Abstract
Vessel wall damage exposes collagen fibres, to which platelets adhere directly via the collagen receptors glycoprotein (GP) VI and integrin α2β1 and indirectly by collagen-bound von Willebrand factor (vWF) via the GPIb-V-IX and integrin αIIbβ3 receptor complexes. Platelet–collagen interaction under shear stimulates thrombus formation in two ways, by integrin-dependent formation of platelet aggregates and by surface exposure of procoagulant phosphatidylserine (PS). GPVI is involved in both processes, complemented by α2β1. In mouse blood flowing over collagen, we investigated the additional role of platelet–vWF binding via GPIb and αIIbβ3. Inhibition of GPIb as well as blocking of vWF binding to collagen reduced stable platelet adhesion at high shear rate. This was accompanied by delayed platelet Ca2+ responses and reduced PS exposure, while microaggregates were still formed. Inhibition of integrin αIIbβ3 with JON/A antibody, which blocks αIIbβ3 binding to both vWF and fibrinogen, reduced PS exposure and aggregate formation. The JON/A effects were not enhanced by combined blocking of GPIb–vWF binding, suggesting a function for αIIbβ3 downstream of GPIb. Typically, with blood from FcR γ-chain +/− mutant mice, expressing 50% of normal platelet GPVI levels, GPIb blockage almost completely abolished platelet adhesion and PS exposure. Together, these data indicate that, under physiological conditions of flow, both adhesive receptors GPIb and αIIbβ3 facilitate GPVI-mediated PS exposure by stabilizing platelet binding to collagen. Hence, these glycoproteins have an assistant procoagulant role in collagen-dependent thrombus formation, which is most prominent at reduced GPVI activity and is independent of the presence of thrombin.
Collagen fibres, exposed upon vessel wall damage, are strongly platelet adhesive. Binding of platelets to collagen triggers a chain of activating events and leads to the assembly of platelet aggregates and the formation of fibrin-containing thrombi. The thrombus-forming reaction is essential in haemostasis, but detrimental in the progression of atherothrombosis and plaque rupture. This process of thrombus formation has been widely studied in vitro, using flow chambers where whole blood is perfused over a collagen-containing surface under (patho)physiological shear conditions, even in the absence of coagulation. From this work, it has appeared that multiple receptors are involved in the interaction of platelets with collagen.
Collagen rapidly adsorbs von Willebrand factor (vWF), which is present in plasma as a multimeric protein (Novak et al. 2002). vWF acts as a bridging molecule in platelet–collagen interactions, since it can bind to both collagen and the receptor complexes glycoprotein (GP)Ib-V-IX and integrin αIIbβ3 through its A3, A1 and C1 domains, respectively. High shear stress induces conformational changes of vWF, which result in a reversible interaction with GPIb (Huizinga et al. 2002). This reduces the velocity of platelets flowing over collagen-bound vWF and results in transient attachment to the collagen surface (Savage et al. 1996). Subsequent, irreversible binding to vWF is mediated by the αIIbβ3 integrin. This integrin also needs conformational changes for ligand interaction (inside-out signalling), which can be achieved, for example, by vWF–GPIb binding (Nesbitt et al. 2002; Arya et al. 2003) or following stimulation of the ADP, thromboxane A2 or collagen receptors (Shattil & Ginsberg, 1997; Jung & Moroi, 2001). Both GPIb and αIIbβ3 also mediate vWF/fibrinogen dependent platelet aggregate formation under shear (Shattil & Ginsberg, 1997; Savage et al. 2002).
Direct platelet–collagen contact is established by the collagen receptors GPVI and integrin α2β1 (Jung & Moroi, 2000; Savage et al. 2002; Nieswandt & Watson, 2003). GPVI acts as a major signalling receptor, while α2β1 is required for stable adhesion to collagen. Ligand-induced clustering of GPVI results in its non-covalent association with the Fc receptor (FcR) γ-chain, which leads to signalling via tyrosine phosphorylation (Gibbins et al. 1997; Tsuji et al. 1997). As a result, phospholipase Cγ2 becomes phosphorylated and activated, which causes a prolonged increase in cytosolic [Ca2+]i (Watson et al. 2001). This Ca2+ response contributes to the release of feedback agonists such as ADP and thromboxane A2, which sustain platelet aggregate formation.
Previous in vivo and in vitro flow studies with mice have indicated that the α2β1 integrin is dispensable for platelet-collagen adhesion and subsequent thrombus formation (Nieswandt et al. 2001a,b). Using mice deficient in α2β1 or GPVI, it was furthermore shown that under high, arterial shear conditions this integrin enhances GPVI signalling and thereby stabilizes the platelet aggregates on collagen (Kuijpers et al. 2003). This has led to a model of interplay between the collagen receptors in which the α2β1 integrin supported by release products functions to enhance GPVI-induced platelet activation. Such a model is now proposed by several groups (Atkinson et al. 2003; Chen & Kahn, 2003; Nieswandt & Watson, 2003; Siljander et al. 2004), although it is still unclear to what extent the synergistic effect of α2β1 on GPVI is due to intracellular signalling by the integrin itself (Jung & Moroi, 2000; Inoue et al. 2003) or to stabilization of collagen–GPVI contact by an activated integrin form.
Apart from aggregate formation, platelet–collagen interaction stimulates the coagulation process. Collagen or collagen-related peptide provokes, in a Ca2+ -dependent way, exposure of phosphatidylserine (PS) at the platelet outer membrane surface (Heemskerk et al. 1997; Siljander et al. 2001). The availability of PS greatly potentiates the conversion of prothrombin into coagulant thrombin and thus enhances thrombin generation (Bevers et al. 1982; Béguin & Kumar, 1997; Heemskerk et al. 2002). PS exposure is one of the early platelet responses in shear-dependent thrombus formation upon perfusion of human or murine blood over vWF–collagen (Kuijpers et al. 2003; Siljander et al. 2004). In both species, it is a consequence of GPVI activity, while α2β1 mainly potentiates the GPVI effect. There is evidence, mostly from experiments with coagulating plasma, that GPIb, αIIbβ3 and also vWF have discrete functions in stimulating platelet-dependent thrombin generation (Béguin et al. 1999; Keuren et al. 2003). However, whether these factors also contribute to the PS-exposing, procoagulant response of platelets interacting with vWF–collagen is still unresolved.
Since platelet–collagen interaction under high shear is dependent on vWF, GPIb and αIIbβ3, we focused here on the contribution of these proteins in the generation of the platelet procoagulant response under flow conditions. Using real-time video imaging techniques, we show that at high shear rates GPIb and vWF – and downstream of them αIIbβ3 – enhance GPVI-mediated PS exposure, especially under conditions of reduced GPVI expression and activity. This novel procoagulant effect of GPIb is independent of thrombin action and is additional to its function in platelet aggregation. It is likely to be of physiological relevance, given the large variation in GPVI expression between humans. As α2β1 also stimulates GPVI activity, our results reveal receptor interplay in the regulation of platelet procoagulant activity that is not limited to collagen receptors, but extends to the GPIb-V-IX complex.
Methods
Animals
Pathogen-free C57Bl/6 mice were obtained from Charles River (Maastricht, the Netherlands); mice deficient in FcR γ-chain (Takai et al. 1994) were obtained from Taconics (Germantown, NY, USA). Heterozygous FcR γ-chain deficient mice were generated by crossing wild-type mice with homozygous deficient mice of C57Bl/6 background (Nieswandt et al. 2001a). Reduced expression of both GPVI and FcR γ-chain was checked at the protein level by Western blots, and by flow cytometric measurement of surface expression of GPVI, as described (Snell et al. 2002). Experimental protocols were ethically approved by the animal care and use committee of the University of Maastricht.
Materials
Fab fragments of rat anti-mouse antibodies were produced and modified in our laboratories (Bergmeier et al. 2000). This involved the p0p/B antibody, which binds to the vWF binding site on GPIbα, and the JON/A antibody, which selectively binds to activated integrin αIIbβ3 and inhibits integrin interaction with both vWF and fibrinogen. Polyclonal rabbit anti-human vWF antibody, cross-reacting with mouse vWF, was obtained from Dako (Glostrup, Denmark), and fluorescein isothiocyanate (FITC)-labelled goat anti-rabbit IgG (GAR-FITC) was from Southern Biotech (Birmingham, AL, USA).
Fibrillar Horm collagen from equine tendon (Horm type-I) was purchased from Nycomed (Munich, Germany). Apyrase, high molecular weight heparin and MRS2179, an antagonist of the P2Y1 purinergic receptor, came from Sigma (St Louis, MO, USA). Annexin V (annexin A5) labelled with FITC was from Nexins Research (Hoeven, The Netherlands); calcein and fluo-3 acetoxymethyl esters, Pluronic F-127, and phalloidin labelled with Texas Red X were from Molecular Probes (Leiden, The Netherlands); H-Phe-Pro-Arg chloromethyl ketone (PPACK) was from Calbiochem (La Jolla, CA, USA). AR-C69931MX, an antagonist of the P2Y12 purinergic receptor, was kindly provided by AstraZeneca R&D (Charnwood, UK). Recombinant saratin was produced in the yeast Hansenula polymorpha, as described (Barnes et al. 2001). Sources of other chemicals are mentioned elsewhere (Heemskerk et al. 1999).
Platelet preparation and labelling
Under full anaesthesia, mice were bled retro-orbitally and killed by cervical dislocation. The blood (1 volume) was collected in 0.5 volumes of saline containing 5 units ml−1 heparin and 40 μm PPACK. Anticoagulated blood (1 volume) was diluted in Hepes buffer (pH 7.4; 0.5 volumes) containing 137 mm NaCl, 5.6 mm glucose, 5 mm Hepes, 2.7 mm KCl, 2 mm MgCl2, 2 mm CaCl2, 0.42 mm NaH2PO4, 0.1% (w/v) bovine serum albumin (BSA), which was supplemented with 1 unit ml−1 heparin. The blood was used within 2 h. Where indicated, blood was incubated with 2.5 μm calcein acetoxymethyl ester for 30 min, as described (Nieswandt et al. 2001a).
Washed murine platelets in Hepes buffer (pH 7.45) were prepared from platelet-rich plasma, as described for rat blood (Heemskerk et al. 1994). For Ca2+ measurements, washed platelets were incubated with fluo-3 acetoxymethyl ester (5 μm) in the presence of Pluronic F-127 (0.2 mg ml−1) and apyrase (0.1 units ml−1 ADPase) at room temperature for 45 min.
Real-time video imaging
Adhesion experiments under flow conditions were performed with mouse blood anticoagulated with PPACK and heparin, basically as described earlier (Nieswandt et al. 2001a). Briefly, coverslips (24 × 60 mm) were partly coated with Horm collagen type I fibres (1.25 mg per 50 mm2), rinsed with saline, and blocked with Hepes buffer containing 1% (w/v) BSA. Coverslips were placed in a parallel-plate transparent flow chamber (slit depth of 50 μm), which was connected by polyethylene tubing (diameter 1 mm), and mounted on an inverted Nikon (Tokyo, Japan) microscope. To prevent coagulation, the chamber and tubing were pre-washed with Hepes buffer containing 1 unit ml−1 heparin. Blood was perfused through the flow chamber using a 1 ml syringe and a pulse-free pump, at the desired shear rate (1000 or 1500 s−1) for 4 min. During perfusion, high-resolution microscopic images of transmission or fluorescence (fluo-3 or calcein) were recorded in real-time with a Visitech imaging system (Sunderland, UK). Digital images were captured with two parallel-placed intensified, CCD cameras, recording infrared (0.3 Hz) and epifluorescence (5 Hz) light at the desired wavelengths (Heemskerk et al. 1997). After perfusion, the flow chamber was rinsed with Hepes buffer supplemented with 1 unit ml−1 heparin at the desired shear rate. Exposure of PS was detected with FITC-labelled annexin A5 (0.5 μg ml−1) added to the rinse buffer.
Where indicated, blood was incubated for 10 min prior to perfusion with a saturating concentration of p0p/B or JON/A Fab fragment (40 μg ml−1), saratin (10 μg ml−1) or MRS2179 (20 μm) plus AR-C69931MX (50 μm). These antagonists were also added to the rinse buffer. To measure changes in cytosolic [Ca2+]i, fluo-3-labelled platelets from mice of the same genotype were added to the anticoagulated blood, so that 5–10% of total platelets were labelled. Labelling a small fraction of the platelets in blood had the advantage that individual, collagen-bound platelets could be distinguished from each other, even when trapped in multiplatelet aggregates.
Real-time changes in fluo-3 fluorescence from single platelets, recorded during the flow experiment, were analysed with Quanticell software. Changes in fluo-3 fluorescence of individual platelets were converted into levels of [Ca2+]i using a pseudo-ratio calibration procedure, exactly as described (Heemskerk et al. 2001). Traces representing averaged Ca2+ responses were constructed from time-adjusted responses of at least 13 single platelets that could be observed for at least 60 s (2–4 mice per condition).
Confocal fluorescence microscopy
Immediately after perfusion, collagen-coated coverslips with aggregates were removed from the flow chamber, fixed with 2% formaldehyde for 15 min, and rinsed with tap water. Further procedures were performed in a humid chamber at room temperature. The samples were first blocked with 15% (w/v) BSA in phosphate-buffered saline (PBS) for 30 min. Samples were then incubated with rabbit anti-human vWF antibody (10 μg ml−1) for 1 h, washed in PBS 3 times for 5 min, and stained with GAR-FITC (10 μg ml−1) for 1 h. Samples were counterstained for actin to stain all platelets. After a triple wash in PBS and permeabilization with 0.005% SDS in PBS for 10 min, they were blocked with 1% BSA in PBS. The samples were then incubated with Texas Red-labelled phalloidin (3 units ml−1) in blocking buffer for 1 h and, after another wash, they were mounted in 9 volumes of glycerol plus 1 volume of 0.2 m Tris-HCl, 0.02% NaN3 with 2% 1,4-diazabicyclo(2,2,2)-octane (Dabco) (pH 8.0). Double-stained aggregates were observed with a Bio-Rad MRC-600 confocal scanning laser microscope (Bio-Rad, Richmond, CA, USA) equipped with a krypton–argon mixed gas laser and a red diode laser (Ion Laser Technology, Salt Lake City, UT, USA) with two separate wavelengths for excitation of fluorescein (488 nm) and Texas Red X (568 nm).
Image analysis
Data were compared off-line from at least nine different randomly chosen, microscopic images, taken at the collagen surface. Platelet deposition was determined from phase-contrast images, as described before (Kuijpers et al. 2003). Area coverage by platelets staining with calcein or FITC-annexin A5 fluorescence was determined with Quanticell software (Visitech). Procoagulant area was corrected for fluorescent glare in the optics by comparison of camera images in fluorescence and transmission modes.
Statistical analysis
Data were tested for significant differences using the statistical package for social sciences (SPSS 11.0, Chicago, IL, USA). Significant differences compared to control condition were evaluated with a Mann–Whitney U test.
Results
Roles of GPIb and vWF in PS exposure in platelets adhering to collagen under shear
Whole blood from wild-type mice, anticoagulated with PPACK–heparin, was used to investigate the contribution of the GPIb–vWF interaction to platelet deposition on collagen and subsequent PS exposure. The blood was perfused over immobilized type-I collagen at an intermediate, arterial wall shear rate (1000 s−1) to achieve GPIb dependency (Kuijpers et al. 2003). Video microscopy showed that most wild-type platelets firmly adhered to the collagen fibres. Some of these platelets assembled to form platelet aggregates, while others remained single and transformed into round, blebbing structures. The latter morphological change is an indicator of PS exposure and procoagulant activity (Siljander et al. 2001; Kuijpers et al. 2003). The deposition of platelets, measured as surface area coverage, increased about linearly for at least 6 min. After 4 min perfusion, 25 ± 2% (mean ± s.e.m., n = 32) of the surface was covered with platelets (Fig. 1A). A similar, linear increase in platelet deposition was observed, when blood containing calcein-labelled platelets was perfused over the collagen surface (data not shown, but see Nieswandt et al. 2001a). Staining with FITC-labelled annexin A5 showed that, at the 4-min end point, a considerable proportion of single, collagen-binding platelets had exposed PS, with an area coverage of fluorescence of 9 ± 1% (n = 20) (Fig. 1B). Experiments where FITC–annexin A5 was added to the blood indicated that PS exposure in deposited platelets started after a lag time of 2 min and then increased continuously up to 10 min.
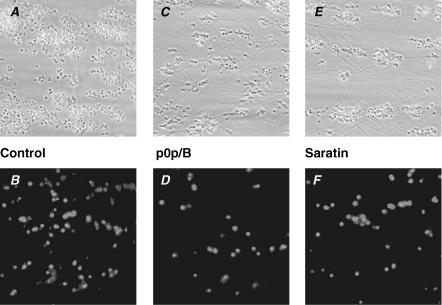
Figure 1. Effect of blocking GPIb on platelet deposition and PS exposure upon flow over vWF/collagen.
Whole blood from C57Bl/6 wild-type mice was perfused over type-I collagen at a wall shear rate of 1000 s−1. A, C and E, phase-contrast microscopic images (120 × 120 μm) after 4 min of perfusion. B, D and F, fluorescence images (150 × 150 μm) after staining with FITC-labelled annexin A5. A and B, untreated control blood. C and D, blood pre-treated with 40 μg ml−1 anti-GPIb p0p/B Fab fragment (10 min). E and F, blood pre-treated with 10 μg ml−1 saratin (10 min).
To block the GPIb–vWF interaction, the wild-type blood was pre-incubated with a saturating concentration of anti-GPIbα p0p/B Fab fragment (40 μg ml−1), which specifically abolishes GPIb binding to mouse vWF (Bergmeier et al. 2000). This treatment led to an increased translocation velocity of platelets over the collagen surface and to diminished stable adhesion, while microaggregates were still formed (Fig. 1C). Platelet deposition was reduced by p0p/B to 66 ± 13% (n = 5) of the control condition. The number of single, PS-exposing platelets (Fig. 1D) was more strongly reduced to about 20% (P = 0.02) of control (Fig. 2A). As an alternative way to eliminate the contribution of vWF, blood was treated with 10 μg ml−1 saratin, a leech protein which blocks vWF binding to collagen (Barnes et al. 2001). Again, platelets were moving over the collagen surface and made fewer stable contacts (Fig. 1E). With some of the platelets still forming aggregates, the total area coverage was 34 ± 6% (n = 11) of control. The number of PS-exposing platelets (Fig. 1F) was similarly reduced to 30 ± 13% of control (Fig. 2A).
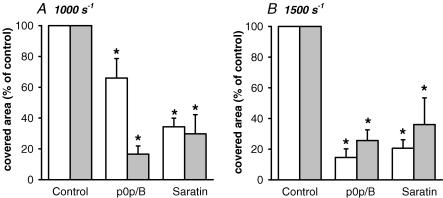
Figure 2. Roles of GPIb and shear stress in platelet deposition and PS exposure.
Whole blood from wild-type mice was perfused over collagen for 4 min at a shear rate of 1000 or 1500 s−1. Effect of pre-treatment with p0p/B Fab (40 μg ml−1) or saratin (10 μg ml−1) on area coverage of all platelets (white bars) and PS-exposing platelets (grey bars). Data are percentages of control condition at indicated shear rate (mean ± s.e.m., n = 6–20). *P < 0.05 compared to control.
To achieve higher GPIb dependency, additional experiments were performed at a higher shear rate of 1500 s−1. In this case, blocking GPIb with p0p/B further reduced the area coverage of platelets to 15 ± 6% and of annexin A5-binding platelets to 26 ± 7% of the control (Fig. 2B). Similarly, the presence of saratin decreased the coverage of platelets to 21 ± 5% and of annexin A5 fluorescence to 36 ± 17% of control. Thus, inhibition of the GPIb–vWF interaction by GPIb blocking (p0p/B) or the absence of vWF (saratin) caused a substantial but incomplete reduction in platelet deposition and PS exposure at shear rates up to 1500 s−1.
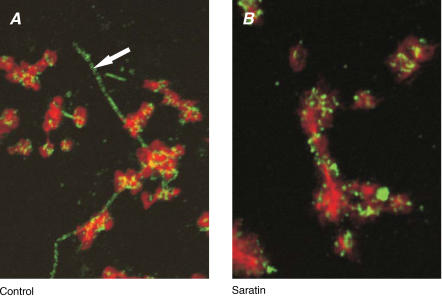
The similarities in effects of p0p/B and saratin treatment suggested that the GPIb interaction with collagen-bound vWF could control PS exposure by allowing stable platelet adhesion. To confirm this, platelets on collagen-coated coverslips were stained post perfusion for vWF and then counterstained with phalloidin to visualize actin filaments. In the control situation, confocal microscopy showed bright vWF staining on the collagen fibres as well as on platelet aggregates, where it appeared as clustered spots (Fig. 3A). With saratin present, the collagen fibres no longer stained for vWF, whereas the platelets in aggregates had unaltered vWF staining (Fig. 3B).
Figure 3. Effect of saratin on vWF binding to collagen and platelets.
Blood from wild-type mice was perfused over collagen in the absence (A) or presence (B) of saratin (10 μg ml−1) at 1000 s−1 for 4 min. Representative confocal fluorescence images are given after fixation with 2% formaldehyde and staining for actin and vWF. Micrographs show contours of platelets (actin) and deposited vWF as brighter punctuated spots; arrow indicates vWF-covered collagen fibre. Image sizes are 20 μm × 25 μm.
Role of GPIb in Ca2+ responses of platelets adhering to vWF–collagen
The activation state of platelets interacting with vWF–collagen was investigated by adding 10% fluo-3-labelled murine platelets to the blood. This labelling of platelets did not influence collagen-induced aggregate formation in vitro, as measured by aggregometry (data not shown). Under control conditions of flow (1000 s−1), the large majority of fluo-3-labelled platelets stably attached to collagen fibrils. The platelets showed a potent and prolonged Ca2+ response (Fig. 4A), as is required for PS exposure (Heemskerk et al. 1997). Traces from single platelets indicated a rapid, prolonged rise in [Ca2+]i that was occasionally preceded by one high spike in [Ca2+]i (Fig. 4B; see also ‘control’ movie in Supplementary material, available online only). As we have shown before, this Ca2+ signal under flow conditions is almost completely dependent on GPVI signalling, with minute, spiking [Ca2+]i increases remaining in the absence of GPVI (Kuijpers et al. 2003).
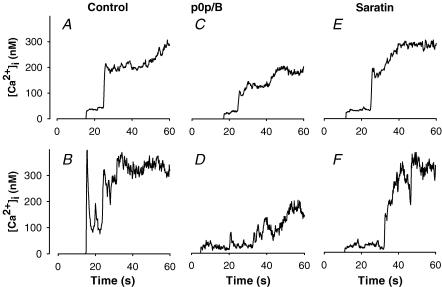
Figure 4. Potentiating role of GPIb in Ca2+ response of collagen-adhering platelets under shear.
Whole blood was supplemented with 10% fluo-3-loaded platelets, and perfused over collagen as indicated for Fig. 1. Upper panels show averaged changes in [Ca2+]i of 13–32 platelets, observed during at least 60 s, from at least two different mice. Lower panels show changes in [Ca2+]i of representative single platelets. Blood was either untreated (A, B) or incubated for 10 min prior to perfusion with 40 μg ml−1 p0p/B Fab (C, D) or 10 μg ml−1 saratin (E, F).
When the blood was treated with anti-GPIb p0p/B, many of the fluo-3-labelled platelets moved over the surface before stopping. Once they had adhered, the Ca2+ signals of many of the p0p/B-treated platelets were reduced in comparison to the control (Fig. 4C and D). Individual cells often showed a series of Ca2+ spikes before reaching a maximal level, which was on average reduced by 35% (n = 13–26 platelets, P = 0.02, unpaired t test, measured after 60 s). This is illustrated in the ‘p0p/B’ movie (see Supplementary material). Treatment of the blood with saratin caused similar but not identical effects. Again, many of the fluo-3-labelled platelets moved over the surface before adhering stably. The Ca2+ responses of these platelets were typically delayed (Fig. 4E and F). The median lag time (time measured from first collagen contact until [Ca2+]i rise) was notably increased from 9 to 13 s compared to control platelets. Maximal [Ca2+]i rises after the delay were unchanged with saratin. These data indicated that the blocking of GPIb and the absence of vWF delayed and destabilized platelet–collagen contact and subsequent signalling. This points to a stimulating role of GPIb and vWF in GPVI-induced Ca2+ signalling. Since PS exposure relies on a persistent [Ca2+]i rise, the reduced or delayed Ca2+ signal with p0p/B or saratin can explain the lower PS exposure under these flow conditions (see Fig. 2A).
Role of integrin αIIbβ3 and GPIb in PS exposure in platelets adhering to vWF–collagen
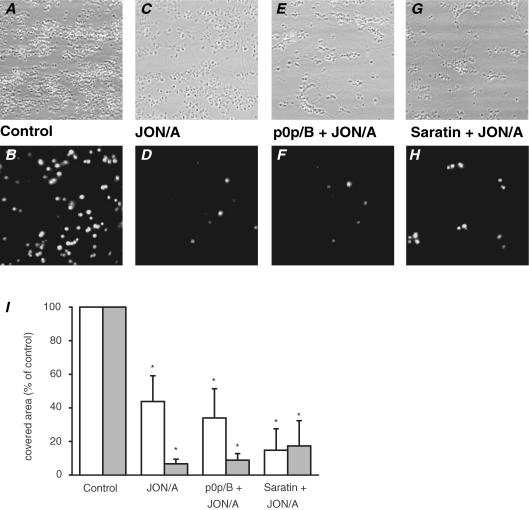
Stable adhesion to vWF requires platelet interaction with both GPIb-V-IX and activated integrin αIIbβ3 (Savage et al. 1998; Wu et al. 2000; Nesbitt et al. 2002; Patel et al. 2003). To interfere with the contribution of αIIbβ3, the murine blood was incubated with Fab fragments (40 μg ml−1) of JON/A, which is a unique, recently characterized antibody that blocks αIIbβ3 binding sites for vWF and fibrinogen (Bergmeier et al. 2002; Grüner et al. 2003). This is in contrast to anti-aggregatory peptide inhibitors of αIIbβ3, which only react with the fibrinogen binding site. Upon perfusion of blood in the presence of JON/A, platelets adhered to vWF–collagen as single cells or two-layered microaggregates (Fig. 5A–D). Surface coverage with platelets was reduced to 44 ± 15%(n = 7) of control (Fig. 5I). PS exposure in platelets on collagen was greatly lowered to 7 ± 3% of control. In comparison, when effects of autocrine ADP were blocked with the P2Y1 and P2Y12 receptor antagonists MRS2179 (20 μm) and AR-C69931MX (50 μm), respectively, this gave a similar decrease in platelet deposition (area coverage with platelets decreased to 48 ± 9% of control; not shown), but a weaker decrease in PS exposure (38 ± 17% of control).
Figure 5. Role of integrin αIIbβ3 in platelet deposition and PS exposure.
Whole blood from wild-type mice was perfused over type-I collagen as indicated for Fig. 1. Upper panels: representative phase contrast images (120 × 120 μm) after 4 min of perfusion. Lower panels: fluorescence images (150 × 150 μm) after staining with FITC-labelled annexin A5. Blood was untreated (A, B), or treated with 40 μg ml−1 anti-αIIbβ3 JON/A Fab fragment alone (C, D), with JON/A plus 40 μg ml−1 p0p/B Fab fragment (E, F), or with 10 μg ml−1 saratin (G, H) 10 min prior to perfusion. I, surface area coverage of all platelets (white bars) and PS-exposing platelets (grey bars) of conditions described in panels A–H. Data are given as percentages of control condition (mean values ± s.e.m., n = 3–11). *P < 0.05 compared to control.
To test whether the inhibitory effects of JON/A were dependent on GPIb and vWF, combined treatments were carried out with either p0p/B or saratin (Fig. 5E–H). The addition of p0p/B did not further reduce platelet deposition nor PS exposure in comparison to JON/A alone (Fig. 5I). Perfusion with saratin plus JON/A resulted in a somewhat lower platelet deposition, and an unchanged reduction in PS-exposing platelets. These data suggest that GPIb and integrin αIIbβ3 influenced platelet activation at least in part via a common route involving vWF.
Increased role of GPIb in PS exposure by platelets with reduced expression of GPVI
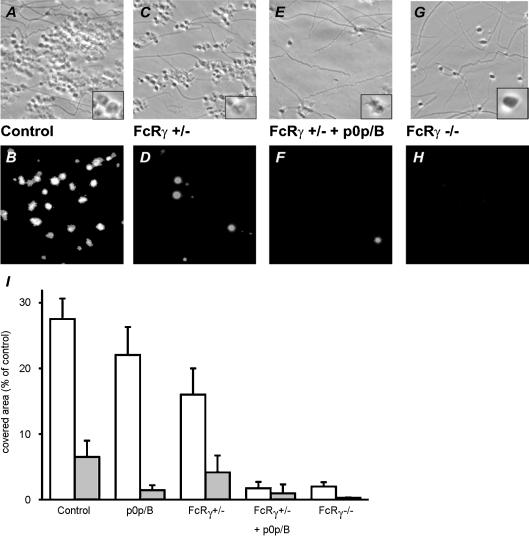
Since GPVI activity is a limiting factor in collagen-dependent platelet activation and aggregation (Chen & Kahn, 2003), the effect of GPIb inhibition was also examined under conditions of reduced GPVI expression. Therefore, blood was used from mice which were partially or completely deficient in FcR γ-chain. As expected, homozygous deficiency resulted in undetectable levels of both FcR γ-chain and GPVI on platelets, while heterozygous deficiency gave a 50% reduction of both proteins (Table 1). Expression levels of GPIb-V-IX and αIIbβ3 were unchanged in these animals. When blood from heterozygous mice was perfused over vWF–collagen (1000 s−1), platelet deposition was about linear with time. In a typical experiment, the increase in surface area coverage was 2.3% min−1 (R2 = 0.44) in comparison to 5.5% min−1 (R2 = 0.93) for wild-type blood. At the 4-min end-point, this resulted in an approximately 42% lower platelet adherence for the FcR γ-chain +/− mice (Fig. 6A–D). Exposure of PS was also about 40% reduced in comparison to wild-type (Fig. 6I). Strikingly, blockage of GPIb with p0p/B fragments in the heterozygous blood nearly completely abolished stable platelet adhesion, while PS-exposing platelets were hardly detectable (Fig. 6E–F). In agreement with earlier results (Nieswandt et al. 2001a; Kuijpers et al. 2003), perfusion of blood from homozygous FcR γ-chain −/− mice under these conditions resulted in diminished platelet adhesion to the collagen. During flow, most FcR γ-chain −/− platelets adhered only transiently to the surface (putatively via GPIb) and then moved on. After 4 min, a limited number of stably adherent platelets was detected (Fig. 6G–H), interestingly especially at sites with a higher density of collagen fibres. These platelets neither showed morphological signs of activation nor exposed PS, confirming the essential role of GPVI in these platelet responses.
Table 1.
Surface expression of different glycoproteins on platelets from wild-type and FcR γ-chain deficient mice
| Glycoprotein(s) | Wild-type | FcRγ +/− | FcRγ −/− |
|---|---|---|---|
| GPVI | 48.3 ± 8.7 | 27.2 ± 9.3* | 6.4 ± 1.9* |
| GPIIa (β1) | 135.6 ± 11.2 | 131.7 ± 13.1 | 133.3 ± 12.8 |
| GPIa (α2) | 47.2 ± 8.5 | 45.5 ± 7.3 | 44.8 ± 7.9 |
| GPIIb/IIIa (αIIbβ3) | 395.8 ± 30.4 | 416.2 ± 32.5 | 399.4 ± 33.7 |
| GPV | 187.2 ± 19.3 | 179.4 ± 16.7 | 185.1 ± 20.5 |
| CD9 | 532.3 ± 38.2 | 535.7 ± 41.4 | 528.7 ± 35.6 |
Expression levels were determined by flow cytometry using fluorophore-labelled monoclonal antibodies, as described (Nieswandt et al. 2000). Platelets were gated by their forward scatter/side scatter characteristics. Results are expressed as mean log fluorescence (arbitrary units) ± s.d. for 6 mice per group.
P < 0.01 compared to wild-type.
Figure 6. Increased role of GPIb in platelet deposition and PS exposure upon reduced expression of GPVI.
Blood from wild-type and FcR γ-chain +/− and −/− mice was perfused over collagen (see Fig. 1). Wild-type and FcR γ-chain +/− blood were pre-incubated with 40 μg ml−1 p0p/B Fab as indicated. Upper panels (A, C, E, G): representative phase contrast images (48 × 48 μm) after 4 min perfusion. Inserts are 200% magnifications showing platelet morphology. Lower panels (B, D, F, H): fluorescence images (150 × 150 μm) after staining with FITC-labelled annexin A5. I, surface area coverage of all platelets (white bars) and PS-exposing platelets (grey bars). Data are mean values ± s.e.m. (n = 3–5).
Discussion
In this study with mouse blood, we have evaluated the contribution of GPIb and collagen-bound vWF to shear-dependent platelet adhesion and procoagulant activity during thrombus formation. The results extend earlier work, demonstrating that GPVI has a conditional role in platelet–collagen interaction and subsequent activation both ex vivo (Nieswandt et al. 2001a; Kuijpers et al. 2003) and in denuded artery in vivo (Massberg et al. 2003). In the present study we find that, at high shear rates up to 1500 s−1 (mimicking those at the arterial wall), the GPIb interaction with vWF deposited on collagen enhances irreversible platelet binding and GPVI-induced PS exposure, along with stimulation of the rise in [Ca2+]i, which is a prerequisite for exposure of procoagulant PS. This response must be regulated separately from platelet aggregate formation, as the PS-exposing cells are usually not found in aggregates. Importantly, at reduced levels of GPVI we find that the procoagulant response is highly dependent on GPIb. Inhibitor studies indicate that αIIbβ3, which also binds to vWF, has an overlapping role with GPIb, underlining the important adhesive function of this integrin, which is mostly considered subordinate to its function in platelet aggregation. As exposed PS potently enhances thrombin generation, in the platelet–collagen interaction either of the two adhesive glycoproteins appears to potentiate the two haemostatic processes contributing to thrombus formation: aggregate formation and coagulant activity.
Human and murine vWF and GPIb-V-IX play an initial role in tethering and adhesion to collagen under high shear flow conditions, and thereby mediate platelet aggregate formation on collagen (Wu et al. 2000; Goto et al. 2002; Savage et al. 2002). In addition, both vWF and the GPIb-V-IX complex are involved in thrombin generation and coagulation, perhaps because of the binding to multiple coagulation proteins, including factor VIII, factor XI, thrombin and fibrin (Beguin et al. 1999; Baird & Walsh, 2002; Andrews et al. 2003; Keuren et al. 2003). Here, we report on a procoagulant effect of vWF and GPIb, i.e. PS exposure, linked to collagen receptor stimulation, which is independent of the formation of thrombin. Whether vWF and GPIb may also contribute to platelet PS exposure in coagulating plasma is still unknown. However, recent data indicate that GPIb can stimulate thrombin formation (in the absence of collagen) in a shear-dependent way (Keuren et al. 2003). It is thus conceivable that the function of GPIb in thrombin formation also involves a shear-dependent stimulation of PS exposure, similar to its function we report here for platelet–collagen interaction.
The data with anti-GPIb p0p/B Fab fragment or saratin (which prevents vWF binding to collagen) show that platelets can still bind to collagen in the absence of the vWF–GPIb interaction up to a shear rate of 1500 s−1. Others have described that GPIb, in the absence of vWF, is still free to bind thrombospondin, which also mediates platelet adhesion under flow (Jurk et al. 2003). Thus, GPIb can still have a role at intermediate shear rates independently of collagen-bound vWF. However, we find that residual, vWF-independent adhesion is mostly due to GPVI or integrin α2β1, as it is abolished in the absence of functional collagen receptors (M. Kuijpers, unpublished results). On the other hand, p0p/B or saratin treatment did lead to a decreased deposition of platelets (Fig. 1), and a reduced or delayed Ca2+ response and PS exposure (Fig. 4). This could indicate that the GPIb-dependent spiking elevations in [Ca2+]i reported in platelets binding to immobilized vWF (Mazzucato et al. 2002; Nesbitt et al. 2002; Mangin et al. 2003) also contribute to the Ca2+ signal in the presence of collagen. We have shown previously that collagen-induced Ca2+ signalling in murine platelets is almost completely dependent on the presence of the GPVI–FcR γ-chain complex, with minute spiking [Ca2+]i increases remaining in FcRγ−/− platelets (Kuijpers et al. 2003), which might be the result of the vWF–GPIb interaction. Accordingly, GPVI must be responsible for the majority of the Ca2+ signal, while GPIb makes only a small contribution. Taken together, these data point to an enhancement of GPVI signalling by GPIb–vWF, by direct signalling and/or allowing full engagement of the GPVI receptors.
The Fab fragment JON/A blocks binding of the αIIbβ3 to fibrinogen and vWF (Gruner et al. 2003), and thus provides a unique tool to study the role of this integrin in adhesion and aggregation. Incubation with JON/A resulted in a stronger inhibition of platelet deposition and PS exposure than the blocking of ADP function induced by receptor antagonists. This confirms the concept that αIIbβ3 is involved in stable adhesion to this surface (Savage et al. 1998). Furthermore, combined blockage of GPIb and integrin αIIbβ3 did not further reduce platelet activation in comparison to αIIbβ3 inhibition alone. This places the function of integrin αIIbβ3 downstream of GPIb, which is in good agreement with the current model of vWF–platelet interaction. This model presumes initial shear-dependent tethering via GPIb-V-IX, subsequent stable adhesion and enhanced signalling via activated integrin αIIbβ3 (Savage et al. 1998; Nesbitt et al. 2002). This is also in line with in vivo findings with mice that platelet adhesion to the vessel wall involves multiple integrin–ligand interactions, none of which are essential by themselves (Grüner et al. 2003), and that β3 deficiency results in defective stable platelet adhesion to the vessel wall (Wagner & Burger, 2003).
For mice with reduced levels of GPVI–FcR γ-chain it is demonstrated that, under static conditions, neither platelet adhesion to immobilized collagen nor protein tyrosine phosphorylation induced by collagen is affected compared to wild-type (Snell et al. 2002). Here we show, with mice expressing half of the normal GPVI–FcR γ-chain levels, that under flow conditions both platelet adhesion on collagen and platelet activation – as apparent from PS exposure – are reduced by about 50%. This indicates that the level of GPVI expression determines (and is a limiting factor for) signalling under shear. This conclusion is in agreement with the findings that collagen-induced Ca2+ responses of murine platelets are dependent on the GPVI expression level (Chen et al. 2002). Similarly, in human tissue, partial blockage of GPVI has been shown to suppress aggregate formation and PS exposure under flow (Siljander et al. 2004). Thus, the regulatory function of this collagen receptor in platelet activation and procoagulant activity appears to be conserved between species.
Interestingly, in FcR γ-chain heterozygous mice, expressing normal GPIb and αIIbβ3 levels on platelets, the blockage of GPIb caused almost complete abolition of stable platelet adhesion and PS exposure under shear (Fig. 6). This indicates that GPIb plays a more prominent role in platelet–collagen interaction at reduced levels of GPVI/FcR γ-chain. Apparently, there is some redundancy in GPIb and GPVI receptor function. It is likely to be of physiological relevance, given the large variation in GPVI expression between humans (Chen et al. 2002; Best et al. 2003; Joutsi-Korhonen et al. 2003). As also α2β1 stimulates GPVI activity, the present results point to receptor interplay in the regulation of platelet procoagulant activity that is not restricted to collagen receptors, but extends to the GPIb-V-IX complex.
Earlier it has been proposed that the FcR γ-chain mediates GPIb-induced platelet activation (Falati et al. 1999; Canobbio et al. 2001). However, our data do not support this concept, as we find that partial knock-out of the γ-chain enhances the GPIb functioning. On the other hand, our findings are fully in line with those of Mangin et al. (2003), who propose that in mice GPIb signalling to Ca2+ flux and filopod extension relies on Src kinases via a pathway distinct from FcR γ-chain derived signalling.
Perfusion of blood from homozygous FcR γ-chain deficient mice resulted in only few adhering platelets, especially at sites where the collagen density was relatively high (Fig. 6). This is in apparent contrast to recent findings with GPVI knockout mice, where full adhesion to collagen was detected under flow conditions (Kato et al. 2003). The difference with the present data is likely explained by differences in methodology (collagen surface, anti-coagulant, mepacrine labelling, etc.). Preliminary data in our laboratory indicate that the few GPVI/FcRγ−/− platelets adhere in a GPIb/α2β1-dependent way (unpublished results). This is similar to the situation with human platelets, where it also has been shown that α2β1 is adhesive in the absence of GPVI signalling (Siljander et al. 2004).
In summary, the current data, together with the earlier established role of integrin α2β1 (Nieswandt et al. 2001a; Kuijpers et al. 2003; Nieswandt & Watson, 2003), confirm that murine GPVI is the primary trigger of activation in platelet–collagen interaction under shear. Further, it is shown that GPVI is facilitated by GPIb-V-IX in addition to integrins α2β1 and αIIbβ3. Platelet adhesion and subsequent activation evolves as a series of multireceptor events with redundancy, facilitation and mutual interactions, rather than as a linear process. Specifically, concerning the procoagulant platelet reaction, both GPIb and αIIbβ3 appear to control PS exposure under these shear conditions, likely by enhancing GPVI-dependent activation. The finding that these adhesive receptors in concert with GPVI have a procoagulant role in collagen-dependent thrombus formation indicates that interplay does not only occur between the collagen receptors, but also extends to the GPIb-V-IX complex.
Supplementary material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2004.062414
http://jp.physoc.org/cgi/content/full/jphysiol.2004.062414v1/DC1 and contains supplementary material consisting of two movies.
This material can also be found at:
http://blackwellpublishing.com/products/journals/suppmat/tjp/tjp341/tjp341sm.htm
References
- Andrews RK, Gardiner EE, Shen Y, Whisstock JC, Berndt MC. Glycoprotein Ib-IX-V. Int J Biochem Cell Biol. 2003;3:1170–1174. doi: 10.1016/s1357-2725(02)00280-7. [DOI] [PubMed] [Google Scholar]
- Arya M, Lopez JA, Romo GM, Cruz MA, Kasirer-Friede A, Shattil SJ, Anvari B. Glycoprotein Ib-IX-mediated activation of integrin alpha(IIb)beta(3): effects of receptor clustering and von Willebrand factor adhesion. J Thromb Haemost. 2003;1:1150–1157. doi: 10.1046/j.1538-7836.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Atkinson BT, Jarvis GE, Watson SP. Activation of GPVI by collagen is regulated by α1β2 and secondary mediators. J Thromb Haemost. 2003;1:1278–1287. doi: 10.1046/j.1538-7836.2003.00245.x. [DOI] [PubMed] [Google Scholar]
- Baird TR, Walsh PN. The interaction of factor XIa with activated platelets but not endothelial cells promotes the activation of factor IX in the consolidation phase of blood coagulation. J Biol Chem. 2002;277:38462–38467. doi: 10.1074/jbc.M205902200. [DOI] [PubMed] [Google Scholar]
- Barnes CS, Krafft B, Frech M, Hofmann UR, Papendieck A, Dahlems U, Gellissen G, Hoylaerts MF. Production and characterization of saratin, an inhibitor of von Willebrand factor-dependent platelet adhesion to collagen. Semin Thromb Hemost. 2001;27:337–348. doi: 10.1055/s-2001-16887. [DOI] [PubMed] [Google Scholar]
- Béguin S, Kumar R. Thrombin, fibrin and platelets, a resonance loop in which von Willebrand factor is a necessary link. Thromb Haemost. 1997;78:590–594. [PubMed] [Google Scholar]
- Béguin S, Kumar R, Keularts I, Seligsohn U, Coller BS, Hemker HC. Fibrin-dependent platelet procoagulant activity requires GPIb receptors and von Willebrand factor. Blood. 1999;93:564–570. [PubMed] [Google Scholar]
- Bergmeier W, Rackebrandt K, Schroder W, Zirngibl H, Nieswandt B. Structural and functional characterization of the mouse von Willebrand factor receptor GPIb-IX with novel monoclonal antibodies. Blood. 2000;95:886–893. [PubMed] [Google Scholar]
- Bergmeier W, Schulte V, Brockhoff G, Bier U, Zirngibl H, Nieswandt B. Flow cytometric detection of activated mouse integrin αIIbβ3 with a novel monoclonal antibody. Cytometry. 2002;48:80–86. doi: 10.1002/cyto.10114. [DOI] [PubMed] [Google Scholar]
- Best D, Senis YA, Jarvis GE, Eagleton HJ, Roberts DJ, Saito T, Jung SM, Moroi M, Harrison P, Green FR, Watson SP. GPVI levels in platelets: relationship to platelet function at high shear. Blood. 2003;102:2811–2818. doi: 10.1182/blood-2003-01-0231. [DOI] [PubMed] [Google Scholar]
- Bevers EM, Comfurius P, van Rijn JLML, Hemker HC, Zwaal RFA. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur J Biochem. 1982;122:429–436. doi: 10.1111/j.1432-1033.1982.tb05898.x. [DOI] [PubMed] [Google Scholar]
- Canobbio I, Bertoni A, Lova P, Paganini S, Hirsch E, Sinigaglia F, Balduini C, Torti M. Platelet activation by von Willebrand factor requires coordinated signaling through thromboxane A2 and FcγIIA receptor. J Biol Chem. 2001;276:26022–26029. doi: 10.1074/jbc.M102639200. [DOI] [PubMed] [Google Scholar]
- Chen H, Kahn ML. Reciprocal signaling by integrin and nonintegrin receptors during collagen activation of platelets. Mol Cell Biol. 2003;23:4764–4777. doi: 10.1128/MCB.23.14.4764-4777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Locke D, Liu Y, Liu C, Kahn ML. The platelet receptor GPVI mediates both adhesion and signaling responses to collagen in a receptor density-dependent fashion. J Biol Chem. 2002;277:3011–3019. doi: 10.1074/jbc.M109714200. [DOI] [PubMed] [Google Scholar]
- Falati S, Edmead CE, Poole AW. Glycoprotein Ib-V-IX, a receptor for von Willebrand factor, couples physically and functionally to the Fc receptor gamma-chain, fyn, and lyn to activate human platelets. Blood. 1999;94:1648–1656. [PubMed] [Google Scholar]
- Gibbins JM, Okuma M, Farndale R, Barnes M, Watson SP. Glycoprotein VI is the collagen receptor in platelets which underlies tyrosine phosphorylation of the Fc receptor γ-chain. FEBS Lett. 1997;413:255–259. doi: 10.1016/s0014-5793(97)00926-5. [DOI] [PubMed] [Google Scholar]
- Goto S, Tamura N, Handa S, Arai M, Kodama K, Takayama H. Involvement of glycoprotein VI in platelet thrombus formation on both collagen and von Willebrand factor surfaces under flow conditions. Circulation. 2002;106:266–272. doi: 10.1161/01.cir.0000021427.87256.7e. [DOI] [PubMed] [Google Scholar]
- Grüner S, Prostredna M, Schulte V, Krieg T, Eckes B, Brakebusch C, Nieswandt B. Multiple integrin–ligand interactions synergize in shear-resistant platelet adhesion at sites of arterial injury in vivo. Blood. 2003;102:4021–4027. doi: 10.1182/blood-2003-05-1391. [DOI] [PubMed] [Google Scholar]
- Heemskerk JWM, Bevers EM, Lindhout T. Platelet activation and blood coagulation. Thromb Haemost. 2002;88:186–193. [PubMed] [Google Scholar]
- Heemskerk JWM, Feijge MAH, Sage SO, Walter U. Indirect regulation of Ca2+ entry by cAMP-dependent and cGMP-dependent protein kinases and phospholipase C in rat platelets. Eur J Biochem. 1994;223:543–551. doi: 10.1111/j.1432-1033.1994.tb19023.x. [DOI] [PubMed] [Google Scholar]
- Heemskerk JWM, Siljander P, Vuist WMJ, Breikers G, Reutelingsperger CPM, Barnes MJ, Knight CG, Lassila R, Farndale RW. Function of glycoprotein VI and integrin α2β1 in the procoagulant response of single, collagen-adherent platelets. Thromb Haemost. 1999;81:78–92. [PubMed] [Google Scholar]
- Heemskerk JWM, Vuist WMJ, Feijge MAH, Reutelingsperger CPM, Lindhout T. Collagen but not fibrinogen surfaces induce bleb formation, exposure of phosphatidylserine, and procoagulant activity of adherent platelets. Blood. 1997;90:2615–2625. [PubMed] [Google Scholar]
- Heemskerk JWM, Willems GM, Rook MB, Sage SO. Ragged spiking of free calcium in ADP-stimulated human platelets: regulation of puff-like calcium signals in vitro and ex vivo. J Physiol. 2001;535:625–635. doi: 10.1111/j.1469-7793.2001.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga EG, Tsuji S, Romijn RAP, Schiphorst ME, de Groot PG, Sixma JJ, Gros P. Structures of glycoprotein Iba and its complex with von Willebrand factor A1 domain. Science. 2002;297:1176–1179. doi: 10.1126/science.107355. [DOI] [PubMed] [Google Scholar]
- Inoue O, Suzuki-Inoue K, Dean WL, Frampton J, Watson SP. Integrin α2β1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCγ2. J Cell Biol. 2003;160:769–780. doi: 10.1083/jcb.200208043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutsi-Korhonen L, Smethurst PA, Rankin A, Gray E, IJsseldijk M, Onley CM, Watkins NA, Williamson LM, Goodall AH, de Groot PG, Farndale RW, Ouwehand WH. The low-frequency allele of the platelet collagen signaling receptor glycoprotein VI is associated with reduced functional responses and expression. Blood. 2003;101:4372–4379. doi: 10.1182/blood-2002-08-2591. [DOI] [PubMed] [Google Scholar]
- Jung SM, Moroi M. Activation of the platelet collagen receptor integrin α2β1. Trends Cardiovasc Med. 2000;10:285–292. doi: 10.1016/s1050-1738(01)00064-0. [DOI] [PubMed] [Google Scholar]
- Jung SM, Moroi M. Platelet collagen receptor integrin α2β1 activation involves differential participation of ADP-receptor subtypes P2Y1 and P2Y12 but not intracellular calcium change. Eur J Biochem. 2001;268:3513–3522. doi: 10.1046/j.1432-1327.2001.02252.x. [DOI] [PubMed] [Google Scholar]
- Jurk K, Clemetson KJ, de Groot PG, Brodde MF, Steiner M, Savion N, Varon D, Sixma JJ, van Aken H, Kehrel BE. Thrombospondin-1 mediates platelet adhesion at high shear via glycoprotein Ib (GPIb): an alternative/backup mechanism to von Willebrand factor. FASEB J. 2003;17:1490–1492. doi: 10.1096/fj.02-0830fje. [DOI] [PubMed] [Google Scholar]
- Kato K, Kanaji T, Russell S, Kunicki TJ, Furihata K, Kanaji S, Marchese P, Reininger A, Ruggeri ZM, Ware J. The contribution of glycoprotein VI to stable platelet adhesion and thrombus formation illustrated by targeted gene deletion. Blood. 2003;102:1701–1707. doi: 10.1182/blood-2003-03-0717. [DOI] [PubMed] [Google Scholar]
- Keuren JF, Ulrichts H, Feijge MAH, Hamulyak K, Deckmyn H, Lindhout T, Heemskerk JWM. Integrin αIIbβ3 and shear-dependent action of glycoprotein Ibα stimulate platelet-dependent thrombin formation in stirred plasma. J Laboratory Clin Med. 2003;141:350–358. doi: 10.1016/S0022-2143(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Kuijpers MJE, Schulte V, Bergmeier W, Lindhout T, Brakebusch C, Offermanns S, Fässler R, Heemskerk JWM, Nieswandt B. Complementary roles of glycoprotein VI and α1β2 integrin in collagen-induced thrombus formation in flowing whole blood ex vivo. FASEB J. 2003;17:685–687. doi: 10.1096/fj.02-0381fje. [DOI] [PubMed] [Google Scholar]
- Mangin P, Yuan Y, Goncalves I, Eckly A, Freund M, Cazenave JP, Gachet C, Jackson SP, Lanza F. Signaling role for phospholipase Cγ2 in platelet glycoprotein Ibα calcium flux and cytoskeletal reorganization. J Biol Chem. 2003;278:32880–32891. doi: 10.1074/jbc.M302333200. [DOI] [PubMed] [Google Scholar]
- Massberg S, Gawaz M, Grüner S, Schulte V, Konrad I, Zohlnhofer D, Heinzmann U, Nieswandt B. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J Exp Med. 2003;197:41–49. doi: 10.1084/jem.20020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucato M, Pradella P, Cozzi MR, De Marco L, Ruggeri ZM. Sequential cytoplasmic calcium signals in a 2-stage platelet activation process induced by the glycoprotein Ibα mechanoreceptor. Blood. 2002;100:2793–2800. doi: 10.1182/blood-2002-02-0514. [DOI] [PubMed] [Google Scholar]
- Nesbitt WS, Kulkarni S, Giuliano S, Goncalves I, Dopheide SM, Yap CL, Harper IS, Salem HH, Jackson SP. Distinct glycoprotein Ib/V/IX and integrin αIIbβ3-dependent calcium signals cooperatively regulate platelet adhesion under flow. J Biol Chem. 2002;277:2965–2972. doi: 10.1074/jbc.M110070200. [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Bergmeier W, Schulte V, Rackebrandt K, Gessner JE, Zirngibl H. Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRγ chain. J Biol Chem. 2000;275:23998–234002. doi: 10.1074/jbc.M003803200. [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Brakebusch C, Bergmeier W, Schulte V, Bouvard D, Mokhtari-Nejad R, Lindhout T, Heemskerk JWM, Zirngibl H, Fässler R. Glycoprotein VI but not α1β2 integrin is essential for platelet interaction with collagen. EMBO J. 2001a;20:2120–2130. doi: 10.1093/emboj/20.9.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieswandt B, Schulte V, Bergmeier W, Mokhtari-Nejad R, Rackebrandt K, Cazenave JP, Ohlmann P, Gachet C, Zirngibl H. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J Exp Med. 2001b;193:459–469. doi: 10.1084/jem.193.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieswandt B, Watson SP. Platelet collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- Novak L, Deckmyn H, Damjanovich S, Harsfalvi J. Shear-dependent morphology of von Willebrand factor bound to immobilized collagen. Blood. 2002;99:2070–2076. doi: 10.1182/blood.v99.6.2070. [DOI] [PubMed] [Google Scholar]
- Patel D, Vaananen H, Jirouskova M, Hoffmann T, Bodian C, Coller BS. Dynamics of GPIIb/IIIa-mediated platelet–platelet interactions in platelet adhesion/thrombus formation on collagen in vitro as revealed by videomicroscopy. Blood. 2003;101:929–936. doi: 10.1182/blood.V101.3.929. [DOI] [PubMed] [Google Scholar]
- Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate–receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–666. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Savage B, Sixma JJ, Ruggeri ZM. Functional self association of von Willebrand factor during platelet adhesion under flow. Proc Natl Acad Sci U S A. 2002;99:425–430. doi: 10.1073/pnas.012459599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, Ginsberg MH. Integrin signaling in vascular biology. J Clin Invest. 1997;100:1–5. [PubMed] [Google Scholar]
- Siljander P, Farndale RW, Feijge MAH, Comfurius P, Kos S, Bevers EM, Heemskerk JWM. Platelet adhesion enhances the glycoprotein VI-dependent procoagulant response: Involvement of p38 MAP kinase and calpain. Arterioscler Thromb Vasc Biol. 2001;21:618–627. doi: 10.1161/01.atv.21.4.618. [DOI] [PubMed] [Google Scholar]
- Siljander PR, Munnix ICA, Smethurst PA, Deckmyn H, Lindhout T, Ouwehand WH, Farndale RW, Heemskerk JWM. Platelet receptor interplay regulates collagen-induced thrombus formation in flowing human blood. Blood. 2004;203:1333–1341. doi: 10.1182/blood-2003-03-0889. [DOI] [PubMed] [Google Scholar]
- Snell DC, Schulte V, Jarvis GE, Arase K, Sakurai D, Saito T, Watson SP, Nieswandt B. Differential effects of reduced glycoprotein VI levels on activation of murine platelets by glycoprotein VI ligands. Biochem J. 2002;368:293–300. doi: 10.1042/BJ20020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcRγ chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Ezumi Y, Arai M, Takayama H. A novel association of Fc receptor γ-chain with glycoprotein VI and their co-expression as a collagen receptor in human platelets. J Biol Chem. 1997;272:23528–23531. doi: 10.1074/jbc.272.38.23528. [DOI] [PubMed] [Google Scholar]
- Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- Watson SP, Asazuma N, Atkinson B, Berlanga O, Best D, Bobe R, Jarvis G, Marshall S, Snell D, Stafford M, Tulasne D, Wilde J, Wonerow P, Frampton J. The role of ITAM- and ITIM-coupled receptors in platelet activation by collagen. Thromb Haemost. 2001;86:276–288. [PubMed] [Google Scholar]
- Wu YP, Vink T, Schiphorst M, van Zanten GH, IJsseldijk MJW, de Groot PG, Sixma JJ. Platelet thrombus formation on collagen at high shear rates is mediated by von Willebrand factor–glycoprotein Ib interaction and inhibited by von Willebrand factor-glycoprotein IIb/IIIa interaction. Arterioscler Thromb Vasc Biol. 2000;20:1661–1667. doi: 10.1161/01.atv.20.6.1661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2004.062414
http://jp.physoc.org/cgi/content/full/jphysiol.2004.062414v1/DC1 and contains supplementary material consisting of two movies.
This material can also be found at:
http://blackwellpublishing.com/products/journals/suppmat/tjp/tjp341/tjp341sm.htm