Abstract
Carbon monoxide (CO) is a biologically active product of haem metabolism that contributes to the normal physiology of the gastrointestinal tract. In this article, we review recent data showing that CO is an integral regulator of gastrointestinal motility and an important factor in the response to gastrointestinal injury. CO is generated by haem oxygenase-2 (HO-2), which is constitutively expressed in many inhibitory neurones of the vertebrate enteric nervous system. The membrane potential gradients along and across the muscle layers of the gastrointestinal tract require the generation of CO by haem oxygenase-2. The presence of CO is also necessary for normal inhibitory neurotransmission in circular smooth muscle and appears to permit nitric oxide-mediated inhibitory neurotransmission. Genetic deletion of the haem oxygenase-2 gene in mice slows gut transit. The other major CO synthetic enzyme, haem oxygenase-1 (HO-1) is induced under conditions of stress or injury. Recent studies have demonstrated that up-regulation of haem oxygenase-1 protects the gut from several types of gastrointestinal injury, suggesting that CO or induction of HO-1 may find therapeutic use in gastrointestinal diseases and injuries. Furthermore, it is anticipated that the understanding of CO-mediated signalling in the gastrointestinal tract will inform studies in other tissues that express haem oxygenases.
General introduction
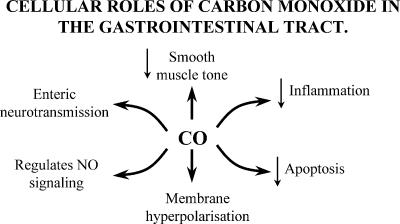
The physiological function of carbon monoxide (CO) has become the subject of intensive research in recent years and studies on the gastrointestinal tract have been at the forefront of these investigations. It is now understood that CO is an important chemical signal that regulates neurotransmission, smooth muscle tone and the response to cellular injury (Fig. 1). In addition, other products of haem degradation, biliverdin and Fe2+, are attracting attention for their physiological effects. There are several comprehensive reviews on the physiology and pathophysiology of CO in general including the proceedings of a recent symposium devoted to the subject (Choi et al. 2002, see also Maines, 1997). However, the role of CO in the gut has not been reviewed since 1999 (Farrugia & Szurszewski, 1999) and the field has advanced significantly since then, with clear demonstrations of CO as a nerve-derived signalling molecule and as a suppressor of cellular injury. There are several products of haem degradation but CO has received most attention in the gut. Therefore, it is the focus of this review. The significance of biliverdin and Fe2+ to gut physiology is still unclear and will not be discussed in detail.
Figure 1.
Cellular roles of carbon monoxide in the gastrointestinal tract.
Synthesis of carbon monoxide
Two haem oxygenase enzymes, haem oxygenase-1 (HO-1) and haem oxygenase-2 (HO-2) are located in the endoplasmic reticulum and catalyse the synthesis of carbon monoxide from Fe protoporphyrin IX (haem) (Table 1, reviewed by Maines, 1997). A third isoform, HO-3, has also been described but it does not generate CO from haem and the functional role of this protein is unclear (McCoubrey et al. 1997). These proteins are structurally quite different but the reaction chemistry is the same, relying on oxidation of NADPH and using molecular oxygen in the cleavage of haem. Carbon monoxide (CO), biliverdin, Fe2+ and H2O2 are generated by this reaction. Until the late 1980s haem oxygenases were considered simply responsible for removal of excess haem. It is now evident that generation of CO, biliverdin, Fe2+ and H2O2 are also important functions of haem oxygenase and that all of the products of haem degradation have biological effects. The anatomical distribution of the synthetic enzymes and the regulation of the enzymes largely determine the contribution of CO to intercellular signalling.
Table 1.
Haem oxygenase isoforms
| HO-1 | HO-2 | HO-3 | |
|---|---|---|---|
| Isoform | Widely inducible | Constitutive, inducible by adrenal glucocorticoids | Unknown |
| Regulation | Regulation of transcription, haem availability | Phosphorylation by ser/thr/tyr kinases, haem availability | Unknown |
| Transcripts | Single 1.8 Kb transcript | 1 widely expressed transcript, 4 add'l transcripts in testis 1.3-2.1 Kb | 2.1-5 Kb |
| Homology | 43% with HO-2 | 43% with HO-1 | 50% with HO-1 |
| 50% with HO-3 | 90% with HO-3 | 90% with HO-2 | |
| Proposed role | Antioxidant and inflammation | Generates signalling molecules | Regulation of haem-dependent genes |
Distribution of haem oxygenases in the gastrointestinal tract.
Haem can be synthesized de novo in all mammalian cells tested to date (Maines, 1980). Under normal, non-diseased conditions, the rate-limiting step to CO production appears to be the activity of the haem oxygenases. HO-1 and HO-2 are regulated by strikingly different mechanisms, which may reflect different physiological and pathological roles.
HO-1 is expressed at very low levels in the gut unless induced by disease, injury and/or inflammation. On the other hand, HO-2 expression has been reported from studies throughout the healthy gut in a number of species. Predictably, there are species differences but the overall pattern of HO-2-like immunoreactivity is similar in the animal models commonly used in studies of motility and enteric neuroscience. A proportion of enteric neuronal cell bodies and fibres in the myenteric plexus and nerve fibres in the deep muscular plexuses express HO-2-like immunoreactivity in human (Miller et al. 2001), mouse (Zakhary et al. 1997; Miller et al. 1998), dog (Farrugia et al. 1998), cat (Ny et al. 1996, 1997), opossum (Battish et al. 2000; Chakder et al. 2000), guinea-pig (Vollerthun et al. 1996) and pig (van Ginneken et al. 2001; Colpaert et al. 2002a). Neurones in the pyloric and ileocaecal sphincters are reported to have particularly high levels of HO-2 (Ny et al. 1997), although other regions of the gut, particularly the stomach and small intestine, also contain an abundance of immunoreactive neurones (e.g. Miller et al. 2001). HO-2 immunoreactivity is typically clearest in cell bodies but is also detected in nerve fibres. Therefore the cell can potentially release CO and bilirubin either at the neurites or from the cell body. Often, HO-2-immunoreactive neurones also express nitric oxide synthase (NOS) and vasoactive intestinal peptide, with the degree of colocalization between HO-2 and NOS varying between 10% for human submucous ganglia and 100% for pig fundus (Colpaert et al. 2002a). Non-neuronal cells in the gastrointestinal tract also express HO-2, including a population of cells in the mucosal epithelium, the smooth muscle of blood vessels, the endothelium of blood vessels and interstitial cells of Cajal (Grozdanovic & Gossrau, 1996; Ny et al. 1996, 1997; Zakhary et al. 1997; Farrugia et al. 1998; Hu et al. 1998; Miller et al. 1998, 2001; Donat et al. 1999; Porcher et al. 1999; Piotrowska et al. 2003). Interstitial cells of Cajal are essential for normal gastrointestinal motility (Ward et al. 1994; Huizinga et al. 1995) and in mouse, interstitial cells of Cajal from the colon and small intestine express HO-2. However, HO-2 is absent from interstitial cells of Cajal in the mouse gastric fundus and fundic smooth muscle is depolarized compared to the rest of the gastrointestinal tract due to lack of CO production. Also the membrane potential gradient observed across the circular smooth muscle of colon and small intestine is not seen in the gastric fundus (Farrugia et al. 2003). HO-2 is expressed in interstitial cells of Cajal identified in rat ileum by morphological criteria (Donat et al. 1999), but the data on human gut are contradictory. Studies identifying HO-2-immunoreactive interstitial cells of Cajal in human gastric antrum (Porcher et al. 1999) and human colon (Piotrowska et al. 2003) have been published, but in another report HO-2 was not detected in interstitial cells of Cajal from either gastric antrum or jejunum (Miller et al. 2001). HO-2 immunoreactivity was absent from the few interstitial cells of Cajal observed in the colon of individuals with Hirschsprung's disease (Miller et al. 2001). The expression of HO-2 in intrinsic blood vessels in the gut and in mesenteric arteries (Naik et al. 2003) indicates that haem catalysis affects vascular tone in these vessels as in other blood vessels (Koehler & Traystman, 2002; Kourembanas, 2002). A role for HO-2 in enteric blood flow has not been reported, but chronic hypoxia does up-regulate HO-1 and cause smooth muscle hyperpolarization in mesenteric arteries from chronically hypoxic rats (Naik et al. 2003). There are no published data on the role of HO-2 in the physiology of epithelial cells from intestinal mucosa.
Regulation of haem oxygenase activity
Regulation of HO-1.
An abundance of data on the regulation of HO-1 throughout the body has been obtained using many different models and many of the preliminary observations were made in tissue and cells from outside the gut. However, studies led by Bauer and colleagues in the gut have identified a number of novel mechanisms for regulation of HO-1 and demonstrated a very important role for regulation of HO-1 in the health of the gastrointestinal tract. HO-1 is predominantly expressed in the spleen, where haemoglobin from senescent erythrocytes is broken down. However, transcription of the gene is readily induced throughout the body by a number of factors associated with cell injury or inflammation. A frequently cited and vivid example is the response to a haematoma in which HO-1 is induced and breaks down the red–purple haemoglobin to green biliverdin and yellow bilirubin (Foresti & Motterlini, 1999). In that situation, HO-1 has a clear role in removal of haem from the site of injury. HO-1 is also induced by hypoxia, UV irradiation, oxidative stress, reactive oxygen species, heavy metals, cytokines, lipopolysaccharide, shear stress and heat shock (Choi & Alam, 1996; Foresti & Motterlini, 1999). In fact, HO-1 is the heat shock protein HSP32 (Keyse & Tyrrell, 1989) and appears to be a generic part of the response to cellular stress regardless of the involvement of haem. In the absence of HO-1, mutant mice (Poss & Tonegawa, 1997) exhibit a greatly reduced resistance to cellular stress, the animals die young and suffer from chronic inflammation and growth retardation. In humans, loss of HO-1 produced similar symptoms (Yachie et al. 1999), and in another study, impaired HO-1 transcription, due to a mutation in the promotor region of the gene, seems to predispose individuals to pulmonary emphysema (Yamada et al. 2000), and coronary artery disease of type 2 diabetics (Chen et al. 2002). The converse is also true; it has been demonstrated that increased HO-1 expression protects many tissues from injury including muscle (Nath et al. 1992), endothelium (Balla et al. 1992) and lung (Choi & Alam, 1996).
The mechanism and effects of inducing HO-1 expression have been studied in many tissues. Hypoxia, CO in low concentrations, haem lysinate, NO in the presence of reactive oxygen species and a number of cytokines, including interleukin-1β and tumour necrosis factor-α, rapidly activate a short-term increase in HO-1 transcription and stabilize existing HO-1 mRNA (Kourembanas, 2002). For example, HO-1 transcription is induced in pulmonary vascular smooth muscle within 6 h of exposure and returns to baseline after 48 h, probably due to feedback inhibition by high concentrations of CO on HO-1 transcription (Morita et al. 1995). During the period of elevated HO-1 activity, a number of inflammatory mediators including NO appear to be suppressed (McQuillan et al. 1994), and this has prompted several studies on the effects of prolonged HO-1 induction. In the lung sustained expression of HO-1, either by repeated haem (McQuillan et al. 1994) administration (Christou et al. 2000) or viral infection with HO-1 cDNA (Minamino et al. 2001) prevented the deleterious pulmonary responses to hypoxic injury. HO-1 and CO increase levels of the anti-inflammatory cytokine IL-10 (Otterbein et al. 2000; Moore et al. 2003), and IL-10 can induce HO-1 (Visner et al. 2003). Other studies have shown that induction of HO-1 suppresses hyper-acute rejection during organ transplantation and that hearts from HO-1 knockout mice are more quickly rejected than organs from wild-type animals (Soares et al. 2001).
The significance of HO-1 induction to gastrointestinal health and disease is only just becoming appreciated. In common with other tissues outside the spleen, the healthy gut does not appear to express significant amounts of HO-1 (Miller et al. 2001), although there are reports of HO-1 expression in nerves and unidentified cells of the smooth muscle layers in the cat gut (Ny et al. 1996, 1997). At the cellular level, HO-1 can be induced in cultured human intestinal epithelial cells and, consistent with a potential anti-inflammatory effect, this inhibits cytokine-mediated inducible NOS (iNOS) expression (Cavicchi et al. 2000). HO-1 activity in the gut is up-regulated by heavy metal administration. Cadmium causes a 300% increase in HO-1 activity in epithelial cells of rat small intestine (Rosenberg & Kappas, 1991), and in guinea-pig stomach, HO-1 is induced in the smooth muscle layers by cobalt administration, resulting in decreased excitability of smooth muscle strips and single cells (Kadinov et al. 2002). For the injured gut, several papers have recently reported that HO-1 can be induced with beneficial effects (Wang et al. 2001; Murthy et al. 2002; Fujii et al. 2003; Moore et al. 2003; Nakao et al. 2003). In animal models of colitis, the condition is associated with increased HO-1 levels (Wang et al. 2001) and further induction of HO-1 results in less inflammation and evidence of more rapid repair (Murthy et al. 2002). In mice with postoperative ileus following mild surgical manipulation of the small intestine, transient expression of HO-1 is detected in macrophages, leucocytes and other cells activated by inflammation. Administration of low concentrations of CO further induces HO-1 expression and improved gut motility and circular muscle contractility in these animals (Moore et al. 2003). In rats injected with lipopolysaccharide, as a model of sepsis, HO-1 is induced in intestinal epithelium and inhibition of HO-1 activity produces much greater mucosal injury in response to the insult (Fujii et al. 2003). CO administration is also effective at reducing inflammatory injury and reducing ileus in transplanted small intestine in rats. Inhalation of CO suppressed production of several inflammatory mediators and lowered blood nitrite levels consistent with reduced induction of iNOS (Nakao et al. 2003).
Clearly, induction of HO-1 represents a surprising and potent anti-inflammatory pathway that acts in many ways to ameliorate tissue injury. Although the toxic effects of chronic CO production or possibly haem depletion are not known, the effects of over-expression of HO-1 in mice are subtle (Maines, 1997). Therefore, it appears that regulation of HO-1 is a promising target for treatment of gastrointestinal diseases that involve oxidative stress and/or inflammatory injury such as diabetic gastroenteropathy, suppression of rejection in organ transplants and inflammatory bowel disease.
Regulation of HO-2.
HO-2 is known as the constitutive form of HO, but HO-2 activity can be increased in response to an acute stimulus such as non-adrenergic non-cholinergic nerve stimulation in the internal anal sphincter of the opossum by vasoactive intestinal peptide (VIP) (Chakder et al. 2000). Studies on the mechanisms of HO-2 induction have demonstrated that Ca2+ influx, activation of protein kinase C (Dore et al. 1999) and activation of tyrosine kinases (Leffler et al. 2003) are all likely mediators of HO-2 activation. These messenger systems increase CO and/or bilirubin production through HO-2 in several tissues, indicating that intracellular second messengers regulate HO-2 activity. The response to glutamate of vascular smooth muscle from pig cerebral microvessels is a particularly robust, receptor-mediated effect that is rapid and involves activation of tyrosine kinases (Leffler et al. 2003). This is one of the clearest demonstrations of regulated CO release, a prerequisite for a genuine intercellular signalling molecule. Recently it has been shown that HO-2 is regulated by the protein kinase CK2. HO-2 is phosphorylated and activated by CK2 resulting in CO-mediated neurotransmission (Boehning et al. 2003). Regulation of HO-2 by CK2 has also been shown in the gut, with the CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) inhibiting murine internal anal sphincter relaxation (Boehning et al. 2003). Regulation of HO-2 by CK2 (also known as casein kinase 2) provides a mechanism for the rapid production of HO-2 in response to an activating stimulus such as Ca2+. In addition, as discussed below, the likely regulation of HO-2 by nitrosylation of cysteines (Ding et al. 1999; Hartsfield, 2002) may have physiological relevance, given the frequent colocalization of NOS and HO-2 in enteric nerves.
Transcriptional regulation of HO-2 expression has been reported for glucocorticoids (Raju et al. 1997), which implies that the level of HO-2 will be increased in times of stress. The connection to stress has not been directly demonstrated, but HO-2 expression does increase in pig duodenum during the first few days after birth when the adrenal cortex matures and glucocorticoids are first synthesized (van Ginneken et al. 2001).
Measurement of haem oxygenase activity
There are a number of methods for the measurement of haem oxygenase activity, including detection of CO in radioactive pulse chase experiments, gas chromatography, UV spectrophotometry (Marks et al. 2002) and spectrophotometric assays for biliverdin-IXα production (Nath et al. 1992; Liang et al. 2000). These assays are not especially sensitive and are off-line, so the data are obtained with some delay. However, it has proven possible to reproducibly measure HO activity in smooth muscle from dog stomach and small intestine using these techniques (Farrugia et al. 2003). One technique for real time measurements of CO production uses laser absorption spectroscopy and holds the promise of rapid and sensitive measurements of haem oxygenase activity from much smaller samples of tissue or from cultured cells (Morimoto et al. 2001). This new technique has not to our knowledge been tested on gut tissue, but may prove very useful in determining the real time changes in haem oxygenase activity and CO production in response to potential regulators of the enzymes.
Physiological effects of CO in the gastrointestinal tract
There is a temptation to regard CO as ‘NO-light’ because of the molecular similarities between CO and NO, common downstream targets and the frequent overlap in the expression of the synthetic enzymes. However, CO has effects that distinguish it from NO, particularly the high affinity of CO for ferrous (but not ferric) haem molecules, the ability of CO to modify histidine residues and the greater stability of CO. CO does not react freely with oxygen or thiols and therefore it has a physiological half-life of minutes compared to seconds for NO. It appears that CO is indeed ‘a paradigm unto itself’, as suggested in a previously published review on the physiological effects of CO (Cary & Marletta, 2001).
CO is required for the membrane potential gradients along and across the gastrointestinal muscle layers.
CO appears to be a hyperpolarizing factor in the gastrointestinal muscle layers. In the mouse and dog, CO production and haem oxygenase activity mirror the smooth muscle membrane potential (Farrugia et al. 2003). In the gastrointestinal tract, there is a large gradient in membrane potential along the long axis of the stomach from the fundus to the pylorus. There is also a membrane potential gradient across the thickness of circular muscle layer (Bauer et al. 1985; Bauer & Sanders, 1985, 1986). In the distal stomach and small intestine, the membrane potential in the circular smooth muscle region next to the longitudinal muscle is approximately 10 mV hyperpolarized compared to circular smooth muscle cells at the inner circular smooth muscle region, next to the submucosa (Bauer et al. 1985; Bauer & Sanders, 1985, 1986). In the large intestine, the gradient is present but it is reversed. CO production and haem oxygenase activity are higher in the hyperpolarized regions of the stomach, small intestine and colon and lower in the more depolarized regions (Farrugia et al. 2003). In regions of the gut with the same smooth muscle mechanical threshold the smooth muscle gradient may allow a graded contractile response to a stimulus, with a weak stimulus recruiting only the more depolarized smooth muscle and a stronger stimulus recruiting more hyperpolarized smooth muscle, suggesting a role for CO in controlling intestinal contractility.
Nitric oxide and carbon monoxide.
Nitric oxide (NO) was described in 1987 as an intercellular messenger (Ignarro et al. 1987; Palmer et al. 1987). The discovery that a gas, NO, can function as a regulator of physiological function led several investigators to look for other gases that may also function as intercellular messengers. Carbon monoxide (CO) was suggested as a second gaseous messenger based on its marked similarities to NO (Marks et al. 1991; Schmidt, 1992). Like NO, CO activates guanylyl cyclase by binding to the haem at the active site of guanylyl cyclase, resulting in increased cyclic GMP (cGMP) levels (Rich et al. 1994). In addition, the frequent colocalization of nitric oxide synthase and haem oxygenase (see Xue et al. 2000; Miller et al. 2001 for examples in the gut) led investigators to study not only CO as a signalling molecule in its own right, but also how CO and NO interact.
Multiple levels of interaction have now been identified. NO appears to increase HO-1 expression by inducing transcription and stabilizing HO mRNA (Hartsfield et al. 1997), although the mechanism of action is still not well understood. Induction of HO-1 by NO may be dependent on their relative concentrations (Hartsfield, 2002). CO regulates NOS activity in a concentration-dependent manner, with high CO levels inhibiting NOS activity and low CO levels stimulating NO production (Ingi et al. 1996; Thorup et al. 1999). In intestinal smooth muscle cells, CO appears to induce NO formation via neuronal and endothelial NOS (nNOS and eNOS) activation (I. Lim, S. J. Gibbons, G. Farrugia et al., unpublished observations). Increased NO levels were not due solely to release of preformed NO by CO, as previously reported (Thorup et al. 1999), as the effects of CO were blocked by inhibitors of NOS.
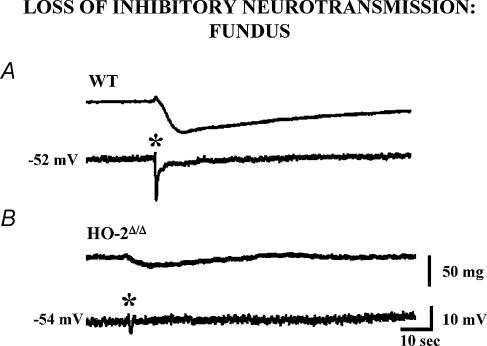
In the enteric nervous system, CO appears to play a crucial role with NO in inhibitory neurotransmission and setting the resting membrane potential in enteric smooth muscle cells (Xue et al. 2000; Farrugia et al. 2003). The resting membrane potential is depolarized in both HO-2 and nNOS knockout mice (Xue et al. 2000). Double knockout mice have even greater depolarization. CO and NO also interact to mediate inhibitory neurotransmission. NO generated from nNOS mediates a significant portion of gastrointestinal inhibitory neurotransmission in several species (Xue et al. 2000). Non-adrenergic non-cholinergic neurotransmission is nearly abolished in HO-2 knockout mice and can be restored by addition of exogenous CO (Fig. 2). L-NNA (NG-nitro-l-arginine), an inhibitor of nNOS, blocks the effect of CO, suggesting that CO is an obligate co-messenger for NO-mediated neurotransmission. Functional gastrointestinal studies reveal different patterns of disturbed transit, with delayed gastric emptying, but apparently normal small and large bowel transit times in nNOS knockouts, while HO-2 knockout animals exhibit overall slowed transit times (Zakhary et al. 1997).
Figure 2. Loss of inhibitory neurotransmission in the gastric fundus of the HO-2 knockout mouse.
A, electrical stimulation of enteric nerves (*) in mouse gastric fundus under non-adrenergic, non-cholinergic conditions evokes a transient hyperpolarization and relaxation of the circular smooth muscle. B, the response to electrical stimulation is significantly reduced in the HO-2 knockout mouse (HO-2Δ(Δ) and is restored by application of CO (see Xue et al. 2000).
The apparent marked overlap between the properties of CO and NO raises the question of why there are two molecules with the same function. An accumulation of data suggests that together with their overlapping functions CO and NO also function as distinct signalling molecules. NO is a free radical with an unpaired electron. Loss of the electron results in the nitrosonium ion, which can participate in oxidative and reductive reactions. CO is a stable compound without an unpaired electron. A major difference between CO and NO is the anti-inflammatory and antiapoptotic effect of CO. CO activates the mitogen-activated protein kinase (MAPK) pathway. The MAPK pathway is involved in proliferation, apoptosis and cytokine release (Morse et al. 2002). CO generated from HO-1 inhibits endothelial cell tumour necrosis factor α-induced apoptosis via a mechanism that includes activation of the p38 mitogen-activated protein kinase signal pathway and nuclear factor-κ ((Brouard et al. 2002; Soares et al. 2002). Both CO and NO directly modify the function of other proteins, but even when acting on the same protein they may have different sites of action. This has been best worked out for calcium-activated large conductance potassium channels. Nitric oxide increases the activity of calcium-dependent potassium channels via modifications to sulfhydryl groups. Carbon monoxide modulates the activity of the same channel by modification of histidine residues. The effects of NO are mediated on the β subunit of the potassium channel while those of CO are on the α subunit, again indicating the divergent mechanisms of action of NO and CO (Wu et al. 2002).
CO as a neurotransmitter.
Gases, unlike conventional neurotransmitters, are not stored in vesicles and therefore neuronal stimulation requires the rapid induction of the biosynthetic enzyme and production of the gas on demand for it to act as a neurotransmitter. For production of NO, neuronal activation results in activation of nNOS by calcium–calmodulin (Bredt & Snyder, 1990). The mechanisms by which CO can be produced on demand have only recently begun to be elucidated. The question of whether CO production can be regulated on demand was addressed in studies of pig cerebellar microvessels (Leffler et al. 2003) and on hippocampal neuronal cultures and the murine internal anal sphincter (Boehning et al. 2003). Boehning and colleagues demonstrated a regulatory pathway following neuronal stimulation in which Ca2+ entry activates protein kinase C (PKC), which in turn phosphorylates CK2, resulting in phosphorylation and activation of HO-2 (Boehning et al. 2003) and providing a mechanism for the regulated release of CO.
CO is the second gas to fulfil many of the criteria for a neurotransmitter. CO is an effective hyperpolarizing factor of enteric smooth muscle and acts on the same molecular targets as the endogenous neurotransmitter. CO is not stored but can be generated by haem oxygenase in enteric neurones. Genetic deletion or pharmacological inhibition of HO-2 reduces the size of the inhibitory junction potential and this effect can be restored by application of exogenous CO. CO is water-soluble, quickly diffuses away from the site of release and is inactivated by binding to haemoglobin. To date the principal problems with calling CO a neurotransmitter have been the issues of regulated release and the overlap between the molecular targets of CO and NO. While the issue of regulated release is now the focus of intense study, it is not possible using the current technology to measure real-time CO release and separate the effects from the effects of NO. The issue of CO as a neurotransmitter is further confounded by the expression of HO-2 in interstitial cells of Cajal, because these cells play a major role in neurotransmission between enteric nerves and smooth muscle. Does the knockout of the HO-2 gene impair neurotransmission because CO generation is lost from nerves or from interstitial cells of Cajal? If the answer is that CO from interstitial cells of Cajal is more significant, then our definition of a non-adrenergic non-cholinergic neurotransmitter in the gut should either exclude CO or also cover signalling molecules generated by non-neuronal cells such as interstitial cells of Cajal, fibroblasts or glia, as has been previously suggested (Snyder & Ferris, 2000).
Mechanism of action of CO
Activation of guanylate cyclase.
CO is a weak activator of soluble guanylate cyclase in vitro with much lower potency and efficacy than NO. However, application of CO to a number of different tissues results in increased cGMP production, activation of type I cGMP-dependent protein kinase and smooth muscle relaxation (Maines, 1997; Denninger & Marletta, 1999), suggesting that in vivo CO does modulate cGMP levels. In the ileum of HO-2 knockout mice, basal levels of cGMP are significantly lower compared to wild type and nNOS knockout animals. In addition, activation of cGMP production by the nicotinic receptor agonist DMPP (1,1-dimethyl-4-phenylpiperazinium) is reduced in HO-2 knockout mice, and levels of cGMP are restored by application of CO or NO (Zakhary et al. 1997). Thus CO does contribute to cGMP production in the gut and several mechanisms have been proposed to explain the enhanced in vivo effects of CO on soluble guanylate cyclase activity. One proposal suggests that CO is particularly potent in some cell types because the conformation of soluble guanylate cyclase is altered by the presence of a hitherto unidentified cofactor similar to the synthetic molecule YC-1 (Stone & Marletta, 1998). YC-1 acts in synergism with CO as activators of soluble guanylate cyclase (Friebe et al. 1996). Another possibility is that CO elevates NO levels, possibly by activation of one of the NOS isozymes or displacement of NO from intracellular stores (see above, Thorup et al. 1999) and that the two molecules act together to increase cGMP production. However, in the mouse ileum CO activates cGMP production in nNOS knockout mice and therefore it appears this isoform of NOS does not mediate the effect. In addition, inhibitors of NOS do not affect CO-stimulated cGMP production in other tissues including vascular smooth muscle (Sammut et al. 1998).
Many cellular responses to CO appear to depend on cGMP production. cGMP-dependent protein kinase I is one target that is an important mediator of smooth muscle relaxation by direct effects on the contractile machinery as well as by altering Ca2+ homeostasis and voltage-gated ion channel activity (Carvajal et al. 2000). In some cells, cGMP also activates cyclic nucleotide-gated ion channels and can regulate the levels of cAMP by inhibition or activation of certain isoforms of phosphodiesterases (Denninger & Marletta, 1999). In pig fundus and guinea-pig ileum, cGMP appears to be required for relaxation of smooth muscle strips (Utz & Ullrich, 1991; Colpaert et al. 2002b). At the cellular level, cGMP-dependent protein kinase mediates activation by CO of voltage-dependent K+ currents in intestinal smooth muscle cells (below).
Activation of potassium channels.
CO has been shown to activate K+ channels in a variety of tissues, including the gastrointestinal tract. In human and canine intestinal smooth muscle, CO activated a delayed rectifier-like K+ current resulting in membrane hyperpolarization (Farrugia et al. 1993, 1998). The current was blocked by quinidine and was also activated by exogenous cGMP, suggesting the cGMP pathway as the mechanism of action of CO. Similar results were seen in rabbit corneal epithelial cells. Exogenous CO activated a non-Ca2+-activated large conductance K+ channel. CO increased intracellular cGMP levels and did not activate the large conductance K+ channel in excised patches, again suggesting that the effects of CO on the K+ channel were through cGMP. CO activates a 70 pS channel in rat thick ascending limb cells (Liu et al. 1999). A direct activation of K+ channels is reported for large conductance Ca2+-activated K+ channels through an interaction with histidine residues on the α subunit. As outlined above this is distinct from the mechanism of action of NO which appears to be through the β subunit.
Binding to other ferrous haem molecules.
The ability of CO to bind ferrous haem is familiar from the effects of this molecule on haemoglobin and the toxic consequences on the oxygen-carrying capacity of erythrocytes. In addition, CO binds to other haem-containing proteins including cytochrome c oxidase, cytochrome P450, NOS and haem-containing transcription factors. Some of these proteins appear to be mediators of the cellular effects of CO. CO regulates circadian gene transcription by binding to the haem-containing transcription factor NPAS2 (Dioum et al. 2002). Cytochrome P450 converts arachidonic acid into a number of eicosanoids that alter vascular smooth muscle tone (Edwards & Weston, 1998) and inhibition of cytochrome P450 appears to be the intermediary for CO-induced vasodilatation in lamb ductus arteriosus (Coceani et al. 1996). CO inhibition of cytochrome c has minor effects in healthy tissue, but under hypoxic conditions CO does cause cellular injury due to oxidative stress in tissues with high O2 requirements (Piantadosi, 2002). The significance of these haem-containing proteins for the effects of CO on the gastrointestinal tract is not clear and requires further investigation.
Mitogen-activated protein kinase (MAPK).
Lipopolysaccharide (LPS) is a constituent of the bacterial cell wall and plays an important role in mediating the effects of sepsis. LPS activates pro-inflammatory mediators and activates MAPKs including ERK1/2, JNK and p38 (Otterbein et al. 2000). HO-1 through CO inhibits the effects of LPS through the MKK–p38 MAPK pathway and IL-10 (Otterbein et al. 2000). A similar mechanism of action may underlie the effects of CO in preventing postoperative ileus.
Effects of other products of haem metabolism
Biliverdin/bilirubin.
The biliverdin produced by haem oxygenases is converted to bilirubin by biliverdin reductase, an enzyme that is often colocalized with haem oxygenases. (e.g. pig fundus enteric neurones (Colpaert et al. 2002a). Bilirubin is generally considered to be a toxic waste product, particularly in the case of neonatal kernicterus (McDonagh, 1990), but it is also a potent antioxidant that scavenges peroxide radicals and inhibits lipid peroxidation (Stocker et al. 1987; Dore et al. 1999). Bilirubin decreases the generation of peroxynitrates and injury of arterial endothelial cells (Foresti & Motterlini, 1999) reduces the contractility of tracheal smooth muscle (Samb et al. 2002) and inhibits migration and adhesion of leucocytes (Ishikawa et al. 1997; Hayashi et al. 1999). There are few published studies on the effects of bilirubin in the gastrointestinal tract, but in the pig fundus bilirubin potentiates inhibitory neurotransmission (Colpaert & Lefebvre, 2000), possibly by preventing the oxidation of NO. These observations indicate that HO-2 activity contributes both a hyperpolarizing factor in CO and a regulator of nitrergic neurotransmission in bilirubin and CO. In addition, bilirubin could be an effective contributor to the anti-inflammatory effects of HO-1 induction. The apparent redundancy of this ‘belts and braces’ mechanism with overlapping roles for CO and bilirubin probably reflects the significant biological hazards due to inflammation and oxidative stress.
Fe2+.
Iron is toxic to cells, it is an effective catalyst for generation of reactive oxygen species and free radicals (Paller, 1988; McCord, 1998). Intracellular iron levels are therefore tightly controlled. Induction of HO-1 occurs in parallel with increased ferritin levels and increased transferrin levels. The results are cells generating large amounts of Fe2+ from haem catalysis that are able to quickly clear this potentially toxic metabolite (Balla et al. 1992; Nath et al. 1992). A physiological function for iron as a messenger has not been described.
Summary
The emerging roles for CO in the gastrointestinal tract now cover an extraordinary range of physiological functions both in health and in disease. CO is now being seriously considered as a therapeutic anti-inflammatory agent. These are indeed exciting times for a molecule once relegated to a useless byproduct of haem metabolism and a much-feared toxic byproduct of incomplete combustion.
Acknowledgments
We thank Gary Stoltz, Peter Strege, Janice Applequist and Kristy Zodrow for excellent technical and secretarial assistance. Our work in this field was funded by grants from the NIH, DK 57061 and DK 52766 to G. Farrugia and DK 17238 to J. H. Szurszewski. We are grateful to Joseph Szurszewski for his continuing support and thank him and Steven Miller for helpful discussions on this manuscript.
References
- Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- Battish R, Cao GY, Lynn RB, Chakder S, Rattan S. Heme oxygenase-2 distribution in anorectum: colocalization with neuronal nitric oxide synthase. Am J Physiol Gastrointest Liver Physiol. 2000;278:G148–G155. doi: 10.1152/ajpgi.2000.278.1.G148. [DOI] [PubMed] [Google Scholar]
- Bauer AJ, Reed JB, Sanders KM. Slow wave heterogeneity within the circular muscle of the canine gastric antrum. J Physiol. 1985;366:221–232. doi: 10.1113/jphysiol.1985.sp015793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AJ, Sanders KM. Gradient in excitation-contraction coupling in canine gastric antral circular muscle. J Physiol. 1985;369:283–294. doi: 10.1113/jphysiol.1985.sp015901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AJ, Sanders KM. Passive and active membrane properties of canine gastric antral circular muscles. Am J Physiol. 1986;251:C268–C273. doi: 10.1152/ajpcell.1986.251.2.C268. [DOI] [PubMed] [Google Scholar]
- Boehning D, Moon C, Sharma S, Hurt KJ, Hester LD, Ronnett GV, Shugar D, Snyder SH. Carbon monoxide neurotransmission activated by CK2 phosphorylation of heme oxygenase-2. Neuron. 2003;40:129–137. doi: 10.1016/s0896-6273(03)00596-8. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem. 2002;277:17950–17961. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000;184:409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Cary SP, Marletta MA. The case of CO signaling: why the jury is still out. J Clin Invest. 2001;107:1071–1073. doi: 10.1172/JCI12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavicchi M, Gibbs L, Whittle BJ. Inhibition of inducible nitric oxide synthase in the human intestinal epithelial cell line, DLD-1, by the inducers of heme oxygenase 1, bismuth salts, heme, and nitric oxide donors. Gut. 2000;47:771–778. doi: 10.1136/gut.47.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakder S, Cao GY, Lynn RB, Rattan S. Heme oxygenase activity in the internal anal sphincter: effects of nonadrenergic, noncholinergic nerve stimulation. Gastroenterology. 2000;118:477–486. doi: 10.1016/s0016-5085(00)70253-8. [DOI] [PubMed] [Google Scholar]
- Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA, Pan WH, Jou YS, Chau LY. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet. 2002;111:1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- Choi AM, Otterbein LE, editors. Emerging role of carbon monoxide in physiologic and pathophysiologic states. Antioxid Redox Signal. 2002;4:227–338. doi: 10.1089/152308602753666271. [DOI] [PubMed] [Google Scholar]
- Christou H, Morita T, Hsieh CM, Koike H, Arkonac B, Perrella MA, Kourembanas S. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res. 2000;86:1224–1229. doi: 10.1161/01.res.86.12.1224. [DOI] [PubMed] [Google Scholar]
- Coceani F, Kelsey L, Seidlitz E. Carbon monoxide-induced relaxation of the ductus arteriosus in the lamb: evidence against the prime role of guanylyl cyclase. Br J Pharmacol. 1996;118:1689–1696. doi: 10.1111/j.1476-5381.1996.tb15593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert EE, Lefebvre RA. Influence of bilirubin and other antioxidants on nitrergic relaxation in the pig gastric fundus. Br J Pharmacol. 2000;129:1201–1211. doi: 10.1038/sj.bjp.0703176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert EE, Timmermans JP, Lefebvre RA. Immunohistochemical localization of the antioxidant enzymes biliverdin reductase and heme oxygenase-2 in human and pig gastric fundus. Free Radic Biol Med. 2002a;32:630–637. doi: 10.1016/s0891-5849(02)00754-2. [DOI] [PubMed] [Google Scholar]
- Colpaert EE, Timmermans JP, Lefebvre RA. Investigation of the potential modulatory effect of biliverdin, carbon monoxide and bilirubin on nitrergic neurotransmission in the pig gastric fundus. Eur J Pharmacol. 2002b;457:177–186. doi: 10.1016/s0014-2999(02)02691-2. [DOI] [PubMed] [Google Scholar]
- Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- Ding Y, McCoubrey WK, Jr, Maines MD. Interaction of heme oxygenase-2 with nitric oxide donors. Is the oxygenase an intracellular ‘sink’ for NO. Eur J Biochem. 1999;264:854–861. doi: 10.1046/j.1432-1327.1999.00677.x. [DOI] [PubMed] [Google Scholar]
- Dioum EM, Rutter J, Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA, McKnight SL. NPAS2: a gas-responsive transcription factor. Science. 2002;298:2385–2387. doi: 10.1126/science.1078456. [DOI] [PubMed] [Google Scholar]
- Donat ME, Wong K, Staines WA, Krantis A. Heme oxygenase immunoreactive neurons in the rat intestine and their relationship to nitrergic neurons. J Auton Nerv Syst. 1999;77:4–12. doi: 10.1016/s0165-1838(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Dore S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D, Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Weston AH. Endothelium-derived hyperpolarizing factor – a critical appraisal. Prog Drug Res. 1998;50:107–133. doi: 10.1007/978-3-0348-8833-2_2. [DOI] [PubMed] [Google Scholar]
- Farrugia G, Irons WA, Rae JL, Sarr MG, Szurszewski JH. Activation of whole cell currents in isolated human jejunal circular smooth muscle cells by carbon monoxide. Am J Physiol. 1993;264:G1184–G1189. doi: 10.1152/ajpgi.1993.264.6.G1184. [DOI] [PubMed] [Google Scholar]
- Farrugia G, Lei S, Lin X, Miller SM, Nath KA, Ferris CD, Levitt M, Szurszewski JH. A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2003;100:8567–8570. doi: 10.1073/pnas.1431233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia G, Miller SM, Rich A, Liu X, Maines MD, Rae JL, Szurszewski JH. Distribution of heme oxygenase and effects of exogenous carbon monoxide in canine jejunum. Am J Physiol. 1998;274:G350–G358. doi: 10.1152/ajpgi.1998.274.2.G350. [DOI] [PubMed] [Google Scholar]
- Farrugia G, Szurszewski JH. Heme oxygenase, carbon monoxide, and interstitial cells of Cajal. Microsc Res Tech. 1999;47:321–324. doi: 10.1002/(SICI)1097-0029(19991201)47:5<321::AID-JEMT3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Foresti R, Motterlini R. The heme oxygenase pathway and its interaction with nitric oxide in the control of cellular homeostasis. Free Radic Res. 1999;31:459–475. doi: 10.1080/10715769900301031. [DOI] [PubMed] [Google Scholar]
- Friebe A, Schultz G, Koesling D. Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J. 1996;15:6863–6868. [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Takahashi T, Nakahira K, Uehara K, Shimizu H, Matsumi M, Morita K, Hirakawa M, Akagi R, Sassa S. Protective role of heme oxygenase-1 in the intestinal tissue injury in an experimental model of sepsis. Crit Care Med. 2003;31:893–902. doi: 10.1097/01.CCM.0000050442.54044.06. [DOI] [PubMed] [Google Scholar]
- Grozdanovic Z, Gossrau R. Expression of heme oxygenase-2 (HO-2)-like immunoreactivity in rat tissues. Acta Histochem. 1996;98:203–214. doi: 10.1016/S0065-1281(96)80040-7. [DOI] [PubMed] [Google Scholar]
- Hartsfield CL. Cross talk between carbon monoxide and nitric oxide. Antioxid Redox Signal. 2002;4:301–307. doi: 10.1089/152308602753666352. [DOI] [PubMed] [Google Scholar]
- Hartsfield CL, Alam J, Cook JL, Choi AM. Regulation of heme oxygenase-1 gene expression in vascular smooth muscle cells by nitric oxide. Am J Physiol. 1997;273:L980–L988. doi: 10.1152/ajplung.1997.273.5.L980. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Takamiya R, Yamaguchi T, Matsumoto K, Tojo SJ, Tamatani T, Kitajima M, Makino N, Ishimura Y, Suematsu M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ Res. 1999;85:663–671. doi: 10.1161/01.res.85.8.663. [DOI] [PubMed] [Google Scholar]
- Hu Y, Yang M, Ma N, Shinohara H, Semba R. Contribution of carbon monoxide-producing cells in the gastric mucosa of rat and monkey. Histochem Cell Biol. 1998;109:369–373. doi: 10.1007/s004180050237. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Ingi T, Cheng J, Ronnett GV. Carbon monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system. Neuron. 1996;16:835–842. doi: 10.1016/s0896-6273(00)80103-8. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Navab M, Leitinger N, Fogelman AM, Lusis AJ. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J Clin Invest. 1997;100:1209–1216. doi: 10.1172/JCI119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadinov B, Itzev D, Gagov H, Christova T, Bolton TB, Duridanova D. Induction of heme oxygenase in guinea-pig stomach: roles in contraction and in single muscle cell ionic currents. Acta Physiol Scand. 2002;175:297–313. doi: 10.1046/j.1365-201X.2002.00995.x. [DOI] [PubMed] [Google Scholar]
- Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler RC, Traystman RJ. Cerebrovascular effects of carbon monoxide. Antioxid Redox Signal. 2002;4:279–290. doi: 10.1089/152308602753666334. [DOI] [PubMed] [Google Scholar]
- Kourembanas S. Hypoxia and carbon monoxide in the vasculature. Antioxid Redox Signal. 2002;4:291–299. doi: 10.1089/152308602753666343. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Balabanova L, Fedinec AL, Waters CM, Parfenova H. Mechanism of glutamate stimulation of CO production in cerebral microvessels. Am J Physiol Heart Circ Physiol. 2003;285:H74–H80. doi: 10.1152/ajpheart.01081.2002. [DOI] [PubMed] [Google Scholar]
- Liang M, Croatt AJ, Nath KA. Mechanisms underlying induction of heme oxygenase-1 by nitric oxide in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2000;279:F728–F735. doi: 10.1152/ajprenal.2000.279.4.F728. [DOI] [PubMed] [Google Scholar]
- Liu H, Mount DB, Nasjletti A, Wang W. Carbon monoxide stimulates the apical 70-pS K+ channel of the rat thick ascending limb. J Clin Invest. 1999;103:963–970. doi: 10.1172/JCI5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM. Iron, free radicals, and oxidative injury. Semin Hematol. 1998;35:5–12. [PubMed] [Google Scholar]
- McCoubrey WK, Jr, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- McDonagh AF. Is bilirubin good for you. Clin Perinatol. 1990;17:359–369. [PubMed] [Google Scholar]
- McQuillan LP, Leung GK, Marsden PA, Kostyk SK, Kourembanas S. Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms. Am J Physiol. 1994;267:H1921–H1927. doi: 10.1152/ajpheart.1994.267.5.H1921. [DOI] [PubMed] [Google Scholar]
- Maines MD. Regional distribution of the enzymes of haem biosynthesis and the inhibition of 5-aminolaevulinate synthase by manganese in the rat brain. Biochem J. 1980;190:315–321. doi: 10.1042/bj1900315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- Marks GS, Brien JF, Nakatsu K, McLaughlin BE. Does carbon monoxide have a physiological function. Trends Pharmacol Sci. 1991;12:185–188. doi: 10.1016/0165-6147(91)90544-3. [DOI] [PubMed] [Google Scholar]
- Marks GS, Vreman HJ, McLaughlin BE, Brien JF, Nakatsu K. Measurement of endogenous carbon monoxide formation in biological systems. Antioxid Redox Signal. 2002;4:271–277. doi: 10.1089/152308602753666325. [DOI] [PubMed] [Google Scholar]
- Miller SM, Farrugia G, Schmalz PF, Ermilov LG, Maines MD, Szurszewski JH. Heme oxygenase 2 is present in interstitial cell networks of the mouse small intestine. Gastroenterology. 1998;114:239–244. doi: 10.1016/s0016-5085(98)70473-1. [DOI] [PubMed] [Google Scholar]
- Miller SM, Reed D, Sarr MG, Farrugia G, Szurszewski JH. Haem oxygenase in enteric nervous system of human stomach and jejunum and co-localization with nitric oxide synthase. Neurogastroenterol Motil. 2001;13:121–131. doi: 10.1046/j.1365-2982.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci U S A. 2001;98:8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Otterbein LE, Turler A, Choi AM, Bauer AJ. Inhaled carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterology. 2003;124:377–391. doi: 10.1053/gast.2003.50060. [DOI] [PubMed] [Google Scholar]
- Morimoto Y, Durante W, Lancaster DG, Klattenhoff J, Tittel FK. Real-time measurements of endogenous CO production from vascular cells using an ultrasensitive laser sensor. Am J Physiol. 2001;280:H483–H488. doi: 10.1152/ajpheart.2001.280.1.H483. [DOI] [PubMed] [Google Scholar]
- Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci U S A. 1995;92:1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D, Sethi J, Choi AM. Carbon monoxide-dependent signaling. Crit Care Med. 2002;30:S12–S17. [PubMed] [Google Scholar]
- Murthy S, Flanigan A, Coppola D, Buelow R. RDP58, a locally active TNF inhibitor, is effective in the dextran sulphate mouse model of chronic colitis. Inflamm Res. 2002;51:522–531. doi: 10.1007/pl00012423. [DOI] [PubMed] [Google Scholar]
- Naik JS, O'Donaughy TL, Walker BR. Endogenous carbon monoxide is an endothelial-derived vasodilator factor in the mesenteric circulation. Am J Physiol Heart Circ Physiol. 2003;284:H838–H845. doi: 10.1152/ajpheart.00747.2002. [DOI] [PubMed] [Google Scholar]
- Nakao A, Kimizuka K, Stolz DB, Seda Neto J, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Bauer AJ, Nalesnik MA, Otterbein LE, Geller DA, Murase N. Protective effect of carbon monoxide inhalation for cold-preserved small intestinal grafts. Surgery. 2003;134:285–292. doi: 10.1067/msy.2003.238. [DOI] [PubMed] [Google Scholar]
- Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny L, Alm P, Ekstrom P, Larsson B, Grundemar L, Andersson KE. Localization and activity of haem oxygenase and functional effects of carbon monoxide in the feline lower oesophageal sphincter. Br J Pharmacol. 1996;118:392–399. doi: 10.1111/j.1476-5381.1996.tb15415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny L, Alm P, Larsson B, Andersson KE. Morphological relations between haem oxygenases, NO-synthase and VIP in the canine and feline gastrointestinal tracts. J Auton Nerv Syst. 1997;65:49–56. doi: 10.1016/s0165-1838(97)00034-9. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Paller MS. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am J Physiol. 1988;255:F539–F544. doi: 10.1152/ajprenal.1988.255.3.F539. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA. Biological chemistry of carbon monoxide. Antioxid Redox Signal. 2002;4:259–270. doi: 10.1089/152308602753666316. [DOI] [PubMed] [Google Scholar]
- Piotrowska AP, Solari V, de Caluwe D, Puri P. Immunocolocalization of the heme oxygenase-2 and interstitial cells of Cajal in normal and aganglionic colon. J Pediatr Surg. 2003;38:73–77. doi: 10.1053/jpsu.2003.50014. [DOI] [PubMed] [Google Scholar]
- Porcher C, Orsoni P, Berdah S, Monges G, Mazet B. Distribution of heme oxygenase 2 in nerves and c-kit (+) interstitial cells in human stomach. Histochem Cell Biol. 1999;112:317–322. doi: 10.1007/s004180050453. [DOI] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju VS, McCoubrey WK, Jr, Maines MD. Regulation of heme oxygenase-2 by glucocorticoids in neonatal rat brain: characterization of a functional glucocorticoid response element. Biochim Biophys Acta. 1997;1351:89–104. doi: 10.1016/s0167-4781(96)00183-2. [DOI] [PubMed] [Google Scholar]
- Rich A, Farrugia G, Rae JL. Carbon monoxide stimulates a potassium-selective current in rabbit corneal epithelial cells. Am J Physiol. 1994;267:C435–C442. doi: 10.1152/ajpcell.1994.267.2.C435. [DOI] [PubMed] [Google Scholar]
- Rosenberg DW, Kappas A. Induction of heme oxygenase in the small intestinal epithelium: a response to oral cadmium exposure. Toxicology. 1991;67:199–210. doi: 10.1016/0300-483x(91)90143-o. [DOI] [PubMed] [Google Scholar]
- Samb A, Taille C, Almolki A, Megret J, Staddon JM, Aubier M, Boczkowski J. Heme oxygenase modulates oxidant-signaled airway smooth muscle contractility: role of bilirubin. Am J Physiol Lung Cell Mol Physiol. 2002;283:L596–L603. doi: 10.1152/ajplung.00446.2001. [DOI] [PubMed] [Google Scholar]
- Sammut IA, Foresti R, Clark JE, Exon DJ, Vesely MJ, Sarathchandra P, Green CJ, Motterlini R. Carbon monoxide is a major contributor to the regulation of vascular tone in aortas expressing high levels of haeme oxygenase-1. Br J Pharmacol. 1998;125:1437–1444. doi: 10.1038/sj.bjp.0702212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HH. NO·, CO and ·OH. Endogenous soluble guanylyl cyclase-activating factors. FEBS Lett. 1992;307:102–107. doi: 10.1016/0014-5793(92)80910-9. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Ferris CD. Novel neurotransmitters and their neuropsychiatric relevance. Am J Psychiatry. 2000;157:1738–1751. doi: 10.1176/appi.ajp.157.11.1738. [DOI] [PubMed] [Google Scholar]
- Soares MP, Brouard S, Smith RN, Bach FH. Heme oxygenase-1, a protective gene that prevents the rejection of transplanted organs. Immunol Rev. 2001;184:275–285. doi: 10.1034/j.1600-065x.2001.1840124.x. [DOI] [PubMed] [Google Scholar]
- Soares MP, Usheva A, Brouard S, Berberat PO, Gunther L, Tobiasch E, Bach FH. Modulation of endothelial cell apoptosis by heme oxygenase-1-derived carbon monoxide. Antioxid Redox Signal. 2002;4:321–329. doi: 10.1089/152308602753666370. [DOI] [PubMed] [Google Scholar]
- Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Stone JR, Marletta MA. Synergistic activation of soluble guanylate cyclase by YC-1 and carbon monoxide: implications for the role of cleavage of the iron-histidine bond during activation by nitric oxide. Chem Biol. 1998;5:255–261. doi: 10.1016/s1074-5521(98)90618-4. [DOI] [PubMed] [Google Scholar]
- Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol. 1999;277:F882–F889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- Utz J, Ullrich V. Carbon monoxide relaxes ileal smooth muscle through activation of guanylate cyclase. Biochem Pharmacol. 1991;41:1195–1201. doi: 10.1016/0006-2952(91)90658-r. [DOI] [PubMed] [Google Scholar]
- van Ginneken C, van Meir F, Sys S, Weyns A. Stereologic description of the changing expression of constitutive nitric oxide synthase and heme oxygenase in the enteric plexuses of the pig small intestine during development. J Comp Neurol. 2001;437:118–128. doi: 10.1002/cne.1274. [DOI] [PubMed] [Google Scholar]
- Visner GA, Lu F, Zhou H, Latham C, Agarwal A, Zander DS. Graft protective effects of heme oxygenase 1 in mouse tracheal transplant-related obliterative bronchiolitis. Transplantation. 2003;76:650–656. doi: 10.1097/01.TP.0000080069.61917.18. [DOI] [PubMed] [Google Scholar]
- Vollerthun R, Hohler B, Kummer W. Heme oxygenase-2 in primary afferent neurons of the guinea-pig. Histochem Cell Biol. 1996;105:453–458. doi: 10.1007/BF01457658. [DOI] [PubMed] [Google Scholar]
- Wang WP, Guo X, Koo MW, Wong BC, Lam SK, Ye YN, Cho CH. Protective role of heme oxygenase-1 on trinitrobenzene sulfonic acid-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G586–G594. doi: 10.1152/ajpgi.2001.281.2.G586. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Cao K, Lu Y, Wang R. Different mechanisms underlying the stimulation of K(Ca) channels by nitric oxide and carbon monoxide. J Clin Invest. 2002;110:691–700. doi: 10.1172/JCI15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Farrugia G, Miller SM, Ferris CD, Snyder SH, Szurszewski JH. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci U S A. 2000;97:1851–1855. doi: 10.1073/pnas.97.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci U S A. 1997;94:14848–14853. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]