | 33.0 ± 3.7§a | |
| ALT3-O2 | 37.4 ± 4.6bc | — | — | — | |
| R | PRE | 1.34 ± 0.06 | 0.98 ± 0.03* | 1.19 ± 0.11*§ | 1.12 ± 0.12* |
| ALT1 | 1.33 ± 0.18 | — | 1.28 ± 0.15 | 1.21 ± 0.06 | |
| ALT2 | 1.28 ± 0.07 | — | 1.21 ± 0.16 | 1.21 ± 0.14 | |
| ALT3 | 1.13 ± 0.08ab | 0.98 ± 0.04 | 1.11 ± 0.16 | 1.14 ± 0.16 | |
| ALT3-O2 | 1.30 ± 0.16d | — | — | — | |
| V̇E (l min−1) | PRE | 121.4 ± 20.5 | 93.5 ± 16.9* | 122.1 ± 21.6§ | 135.4 ± 16.8§ |
| ALT1 | 135.6 ± 19.2 | — | 145.4 ± 30.9 | 144.3 ± 37.0 | |
| ALT2 | 124.1 ± 24.5 | — | 152.2 ± 20.8 | 162.3 ± 58.9 | |
| ALT3 | 121.3 ± 27.9 | 112.5 ± 24.0a | 147.0 ± 32.5 | 150.9 ± 46.8a | |
| ALT3-O2 | 137.4 ± 15.3 | — | — | — | |
| HR (beats min−1) | PRE | 188 ± 13 | 167 ± 10* | 178 ± 13 | 190 ± 7§ |
| ALT1 | 175 ± 8a | — | 152 ± 15*a | 161 ± 10a | |
| ALT2 | 180 ± 10 | — | 144 ± 10*a | 159 ± 4*a | |
| ALT3 | 179 ± 9a | 171 ± 4 | 148 ± 11*§a | 149 ± 7*§a | |
| ALT3-O2 | 182 ± 11 | — | — | — | |
| SaO2 (%) | PRE | 96.1 ± 1.1 | 96.1 ± 1.0 | 97.8 ± 0.5 | 96.1 ± 1.1 |
| ALT1 | 83.4 ± 2.8a | — | 78.2 ± 9.4a | 71.8 ± 7.2*a | |
| ALT2 | 82.3 ± 7.1a | — | 79.7 ± 6.5a | 75.6 ± 6.1a | |
| ALT3 | 81.6 ± 5.8a | 80.0 ± 7.0a | 76.0 ± 5.6a | 72.7 ± 5.4a | |
| ALT3-O2 | 97.3 ± 1.8bcd | — | — | — |
Values are means ±s.d. Significantly different from Tib 2
Values are means ±s.d. Significantly different from Sh
Values are means ±s.d. Significantly different from UT.
Significantly different from PRE
Significantly different from ALT1
Significantly different from ALT2
Significantly different from ALT3.
Peak power output (Ẇpeak, W) developed by Tibetan lowlanders and Sherpas was almost independent of the environmental conditions, being only 7% and 8%, respectively, lower at ALT3 than the corresponding PRE value. By contrast, Ẇpeak of untrained and trained Caucasians underwent a sizeable reduction, being 26% and 37% less at ALT3 than at PRE (P < 0.01).
At PRE, average V̇O2peak values of Tibetan lowlanders and untrained Caucasians were similar (38.0 ± 4.6 and 39.0 ± 6.0 ml kg−1 min−1, respectively). By contrast, trained Caucasians were characterized by the highest value (60.7 ± 3.2 ml kg−1 min−1), whereas Sherpas' V̇O2peak was in-between (48.9 ± 6.8 ml kg−1 min−1). At ALT1 and ALT2 V̇O2peak of the investigated groups underwent a significant decrease (P < 0.05) compared to PRE. At ALT3, Tibetans recovered almost entirely the PRE value (35.0 ± 3.7 ml kg−1 min−1), wheras V̇O2peak of trained Caucasians dropped to 33.0 ± 3.7 ml kg−1 min−1. Also V̇O2peak of Sherpas and untrained Caucasians was significantly (P < 0.05) reduced compared to PRE (41.1 ± 2.9 and 26.9 ± 4.5 ml kg−1 min−1). In all conditions (from PRE to ALT3), gas exchange ratio (R) was in most cases ≥ 1, showing that all subjects had attained exhaustion.
Average V̇Epeak of Tibetans was unaffected by altitude. At ALT3 V̇Epeak of Tib 2 and Sherpas was similar (121.3 ± 27.9 and 112.5 ± 24.0 l min−1, respectively). Average V̇Epeak increased slightly in untrained and trained Caucasians. In the latter it was 150.9 ± 46.8 l min−1, i.e significantly (P < 0.05) higher than the PRE value (135.4 ± 16.8 l min−1).
The HRpeak levels of Tibetan lowlanders were slightly lower at ALT1, ALT2 and ALT3 (175 ± 8, 180 ± 10 and 179 ± 9 beats min−1, respectively) than at PRE (188 ± 13 beats min−1). The ALT3 value was not statistically different from that of the Sherpas (171 ± 4 beats min−1). During O2 breathing at ALT3, HRpeak of Tibetans was similar to the value found at PRE. By contrast, at ALT1, HRpeak of untrained and trained Caucasians decreased significantly compared to PRE levels, on average, from 178 ± 13 to 151 ± 15 and from 190 ± 7 to 161 ± 10 beats min−1, respectively. Thereafter, HRpeak kept further decreasing and attained 148 ± 11 and 149 ± 7 beats min−1, respectively, at ALT3. HRpeak of Tibetan lowlanders during the sojourn was always significantly (P < 0.01) higher than the corresponding values for untrained and trained Caucasians.
At peak exercise, average SaO2 (SaO2peak) of each group was significantly lower during the sojourn at 5050 m than during the control tests (PRE). No differences were found from ALT1 to ALT3. At ALT1, average SaO2peak of Tib 2 (83 ± 3%) was significantly (P < 0.05) higher than that of trained Caucasians (72 ± 7%).
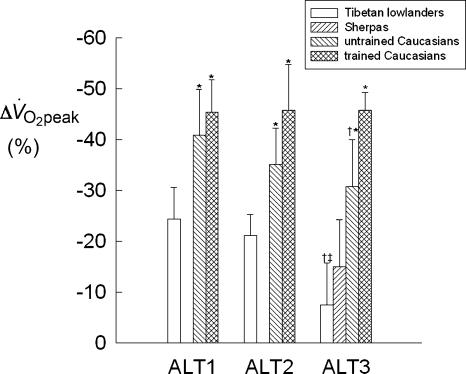
The percentage change of peak oxygen consumption (ml kg−1 min−1) compared to PRE values (Δ V̇O2peak) of the investigated groups during the exposure to 5050 m is shown in Fig. 2. In Tib 2 at ALT1, Δ V̇O2peak was −24.4 ± 6.3% (P < 0.001). However, in the course of the sojourn, V̇O2peak kept increasing steadily and at ALT3 it was not significantly different from the PRE value (∼−8%) and from the value determined during acute normoxia at ALT3 (ALT3-O2, 37.4 ± 4.6 ml kg −1 min−1). Similarly to Tibetans, untrained (UT) and trained (T) Caucasians underwent the greatest Δ V̇O2peak (−40.9 ± 8.9% and −45.4 ± 6.3%, respectively) (P < 0.001) at ALT1. At ALT2, V̇O2peak of UT started recovering slightly, although non-significantly, whereas it did not change in T. At ALT3, Δ V̇O2peak average values of untrained and trained Caucasians were −30.7 ± 9.3% and −45.7 ± 3.5%, respectively. During acclimatization Δ V̇O2peak was significantly (P < 0.05) greater in Caucasians than in Tib 2. At ALT3, Δ V̇O2peak of Sherpas was ∼−15%, i.e. slightly higher (P < 0.05) than the value for Tib 2.
Figure 2. Time course of the percentage change of peak oxygen consumption expressed per kg of body mass (Δ V̇O2peak, the PRE value made equal to 0) of Tibetan lowlanders, Sherpas, and untrained and trained Caucasians during the sojourn at 5050 m.
*P < 0.05 when comparing Caucasians with Tibetans and Sherpas at a given condition. †,‡P < 0.05 when comparing ALT3 with ALT1 and ALT2, respectively.
Discussion
The present study was designed to investigate the main metabolic, respiratory, and cardiovascular responses to peak exercise of subjects with different ethnic background, altitude exposure history and training conditions, in the course of a standardized sojourn at 5050 m. To the authors' knowledge this is the first comparative study carried out on homogeneous groups of selected subjects, in identical environmental conditions, using the same protocols and experimental set-up, i.e. eliminating most of the common confounding factors.
Two findings stemming from this investigation are novel. The first is that Tibetan lowlanders (Tib 2) never exposed to high altitude before, compared to untrained and trained Caucasians, were able to retain almost entirely their PRE V̇O2peak (Δ V̇O2peak: −8%versus−30% and −45%, respectively). The second is that, within the investigated ethnic groups (Caucasians and Tibetans), subjects with higher aerobic power in control conditions lost at ALT3 a greater fraction of their PRE V̇O2peak. This finding may explain in part the dissociation found in elite Caucasian mountaineers between climbing performance and sea-level V̇O2peak. Indeed, one of the greatest climbers of the last century (Reinhold Messner), the first man ever to reach the summit of Mt Everest (8848 m) without supplementary oxygen, was characterized at sea level by a V̇O2peak of only 49 ml kg−1 min−1 (Oelz et al. 1986).
Respiratory, metabolic and heart rate measurements
At PRE, resting V̇O2 level appears to be significantly higher in Sherpas than in all the other groups (see Table 2). Excitement and/or metabolic alterations from changing nutritional habits might be at the basis of this unexpected finding. Both Tibetan groups were characterized by higher resting V̇E levels compared to Caucasians. These results are most probably explained by the discomfort due to breathing through a mouthpiece. Nevertheless, Sherpas, like acclimatized Caucasians, might have been characterized also by a persistent adaptation-induced hyperventilation, at least in the early phase of acute normoxia (Weil, 1991). It may also be noticed that at PRE and ALT3 resting gas exchange ratio (R) of Tibetan lowlanders and Sherpas is close to 1, being greater than in Caucasians. Higher R levels, when reflecting higher respiratory quotients, may be advantageous for Tibetans and Sherpas, particularly at altitude. In fact, for any given CO2 tension, high R would be accompanied by higher O2 tension in the alveolar air, thus enhancing O2 diffusion through the alveolar–capillary barrier (Ward et al. 1990). Resting HR values at PRE were similar among groups. Only trained Caucasians had significantly lower resting HR and this was likely the result of their training history.
As shown in Table 3, average peak aerobic power was significantly (P < 0.05) different in Tib 2 and Sherpas both at 1300 m (38 versus 49 ml kg−1 min−1) and at 5050 m (35 versus 41 ml kg−1 min−1), respectively. This finding reflects the better fitness of Sherpas, who were more active than Tibetan lowlanders.
Relationships between Δ V̇O2peak at altitude and control PREV̇O2peak values
As shown in Fig. 2, after 26–28 days at 5050 m Tibetan lowlanders, differently from Caucasians, recovered almost entirely their PRE V̇O2peak. This feature may be considered the result of a genetic adaptation enhancing acclimatization. In fact, as shown by Sun et al. (1990) and Niu et al. (1995), at 3680 m the recovery of sea level V̇O2peak by Han individuals without such adaptation may take years.
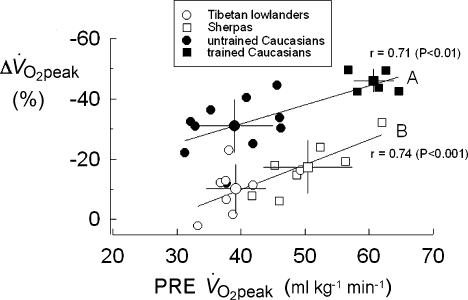
In Fig. 3, individual Δ V̇O2peak data of all subjects at ALT3 are plotted versus the corresponding V̇O2peak control values (PRE). Two distinct linear functions can be identified by interpolating data from untrained and trained Caucasians (A; r= 0.71; P < 0.01), and from Tibetan lowlanders and Sherpas (B; r= 0.74; P < 0.001), respectively. Function B is parallel, down-shifted, and significantly different (P < 0.05) from A. It appears that the percentage loss of V̇O2peak at altitude (Δ V̇O2peak, %) is positively correlated, both in Caucasians (UT and T) and in Tibetans (Tib 2 and Sh) with the V̇O2peak control values (PRE). The greater loss of V̇O2peak in trained compared with untrained individuals might be the consequence of the greater impairment of O2–blood equilibration due to a shorter red blood cell alveolar–capillary transit time (Dempsey et al. 1984; Hopkins et al. 1996; Dempsey & Wagner, 1999). The vertical difference between functions A and B, i.e. an index of the capacity of Tibetans to preserve at altitude a greater fraction of their control PRE V̇O2peak compared to Caucasians, probably depends on ethnic characteristics. These novel findings raise a major issue concerning the mechanism by which Tibetans preserve more of their PRE V̇O2peak at altitude.
Figure 3. Individual loss of peak aerobic power expressed per kg of body mass (Δ V̇O2peak, %)after 26–28 days (ALT3) at 5050 m, as a function of the corresponding PRE peak aerobic power (V̇O2peak, ml kg− min−).
Mean values ±s.d. of the 4 groups of subjects are also shown (large symbols). Continuous lines (A and B), fitted through individual values, differ significantly from each other (P < 0.05).
Factors allowing Tibetans to preserve their peak aerobic power at altitude
Determinants of V̇O2peak.
As is well known, in Caucasians at sea level V̇O2peak depends mainly (∼70%) on peak cardiac output (Q̇peak). The latter is the major determinant of the maximum circulatory convective O2 flow, i.e. the product of Q̇peak and arterial O2 content (CaO2 = [HB] × SaO2 %), to exercising muscles. In acute hypoxia the role of the cardiovascular factors tends to decrease, whereas the influence of alveolar ventilation, O2 diffusion across the alveolar–capillary barrier and of the tissue factors (from the capillary down to the respiratory chain) progressively increases (di Prampero & Ferretti, 1990; Wagner, 1996). At altitudes above 5500 m, the weight of tissue factors, due to muscle wasting, is expected to play an even greater role, mainly in Caucasians (Martinelli et al. 1990; Hoppeler et al. 2003; Gelfi et al. 2004). The role of the above determinants may be different in acclimatized Tibetan lowlanders as a consequence of genetic adaptations.
Haemoglobin concentration
Tibetan lowlanders and altitude Sherpas share a low blood haemoglobin concentration. At any given altitude below 5000 m, [Hb] has generally been reported to be lower in Tibetan populations than in Andean highlanders (Beall, 2001) and in acclimatized Caucasian or Asian lowlanders. Indeed, after 26–28 days at 5050 m, [Hb] of Tib 2 and Sherpas was similar, 16.6 and 17.4 g dl−1, respectively, i.e. significantly (P < 0.05) lower than values for acclimatized Caucasians (18.6–19.4 g dl−1). Apart from the possible positive effects on the cardiac function of the concurrent drop in haematocrit and blood viscosity, low [Hb] may be advantageous for peripheral O2 transport. In fact, as was recently shown by Calbet et al. (2002), leg blood flow and vascular conductance of lowlanders acclimatized to 5260 m were systematically higher when [Hb] was artificially reduced.
Arterial O2 saturation
At peak exercise carried out at altitude, individual arterial O2 saturation (SaO2peak) ranged from ∼65% to ∼90%. As shown in Fig. 4, at ALT3 most Tibetans and Sherpas were characterized by SaO2peak values higher than Caucasians. The large variability in SaO2peak is likely to be attributable to differences in lung maximum O2 diffusing capacity (DLO2,max), which is usually lower in acclimatized Caucasians than in altitude natives (Wagner et al. 2002). Exercise gas exchange at the alveolar level, and therefore SaO2, may be less impaired in altitude Tibetans than in Caucasians by lesser extravascular accumulation of fluids in the lung (see Anholm et al. 1999; Cremona et al. 2002), reduced hypoxic pulmonary vasoconstriction (for a review, see Moore et al. 1998), and more limited ventilation–perfusion mismatch. Due to genetic adaptations, such characteristics might still be present in some Tibetan lowlanders, thus generating higher SaO2peak values. In fact, in the absence of a genetic adaptation, SaO2peak of acclimatized lowlanders may take years to approach the values found in altitude natives (Sun et al. 1990).
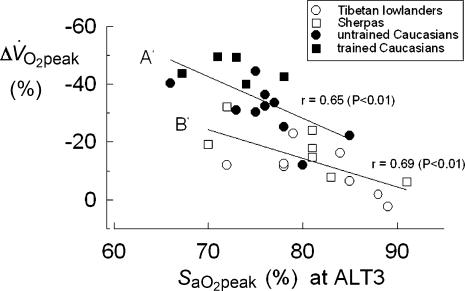
Figure 4. Individual loss of peak aerobic power expressed per kg of body mass (Δ V̇O2peak,%) after 26–28 days (ALT3) at 5050 m, as a function of the corresponding value of arterial O2 saturation (SaO2peak,%) at peak exercise.
Regression equations were determined for untrained and trained Caucasians (A′, P < 0.01) and for Tibetan lowlanders and Sherpas (B′, P < 0.01). The difference between the two equations is statistically significant (P < 0.01).
Δ V̇O2peak values of the investigated subjects appear to be correlated also with SaO2peak. In Fig. 4, individual Δ V̇O2peak values at ALT3 are plotted versus the corresponding SaO2peak values. Again, two linear functions can be identified interpolating the individual data of trained and untrained Caucasians (A′, r= 0.65; P < 0.01), and those of Tibetan lowlanders and Sherpas (B′, r= 0.69; P < 0.01), respectively. It appears that (i) the subjects characterized by the lowest SaO2peak are those undergoing the largest loss of V̇O2peak (trained Caucasians); (ii) at peak exercise, and with the same SaO2peak, Tibetans (Tib 2 and Sherpas) preserve V̇O2peak better than Caucasians. That is to say that SaO2peak is not the sole factor affecting Tibetans' V̇O2peak at altitude.
Peak heart rate and cardiac output
As shown in Table 3, following 28–30 days' altitude exposure, HRpeak of T and UT Caucasians decreased, on average, from ∼180–190 to ∼150 beats min−1. This is likely to be a consequence of a down-regulation of β-adrenergic cardiac receptors (Richalet et al. 1988) and/or of enhanced parasympathetic activity (Hartley et al. 1974; Boushel et al. 2001). In this context, however, it was recently shown that parasympathetic blockade, while increasing HRpeak, had no effect on maximal cardiac output and exercise capacity of acclimatized lowlanders, at least up to 3800 m (Bogaard et al. 2002) and therefore should not influence V̇O2peak. By contrast, HRpeak of acclimatized Tibetan lowlanders changed only slightly from control values, attaining, on average, ∼180 beats min−1, i.e. almost the same value found during acute normoxia. The latter finding indicates that, despite the fact that they were born at low altitude, Tibetan lowlanders, as is the case for Tibetan highlanders (Zhuang et al. 1993) and altitude Sherpas, apparently do not undergo desensitization of β-adrenergic cardiac receptors or increase of the parasympathetic tone upon exposure to chronic hypoxia.
Cardiac output was not determined in the present study. However, it is likely that maximal O2 convective flow (Q̇peak × CaO2) was almost preserved in all subjects, independent of their ethnicity. In fact, it is known that at altitudes between 3800 and 5800 m, peak or near-peak Q̇ of physically active acclimatized Caucasians (for a review see Wagner, 2000) may range from ∼14 to ∼20 l min−1 (Pugh, 1964; Vogel et al. 1967; Saltin et al. 1968; Cerretelli, 1976; Bogaard et al. 2002; Calbet et al. 2002), being ∼15% lower than sea level control values. Nevertheless, in acclimatized lowlanders as well as in Andean populations, the decrease of maximum convective O2 flow, depending on the decrease of Q̇peak is more than compensated for by a ∼30% increase in [Hb] and arterial O2 content (Cerretelli, 1976; Calbet et al. 2002, 2003). On the other hand, haemoglobin O2 affinity of acclimatized lowlanders, altitude Sherpas and Andean populations was found to be close to sea-level standards or only slightly increased (Samaja et al. 1979; Moore et al. 1992, 1998). Thus, in acclimatized lowlanders there seems to be a dissociation between the almost constant maximal O2 delivery to the working muscles and the drop of V̇O2peak during exercise at altitude. Although the underlying mechanisms are still unknown, the hypothesis can be put forward that, due to high [Hb] values, a relatively lower fraction of nutritional blood flow may perfuse exercising muscles at altitude (Calbet et al. 2003). This may not be the case for acclimatized Tibetan lowlanders. In fact, as mentioned before, their higher HRpeak and lower blood viscosity are likely to favour adequate Q̇peak values (Chen et al. 1997) and to increase nutritional blood flow to working muscles, respectively. The latter, along with an apparently larger oxygen extraction (Pugh, 1964) may account for the greater preservation at altitude of PRE V̇O2peak in acclimatized Tibetans compared to Caucasians. On the other hand, apart from a slight reduction in body weight observed in untrained Caucasians, there are no hints of structural and functional deterioration of the muscle mass after the sojourn at the Pyramid-Laboratory that could account for the loss of different fractions of PRE V̇O2peak in the investigated groups (Kayser et al. 1993). Relevant for the interpretation of the present results may be the finding that Tibetan lowlanders are characterized by smaller muscle fibre cross-sectional area than non-Tibetan controls (Kayser et al. 1996). Since the muscle capillary density is the same, this adaptive change may result in a shorter diffusion path for O2 at the muscle level. The above feature may be one of the factors contributing to preserve V̇O2peak of Tibetan lowlanders upon hypoxia exposure.
Acknowledgments
The authors are indebted to A. Da Polenza, G. P. Verza, Hari Shresta, and Nima Nuru Sherpa, who organized the scientific expeditions to the Pyramid Laboratory, to the Royal Nepal Academy of Science and Technology, to the volunteers, and the several postgraduate students who made this project possible. The work was partially supported by the Ev-K2-CNR Strategic Project of the Consiglio Nazionale delle Ricerche of Italy.
B. Basnyat has been appointed by the Royal Nepal Academy of Science and Technologies, Kathmandu, Nepal.
References
- Anholm JD, Milne ENC, Stark P, Bourne JC, Friedman P. Radiographic evidence of interstitial pulmonary edema after exercise at altitude. J Appl Physiol. 1999;86:503–509. doi: 10.1152/jappl.1999.86.2.503. [DOI] [PubMed] [Google Scholar]
- Beall CM. Adaptations to altitude: a current assessment. Ann Rev Anthropol. 2001;30:423–456. [Google Scholar]
- Beall CM, Brittenham GM, Strohl KP, Blangero J, Williams-Blangero S, Goldstein MC, Decker MJ, Vargas E, Villena M, Soria R, Alarcon AM, Gonzales C. Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol. 1998;106:385–400. doi: 10.1002/(SICI)1096-8644(199807)106:3<385::AID-AJPA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Bogaard HJ, Hopkins SR, Yamaya Y, Niizeki K, Ziegler MG, Wagner PD. Role of autonomic nervous system in the reduced maximal cardiac output at altitude. J Appl Physiol. 2002;93:271–279. doi: 10.1152/japplphysiol.00323.2001. [DOI] [PubMed] [Google Scholar]
- Boushel R, Calbet JAL, Rådegran G, Søndergaard H, Wagner PD, Saltin B. Parasympathetic neural activity accounts for the lowering of exercise heart rate at high altitude. Circulation. 2001;104:1785–1791. doi: 10.1161/hc4001.097040. [DOI] [PubMed] [Google Scholar]
- Calbet JAL, Boushel R, Rådegran G, Søndergaard H, Wagner PD, Saltin B. Why is VO2max after altitude acclimatization reduced despite normalization of arterial O2 content. Am J Physiol Regul Integr Comp Physiol. 2003;284:R304–R316. doi: 10.1152/ajpregu.00156.2002. [DOI] [PubMed] [Google Scholar]
- Calbet JAL, Rådegran G, Boushel R, Søndergaard H, Saltin B, Wagner PD. Effect of blood haemoglobin concentration on VO2max and cardiovascular function in lowlanders acclimatised to 5260 m. J Physiol. 2002;545:715–728. doi: 10.1113/jphysiol.2002.029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerretelli P. Limiting factors to oxygen transport on Mount Everest. J Appl Physiol. 1976;40:658–667. doi: 10.1152/jappl.1976.40.5.658. [DOI] [PubMed] [Google Scholar]
- Cerretelli P, Hoppeler H. Morphologic and metabolic response to chronic hypoxia: the muscle system. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, section 4, Environmental Physiology. New York: Oxford University Press; 1996. pp. 1155–1181. [Google Scholar]
- Chen QH, Ge RL, Wang XZ, Chen HX, Wu TY, Kobayashi T, Yoshimura K. Exercise performance of Tibetan and Han adolescents at altitude of 3,417 and 4,300 m. J Appl Physiol. 1997;83:661–667. doi: 10.1152/jappl.1997.83.2.661. [DOI] [PubMed] [Google Scholar]
- Cremona G, Asnaghi R, Baderna P, Brunetto A, Brutsaert T, Cavallaro C, Clark TM, Cogo A, Donis R, Lanfranchi P, Luks A, Novello N, Panzetta S, Perini L, Putnam M, Spagnolatti L, Wagner H, Wagner PD. Pulmonary extravascular fluid accumulation in recreational climbers: a prospective study. Lancet. 2002;359:303–309. doi: 10.1016/s0140-6736(02)07496-2. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Hanson P, Henderson K. Exercise induced arterial hypoxemia in healthy human subjects at sea level. J Physiol. 1984;355:161–175. doi: 10.1113/jphysiol.1984.sp015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol. 1999;87:1997–2006. doi: 10.1152/jappl.1999.87.6.1997. [DOI] [PubMed] [Google Scholar]
- di Prampero PE, Ferretti G. Factors limiting maximal oxygen consumption in humans. Respir Physiol. 1990;80:113–127. doi: 10.1016/0034-5687(90)90075-a. [DOI] [PubMed] [Google Scholar]
- Frisancho AR, Sanchez J, Pallardel D, Yanez L. Adaptive significance of small body size under poor socio-economic conditions in southern Peru. Am J Phys Anthropol. 1973;39:255–262. doi: 10.1002/ajpa.1330390216. [DOI] [PubMed] [Google Scholar]
- Gelfi C, De Palma S, Ripamonti M, Wait R, Eberini I, Bajracharya A, Marconi C, Schneider A, Hoppeler H, Cerretelli P. New aspects of altitude adaptation in Tibetans: a proteomic approach. FASEB J. 2004 doi: 10.1096/fj.03-1077fje. (in press; doi; 10.1096/fj.03-1077fje) [DOI] [PubMed] [Google Scholar]
- Gore CJ, Hahn AG, Scroop GC, Watson DB, Norton KI, Wood RJ, Campbell DP, Emonson DL. Increased arterial desaturation in trained cyclists during maximal exercise at 580 m altitude. J Appl Physiol. 1996;80:2204–2210. doi: 10.1152/jappl.1996.80.6.2204. [DOI] [PubMed] [Google Scholar]
- Grassi B, Marzorati M, Kayser B, Bordini M, Colombini A, Conti M, Marconi C, Cerretelli P. Peak blood lactate and blood lactate vs. workload during acclimatization to 5,050 m and in deacclimatization. J Appl Physiol. 1996;80:685–692. doi: 10.1152/jappl.1996.80.2.685. [DOI] [PubMed] [Google Scholar]
- Greska LP, Spielvogel H, Paredes Fernandez L. Maximal exercise capacity in adolescent European and Amerindian high-altitude natives. Am J Phys Anthropol. 1985;67:209–216. doi: 10.1002/ajpa.1330670306. [DOI] [PubMed] [Google Scholar]
- Groves BM, Droma T, Sutton JR, McCullough RG, McCullough RE, Zhuang J, Rapmund G, Sun S, Janes C, Moore LG. Minimal hypoxic pulmonary hypertension in normal Tibetans at 3,658 m. J Appl Physiol. 1993;74:312–318. doi: 10.1152/jappl.1993.74.1.312. [DOI] [PubMed] [Google Scholar]
- Hartley LH, Vogel JA, Cruz JC. Reduction of maximal exercise heart rate at altitude and its reversal with atropine. J Appl Physiol. 1974;36:362–365. doi: 10.1152/jappl.1974.36.3.362. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, Belzberg AS, Wiggs BR, McKenzie DC. Pulmonary transit time and diffusion limitation during heavy exercise in athletes. Respir Physiol. 1996;103:67–73. doi: 10.1016/0034-5687(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Vogt M, Weibel ER, Flück M. Response of skeletal muscle mitochondria to hypoxia. Exp Physiol. 2003;88:109–119. doi: 10.1113/eph8802513. [DOI] [PubMed] [Google Scholar]
- Kayser B, Hoppeler H, Desplanches D, Marconi C, Broers B, Cerretelli P. Muscle ultrastructure and biochemistry of lowland Tibetans. J Appl Physiol. 1996;81:419–425. doi: 10.1152/jappl.1996.81.1.419. [DOI] [PubMed] [Google Scholar]
- Kayser B, Narici M, Milesi S, Grassi B, Cerretelli P. Body composition and maximum alactic anaerobic performance during a one month stay at high altitude. Int J Sports Med. 1993;14:244–247. doi: 10.1055/s-2007-1021171. [DOI] [PubMed] [Google Scholar]
- Koistinen P, Takala T, Martikkala V, Leppaluoto J. Aerobic fitness influences the response of maximal oxygen uptake and lactate threshold in acute hypobaric hypoxia. Int J Sports Med. 1995;26:78–81. doi: 10.1055/s-2007-972968. [DOI] [PubMed] [Google Scholar]
- Lawler J, Powers SK, Thompson D. Linear relationship between VO2max and VO2max decrement during exposure to acute hypoxia. J Appl Physiol. 1988;64:1486–1492. doi: 10.1152/jappl.1988.64.4.1486. [DOI] [PubMed] [Google Scholar]
- Martin D, O'Kroy J. Effects of acute hypoxia on theVO2max of trained and untrained subjects. J Sports Sci. 1993;11:37–42. doi: 10.1080/02640419308729961. [DOI] [PubMed] [Google Scholar]
- Martinelli M, Howald H, Hoppeler H. Muscle lipofuscin content and satellite cell volume is increased after high altitude exposure in humans. Experientia. 1990;46:672–676. doi: 10.1007/BF01939930. [DOI] [PubMed] [Google Scholar]
- Marzorati M, Marconi C, Grassi B, Colombini A, Conti M, Caspani E, Cerretelli P. VO2max in chronic hypoxia: greater reduction in athletes than in sedentary subjects. FASEB J. 1995;9:A648. [Google Scholar]
- Moore LG. Human genetic adaptation to high altitude. High Alt Med Biol. 2001;2:257–279. doi: 10.1089/152702901750265341. [DOI] [PubMed] [Google Scholar]
- Moore LG, Curran-Everett L, Droma TS, Groves BM, McCullough RE, McCullough RG, Sun SF, Sutton JR, Zamudio S, Zhuang JG. Are Tibetans better adapted? Int J Sports Med. 1992;13(Suppl. 1):S86–S88. doi: 10.1055/s-2007-1024605. [DOI] [PubMed] [Google Scholar]
- Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives. Yrbk Phys Anthropol. 1998;41:25–64. doi: 10.1002/(sici)1096-8644(1998)107:27+<25::aid-ajpa3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Niu W, Wu Y, Li B, Chen N, Song S. Effects of long-term acclimatization in lowlanders migrating to high altitude: comparison with high altitude residents. Eur J Appl Physiol. 1995;71:543–548. doi: 10.1007/BF00238558. [DOI] [PubMed] [Google Scholar]
- Oelz O, Howald H, di Prampero PE, Hoppeler H, Claassen H, Jenni R, Buhlmann A, Ferretti G, Bruckner J-C, Veicsteinas A, Gussoni M, Cerretelli P. Physiological profile of world-class high-altitude climbers. J Appl Physiol. 1986;60:1734–1742. doi: 10.1152/jappl.1986.60.5.1734. [DOI] [PubMed] [Google Scholar]
- Pugh LGCE. Cardiac output in muscular exercise at 5,800 m (19,000 ft) J Appl Physiol. 1964;19:441–447. [Google Scholar]
- Richalet JP, Larmignat P, Rathat C, Kéromès A, Baud P, Lhoste F. Decreased cardiac response to isoproterenol infusion in acute and chronic hypoxia. J Appl Physiol. 1988;65:1957–1961. doi: 10.1152/jappl.1988.65.5.1957. [DOI] [PubMed] [Google Scholar]
- Saltin B, Grover RF, Blomquist CG, Hartley LH, Johnson RL., Jr Maximal oxygen uptake and cardiac output after 2 weeks at 4,300 m. J Appl Physiol. 1968;25:400–409. [Google Scholar]
- Samaja M, Veicsteinas A, Cerretelli P. Oxygen affinity of blood in altitude Sherpas. J App Physiol. 1979;47:337–341. doi: 10.1152/jappl.1979.47.2.337. [DOI] [PubMed] [Google Scholar]
- Shephard RJ, Bouhlel E, Vandewalle H, Monod H. Peak oxygen intake and hypoxia: influence of physical fitness. Int J Sports Med. 1988;9:279–283. doi: 10.1055/s-2007-1025022. [DOI] [PubMed] [Google Scholar]
- Sun SF, Droma TS, Zhang JG, Tao JX, Huang SY, McCullough RG, McCullough RE, Reeves CS, Reeves JT, Moore LG. Greater maximal O2 uptakes and vital capacities in Tibetan than Han residents of Lhasa. Respir Physiol. 1990;79:151–162. doi: 10.1016/0034-5687(90)90015-q. [DOI] [PubMed] [Google Scholar]
- Terrados N, Mizuno M, Andersen H. Reduction in maximal oxygen uptake at low altitudes: role of training status and lung function. Clin Physiol. 1985;5(Suppl. 3):75–79. doi: 10.1111/j.1475-097x.1985.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Vogel JA, Hansen JA, Harris CW. Cardiovascular responses in man during exhaustive work at sea level and high altitude. J Appl Physiol. 1967;23:531–539. doi: 10.1152/jappl.1967.23.4.531. [DOI] [PubMed] [Google Scholar]
- Wagner PD. A theoretical analysis of factors determining VO2max at sea level and altitude. Respir Physiol. 1996;106:329–343. doi: 10.1016/s0034-5687(96)00086-2. [DOI] [PubMed] [Google Scholar]
- Wagner PD. Reduced maximal cardiac output at altitude – mechanisms and significance. Respir Physiol. 2000;120:1–11. doi: 10.1016/s0034-5687(99)00101-2. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Araoz M, Boushel R, Calbet JAL, Jessen B, Rådegran G, Spielvogel H, Söndegaard H, Wagner H, Saltin B. Pulmonary gas exchange and acid-base state at 5,260 m in high-altitude Bolivians and acclimatized lowlanders. J Appl Physiol. 2002;92:1393–1400. doi: 10.1152/japplphysiol.00093.2001. [DOI] [PubMed] [Google Scholar]
- Ward MP, Milledge JS, West JB. High Altitude Medicine and Physiology. Philadelphia: UPP; 1990. p. 289. [Google Scholar]
- Weil JV. Control of ventilation in chronic hypoxia. Role of peripheral chemoreceptors. In: Lahiri S, Cherniack NS, Fitzgerald RS, editors. Response and Adaptation to Hypoxia. Organ to Organelle. New York, Oxford: Oxford University Press; 1991. pp. 122–132. [Google Scholar]
- Young AJ, Cymerman A, Burse RL. The influence of cardiorespiratory fitness on the decrement in maximal aerobic power at high altitude. Eur J Appl Physiol. 1985;54:12–15. doi: 10.1007/BF00426291. [DOI] [PubMed] [Google Scholar]
- Zhuang J, Droma T, Sutton JR, Groves BM, McCullough RE, McCullough RG, Sun S, Moore LG. Smaller alveolar-arterial O2 gradients in Tibetan than Han residents of Lhasa (3658 m) Respir Physiol. 1996;103:75–82. doi: 10.1016/0034-5687(95)00041-0. [DOI] [PubMed] [Google Scholar]
- Zhuang J, Droma T, Sutton JR, McCullough RE, McCullough RG, Groves BM, Rapmund G, Janes C, Sun S, Moore LG. Autonomic regulation of HR response to exercise in Tibetans and Han residents of Lhasa (3,658 m) J Appl Physiol. 1993;75:1968–1973. doi: 10.1152/jappl.1993.75.5.1968. [DOI] [PubMed] [Google Scholar]