Abstract
Simultaneous intracellular recordings were made from myenteric neurons and circular muscle (CM) cells in isolated, stretched segments of guinea-pig distal colon. We have shown previously that maintained stretch generates a repetitive and coordinated discharge of ascending excitatory and descending inhibitory neuronal reflex pathways in the distal colon. In the presence of nifedipine (1–2 μm) to paralyse the muscle, simultaneous recordings were made from 25 pairs of AH (after-hyperpolarization)-neurons and CM cells separated by 100–500 μm. In all 25 AH-neurons, proximal process potentials (PPPs) were never recorded, even though at the same time, all recordings from neighbouring CM cells showed an ongoing discharge of inhibitory junction potentials (IJPs) anally, or excitatory junction potentials (EJPs) orally. In fact, 24 of 25 AH-neurons were totally silent, while in one AH-cell, some spontaneous fast excitatory postsynaptic potentials (FEPSPs) were recorded. All 10 electrically silent AH-cells that were injected with neurobiotin were found to be multipolar Dogiel type II neurons. In contrast, when recordings were made from myenteric S-neurons, two distinct electrical patterns of electrical activity were recorded. Recordings from 25 of 48 S-neurons showed spontaneous FEPSPs, the majority of which (22 of 25) showed periods when discrete clusters of FEPSPs (mean duration 88 ms) could be temporally correlated with the onset of EJPs or anal IJPs in the CM. Nine S-neurons were electrically quiescent. The second distinct electrical pattern in 14 S-neurons consisted of bursts, or prolonged trains of action potentials, which could be reduced to proximal process potentials (PPPs) in six of these 14 neurons during membrane hyperpolarization. Unlike FEPSPs, PPPs were resistant to a low Ca2+ –high Mg2+ solution and did not change in amplitude during hyperpolarizing pulses. Mechanosensory S-neurons were found to be uniaxonal or pseudounipolar filamentous neurons, with morphologies consistent with interneurons. No slow EPSPs were ever recorded from AH- or S-type neurons when IJPs or EJPs occurred in the CM. In summary, we have identified a population of mechanosensory S-neurons in the myenteric plexus of the distal colon which appear to be largely stretch sensitive, rather than muscle-tension sensitive, since they generate ongoing trains of action potentials in the presence of nifedipine. No evidence was found to suggest that in paralysed preparations, the repetitive firing in ascending excitatory or descending inhibitory nerve pathways was initiated by myenteric AH-neurons, or slow synaptic transmission.
More than 100 years ago, Bayliss & Starling (1899, 1900) first reported that polarized neuronal reflexes could be evoked in the intestine of dogs by a variety of stimuli including luminal stretch, mucosal stimulation and pinching of the gut wall. Since these reflexes occurred in isolated segments of bowel and were preserved following cutting of the extrinsic nerves, it was assumed that the complete neural reflex arcs were intrinsic to the gut wall (Bayliss & Starling, 1900; Bülbring et al. 1958; Hirst et al. 1974, 1975; Costa & Furness, 1976; Smith et al. 1991, 1992b). Later, it was proposed that intestinal stretch reflexes and mucosal reflexes, which give similar responses in enteric neurons and in the smooth muscle of the guinea-pig ileum, were mediated by two different intrinsic sensory neurons (Smith et al. 1991, 1992a). Others have proposed, however, that stretch and mucosal reflexes in the colon of rats are mediated via both extrinsic and intrinsic sensory neurons, respectively (Grider & Jin, 1994). Although, in the guinea-pig small and large intestine both mucosal and distension reflexes are intrinsic to the intestinal wall, since they are preserved following extrinsic denervation (Furness et al. 1995) or following capsaicin pretreatment (Spencer et al. 2002).
Nishi & North (1973) and Hirst et al. (1974) found two major electrophysiological classes of myenteric neuron called S/type I and AH/type II neurons. Hirst et al. (1974) called one class ‘… synaptic or S cells since they received extensive [fast] excitatory synaptic input’. The AH/type II neuron, on the other hand exhibited a prolonged after-hyperpolarization that ensued following a single somal action potential. S-neurons situated away from the site of stimulation respond to distension or mucosal stimulation with bursts of fast excitatory postsynaptic potentials (FEPSPs), whereas, AH-neurons at the same location do not respond to reflex stimulation (Hirst et al. 1975; Bornstein et al. 1991; Smith et al. 1992a). S-neurons comprise the interneurons and motor neurons in the myenteric plexus (Bornstein et al. 1991; Smith et al. 1992a). However, more recent studies have shown that myenteric AH-neurons, unlike S-neurons, respond directly to chemical stimulants applied to the mucosa, such as serotonin in the guinea-pig proximal colon (Smith, 1994, 1996), or adenosine triphosphate (Bertrand & Bornstein, 2002) and HCl (Kunze et al. 1995) in the guinea-pig small intestine applied to the mucosa directly adjacent to the recording site. In addition, AH-neurons in the guinea-pig small intestine respond to increases in smooth muscle tension with an ongoing action potential discharge (Kunze et al. 1998). As a result of these findings, it has been proposed that some myenteric AH-neurons are intrinsic mechanosensory and/or chemosensory neurons that may be responsible for initiating ascending excitatory and descending inhibitory peristaltic reflexes (Kunze & Furness, 1999). In the guinea-pig ileum and distal colon, most myenteric AH-neurons have a distinctive Dogiel type II morphology with large smooth cell bodies and multipolar processes (Hodgkiss & Lees, 1983; Wade & Wood, 1988; Wood, 1989; Lomax et al. 1999; Neunlist et al. 1999; Smith et al. 1999). In contrast, most myenteric S-neurons (i.e interneurons or motor neurons) typically have a distinctive Dogiel type I morphology and are usually uniaxonal (Bornstein et al. 1991; Smith et al. 1992a; Costa et al. 1996; Brookes et al. 1997; Lomax et al. 1999; Smith et al. 1999; Brookes, 2001). However, a few myenteric AH-neurons in the small and large intestine are filamentous, uniaxonal neurons that have FEPSPs (Song et al. 1997; Lomax et al. 1999; Tamura et al. 2001; Nurgali et al. 2003). Furthermore, at present, there is a limited knowledge of the functional roles of AH- or S-type neurons during motility reflexes in the distal colon and which classes of neuron may exhibit mechanosensory properties.
The motility of the guinea-pig distal colon is complex. Recently, D'antona et al. (2001) used video imaging of the distal colonic wall to study fecal pellet propulsion. They observed two nerve-mediated patterns of motility: peristalsis induced by a fecal pellet and ‘clusters of annular circular muscle contractions separated by short dilated regions’ that propagated aborally. The latter activity occurred during the intervals between pellet propulsion and in an empty segment of colon. Later, we found that even local maintained distension of the guinea-pig distal colon could activate two distinct motor patterns that are likely to underlie fecal pellet propulsion (Spencer et al. 2002, 2003; Smith et al. 2003). The first motor pattern consisted of low frequency, rhythmically occurring peristaltic waves that had a long duration (∼40 s) and propagated along the colon in response to a fixed distension stimulus provided by an artificial fecal pellet. This strongly propulsive motor pattern was highly dependent upon smooth muscle tone (Smith et al. 2003). The second stretch-activated motor pattern consisted of a faster rhythmic motor pattern, when a repetitive discharge of EJPs occurred in the CM at the oral end of colon that were synchronized in time with the onset of IJPs in the CM some 2 cm anally (Spencer et al. 2002, 2003). That is, ascending excitatory and descending inhibitory neuronal pathways fired simultaneously and repetitively to the LM and CM layers. A most notable observation of this rhythmic motor pattern was that it occurred in the presence of nifedipine, when smooth muscle tone and tension is abolished (Spencer et al. 2002, 2003). This result is opposite to what would be expected if myenteric AH-neurons generate this ongoing rhythmic motor pattern in the distal colon; since it has been shown that mechanosensory AH-neurons in the small intestine become inactivated by L-type Ca2+ blockers that abolish muscle tone by blocking action potentials in the muscle (Kunze et al. 1998). This suggests then, that the rhythmic motor pattern we have described in the distal colon is generated by a population of mechanosensory neurons that are stretch dependent, rather than muscle-tone or tension dependent (Spencer et al. 2002). This raises two important questions. (1) Do myenteric AH-neurons have a role in generating ongoing reflex activity in paralysed, stretched preparations of distal colon? If so, then the AH-neurons in the distal colon must have properties different from tone-sensitive AH-neurons in the small intestine. (2) Are there other mechanosensory neurons in the colon that are sensitive to stretch rather than muscle tone?
A major development in our understanding of the neural control of colonic motility would occur if we could correlate the activity of myenteric neurons and smooth muscle cells during peristaltic activity. Therefore, we have developed a technique to record simultaneously from either AH- or S-type neurons and circular smooth muscle cells in close apposition during repetitive and coordinated firing of stretch-activated ascending excitatory and descending inhibitory reflex pathways in the distal colon (see Spencer et al. 2002, 2003). Our findings show that the maintained stretch-activated ongoing reflex activity in the distal colon, which is independent of muscle tone, is likely to be driven by a population of stretch-sensitive myenteric S-interneurons, rather than AH-neurons. Also, neural activity in S-motor neurons is well correlated with junction potentials in the circular muscle. A preliminary account of these studies has been previously published in abstract form (Spencer & Smith, 2003).
Methods
Preparation of tissues
Guinea-pigs (150–300 g) of either sex were killed by inhalation anaesthetic (IsoSol-Isoflurane-Vedco, Bristol, UK), using protocols approved by the Animal Ethics Committee of the University of Nevada School of Medicine. The terminal distal colon (2–4 cm oral to the anal sphincter) was removed and placed immediately into a Sylgard-lined Petri dish containing oxygenated Krebs solution at room temperature. Segments (∼20 mm in length) of distal colon were then incised along the mesenteric border and pinned flat to the base of the dissecting dish with the mucosa facing uppermost. The mucosa, submucosa and submucous plexus were sharp dissected away to expose the underlying circular muscle layer. Then two different preparations were created: (1) the longitudinal muscle, circular muscle–myenteric plexus (LMCM-MP) preparation and (2) the circular muscle–myenteric plexus (CM-MP) preparation.
In the LMCM-MP-preparation the LM remained intact with the myenteric plexus, and small strips of CM (∼2–3 mm wide) were sharp dissected off the myenteric plexus to expose the underlying ganglia at either the oral or anal end of colon. In all, 10% of the CM was removed from the myenteric plexus. In some experiments, small strips of CM were also removed so that recordings could be made from myenteric neurons in the middle of the preparation. If the CM had been completely removed off the myenteric plexus, then stretch-activated junction potentials and neuronal activities in myenteric S-neurons are greatly reduced, or abolished (N. J. Spencer & T. K. Smith, unpublished observations). Following removal of the CM, preparations were then transferred to an organ bath and pinned taut under maintained circumferential stretch with the CM facing uppermost. Under these pinned conditions the maximum distance between either circumferential edge was between 10 and 13 mm.
The CM-MP preparation contained all the CM, but no LM. That is, the CM was completely intact with the myenteric plexus, but with the LM removed to expose the myenteric plexus. To form CM-MP preparations strips of LM were peeled away from the myenteric plexus, using a protocol first introduced by Furukawa et al. (1986) for the murine colon. CM-MP preparations were also pinned taut under maintained circumferential stretch, similar to LMCM-MP preparations, to the floor of the organ bath.
The base of the organ bath recording chamber (∼8 ml capacity) consisted of a microscope coverslip that was laminated with a fine layer (∼2–3 mm deep) of Sylgard silicon (Dow Corning Corp. Midland, MI, USA). The organ bath, which was mounted upon a fixed stage spanning an independently moveable inverted microscope (Nikon TS100), was continuously perfused with oxygenated warm Krebs solution (see below) at 36°C at a flow rate of 2 ml min−1. The Krebs solution always contained nifedipine (1–2 μm) to paralyse the muscle in order to facilitate intracellular recordings from neurons and muscle.
Protocol for simultaneous intracellular recordings from myenteric neurons and circular muscle cells
Simultaneous intracellular recordings were made from myenteric neurons and CM cells using two independently mounted micromanipulators (WPI; World Precision Instruments, Inc. Sarasota, FL, USA, model M3301R). The position of the microelectrodes could be readily adjusted to record from individual ganglion cells located within 500 μm from the CM cell recording site. Microelectrodes (i.d. 0.5 mm) were filled with 1.5 m KCl solution and had tip resistances of about 130–220 MΩ. Electrical signals were amplified using a dual input Axoprobe 1A amplifier (Axon Instruments, Foster City, CA, USA) and digitized at 2–5 kHz for neuronal recordings and 500 Hz to 1 kHz for smooth muscle recordings on a PC running Axoscope software (version 8.0; Axon Instruments).
Protocol for neurobiotin injection into myenteric neurons
Short (400 ms) depolarizing current pulses at frequencies of between 2 and 3 Hz were used to inject neurobiotin into myenteric neurons for periods of between 5 and 30 min. Following neuronal recordings, Zamboni's solution was used to fix preparations overnight. The next morning DMSO solution was used to wash out the Zamboni's solution and an immunoperoxidase reaction using DAB as a substrate was performed to visualize neurons, using previously described techniques (see Smith et al. 1992a, 1999).
Drugs and solutions
The composition of the modified Krebs solution was (mm): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; and glucose, 11.5. Hexamethonium bromide and nifedipine were obtained from Sigma Chemical Co. (St Louis, MO, USA). In some experiments, the composition of the Krebs solution was modified to block all synaptic transmission in the colon (see Spencer et al. 1999). In this solution, extracellular Ca2+ was reduced 10-fold and extracellular Mg2+ was raised 10-fold. This solution contained (mm): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgSO4, 12.0; CaCl, 0.25; and glucose,11.5. Neurobiotin tracer was obtained from Vector Laboratories (Burlingame, CA, USA), as was the ABC Vectastain kit or immunoperoxidase reaction.
Analysis of data
Measurements of neuronal action potential amplitudes, half-durations, after-hyperpolarization durations, after-hyperpolarization amplitudes, and resting membrane potentials were made using Axoscope (Version 8.0, Axon Instruments). The amplitudes and durations of IJPs and EJPs and the intervals between them were also recorded. The use of n in the Results section always refers to the number of animals on which experiments were performed. In tissue from some animals, more than 1 impalement (recording) was made, and in these cases, the number of neurons recorded from is also stated. Student's paired and unpaired t tests were used where appropriate and a P value < 0.05 was considered statistically significant.
Results
General observations
When recordings were made from CM cells in all stretched preparations of distal colon (both CM-MP and LMCM-MP), an ongoing discharge of tetrodotoxin-sensitive junction potentials was always recorded (n = 68). When impalements were made from CM cells adjacent to (≤ 4 mm) the oral cut end of distal colon, the predominant electrical event recorded was an ongoing discharge of EJPs, which showed large fluctuations in amplitude. Similarly, when recordings were made adjacent to (≤ 4 mm) the anal cut end of distal colon, the predominant electrical response was an ongoing discharge of IJPs (Fig. 1; see also Spencer et al. 2002). The intervals between each oral EJP and anal IJP were highly variable, as were the amplitudes of each individual junction potential (see Figs 1 and 2). There was, however, no significant difference between the amplitudes or intervals between oral EJPs or anal IJPs recorded in CM-MP and LMCM-MP preparations (Student's unpaired t test; P > 0.05; n = 68). When recordings were made ≤ 4 mm from the anal end of CM-MP or LMCM-MP preparations, the mean amplitudes of IJPs were 8.2 ± 0.6 mV (range 1–23 mV) and 9.1 ± 0.8 mV (range 2–24 mV), respectively, and the mean intervals between IJPs were 4.1 ± 0.5 s (range 1.9–5.3 s) in CM-MP preparations and 2.7 ± 0.5 s (range 1.3–6.1 s) in LMCM-MP preparations. The mean amplitudes of EJPs recorded ≤ 4 mm from the oral cut end and the mean intervals between them were, respectively, 12.2 ± 0.8 mV (range 3–20 mV) and 3.3 ± 0.7 s (range 1.9–6.1 s) for CM-MP preparations, and 12.5 ± 0.6 mV (range 3–27 mV) and 3.9 ± 0.3 s (range 2.6–5.6 s) for LMCM-MP preparations. A maintained discharge of oral EJPs and anal IJPs was regularly recorded from the CM layer in each stretched preparation for periods of 6–8 h. We have previously shown using simultaneous recordings from CM cells located at both ends of the preparation that the ongoing oral EJPs occur at the same time as the anal IJPs (Spencer et al. 2002, 2003). Since these preparations were paralysed, but yet still showed repetitive firing of ascending excitatory and descending inhibitory nerve pathways, this gave us a unique opportunity to determine the activities of myenteric AH- and S-type neurons and correlate it with activity in the underlying muscle.
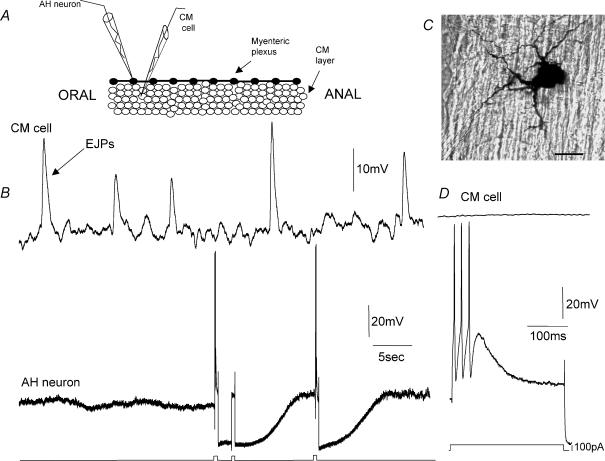
Figure 1. Inactivity in a myenteric AH neuron during ascending excitation.
A, diagrammatic representation of the preparation used for simultaneous recording from myenteric neurons and CM cells in preparations where the CM was intact, but the LM removed (CM-MP preparation; see Methods). B shows a typical simultaneous recording from a myenteric AH-neuron and CM cell located < 500 μm apart and within 1 mm of the oral cut end of colon. An ongoing discharge of EJPs occurs in the CM at the oral end of the colon. At the same time as EJPs occurred, this AH-neuron in a neighbouring ganglia was electrically quiescent, until depolarizing current was injected into the neuron on two occasions to evoke action potentials. Following the evoked action potentials a prolonged membrane hyperpolarization ensued. Evoked action potentials in this neuron did not cause any detectable membrane potential change in the CM cell. C shows that the AH-neuron recorded in B was a multipolar Dogiel type II neuron. D, an expanded portion of the recording shown in B, showing that the evoked action potentials in this neuron did not change CM membrane potential. The calibration bar in C represents 30 μm. The resting membrane potentials of the CM cell and the AH-neuron were −45 and −61 mV.
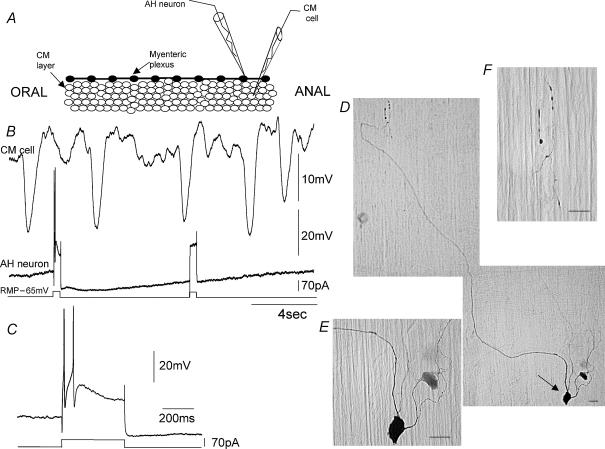
Figure 2. Morphology and projections of an electrically silent myenteric AH-neuron recorded from the anal end of distal colon in a CM-MP preparation.
A, diagrammatic representation of the relative locations of the two recording electrodes at the anal end of colon. The LM was removed while the CM remained intact. B shows a simultaneous recording from an AH-neuron and a closely apposed CM cell (500 μm apart). An ongoing discharge of large amplitude IJPs occurred in the CM cell, even though at the same time the AH-neuron was silent. Depolarizing current injection evoked two action potentials in the soma, followed by a prolonged membrane after-hyperpolarization. C shows an expanded portion of the evoked action potential shown in B. D, the neuron in A was found to be a multipolar Dogiel type II neuron (see arrow) with a long process that appeared to enter the CM layer. E, the cell body of the AH-neuron from which the recording was made (see arrow in D). F shows an enlarged image of the nerve endings in the CM layer. The cell body was measured 40 μm (major axis) and 23 μm (minor axis). The calibration bars in D–F represent 25 μm. The resting membrane potentials of the CM cell and the AH-neuron were −49 and −62 mV.
Simultaneous recordings from myenteric neurons and CM cells
In total, simultaneous recordings were made from 73 myenteric neurons and CM cells (n = 68 animals). Of these, 25 neurons were classified electrophysiologically as AH-neurons (with after-hyperpolarizations longer than 4 s), while the remaining 48 neurons were characterized electrophysiologically as ‘S-neurons’ because they received prominent spontaneous and/or evoked FEPSPs in response to nerve stimulation (as described by Hirst et al. 1974). Of the 48 S-neurons recorded, 14 S-neurons were found to generate their own intrinsic action potentials in stretched preparations, which persisted when synaptic transmission within the colon was prevented (see below). The majority of the remaining 34 S-neurons showed ongoing spontaneous FEPSPs. No slow EPSPs were ever recorded in any myenteric neuron (n = 78).
Simultaneous recordings from myenteric AH-neurons and circular muscle cells
Recordings in preparations partially devoid of circular muscle (LMCM-MP)
We were interested in whether myenteric AH-neurons were involved in the generation of stretch-activated ongoing ascending excitatory (EJPs) and descending inhibitory (IJPs) in the CM layer. To test this, simultaneous recordings were made from 25 pairs of myenteric AH-neurons and CM cells in (n = 23 guinea-pigs). Simultaneous recordings were made from 11 AH-neurons and CM cells in LMCM-MP preparations, and 14 AH-neurons and CM cells in CM-MP preparations. The recordings made from AH-neurons were at a distance of less than 500 μm from the CM cell recording site, which usually represented a distance of one to three ganglia from the CM cell recording site. The major finding was that all AH-neurons in either type of preparation failed to reveal spontaneous action potential or proximal process potentials, even though at the same time, recordings from neighbouring CM cells consistently always showed an ongoing discharge of anal IJPs or oral EJPs. In all recordings from CM cells at the oral end of the colon, an ongoing discharge of EJPs was consistently recorded (Fig. 1), whereas at the anal end of the colon, recordings from all CM cells consistently showed an ongoing discharge of IJPs (see Fig. 2).
We were particularly interested in whether evoking action potentials in electrically silent AH-neurons would cause a change in membrane potential of closely apposed CM cells. When depolarizing currents (100–300 pA) were passed down the electrode, all 25 AH-neurons typically responded with one or two somal action potentials. On occasions, with larger depolarizing current, up to four action potentials could be evoked in AH-neurons, before a prolonged membrane after-hyperpolarization ensued. Under no circumstances did evoked action potentials in AH-neurons cause any detectable change in membrane potential of neighbouring CM cells, or disrupt ongoing junction potentials (Figs 1 and 2). In LMCM-MP preparations, myenteric AH-neurons had a resting membrane potential of −56.3 ± 1.1 mV (range 51 to −63 mV, n = 11). The mean peak amplitude of action potentials in AH-neurons evoked by direct current injection was 45.1 ± 2.9 mV (range 31–65 mV; n = 11), and the mean spike half-duration was 1.7 ± 0.1 ms (range 1–2 ms; n = 11). Following evoked somal action potentials, the mean AH amplitude and duration were 11.3 ± 0.9 mV (range 6–15 mV; n = 11) and 6.7 ± 0.9 s (range 4–14 s; n = 11), respectively (see Table 1).
Table 1.
Characteristics of myenteric AH-neurons
| RMP (mV) | n | Action potential amplitude (mV) | n | Action potential half-duration (ms) | n | AH amplitude (mV) | n | AH duration (s) | n | |
|---|---|---|---|---|---|---|---|---|---|---|
| CMMP preparation | − 64.1 ± 1.9* | 12 | 53.6 ± 10.2 | 12 | 1.9 ± 0.6 | 12 | 11.5 ± 3.9 | 12 | 8.9 ± 0.9 | 12 |
| LMCM-MP preparation | − 56.3 ± 1.1 | 11 | 45.1 ± 2.9 | 11 | 1.7 ± 0.1 | 11 | 11.3 ± 0.9 | 11 | 6.7 ± 0.9 | 11 |
Values are means ± s.e.m. The characteristics of myenteric AH-neurons are shown in preparations with the LM removed from the myenteric plexus (i.e CM-MP preparations), or partial removal of the CM from the myenteric plexus (LMCM-MP preparations).The only significant difference found between AH-neurons recorded from CM-MP preparations and LMCM-MP preparations was that the resting membrane potentials of AH-neurons of CM-MP preparations were significantly depolarized(
P = 0.015; n = 10; Student's unpaired t test).
Recordings in preparations devoid of longitudinal muscle (CM-MP)
It might have been argued that AH-neurons were electrically silent during recordings from LMCM-MP preparations because the CM had been dissected off the myenteric plexus at the site where neuronal recordings were made. Therefore, we developed a preparation where the CM remained completely intact with the myenteric plexus, but strips of LM were removed to expose the myenteric plexus (see Fig. 1A). Using this CM-MP preparation, simultaneous recordings were made from 14 AH-neurons and CM cells in 12 different animals. Simultaneous recordings were made from CM cells and AH-neurons at distances < 500 μm from each other. It was found that 13 of 14 AH-neurons in CM-MP preparations were totally quiescent at the same time as all CM cell impalements showed an ongoing discharge of oral EJPs and anal IJPs (Figs 1 and 2). In one AH-neuron, some spontaneous FEPSPs were recorded that occasionally reached action potential threshold. This neuron fired discrete clusters of FEPSPs, but was not filled sufficiently well to determine its morphology. All AH-neurons injected with neurobiotin had Dogiel type II morphologies and were always totally quiescent in the presence of nifedipine (n = 10).
In response to outward (depolarizing) current clamp (50–300 pA), AH-neurons typically fired a single action potential, or on occasion up to a maximum of four action potentials, which were immediately followed by a prolonged membrane hyperpolarization (see Hirst et al. 1974). The only significant difference found between AH-neurons recorded from CM-MP preparations and those from LMCM-MP preparations was that the resting membrane potentials of AH-neurons in CM-MP preparations were significantly more hyperpolarized (P = 0.015; n = 10; Student's unpaired t test; see Table 1). The mean action potential amplitude and half-duration of evoked action potentials in AH-neurons from CM-MP preparations were 53.6 ± 10.2 mV (range 38–70 mV; n = 12) and 1.9 ± 0.6 ms (range 1–3 ms; n = 12). Typical recordings from myenteric AH-neurons and CM cells in CM-MP preparations are shown in Figs 1 and 2. The mean amplitude and duration of the AH were 11.5 ± 3.9 mV (range 5–20 mV; n = 12) and 8.9 ± 0.9 s (range 5.0–14.1 s; n = 12), respectively. AH-neurons in CM-MP preparations had a mean resting membrane potential of −64.1 ± 1.9 mV (range −51 to −80 mV; n = 12; see Table 1). As with recordings from AH-neurons in LMCM-MP preparations, when action potentials were evoked in all AH-neurons of CM-MP preparations, no detectable change in membrane potential was detected in closely apposed CM cells (Figs 1 and 2).
Response of AH-neurons to ganglionic compression
During simultaneous recordings from both AH-neurons and CM, a fine artist's paint brush was used to press on myenteric ganglia, in an attempt to activate putative sensory nerve endings. Ganglia were stimulated ∼5 mm from the AH-neuron recording site. In response to ganglionic compression, no action potentials or proximal process potentials were recorded (n = 4), although an IJP was consistently evoked anal to the stimulus in the CM cell. Eventually each impalement was lost due to movement.
Morphologies of myenteric AH-neurons
In the guinea-pig distal colon, myenteric neurons with AH-electrophysiology have been shown to have either Dogiel type I or Dogiel type II morphologies (Lomax et al. 1999; Tamura et al. 2001; Nurgali et al. 2003). However, all Dogiel type II neurons were reported to have AH-neuron electrophysiology (Nurgali et al. 2003). Therefore, we were interested in the morphologies of the AH-neurons that we recorded from during our recordings. In 10 animals, AH-neurons were injected with neurobiotin and sufficiently labelled to identify the cell bodies and/or dendritic processes. AH-neurons were found to have large smooth cell somas with multipolar processes, examples of which are shown in Figs 1 and 2. The cell bodies of AH-neurons had dimensions of 39.7 ± 3.1 μm in their major axis (range 31.4–51 μm) and 22.6 ± 1.4 μm (range 18.0–27 μm) in their minor axis.
Previous studies have only identified AH-neurons when the CM had been removed from the myenteric plexus (Lomax et al. 1999; Tamura et al. 2001; Nurgali et al. 2003). However, in this study, we preserved the CM layer during recordings from AH-neurons. In these preparations, we found that in two AH-cells where the CM remained attached to the myenteric plexus, AH-neurons had at least one axon that projected into the CM layer itself (Fig. 2D and F). These swellings along the nerve endings may represent expansion bulbs of cut axons that normally projected into the mucosa.
Identification of mechanosensory S-neurons
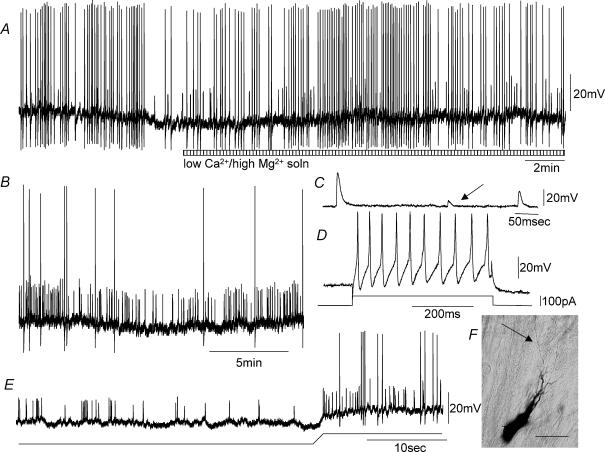
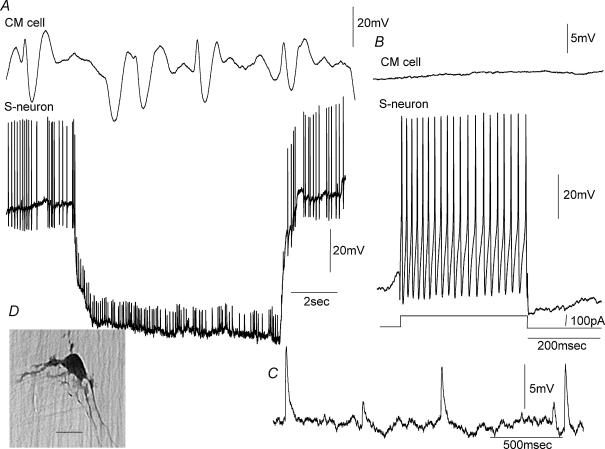
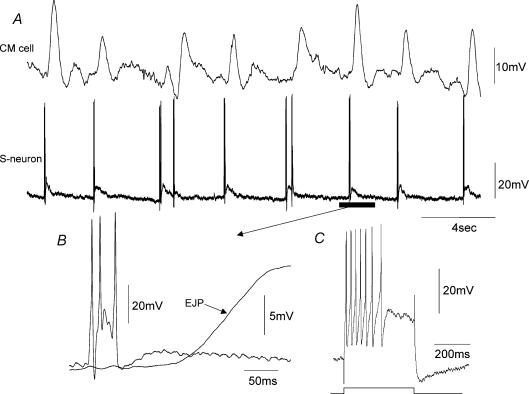
When recordings were made from myenteric ganglia with the CM layer attached to the myenteric plexus, a population of S-neurons were recorded which generated an ongoing discharge of action potentials in stretched preparations (Figs 3–6). These S-neurons fired ongoing action potentials at a mean frequency of 4.2 Hz (range 1.8–14.1 Hz, n = 14). To test whether the ongoing firing of action potentials in these S-neurons was generated via synaptic inputs from other neurons, we replaced the normal Krebs solution with a low Ca2+ –high Mg2+ solution (see Methods). This solution abolished all fast synaptic inputs in all S-neurons and all junction potentials in the CM cells, but it did not affect the ongoing discharge of action potentials in this population of S-neurons (n = 8; Fig. 3A). These neurons were classified as ‘S-neurons’ because they (1) received prominent FEPSPs either spontaneously, or in response to single electrical stimuli, and (2) they always fired tonically to injection of depolarizing current (Figs 3–6). The resting membrane potential of mechanosensory S-neurons was−47.6 ± 0.7 mV (range −45 to −51 mV; n = 9), and the mean action potential amplitude and half-duration were 43 ± 2.1 mV (range 31–58 mV; n = 14) and 1.8 ± 0.1 ms (range 1–2.4 ms; n = 14). When a large membrane hyperpolarization was imposed on the cell soma of mechanosensory S-neurons, the ongoing discharge of action potentials was reduced to proximal process potentials in six of 14 S-neurons (Figs 4 and 6). Proximal process potentials were readily identified over fast synaptic inputs because (1) they were unaffected by the low Ca2+ –high Mg2+ solution, (2) their amplitudes remained constant, and (3) they did not increase in amplitude with membrane hyperpolarization (Fig. 6D). Process potentials reflect the electrotonic conduction of action potentials into the soma that are generated in dendritic processes or axons (sensory endings) at some unknown distance away from the cell body, and have failed to trigger an action current in the soma. This is why their amplitudes remain constant and persist in a low Ca2+ –high Mg2+ solution (Fig. 3 B). In the remaining eight mechanosensory S-neurons, the intrinsic discharge of action potentials was extinguished when a large (∼50 mV) conditioning membrane hyperpolarization was imposed on the soma (Fig. 5 B). It is likely that these action potentials also arose at sites either in the soma itself, or in short dendritic processes that lie close to the cell body. These neurons showed a characteristic pattern of membrane noise between action potentials (Figs 5A and 7B). Three of these myenteric S-neurons were found to respond to compression of the CM at a site circumferential to the ganglia from which recordings were made with brief bursts of action potentials. In one of these S-neurons where a low Ca2+ –high Mg2+ solution was applied, the evoked action potentials in response to CM compression persisted (Fig. 7Ai).
Figure 3. Electrical activity recorded from a mechanosensory S-neuron in the myenteric plexus, when pinned under maintained circumferential stretch.
A, spontaneous action potentials and proximal process potentials occurred in this neuron when recorded in the presence of nifedipine, and were unaffected by the application of a low Ca2+ –high Mg2+ solution to block all synaptic transmission (see hatched bar). B shows an expanded portion of the recording in the low Ca2+ –high Mg2+ solution. Note, the similarity in amplitude of process potentials recorded in the low Ca2+ solution. Some process potentials were sufficient to trigger a full somatic action potential. C, some spontaneous FEPSPs were recorded in this S-neuron prior to the application of the low Ca2+ –high Mg2+ solution. These are identified by their irregular amplitudes (see arrow). D, this neuron fired tonically in response to depolarizing current. E, in the presence of the low Ca2+ –high Mg2+ solution, removal of hyperpolarizing holding current increased the discharge of process potentials, suggesting that some mechanosensory ion channels may exist close to, or even in the cell soma itself. F, this neuron was an orally projecting uniaxonal filamentous neuron. The calibration bar represents 20 μm. The resting potential of this neuron was −53 mV.
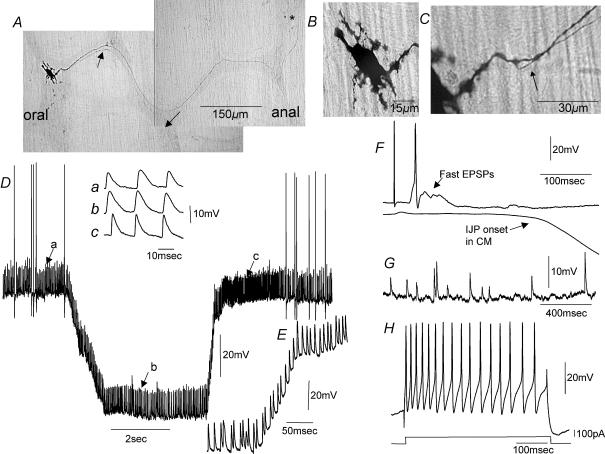
Figure 6. Electrical and morphological characteristics of a mechanosensory descending interneuron.
A, morphology of a pseudounipolar descending neuron that gave rise to two axons (more oral arrow). The thin axon appeared to provide synaptic outputs in the second row of ganglia (more anal arrow). The thicker darker axon appeared to leave a ganglia and enter the neighbouring CM (see *). B shows the cell body of this neuron with filamentous dendritic processes, one of which runs parallel to the CM fibres. C, this neuron had two bifurcating axons close to the axon hillock; see arrow. D, spontaneous proximal process potentials and action potentials occurred in this neuron under stretch and in the presence of nifedipine. When the membrane was artificially hyperpolarized by ∼50 mV the amplitude and interval between process potentials did not change, suggesting that these events were electrotonically invading the soma from a site distant to the cell body. E shows removal of hyperpolarizing holding current, where process potentials do not change characteristics. F, when simultaneous recordings were made from a CM cell and an S-neuron, single pulse transmural stimulation evoked FEPSPs (see arrow) and a single action potential, followed by a fast IJP in the CM. G, in addition to proximal process potentials, this neuron showed ongoing spontaneous fast EPSPs. These were identified because their amplitudes were highly variable. H, this neuron fired action potentials tonically in response to depolarizing current injection. The resting membrane potential of this neuron was −48 mV.
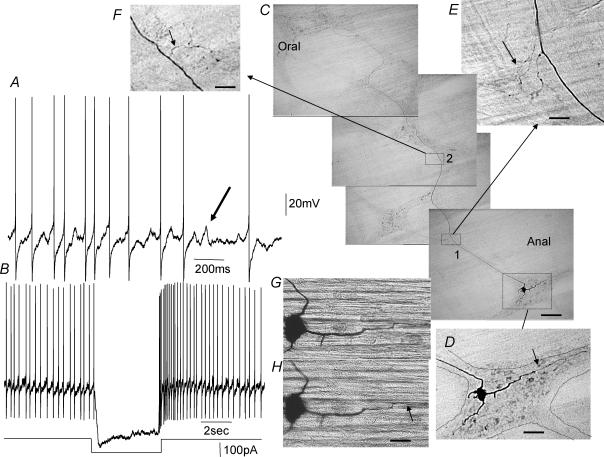
Figure 4. Simultaneous recording from a mechanosensory S-neuron and a circular muscle cell.
A, ongoing IJPs occur in the CM cell at the same time as action potentials discharge spontaneously in this S-neuron. No correlation was observed between spontaneous action potentials in this S-neuron and the IJPs in the CM. When a conditioning hyperpolarization of ∼50 mV was imposed on the cell soma, action potentials were converted into proximal process potentials. When the hyperpolarizing current was withdrawn, process potentials were converted back to full somatic action potentials. B, in response to depolarizing current, this was a tonic firing cell, but the train of action potentials did not cause any change in membrane potential in the neighbouring CM. C, in addition to process potentials and spontaneous action potentials, this neuron also received prominent FEPSPs, distinguishable by their irregular amplitudes. D shows the filamentous processes of this Dogiel type I neuron. This neuron appeared to be uniaxonal and projected anally for at least one row of ganglia, but was not filled sufficiently to trace its ending. The calibration bar represents 20 μm. The resting membrane potential of this neuron was −50 mV.
Figure 5. Intrinsically active ascending interneuron in a stretched segment of distal colon.
A, ongoing action potentials and membrane noise recorded from a stretched preparation, while in the presence of nifedipine. Note the membrane noise during the interspike intervals. B, ongoing action potentials and noise are abolished by membrane hyperpolarization. This suggests that the action potentials arise close to or within the cell soma. C, morphology of this ascending interneuron, showing fine varicose synaptic outputs in the first and second rows of ganglia (see boxes 1 and 2, shown on enlarged scale in E and F). This neuron had a long circumferentially projecting dendrite that arose from the soma. D, enlarged image of the neuronal soma and processes. E and F show the synaptic outputs of this ascending interneuron, expanded from boxes 1 and 2 in C. G and H show a short process that appears to enter the CM layer. G, when the soma is in focus, the end of this process is out of focus, suggesting it is not in the plane of the soma. H shows this process in focus (see arrow) and entering the CM layer, while the soma is now out of focus compared with G. The long axon of this neuron eventually left a ganglion (most oral ganglion shown in the top panel in C) and entered and dived down through the CM ending in an expansion bulb on the submucosal surface of the CM (not shown), suggesting that it may terminate in the submucous plexus or the mucosa. Calibration bars represent 100 μm in C, 35 μm in D, 13 μm in E and F, and 17 μm in G and H.
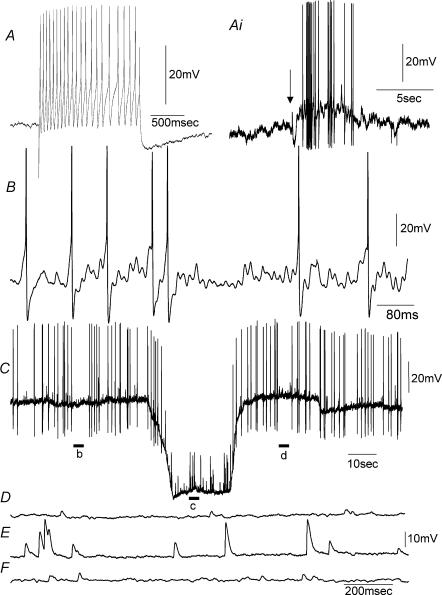
Figure 7. Properties of myenteric S-neurons in stretched preparations of distal colon while in the presence of nifedipine.
A, a tonic S-neuron firing in response to depolarizing current. Ai, while in the presence of a low Ca2+ –high Mg2+ solution, the neuron shown in A responded with a burst of action potentials in response to a ganglionic compression stimulus to the CM. B, in a different animal, an intrinsically active S-neuron showed an ongoing discharge of membrane noise represented by the unstable membrane potential. C, effects of membrane hyperpolarization on an S-neuron that had spontaneous action potentials and fast EPSPs. When the membrane potential was artificially hyperpolarized, the action potentials ceased, and only fast EPSPs were recorded. Fast EPSPs were detected because they increased in amplitude and had irregular amplitudes. Compare panels b, c and d, shown on an expanded scale in D–F. Recordings shown in A–C are all from different animals.
Neurobiotin was injected sufficiently into six of the 14 mechanosensory S-neurons to determine their morphologies. In all six neurons, these cells were found to be uniaxonal or biaxonal with filamentous dendrites (Figs 3–6). In three of these six neurons, the axons could be traced sufficiently to at least the first row of ganglia, where synaptic outputs were revealed (see Figs 5 and 6). These neurons were likely to be interneurons, two of which projected orally while the third projected anally (Figs 5 and 6). The cell bodies of filamentous S-neurons that showed proximal process potentials measured 29.3 ± 3.9 μm (range 20–37 μm) in the major axis and 12.14 ± 1.3 μm (range 8.6–14.2 μm) in their minor axis. Two orally projecting, uniaxonal, filamentous neurons had at least one dendrite that appeared to project from the cell body to the CM lying under the ganglion (Fig. 5G and H). Furthermore, the ending of its process left the ganglia and penetrated onto the inner surface of the CM where it ended in an expansion bulb, suggesting that it may have projected to the submucous plexus or mucosa. Also, a descending filamentous neuron was pseudounipolar, with one long process ending in a nearby ganglia and another process ending in the CM (Fig. 6A–C).
Activity in S-neurons and CM cells during ongoing reflex activity
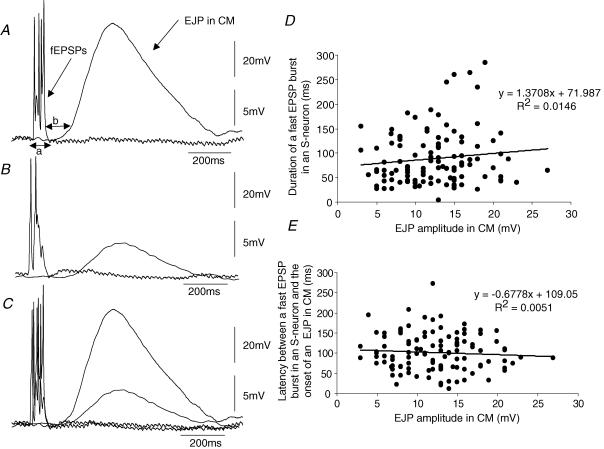
Since myenteric AH-neurons were found to be electrically quiescent during ongoing EJPs and IJPs in the CM, we were interested in the firing patterns and synaptic inputs in all other myenteric S-neurons that did not show mechanosensory properties. In CM-MP preparations, simultaneous recordings were made from 34 pairs of myenteric S-neurons and CM cells (n = 26 guinea-pigs). The two recording electrodes were < 500 μm apart and both impalements were made ≤ 4 mm from the oral cut end of the colonic preparation. In all 34 CM cells impaled at the oral end of the colon, ongoing EJPs of variable amplitude and interval were recorded from CM-MP preparations: mean amplitude 12.2 ± 0.8 mV (range 3–20 mV) and mean interval 3.3 ± 0.7 s (range 1.9–6.1 s). At the same time as EJPs occurred in the CM, recordings from 25 of the 34 S-neurons showed ongoing fast synaptic inputs (FEPSPs) see Figs 8 and 9. In nine of the 34 S-neurons, no ongoing synaptic activity was recorded. During the recordings from S-neurons that showed spontaneous FEPSPs, the majority of S-neurons (22 of 25) showed periods whereby discrete clusters of FEPSPs could be temporally correlated with the onset of EJPs in the CM, examples of which are shown in Figs 8 and 9. The mean duration of a burst of FEPSPs in S-neurons that were involved in EJP generation was 88.9 ± 5.2 ms (n = 18; see Table 2). Interestingly, there was no correlation between the duration of a burst of FEPSPs in any given S-neuron, when compared to the amplitude of the EJP that was generated in the CM layer (R2 = 0.015; n = 18; Fig. 10D). Also, there was no correlation found between the amplitude of any individual EJP, when compared to the latency between end of a burst of FEPSPs and on the onset of the EJP in the CM (R2 = 0.005; Fig. 10E). The mean latency between the end of a burst of FEPSPs in an S-neuron and the onset of an EJP in the CM was 100.6 ± 4.4 ms (n = 26; Table 2). When depolarizing current was passed into all S-neurons, trains of action potentials could be readily evoked, but no detectable change in CM membrane potential was ever observed. Interestingly, no slow EPSPs were ever recorded in any S-neuron of these stretched preparations, when EJPs and IJPs occurred in the CM cells. The mean resting membrane potential of S-neurons that showed discrete bursts of FEPSPs was −53.2 ± 2.6 mV (n = 15), and the mean action potential amplitudes and half-durations were 39.1 ± 1.4 mV (n = 27) and 2.1 ± 0.2 ms (n = 27), respectively.
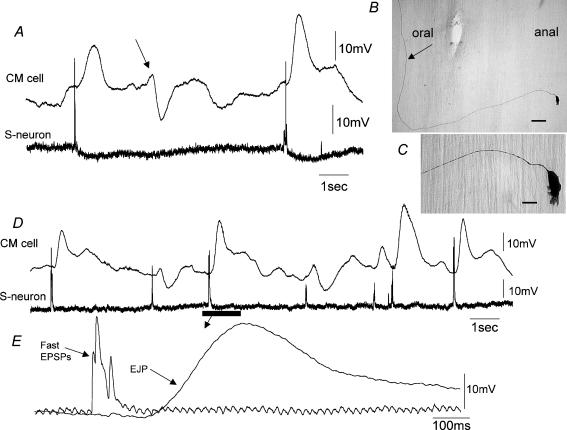
Figure 8. Morphology and synaptic inputs to a myenteric S-neuron involved in repetitively discharging ascending excitatory nerve pathways.
A, simultaneous recording from a myenteric S-neuron and CM at the oral end of a CM-MP preparation. Prior to the onset of each EJP in the CM, a brief burst of fast EPSPs occurred in this neuron. Note that during the IJPs that occurred in the CM, this S-neuron did not receive fast EPSPs, suggesting that it may have been an excitatory motor neuron. B, this neuron was injected with neurobiotin and found to be a uniaxonal Dogiel type I neuron with short lamellar dendrites. C, an expanded image of the cell body of the neuron recorded from in A. D shows another simultaneous recording from an S-neuron and CM cell in a different animal. Note that prior to each EJP a brief burst of fast EPSPs occurred in the S-cell. E, the recording period represented by the bar in D is shown on an expanded time scale. Fast EPSPs and the single EJP are shown. Note that the discrete burst of fast EPSPs immediately precedes the EJP by approximately 150 ms. The calibration bar represents 40 μm in B and 15 μm in C. The resting membrane potentials of the CM cell and the S-neuron were −36 and −45 mV.
Figure 9. Firing patterns of a myenteric S-neuron during repetitively discharging ascending excitatory nerve pathways.
A, simultaneous recording from an S-neuron and CM cell at the oral end of a CM-MP preparation. Note that the duration of the fast EPSPs and action potentials that precede each EJP are similar, but the amplitudes of each EJP in the CM are dissimilar. B, expanded trace from the period represented by the black bar in A. C, this neuron fired a brief burst of action potentials in response to depolarizing current. The resting membrane potential of this neuron was −47 mV.
Table 2.
Characteristics of fast synaptic inputs in myenteric S-neurons that underlie EJP generation in circular muscle
| EJP amplitude in CM (mV) | FEPSP duration in S-neurons (ms) | Latency between end of FEPSP burst and onset of EJP (ms) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± S.E.M | Range | n | Mean ± S.E.M | Range | n | Mean ± S.E.M | Range | n |
| 12.4 ± 0.45* | 3–27 | 26 | 89.0 ± 5.2 | 4–284 | 26 | 100.6 ± 4.4 | 11–271 | 26 |
Table 2 shows the characteristics of fast synaptic inputs recorded from a population of myenteric S-neurons which showed time-locked FEPSPs prior to the onset of EJPs in the CM layer. The mean duration of a burst of fast EPSPs (88 ms) was the time in which a discrete discharge of FEPSPs occurred in S-neurons immediately prior to the activation of an EJP in the CM layer. The mean latency between the onset of an EJP in the CM and the end of a fast synaptic burst in an S-neuron was ∼100 ms, which is highly consistent with the time courses known for the activation of second messenger systems known to generate a junctional current in gastrointestinal smooth muscle.
Figure 10. Characteristics of fast synaptic potentials in a myenteric S-neuron that underlies EJP generation in circular muscle.
A and B show a simultaneous recordings from the same S-neuron and CM cell as in Fig. 9 at the oral end of a CM-MP preparation. Note the EJPs vary widely in amplitude in A and B, yet the duration of the fast EPSP burst in the S-neuron is similar. Arrows a and b (see A) indicate how the duration of FEPSPs and the latency of EJP onset were measured. C, recordings from A and B are superimposed to show the similarity in duration of fast EPSP bursts, despite large differences in EJP amplitude. D, summarized data from 26 different S-neurons showing that there was no temporal correlation between the duration of the fast synaptic inputs in an individual S-neuron compared with the amplitude of the EJP in the CM (R2 = 0.01). E, similarly, there was no correlation between the latency of a fast EPSP burst in an S-neuron (see arrow b in A) when compared to the amplitude of an EJP in the CM (R2 = 0.005). The resting membrane potential of this neuron was −47 mV.
Discussion
In this study, we developed a technique to record the electrical activities in both myenteric neurons and CM cells simultaneously, in stretched preparations of guinea-pig distal colon, where ascending excitatory and descending inhibitory nerve pathways discharge repetitively (see Spencer et al. 2002, 2003). During these recordings, we have identified the activity in three different functional classes of myenteric neurons:
Myenteric AH-neurons which were found to be electrically silent in preparations where the smooth muscles were paralysed by nifedipine, despite the fact that circumferential stretch activated a rhythmic and coordinated discharge of ascending excitatory (EJPs) and descending inhibitory (IJPs) nerve pathways to the CM layer.
Mechanosensory S-neurons whose ongoing activity appears to be largely stretch-sensitive, rather than dependent upon muscle tone. These S-neurons fire ongoing trains of action potentials even when synaptic transmission within the colon is abolished; and even when the smooth muscles are paralysed by nifedipine. These mechanosensitive S-neurons appear to comprise some ascending and descending interneurons that likely have mechanosensitive processes in the CM.
S-neurons that fire short bursts of fast EPSPs, or action potentials that precede oral EJPs or anal IJPs in the CM. Most S-neurons that received discrete bursts of fast EPSPs that were time-locked with junction potentials in the CM appear to be largely motor neurons to the LM or CM (N. J. Spencer and T. K. Smith, unpublished observations).
Simultaneous recordings from AH-neurons and CM during ongoing reflex activity
In the guinea-pig small intestine, myenteric AH-neurons have been proposed as intrinsic mechanosensory neurons (Kunze et al. 1998; Kunze & Furness, 1999), as first postulated by Hirst et al. (1974). In light of this suggestion, we assumed that in our stretched preparations of distal colon, AH-neurons must be active and responsible for the ongoing repetitive firing of ascending EJPs and descending IJPs that occurred in the CM layer (see Spencer et al. 2002, 2003). This assumption was not supported by our findings. What we did find was that when simultaneous recordings were made from CM cells and myenteric AH-neurons, 24 of 25 AH-neurons were found to be electrically silent, at the same time as an ongoing discharge of anal IJPs and oral EJPs occurred in the CM. One AH-neuron showed some spontaneous fast EPSPs. However, no AH-neuron ever showed either proximal process potentials or full somatic action potentials (n = 25). If myenteric AH-neurons are mechanosensory neurons in the distal colon, then this raises the question of why these neurons were electrically silent in our stretched preparations. Our findings suggest one of two possibilities. Firstly, AH-neurons in the distal colon are sensitive to changes in muscle tension or tone, and become inactivated in paralysed preparations, as they do in the guinea-pig ileum (Kunze et al. 1998, 2000). Alternatively, it is possible that AH-neurons in the distal colon are rapidly adapting mechanoreceptors and become inactivated in response to maintained stretch. Indeed, if the conclusions of Kunze et al. (1998, 2000) in the guinea-pig ileum apply to the guinea-pig distal colon, then we should not have expected any activity in AH-neurons under our recording conditions, because as mentioned, AH-neurons become inactivated by muscle paralysis and all our recordings were made in the presence of nifedipine. It is entirely possible that in the distal colon, myenteric AH-neurons are mechanosensory, but, simply require muscle tension or tone for their activation. In support of this, we have recently described another motor pattern in the guinea-pig distal colon that consists of rhythmic peristaltic waves in response to maintained distension provided by a fixed balloon or an artificial fecal pellet (Smith et al. 2003). These highly propulsive contractile waves are highly sensitive to nifedipine, or other antagonists of smooth muscle tone, suggesting that a population of tone-sensitive sensory neurons exists in the distal colon (Smith et al. 2003).
Identification of mechanosensory S-neurons
A major finding of the current study was that a population of myenteric S-neurons fired ongoing bursts or trains of action potentials that were unaffected by a low Ca2+ –high Mg2+ solution, or the presence of nifedipine, suggesting that their own intrinsic excitability was not dependent upon synaptic inputs from other neurons, or smooth muscle tension (contractility) or tone. In our study, all mechanosensory neurons recorded from the distal colon were classified as S-neurons because they received prominent spontaneous and/or evoked fast EPSPs in response to nerve stimulation, consistent with the original classification of an ‘S-neuron’ (Hirst et al. 1974). These mechanosensory S-neurons also always discharged tonically in response to injection of depolarizing current. In addition to fast EPSPs and ongoing action potentials, when a large conditioning hyperpolarization was applied to the cell soma, ongoing action potentials in mechanosensory S-neurons could be reduced to proximal process potentials.
In the current study, eight of 14 mechanosensory S-neurons showed an ongoing discharge of membrane noise during the intervals between action potentials (see Fig. 7B). This noise persisted in the presence of a low Ca2+ –high Mg2+ solution. Membrane noise in these mechanosensitive neurons, which is also commonly observed in many CNS neurons, is a common feature of stochastic resonance. Stochastic resonance is important in some sensory neurons since it increases the background level of excitability, so that a neuron can more readily respond to small sensory inputs. This phenomenon is also well described for intrinsic cardiac sensory neurons (Kember et al. 2000).
When neurobiotin was injected into these mechanosensory S-neurons, these cells were found to be uniaxonal or pseudounipolar interneurons with filamentous dendrites, similar to the filamentous interneurons described by Lomax et al. (1999). Furthermore, since we preserved the CM in our preparations, we were able identify dendrites or processes of these neurons that appeared to enter the CM. If the CM is the site of sensory transduction for the ongoing stretch-activated reflex in the colon, then this would explain why stretch-activated synaptic activity in myenteric neurons is substantially reduced or absent in classic stretched LM-MP preparations where the CM muscle is removed from the myenteric plexus (see Lomax et al. 1999).
Mechanosensory interneurons have been identified and well described in a variety of invertebrates, such as locusts (Kalogianni, 1996), crayfish (Wilkens & Marzelli, 1979), crickets and blow flies (Jacobs et al. 1986; Mitchell & Itagaki, 1992). For example, in the abdominal ganglion of locusts, Kalogianni (1996) identified a population of descending mechanosensory interneurons that responded directly to wind and tactile inputs. Also, a first order mechanosensory interneuron has also been located in the crayfish tailfin that has been shown to be directly activated by water currents and distortion (Wilkens & Marzelli, 1979). It is perhaps not surprising then that a population of myenteric interneurons exist in the mammalian gastrointestinal tract that also exhibit mechanosensory properties. Mechanosensory S-neurons recorded in the current study exhibit properties that may differ from other intrinsic mechanosensory neurons described in the small bowel (Kunze et al. 1998). This is because in the distal colon, mechanosensory S-neurons maintain ongoing firing, even in the presence of nifedipine. In light of these observations, we suggest that mechanosensory S-neurons, at least in the distal colon, appear to be largely stretch sensitive, rather than muscle-tension or tone sensitive. Interestingly, in the mouse colon, Miller & Szurszewski (2003) have recently found that the mechanosensitivity of intestinofugal neurons, which project from the colon to the superior mesenteric ganglia, were also insensitive to muscle paralysis.
Proximal process potentials in myenteric S-neurons
Wood & Mayer (1978) first reported proximal process potentials in six myenteric neurons of isolated guinea-pig jejunum. They found these events ‘… are distinguished also by absence of any change in amplitude as the membrane potential is experimentally hyperpolarized.’ Using this criteria, and by applying a low Ca2+ –high Mg2+ solution to the colon, mechanosensory S-neurons were readily distinguished from other S-neurons that received only FEPSPs. Proximal process potentials are presumably being generated away from the cell body in the mechanosensitive processes of these neurons.
Twenty years later, Kunze et al. (1998) reported that when electrical hyperpolarization was imposed on myenteric AH-neurons in unparalysed preparations, the stretch-activated firing of action potentials was extinguished and no process potentials were recorded in four out of 14 AH-neurons studied. In the other 10 neurons studied, process potentials, or action potentials persisted. In our study, during recordings from six of 14 mechanosensory S-neurons, the ongoing firing of action potentials was reduced to proximal process potentials, whose amplitudes remained constant and persisted in the presence of a low Ca2+ solution. In the other eight S-neurons, action potential firing was extinguished when a large (∼50 mV) conditioning hyperpolarization was imposed on the cell soma. Currently, the location of the mechanosensory ion channels in myenteric S-neurons of the distal colon is unknown. It is quite possible that some mechanosensory ion channels exist either in the neuronal cell soma, or more likely in dendritic processes that appear to lie within the CM. The latter explanation could explain why a large (∼50 mV) hyperpolarization applied to the soma may suppress channel activation underlying action potential firing in dendrites. In support of this, it was possible to observe in the presence of a low Ca2+ –high Mg2+ solution, that upon withdrawal of a hyperpolarizing potential, an increase in process potential discharge occurred (Fig. 3E). Future studies will be required to determine the exact sites of mechanosensory ion channels within myenteric S-neurons.
The frequency of firing of action potentials in mechanosensory S-neurons was typically of the order of 4–10 Hz, yet the frequency of EJPs in the CM was of the order of 0.3 Hz. It is not clear at this point how tonic firing of mechanosensory S-neurons may encode for the rhythmic, low frequency generation of EJPs in the CM. Interestingly, the coordinated low frequency muscle contractions of many vertebrates and invertebrates, such as swimming movements in marine mollusk Aplysia, also have a substantially high sensory neuron firing frequency (Phares et al. 2003) that encodes for a substantially lower frequency of muscle contractions during swimming (Gamkrelidze et al. 1995).
Simultaneous recordings from myenteric S-neurons and CM during ongoing reflex activity
In contrast to AH-neurons, when recordings were made from myenteric S-neurons that did not show mechanosensory properties, the majority of neurons showed ongoing fast EPSPs. In fact, when simultaneous recordings were made from myenteric S-neurons and CM cells, 22 of 34 S-neurons showed discrete bursts of fast FEPSPs that were found to temporally correlate with the onset of EJPs in the CM layer. These S-neurons, which appear to include motor neurons, were clearly a part of a large neural network that was responsible for the generation each individual single EJP in the CM, since when recordings were made from many S-neurons within a given preparation, many S-neurons within that preparation showed discrete bursts of FEPSPs prior to each EJP onset. Interestingly, in the S-neurons which showed time-locked bursts of fast EPSPs preceding EJPs in the CM, no correlation was found between the duration of each burst of fast EPSPs when compared to the amplitudes of EJPs. This suggests that the duration of a synaptic burst of fast EPSPs in any given S-neuron is not a factor in determining the amplitude of a junction potential evoked in colonic CM. We suggest that the ongoing fluctuations in EJP amplitudes in the CM are related to the number of excitatory motor neurons that have been synaptically recruited at the same point in time (Spencer et al. 2001) rather than ongoing variability in the number of quanta released from a single motor neuron.
Conclusion
We have identified a population of mechanosensory S-neurons in the distal colon which have morphologies similar to filamentous interneurons. These mechanosensory S-neurons appear to be largely stretch sensitive, rather than muscle-tension or tone sensitive, since their activation is resistant to nifedipine and muscle paralysis. We also show that repetitive firing in ascending excitatory nerve pathways to the CM is generated by discrete bursts of FEPSPs that last for ∼100 ms in individual myenteric S-neurons. In paralysed preparations of distal colon, we found no evidence to suggest that myenteric AH-neurons, or slow synaptic transmission were involved in the repetitive firing of ascending excitatory and descending inhibitory neuronal pathways to the CM layer.
Acknowledgments
This study was supported by a grant from the National Institute of Health (USA) (No. RO1 NIDDK 45713) awarded to T.K.S. and N.J.S.
References
- Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM, Starling EH. The movements and innervation of the large intestine. J Physiol. 1900;26:107–118. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Bornstein JC. ATP as a putative sensory mediator: activation of intrinsic sensory neurons of the myenteric plexus via P2X receptors. J Neurosci. 2002;22:4767–4775. doi: 10.1523/JNEUROSCI.22-12-04767.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Smith TK, Trussell DC. Synaptic responses evoked by mechanical stimulation of the mucosa in morphologically characterized myenteric neurons of the guinea-pig ileum. J Neurosci. 1991;1:505–518. doi: 10.1523/JNEUROSCI.11-02-00505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ. Classes of enteric nerve cell. Anat Record. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Brookes SJH, Medeeniya A, Jobling P, Costa M. Orally projecting interneurons in the guinea-pig small intestine. J Physiol. 1997;505:473–491. doi: 10.1111/j.1469-7793.1997.473bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Lin RCY, Schofield G. An investigation of the peristaltic reflex in relation to anatomical observations. Quart J Exp Physiol. 1958;43:26–37. doi: 10.1113/expphysiol.1958.sp001305. [DOI] [PubMed] [Google Scholar]
- Costa M, Brookes SJ, Steele PA, Gibbins I, Burcher E, Kandiah C. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol. 1976;294:47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- D'antona G, Hennig GW, Costa M, Humphreys CM, Brookes SJ. Analysis of motor patterns in the isolated guinea-pig large intestine by spatio-temporal maps. Neurogastroenterol Mot. 2001;13:483–492. doi: 10.1046/j.1365-2982.2001.00282.x. [DOI] [PubMed] [Google Scholar]
- Furness JB, Johnson PJ, Pompolo S, Bornstein JC. Evidence that enteric motility reflexes can be initiated through entirely intrinsic mechanisms in the guinea-pig small intestine. Neurogastroenterol Mot. 1995;7:89–96. doi: 10.1111/j.1365-2982.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Taylor GS, Bywater RAR. An intracellular study of myenteric neurons in the mouse colon. J Neurophysiol. 1986;55:1395–1406. doi: 10.1152/jn.1986.55.6.1395. [DOI] [PubMed] [Google Scholar]
- Gamkrelidze GN, Laurienti PJ, Blankenship JE. Identification and characterization of cerebral ganglion neurons that induce swimming and modulate swim-related pedal ganglion neurons in Aplysia brasiliana. J Neurophysiol. 1995;74:1444–1462. doi: 10.1152/jn.1995.74.4.1444. [DOI] [PubMed] [Google Scholar]
- Grider JR, Jin JG. Distinct populations of sensory neurons mediate the peristaltic reflex elicited by muscle stretch and mucosal stimulation. J Neurosci. 1994;14:2854–2860. doi: 10.1523/JNEUROSCI.14-05-02854.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Holman ME, McKirdy HC. Two descending nerve pathways activated by distension of guinea-pig small intestine. J Physiol. 1975;244:113–127. doi: 10.1113/jphysiol.1975.sp010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Holman ME, Spence I. Two types of neurons in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974;236:303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkiss JP, Lees GM. Morphological studies of electrophysiologically-identified myenteric plexus neurons of the guinea-pig ileum. Neuroscience. 1983;8:593–608. doi: 10.1016/0306-4522(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Jacobs GA, Miller JP, Murphey RK. Integrative mechanisms controlling directional sensitivity of an identified sensory interneuron. J Neurosci. 1986;6:2298–2311. doi: 10.1523/JNEUROSCI.06-08-02298.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogianni E. Morphology and physiology of abdominal projection interneurones in the locust with mechanosensory inputs from ovipositor hair receptors. J Comp Neurol. 1996;18:656–673. doi: 10.1002/(SICI)1096-9861(19960318)366:4<656::AID-CNE7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kember GC, Fenton GA, Collier K, Armour JA. Aperiodic stochastic resonance in a hysteretic population of cardiac neurons. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 2000;61:1816–1824. doi: 10.1103/physreve.61.1816. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Bornstein JC, Furness JB. Identification of sensory nerve cells in a peripheral organ (the intestine) of a mammal. Neuroscience. 1995;66:1–4. doi: 10.1016/0306-4522(95)00067-s. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Clerc N, Furness JB, Gola M. The soma and neurites of primary afferent neurons in the guinea-pig intestine respond differentially to deformation. J Physiol. 2000;526:375–385. doi: 10.1111/j.1469-7793.2000.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WAA, Furness JB. The enteric nervous system and regulation of intestinal motility. Ann Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Furness JB, Bertrand PP, Bornstein JC. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. J Physiol. 1998;506:827–842. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax AEG, Sharkey KA, Bertrand PP, Low AM, Bornstein JC, Furness JB. Correlation of morphology, electrophysiology and chemistry of neurons in the myenteric plexus of the guinea-pig distal colon. J Auton Nerv System. 1999;76:45–61. doi: 10.1016/s0165-1838(99)00008-9. [DOI] [PubMed] [Google Scholar]
- Miller SM, Szurszewski JH. Circumferential, not longitudinal, colonic stretch increases synaptic input to mouse prevertebral ganglion neurons. Am J Physiol. 2003;285:G1129–G1138. doi: 10.1152/ajpgi.00292.2003. [DOI] [PubMed] [Google Scholar]
- Mitchell BK, Itagaki H. Interneurons of the subesophageal ganglion of Sarcophaga bullata responding to gustatory and mechanosensory stimuli. J Comp Physiol. 1992;171:213–230. doi: 10.1007/BF00188929. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Dobreva G, Schemann M. Characteristics of mucosally projecting myenteric neurons in the guinea-pig proximal colon. J Physiol. 1999;517:533–546. doi: 10.1111/j.1469-7793.1999.0533t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S, North RA. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973;231:471–479. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurgali K, Furness JB, Stebbing MJ. Correlation of electrophysiology, shape and synaptic properties of myenteric AH neurons of the guinea-pig distal colon. Auton Neuroscience. 2003;103:50–63. doi: 10.1016/s1566-0702(02)00212-6. [DOI] [PubMed] [Google Scholar]
- Phares GA, Antzoulatos EG, Baxter DA, Byrne JH. Burst-induced synaptic depression and its modulation contribute to information transfer at Aplysia sensorimotor synapses: empirical and computational analyses. J Neurosci. 2003;10:8392–8401. doi: 10.1523/JNEUROSCI.23-23-08392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK. Myenteric AH neurons are sensory neurons in the guinea-pig proximal colon: an electrophysiological analysis in intact preparations. Gastroenterology. 1994;862:A-216. (abstract) [Google Scholar]
- Smith TK. An electrophysiological identification of intrinsic sensory neurons responsive to 5-HT applied to the mucosa that underlie peristalsis in the guinea-pig proximal colon. J Physiol. 1996;495:102P. [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Interactions between reflexes evoked by distension and mucosal stimulation: electrophysiological studies of guinea-pig ileum. J Auton Nerv Syst. 1991;34:69–75. doi: 10.1016/0165-1838(91)90009-r. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea-pig small intestine. J Neurosci. 1992a;12:1502–1510. doi: 10.1523/JNEUROSCI.12-04-01502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Burke EP, Shuttleworth CWR. Topographical and electrophysiolgical characteristics of highly excitable S neurons in the myenteric plexus of the guinea-pig ileum. J Physiol. 1999;517:817–830. doi: 10.1111/j.1469-7793.1999.0817s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Bywater RAR, Taylor GS, Holman ME. Electrical responses of the muscularis externa to distension of the isolated guinea-pig distal colon. J Gastrointest Mot. 1992b;4:145–156. [Google Scholar]
- Smith TK, Oliver GR, Hennig GW, O'Shea D, Vanden Berghe P, Kang SK, Spencer NJ. A smooth muscle tone-dependent migrating motor pattern in guinea-pig distal colon. J Physiol. 2003;551:955–969. doi: 10.1113/jphysiol.2003.049163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z-M, Brookes SJH, Ramsay GA, Costa M. Characterization of myenteric interneurons with somatostatin immunoreactivity in the guinea-pig small intestine. Neuroscience. 1997;80:907–923. doi: 10.1016/s0306-4522(96)00605-7. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. Spatial and temporal coordination of junction potentials in circular muscle of guinea-pig distal colon. J Physiol. 2001;535:565–578. doi: 10.1111/j.1469-7793.2001.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. A rhythmic motor pattern activated by circumferential stretch in guinea-pig distal colon. J Physiol. 2002;545:629–648. doi: 10.1113/jphysiol.2002.028647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. Stretch activated neuronal pathways in longitudinal and circular muscle of guinea-pig distal colon. Am J Physiol. 2003;284:G231–G241. doi: 10.1152/ajpgi.00291.2002. [DOI] [PubMed] [Google Scholar]
- Spencer N, McCarron SL, Smith TK. Sympathetic inhibition of ascending and descending interneurons during the peristaltic reflex in the guinea-pig distal colon. J Physiol. 1999;519:539–550. doi: 10.1111/j.1469-7793.1999.0539m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. The circular muscle is essential for mechanosensory transduction underlying stretch-activated neural pathways in distal colon. Neurogastroenterol Mot. 2003;15:208. (abstract) [Google Scholar]
- Tamura K, Ito H, Wade PR. Morphology, Electrophysiology and Calbindin Immunoreactivity of myenteric neurons in the guinea-pig distal colon. J Comp Neurol. 2001;437:423–437. doi: 10.1002/cne.1293. [DOI] [PubMed] [Google Scholar]
- Wade PR, Wood JD. Electrical behavior of myenteric neurons in guinea pig distal colon. Am J Physiol. 1988;254:G522–G530. doi: 10.1152/ajpgi.1988.254.4.G522. [DOI] [PubMed] [Google Scholar]
- Wilkens LA, Marzelli GA. Central inhibition of an identified mechanosensory interneuron in the crayfish. J Neurobiol. 1979;10:247–254. doi: 10.1002/neu.480100305. [DOI] [PubMed] [Google Scholar]
- Wood JD. Electrical and synaptic behaviour of enteric neurons. In: Schultz SG, Wood JD, Ranner BB, editors. Handbook of Physiology. Bethesda: American Physiological Society; 1989. pp. 465–516. [Google Scholar]
- Wood JD, Mayer CJ. Intracellular study of electrical activity of Auerbach's plexus in guinea-pig small intestine. Pflugers Arch. 1978;374:265–275. doi: 10.1007/BF00585604. [DOI] [PubMed] [Google Scholar]