Abstract
The M1 and M3 subtypes are the major muscarinic acetylcholine receptors in the salivary gland and M3 is reported to be more abundant. However, despite initial reports of salivation abnormalities in M3-knockout (M3KO) mice, it is still unclear which subtype is functionally relevant in physiological salivation. In the present study, salivary secretory function was examined using mice lacking specific subtype(s) of muscarinic receptor. The carbachol-induced [Ca2+]i increase was markedly impaired in submandibular gland cells from M3KO mice and completely absent in those from M1/M3KO mice. This demonstrates that M3 and M1 play major and minor roles, respectively, in the cholinergically induced [Ca2+]i increase. Two-dimensional Ca2+-imaging analysis revealed the patchy distribution of M1 in submandibular gland acini, in contrast to the ubiquitous distribution of M3. In vivo administration of a high dose of pilocarpine (10 mg kg−1, s.c.) to M3KO mice caused salivation comparable to that in wild-type mice, while no salivation was induced in M1/M3KO mice, indicating that salivation in M3KO mice is caused by an M1-mediated [Ca2+]i increase. In contrast, a lower dose of pilocarpine (1 mg kg−1, s.c.) failed to induce salivation in M3KO mice, but induced abundant salivation in wild-type mice, indicating that M3-mediated salivation has a lower threshold than M1-mediated salivation. In addition, M3KO mice, but not M1KO mice, had difficulty in eating dry food, as shown by frequent drinking during feeding, suggesting that salivation during eating is mediated by M3 and that M1 plays no practical role in it. These results show that the M3 subtype is essential for parasympathetic control of salivation and a reasonable target for the drug treatment and gene therapy of xerostomia, including Sjögren's syndrome.
Muscarinic acetylcholine receptors (mAChR) consist of five genetically distinct subtypes, M1–M5, and are thought to play important roles in the regulation of many fundamental functions in the central and peripheral nervous systems. The importance of the different subtypes in specific functions has been difficult to determine because of the lack of muscarinic ligands that are specific for each subtype and the mixed expression of multiple subtypes in tissues (for reviews, see Caulfield & Birdsall, 1998; Wess, 2003). However, the recent availability of mutant mice deficient in specific mAChR subtypes has made this possible. For example, Matsui et al. (2000) demonstrated that M3 receptors play key roles in peripheral autonomic organs, as evidenced by multiple abnormalities in M3KO mice, including growth retardation starting during weaning. Subsequently, Yamada et al. (2001) suggested that this phenotype was due to appetite loss resulting from dysfunction of the hypothalamic hunger centre.
Salivary secretion is under the control of autonomic nervous activity, the rate of fluid output being regulated by parasympathetic activity mediated by mAChRs in the acinar cells, the salivary gland cells responsible for saliva secretion (Baum, 1993; Cook et al. 1994). In terms of mAChR subtype, both the M1 and M3 subtypes have been reported to be present in salivary glands (e.g. Maeda et al. 1988; Yamamoto et al. 1996; for reviews, see Baum, 1993; Caulfield, 1993; Levey, 1993), and some reports have demonstrated predominant or exclusive expression of the M3 subtype in the major salivary glands (Dehaye et al. 1988; Dai et al. 1991; Watson et al. 1996; Moriya et al. 1999; Bockman et al. 2001). However, few physiological studies have been carried out to determine the functional relevance of specific mAChR subtypes in physiological salivation. In addition, there is a discrepancy regarding salivation data between the two above-mentioned reports using M3KO mice, with Matsui et al. (2000) reporting severe hyposalivation, which presumably led to growth failure due to eating difficulties, and Yamada et al. (2001) claiming that salivation was almost intact and questioning the significance of the M3 subtype. Thus, it remains controversial whether the M3 receptors play the major role in salivation. Moreover, in previous studies on salivation in M3KO mice, including another demonstrating impaired salivation in M3KO mice (Bymaster et al. 2003), cholinergic sialogogues were used to increase salivary secretion to detectable levels; however, this does not necessarily reflect the physiological role of mAChRs, as it is not clear what dose of the drugs represents physiological parasympathetic stimulation. In summary, there is no general agreement regarding which mAChR subtypes contribute to cholinergic control of salivation and to what extent, especially under physiological conditions.
In order to determine the functional relevance of mAChR subtypes in salivary secretion, we used enzymatically dispersed submandibular gland (SMG) cells from specific mAChR KO mice to examine the cholinergically induced intracellular Ca2+ concentration ([Ca2+]i) increase which is mediated by inositol-1,4,5-trisphosphate (IP3) production and activation of IP3 receptors. This [Ca2+]i increase in acinar cells is part of the fluid secretion mechanism (Baum & Wellner, 1999; Gallacher & Smith, 1999) and results in the activation of apical Cl− and basolateral K+ channels, transepithelial Na+ transportation to the lumen, the movement of water through the apical water channel, aquaporin-5 (Raina et al. 1995; Nielsen et al. 1997; Funaki et al. 1998), and the creation of the isotonic primary saliva in the apical lumen (Ma et al. 1999; Krane et al. 2001). In assessing cholinergic activation of salivary gland cells, the measurement of [Ca2+]i signalling in enzymatically dispersed SMG cells is more precise than the measurement of salivary secretion stimulated by sialogogues in vivo and has other advantages in that (1) the response represents a direct action of stimulants on salivary gland cells, (2) a wide range of concentration of stimulants can be tested because of the higher sensitivity of the detection method for low drug concentrations and the capacity of the in vitro preparation to permit application of high drug concentrations without the side-effects associated with systemic administration, (3) various drugs can be applied in combination, while controlling the timing of application and the concentrations of these drugs, and (4) the response is not affected by the general condition of the mice, such as changes in respiration, body temperature, depth of anaesthesia, etc. In the present study, [Ca2+]i changes in response to cholinergic stimulation were compared in mice lacking the M1, M3, or M5 subtypes, each of which can potentially couple to the IP3-mediated Ca2+ signalling pathway. Furthermore, in order to assess salivation induced by physiological parasympathetic stimuli, we analysed the eating behaviour of M3KO mice, since it is known that rodents with insufficient salivation show increased prandial drinking (Epstein et al. 1964). Using these physiological approaches, we conclusively demonstrate that the M3 subtype plays a central role in the control of salivary fluid secretion by the parasympathetic nervous system under physiological conditions.
Methods
All experiments were carried out in accordance with the guidelines approved by the animal welfare committees of The Institute of Medical Science at The University of Tokyo.
M1KO, M3KO and M1/M3KO mice
The generation and characterization of these mice have been previously described (Matsui et al. 2000; Ohno-Shosaku et al. 2003).
Generation of M5KO mice
A targeting vector, pChrm5-N2 (Fig. 1A), was constructed from 129/SvJ-derived genomic fragments of Chrm5 (Matsui et al. 1999). The BamHI-SspI (1.1 kb) and BamHI-EcoRI (8.2 kb) fragments were placed upstream and downstream of a PGK-neo-bpA cassette (Soriano et al. 1991), respectively. A PGK-DTA cassette (Yagi et al. 1990) was inserted at the upstream end in the reverse orientation.
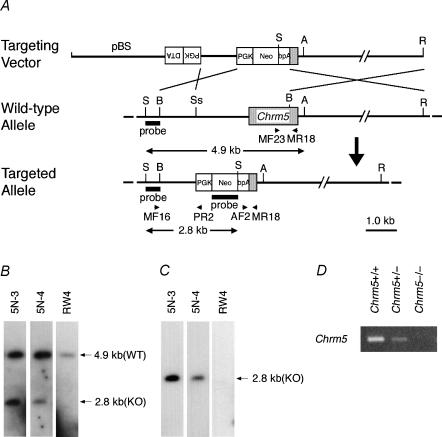
Figure 1. Generation of Chrm5-deficient mice.
A, targeting strategy by homologous recombination in ES cells. The targeting vector pChrm5-N2 contained the neo gene and the diphtheria toxin α-subunit gene (DTA) driven by the phosphoglycerate kinase I promoter (PGK). The arrowheads marked MF16 and PR2 indicate the PCR primers used for homologous recombinant screening, and those marked MF23, AF2 and MR18 indicate the primers used for genotyping. ApaI (A), BamHI (B), HindIII, EcoRI (R), SacI (S), and SspI (Ss) sites relevant to the identification of homologous recombinant ES cell clones are shown, as well as the expected size of the bands hybridizing with the Chrm5 and neo probes (indicated by 4.9 and 2.8 kb bands and arrows). B and C, confirmation of homologous recombination in ES cell clones by Southern hybridization. B, hybridization with the Chrm5 probe showing the 2.8 kb band specific for the targeted allele and the 4.9 kb band derived from the wild-type allele. 5N-3 and 5N-4 are representative homologous recombinant clones, whereas RW4 is a parental ES cell line. C, hybridization with the neo probe showing the 2.8 kb band specific for the targeted allele. D, RT-PCR analysis showing Chrm5 mRNA levels in the brains of the wild-type and heterozygous and homozygous mutant mice. The levels were lower in the heterozygous brain than in the wild-type brain. No PCR product was seen with the homozygous mutant.
Embryonic stem (ES) cells (2.0 × 107 cells; RW4 clone; Genome Systems, St Louis, MO, USA) were electroporated with the targeting vector linearized at the unique SalI site. G418-resistant homologous recombinant candidates were screened by 43 cycles of PCR (each 30 s at 94°C, 30 s at 55°C, and 60 s at 72°C), after activation of AmpliTaq Gold polymerase (Perkin-Elmer Biosystems, Foster City, CA, USA) by heating for 10 min at 95°C, using primers MF16 (5′-GCT GTT GAC CAG GTT TTC TG-3′) and PR2 (5′-TAA AGC GCA TGC TCC AGA CT-3′) to amplify the 1.1 kb fragment of the recombinant DNA. The PCR mixture consisted of both primers (each 0.2 μm), 200 μm dNTPs, 1.5 mm MgCl2, and 0.5 U of AmpliTaq Gold polymerase in PCR Buffer II (Perkin Elmer Biosystems) in a final volume of 20 μl. Homologous recombination of candidate clones was verified by Southern hybridization (Fig. 1B and C). To generate chimeric mice, the homologous recombinant ES cells were injected into C57BL/6 blastocysts, which were transferred into pseudopregnant MCH females (CLEA, Tokyo, Japan). The lights in the animal room were turned on between 7.00 am and 7.00 pm. The mice were fed standard dry pellets (CA-1; CLEA, Tokyo, Japan) and water ad libitum. The mice used to generate M5KO mice were backcrossed to the C57BL/6 J strain (CLEA, Tokyo, Japan) for 9–11 generations.
The genotype of the mice was determined by PCR using the primers MR18 (5′-GGG GTT GAT GGT GCT GTT GA-3′), MF23 (5′-CCC TCG GAC TGA AAA CAA TG-3′), and AF2 (5′-GGG AAG ACA ATA GCA GGC AT-3′). The PCR conditions were the same as those used for M3 genotyping (Matsui et al. 2000). To measure M5 mRNA levels in the mutant mice, total RNA was extracted from the whole brain tissue of 3-month-old male mice using an RNA isolation kit (ISOGEN, NipponGene, Toyama, Japan) according to the manufacturer's instruction. Complementary DNA was synthesized from 5 μg of the total RNA in a 33 μl reaction volume using a First Strand cDNA synthesis kit (Amersham Pharmacia Biotech). One microlitre of the cDNA synthesis solution was used as the template for PCR. The primers used in the PCR were MF47 (5′-GGC TGA CCT CCA AGG TTC CG-3′) and MR45 (5′-GAG TCT GTG AGC AGC ACC TG-3′). The PCR conditions were the same as those used in the genotyping PCR, except that the cycle number was 30. The PCR products were electrophoresed in a 1.5% agarose gel and visualized by ethidium bromide staining. As shown in Fig. 1D, M5 mRNA levels were reduced in Chrm5+/− tissue and undetectable in Chrm5−/− tissue. The M1/M5 double KO mice were generated by the successive crossings of the M1 and M5 mutants. Their genetic background was equivalent to that obtained between N7 and N8 backcross generations to C57BL/6 J.
SMG cell preparation
Three- to four-month-old female mice were used for the experiments. Following deep anaesthesia with Nembutal (60–70 mg kg−1, i.p.) and kill by heart puncture, the bilateral SMGs from one mouse were immediately removed, placed in cold balanced salt solution (BSS) containing (mm): 115 NaCl, 5.4 KCl, 2 Ca2+, 1 Mg2+, 20 Hepes, 10 glucose (pH 7.4), supplemented with 1.25% bovine serum albumin (BSS-BSA), and rapidly minced. The material was then digested for 20 min at 37°C with 2 mg ml−1 of collagenase type-2 (Worthington, Malvern, PA, USA) in BSS-BSA, the suspension being gently passed through a pipette 20 times every 10 min. After digestion, the preparation was centrifuged at 70 g for 1 min, and the pellet was resuspended in 10 ml of BSS-BSA, rinsed twice, and filtered through a 100 μm nylon mesh, to generate a batch of SMG cells.
Measurement of the intracellular Ca2+ concentration
The isolated SMG cell preparation was loaded with fura-2 by incubation for 45 min at room temperature with 3 μm fura-2 AM (Dojindo, Kumamoto, Japan) suspended in BSS-BSA, rinsed twice, resuspended in 4 ml of BSS, and stored at 4°C. Ratiometric measurement of fura-2 fluorescence was made using a spectrofluorometer (CAF-110, Jasco, Tokyo, Japan). A 500 μl sample of fura-2-loaded SMG cells was transferred to a glass cuvette and alternately illuminated with 340 and 380 nm excitation light, the resultant fluorescence (510 ± 10 nm) being collected at 25 Hz. Drugs (0.5–1.5 μl) were added directly to the cell suspension during fluorescence recordings. At the end of each experiment, the maximum fluorescence ratio (Rmax) was determined by adding 0.2% Triton X-100 to the cuvette, then the minimum fluorescence ratio (Rmin) was determined by adding 10 mm EGTA; these values then used to calculate the [Ca2+]i using Grynkiewicz's equation (Grynkiewicz et al. 1985). The fluorescence intensities excited by 340 and 380 nm wavelength light (F340 and F380, respectively) and the ratio (F340/F380) were digitized with 12-bit resolution and stored and displayed in a personal computer using the MacLab4/s system (ADInstruments-Japan, Tokyo, Japan).
For two-dimensional measurement of [Ca2+]i changes, a small aliquot (20–50 μl) of fura-2-loaded SMG cell suspension was dispersed on the Cell-Tak (BD Biosciences, Bedford, MA, USA)-coated glass that formed the bottom of the recording chamber, mounted on the stage of an inverted fluorescence microscope (IX70, Olympus, Tokyo, Japan) and perfused with BSS at a rate of 2 ml min−1 at room temperature. Excitation of fura-2 was made every 5 s by an alternate illumination of 340 and 380 nm light, and the resultant fluorescence (510–550 nm; F340 and F380) was collected using an objective lens (UPlanApo 20×/340, Olympus) and silicon-intensified target camera (Hamamatsu Photonics, Hamamatsu, Japan), processed to obtain pseudo-coloured images of F340/F380 and stored in a personal computer using the software ARGUS50/CA (Hamamatsu Photonics).
Measurement of saliva secretion
Under anaesthesia with Nembutal (40 mg kg−1, i.p.), female mice, fasted for 5–7 h, were injected subcutaneously (s.c.) with pilocarpine-HCl (1 or 10 mg kg−1) on a 37°C plate. The saliva secreted into the oral cavity during each 5 min period after injection was carefully collected using a manual pipetter and transferred to a pre-weighed centrifuge tube and the net weight of secreted saliva calculated by subtracting the weight of the empty tube from the weight of the tube containing saliva.
Assessment of prandial drinking
Mice were fasted overnight with free access to water. In the morning, each mouse was fed with about 3 g of dry pelleted food (CA-1, CLEA, Japan) or paste food, prepared by mixing the powder form of CA-1 with 1.5 × its weight of water, and eating behaviour was recorded for 2 h using a video camera. The number of approaches to the water nozzle preceded by eating was counted. Changes in the body weight of the mouse, the food pellet, and the water bottle were recorded.
Results
Carbachol-induced [Ca2+]i changes in SMG cell suspension
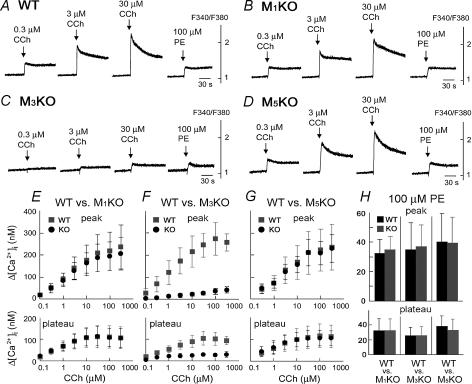
In order to examine Ca2+ signalling induced by cholinergic stimulation in SMG cells, the [Ca2+]i in enzymatically dispersed SMG cells from WT, M1KO, M3KO, and M5KO mice was measured ratiometrically using fura-2. Figure 2A–D shows typical [Ca2+]i increases in SMG cells from each genotype in response to stimulation with carbachol (CCh), a non-selective cholinergic agonist, at 0.3, 3 and 30 μm, while Fig. 2E–G shows the summarized results over a wider range. In all genotypes, the SMG cells showed a concentration-dependent CCh-induced [Ca2+]i increase; however, the response in M3KO mice was greatly reduced, the maximal response at 300 μm CCh being equivalent to those induced in the other genotypes by concentrations of 0.1–0.3 μm. The [Ca2+]i increases in WT, M1KO, and M5KO mice were indistinguishable. These results show that no compensation by other subtypes, such as M1 and M5, was seen in the M3 mutants in terms of the CCh-induced [Ca2+]i response. In contrast, phenylephrine, an α1-adrenergic agonist, induced similar [Ca2+]i responses in all four genotypes (Fig. 2A–D), indicating that the signal transduction pathway activated by α1-adrenergic stimulation that leads to salivation in vivo was intact in M3KO SMG cells. Figure 2E–H summarizes the results for the CCh- and phenylephrine-induced [Ca2+]i increases at the peak and plateau (2 min after drug application) in all SMG cells (n = 4 for each genotype) and demonstrates that Ca2+ signalling in response to CCh in M3KO mice was severely impaired. The plateau level of the CCh-induced [Ca2+]i increase was also reduced in M3KO mice; however, this fact does not necessarily mean that Ca2+ channels mediating the Ca2+ influx component in CCh-induced [Ca2+]i increases in SMG cells were defective in M3KO mice, as this component is mediated by the store-operated Ca2+ channels, which are shown to be intact in M3KO mice in Fig. 4, and/or the receptor-operated Ca2+ channels, the activation of which is dependent on the extent of receptor activation in the plasma membrane (i.e. mAChR activation in this preparation).
Figure 2. Carbachol (CCh)- and phenylephrine (PE)-induced [Ca2+]i changes in SMG cells from wild-type and KO mice.
A–D, responses in individual representative mice. CCh or PE was applied to the SMG cells at the time point indicated by the arrow. The results shown are representative of 4 experiments. E–H, summarized peak [Ca2+]i (upper panel) and plateau [Ca2+]i (lower panel) increases induced by CCh (E–G; grey square: WT, black circle: KO) or PE (H; black column: WT, grey column: KO) in M1-, M3-, or M5-deficient mice (mean ± s.d.; n = 4 for each genotype). The [Ca2+]i was calculated using the equation described by Grynkiewicz et al. (1985). Control responses were measured in WT controls for each genotype.
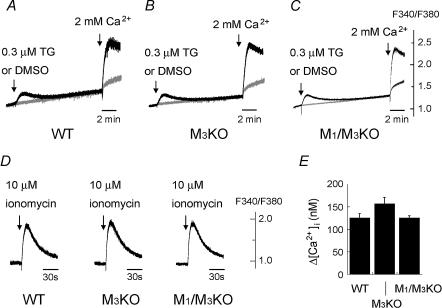
Figure 4. Effect of thapsigargin (TG) and induction of capacitative Ca2+ entry (A–C), and Ca2+ release from the internal Ca2+ store by ionomycin (D and E) in WT, M3KO and M1/M3 double KO SMG cells.
Drugs were applied to the SMG cell suspension at the time point indicated by the arrow. A–C, [Ca2+]i change in the nominal absence of external Ca2+ in response to 0.3 μm thapsigargin (black trace) or control dimethylsulfoxide (DMSO; grey trace) and that caused by addition of 2 mm Ca2+. Note the large [Ca2+]i increase induced in all of the WT (A), M3KO (B) and M1/M3 double KO (C) SMG cells by adding external Ca2+ following TG application (capacitative Ca2+ entry). D, [Ca2+]i change in the absence of external Ca2+ in response to 10 μm ionomycin in the WT, M3KO and M1/M3 double KO SMG cells. 1 mm EGTA was added to the external solution. E, summarized peak [Ca2+]i increases induced by 10 μm ionomycin in WT, M3KO, or M1/M3 double KO SMG cells (mean ± s.e.m.; n = 4 for each genotype). All the results shown are representative of 4 experiments.
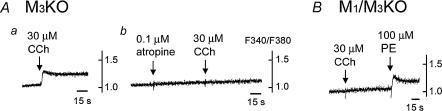
We then examined the mechanism involved in the small CCh-induced [Ca2+]i increase seen in M3KO SMG cells. This increase was blocked by 0.1 μm atropine (n = 4), a muscarinic antagonist (Fig. 3Aa and b), showing that it was attributable to mAChR activation. As shown in Fig. 3B, in M1/M3 double KO mice, no CCh-induced [Ca2+ ]i increase was seen, whereas the phenylephrine-induced [Ca2+]i increase was comparable to that in the other genotypes (n = 4, compare with Fig. 2), indicating that the small CCh-induced [Ca2+]i increase seen in M3KO SMG cells was mediated by M1.
Figure 3. CCh-induced [Ca2+]i increase in M3KO mice, its block by a muscarinic antagonist, atropine, and absence of the CCh-induced [Ca2+]i increase in M1/M3 double KO mice.
A, SMG cells from M3KO mice; a, effect of CCh on the [Ca2+]I; b, block by atropine. The results shown in a and b were obtained using the same batch of SMG cells. B, effect of CCh or PE on the [Ca2+]i in SMG cells from M1/M3 double KO mice. Note the absence of CCh-induced [Ca2+]i increase. All the results shown are representative of 4 experiments.
To exclude the possibility that this reduction in Ca2+ signalling resulted from dysfunction of the internal Ca2+ stores (empty stores or non-functional sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) or capacitative Ca2+ entry (CCE)), we then examined whether the internal Ca2+ stores were functional in M3KO and M1/M3 double KO SMG cells. It has been known that SMG cells use the CCE pathway to refill depleted internal Ca2+ stores (Watson et al. 1999; Liu & Ambudkar, 2001). In WT SMG cells (n = 4), when 0.3 μm thapsigargin (TG), a SERCA inhibitor, was applied in the nominal absence of external Ca2+, a small [Ca2+]i increase was induced (Fig. 4A), suggesting that the internal Ca2+ store was loaded, since this TG-induced response is thought to reflect leakage of Ca2+ from the store revealed when Ca2+ uptake into the store by SERCA is inhibited by TG (Smith & Reed, 1996; Mogami et al. 1998; Lomax et al. 2002). Furthermore, when the external Ca2+ concentration was increased to 2 mm 10 min after TG addition, a large [Ca2+]i increase, i.e. CCE, was seen (Fig. 4A). The TG-induced [Ca2+]i increase and CCE in M3KO and M1/M3 double KO SMG cells (Fig. 4B and C) were indistinguishable from those in WT SMG cells, indicating that SERCA and CCE, both of which are involved in filling the internal Ca2+ store, were functional in M3KO and M1/M3 double KO SMG cells. The filling state of the internal Ca2+ store was next assessed by applying ionomycin, a Ca2+ ionophore, in the absence of external Ca2+ (Smith & Reed, 1996). The ionomycin-induced [Ca2+]i increase in M3KO or M1/M3 double KO SMG cells was indistinguishable from that in WT SMG cells (Fig. 4D and E), indicating that the internal Ca2+ store was not emptied in M3KO and M1/M3 double KO SMG cells and, thus, that the impaired CCh-induced [Ca2+]i increase in those SMG cells was not attributable to a depleted Ca2+ store. Altogether, these results clearly demonstrate that, in WT mice, M3 is the major receptor subtype responsible for parasympathetic activation of Ca2+ signalling, with the M1 subtype making a smaller contribution.
Ca2+ imaging in individual acinar cell clusters
SMG cell suspensions contain nonacinar cells, e.g. ductal cells, in addition to acinar cells (Xu et al. 1996), and the population of ductal cell clusters in our SMG cell suspension was approximately 10%. Therefore, it could be argued that CCh-induced [Ca2+]i increases in SMG cell suspensions, especially the small, M1-mediated responses seen in M3KO SMG cell suspension, might represent those in nonacinar cells rather than those in acinar cells. In addition, we questioned, with regard to the M1-mediated small responses, whether M1 receptors are expressed in most of the acinar cells at low levels, or whether they are expressed in a small population of the acinar cells at high levels. To address these issues, we carried out two-dimensional analysis of [Ca2+]i changes in individual acinar cell clusters using fluorescent videomicroscopy technique, in which ductal cells could be excluded from the measurement based on their longitudinally elongated morphology.
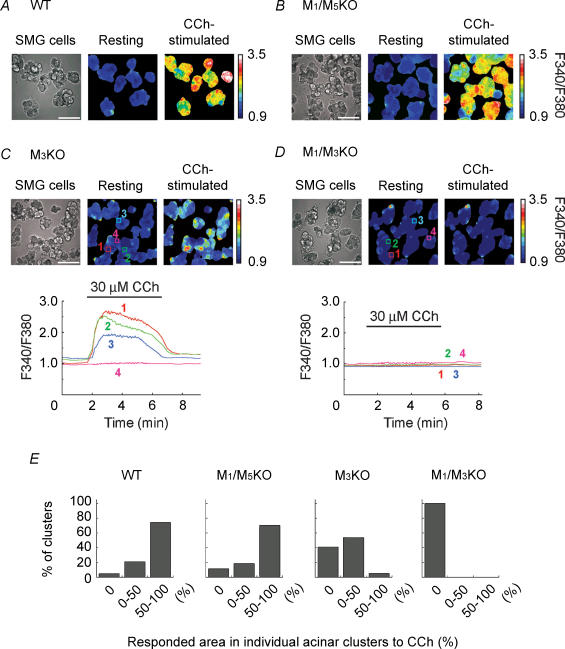
Figure 5 shows the [Ca2+]i changes in response to 30 μm CCh in individual acinar cell clusters prepared from WT (Fig. 5A), M1/M5KO (Fig. 5B), M3KO (Fig. 5C) and M1/M3KO (Fig. 5D) mice. In WT (Fig. 5A) and M1/M5KO (Fig. 5B) SMG acinar cell clusters, almost the whole region of each acinar cluster strongly responded to CCh, while no [Ca2+]i increase was seen in all the acinar cell clusters from M1/M3KO (Fig. 5D) SMG, indicating that M1 and M3 mediated the CCh-induced [Ca2+]i increase in SMG acinar cells. Interestingly, in acinar clusters from M3KO mice (Fig. 5C), part of the clusters responded to CCh, with some of them showing a large [Ca2+]i increase comparable to WT and M1/M5KO acinar cell clusters. In order to quantify these findings, we categorized acinar clusters into three groups: (1) acinar clusters without any response (responding area, 0%) (2) clusters which partly responded (responding area, 0–50%), and (3) clusters in which almost the whole region responded (responding area, 50–100%), and the results were summarized in Fig. 5E. In approximately 50% of M3KO acinar clusters, only part of the individual clusters showed a CCh-induced [Ca2+]i increase. These imaging results demonstrate that some M3KO acinar cells responded to CCh via M1 activation, strongly suggesting that distribution of M1 in SMG acini is not ubiquitous and that some acinar cells express M1 at a high level.
Figure 5. Ca2+ imaging in individual SMG acinar cell clusters and effect of CCh.
A–D, image of SMG acinar cell clusters and pseudo-colour images of F340/F380 under resting and CCh-stimulated (30 μm) conditions are shown (A, WT; B, M1/M5 double KO; C, M3KO; D, M1/M3 double KO SMG). Scale bars indicate 100 μm. Lower panels in C and D show the [Ca2+]i changes at regions 1–4 depicted in the upper panels. The results shown in A–D represent at least 8 separate measurements for each genotype. E, summarized responses of individual acinar cell clusters induced by 30 μm CCh in WT, M1/M5 double KO, M3KO, or M1/M3 double KO SMG acinar cell clusters. Proportion of clusters categorized into three groups based on the responding area (0, 0–50 and 50–100%) as a precentage of the total number of clusters for each genotype was shown.
Pilocarpine-induced salivation
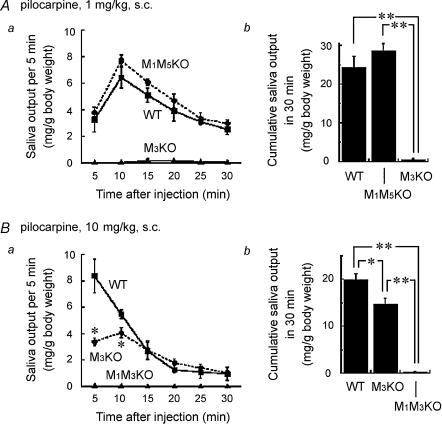
The impairment of cholinergically stimulated Ca2+ signalling in M3KO SMG cells strongly suggested that M3KO mice would exhibit hyposalivation. Matsui et al. (2000) showed that M3KO mice did not produce detectable amounts of saliva following s.c. injection of 1 mg kg−1 of pilocarpine, while Yamada et al. (2001) subsequently showed that M3KO mice do produce significant amounts of saliva when injected with the higher dose of 15 mg kg−1 of pilocarpine. In the present study, we modified our experimental protocol to allow stronger cholinergic stimulation. To prevent death from respiratory distress and to protect mice from hypothermia resulting from anaesthesia and central muscarinic action, the concentration of the anaesthetic used was reduced from 50 to 40 mg kg−1 and the body temperature was controlled during saliva collection. When 1 mg kg−1 of pilocarpine was injected s.c. into anaesthetized WT, M1/M5KO and M3KO mice, copious salivation was induced in WT and M1/M5KO mice, but only slight salivation was detected in M3KO mice. Figure 6A shows the saliva output in each 5 min period (a) and the cumulative amount secreted in 30 min (b) following pilocarpine injection. The cumulative amounts of saliva secreted in 30 min were quite different between WT or M1/M5KO and M3KO mice, being, respectively (average ± s.e.m.): 24.4 ± 2.8 mg (g body weight)−1 (n = 5), 28.6 ± 1.8 mg (g body weight)−1 (n = 4) and 0.57 ± 0.26 mg (g body weight)−1 (n = 5; **P < 0.01, compared with WT or M1/M5KO). There was no statistically significant difference in salivation between WT or M1/M5KO mice. Statistical significance was determined using Scheffe's multiple comparisons following ANOVA. The results mean that the presence of M3 is essential and sufficient for induction of salivation by 1 mg kg−1 of pilocarpine. When the dose was increased to 10 mg kg−1, pilocarpine induced considerable salivation in M3KO mice, although the amount of saliva secreted was smaller than in WT mice, the cumulative amounts being for WT and M3KO mice, respectively: 19.9 ± 1.3 mg (g body weight)−1 (n = 5) and 14.7 ± 1.3 mg (g body weight)−1 (n = 5; Fig. 6Bb; *P < 0.05, compared with WT). The rate of saliva secretion was significantly lower in M3KO mice than in WT mice during the early period (5–10 min) after pilocarpine administration (Fig. 6Ba; *P < 0.05, Scheffe's test comparing M3KO mice to WT mice). In contrast, in M1/M3KO mice, this high dose of pilocarpine induced almost no salivation, the cumulative amount of saliva secreted in 30 min being 0.2 ± 0.1 mg (g body weight)−1 (Fig. 6Bb; n = 4; **P < 0.01, Scheffe's multiple comparisons), indicating that the salivation induced by injection of 10 mg kg−1 of pilocarpine in M3KO mice was mediated by the M1 subtype. These results show that both the M1 and M3 receptors have the potential to induce salivation and that the threshold for salivation mediated by the M3 subtype is lower than that mediated by the M1 subtype.
Figure 6. Cholinergically stimulated salivation in WT, M1/M5KO, M3KO and M1/M3KO mice.
The saliva output in each 5 min period after stimulation is represented by the symbols and lines in the left panel (a) and the cumulative amount in 30 min in the right panel (b). A, salivation in response to 1 mg kg−1 of pilocarpine (s.c.) in WT (n = 5), M1/M5 double KO (n = 4) and M3KO (n = 5) mice. B, salivation in response to 10 mg kg−1 of pilocarpine (s.c.) in WT (n = 5), M3KO (n = 5), and M1/M3KO (n = 4) mice. The results are presented as the mean ± s.e.m.; some of the error bars are hidden by symbols. Statistical analysis was made using Scheffe's multiple comparisons following one-way ANOVA. In Aa, the values at all time points in M3KO mice showed a statistically significant difference (P < 0.01), compared to WT or M1/M5 double KO mice. In Ba, significantly smaller values in M3KO mice are depicted by asterisks (*P < 0.05, compared to WT), while the values at all time points in M1/M3 KO mice show statistically significant difference (P < 0.01, Student's t test), compared to WT or M3KO mice. In b, significantly smaller values were depicted by asterisks (*P < 0.05 and **P < 0.01).
Prandial drinking
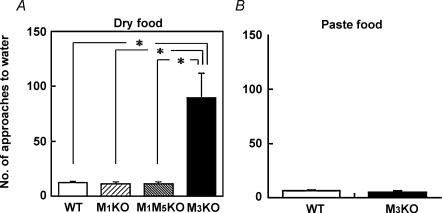
In order to determine the relevance of the M1 and the M3 subtypes in physiological salivation, we next studied the eating behaviour of WT, M1KO, M1/M5KO and M3KO mice and found that this was markedly different in M3KO mice, which showed behavioural features similar to those of salivarectomized rats (Epstein et al. 1964). The results using pelleted food are shown in Fig. 7A. During eating, the WT, M1KO and M1/M5KO mice rarely approached the water bottle nozzle, showing only 12.3 ± 1.0 (n = 14) and 11.3 ± 1.8 (n = 7), 12.5 ± 1.2 (n = 11) approaches in 120 min for WT, M1KO and M1/M5KO mice, respectively (no significant difference with Scheffe's multiple comparisons following one-way ANOVA). The fact that M1/M5KO mice, which bear only M3, did not show frequent prandial drinking indicates that the presence of M3 is sufficient for salivation during feeding. In contrast, the eating behaviour in M3KO mice was frequently interrupted by drinking, resulting in a significantly greater number of approaches to the water bottle nozzle (89.5 ± 22.2 approaches in 120 min; n = 14, *P < 0.01, Scheffe's test). Like salivarectomized rats (Epstein et al. 1964), the M3KO mice approached the water nozzle after a few bites and stayed there for only a moment (less than 1 s), as if they just licked the nozzle. The consumed amounts of dry food and water during the observation period were, respectively, 0.88 ± 0.1 g and 1.8 ± 0.2 ml in WT mice (n = 14, average ± s.e.m.), 0.77 ± 0.1 g and 1.2 ± 0.1 ml in M1KO (n = 7), 0.90 ± 0.1 g and 1.4 ± 0.1 ml in M1/M5KO mice (n = 9), and 0.50 ± 0.1 g and 1.3 ± 0.2 ml in M3KO mice (n = 14). As shown in Fig. 7B, the increased frequency of prandial drinking in M3KO mice was solely due to the dryness of the food, as this effect was not seen with hydrated paste food when only 6.0 ± 1.0 and 4.6 ± 1.8 approaches were made (no significant difference with Student's t test; n = 5 for both) in 120 min for WT and M3KO mice, respectively. The consumed amounts of hydrated paste food and water were, respectively, 3.2 ± 0.7 g and 0.7 ± 0.2 ml in WT mice (n = 5), and 2.4 ± 0.3 g and 0.3 ± 0.2 ml in M3KO mice (n = 5). The results clearly demonstrate that presence of M3 is necessary and sufficient for physiological salivation during eating.
Figure 7. Prandial drinking behaviour during food intake.
Approaches to the water nozzle in 120 min during eating were measured in WT (n = 14), M1KO (n = 7), M1/M5KO (n = 11) and M3KO (n = 14) mice for dry food (A), or in WT (n = 5) and M3KO (n = 5) mice for hydrated food (B) (mean ± s.e.m.; *P < 0.01, Scheffe's multiple comparisons following one-way ANOVA).
Discussion
The present study demonstrated the critical role of the M3 mAChR in the cholinergically activated [Ca2+]i increase in SMG cells, cholinergic stimulant-induced salivation, and physiological salivary secretion during eating. These results agree with a previous study (Matsui et al. 2000) showing that deficiency of the M3 mAChR causes hyposalivation in mice.
Using enzymatically dispersed SMG cells, we found that the [Ca2+]i increase in response to the cholinergic agonist CCh, which substitutes for parasympathetic activation, was reduced by approximately 85% in M3KO mice, compared to WT, M1KO, and M5KO mice. The results indicate the major role of the M3 subtype in inducing Ca2+ signalling in SMG cells. The [Ca2+]i increase induced by high concentrations (100–300 μm) of CCh in M3KO mice was only equivalent to that induced by low concentrations (0.1–0.3 μm) of CCh in the other genotypes. This residual, small CCh-induced [Ca2+]i increase in M3KO mice was completely blocked by atropine, showing that it was mediated by mAChRs. Furthermore, no small [Ca2+]i increase was seen in SMG cells from M1/M3 double KO mice, showing that the M1 subtype is also involved in cholinergically activated Ca2+ signalling in SMG cells, but much stronger stimulation is required to induce Ca2+ signalling through this subtype. The great reduction of Ca2+ signalling in the M3KO SMG cells was presumably attributable to the predominance of M3 over M1 in mAChR populations (Dehaye et al. 1988; Dai et al. 1991; Watson et al. 1996; Moriya et al. 1999; Bockman et al. 2001), since the ability of the M1 and M3 subtypes to produce IP3 in response to CCh is approximately equivalent (Burford et al. 1995). The M5 subtype seems not to be involved in induction of Ca2+ signalling. In summary, in terms of the CCh-induced [Ca2+]i increase, M3 is the major receptor subtype responsible for parasympathetic control of the SMG, while the M1 subtype makes a much smaller contribution.
It is notable that a small population of M3KO acinar cells showed a large [Ca2+]i increase comparable to that in WT acinar cells in response to CCh (Fig. 5C) and that no responses were seen in M1/M3 KO acinar cells (Fig. 5D). These results strongly suggest that M1 is expressed in a heterogeneously scattered fashion in SMG acini, showing a patchy distribution pattern. In contrast, the imaging results in WT and M1/M5 KO mice are strongly suggestive of the ubiquitous expression of M3 in SMG acinar cells. Since no antibodies against M1 or M3 are available for immunocytochemical analysis, this is the first demonstration of the distribution patterns of these receptors in SMG.
The results of pilocarpine-induced salivation also demonstrated the involvement of the M1 and M3 mAChR subtypes in salivation. Our result showing that a low dose of pilocarpine (1 mg kg−1, s.c.) effectively induced salivation in WT mice is consistent with those of many studies using low doses of pilocarpine in normal rodents (e.g. see Murai et al. 1996; Kawaguchi et al. 1997; Matsui et al. 2000; Takeuchi et al. 2002; for 1 mg kg−1 or lower doses given s.c. in mice); but, notably, this dose failed to induce salivation in M3KO mice. Increasing the dose of pilocarpine to 10 mg kg−1 induced salivation in M3KO mice, but failed to induce salivation in M1/M3KO mice, indicating that the salivation stimulated by the high dose of pilocarpine in M3KO mice was mediated by activation of the M1 subtype. These observations suggest that M3-mediated salivation has a lower threshold than M1-mediated salivation in mice when systemically stimulated by a cholinergic stimulant. The fact that as an average response, the M1-mediated [Ca2+]i increase induced by high CCh concentrations (100–300 μm) in M3KO SMG cells was only equivalent to that induced by low CCh concentrations (0.1–0.3 μm) in genotypes bearing the M3 subtype (Fig. 2) means that a submaximal [Ca2+]i increase is sufficient to induce salivation, in line with the result of Dai & Baum (1993) showing that a maximal functional response can be achieved in acinar cells without activating all of the muscarinic receptors. There was a difference in temporal profiles between salivations in WT mice induced by low and high doses of pilocarpine: 1 mg kg−1 (s.c.) caused a lasting salivation over the observation period of 30 min, while 10 mg kg−1 (s.c.) caused a transient salivation that was copious between 5 and 10 min after injection but had ceased by 20–30 min. The different profiles resulted in a greater amount of saliva output after 1 mg kg−1 pilocarpine than after 10 mg kg−1. It has been shown, in SMG acinar cells, that low concentrations of muscarinic agonist induced long-lasting, oscillating Ca2+ signalling which could support sustained fluid secretion, while high concentrations of agonist caused extensive, but transient, Ca2+ signalling (Smith & Gallacher, 1992; Zhang et al. 1996), which presumably resulted from desensitization of the M3 receptor (Edwardson & Szekeres, 1999; Willets et al. 2003). The difference in the temporal profiles of salivation caused by low and high concentrations of pilocarpine may be explained by this difference in Ca2+ signalling pattern which involves M3 receptor desensitization as the underlying mechanism.
As described above, the considerable salivation in M3KO mice induced by a high dose of pilocarpine was mediated by activation of M1. This salivation could be explained by the presence of the M1 subtype in acinar cells, which could cause a large [Ca2+]i increase in the acinar cells, although the distribution of the receptor is heterogeneous and sparse in SMG. Another explanation could be that systemic administration of pilocarpine caused activation of sympathetic nerves by inhibiting M current in the superior cervical ganglion, resulting in salivation via adrenergic activation. We consider the latter unlikely based on the following facts: (1) adrenergic stimulation does not cause as large an output of saliva as cholinergic stimulation (e.g. Murai et al. 1996; Kawaguchi et al. 1997; Matsui et al. 2000), and (2) low doses of pilocarpine, which should activate the M1 subtype abundantly expressed in the ganglion (Caulfield & Birdsall, 1998), did not cause salivation in M3KO mice. However, we have not, so far, elucidated the precise mechanism of the M1-mediated salivation in M3KO mice.
Regardless of the M1-mediated mechanism, the present results clearly demonstrate that the M3 subtype plays the major role in cholinergically stimulated salivation. It is likely that this functional predominance of the M3 subtype applies to another major salivary gland, the parotid gland, because salivation induced by systemic administration of pilocarpine at low dose, which was abolished in M3KO mice, should include the salivary flow from both major glands.
There has been a controversy concerning the hyposalivation phenotype of M3KO mice (Matsui et al. 2000; Yamada et al. 2001). When the dose dependence of salivation by pilocarpine is compared among these reports, the major discrepancy lies in the difference in salivation responses in WT mice, rather than those in M3KO mice. Yamada et al. (2001) reported that slight salivation was only induced by low doses of pilocarpine (1 mg kg−1, s.c.) in WT mice and concluded that there was no significant difference in pilocarpine-induced salivation between WT and M3KO mice (Yamada et al. 2001; Wess, 2003), whereas data in Matsui et al. (2000) and the present study show copious salivary flow in WT and M1/M5KO mice, both of which bear M3, in response to the same dose of pilocarpine with the same route of application. As there have been a number of reports showing that this dose, or even smaller doses, of pilocarpine could induce considerable salivation in normal animals (e.g. for mice, see Murai et al. 1996; Kawaguchi et al. 1997; Matsui et al. 2000; Takeuchi et al. 2002; and we have reproducibly obtained the same results in WT mice (n > 20) in separate experiments for other purposes), it is very likely that low doses of pilocarpine are capable of inducing M3-mediated salivation in normal mice. Although we do not know the precise underlying reasons, differences in the techniques used to assess saliva output might be responsible for the different results in WT mice and, hence, for the different conclusions. It could also be argued that sex difference might contribute to the discrepancy: we measured the saliva output in female mice, whereas Yamada et al. (2001) used males. However, we have already shown that both males and females secrete saliva in a similar manner in response to 1 mg kg−1 pilocarpine (Matsui et al. 2000). Therefore, it seems unlikely that the sex difference accounts for this discrepancy.
Changes in drinking behaviour as represented by an increase in water intake are characteristic of animals with severe hyposalivation (Epstein et al. 1964; Blazsek & Varga, 1999; Hamada et al. 2000) and are thought to be a form of compensatory behaviour for insufficient salivation, designed to moisten dry food, since feeding behaviour is frequently interrupted by drinking (Epstein et al. 1964; Blazsek & Varga, 1999) and such prandial drinking is not seen in animals fed hydrated paste food (Epstein et al. 1964). The present result demonstrating that only M3KO mice showed frequent prandial drinking suggests that deficiency of the M3 receptor subtype, but not the M1 subtype, causes severe hyposalivation under the physiological condition of eating. Despite the potential of the M1 subtype to induce salivation, we did not find evidence for any physiological significance of M1-mediated salivation. Presumably, physiological stimulation is not strong enough to induce M1-mediated salivation. We therefore conclude that the M3 subtype is essential for salivation during eating. From the pharmacological point of view, it should be noted that the response produced by the low dose of pilocarpine (1 mg kg−1, s.c.), rather than the high dose, is closer to that caused by the physiological stimulation of eating. It should be also noted that the M3KO mice consumed significantly less dry food than WT mice in our behavioural test (0.50 ± 0.1 g versus 0.88 ± 0.1 g; P < 0.01, Student's t test). When fed with paste food, however, the decrease in food intake by M3KO mice was less significant (2.4 ± 0.3 g versus 3.2 ± 0.7 g; P = 0.33, Student's t test). This suggests that the reduced salivation not only resulted in the frequent drinking behaviour but also hampered eating dry food. Furthermore, in spite of the increased frequency in approaching the water bottle, the amount of water consumed by M3KO mice was not different compared to the other animals. This suggests that the frequent drinking was intended solely as a substitute for the reduced salivation and should not be associated with an increased demand for water intake.
In M3KO mice, growth failure first becomes evident during the weaning period at 3 weeks after birth (Matsui et al. 2000; Yamada et al. 2001). A similar association between hyposalivation and postweaning growth retardation is seen in mice lacking aquaporin-5, a water channel in the salivary gland (Ma et al. 1999). Given that growth failure in both aquaporin-5-deficient and M3-deficient mice is greatly improved by feeding hydrated paste food (Ma et al. 1999; Matsui et al. 2000; Yamada et al. 2001), it is very likely that hyposalivation exaggerates postweaning dysphagia, raising the concept that normal salivation mediated by the M3 mAChR–IP3 receptor–aquaporin-5 signalling pathway is critical to the smooth transition from consumption of milk to dry food and the normal growth of the infant during weaning. Reduced salivary secretion in the oral cavity during eating probably prevents weanling animals with hyposalivation from eating dry food until they learn prandial drinking behaviour. It should be noted that a deficiency of the ion transport system (Na+–K+–2 Cl− cotransporter or Na+–H+ exchanger) in the salivary gland acinar cells also caused hyposalivation and growth failure, and some of the mutations were even lethal around weaning (Bell et al. 1999; Flagella et al. 1999; Evans et al. 2000; Park et al. 2001).
Because neither pharmacological ligands nor specific antibodies against each receptor subtype are available, the precise contribution of each subtype to the salivary gland function remains unknown in other species, including humans. Preliminary pharmacological studies have suggested that the main mAChR subtype in the human submandibular gland is M3 (Giraldo et al. 1988; Vanderheyden et al. 1990). In addition, the pharmacological profile of mAChR-mediated Ca2+ responses in the human salivary gland cell line, HSG, matched that of M3 (Poronnik et al. 1999). These data are consistent with our present results in the mouse, although the functional predominance of M3 in the control of salivation was not shown in these previous studies. The present results therefore provide a molecular basis for the development of specific therapies, including gene therapy, for the clinical treatment of hyposalivation. Xerostomia, a symptom caused by lack of saliva in the oral cavity, can be induced by various factors, such as diseases, medications and therapeutic irradiation, and results in frequent thirst, mucosal vulnerability, and difficulties in speaking, swallowing, and eating. Since salivary secretion is stimulated by parasympathetic activation, mAChR stimulants have been used for the treatment of xerostomia, including Sjögren's syndrome, a chronic autoimmune disorder of the exocrine glands leading to functional impairment, such as a dry mouth and eyes (for reviews, see Fox et al. 2001). The present results clearly indicate that the M3 mAChR is a reasonable target for drug development for xerostomia, as selective agonists can efficiently stimulate salivary secretion at low doses with minimal side-effects. Our finding that a higher dose (10 mg kg−1) of pilocarpine did not induce more saliva than a lower dose (1 mg kg−1) in WT mice underlines the clinical importance of optimizing the regimen of muscarinic agonist administration for the treatment of dry mouth patients. Considering that exocrine acinar cells may become functionally quiescent as a result of chronic stimulation by agonistic autoantibodies (Bacman et al. 1996), strong and long-term stimulation with muscarinic agonists might even impair salivary gland function, possibly by desensitization of the receptor. The use of specific mAChR KO mice should facilitate the development of mAChR subtype-selective drugs and gene therapy, regardless of the tissues targeted.
Acknowledgments
We thank I. Ishii, A. Matsunaga, A. Yokoi, Y. Araki, S. Kobayashi, N. Matsubara, H. Karasawa, S. Takahashi and D. Motomura for technical assistance in generating M5KO mice. This study was supported by the JST and by Grants-in-Aid for scientific research (M.M., T.M. and K.M.), Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Science, Sports, Culture and Technology of Japan (T.M.), Industrial Technology Research Grant Programs from the New Energy and Industrial Technology Development Organization (NEDO) of Japan (M.M.), Health and Labour Sciences Research Grants for Research on Measures for Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan (M.M.), and the Terumo Life Science Foundation, the Naito Foundation and the Sumitomo Foundation (T.M.).
References
- Bacman S, Sterin-Borda L, Camusso JJ, Arana R, Hubscher O, Borda E. Circulating antibodies against rat parotid gland M3 muscarinic receptors in primary Sjogren's syndrome. Clin Exp Immunol. 1996;104:454–459. doi: 10.1046/j.1365-2249.1996.42748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum BJ. Principles of saliva secretion. Ann NY Acad Sci. 1993;694:17–23. doi: 10.1111/j.1749-6632.1993.tb18338.x. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Wellner RB. Receptors in salivary glands. In: Garett JR, Ekström J, Anderson LC, editors. Neural Mechanisms of Salivary Gland Secretion. Karger, Basel: 1999. pp. 44–58. [Google Scholar]
- Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol. 1999;276:C788–C795. doi: 10.1152/ajpcell.1999.276.4.C788. [DOI] [PubMed] [Google Scholar]
- Blazsek J, Varga G. Secretion from minor salivary glands following ablation of the major salivary glands in rats. Arch Oral Biol. 1999:S45–48. doi: 10.1016/s0003-9969(99)90016-x. [DOI] [PubMed] [Google Scholar]
- Bockman CS, Bradley ME, Dang HK, Zeng W, Scofield MA, Dowd FJ. Molecular and pharmacological characterization of muscarinic receptor subtypes in a rat parotid gland cell line: comparison with native parotid gland. J Pharmacol Exp Ther. 2001;297:718–726. [PubMed] [Google Scholar]
- Burford NT, Tobin AB, Nahorski SR. Differential coupling of m1, m2 and m3 muscarinic receptor subtypes to inositol 1,4,5-trisphosphate and adenosine 3′,5′-cyclic monophosphate accumulation in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1995;274:134–142. [PubMed] [Google Scholar]
- Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci. 2003;17:1403–1410. doi: 10.1046/j.1460-9568.2003.02588.x. [DOI] [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors – Characterization, coupling and function. Pharmac Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJ. International union of pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- Cook DI, Van Lennep EW, Roberts ML, Young JA. Secretion by the major salivary glands. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Raven, New York: 1994. pp. 1061–1117. [Google Scholar]
- Dai YS, Ambudkar IS, Horn VJ, Yeh CK, Kousvelari EE, Wall SJ, Li M, Yasuda RP, Wolfe BB, Baum BJ. Evidence that M3 muscarinic receptors in rat parotid gland couple to two second messenger systems. Am J Physiol. 1991;261:C1063–C1073. doi: 10.1152/ajpcell.1991.261.6.C1063. [DOI] [PubMed] [Google Scholar]
- Dai Y, Baum BJ. Relationship between muscarinic receptor occupancy and response in rat parotid acinar cells. Am J Physiol. 1993;265:G1122–G1127. doi: 10.1152/ajpgi.1993.265.6.G1122. [DOI] [PubMed] [Google Scholar]
- Dehaye JP, Marino A, Soukias Y, Poloczek P, Winand J, Christophe J. Functional characterization of muscarinic receptors in rat parotid acini. Eur J Pharmacol. 1988;151:427–434. doi: 10.1016/0014-2999(88)90539-0. [DOI] [PubMed] [Google Scholar]
- Edwardson JM, Szekeres PG. Endocytosis and recycling of muscarinic receptors. Life Sci. 1999;64:487–494. doi: 10.1016/s0024-3205(98)00592-x. [DOI] [PubMed] [Google Scholar]
- Epstein AN, Spector D, Samman A, Goldblum C. Exaggerated prandial drinking in the rat without salivary glands. Nature. 1964;201:1342–1343. doi: 10.1038/2011342a0. [DOI] [PubMed] [Google Scholar]
- Evans RL, Park K, Turner RJ, Watson GE, Nguyen HV, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE. Severe impairment of salivation in Na+/K+/2Cl− cotransporter (NKCC1)-deficient mice. J Biol Chem. 2000;275:26720–26726. doi: 10.1074/jbc.M003753200. [DOI] [PubMed] [Google Scholar]
- Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithel ial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- Fox RI, Konttinen Y, Fisher A. Use of muscarinic agonists in the treatment of Sjögren's Syndrome. Clin Immunol. 2001;101:249–263. doi: 10.1006/clim.2001.5128. [DOI] [PubMed] [Google Scholar]
- Funaki H, Yamamoto T, Koyama Y, Kondo D, Yaoita E, Kawasaki K, Kobayashi H, Sawaguchi S, Abe H, Kihara I. Localization and expression of AQP5 in cornea, serous salivary glands, and pulmonary epithelial cells. Am J Physiol. 1998;275:C1151–C1157. doi: 10.1152/ajpcell.1998.275.4.C1151. [DOI] [PubMed] [Google Scholar]
- Gallacher DV, Smith PM. Autonomic transmitters and Ca2+-activated cellular responses in salivary glands in vitro. In: Garett JR, Ekström J, Anderson LC, editors. Neural Mechanisms of Salivary Gland Secretion. Karger, Basel: 1999. pp. 80–93. [Google Scholar]
- Giraldo E, Martos F, Gomez A, Garcia A, Vigano MA, Ladinsky H, Sanchez de La Cuesta F. Characterization of muscarinic receptor subtypes in human tissues. Life Sci. 1988;43:1507–1515. doi: 10.1016/0024-3205(88)90398-0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hamada A, Inenaga K, Nakamura S, Terashita M, Yamashita H. Disorder of salivary secretion in inbred polydipsic mouse. Am J Physiol. 2000;278:R817–R823. doi: 10.1152/ajpregu.2000.278.4.R817. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Murai S, Saito H, Itoh T. Changes in the noradrenaline and acetylcholine content of three major salivary glands and in the salivation and protein component patterns of whole saliva in chronically isoprenaline administered mice. Arch Oral Biol. 1997;42:225–234. doi: 10.1016/S0003-9969(96)00107-0. [DOI] [PubMed] [Google Scholar]
- Krane CM, Melvin JE, Nguyen HV, Richardson L, Towne JE, Doetschman T, Menon AG. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell Volume regulation. J Biol Chem. 2001;276:23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- Levey AI. Immunological localization of m1-m5 muscarinic acetylcholine receptors in peripheral tissue and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- Liu X, Ambudkar IS. Characteristics of a store-operated calcium-permeable channel. J Biol Chem. 2001;276:29891–29898. doi: 10.1074/jbc.M103283200. [DOI] [PubMed] [Google Scholar]
- Lomax RB, Camello C, Coppenolle FV, Petersen OH, Tepkin AV. Basal and physiological Ca2+ leak from the endoplasmic reticulum of pancreatic acinar cells. J Biol Chem. 2002;277:26479–26485. doi: 10.1074/jbc.M201845200. [DOI] [PubMed] [Google Scholar]
- Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- Maeda A, Kubo T, Mishina M, Numa S. Tissue distribution of mRNAs encoding muscarinic acetylcholine receptor subtypes. FEBS Lett. 1988;239:339–342. doi: 10.1016/0014-5793(88)80947-5. [DOI] [PubMed] [Google Scholar]
- Matsui M, Araki Y, Karasawa H, Matsubara N, Taketo MM, Seldin MF. Mapping of five subtype genes for muscarinic acetylcholine receptor to mouse chromosomes. Genes Genet Syst. 1999;74:15–21. doi: 10.1266/ggs.74.15. [DOI] [PubMed] [Google Scholar]
- Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci U S A. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami H, Tepkin AV, Petersen OH. Termination of cytosolic Ca2+ signals: Ca2+ reuptake into intracellular stores is regulated by the free Ca2+ concentration in the store lumen. EMBO J. 1998;17:435–442. doi: 10.1093/emboj/17.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya H, Takagi Y, Nakanishi T, Hayashi M, Tani T, Hirotsu I. Affinity profiles of various muscarinic antagonists for cloned human muscarinic acetylcholine receptor (mAChR) subtypes and mAChRs in rat heart and submandibular gland. Life Sci. 1999;64:2351–2358. doi: 10.1016/s0024-3205(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Murai S, Saito H, Masuda Y, Nakamura K, Michijiri S, Itoh T. Effects of short-term (2 weeks) streptozotocin-induced diabetes on acetylcholine and noradrenaline in the salivary glands and secretory responses to cholinergic and adrenergic sialogogues in mice. Arch Oral Biol. 1996;41:673–677. doi: 10.1016/s0003-9969(96)00042-8. [DOI] [PubMed] [Google Scholar]
- Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol. 1997;273:C1549–C1561. doi: 10.1152/ajpcell.1997.273.5.C1549. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signaling in the hippocampus. Eur J Neurosci. 2003;18:109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- Park K, Evans RL, Watson GE, Nehrke K, Richardson L, Bell SM, Schultheis PJ, Hand AR, Shull GE, Melvin JE. Defective fluid secretion and NaCl absorption in the parotid glands of Na+/H+ exchanger-deficient mice. J Biol Chem. 2001;276:27042–27050. doi: 10.1074/jbc.M102901200. [DOI] [PubMed] [Google Scholar]
- Poronnik P, O'Mullane LM, Conigrave AD, Cook DI. Use of replication-deficient adenoviruses to study signal transduction pathways. Muscarinic responses in HSG and HT29 epithelial cell lines are mediated by G protein βγ-subunits. Pflugers Arch. 1999;438:79–85. doi: 10.1007/s004240050882. [DOI] [PubMed] [Google Scholar]
- Raina S, Preston GM, Guggino WB, Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995;270:1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- Smith PM, Gallacher DV. Acetylcholine- and caffeine-evoked repetitive transient Ca2+-activated K+ and C1− currents in mouse submandibular cells. J Physiol. 1992;449:109–120. doi: 10.1113/jphysiol.1992.sp019077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Reed HE. Amplification of the thapsigargin-evoked increase in the cytosolic free Ca2+ concentration by acetylcholine in acutely isolated mouse submandibular acinar cells. Biochem J. 1996;317:779–783. doi: 10.1042/bj3170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Takeuchi J, Fulton J, Jia Z, Abramov-Newerly W, Jamot L, Sud M, Coward D, Ralph M, Roder J, Yeomans J. Increased drinking in mutant mice with truncated M5 muscarinic receptor genes. Pharmacol Biochem Behav. 2002;72:117–123. doi: 10.1016/s0091-3057(01)00725-0. [DOI] [PubMed] [Google Scholar]
- Vanderheyden P, Gies JP, Ebinger G, De Keyser J, Landry Y, Vauquelin G. Human M1-, M2- and M3-muscarinic cholinergic receptors: binding characteristics of agonists and antagonists. J Neurol Sci. 1990;97:67–80. doi: 10.1016/0022-510x(90)90099-9. [DOI] [PubMed] [Google Scholar]
- Watson EL, Abel PW, DiJulio D, Zeng W, Makoid M, Jacobson KL, Potter LT, Dowd FJ. Identification of muscarinic receptor subtypes in mouse parotid gland. Am J Physiol. 1996;271:C905–C913. doi: 10.1152/ajpcell.1996.271.3.C905. [DOI] [PubMed] [Google Scholar]
- Watson EL, Jacobson KL, Singh JC, Ott SM. Nitric oxide acts independently of cGMP to modulate capacitative Ca2+ entry in mouse parotid acini. Am J Physiol. 1999;277:C262–C270. doi: 10.1152/ajpcell.1999.277.2.C262. [DOI] [PubMed] [Google Scholar]
- Wess J. Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends Pharmacol Sci. 2003;24:414–420. doi: 10.1016/S0165-6147(03)00195-0. [DOI] [PubMed] [Google Scholar]
- Willets JM, Mistry R, Nahorski SR, Challiss RA. Specificity of G protein-coupled receptor kinase 6-mediated phosphorylation and regulation of single-cell M3 muscarinic acetylcholine receptor signaling. Mol Pharmacol. 2003;64:1059–1068. doi: 10.1124/mol.64.5.1059. [DOI] [PubMed] [Google Scholar]
- Xu X, Diaz J, Zhao H, Muallem S. Characterization, localization and axial distribution of Ca2+ signaling receptors in the rat submandibular salivary gland ducts. J Physiol. 1996;491:647–662. doi: 10.1113/jphysiol.1996.sp021246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T, Ikawa Y, Yoshida K, Shigetani Y, Takeda N, Mabuchi I, Yamamoto T, Aizawa S. Homologous recombination at c-fyn locus of mouse embryonic stem cells with use of diphtheria toxin A-fragment gene in negative selection. Proc Natl Acad Sci U S A. 1990;87:9918–9922. doi: 10.1073/pnas.87.24.9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, Felder CC, Deng CX, Wess J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Sims NE, Macauley SP, Nguyen KH, Nakagawa Y, Humphreys-Beher MG. Alterations in the secretory response of non-obese diabetic (NOD) mice to muscarinic receptor stimulation. Clin Immunol Immunopathol. 1996;78:245–255. doi: 10.1006/clin.1996.0036. [DOI] [PubMed] [Google Scholar]
- Zhang W, Fukushi Y, Nishiyama A, Wada J, Kamimura N, Mio Y, Wakui M. Role of extracellular Ca2+ in acetylcholine-induced repetitive Ca2+ release in submandibular gland acinar cells of the rat. J Cell Physiol. 1996;167:277–284. doi: 10.1002/(SICI)1097-4652(199605)167:2<277::AID-JCP11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]