Abstract
Norway spruce (Picea abies L. Karst) produces an oleoresin characterized by a diverse array of terpenoids, monoterpenoids, sesquiterpenoids, and diterpene resin acids that can protect conifers against potential herbivores and pathogens. Oleoresin accumulates constitutively in resin ducts in the cortex and phloem (bark) of Norway spruce stems. De novo formation of traumatic resin ducts (TDs) is observed in the developing secondary xylem (wood) after insect attack, fungal elicitation, and mechanical wounding. Here, we characterize the methyl jasmonate-induced formation of TDs in Norway spruce by microscopy, chemical analyses of resin composition, and assays of terpenoid biosynthetic enzymes. The response involves tissue-specific differentiation of TDs, terpenoid accumulation, and induction of enzyme activities of both prenyltransferases and terpene synthases in the developing xylem, a tissue that constitutively lacks axial resin ducts in spruce. The induction of a complex defense response in Norway spruce by methyl jasmonate application provides new avenues to evaluate the role of resin defenses for protection of conifers against destructive pests such as white pine weevils (Pissodes strobi), bark beetles (Coleoptera, Scolytidae), and insect-associated tree pathogens.
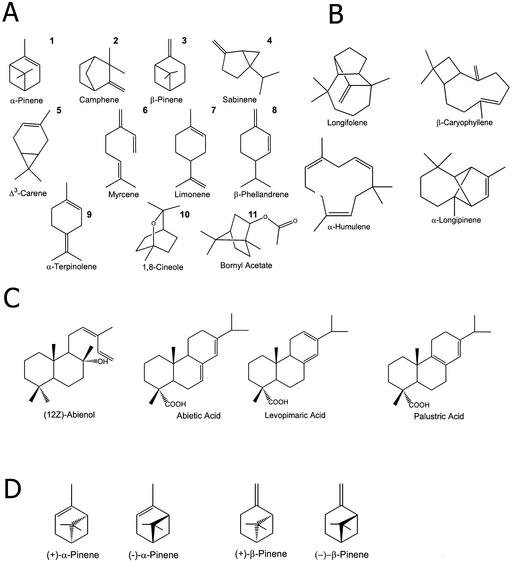
Conifers produce extensive terpenoid-based resins that have long been studied for their industrial importance and role in defense against herbivores and pathogens (Bohlmann and Croteau, 1999; Phillips and Croteau, 1999; Trapp and Croteau, 2001). Composed of approximately equal molar amounts of monoterpenes (10 carbon atoms) and diterpenes (20 carbon atoms), conifer resin also contains a smaller proportion of sesquiterpenes (15 carbon atoms; Fig. 1). The monoterpenes and sesquiterpenes constitute the volatile turpentine fraction of conifer oleoresin, whereas the diterpene resin acids form the rosin. Conifers have specialized anatomical structures for accumulation of resin terpenes, which can be as simple as the resin blisters found in species of true fir (Abies spp.), or more complex such as the resin-filled canals of spruce (Picea spp.) and pine (Pinus spp.) that are interconnected in a three-dimensional reticulate system (Bannan, 1936; Fahn, 1979).
Figure 1.
Representative structures of terpenoids of Norway spruce (Picea abies L. Karst). A and D, Monoterpenes (10 carbon atoms). B, Sesquiterpenes (15 carbon atoms). C, Diterpene resin acids (20 carbon atoms). Monoterpenes are numbered corresponding to peak numbers in Figure 9.
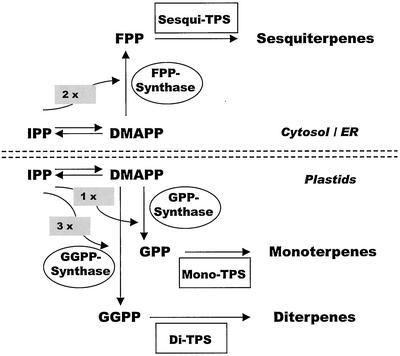
The study of plant terpenoid biosynthesis has made rapid progress in recent years (Chappell, 1995; McGarvey and Croteau, 1995; Gershenzon and Kreis, 1999; Bohlmann et al., 2000; Fig. 2). Two pathways exist for the formation of the five-carbon biosynthetic building block, IPP. The mevalonate pathway is found in the cytosol/endoplasmic reticulum and the 2-C-methylerythritol-4-phosphate pathway, which proceeds via 1-deoxyxylulose-5-phosphate, occurs in plastids (Eisenreich et al., 1998; Lichtenthaler, 1999). Condensation of IPP and its isomer, DMAPP, by class-specific prenyltransferases (PTs) supplies the three central intermediates of the isoprenoid pathway, GPP, FPP, and GGPP (Alonso and Croteau, 1993). The basic terpene skeletons are then formed from GPP, FPP, or GGPP by catalysis of terpene synthases (TPS) resulting in monoterpenes (10 carbon atoms), sesquiterpenes (15 carbon atoms), and diterpenes (20 carbon atoms), respectively (Bohlmann et al., 1998b; Davis and Croteau, 2000). These enzymes function through the divalent metal ion-assisted generation of carbocation intermediates from the prenyl diphosphate precursor and give rise to the hundreds of cyclic and acyclic parent skeletons typical of plant terpenoids. Many TPS yield only one or a few closely related products, whereas some TPS form complex product mixtures (Bohlmann et al., 1998b; Steele et al., 1998a).
Figure 2.
Scheme of the pathways of terpenoid biosynthesis in conifers. The five-carbon precursors, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), are formed via two pathways, the mevalonate pathway in the cytosol/endoplasmic reticulum and the 2-C-methylerythritol-4-phosphate pathway (via 1-deoxyxylulose-5-phosphate) in plastids. Prenyltransferases (PTs) catalyze (1′-4) head-to-tail condensations of DMAPP with one, two, or three molecules of IPP to form geranyl diphosphate (GPP; GPP synthase), farnesyl diphosphate (FPP; FPP synthase), and geranylgeranyl diphosphate (GGPP; GGPP synthase), respectively. Terpene synthases (TPS; cyclases) of three classes (mono-TPS, sesqui-TPS, and di-TPS) convert the three prenyl diphosphate intermediates into the hundreds of cyclic and acyclic terpenoids characteristic of conifers.
Most of the research on the biosynthesis of conifer terpenoids to date has focused on the TPS enzymes and their genetic regulation in grand fir (Abies grandis; Lewinsohn et al., 1991; Stofer Vogel et al., 1996; Bohlmann et al., 1997, 1998a, 1998b, 1999; Steele et al., 1998a, 1998b; Bohlmann and Croteau, 1999). These studies revealed a wound-induced resin response in stem tissues based on up-regulation of the TPS genes and enzymes. However, the possible role of PTs for regulation of induced resin formation has not been as thoroughly studied (Tholl et al., 2001). In addition, very little is known about the biochemical processes of induced terpenoid formation in other conifer species.
The genus Picea includes some of the economically most important species of forest trees. In Picea spp., stem resin accumulates constitutively in axial resin canals in the cortex and in axial traumatic resin ducts (TDs), which appear within the developing xylem after mechanical wounding, insect feeding, or fungal elicitation (Fig. 3). Recent microscopic work with Norway spruce has described details of the TD formation (Nagy et al., 2000). The production of TDs is due to a change in the developmental program of cambial activity whereby some of the xylem mother cells initiate epithelial cells (which eventually come to surround the TD lumen) in lieu of tracheids. However, it is not known whether duct formation is associated with de novo biosynthesis of resin terpenoids in the xylem, or if preformed resin stored under pressure in constitutive resin canals in the bark tissue is mobilized to these new sites for resin accumulation. De novo formation could result in an increase in total resin quantity and a change in resin composition.
Figure 3.
Light microscopy of induced TD differentiation in cross sections of Norway spruce stems. A and E, Unsprayed controls show the absence of resin ducts in the constitutive xylem (X) and phloem (P), but large constitutive resin ducts (CD) are present in the cortex of control trees. B, Nine days after 10 mm methyl jasmonate (MeJA) treatment, early stage of induced development of TDs in the xylem next to the vascular cambium. C, Twenty-five days after MeJA treatment, showing accumulation of resin in the lumen of the fully differentiated TD visualized by staining with copper acetate. D, Two months after treatment, lumen (L) of TD with resin droplet (blue) and remainder of epithelial resin duct cells (E). F, Two months after treatment, showing a ring of fully formed traumatic ducts in the newly developed xylem.
Previous studies of the anatomy and resin chemistry of TDs in spruce involved mechanical wounding or fungal inoculation of trees as a means to induce TD formation (Alfaro, 1995; Tomlin et al., 1998, 2000; Franceschi et al., 2000). In this study, we developed a noninvasive procedure for TD induction in Norway spruce based on topical application of methyljasmonate (MeJA). This treatment enabled a detailed chemical and biochemical analysis of the traumatic resin response.
RESULTS
Induced Formation of TDs
Formation of TDs in spruce is elicited by stem-boring insects and microbial pathogens as a defense response that can also be induced by mechanical wounding or by wounding and fungal inoculation of trees (Alfaro, 1995; Tomlin et al., 1998, 2000; Franceschi et al., 2000). Because wounding of trees can cause massive bleeding and volatilization of oleoresin and disruption of the tissues that are possibly involved in de novo resin formation, it was important to develop a noninvasive method for TD induction to enable a detailed chemical and biochemical analysis of the traumatic resin response. To determine if MeJA induces the formation of TDs, 2-year-old Norway spruce saplings were sprayed with MeJA in aqueous solution and stem samples were examined by light microscopy over a period of 2 months after treatment. Copper acetate staining was used to visualize the terpenes accumulating in the constitutive resin ducts and TDs (Fig. 3).
In unsprayed saplings, axial resin ducts were largely restricted to the bark (all tissues outside of the cambium, i.e. phloem, cortex, and periderm). However, after MeJA treatment, striking morphological changes became apparent as early as 6 to 9 d after application. As seen in Figure 3B, some new xylem cells immediately adjacent to the cambium had denser cytoplasm and thinner walls than surrounding xylem cells and appeared to constitute the epithelial cells of nascent TDs which surround the terpene-rich lumen. Within 15 d after treatment, lumens of the developing ducts were clearly discernible in a ring within the youngest portion of the xylem. Starting at this time, the lumen began filling with resin, presumed to be secreted from the epithelial cells, that were still visible at d 25 (Fig. 3C). Two months after induction, the lumen had enlarged further and the epithelial cells had disappeared or diminished in size considerably (Fig. 3, D and F). During the same period, resin ducts in the bark were not visibly affected.
A range of concentrations of MeJA (1–100 mm) applied as a surface spray was shown to induce TD formation in spruce stems. Response at 10 mm was greater than that at 1 mm, but at 100 mm MeJA xylem development was minimal after TD initiation and several of the treated saplings shed needles and suffered severely reduced growth. Thus, 10 mm was routinely employed in further experiments. However, the MeJA concentration that is effective in the responding tissues is probably much lower than that of the applied surface spray because this elicitor is unlikely to easily penetrate the thick cortex of spruce stems. This anatomical feature precludes any direct comparison of effective MeJA concentration to that previously used in studies of herbaceous angiosperms or plant cell suspension cultures. A full TD response was also induced by much lower concentrations of MeJA (100–500 μm MeJA) when 0.1% (v/v) Tween 20 was added to the spray solution (data not shown).
Induced Accumulation of Monoterpenes and Diterpenes
The initiation of TD formation in the developing xylem by MeJA suggested that increased accumulation of resin terpenoids would also be observed. To test this possibility, the effect of MeJA on resin terpenoid composition was evaluated in both wood and in bark. Saplings were treated with 1, 10, or 100 mm MeJA as above and the saplings were harvested for resin analysis 2 months later when TDs were fully developed.
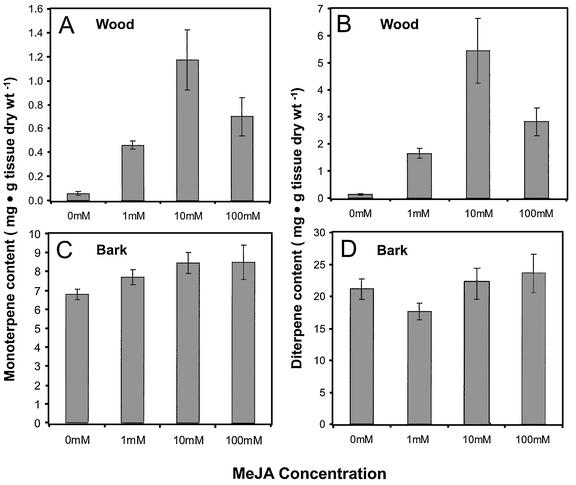
The three MeJA treatments significantly increased the total accumulation of monoterpenes (Table I) and diterpenes (Table III) within the wood tissue of sapling stems (Fig. 4), but sesquiterpene (Table II) concentrations were unchanged. Monoterpenes showed a 5-fold increase at 1 mm MeJA, a 12-fold increase at 10 mm MeJA, and a 7-fold increase at 100 mm MeJA compared with control saplings. Diterpene accumulations in the wood reflected the same trend with an 11-fold increase at 1 mm, a 38-fold increase at 10 mm, and a 20-fold increase at 100 mm. The lower accumulation levels in the saplings treated with 100 mm MeJA as compared with 10 mm MeJA may reflect the negative effect this high concentration had on growth and development as described above. In bark tissue, there was only a minor relative increase in monoterpene accumulation (Fig. 4C; Table I), and no consistent change in diterpene levels (Fig. 4D; Table III). It should be mentioned that clonal trees of Norway spruce with low constitutive amounts of monoterpenes and diterpenes in the xylem (clone 1015-903, Fig. 4) revealed a stronger relative induction than clonal trees with higher constitutive amounts of monoterpenes and diterpenes (clone 3166-728; Fig. 5). However, the absolute amounts of induced monoterpenes and diterpenes were similar in these clones.
Table I.
Monoterpene composition of constitutive and induced resin in wood and bark of Norway spruce
| Monoterpene | Control Bark | MeJA Bark | Control Wood | MeJA Wood |
|---|---|---|---|---|
| μg g−1 dry wt | ||||
| Santene | 19.2 | 19.0 | nda | nd |
| Tricyclene | 14.9 | 21.4 | 6.0 | 5.8 |
| α-Pinene | 1,609.3 | 2,744.8 | 212.6 | 522.6 |
| Camphene | 68.0 | 127.7 | 15.9 | 22.0 |
| β-Pinene | 4,586.3 | 6,388.1 | 225.6 | 836.8 |
| Sabinene | 22.5 | 34.0 | 1.7 | 7.0 |
| Δ3-Carene | 109.8 | 123.2 | nd | 4.3 |
| α-Phellandrene | 11.8 | 15.3 | nd | nd |
| Myrcene | 240.8 | 337.8 | 15.7 | 56.1 |
| Limonene | 100.5 | 463.1 | 10.0 | 35.6 |
| β-Phellandrene | 623.5 | 845.9 | 38.3 | 142.6 |
| 1,8-Cineole | nd | 31.1 | 3.4 | 1.8 |
| α-Terpinolene | 31.5 | 41.0 | 1.5 | 6.4 |
| α-Fenchone | 31.6 | 44.9 | 3.1 | 2.1 |
| Bornyl acetate | 37.1 | 62.3 | 0.8 | 6.6 |
| Other | 37.5 | 54.4 | 1.4 | 1.5 |
| Total | 7,544.3 | 11,354.0 | 536.0 | 1,651.2 |
nd, Not detected.
Table III.
Diterpene composition of constitutive and induced resin in wood and bark of Norway spruce
| Diterpene | Control Bark | MeJA Bark | Control Wood | MeJA Wood |
|---|---|---|---|---|
| μg g−1 dry wt | ||||
| Manoyl oxide | 460.1 | 1,004.0 | nd | nd |
| Abienol | 4,674.3 | 4,528.4 | 301.3 | 365.1 |
| Pimaric acid | 373.5 | 374.5 | 66.3 | 160.8 |
| Sandaracopimarate | 377.7 | 506.2 | 83.7 | 171.7 |
| Dehydroabietinal | 145.7 | 145.1 | nd | nd |
| Isopimaric acid | 1,712.7 | 1,918.2 | 195.4 | 208.2 |
| Palustic acid | 447.7 | 400.3 | 83.1 | 43.6 |
| Levopimaric acid | 3,623.7 | 5,046.9 | 443.0 | 2,309.4 |
| Dehydroabietate | 703.3 | 970.0 | 72.7 | 47.5 |
| Abietic acid | 2,837.2 | 2,713.5 | 119.8 | 241.8 |
| Neoabietic acid | 1,881.1 | 2,127.0 | 74.0 | 181.9 |
| Other | 993.2 | 1,042.0 | 66.3 | 160.8 |
| Total | 18,230.2 | 20,776.1 | 1,505.6 | 3,890.8 |
nd, Not detected.
Figure 4.
Tissue-specific and dose-dependent changes in monoterpene and diterpene accumulation in wood and bark after treatment of trees with MeJA. A, Monoterpenoids, wood. B, Diterpenoids, wood. C, Monoterpenoids, bark. D, Diterpenoids, bark. Each concentration was tested on four trees. Analysis was performed in duplicate and results are presented as the mean with se. Wood denotes the entire xylem tissue of a stem section. Bark denotes all tissues outside the vascular cambium, including phloem, cortex, and periderm. Lower concentrations of MeJA of 100 to 500 μM induced chemical changes of wood resin terpenoids similar to those induced with 10 mm MeJA when Tween 20 was added at 0.1% (v/v) to the surface spray.
Table II.
Sesquiterpene composition of constitutive and induced resin in wood and bark of Norway spruce
| Sesquiterpene | Control Bark | MeJA Bark | Control Wood | MeJA Wood |
|---|---|---|---|---|
| μg g−1 dry wt | ||||
| Longipinene | 14.8 | 25.3 | 0.9 | 0.4 |
| Longifolene | 38.4 | 52.5 | 2.5 | 1.2 |
| α-Cedrene | 14.4 | 17.5 | 1.2 | 0.0 |
| β-Caryophyllene | 134.1 | 189.5 | 1.3 | 5.5 |
| (Z)-β-Farnesene | 23.2 | 23.6 | 0.7 | 1.5 |
| α-Humulene | 25.9 | 41.5 | 0.5 | 1.5 |
| (Z,E)-α-Farnesene | 12.6 | 17.7 | 0.3 | 0.8 |
| (E)-β-Farnesene | 5.7 | 8.0 | nd | nd |
| (Z)-α-Bisabolene | 3.9 | 3.7 | 2.2 | 3.4 |
| (E)-α-Bisabolene | 4.9 | 5.8 | 1.3 | 0.9 |
| Other | 20.0 | 25.5 | 4.5 | 7.0 |
| Total | 297.9 | 410.6 | 15.4 | 22.2 |
nd, Not detected.
Figure 5.
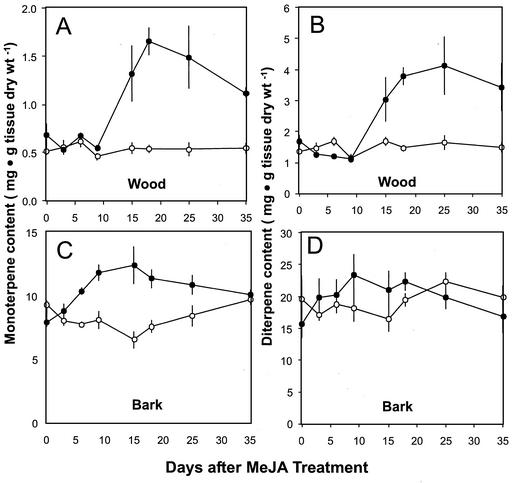
Time course of total monoterpenoid and diterpenoid content in wood and bark after treatment with MeJA. A, Monoterpenoids, wood. B, Diterpenoids, wood. C, Monoterpenoids, bark. D, Diterpenoids, bark. Data are presented as the means with se of duplicate or triplicate assays of extracts from treated (●) and control (○) trees.
Time Courses of Induced Monoterpenoid and Diterpenoid Accumulation
The temporal pattern of monoterpenoid, sesquiterpenoid, and diterpenoid accumulation in wood and bark tissue was analyzed over a 5-week period after treatment with 10 mm MeJA (Fig. 5). There was a significant increase in the accumulation of both monoterpenoids and diterpenoids in the wood compared with untreated controls starting 10 to 15 d after treatment (Fig. 5). Monoterpene concentrations peaked (1.7 mg g−1 tissue dry weight) at 18 d posttreatment (Fig. 5A) and then fell slightly over the remainder of the 35-d time course, although they remained greater than those seen in the controls. A similar pattern was seen for wood diterpenes, with the first significant increase over controls becoming evident at d 15 (Fig. 5B). The maximum diterpene concentration was observed at d 25 (4 mg g−1 tissue dry weight), slightly later than the peak for monoterpene accumulation. Thus, unlike other JA-induced plant defense responses, the increase in resin terpenoids after MeJA treatment persists over an extended period, consistent with the persistence of the TDs.
In contrast, the bark tissue, already rich in resin from constitutive ducts, exhibited much smaller relative changes in terpene concentrations over the 35-d time course after MeJA treatment. Monoterpenes reached a maximum concentration (12 mg g−1 tissue dry weight) at d 15 after MeJA treatment (Fig. 5C), and decreased to approximately the same levels as the controls by d 35. Significant increases were already seen by d 6, indicating that the bark responds more rapidly to MeJA treatment than the wood, possibly because the response in the bark does not require de novo differentiation of resin producing cells as with TDs in the xylem. For bark diterpenes, MeJA treatment had no significant effects over the period of this time course (Fig. 5D).
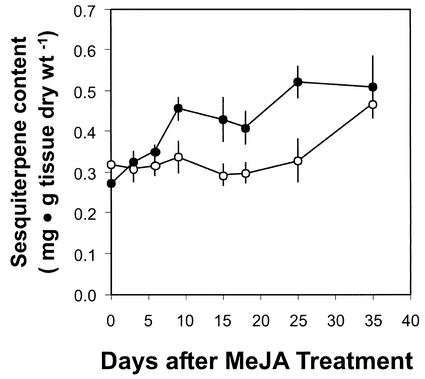
For the third class of resin terpenoids, the sesquiterpenoids, MeJA treatment had no effect on their accumulation in wood tissue and resulted in a weak response in the bark (less than 2-fold) over the monitored time course with maximum increase in accumulation between d 10 and 25 (Fig. 6). The time course of induction of sesquiterpenoid accumulation in the bark lacks a clear peak and appears more transient than that seen for the monoterpenoid and diterpenoid resin components, which likely explains why sesquiterpenoid induction was not observed when resin accumulation was monitored at the end of the 2-month experiment with the 1, 10, and 100 mm MeJA-treated saplings.
Figure 6.
Time course of total sesquiterpenoid content in bark after treatment with MeJA. Data are presented as the means with se of duplicate or triplicate assays of extracts from treated (●) and control (○) trees.
Composition of Constitutive and Induced Resin Terpenoids
Analyses of resin from MeJA-treated and control saplings 18 d after treatment revealed a number of differences in composition. The seven most abundant monoterpenoids in both bark and wood tissues were, in order of decreasing abundance in induced tissues, β-pinene, α-pinene, β-phellandrene, limonene, myrcene, Δ3-carene, and camphene (Table I; Fig. 1). In the bark, most of these abundant compounds increased 1.4- to 2-fold upon MeJA treatment with a higher increase (4-fold) for limonene and a lower increase (1.13-fold) for Δ3-carene accumulation in induced bark tissue.
In the wood, the site of TD formation, changes in individual monoterpene concentrations were more pronounced. Whereas α-pinene and camphene increased by only 2.5- and 1.5-fold, respectively, the concentrations of most of the remaining major monoterpenes (β-pinene, myrcene, limonene, and β-phellandrene) increased by 3.7- to 4.3-fold. Δ3-Carene was not detectable before treatment. The two major monoterpene resin constituents in the wood, α-pinene and β-pinene, were differentially affected by MeJA. These two compounds are found in the wood in nearly equal amounts in control saplings, but the proportion of β-pinene to α-pinene increases upon MeJA treatment reaching a ratio of 2:1 at d 15. In the bark, this proportion is 3:1 regardless of treatment. Several of the oxygenated monoterpenoids and minor hydrocarbons also exhibited altered concentrations upon MeJA application, including 1,8-cineole (not present in the control), α-fenchone (1.4-fold increase), and bornyl acetate (1.7-fold increase). In the wood, increases of bornyl acetate (8-fold) and α-terpinolene (4.3-fold) were found. Chiral analysis of wood extracts showed an increase in the relative amounts of (−)-α-pinene and (−)-β-pinene in relation to their respective (+)-enantiomers (Fig. 1D) (data not shown), indicating the existence of at least two different pinene synthases in the stem tissue of Norway spruce, as in grand fir (Bohlmann et al., 1997, 1999) and in Pinus taeda (Phillips et al., 1999).
In both bark and wood, most of the individual sesquiterpenes increased by 1.4-fold after MeJA treatment (Table II). However, wood extracts showed a 4.2-fold increase in β-caryophyllene and a 2- to 3-fold increase in (Z)-β-farnesene, α-humulene, and (E)-β-farnesene (Fig. 1).
Although diterpene concentrations did not differ between control and MeJA-treated bark extracts, there was an overall 2.6-fold induction in the wood (Table III). Concentrations of levopimaric acid (Fig. 1) showed the highest increase (5.2-fold), whereas neoabietic acid, pimaric acid, and three unidentified peaks exhibited approximately 2.5-fold increases in treated over control saplings. Noted also in this analysis was the constant concentration of abienol, the major diterpene alcohol found in this tissue in induced and control trees. Another interesting observation is the decrease in dehydroabietic acid (1.5-fold) seen in treated versus control trees.
Effect of Induction on PT Activities
Accumulation of resin terpenoids in developing xylem after MeJA treatment may reflect de novo synthesis or mobilization of resin from sites of constitutive accumulation in the bark. To test for induced de novo biosynthesis, protein extracts from bark and wood of saplings were assayed in vitro for activities of PTs and TPS, two principal steps in terpene biosynthesis (Fig. 2).
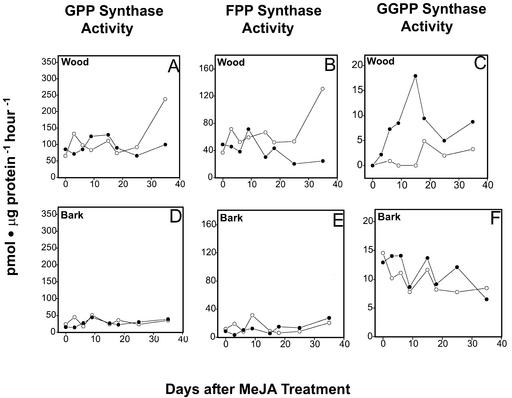
Three different types of PTs were measured, GPP synthase, FPP synthase, and GGPP synthase, which provide the precursors for all monoterpene, sesquiterpene, and diterpene formation, respectively. All three activities were detectable in bark extracts but were unaffected by treatment with 10 mm MeJA over the time course of 35 d (Fig. 7). In the xylem, the activities of GPP synthase and FPP synthase also did not differ between MeJA-treated and control trees (Fig. 7, A and B). However, GGPP synthase activity, which was barely detectable in constitutive wood extracts, was strongly induced by treatment with MeJA starting 3 d posttreatment and reaching a maximum specific activity (10 pmol μg protein−1 h−1) at d 15 (Fig. 7C). At d 18 and 25, the activity declined, but remained at higher levels relative to the control. The induction of GGPP synthase in the xylem reflects the tissue specific response to MeJA previously observed at the microscopic structural level and by terpenoid analysis.
Figure 7.
Time course of PT activity in wood and bark after treatment with MeJA. A, GPP synthase activity, wood. B, FPP synthase activity, wood. C, GGPP synthase activity, wood. D, GPP synthase activity, bark. E, FPP synthase activity, bark. F, GGPP synthase activity, bark. Values are the means of duplicate or triplicate assays of extracts from treated (●) and control (○) trees. A rapid increase of enzyme activity was found only for GGPP synthase in induced wood samples. The apparent increase in specific activities of GPP synthase and FPP synthase at d 35 reflect on a decrease of total protein in these samples. Ranges of duplicate assays were normally 1% to 25% of the mean but were 45% to 60% of the mean in control d 35 in A and B and in MeJA d 25 and 35 in B.
Effect of Induction on TPS Activities
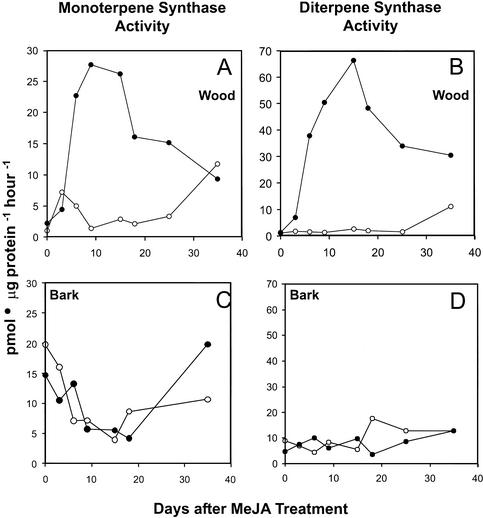
Two classes of TPS, mono-TPS, and di-TPS, were measured. In unsprayed saplings, these activities were at best barely detectable in wood extracts, but revealed strong activity in the bark (Fig. 8). Treatment with MeJA did not affect the activity of bark mono-TPS over a time course of 35 d. In contrast, mono-TPS activity rose rapidly in wood with increased specific activity detectable 3 d after treatment with 10 mm MeJA. The peak at 9 d (28 pmol μg protein−1 h−1; Fig. 8A) corresponded to a 28-fold increase in activity over the control, and was 5-fold higher than the specific mono-TPS activities in bark, the site of constitutive resin formation and resin accumulation. By d 18 and 25, the activity in induced wood had dropped but remained approximately 12 times that of the control until d 35, when similar levels of activity were found in treated and control trees.
Figure 8.
Time course of monoterpenoid synthase activity and diterpenoid synthase activity in wood and bark after treatment with MeJA. A, Monoterpenoid synthase activity, wood. B, Monoterpenoid synthase activity, bark. C, Diterpenoid synthase activity, wood. D, Diterpenoid synthase activity, bark. Data are the means of duplicate or triplicate assays of extracts from treated (●) and control (○) trees.
This transient increase of enzyme activity after MeJA application was also observed for di-TPS in wood, which exhibited the same rapid induction detectable 3 d posttreatment (Fig. 8B). Induced di-TPS activity reached a maximum at d 15 with a 22-fold increase (66 pmol μg protein−1 h−1) in activity over the control. This activity exceeds the specific activity of di-TPS from bark tissue at the same time point by more than 10-fold. After the maximum at 15 d, di-TPS activity remained at an elevated level over the entire 35-d time course. This transient induction of enzyme activities in terpene-accumulating wood tissue coupled with the appearance of TDs is highly suggestive of the occurrence of MeJA-induced de novo synthesis.
Products of the Induced TPS Activities
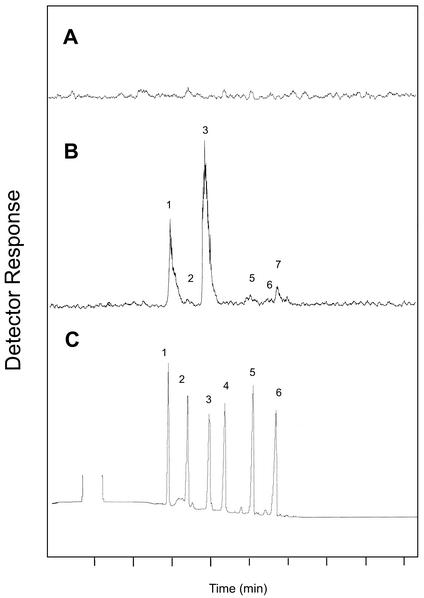
Product analysis of the mono-TPS assays of induced wood tissue by radio-gas chromatography (GC; Fig. 9) revealed the presence of the six major monoterpene components that are all found in xylem resin: α-pinene (peak 1), camphene (peak 2), β-pinene (peak 3), myrcene (peak 5) limonene (peak 6), and β-phellandrene (peak 7). The individual components were found in approximately the same ratios as they occur in the xylem resin (Table I). In addition, an increase of β-pinene relative to α-pinene paralleled that observed in the resin extracts from induced xylem.
Figure 9.
Analysis of products formed in vitro by constitutive and induced monoterpenoid synthase activity from wood tissue. A, Radio-GC traces for monoterpenoid synthase assay products from wood of control saplings. B, Radio-GC trace for monoterpenoid synthase assay products from wood of saplings treated with 10 mm MeJA. C, Thermal conductivity detector (TCD) trace for monoterpene standards. Standard for β-phellandrene not shown. Peak 1, α-Pinene; 2, camphene; 3, β-pinene; 4, Δ3-carene; 5, myrcene; 6, limonene; 7, β-phellandrene.
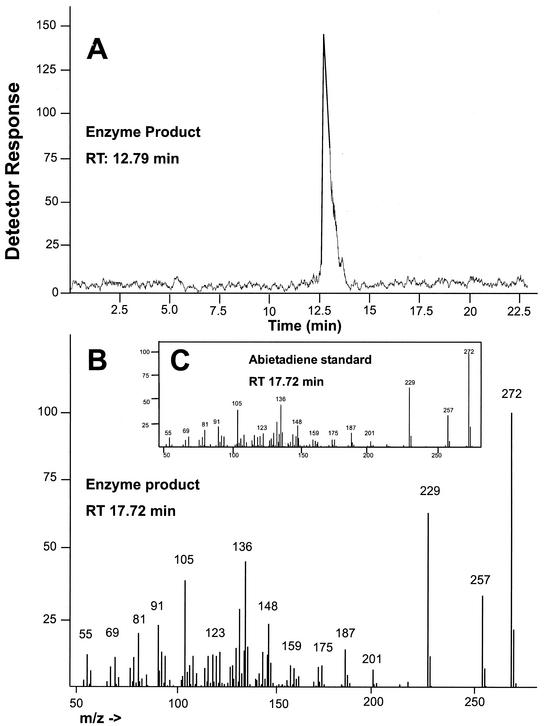
Products of the di-TPS assays analyzed by radio-GC and GC-mass spectroscopy (MS) revealed the presence of a single peak (Fig. 10), identified as abietadiene by comparing the mass spectrum with that of an authentic standard. The result is consistent with the activity of the monospecific abietadiene-producing grand fir di-TPS described previously (LaFever et al., 1994). Peters et al. (2000) recently found that under modified conditions, the cloned grand fir abietadiene synthase actually produces abietadiene, levopimaradiene, and neoabietadiene in nearly equal ratios as well as three other minor products. The Norway spruce enzyme might also produce additional products under similar conditions, which will be tested in future work once the corresponding spruce di-TPS gene has been cloned.
Figure 10.
Analysis of products formed in vitro by induced diterpenoid synthase activity from wood tissue. A, Radio-GC trace for di-TPS assay products. B, GC-MS fragmentation pattern for major di-TPS assay product. C, GC-MS fragmentation pattern for authentic abietadiene standard.
DISCUSSION
Conifers include some of the longest living of all organisms, which, during their extended lifetimes, must survive countless challenges by a diverse array of herbivore and pathogen species (Seybold et al., 2000). Constitutive and inducible resin terpenoids are characteristic defense compounds of many conifer genera of the pine family (Bohlmann and Croteau, 1999; Phillips and Croteau, 1999; Trapp and Croteau, 2001). Species of Picea, like other conifers, produce copious amounts of resin terpenoids in the bark as a constitutive outer defense barrier, but do not accumulate significant amounts of resin in the constitutive wood and developing xylem in contrast to some other conifers. However, induced resin terpenoids accumulate in newly initiated axial resin ducts (TDs) in the developing xylem of Picea spp. (spruce; Nagy et al., 2000). Induced terpenoid defense responses in Picea spp. are activated by stem-boring insects, such as coniferophagous bark beetles (Scolytidae) and the white pine weevil (Pissodes strobi), which are among the most destructive insect pests of conifer forests worldwide, as well as by insect-associated fungi, such as the blue stain fungus Ceratocystis polonica (Alfaro, 1995; Tomlin et al., 1998; Franceschi et al., 2000). De novo formation of resin ducts in the developing xylem has been described as a possible resistance mechanism against insects and pathogens in white spruce (Picea glauca), Sitka spruce (Picea sitchensis), and Norway spruce (Alfaro, 1995; Tomlin et al., 1998; Franceschi et al., 2000).
In an effort to characterize the induced changes in terpene chemistry as well as changes in terpenoid biosynthetic activities during the development of traumatic resinosis in Norway spruce, we first tested the effect of MeJA to establish a nondestructive method of inducing the resin defense response. Whereas the effect of exogenous MeJA and the role of endogenous octadecanoids have been well characterized in herbaceous angiosperm species (Farmer and Ryan, 1992; Creelman and Mullet, 1997; Baldwin, 1999), the effect of jasmonates has been demonstrated in only a few conifers, mainly in cell cultures. MeJA induces the transformation of E-α-bisabolene into todomatuic acid, a precursor of juvabione type insect juvenile hormone analogs, in suspension culture of grand fir (Bohlmann et al., 1998a). In cell cultures of Taxus canadensis, MeJA induces production of paclitaxel (taxol) and other taxoids with the concomitant increase in GGPP synthase and acetyl-CoA:taxadienol-O-acetyl transferase enzyme activities, both of which are involved in taxol biosynthesis (Yukimune et al., 1996; Hefner et al., 1998; Ketchum et al., 1999). Spruce cell cultures treated with MeJA show transcript accumulations of chalcone synthase and a 14-3-3 protein (Lapointe et al., 2001). In Douglas fir, MeJA was found to induce the expression of two low-Mr heat shock proteins in dormant seeds (Kaukinen et al., 1996). Other work with jasmonates in conifers has focused on jasmonate-induced effects on interactions with fungi. Jasmonates have been implicated in both symbiotic and antagonistic relationships of seedlings with fungi. Apparently, MeJA application assists ectomycorrhizal colonization of Norway spruce roots (Regvar et al., 1997). MeJA application to Norway spruce seedlings also enhances their survival rate when they were challenged by Pythium ultimun (Kozlowski et al., 1999).
Compared with the numerous effects of jasmonates reported for angiosperms and the effects previously described for conifers, the induction of terpenoid defenses in Norway spruce is probably among the most complex responses. Similar to a stem-boring insect attack, treatment with MeJA alters the developmental program of xylem mother cells within the vascular cambium switching differentiation from tracheids to that of TD cells, two cell types with enormous contrasts in structure and function (Esau, 1977). In addition, induced de novo differentiation of resin ducts in the xylem is also associated with resin terpenoid accumulation and de novo resin biosynthesis. Interestingly, this effect is strictly tissue specific because existing resin canals in the phloem and cortex are not affected in the young trees of this study. Unlike older trees, young Norway spruce trees do not accommodate significant radial resin duct formation in the phloem. Because the phloem of older trees is the primary site of attack by bark beetles, the possibility that radial ducts in the phloem of older trees could also be activated by MeJA will be tested in future work.
Differential effects of MeJA in Norway spruce stem tissues are further observed at the level of terpenoid biosynthesis and terpenoid accumulation. Induced accumulation of resin terpenoids is most apparent in the wood and barely detectable in the bark, where high constitutive resin levels already exist. Among the resin terpenoids, levels of the two major classes of resin components, monoterpenes and diterpenes, are strongly increased, whereas the sesquiterpenes, the least abundant class of resin terpenoids, are only weakly affected in the induced xylem. The formation of traumatic resin occurs on a much longer time scale than that of most other induced defenses. However, it has been demonstrated that the site of TD development not only corresponds to the area in which bark beetle or weevil larvae could potentially develop, but that the time of TD development is synchronized with the time during which these insects would be emerging from their eggs, thereby flooding the brood with toxic and immobilizing resin constituents (Alfaro, 1995).
In measuring terpenoid biosynthetic enzymes, we observed a dramatic response in both monoterpenoid and diterpenoid pathway activities in wood tissue, whereas sesquiterpene biosynthesis was only weakly and transiently affected in the bark. Of the three PTs of resin terpenoid biosynthesis (Fig. 2), only GGPP synthase was induced, but not GPP synthase and FPP synthase. The lack of FPP synthase activation reflects the weak effect of MeJA on sesquiterpene accumulation associated with TD development. Similarly, sesqui-TPS activities in these tissues are below detection limits (data not shown). However, despite a strong induction of monoterpene accumulation and mono-TPS induction in developing xylem, GPP synthase is not induced, suggesting that GPP synthase activity is not limiting for induced monoterpene formation and accumulation. Similar results were obtained from grand fir saplings, which showed no increase in GPP synthase activity over a 20-d period after mechanical wounding of the stem (Tholl et al., 2001), even though mono-TPS enzyme activity and gene expression increased strongly during this same period (Bohlmann et al., 1997; Steele et al., 1998b). In contrast, GGPP synthase is active at a very low level in the constitutive xylem and induced accumulation of diterpene resin acids is preceded by coordinately increased activities of both GGPP synthase and di-TPS in xylem of MeJA-treated spruce trees. These results suggest that unlike monoterpene accumulation, the induction of diterpenes is controlled not only by enzyme activity at the level of TPS but also at the level of the PT that yields the 20-carbon precursor. The differential enzymatic regulation of the three classes of resin terpenoids (monoterpenoids, sesquiterpenoids, and diterpenoids) has important implications for attempts to alter resin composition in spruce. For instance, increased monoterpenoid formation may not require altering expression of GPP synthase, whereas altering GGPP synthase together with di-TPS may be necessary to alter diterpene formation.
This is the first study, to our knowledge, that demonstrates the constitutive and induced activity of terpenoid synthases at a tissue-specific level in a gymnosperm system. Enzymatic and molecular studies of terpenoids at the tissue-specific level have previously been reported only in some angiosperm systems, the glandular trichomes of Mentha × piperita (McConkey et al., 2000), the trichomes of Nicotiana glutinosa (Guo and Wagner, 1995), or the scent-producing floral tissues of Clarkia breweri (Dudareva et al., 1996). The results described here justify future analysis of MeJA-induced TD differentiation and regulation of terpenoid biosynthesis in spruce at the cellular and subcellular level to elucidate the control mechanisms responsible for such localized expression.
This study did not intend to investigate the role of jasmonates as endogenous signal molecules in conifers. Treatment of trees with MeJA was tested and developed solely as a procedure to study in detail the chemical, biochemical, and anatomical processes of traumatic resin defense in spruce. Nevertheless, our results could imply that octadecanoids are involved in insect-induced defense in conifers, a possibility which will be tested in future work. Because this is the first demonstration of TD formation by a signal molecule, to our knowledge, we will also evaluate the effect of other signal molecules to elucidate the endogenous signal cascade active in xylem-specific resinosis and TD development in response to insects, pathogens, or wounding. The noninvasive and dose-dependent activation of defenses in spruce by MeJA treatment provides new opportunities to evaluate the significance of induced responses in conifers in bioassays with insects and pathogens, as recently demonstrated for agricultural crops (Thaler, 1999). Considering restrictions on use of pesticides for insect and pathogen control in forest systems, pretreatment of trees with elicitors can be explored as a strategy for tree protection. Furthermore, nondestructive induction of traumatic resinosis in spruce provides a superb system for discovery of genes associated with resin canal differentiation in the developing xylem and genes of induced terpenoid formation and accumulation, a paramount characteristic of conifers.
MATERIALS AND METHODS
Plant Materials
Norway spruce (Picea abies L. Karst) trees of clonal lines 3166-728 and 1015-903 were propagated from lateral branches of current and previous year growth at the Niedersächsische Forstliche Versuchsanstalt (Escherode, Germany). Fully regenerated, 2-year-old, rooted saplings were grown in 2-L pots in a 2:3 (v/v) ratio of peat:universal planting mix for at least 6 months before experiments. Trees were fertilized with Osmocoat and maintained in growth chambers under illumination with high-pressure sodium vapor lamps. The photoperiod and ambient temperature cycled from 1 h at 220 μmol m−2 s−1 (20°C), 4 h at 440 μmol m−2 s−1 (20°C), 3 h at 660 μmol m−2 s−1 (22°C for 2 h and 24°C for 1h), 7 h at 440 μmol m−2 s−1 (24°C for 1 h, 22°C for 2 h, and 20°C for 4 h), and 1 h at 220 μmol m−2 s−1 (18°C). This was followed by 8 h of darkness (18°C). The relative humidity was maintained at 50% throughout the entire cycle. Saplings were introduced to these growth conditions 4 weeks before use in experiments to ensure a complete break of dormancy.
Substrates, Standards, and Reagents
Chemical reagents were from Sigma-Aldrich (Steinheim, Germany) or Roth (Karlsruhe, Germany). Terpene standards were from Sigma-Aldrich, Roth, Bedoukian Research (Danbury, CT), and Helix Biotech (Richmond, BC) and were of the highest purity available. All solvents were GC grade. The substrates, [1-14C] IPP (54 Ci mol−1), [1-3H]GPP (20 Ci mol−1), and all-trans-[1-3H]GGPP (58 Ci mol−1) were from Biotrend (Köln, Germany). [1-3H]FPP (125 Ci mol−1) was the gift of Rodney Croteau (Washington State University, Pullman). Unlabeled DMAPP, GPP, and FPP were from Echelon Research Laboratories Inc. (Salt Lake City). Diazomethane was made fresh from Diazald (Aldrich, Milwaukee, WI) by a standard procedure (Aldrich technical information bulletin no. 180).
MeJA Treatment and Harvest of Tissues
To test dose-dependent effects of MeJA, saplings (clone 1015-903) were sprayed with 1, 10, or 100 mm solutions of MeJA (95% [w/w] pure, Sigma-Aldrich) dissolved in distilled water. Time course experiments were done with saplings (clone no. 3166-728) sprayed with 10 mm MeJA dissolved in distilled water. Saplings were placed in a ventilated fume hood and each tree was sprayed with 150 mL of MeJA solution over a period of 30 min to obtain a complete and even coating. Saplings were kept in fume hoods for 1 h after treatment to allow evaporation of excess MeJA solution before transferring to growth chambers. Control saplings (four for dose-dependent experiment and four for each time point for the time course) were sprayed with water. Control and MeJA-treated saplings were kept in separate growth chambers. To determine dose-dependent effects of MeJA, trees were harvested after 42 d by cutting the stem above the ground and just below the upper internode and freezing the entire section in liquid nitrogen. Needles were removed and the tissue was stored at −80°C. The lowest 3 cm of the stem section was discarded. The next 3 to 4 cm of stem tissue was prepared for resin extractions. This section was cut into two pieces of equal length. The bark of these stem sections was sliced longitudinally with a razor blade, and while still frozen, was peeled away from the wood. Bark and wood were extracted separately for resin analysis. An additional 2 cm of the stem was used for light microscopy analysis of TD development. Saplings for each of the time points were harvested in the same manner for microscopy and resin analysis. An extra 7 cm of the harvested stem was used for enzyme preparations. For enzyme extracts, bark and wood tissues were separated as indicated and the similar tissues of four saplings for each time point were combined into one protein extract.
Light Microscopy
Samples were prepared for cryosectioning by soaking small sections (2–3 cm long) of the stems in a solution of 4% (w/v) formaldehyde and 100 mm K2HPO4 (pH 7.5) for 4 h. The samples were then washed with distilled water and submerged in saturated (aqueous) copper acetate overnight. Before sectioning, the samples were removed from the copper acetate solution and frozen at −20°C. Cryosectioning of the samples took place at −20°C and each section was sliced into 18-μm cross sections. A polyvinyl alcohol-based glue was used to attach the coverslip immediately after sectioning. After visualizing by light microscopy (Axiophot, Zeiss GmbH, Jena, Germany), digital images were taken of the samples.
Extraction of Resin Terpenes
Extraction of terpene constituents was modified from Lewinsohn et al. (1993). All steps of this procedure were carried out in 2-mL vials (glass with a teflon-coated screw cap, Hewlett-Packard, Palo Alto, CA). Bark and wood tissue samples of approximately 1 cm to 1.5 cm in length were submerged separately into 1.5 mL of tert-butyl methyl ether in a 2-mL vial containing 150 μg mL−1 isobutylbenzene and 200 μg mL−1 dichlorodehydroabietic acid as internal standards. The tissue samples were extracted over 14 h with constant shaking at room temperature. To purify extracted terpenes from other small organic acids, the ethereal extract (approximately 1.5 mL) was transferred to a fresh vial and washed with 0.3 mL of 0.1 m (NH4)2CO3 (pH 8.0). Diterpene acids were then methylated by adding 0.4 mL of the washed etheral extract to 0.16 mL of methanol and 0.15 mL of diazomethane in a separate vial, which was then capped and left at room temperature for 30 min to allow the methylation reaction to go to completion. Then, the solvent was evaporated under nitrogen, leaving the residual diterpene fraction. The monoterpenes, sesquiterpenes, and diterpenes were then recombined by dissolving the methylated diterpene residue in 0.6 mL of the washed etheral extract. The extract was prepared for capillary GC or GC-MS analysis by filtering through a Pasteur pipette column filled with 0.3 g of silica gel (Sigma 60 Å) overlaid with 0.2 g of anhydrous MgSO4. The column was further eluted with 1 mL of diethyl ether to release bound oxygenated terpenes and both eluants were collected in a fresh vial. Finally, the sample was evaporated to an approximate volume of 100 μL that was stored at −20°C. The dry weights of each extracted tissue were determined after drying at 70°C for 20 h to calculate terpene constituent concentrations on a mg g−1 dry weight basis. ses were calculated from eight independent extracts per treatment.
Analysis of Monoterpenes, Sesquiterpenes, and Diterpenes
For monoterpene and sesquiterpene analysis, a Hewlett-Packard 6890 GC was equipped with a flame ionization detector (FID) fitted with a DB-WAX column (0.25 mm × 0.25 μm × 30 m, J&W Scientific, Folsom, CA). The flow rate was 2 mL H2 min−1 and the FID was operated at 300°C. One microliter of extract was introduced into the injection port at 220°C and was split in either a 10:1 ratio for the bark extracts or a 5:1 ratio for the wood extracts. The GC was programmed with an initial oven temperature of 40°C (3-min hold), and temperature increased at a rate of 3°C min−1 until 80°C, followed by 5°C min−1 until 180°C and then 15°C min−1 up to 240°C (5-min hold). GC-MS analysis was accomplished with a Hewlett-Packard 6890 GC-MSD system (70 eV), using a DB-WAX column as described above. Split injections (1-μL etheral extract) were made at a ratio of 5:1 (bark extracts) or 3:1 (wood extracts) with an injector temperature of 220°C. The instrument was programmed from initial temperature of 40°C (3-min hold) and increased at a rate of 1.5°C min−1 until 45°C, then increasing at 3°C min−1 up to 80°C, 5°C min−1 until 180°C, followed by an additional ramp of 10°C min−1 up to 240°C (5-min hold). Helium was used at a constant flow of 1 mL min−1. Chiral analysis of monoterpene constituents utilized the same GC-FID equipped with a Cyclodex-B (0.25 mm × 0.25 μm × 30 m, J&W Scientific). The same etheral samples were injected in a split ratio of 20:1 (220°C injection port). The oven was programmed initially at 40°C, increasing at 1°C min−1 until 45°C, then at 5°C min−1 until 65°C, followed by 20°C min−1 until 230°C (2-min hold). All other conditions were identical to those used in analysis with the DB-Wax column mentioned above.
Analysis of diterpene constituents was performed on the same GC-FID and GC-MS instruments fitted with an HP-5 column (0.25 mm × 0.25 μm × 30 m, Hewlett-Packard). Injections were 1 μL of the etheral extracts. For GC-FID analysis, the split ratios were 40:1 or 20:1 for bark and wood extracts, respectively. The injection port was operated at 250°C and the FID was maintained at 300°C. The oven was programmed from an initial temperature of 120°C to 150°C at a rate of 1°C min−1 followed by 5°C min−1 until 280°C (5-min hold). GC-MS split ratios were 20:1 (bark extracts) or 10:1 (wood extracts) with an injector temperature of 220°C. The instrument was programmed from an initial temperature of 120°C and increased at a rate of 1°C min−1 until 150°C, followed by 5°C min−1 up to 280°C (6-min hold). Helium was at a constant flow of 1 mL min−1.
GC-FID-generated peaks were integrated using Hewlett-Packard Chemstation software. Terpene concentrations were calculated by comparing the integrated peak area to that of the internal standard. Isobutylbenzene was used as the internal standard for both monoterpenes and sesquiterpenes. Methylated dichlorodehydroabietic acid was employed as an internal standard to calculate diterpene concentrations. Identification of terpenes was based on comparison of retention times and mass spectra with authentic standards or with mass spectra in the Wiley or National Institute of Standards and Technology libraries.
Protein Extraction
Tissue samples (bark or wood) from each of the four saplings per time point were combined into one protein preparation and extracted as previously described (Lewinsohn et al., 1991). Using an analytical grinding mill (A10, IKA WORKS, Cincinnati), the tissue was ground to a fine powder in liquid nitrogen and combined with extraction buffer {50 mm MOPSO [3-(N-morpholino)-2-hydroxypropanesulfonic acid], pH 6.8; 5 mm ascorbic acid; 5 mm sodium bisulfite; 5 mm dithiothreitol (DTT); 10 mm MgCl2; 1 mm EDTA; 10% [v/v] glycerol; 1% [w/v] polyvinylpyrrolidone [Mr 10,000]; 4% [w/v] polyvinylpolypyrrolidone, 4% [w/v] amberlite XAD-4; and 0.1% [v/v] Tween 20) in a ratio of 1:10 (g tissue:mL buffer). The preparations were allowed to shake at 4°C for 30 min and were then centrifuged at 10,000g for 30 min. The supernatant was then filtered through two layers of no. 1 filter paper (Whatman, Kent, UK), divided into 4-mL aliquots, frozen in liquid nitrogen, and kept at −80°C. Extracts were thawed only once before enzyme assay. Total protein concentration of each protein extract was determined using the Coomassie reagent and protocol (Bio-Rad Laboratories, Hercules, CA).
PT Enzyme Assays
Wood and bark protein extracts were desalted into a buffer containing 20 mm MOPSO, pH 7.0, 10 mm MgCl2, 10% (v/v) glycerol, and 2 mm DTT. Assays were carried out in duplicate or triplicate in a final volume of 500 μL containing 40 μm [1-14C]IPP (54 Ci mol−1) and 40 μm DMAPP. To reduce competing IPP isomerase activity, 5 mm iodoacetamide was added. After the reaction was initiated by addition of the enzyme preparation, the assay mixture was immediately overlaid with 1 mL of pentane and incubated for 1 h at 30°C. To stop the assay and hydrolyze all diphosphate esters (both unreacted substrate as well as products), a 500-μL solution of calf intestine alkaline phosphatase (Sigma, 4 units) and potato apyrase (Sigma, 4 units) in 0.2 m Tris-HCl, pH 9.5, was added to each assay and incubated at 30°C for 8 to 12 h. After enzymatic hydrolysis, the resulting prenyl alcohols were extracted into 2 mL of diethyl ether and, after addition of a mixture of terpene standards, the organic extract was prepared for radio-GC as described previously (Burke et al., 1999). Radio-GC analysis was performed on a Hewlett-Packard HP6890 gas chromatograph (injector at 220°C and TCD at 250°C) in combination with a Raga radio detector (Raytest, Giessen, Germany). The concentrated organic phase (1–2 μL) was injected on a DB-wax capillary column (30 m × 0.25 mm with 0.25-μm phase coating; J&W Scientific). Separation was achieved under a He flow rate of 2 mL min−1 with a temperature program of 3 min at 40°C, a ramp to 70°C at 3°C min−1 (1-min hold), and a second ramp from 70°C to 240°C at 6°C min−1 (30-min hold). Both mass and radioactivity traces were monitored simultaneously. Products were identified by comparison of retention times with those of co-injected authentic standards.
Preliminary enzyme assays had shown that 3H-labeled GPP and FPP were not incorporated into longer C-15 or C-20 prenyl diphosphate products, when GPP or FPP concentrations of 2 to 5 m were used in the presence of 40 m IPP and 40 m DMAPP. Thus, individual PT activities could be clearly distinguished.
TPS Enzyme Assays
TPS activities were determined by published procedures (Lewinsohn et al., 1991; LaFever et al., 1994; Bohlmann et al., 1997) with minor modifications. Before assaying enzyme activity, the frozen protein extracts were placed at 37°C until just thawed. The protein extracts were desalted in Bio-Rad Econo PacI0DG sizing columns pre-equilibrated with appropriate assay buffers: mono-TPS buffer (25 mm HEPES, pH 7.5; 5 mm DTT; 10% [v/v] glycerol; 1 mm MnCl2; and 100 mm KCl), sesqui-TPS buffer (25 mm HEPES, pH 7.3; 10 mm MgCl2; 10 mm DTT; and 10% [v/v] glycerol), or di-TPS buffer (30 mm HEPES, pH 7.2; 7.5 mm MgCl2; 20 μm MnCl2; 5% [v/v] glycerol; and 5 mm DTT). Enzyme activity was assessed with 1 mL of the desalted extracts with the addition of 10 μm GPP (with 1 μCi 3H-GPP) for mono-TPS activities, or 10 μm GGPP (0.5 μCi 3H-GGPP) as substrate for di-TPS assays. All enzyme assays were done in duplicate, overlaid with 1 mL of pentane to collect released volatiles, and incubated at 30°C for 1.5 h. To stop all enzyme activity, the extracts were immediately frozen. After thawing, the aqueous assay fraction was rapidly extracted with the pentane fraction by vortexing, and separation of the aqueous and organic fractions was achieved by centrifugation at 2,500g for 2 min. The 1-mL pentane overlay was removed and filtered through a Pasteur pipette filled with 0.4 g of silica gel (Sigma 60 Å) overlaid with 0.6 g of MgSO4 to remove nonspecific substrate hydrolysis products and to dry the pentane extract. Each enzyme assay was extracted with an additional two portions of pentane, vortexed, and centrifuged as before. These sequential extractions were also passed over the same column and pooled with the initial column eluent. Subsequently, the column was washed with pentane (2 × 1 mL) and the total volume was determined. The extracts were analyzed by liquid scintillation counting, 0.1 mL in 0.3 mL of Lipoluma (J.T. Baker, Deventer, The Netherlands) and GC.
The conditions for all enzyme assays, including pH optimum, incubation time, substrate concentration, and temperature optimum, were optimized for this system such that maximum activity was achieved in a linear range of product generation. In addition, the possibility that enzyme activities in induced tissues might have been inhibited by additional resin or phenolic substances was ruled out by experiments in which extracts from different stages of the time course were mixed together. In all cases, the resulting enzyme activity was additive, implying that additional compounds found in induced tissues had no effect on enzyme activity.
Analysis of TPS Assay Products
The extracts of duplicate assays were combined and evaporated on ice to 50 to 100 μL. From this, 2 μL was analyzed by radio-GC coupled with a TCD. The radio detector enabled the detection of radioactive substances and the TCD provided a nondestructive method to determine retention times of co-injected, unlabeled standards. For the mono-TPS assays, the Hewlett-Packard 6890 GC was fitted with a DB-WAX column (described above). The column flow rate was 2 mL H2 min−1, and the TCD was kept at 250°C with an additional 7 mL H2 min−1 (make-up gas). Parameters for the RAGA radio-detector (Radiomatic, Giessen, Germany) were as follows: The platinum catalyst was operated at 740°C, the total H2 flow rate including the make-up gas was 20 mL min−1, and methane quench gas flowed through a 2-mL counting tube at 5 mL min−1. The samples were introduced to the injection port at 220°C and were split in either a 5:1 ratio for the bark assay extractions or a 3:1 ratio for the wood assay extractions. The oven was programmed with an initial temperature of 40°C (3-min hold), and increased at a rate of 1.5°C min−1 until 70°C, followed by an increase of 15°C min−1 until 240°C (3-min hold). Monoterpenes were identified by comparing retentions times with those of known standards.
Extracts of di-TPS assays were analyzed by GC-MS employing a DB-WAX column (described above). Extracts from the bark and wood assays were split 10:1 or 5:1, respectively. The injection port was operated at 250°C and the column flow and the radio detector were maintained as for the monoterpene analysis. The oven was programmed from an initial temperature of 180°C to 240°C at a rate of 4°C min−1, which was followed by a 6-min hold. The extracts were further analyzed by GC-MS. For this analysis, the same DB-WAX column was used. A splitless injector was kept at 220°C and a flow rate of 1 mL He min−1 was maintained. The initial temperature was 50°C and the rate increased at a constant 10°C min−1 up to 280°C (3-min hold). All other parameters were as described for the GC-MS diterpene resin analysis. For peak identification, the 70-eV mass spectra generated were compared with an authentic abietadiene standard (Rodney Croteau). All analysis was performed with Hewlett-Packard Chemstation software.
ACKNOWLEDGMENTS
We thank Juergen Schmidt for trees, Rodney Croteau for substrates and the abietadiene standard, and Tina Letsch and Nadine Gallitschke for excellent technical assistance.
Footnotes
This work was supported by the Natural Sciences and Engineering Research Council (funds to J.B.), by the Canadian Foundation for Innovation (funds to J.B.), by the British Columbia Ministry of Forests (funds to J.B.), and by the Max Planck Society (funds to J.G. and fellowships to D.M. and D.T.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.011001.
LITERATURE CITED
- Alfaro RI. An induced defense reaction in white spruce to attack by the white pine weevil, Pissodes strobi. Can J For Res. 1995;25:1725–1730. [Google Scholar]
- Alonso WR, Croteau R. Prenyltransferases and cyclases. Methods Plant Biochem. 1993;9:239–260. [Google Scholar]
- Baldwin IT. The jasmonate cascade and the complexity of the induced defense against herbivore attack. Ann Plant Rev. 1999;3:155–186. [Google Scholar]
- Bannan MW. Vertical resin ducts in the secondary wood of the abietineae. New Phytol. 1936;35:11–46. [Google Scholar]
- Bohlmann J, Crock J, Jetter R, Croteau R. Terpenoid-based defenses in conifers: cDNA cloning, characterization, and functional expression of wound-inducible (E)-α-bisabolene synthase from grand fir (Abies grandis) Proc Natl Acad Sci USA. 1998a;95:6756–6761. doi: 10.1073/pnas.95.12.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann J, Croteau R. Diversity and variability of terpenoid defenses in conifers: molecular genetics, biochemistry and evolution of the terpene synthase gene family in grand fir (Abies grandis) In: Chadwick DJ, Goode JA, editors. Insect Plant Interactions and Induced Plant Defense. West Sussex, UK: John Wiley and Sons Ltd.; 1999. pp. 132–146. [DOI] [PubMed] [Google Scholar]
- Bohlmann J, Gershenzon J, Aubourg S. Biochemical, molecular genetic and evolutionary aspects of defense-related terpenoid metabolism in conifers. Rec Adv Phytochem. 2000;34:109–150. [Google Scholar]
- Bohlmann J, Meyer-Gauen G, Croteau R. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA. 1998b;95:4126–4133. doi: 10.1073/pnas.95.8.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann J, Phillips M, Ramachandiran V, Katoh S, Croteau R. cDNA cloning, characterization, and functional expression of four new monoterpene synthase members of the Tpsd gene family from grand fir (Abies grandis) Arch Biochem Biophys. 1999;368:232–243. doi: 10.1006/abbi.1999.1332. [DOI] [PubMed] [Google Scholar]
- Bohlmann J, Steele CL, Croteau R. Monoterpene synthases from grand fir (Abies grandis): cDNA isolation, characterization, and functional expression of myrcene synthase, (−)-(4S)-limonene synthase, and (−)-(1S, 5S)-pinene synthase. J Biol Chem. 1997;272:21784–21792. doi: 10.1074/jbc.272.35.21784. [DOI] [PubMed] [Google Scholar]
- Burke CC, Wildung MR, Croteau R. Geranyl diphosphate synthase: cloning, expression, and characterization of the prenyltransferase as a heterodimer. Proc Natl Acad Sci USA. 1999;96:13062–13067. doi: 10.1073/pnas.96.23.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. Biochemistry and molecular biology of the isoprenoid biosynthetic pathway in plants. Ann Rev Plant Phys Plant Mol Biol. 1995;46:521–547. [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Ann Rev Plant Phys Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Davis EM, Croteau R. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. Top Curr Chem. 2000;209:53–95. [Google Scholar]
- Dudareva N, Cseke L, Blanc VM, Pichersky E. Evolution of floral scent in Clarkia: novel patterns of S-linalool synthase gene expression in the C. breweri flower. Plant Cell. 1996;8:1137–1148. doi: 10.1105/tpc.8.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk MH, Bacher A. The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chem Biol. 1998;5:221–233. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- Esau K. Anatomy of Seed Plants. New York: John Wiley and Sons; 1977. [Google Scholar]
- Fahn A. Secretory Tissues in Plants. New York: Academic Press; 1979. [Google Scholar]
- Farmer EE, Ryan CA. Ocadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi VR, Krokene P, Krekling T, Christiansen E. Phloem parenchyma cells are involved in local and distant defense responses to fungal inoculation or bark beetle attack in Norway spruce (Pinaceae) Am J Bot. 2000;87:314–326. [PubMed] [Google Scholar]
- Gershenzon J, Kreis W. Biochemistry of terpenoids: monoterpenes, sesquiterpenes, diterpenes, sterols, cardiac glycosides, and steroid saponins. Ann Plant Rev. 1999;3:222–299. [Google Scholar]
- Guo Z, Wagner GJ. Biosynthesis of labdenediol and sclareol in cell-free extracts from trichomes of Nicotiana glutinosa. Planta. 1995;197:627–632. [Google Scholar]
- Hefner J, Ketchum RE, Croteau R. Cloning and functional expression of a cDNA encoding geranylgeranyl diphosphate synthase from Taxus canadensis and assessment of the role of this prenyltransferase in cells induced for taxol production. Arch Biochem Biophys. 1998;360:62–74. doi: 10.1006/abbi.1998.0926. [DOI] [PubMed] [Google Scholar]
- Kaukinen KH, Tranbarger TJ, Misra S. Post germination induced and hormonally dependent expression of low molecular weight heat shock protein genes in Douglas fir. Plant Mol Biol. 1996;30:1115–1128. doi: 10.1007/BF00019546. [DOI] [PubMed] [Google Scholar]
- Ketchum RE, Gibson DM, Croteau RB, Shuler ML. The kinetics of taxoid accumulation in cell suspension cultures of Taxus following elicitation with methyl jasmonate. Biotechnol Bioeng. 1999;62:97–105. doi: 10.1002/(sici)1097-0290(19990105)62:1<97::aid-bit11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Kozlowski G, Buchala A, Metraux JP. Methyl jasmonate protects Norway spruce [Picea abies (L.) Karst.] seedlings against Pythium ultimum Trow. Physiol Mol Plant Pathol. 1999;55:53–58. [Google Scholar]
- LaFever RE, Stofer Vogel B, Croteau R. Diterpenoid resin acid biosynthesis in conifers: enzymatic cyclization of geranylgeranyl pyrophosphate to abietadiene, the precursor of abietic acid. Arch Biochem Biophys. 1994;313:139–149. doi: 10.1006/abbi.1994.1370. [DOI] [PubMed] [Google Scholar]
- Lapointe G, Luckevich MD, Seguin A. Investigation on the induction of 14-3-3 in white spruce. Plant Cell Rep. 2001;20:79–84. doi: 10.1007/s002990000275. [DOI] [PubMed] [Google Scholar]
- Lewinsohn E, Gijzen M, Savage TJ, Croteau R. Defense mechanisms of conifers: relationship of monoterpene cyclase activity to anatomical specialization and oleoresin monoterpene content. Plant Physiol. 1991;96:38–43. doi: 10.1104/pp.96.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn E, Savage TJ, Gijzen M, Croteau R. Simultaneous analysis of monoterpenes and diterpenoids of conifer oleoresin. Phytochem Anal. 1993;4:220–225. [Google Scholar]
- Lichtenthaler HK. The 1-deoxy-d-xylulose 5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:47–65. doi: 10.1146/annurev.arplant.50.1.47. [DOI] [PubMed] [Google Scholar]
- McConkey ME, Gershenzon J, Croteau R. Developmental regulation of monoterpene biosynthesis in glandular trichomes of peppermint. Plant Physiol. 2000;122:215–223. doi: 10.1104/pp.122.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarvey DJ, Croteau R. Terpenoid metabolism. Plant Cell. 1995;7:1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy NE, Franceschi VR, Solheim H, Krekling T, Christiansen E. Wound induced traumatic resin duct development in stems of Norway spruce (Pinaceae): anatomy and cytochemical traits. Am J Bot. 2000;87:302–313. [PubMed] [Google Scholar]
- Peters RJ, Flory JE, Jetter R, Ravn MM, Lee HJ, Coates RM, Croteau R. Abietadiene synthase from grand fir (Abies grandis): characterization and mechanism of action of the “pseudomature” recombinant enzyme. Biochem. 2000;39:15592–15602. doi: 10.1021/bi001997l. [DOI] [PubMed] [Google Scholar]
- Phillips MA, Croteau R. Resin based defenses in conifers. Trends Plant Sci. 1999;4:184–190. doi: 10.1016/s1360-1385(99)01401-6. [DOI] [PubMed] [Google Scholar]
- Phillips MA, Savage TJ, Croteau R. Monoterpene synthases of loblolly pine (Pinus taeda) produce pinene isomers and enantiomers. Arch Biochem Biophys. 1999;372:197–204. doi: 10.1006/abbi.1999.1467. [DOI] [PubMed] [Google Scholar]
- Regvar M, Gogala N, Znidarsic N. Jasmonic acid effects mycorrhization of spruce seedlings with Laccaria laccata. Trees. 1997;11:511–514. [Google Scholar]
- Seybold SJ, Bohlmann J, Raffa KF. Biosynthesis of coniferophagous bark beetle pheromones and conifer isoprenoids: evolutionary perspective and synthesis. Can Entomol. 2000;132:697–753. [Google Scholar]
- Steele CL, Crock J, Bohlmann J, Croteau R. Sesquiterpene synthases from grand fir (Abies grandis): comparison of constitutive and wound induced activities, and cDNA isolation, characterization, and bacterial expression of δ-selinene synthase and γ-humulene synthase. J Biol Chem. 1998a;273:2078–2089. doi: 10.1074/jbc.273.4.2078. [DOI] [PubMed] [Google Scholar]
- Steele CL, Katoh S, Bohlmann J, Croteau R. Regulation of oleoresinosis in grand fir (Abies grandis): differential transcriptional control of monoterpene, sesquiterpene, and diterpene synthase genes in response to wounding. Plant Physiol. 1998b;116:1497–1504. doi: 10.1104/pp.116.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stofer Vogel B, Wildung MR, Vogel G, Croteau R. Abietadiene synthase from grand fir (Abies grandis): cDNA isolation, characterization, and bacterial expression of a bifunctional diterpene cyclase involved in resin acid biosynthesis. J Biol Chem. 1996;271:23262–23268. doi: 10.1074/jbc.271.38.23262. [DOI] [PubMed] [Google Scholar]
- Thaler JS. Jasmonate-inducible plant defenses cause increased parasitism of herbivores. Nature. 1999;399:686–688. [Google Scholar]
- Tholl D, Croteau R, Gershenzon G. Partial purification and characterization of the short-chain prenyltransferases, geranyl diphosphate synthase and farnesyl diphosphate synthase, from Abies grandis (grand fir) Arch Biochem Biophys. 2001;386:233–242. doi: 10.1006/abbi.2000.2212. [DOI] [PubMed] [Google Scholar]
- Tomlin ES, Alfaro RI, Borden JH, He FL. Histological response of resistant and susceptible white spruce to simulated white pine weevil damage. Tree Physiol. 1998;18:21–28. doi: 10.1093/treephys/18.1.21. [DOI] [PubMed] [Google Scholar]
- Tomlin ES, Antonejevic E, Alfaro RI, Borden JH. Changes in volatile terpene and diterpene resin acid composition of resistant and susceptible white spruce leaders exposed to simulated white pine weevil damage. Tree Physiol. 2000;20:1087–1095. doi: 10.1093/treephys/20.16.1087. [DOI] [PubMed] [Google Scholar]
- Trapp S, Croteau R. Defensive resin biosynthesis in conifers. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:689–724. doi: 10.1146/annurev.arplant.52.1.689. [DOI] [PubMed] [Google Scholar]
- Yukimune Y, Tabata H, Higashi Y, Hara Y. Methyl-jasmonate induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat Biotechnol. 1996;14:1129–1132. doi: 10.1038/nbt0996-1129. [DOI] [PubMed] [Google Scholar]