Abstract
Short-term depression strongly influences neuronal activity in cerebral circuits and contributes to low-pass temporal filtering of information. In this work, we show that synaptic depression evoked by stimulation of commissural–Schaffer collateral afferents at 10 Hz is associated with a reduction of the fibre volley. This depression of action potentials is also evident in the absence of extracellular Ca2+, which underlies its release-independent nature. In addition, this reduction of the excitability is independent of failures in action potential propagation since increasing the distance between the stimulus and recording electrodes does not alter this effect. Whole-cell recordings show that tetanic stimulation at supraminimal intensity induces action potential failures preceded by changes in the repolarization rate of the action potentials leading the membrane potential to hyperpolarized values. This activity-dependent hyperpolarization was blocked by ouabain, an indication of the important role of the Na+ –K+-ATPase in this process. Then again, an alteration of the firing threshold was observed when action potentials were elicited either by somatic current injection or by synaptic stimulation, which indicates that this mechanism could alter the EPSP–spike coupling in these cells. The results suggest that these factors act together to reduce gradually the safety factor for action potential generation and to produce failures in action potential initiation; in fact, experiments made at twice the supraminimal intensity show a dramatic decrease in the rate of these failures. Taken together, the results suggest the existence of a release-independent component of short-term depression that is related to failures in action potential initiation.
Short-term synaptic depression is a form of plasticity that has been widely studied in recent years. This activity-dependent plasticity can last from milliseconds to minutes (Thomson, 2000; Zucker & Regehr, 2002) and has been implicated in the regulation of neuronal synchronization by conferring low-pass filtering properties to synapses (Abbott et al. 1997; Thomson, 2000). According to this, short-term depression has been postulated to be essential in the regulation of many cognitive functions including habituation (Chung et al. 2002; Nicolelis, 2002) and coordination of motor control (Nadim & Manor, 2000).
The mechanisms of short-term depression seem to be mediated mainly by the balance between vesicle depletion and refilling of the neurotransmitter in the synaptic terminal (Südhof, 2000; Harata et al. 2001). In addition, a decrease in synaptic strength can arise from the release of modulatory substances from the activated presynaptic terminals, postsynaptic cells, or neighbouring cells (Deisz & Prince, 1989; Von Gersdorff et al. 1997; Wu & Saggau, 1997; Micheva et al. 2003). Other processes such as inactivation of presynaptic Ca2+ channels (Forsythe et al. 1998; Patil et al. 1998) and postsynaptic mechanisms such as desensitization of ligand-gated receptors (Brenowitz & Trussell, 2001; Wong et al. 2003) have been found to contribute to short-term depression. However, sodium action potentials (APs) have received little attention as potential regulators of synaptic depression. Indeed, although sodium APs are generally considered all-or-nothing events, the state of inactivation or the local density of voltage-gated Na+ channels could influence synaptic efficacy by altering the action potential waveform or by promoting failure (Brody & Yue, 2000; Prakriya & Mennerick, 2000; He et al. 2002; Carr et al. 2003; Meeks & Mennerick, 2004).
Several reports have shown that backpropagated APs present an activity-dependent reduction of their amplitude when they propagate along the dendritic arbor (Colbert et al. 1997; Jung et al. 1997), and it has been suggested that this effect is mediated by a down-regulation of sodium channel activity. Nevertheless, no similar results have been found at somata level (Colbert et al. 1997; Mickus et al. 1999), which indicates a subcellular specialization for this mechanism. On the other hand, conduction failures have been found to produce frequency-dependent synaptic depression in various types of neurones, supposedly produced by local depolarizations or by increases in the membrane conductance (Hatt & Smith, 1976; Lüscher et al. 1994). Another form of conduction failure that occurs after a hyperpolarization and requires the activation of a fast A-type K+-current has been reported in CA3 pyramidal cells in vitro (Debanne et al. 1997; Kopysova & Debanne, 1998). This conduction block would depend on the recent activity of the cell and may produce failures during an AP train, giving axons a strong modulatory capability in the regulation of information processing.
In addition to conduction failures, myelinated and unmyelinated axons show activity-dependent hyperpolarization at frequencies within their physiological range (Morita et al. 1993; Kobayashi et al. 1997). Recently, Soleng et al. (2003a) have contributed evidence of this activity-dependent hyperpolarization, which could produce failures in Schaffer collaterals, but in contrast to the work of the Debanne group, they propose that these hyperpolarization-dependent failures might occur by changes in AP gating rather than by the block of AP conduction. However, Soleng's work provides only indirect evidence of this activity-dependent hyperpolarization, and the role of this phenomenon in short-term depression has not been assessed.
In an attempt to resolve these questions, we investigated whether changes in intrinsic excitability could be induced by a protocol of repetitive stimulation that usually induces synaptic depression in the terminals of CA3 cell axons of the hippocampus. We report that the compound action potential of these axons decreases during this protocol of repetitive stimulation, and that this decrease is produced mainly by failures in AP generation rather than by a reduction in AP amplitude or failures in AP propagation. We have also observed that these AP generation failures can be induced by changes in the firing threshold and by activity-dependent hyperpolarization of the membrane potential (Vm).
Methods
Hippocampal slice preparation
All experimental procedures described below were performed in accordance with the directives of the European Union (86/609/EEC) and Spanish legislation for the use and care of laboratory animals (BOE 65/8509-12, 1988). Transverse hippocampal slices (400 μm) were obtained by standard methods from neonatal (14- to 21-day-old) Wistar rats. Animals were killed by decapitation, and the hippocampi were quickly dissected in cold Krebs buffer. Slices were cut and placed in a humidified holding chamber for at least 1 h. A single slice was transferred to the recording chamber where it was submerged in a continuously superfusing solution saturated with 95% O2–5% CO2. The composition of the standard ACSF was (mm): 120 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 26.2 NaHCO3, 1 NaH2PO4, 11 glucose, 0.1 picrotoxin toxin (PTX), 0.05 d-aminophosphonovalerate (APV), pH 7.4 when equilibrated with 95% O2–5% CO2. In the experiments of Figs 1C, D performed in a calcium-containing Ringer solution, a concentration of 1–2 μm CNQX was added to the standard ACSF to reduce the field excitatory postsynaptic potential (fEPSP) and ensure a correct measurement of the presynaptic fibre volley. In calcium-free experiments, CaCl2 was omitted and replaced by an equimolar concentration of MgCl2; 2 mm EGTA were added and APV was omitted. A peristaltic pump (Gilson, Villiers le Bel, France) was used to circulate the solution (1.5–2 ml min−1) through the recording chamber, keeping the flow rate constant and avoiding any flow artefacts. Experiments were performed at 28–30°C.
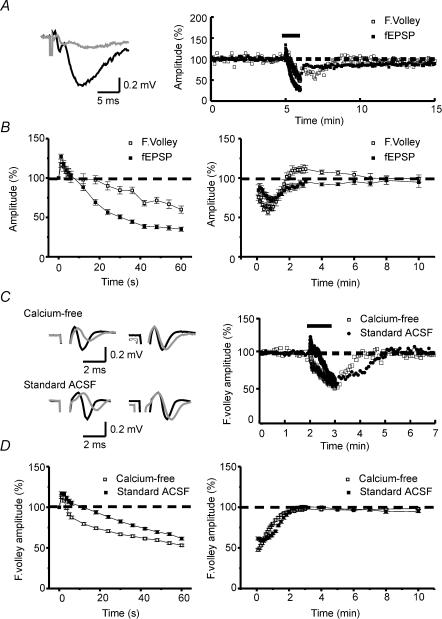
Figure 1. Fibre volley is depressed by repetitive stimulation.
A, averaged traces (left) of three consecutive recordings before (black line) and at the end (grey line) of the 10 Hz tetanization. Note the increase of latency and the decrease of amplitude in both the fibre volley and the excitatory postsynaptic potential (fEPSP) at the end of the tetanus. Sample experiment (right) showing the time course of normalized amplitudes of the fEPSP and the fibre volley. B, summary plot of normalized fibre volley amplitudes in representative pulses during (left) and after (right) the tetanic stimulation (n = 7). C, averaged traces (left panel) of three consecutive recordings before (black line) and at the end (grey line) of the 10 Hz tetanization. The left-hand traces show the increase of latency and the decrease of the fibre volley amplitude in both calcium-free and standard ACSF (plus 1–2 μm CNQX) experiments. Depression of the negative peak of fibre volley is greater than that of the positive peak. The right-hand traces show amplitude normalizations of the left traces. In these traces, differences in rise time and half-width appear in both calcium-free and standard ACSF experiments. The sample experiments in the right panel show the time course of normalized amplitudes of the fibre volley in both calcium-free and standard ACSF experiments. D, summary plot of representative pulses during (left) and after (right) tetanic stimulation in calcium-free (n = 46) and standard ACSF (n = 22) experiments. The absence of calcium does not block the short-term depression of the fibre volley. The bars above the plots in A (right) and C (right) represents the moment at which the 10 Hz (600 pulses) tetanization was delivered.
Extracellular field potential recording
Extracellular field potentials were recorded with a glass micropipette (filled with 3 m NaCl) placed in the middle of stratum radiatum of CA1. Cathodal stimuli were delivered to the commissural–Schaffer collateral afferents at 0.2 Hz with concentric bipolar stainless steel electrodes (FHC, Brunswick, ME, USA), and were generated using a Master-8 programmable pulse generator (AMPI, Jerusalem, Israel). Stimulus intensities were adjusted to generate peak-to-peak fibre volley amplitudes of 0.5 ± 0.3 mV at the beginning of the experiments. We chose these amplitudes because they were in the range of those used in experiments dealing with long-term and short-term plasticities (Vara et al. 2002, 2003). The intensities ranged between 0.1 and 0.4 mA (0.06 ms) in all extracellular experiments except those from Fig. 2 and those in which the depression of the positive peak was measured independently from that of the peak-to-peak amplitude, which were made using a wider range of intensities (0.25–1.25 mA, 0.06 ms). Short-term depression was induced by delivering a low-frequency stimulation protocol (LFS; 600 pulses at 10 Hz) only after at least 5 min of a stable baseline recording of both fEPSP and fibre volley amplitudes.
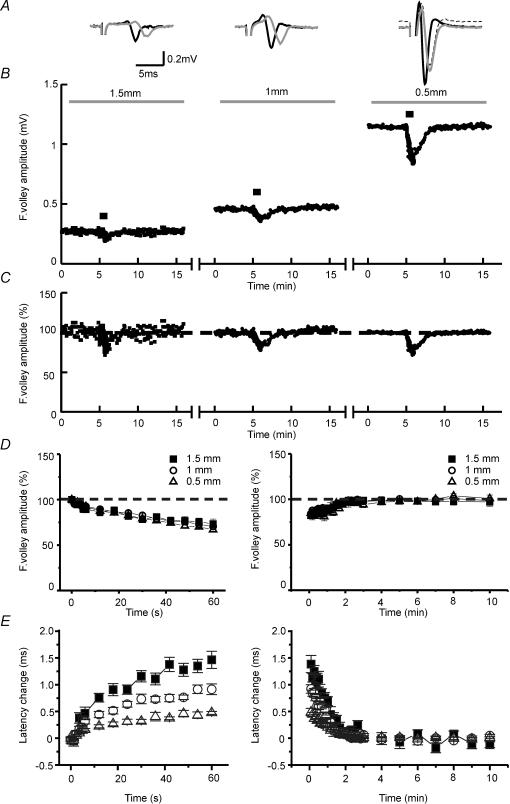
Figure 2. Short-term depression of the fibre volley is independent of axonal propagation failures.
A, averaged traces of three consecutive recordings before (black line) and at the end (grey line) of the 10 Hz tetanization at 1.5 mm (left), 1 mm (centre) and 0.5 mm (right) from the stimulus electrode. At high fibre volley amplitudes the baseline of the recordings shifts to positive values during the tetanus (dashed trace in 0.5 mm), indicating a higher ratio of cations to anions in the extracellular space. To allow a better comparison of the waveform of fibre volleys, we have normalized these traces to the baseline (grey line). B, sample experiment showing the time course of fibre volley amplitudes at different distances between stimulus and recording electrodes. The bar above the plot represents the moment at which the 10 Hz (600 pulses) tetanization was delivered. For clarity, the times are reset for each condition since real times are usually longer. Note how fibre volley amplitude increases as distance between electrodes decreases. C, the same experiment as B but with fibre volley amplitudes normalized to allow a better comparison between conditions. D, summary plot of normalized fibre volley amplitudes in representative pulses during (left) and after (right) the tetanus at different distances between stimulus and recording electrodes (n = 7). No differences are detected when distances between electrodes vary. E, summary plot of latency changes in representative pulses during (left) and after (right) the tetanus at different distances between stimulus and recording electrodes (n = 7). Latency changes induced by 10 Hz stimulation increase with the distance between electrodes, indicating changes in conduction velocity.
Whole-cell recordings
Whole-cell recordings were obtained from CA3 pyramidal cells using the ‘blind’ method. The patch pipettes were filled with a solution containing (mm): 125 potassium gluconate, 10 EGTA, 10 Hepes, 8 NaCl, 3 Tris-ATP, 0.3 GTP, and 2 MgCl2, except in experiments performed in a calcium-containing Ringer solution in which EGTA were excluded to avoid any interference with calcium-dependent currents. When filled with these solutions, the pipettes presented resistances of 5–10 μΩ. All the whole-cell experiments were performed in calcium-free media, except those of Figs 4D and 8, which were performed in standard ACSF. Cells were recorded with an Axoclamp 2A amplifier (Axon Instruments, Foster City, CA, USA) in the current-clamp bridge mode. Only cells with a stable resting membrane potential more negative than −50 mV and action potential amplitudes over 70 mV were used for recording. Occasionally, spontaneous bursts of action potentials appeared during the 10 Hz tetanization. Whenever this occurred, the recordings were not included in the analysis.
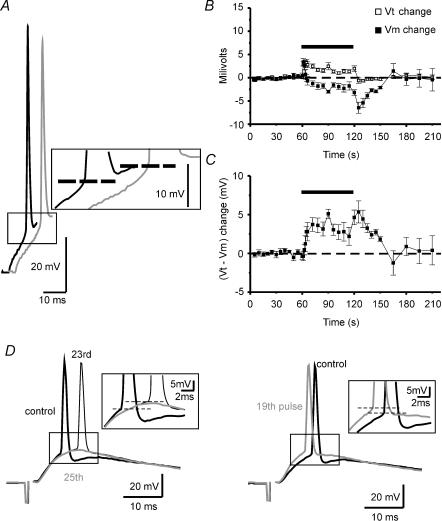
Figure 4. Changes in firing threshold during somatic current injection and synaptically induced EPSPs at 10 Hz stimulation.
A, sample traces of APs induced by somatic current injection recorded at pre-tetanus (black trace) and at the 372nd pulse (grey trace) of the tetanus, recordings at which the resting membrane potential was the same. Note how the firing threshold (dashed line) shifts to depolarized values during the tetanus. B, summary plot showing the time course of firing threshold (Vt) and membrane potential (Vm) changes (n = 10). Note how changes in Vt are developed and extinguished rapidly while those of Vm are maximal after the end of the tetanus. C, summary plot showing the time course of the changes in voltage jump (Vt–Vm) necessary to reach the firing threshold (n = 10). At the onset of the tetanus there is no net change in this value, but after a few pulses (coinciding with Vm hyperpolarization; see B), it increases rapidly and reaches its maximal value once the tetanus has ceased. D, sample traces of APs induced by synaptic EPSPs evoked by stimulation of commissural fibres. Thick black traces (control) show EPSPs recorded in current clamp at a Vm of ∼−10 mV from the resting membrane potential before the tetanic stimulation (see Methods). Thin black trace (23rd pulse) and grey trace (25th pulse) in the left panel show EPSPs recorded at resting membrane potential during the tetanic stimulation. Grey trace in the right panel represents the 19th EPSP of the 10 Hz tetanus recorded at resting membrane potential. Both panels show how the firing threshold (dashed line) shifts to depolarized values during the tetanus, inducing failures in AP generation at the same Vm and EPSP slope at which the cell previously fired (left panel). Interestingly, shifts in firing threshold can be observed even in faster slopes of the EPSP and shorter latencies from the stimulus onset (right panel). To allow a better comparison, all the recordings are amplified at the moment of the AP onset (see boxes in A and D). The bars in the summary plots represent the moment at which the 10 Hz (600 pulses) tetanization was delivered.
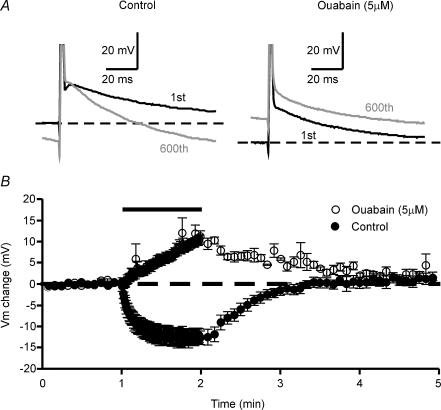
Figure 8. Activity-dependent hyperpolarization is blocked by ouabain.
A, sample traces of APs evoked by 1-ms 2 × SM somatic current pulses before (black traces) and at the end (grey traces) of the tetanus. B, summary graph of the time courses of Vm changes during tetanization in both standard ACSF (n = 6) and standard ACSF plus 5 μm ouabain (n = 4). Note how experiments performed under ouabain-containing Ringer solution developed an activity-dependent depolarization instead of an activity-dependent hyperpolarization. The bar above the plot represents the moment at which the 10 Hz (600 pulses) tetanization was delivered.
Antidromic action potentials were evoked by stimulating Schaffer collaterals in stratum radiatum near the CA2 area with cathodal stimuli (0.06 ms pulse width) delivered by monopolar stainless steel electrodes (FHC, Brunswick, ME, USA). Supraminimal stimulation was defined as the minimal intensity that evokes APs in all the baseline stimuli. To obtain these intensities we stimulated the Schaffer collaterals at high intensity (usually 0.15–0.3 mA); once an AP was evoked, we decreased the intensity of the stimulus electrode until no AP could be elicited; then it was increased gradually until 12 consecutive action potentials were induced.
In some experiments, APs were elicited by somatic depolarizing current injection through the recording electrode once every 5 s. For experiments in Fig. 4A–C, current pulses (0.1–0.5 nA, of 20 ms duration) were adjusted to induce an AP near the middle of the pulse width. For the experiments in Fig. 8, somatic current pulses of 1 ms of duration were delivered at high suprathreshold intensity, by obtaining the values of the somatic current pulses needed to depolarize the cell at membrane potentials slightly positive to the firing threshold, and then injecting twice this current to ensure that they were always suprathreshold.
Synaptically induced EPSPs were obtained by stimulating the commissural fibres in the stratum radiatum near the CA1 area with cathodal stimuli (0.06 ms pulse width) delivered by monopolar stainless steel electrodes. Since we placed the electrodes near the Schaffer collateral pathway, we occasionally recorded antidromic APs. When this occurred we moved the stimulus electrode to ensure that these APs did not alter the EPSP waveform and/or the EPSP-spike coupling. We observed in these experiments that during tetanization the membrane potential hyperpolarized initially and the EPSP slope varied. Since it has been observed that changes in these parameters alter the EPSP-spike coupling (Fricker et al. 1999; Axmacher & Miles, 2004), we made stimulations at different intensities and at different membrane potentials before tetanization to ensure that we obtained EPSPs with comparable membrane potential and slopes between the pretetanic and tetanic conditions.
Data analyses
The amplitude of the fEPSP was used as a measure of synaptic activity. The presynaptic fibre volley was triphasic and its amplitude was measured from the first positive peak that appears after the stimulus to the following negative peak. In whole-cell recordings, we defined AP onset for the ‘antidromic’ experiments and firing threshold for the experiments of Fig. 4, as the measured membrane potential when dV/dt exceeded 10 V s−1 (Fricker et al. 1999). The AP depolarization rate was measured as the slope between 15 and 85% of the AP amplitude. The fast repolarization rate was measured using the slope of the first third of the repolarizing phase, from 300 μs after the AP peak. We chose this value because fast repolarization K+ currents (A and D type) in CA3 pyramidal neurones have been found to reach their maximal activation at about this time (Mitterdorfer & Bean, 2002). Experiments in which either depolarization or fast repolarization rates varied above ± 10% during the last 12 pulses of the baseline were excluded from the analyses of these parameters. For slow repolarization rate, we measured the slope between 25 and 75 ms after the stimuli shock. To clarify and to facilitate the analysis of the data in the summary graphs, we plotted only some representative values. In the whole-cell experiments dealing with changes in AP waveform and latency, when an AP failure coincided with the time at which we took the values, we searched for a successful AP in the preceding or following 10 pulses. If no successful AP was produced at this time, the value was not taken into account for analysis. Recordings were digitized at 20 kHz and analysed with a MINTRA program. Data were analysed using appropriate one-way ANOVA and/or (paired or unpaired) Student's t tests, with P < 0.05 considered as statistically significant.
Pharmacology
All the drugs used were purchased from Sigma (St Louis. MO, USA) and made up as stock solutions in bidistilled H2O. Finally, they were diluted to their final concentrations in the external solution before use. The compounds were applied via bath perfusion through a peristaltic pump.
Results
Synaptic and fibre volley depression during low-frequency stimulation in Schaffer collaterals
Repetitive stimulation of synapses leads to a rundown in synaptic response, which may reflect a depletion of the vesicles available for release and/or an active reduction of release probability (Zucker & Regehr, 2002). We used a protocol of repetitive stimulation at physiological frequencies (10 Hz for 60 s) to obtain a strong synaptic depression. During tetanus, the fEPSP amplitude showed an initial facilitation/augmentation of +27.18 ± 3.15% (n = 7) and then was overwhelmed by a synaptic depression which, in the last stimulus of the tetanus, was −64.55 ± 2.77% (n = 7, Fig. 1B). To determine the contribution of presynaptic action potentials as regulators of short-term plasticity we measured the compound action potential of the commissural–Schaffer collateral axons evoked by the same protocol of repetitive stimulation. As with fEPSP amplitudes, this protocol produced an initial potentiation followed by the subsequent depression, although the initial augmentation was slightly greater for synaptic amplitude (fEPSP amplitude: +27.18 ± 3.15%; fibre volley amplitude: +16.7 ± 3.11%; n = 7, P < 0.05, paired t test; Fig. 1B). In addition, the magnitude of the depression at the end of the tetanus was also greater in the synaptic component (fEPSP amplitude: −64.55 ± 2.77%; fibre volley amplitude: −38.23 ± 4.69%; n = 7, P < 0.001, paired t test; Fig. 1B). After the tetanus, both components recovered their initial values in 2–3 min, and as occurred during the tetanus, the recovery of the depression showed similar kinetics but more pronounced in the fibre volley component, i.e. amplitudes for fibre volley and fEPSP 30 s after tetanus were reduced by 36.05 ± 1.6 and 22.27 ± 4.07%, respectively (n = 7, P < 0.01, paired t test, Fig. 1B). Taken together, these data suggest a modulatory axonal component playing an important role in short-term depression, although this phenomenon does not exclude the implication of other mechanisms since the magnitude of the changes observed during and after the LFS was different in the two components.
To determine whether this AP short-term depression was calcium dependent, we performed a second set of experiments using the same protocol but in calcium-free Ringer solution (see Fig. 1C and D). When we compared the results obtained in the standard ACSF with those in the calcium-free Ringer solution, we observed that the peak of the initial potentiation during the protocol of repetitive stimulation was greater in standard ACSF (+ 21.62 ± 2.08%, n = 22, versus +15.14 ± 1.29%, n = 46; P < 0.01, unpaired t test; Fig. 1D), and long-lasting (15.19 ± 2.01 s, n = 22, versus 3.85 ± 0.33 s, n = 46; P < 0.0001, unpaired t test; Fig. 1D). In addition, we observed that the final depression was slightly greater in calcium-free Ringer solution (−46.79 ± 1.5%, n = 46, versus −38.51 ± 1.87%, n = 22; P < 0.01 unpaired t test; Fig. 1D). In short, although we appreciate that there were some differences between the treatments, the results show that the absence of calcium not only fails to block the short-term depression of APs, but potentiates it. From these data, we conclude that although calcium can regulate part of the effect of repetitive stimulation on presynaptic excitability, the main mechanisms involved in the depression of APs must be calcium independent and therefore release independent.
We found another important effect of the repetitive stimulation when we compared the waveforms of the pretetanic fibre volleys with those at the end of the tetanus. Previous investigators had found that several intracellular parameters can be deduced from extracellular spike waveforms (Hubbard et al. 1969; Henze et al. 2000). In the case of a field potential generated by an action potential travelling along a nerve fibre, the extracellular recording electrode shows a triphasic wave. The first component is a small positive current induced by the passive source, which corresponds to the leading outward passive current as the impulse approaches. Once the action potential invades the recording area, the electrode registers the negative extracellular field potential produced by the active current sink, which corresponds to the current generated by the net inward currents flowing into the cell during the depolarizing phase of the action potential. Then, the recording electrode detects a more slowly rising positive current corresponding to the net outward currents during the repolarization (active current source) (Hubbard et al. 1969).
In our experiments, the negative field produced by the entry of sodium ions and the following positive deflection induced by repolarizing currents were slowed at the end of the tetanus, which suggests that both the rise time and the repolarization rate of the population of APs were altered by the repetitive stimulation (Fig. 1C). The latency to the first peak of the extracellular fibre volley was increased, indicating changes in the velocity of conduction and/or changes in the activation threshold (Fig. 1C). In addition, the depression of each peak of the fibre volley appeared asymmetric. To analyse this in detail, we measured independently the depression of both the positive peak and the peak-to-peak amplitude of the fibre volley. We minimized the contribution of the electrical noise in the measurements of these parameters by excluding from analysis those experiments in which the amplitude of the positive peak was below 0.15 mV and by performing some new experiments at larger fibre volley amplitudes. Under these conditions, we found that at the end of the tetanus the depression of the positive peak was slightly smaller than that observed for the peak-to-peak amplitude (positive peak depression: −26.89 ± 2.62%; peak-to-peak depression: −30.679 ± 1.69%, n = 21, P < 0.05, paired t test). Since the extracellular first positive wave represents a passive source of the active sink, it depends directly on the amplitude of the AP and on the resting membrane potential. Considering that the main contribution to the depression that we observed should be related either to changes in action potential amplitude or to action potential failures, or to both, this reduction should be equivalent for the peak-to-peak and positive peak amplitudes. The disparity we found would imply that another component, such as a hyperpolarization of the membrane potential, might be responsible for the mild asymmetric reduction of these values.
APs short-term depression is independent of failures in propagation
One of the mechanisms proposed for the depression of the excitability is the block of conduction of APs along the axon (Hatt & Smith, 1976; Lüscher et al. 1994; Debanne et al. 1997; Kopysova & Debanne, 1998). To determine whether the action potential fails to propagate successfully along the axon during the tetanus, we compared the depression of the compound action potential amplitude at three different points along the axon by placing the stimulus electrode 0.2 mm from CA3, and moving the recording electrode to 1.5, to 1 and to 0.5 mm, from the stimulus. The same stimulus strength was used for each distance, and the slices were perfused with calcium-free medium. On analysing the baseline amplitude of the fibre volley at each distance, we observed that the shorter the distance between the electrodes the larger the amplitude (Fig. 2B). This phenomenon has been seen previously (Raastad & Shepherd, 2003) and could be due to many circumstances, including failures in propagation of the AP under basal conditions, or loss of fibres as a result of the slice preparation. Since AP has been found to propagate reliably along the Schaffer collateral (Raastad & Shepherd, 2003), and since these axons travel in all directions along the stratum radiatum (Shepherd & Harris, 1998; Soleng et al. 2003b), it seems likely that when we move away from the stimulus electrode, the amplitude of the fibre volley is reduced because of a reduction in the number of intact fibres due to the slice dissection angle. When comparing the effects of the 10 Hz tetanization at different distances between electrodes, we observed no differences during or after the LFS (Fig. 2C and D), which shows that the depression of the compound action potential is independent of the number of fibres recruited and of the distance between electrodes, so the data suggest that this plasticity is not related to conduction failures.
As mentioned above, the latency to the first peak of the fibre volley increased at 10 Hz stimulation (see Figs 1C and 2A). To ascertain whether the latency changes were due to a decrease in the conduction velocity of the APs we analysed these changes at different distances during the repetitive stimulation at 10 Hz. The results show that the changes in latency increased with the distance (latency changes at 0.5 mm: 0.47 ± 0.03 ms; 1 mm: 0.91 ± 0.1 ms; and 1.5 mm: 1.46 ± 0.16 ms; n = 7, F = 20.443, P < 0.0001, one-way ANOVA; Fig. 2E), even when the depression of the fibre volley amplitude was the same (Fig. 2D). Similar results were reported recently by Soleng et al. (2003a), who suggested that these changes in latency were due to an activity-dependent hyperpolarization. Interestingly, we also observed that in experiments with high fibre volley amplitudes (i.e. at 0.5 mm distances), the baseline of the recordings shifts to positive values during the tetanus (dashed trace in 0.5 mm). Since the baseline extracellular potential is produced by the difference between the concentration of some ions at the tip of the recording pipette and those at the level of the ground electrode, the changes that we observed could be indicative of a higher ratio of cation to anions in the extracellular space near the recorded fibres due to a hyperpolarization of the axons during the tetanus. Similar results were obtained in experiments with extracellular recording pipettes filled with the extracellular media instead of 3 m NaCl (data not shown). The need to stimulate a high number of fibres simultaneously to detect these changes could be related to the low sensitivity of the electrodes.
AP short-term depression is not produced by changes in AP amplitude
Several reports have shown that in dendrites, sodium channel activity can be down-regulated in an activity-dependent manner leading to smaller AP amplitudes (Colbert et al. 1997; Mickus et al. 1999), but we found no evidence of this mechanism in somatic sodium channel APs. Since the trains of stimuli used in the above-mentioned works were shorter than those used in our experiments, we next investigated whether antidromically elicited action potentials recorded from CA3 somata in calcium-free medium were reduced in a similar way under our conditions. As in the above reports, our whole-cell recordings showed that AP amplitudes at the end of the tetanus did not differ very much from those recorded before the tetanization (pre-tetanus: 100.39 ± 3.79 mV; 600th pulse: 101.76 ± 4.32 mV; n = 10, P > 0.3, paired t test). Interestingly, we observed that the latency of action potentials was gradually increased in all the cells recorded (Fig. 3A) with failures in 13 out of 20 cells (Fig. 3D). Similar results had been observed with extracellular recordings (Soleng et al. 2003a; see also Fig. 1C) and had been related to membrane potential hyperpolarization during repetitive stimulation. In studying the membrane potential changes during tetanus, we observed that the cells did in fact become hyperpolarized (pre-tetanus: −68.43 ± 1.3 mV; 600th pulse: −75.0 ± 1.8 mV; n = 16, P < 0.001, paired t test; Fig. 3B), and that latency and membrane potential changes were closely correlated during the tetanus (Fig. 3B and C). Figure 3C shows that 11 out of 15 cells presented correlations above −0.7. The remaining four cells presented no significant correlations and in fact these cells presented the lowest variations in membrane potential and latency changes, which made it difficult to measure their correlations. Taken together, these results suggest that the development of the hyperpolarization moved the resting membrane potential away from the firing threshold, giving longer times for AP conduction and producing the latency increase observed during the tetanus.
Figure 3. Changes in AP latency, failures and waveform during 10 Hz stimulation.
A, sample traces of APs recorded at pre-tetanus (black trace) and the beginning of the tetanus (grey lines). For clarity, only one of every two APs is shown. Note how the AP latency increases before a failure occurs. B, summary graphs of the time courses of Vm (▪, n = 20) and latency (□, n = 16) changes. Note the similar time courses, although of opposite sign, of the parameters. C, graph showing the values of the correlation between the changes in Vm and latency during the tetanus. Each circle represents the value of one experiment. •, significant correlations (P < 0.05); ○, non-significant correlations. D, summary graph of the averaged failures during the tetanus (n = 20). Zero value indicates the onset of the tetanus, so at this time no failures are produced. The rest of the values indicate the percentage of AP failures every 60 pulses (i.e AP failures between pulses 1–60, 61–120, 121–180 and so on). E, left: sample traces of APs recorded at pre-tetanus (black trace) and at the 486th pulse (grey trace) of the tetanus. These recordings were chosen because membrane potential is the same for both APs. For clarity, the recordings are normalized to the AP onset. E, right: summary plot indicating the time course of depolarization rate (n = 16). Note how changes in this parameter are developed and extinguished rapidly. F, graph showing the values of the correlation between the changes in depolarization rate and latency during the tetanus. Each circle represents the value of one experiment. •, significant correlations (P < 0.05); ○, non-significant correlations. Note the high variability of correlation values. G, left: the same sample traces as in E but, on this occasion, the test AP (grey trace) is normalized to the amplitude of the pre-tetanic AP (black trace). G, right: summary plot indicating the time course of fast repolarization rate (n = 16). H, left: the same traces as in E but with the test AP (grey trace) normalized to the amplitude of the pre-tetanic AP (black trace) and the time scale increased to allow a better comparison of the slow repolarizing component. G, right: summary plot indicating the time course of slow repolarization rate (n = 16) showing how this parameter increases gradually during the tetanus and decreases slowly at the end of the 10 Hz stimulation. The bars in all summary plots represent the moment at which the 10 Hz (600 pulses) tetanization was delivered.
Effects of repetitive stimulation on AP waveform
To study in depth the effects of the 10 Hz tetanization on the action potentials, we evaluated the changes in several parameters: the depolarization rate, fast repolarization rate and slow repolarization rate. Changes in the depolarization rate are known to reflect changes in sodium channel density (Madeja, 2000); on measuring this parameter we observed that at the end of the tetanus, it was reduced by −13.35 ± 2.11% (n = 13, P < 0.001, paired t test; Fig. 3E). In addition, this reduction in the rate of depolarization started abruptly at the beginning and remained stable during the rest of the repetitive stimulation, recovering completely 5 s after the tetanus (Fig. 3E). It is known that changes in sodium channel activity could alter the latency of APs (Hodgkin & Katz, 1949), so to study in detail whether these changes might form the basis of the latency delays observed with repetitive stimulation, we analysed the correlations between changes in the depolarization rate and latency during the tetanus. Figure 3F shows that six out of 15 cells presented significant negative correlations, two out of 15 presented positive correlations and the remaining seven cells did not correlate significantly. These results suggest that although changes in sodium channel functioning could contribute to AP delay, in our experiments the main mechanism responsible for these latency changes was the hyperpolarization of the cell.
When we measured the fast repolarization rate, an indicator of fast-repolarizing K+ currents, we found a reduction of −23.77 ± 3.56% (n = 13, P < 0.001, paired t test. Fig. 3G) at the end of the repetitive stimulation; this suggests that fast-repolarizing currents were not responsible for the activity-dependent hyperpolarization. Interestingly, in contrast to changes in the rise time of the APs, the reduction of the fast repolarization rate was developed gradually during the tetanus and recovered slowly to baseline values (Fig. 3G), an indication that several mechanisms affecting the AP waveform could be activated during tetanization.
In contrast to the depolarization and fast repolarization rate, the slow repolarization rate was accelerated during the tetanus (+ 98.61 ± 1.24%, n = 15, P < 0.0001; Fig. 3H). As with fast repolarization, the build-up and the recovery of this effect were gradual, with kinetics similar to those of the latency changes (Fig. 3B and H), so changes in membrane potential and latency seem to be related by a slow hyperpolarizating agent rather than by a faster one.
Repetitive stimulation alters firing threshold
As mentioned above, the failures in action potential generation that we observed could be explained by the activity-dependent hyperpolarization induced by the tetanic stimulation. However, other mechanisms must be taken into account, such as variations in the firing threshold. To determine this threshold, we recorded action potentials in the whole-cell configuration in calcium-free medium using orthodromic pulses of 20 ms and defining the threshold as the membrane potential at which dV/dt exceeded 10 V s−1. Under these conditions, the firing threshold during the baseline was −51.95 ± 1.46 mV, and shifted towards depolarization at the onset of the tetanus (12th pulse: −48.93 ± 1.57 mV; n = 10, P < 0.001, paired t test; Fig. 4B). After tetanus, the firing threshold returned to baseline levels immediately.
To determine whether these changes in firing threshold were also obtained in response to an excitatory postsynaptic potential (EPSPs) we stimulated the commissural fibres to induce suprathreshold EPSPs in standard ACSF. When we delivered the tetanization, we observed that in most of the cells the membrane potential became hyperpolarized and the EPSP slope increased at the beginning of the tetanus, then the EPSP slope started to decrease to subthreshold values and no comparison between tetanic and pre-tetanic firing threshold could be made. Since it has been shown that faster slopes and hyperpolarized resting potentials decrease the firing threshold (Fricker et al. 1999; Axmacher & Miles, 2004), we compared only the threshold for action potentials coupled to EPSPs of similar slope and resting potential before and during the tetanus (see Methods). Under these conditions, we observed that the firing threshold shifted to depolarized values during the repetitive stimulation (pre-tetanus: −41.0 ± 1.2 mV; tetanus: −39.3 ± 1.0 mV; n = 7, P < 0.01, paired t test), and these changes could lead to failures in action potential generation (Fig. 4D). In addition, this alteration on EPSP–spike coupling was also evident when the depolarization rate of the tetanic EPSP was faster than the pre-tetanic one (Fig. 4D, right), so the real alteration of this parameter could be even larger than the values that we have reported. Taken together, these data suggest that both hyperpolarization and changes in firing threshold can act in coordination to increase the voltage jump necessary to generate an action potential (see Fig. 4C).
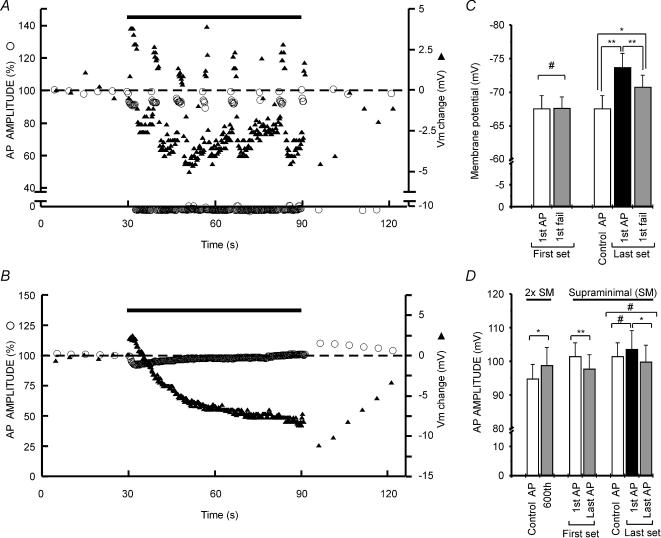
It has been reported that in normal conditions, the AP is generated in the axon of the neurones (Stuart & Sakmann, 1994; Colbert & Johnston, 1996; Stuart et al. 1997), so the AP recorded in whole-cell experiments is the back-propagated AP when it reaches the soma (Colbert & Johnston, 1996). This would imply that the somatic depolarization that we induced, whether by somatic current injection or by synaptic EPSPs to produce the AP in the axon could inactivate some sodium channels of the soma before the AP was backpropagated, altering the values we obtained for the firing threshold. To sidestep this problem, we proceeded to investigate whether those changes in the safety factor for AP generation were also involved in AP failures when axons are directly stimulated. We made whole-cell recordings of CA3 pyramidal cells and induced action potentials antidromically by stimulating Schaffer collaterals at two different intensities: supraminimal (SM) and twice supraminimal (2 × SM) in calcium-free medium. The ideal configuration should include patch-clamp recordings of Schaffer collaterals, but unfortunately these fibres are too thin to be recorded directly. Under these conditions, the tetanization at 10 Hz should induce more failures at supraminimal intensities than at 2 × SM, and this is what we found. At SM intensity, the latency of APs increased gradually during the tetanus up to a point at which the APs began to fail. After some failures the cell became reactivated and fired some more APs until they began to fail again. This phenomenon was cyclic (see Fig. 6A) and was observed in 11 of 13 cells. As shown in Fig. 5C, in the interval between six and 12 s of SM tetanic stimulation, 64.24 ± 8.91% of the stimuli (n = 13, Fig. 5C) did not generate any action potential, and this proportion of failures remained constant during the tetanus. In contrast, at 2 × SM tetanic stimulation, very few AP failures were observed (n = 6, Fig. 5C), and the delay in AP appearance increased further (Fig. 5B).Similar data had been observed with extracellular recordings (Soleng et al. 2003a) and, together with our results, they clearly indicate that, during a sustained train of stimuli, these changes of membrane potential and firing threshold could reduce the safety factor for AP generation.
Figure 6. Patterns of AP failures during 10 Hz stimulation.
A, representative experiment showing the time course of AP amplitude and membrane potential changes at SM intensity. Note how successful APs are grouped in sets resembling bursts. Changes in Vm are also evident during ‘burst’ and ‘interburst’ periods. B, representative experiment showing the time course of AP amplitude and membrane potential changes at 2 × SM intensity. AP amplitudes hardly vary and no AP failures are observed. In these experiments, hyperpolarizations are stronger than those observed in SM experiments, indicating an accumulative effect of APs on this mechanism. C, summary graph of Vm values at different moments of the tetanus at SM intensity (n = 8). White bars represent control (last pulse prior to tetanic stimulation) Vm values. Note that these values are the same as those we called 1st AP of the 1st set. The first set refers to the first group of successful action potentials and the last set to the last complete group of successful APs. D, summary graph of AP amplitude values at different moments of the tetanus at SM (n = 8) and 2 × SM (n = 7) intensities. White bars represent baseline values. Baseline values and sets of AP are defined as in C. The bars above the plots in A and B represent the moment at which the 10 Hz (600 pulses) tetanization was delivered. *P < 0.05, and **P < 0.01, significant differences (Student's paired t test); # non-significant differences.
Figure 5. Changes in AP waveform and block of AP failures induced by 10 Hz stimulation at 2 × SM intensity.
A, sample whole-cell recordings of experiments at SM and 2 × SM intensities. The vertical dashed lines show pre-tetanic AP onset, and the horizontal ones the resting membrane potential. B, summary plot of time course of the latency changes at both SM (n = 10) and 2 × SM (n = 6) intensities. C, summary graph of the averaged failures during the tetanus at both SM (n = 10) and 2 × SM (n = 6) intensities. Zero value indicates the onset of the tetanus so at this time no failures occur. The remaining values indicate the percentage of AP failures every 60 pulses (i.e. AP failures between pulses 1–60, 61–120, 121–180 and so on). Note the small proportion of failures at 2 × SM intensity. D, sample traces of whole-cell recordings plotted at wide time scale to allow a better observation of the slow repolarization rate. E, summary plot indicating the time course of slow repolarization rate at SM (n = 13) and 2 × SM (n = 6) intensities. F, summary graph of the time courses of Vm changes at SM (n = 13) and 2 × SM (n = 6) intensities. Note how membrane potential becomes more hyperpolarized in the high intensity protocol. G, top: the same sample traces as in D but the test APs is normalized to the amplitude of the pre-tetanic APs and the time scale is reduced. G, bottom: summary plot indicating the time course of fast repolarization rate at SM (n = 9) and 2 × SM (n = 6) intensities. H, top: the same sample traces as in D but for clarity the recordings are normalized to the AP onset and the time-scale has been reduced. H, bottom: summary plot indicating the time course of depolarization rate at SM (n = 9) and 2 × SM (n = 6) intensities. All recordings represented by black lines show pre-tetanic APs, and those by grey lines show the last AP of the tetanus. The bars in all summary plots represent the moment at which the 10 Hz (600 pulses) tetanization was delivered.
Effects of repetitive stimulation on action potentials are accumulative
To test whether changes in action potentials are dependent on the number of successful APs, we compared the effects of tetanization on the AP waveform at SM and at 2 × SM intensities. At high intensity, the rate of slow repolarization observed at the end of the tetanus was considerably faster than at SM intensity (2 × SM: +141.68 ± 11.39%, n = 6; SM: +68.98 ± 13.12%, n = 8; P < 0.01, unpaired t test; Fig. 5D and E). Consequently, these cells were more hyperpolarized than those that received SM stimulation (Vm change: −10.75 ± 1.41 mV, n = 6; −3.18 ± 1.14 mV, n = 9; P < 0.01, unpaired t test; Fig. 5D and F), so latency changes were also larger (2 × SM: 1.09 ± 0.12 ms, n = 6; SM: 0.44 ± 0.16 ms, n = 8; P < 0.05, unpaired t test; Fig. 5A and B). In the case of the fast repolarization rate, we also found a greater effect when cells were forced to fire with the 2 × SM protocol (2 × SM: −31.66 ± 4.18%, n = 6; SM: −14.63 ± 4.16%, n = 6; P < 0.05, unpaired t test; Fig. 5G). Taken together, these results suggest that both the reduction in the fast repolarization rate and the hyperpolarization induced by tetanic stimulation clearly depend on an accumulative process.
In contrast, the rate of depolarization did not differ significantly when a 2 × SM protocol was applied (2 × SM: −15.57 ± 2.09%, n = 6; SM: −9.03 ± 2.98, n = 6; P > 0.1, unpaired t test; Fig. 5H). Since the rate of depolarization is an indicator of sodium channel availability (Madeja, 2000) and the firing threshold depends directly on the number of these channels ready to be opened (Fricker et al. 1999), we concluded that firing threshold changes are saturated early during the tetanus (see Fig. 3E, and also Figs 4B and 5H). However, it could also be that this effect is indeed accumulative and non-saturating, and that it is counteracted by an acceleration of the recovery from inactivation of these channels produced by the hyperpolarization of the membrane potential.
The time course of AP failures follows burst-like patterns
As mentioned above, at SM intensity the latency of APs increases gradually during the tetanus until they begin to fail, then, after some failures, the cell becomes reactivated and fires some more APs until they began to fail again. As shown in Fig. 6A, these patterns are cyclic and start after the depolarization of the membrane potential at the onset of the tetanus. Then the membrane potential becomes hyperpolarized gradually until the first failure in AP generation appears. After some failures, the cell starts to recover towards the resting membrane potential until a new AP is induced and the process is repeated. This oscillatory behaviour of membrane potential is not observed at 2 × SM since AP failures are not induced (Fig. 6B).
Taking advantage of these patterns of response, we tested the mechanism responsible of AP failures. One role is played by the activity-dependent hyperpolarization and the other by the change in the firing threshold. To study the contribution of each factor, we checked the potential at which the first failure occurred during the tetanus and compared it to the first AP of the tetanus. The results show that the failures appear at membrane potentials similar to those of the baseline (Vm baseline: −67.56 ± 1.94 mV; Vm 1st fail: −67.63 ± 1.67 mV; n = 8, P > 0.9, paired t test; Fig. 6C). When we repeated this test in the last set of successful APs of the tetanus, we observed that the failure appeared at a membrane potential more depolarized than those of the first AP of this set (Vm 1st AP: −73.67 ± 2.11 mV; Vm 1st fail: −70.77 ± 1.78 mV; n = 8, P < 0.01, paired t test; Fig. 6C). It appears, then, that during the tetanus, although a hyperpolarization is needed to take the membrane potential far away from the firing threshold, a previous change of this threshold could be necessary to induce AP failures. Indeed, the correct sequence of events could be as follows: after a few action potentials, the cell depolarizes and the firing threshold changes rapidly. Then an activity-dependent hyperpolarization is produced and a failure in AP initiation occurs (see Figs 4B and 6A). However, once the tetanization ceased, the sequence of events underlying failures would be different since no changes in the firing threshold were observed, but in contrast, the hyperpolarization of membrane potential reached its maximal value at this moment (see Fig. 4B), which suggests that changes in membrane potential can be strong enough to induce failures in action potential initiation.
Time course of the changes in action potentials during tetanus
In an overall analysis, we mention above that AP amplitudes did not vary during tetanus. However, the experiments illustrated in Fig. 6 show how AP amplitudes increase or decrease depending on the stimulation intensity. On careful analysis, we detected that AP amplitudes decreased slightly at the beginning of 2 × SM tetanic stimulation and then increased slowly, giving larger AP amplitudes at the end of the tetanus (baseline AP amplitude: 94.78 ± 4.3 mV; 600th pulse amplitude: 98.77 ± 5.34 mV; n = 7, P < 0.05, paired t test; Fig. 6D). Conversely, in SM experiments, the AP amplitude decreased slightly but consistently during each set of successful APs (baseline AP amplitude: 102.91 ± 3.81 mV; amplitude of the last AP of the 1st set: 99.12 ± 3.9 mV; n = 8, P < 0.01, paired t test; Fig. 6D). Interestingly, the amplitude of the 1st AP after a period of failures was fully restored to its initial values (baseline AP amplitude: 101.42 ± 4.06 mV; amplitude of the 1st AP of the last set: 103.59 ± 5.53 mV; n = 7, P > 0.3, paired t test; Fig. 6D).
Changes in AP amplitude indicate variations in sodium channel availability, although alterations in these channels could occur without any effect on this parameter. To avoid this limitation, we measured the rate of depolarization since it is a reliable reflection of changes in sodium channel functioning (Madeja, 2000). The results agreed perfectly with AP amplitude data, and the time courses were similar (see Fig. 7A). However, it must be taken into consideration that the recovery observed in these parameters after some failures actually occurred in APs that started at hyperpolarized resting potentials (Fig. 6C), so a restoration of either AP amplitude or AP rise time did not necessarily imply a complete restoration of sodium channel functioning. Taken together, all these data show that, although changes in sodium channel functioning during the tetanus are mild, they are important enough to induce changes in the firing threshold, and as stated previously, they could be very important in the induction of the failures in AP generation.
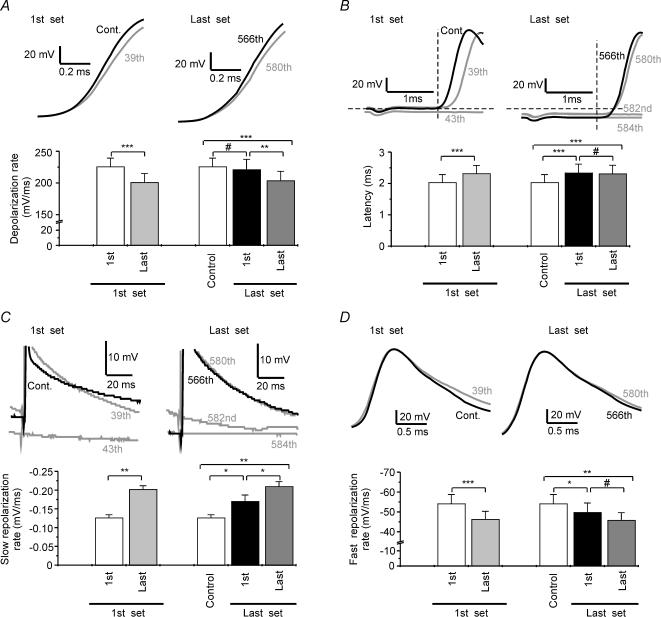
Figure 7. Patterns of changes in AP waveform during 10 Hz stimulation.
A, top: sample traces of the first (black lines) and the last (grey lines) AP of the first and the last set of APs normalized to the AP onset. A, bottom: summary graph of the depolarization rate at different moments of the tetanus (n = 7) in experiments with AP failures. B, top: traces of the same cell as in A but without normalization to the AP onset and at different time scales. B, bottom: summary graph of the latency values at different moments of the tetanus (n = 7) in experiments with AP failures. C, top: the same traces as in B but AP amplitudes have been truncated. C, bottom: summary graph of the slow repolarization rate values at different moments of the tetanus (n = 7) in experiments with AP failures. D, top: sample traces normalized to the amplitude of the first AP of the corresponding set of APs to allow a better comparison of the repolarizing rate. D, bottom: summary graph of the fast repolarization rate values at different moments of the tetanus (n = 7) in experiments with AP failures. White bars in summary graphs represent control (last pulse before tetanic stimulation) values. Note that these values coincide with those we also called 1st AP of the 1st set. The first set refers to the first group of successful action potentials and the last set to the last group of successful APs that precedes an AP failure. All recordings plotted with black lines show the first AP of each set of successful APs. For clarity, each AP is labelled with its ordinal number in the tetanus. 39th and 580th pulses represent the last AP of each set of successful APs. * P < 0.05, ** P < 0.01, *** P < 0.001, significant differences (Student's paired t test); # non-significant differences.
A report from Soleng et al. (2003a) has shown that repetitive stimulation generates changes in latency which reset when a failure in AP generation is produced. Since the tetanic stimulation used by these authors is considerably shorter than those used in our protocols, we tested whether a similar resetting of this parameter occurs under our conditions. We found that as noted above, the latency for AP induction was increased during the first set of APs (1st AP: 2.03 ± 0.25 ms; last AP of the 1st set: 2.31 ± 0.26 ms; n = 7, P < 0,001, paired t test; Fig. 7B). However, when we compared the 1st AP of the tetanus with the 1st AP of the last set of successful APs, we found a sustained delay in the appearance of the AP (1st AP of the 1st set: 2.03 ± 0.25 ms; 1st AP of the last set: 2.34 ± 0.28 ms; n = 7, P < 0,001, paired t test; Fig. 7B). A plausible explanation of this effect is that the membrane potential in the last set of APs was still hyperpolarized (Vm 1st AP of the 1st set: −67.56 ± 1.94 mV; Vm 1st AP of the last set: −73.67 ± 2.11 mV; n = 8, P < 0,01, paired t test; Fig. 6C).
In the case of the fast repolarization rate, we observed an initial reduction of the slope that was maximal at the end of the first set of successful APs (1st AP of the 1st set: 54.12 ± 4.63 mV ms−1; last AP of the 1st set: 46.25 ± 4.06 mV ms−1; n = 7, P < 0,001, paired t test; Fig. 7D) and that did not recover (1st AP of the 1st set: 54.12 ± 4.63 mV ms−1; 1st AP of the last set: 49.71 ± 4.78 mV ms−1, n = 7, P < 0,05, paired t test; Fig. 7D), which indicates a long-lasting mechanism underlying this effect.
Na+–K+-ATPase blockade prevents the activity-dependent hyperpolarization
We observed that the membrane potential hyperpolarizes when Schaffer collaterals are stimulated at physiological frequencies; several mechanisms could underly this effect. The main mechanism proposed involves the activation of an electrogenic Na+–K+-ATPase which would introduce two K+ ions and extrude three Na+ ions, when [K+]o and [Na+]i begin to increase during the tetanization (Rang & Ritchie, 1968; Morita et al. 1993; Kobayashi et al. 1997; Beaumont et al. 2002). To test this possibility we evoked the APs by administering suprathreshold somatic current pulses of 1 ms duration (see Methods) in the presence (Fig. 8B) and absence (Fig. 8A) of the Na+–K+-ATPase inhibitor ouabain (5 μm). As would be expected from the increase in [K+]o and [Na+]i ions produced by Na+–K+-ATPase blockade during repetitive stimulation, the activity-dependent hyperpolarization shifted to an activity-dependent depolarization in the presence of ouabain (Vm change in standard ACSF: −12.40 ± 1.96 mV, n = 7; Vm change in ouabain-containing Ringer solution: +11.27 ± 1.04 mV; n = 4, P < 0.0001, unpaired t test; Fig. 8C).
Discussion
We investigated a release-independent mechanism of presynaptic depression using a protocol of repetitive stimulation that induces synaptic depression in terminals of CA3 cell axons of hippocampus. The results of our extracellular experiments indicate that the amplitude of the action potential population is noticeably altered during repetitive stimulation at physiological frequencies. At the onset of the tetanus some initial potentiation emerges and then a depression of the fibre volley amplitude. These effects can be observed even with calcium-free Ringer solution or in the presence of antagonists of glutamate and GABA ionotropic receptors, an indication of their release-independent nature. In addition to this modulation of amplitude, we observed changes in waveform and delays in the AP latency. Similar results have been observed in whole-cell experiments, and together with reports from other authors (Debanne et al. 1999; Brody & Yue, 2000) they suggest that short-term plasticity is regulated to some degree by changes in spike activity and waveform.
Several mechanisms could underlie this short-term depression of the action potentials: one could be the reduction of AP amplitude because of a down-regulation of sodium channel activity, and another the induction of failures by alterations in the action potential generation and/or propagation. Changes in sodium action potentials have been described in dendrites of pyramidal neurones, but no similar results were observed in soma or axons (Colbert et al. 1997; Mickus et al. 1999). In our experiments, the amplitude of the APs hardly varied, but we saw how failures were produced when tetanic stimulation was delivered at supraminimal intensities.
Mechanisms underlying action potential failures
Many reports have described blocks of AP conduction after repetitive stimulation in various cell types (Hatt & Smith, 1976; Lüscher et al. 1994; Debanne et al. 1997; Kopysova & Debanne, 1998), and several mechanisms underlying these effects have been proposed. On the one hand, some authors argue that conduction failures are produced by local depolarizations or by increases in the membrane conductance (Hatt & Smith, 1976; Lüscher et al. 1994), whereas conduction failures that occur after a hyperpolarization and require the activation of a fast A-type K+ current have been reported in CA3 pyramidal cells in vitro (Debanne et al. 1997; Kopysova & Debanne, 1998). In relation to the first hypothesis, we observed that during repetitive stimulation, membrane potentials are hyperpolarized rather than depolarized (see Fig. 3B and also Figs 4B and 5F). A possible explanation of these differences is that the previous studies were made in neuromuscular junctions of crayfish (Hatt & Smith, 1976) and dorsal root ganglion cells (Lüscher et al. 1994), structures with architectures, cell types and environments very different from those of the hippocampus. In relation to the Debanne group hypothesis, a mechanism dependent on A-type K+ current seems unlikely since, although a hyperpolarization of the membrane potential was observed, we detected a down-regulation of fast-repolarizing K+ currents during tetanus. The difference may lie in the protocols used (paired stimulation versus extensive repetitive stimulation). Further evidence against the conduction block hypothesis comes from our experiments showing that at Schaffer collaterals, the depression of the compound action potential is not due to a block of AP propagation; increasing the distance between the stimulus and the recording site did not alter the degree of AP short-term depression. Moreover, these activity-dependent failures decreased at 2 × SM intensity whereas if they had been produced by a block of AP conduction, they should have been independent of the stimulus intensity. All these data, together with those from Raastad & Shepherd (2003), who found that action potentials propagate faithfully throughout axon arbors of stratum radiatum, demonstrate that activity-dependent failures of APs are not due to blocks in axonal propagation.
Therefore, a failure in action potential initiation seems to be the most likely hypothesis. It has been suggested that such a failure is produced by an activity-dependent hyperpolarization of membrane potential (Soleng et al. 2003a), although other mechanisms such as alterations of sodium channel functioning should be taken into account (Fleidervish et al. 1996).
Comparison of changes in APs recorded in whole-cell configuration with axonal recordings of APs obtaining by extracellular techniques
Before we discuss changes in firing threshold or waveform of the AP recorded with the whole-cell technique, we must mention some limitations of this approach. The principal problem of these recordings is that in normal conditions the AP is generated in the axon (Stuart & Sakmann, 1994; Colbert & Johnston, 1996; Stuart et al. 1997) so we recorded the back-propagated AP when it reaches the soma (Colbert & Johnston, 1996). We cannot be sure that the changes induced by repetitive stimulation that we measured are produced at axon and/or at soma level. This would mean that, if the AP changes that we measured were the somatic transformation of the AP generated in the axon, we cannot be sure that these changes in the threshold and/or the waveform of these action potentials are be the same in the axon.
This limitation could be overcome by making patch-clamp recordings of the Schaffer collaterals, but this is technically impossible because they are too thin to be recorded, so we can only infer that these alterations in APs also occurred in the axon from extracellular recordings. In the present study, we observed that the changes in latency, rise time and repolarization rate that we detected in whole-cell recordings (Fig. 3) were also observed in extracellular recordings (Fig. 1). In addition, the activity-dependent hyperpolarization evoked by repetitive stimulation could also be inferred from the fact that during tetanization the baseline of the recordings shifted to positive values during the tetanus (Fig. 2, dashed trace in 0.5 mm), indicating a higher ratio of cations to anions in the extracellular space near the recorded fibres and a hyperpolarization of the axons during the tetanus. Taken together, these results suggest that the effects observed in the somatic APs could be generalized to axons.
Changes in Na+ channels during repetitive stimulation
Voltage-sensitive Na+ channels determine the threshold of action potential generation and affect the upstroke of the action potential (Hodgkin & Huxley, 1952a). The fraction of Na+ channels available for opening at a certain time is modulated to some extent by the balance between two voltage-dependent mechanisms: the inactivation and the recovery from inactivation of these channels. Since the time for recovery from inactivation of Na+ channels increases during a long steady depolarization (Hodgkin & Huxley, 1952b; Fricker et al. 1999), we expect that during repetitive stimulation, the fraction of available Na+ channel decreases, raising the firing threshold and lowering the rate of depolarization of the upstroke of action potentials. This kind of slow cumulative Na+ channel inactivation has been related to the regulation of dendritic electrogenesis, repetitive discharge and spike patterning, and usually leads to decreases in AP amplitude (Colbert et al. 1997; Johnston et al. 1999; Mickus et al. 1999). Although we have shown that changes in AP amplitude do not account for the changes observed in extracellular fibre volley, mild alterations in the recovery from inactivation of sodium channels can alter the firing threshold and therefore the generation of the action potential. In this sense, persistent Na+ currents show interesting gating kinetics such as their slow and long-lasting inactivation (Fleidervish et al. 1996). These currents are thought to be generated by the same Na+ channels that open transiently during action potentials (Alzheimer et al. 1993). Since they are activated at voltages more negative than those necessary to activate the transient current, they should have an important role in setting the spike threshold and determining the repetitive firing effects on the APs (Fleidervish et al. 1996). In our experiments, we saw some afterdepolarization that disappeared during the tetanus, but we cannot be sure that it was due to a persistent Na+ current, nor that its disappearance was the result of a reduction of this current. In addition, the disappearance of the afterdepolarization that we observed during the tetanus followed time courses very different from those of the changes in sodium channels measured from the rate of depolarization. This does not exclude a possible role for a persistent Na+ current in some of the effects that we report in this paper, but other possibilities such as the activation of some repolarizing agents (i.e. a K+ current or an electrogenic pump) could be responsible for the reduction of this afterdepolarization.
Activity-dependent hyperpolarization of membrane potential
As noted above, several reports have shown that repetitive stimulation of myelinated (Morita et al. 1993) and unmyelinated axons (Rang & Ritchie, 1968) could lead to activity-dependent hyperpolarization. However, only one indirect measure of this phenomenon has been made in Schaffer collaterals (Soleng et al. 2003a). In our experiments, we found that the membrane potential does hyperpolarize when Schaffer collaterals are stimulated at physiological frequencies. In addition, this activity-dependent hyperpolarization was completely blocked by the Na+–K+-ATPase antagonist ouabain (see Fig. 8), and similar results have been obtained by others in the axons of lizards (Morita et al. 1993) and crayfishes (Beaumont et al. 2002). Although our activity-dependent hyperpolarization was blocked by ouabain, this does not exclude a role for a K+ conductance in these hyperpolarizations since the block of the Na+–K+-ATPase could increase the [K+]o to levels at which the electrochemical gradient for K+ currents was near equilibrium. Several currents, such as the Na+-dependent K+ current that is activated by the accumulation of [Na+]i (Safronov & Vogel, 1996; Bhattacharjee et al. 2003; Franceschetti et al. 2003) and/or the ATP-sensitive K+ current that could be activated by the drop in intracellular ATP (Griesemer et al. 2002), fit well with the cumulative effect of APs on the hyperpolarization that we observed (see experiments at SM and 2 × SM stimulation intensities) since they can be regulated in an activity-dependent manner. Direct studies are needed, however, to elucidate this question.
Sequence of processes leading to AP failure
AP failures do not develop in a constant manner, and in fact the patterns of these failures are cyclic. Tetanus starts with a rapid depolarization of the membrane potential, probably dependent on the K+ efflux and Na+ influx that follow the first APs and could lead to an increment of the density of Na+ channels in an inactivated state. Indeed, it has been shown that both extracellular accumulation of K+ and depolarizations of membrane potential increase the time of recovery from inactivation of these channels (Hodgkin & Huxley, 1952b; Hille, 2001; Meeks & Mennerick, 2004). Together, these effects could lead to the rapid shift of the voltage threshold that we observed in our experiments, although these changes were not strong enough to cause any failure during this period. To induce failures, another change should occur, and it appears later in the form of an activity-dependent hyperpolarization. As stated above, other mechanisms, such as an activation of either the Na+–K+-ATPase and/or a K+ current, could underlie this process and the final effect is the development of AP failures. Interestingly, the cell remains hyperpolarized even after the first failures appear, an indication that the hyperpolarizing agent presents slow inactivation kinetics. After a few failures, the cell depolarizes slowly towards its resting potential. This depolarization could be due to inactivation of the hyperpolarizing agent and/or to the activation of an inwardly rectifying current such as the Ih current. The latter hypothesis would be supported by the results of Soleng et al. (2003a), who found that antagonists of this current could facilitate the activity-dependent hyperpolarization induced by repetitive stimulation. Together with this depolarization, Na+ channels would also increase their activity since their recovery of inactivation would be speeded up by the prior hyperpolarization, leading to the appearance of a new action potential and the repetition of the process.
Methodological implications
The consequences of the activity-dependent failures that we describe might be important in the interpretation of results of experiments that utilize prolonged repetitive stimulations. Usually, these studies consider the AP generation and waveform as constant. Indeed, many reports based on the study of short-term depression discuss the results only in terms of Ca2+-dependent mechanisms (Liley & North, 1953; Dobrunz & Stevens, 1997; Hanse & Gustafsson, 2001a,b; Dobrunz, 2002). It must be noted that our results do not exclude the importance of these mechanisms in the development of short-term synaptic plasticity since, as we have reported, the changes observed in the presynaptic fibre volley during the 10 Hz tetanus, did not mimic perfectly the changes in the postsynaptic response. However, the changes that we describe here are important enough to be taken into consideration in studies of short-term synaptic plasticity and in those that make use of minimal stimulation to study the probability of release of synaptic terminals. Some authors (Dobrunz & Stevens, 1997; Hanse & Gustafsson, 2001a,b; Dobrunz, 2002) use this method with stimulus intensities and frequencies close to those we use in the supraminimal protocol, but the synaptic failures are interpreted only through changes in probability of release without considering the effects of repetitive stimulation on AP generation. An easy way to solve this problem is to use minimal stimulation protocols at lower frequencies so that AP failures do not occur and the waveform of the APs remain constant.
Functional implications
It is widely known that hippocampal cells can fire rhythmically at frequencies close to those we used in this work. Therefore, the block of AP generation that we describe underscores the importance of this new form of plasticity that can last minutes and helps neurones to filter stimuli with low safety factors for AP induction, allowing axons to transmit information at this rate only when generated by strong inputs. Moreover, this plasticity is input specific, so it allows neuronal circuits to refine the information accurately at a stage previous to neurotransmitter release. In addition, the waveform of successful action potentials is altered during tetanus, giving wider half-widths. Agents that increase the half-width of the APs could also alter calcium entry to presynaptic terminals and therefore the neurotransmitter release capabilities (Colino et al. 1998; Wheeler et al. 1996), an additional way to regulate the kinetics of release- and calcium-dependent short-term depression.
Acknowledgments
We are grateful to Dr D. K. Selig who donated the MINTRA program. This research was supported by the Dirección General de Investigación Científica y Técnica (BFI2001-1185) and by the Comunidad Autónoma de Madrid (08.5/0054/2003 2).
References
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Alzheimer C, Schwindt PC, Crill WE. Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J Neurosci. 1993;13:660–673. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Miles R. Intrinsic cellular currents and the temporal precision of EPSP-action potential coupling in CA1 pyramidal cells. J Physiol. 2004;555:713–725. doi: 10.1113/jphysiol.2003.052225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont V, Zhong N, Froemke RC, Ball RW, Zucker RS. Temporal synaptic tagging by I(h) activation and actin: involvement in long-term facilitation and cAMP-induced synaptic enhancement. Neuron. 2002;33:601–613. doi: 10.1016/s0896-6273(02)00581-0. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci. 2003;23:11681–11691. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz S, Trussell LO. Minimizing synaptic depression by control of release probability. J Neurosci. 2001;21:1857–1867. doi: 10.1523/JNEUROSCI.21-06-01857.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DL, Yue DT. Release-independent short-term synaptic depression in cultured hippocampal neurons. J Neurosci. 2000;20:2480–2494. doi: 10.1523/JNEUROSCI.20-07-02480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer JT, Catterall WA, et al. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron. 2003;39:793–806. doi: 10.1016/s0896-6273(03)00531-2. [DOI] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34:437–446. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Johnston D. Axonal action-potential initiation and Na+ channel densities in the soma and axon initial segment of subicular pyramidal neurons. J Neurosci. 1996;16:6676–6686. doi: 10.1523/JNEUROSCI.16-21-06676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Magee JC, Hoffman DA, Johnston D. Slow recovery from inactivation of Na+ channels underlies the activity-dependent attenuation of dendritic action potentials in hippocampal CA1 pyramidal neurons. J Neurosci. 1997;17:6512–6521. doi: 10.1523/JNEUROSCI.17-17-06512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colino A, Garcia-Seoane JJ, Valentin A. Action potential broadening induced by lithium may cause a presynaptic enhancement of excitatory synaptic transmission in neonatal rat hippocampus. Eur J Neurosci. 1998;10:2433–2443. doi: 10.1046/j.1460-9568.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Action-potential propagation is gated by an IA-like potassium conductance in hippocampus. Nature. 1997;389:286–289. doi: 10.1038/38502. [DOI] [PubMed] [Google Scholar]
- Debanne D, Kopysova IL, Bras H, Ferrand N. Gating of action potential propagation by an axonal A-like potassium conductance in the hippocampus: a new type of non-synaptic plasticity. J Physiol (Paris) 1999;93:285–296. doi: 10.1016/s0928-4257(00)80057-1. [DOI] [PubMed] [Google Scholar]
- Deisz RA, Prince DA. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE. Release probability is regulated by the size of the readily releasable vesicle pool at excitatory synapses in hippocampus. Int J Dev Neurosci. 2002;20:225–236. doi: 10.1016/s0736-5748(02)00015-1. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Fleidervish IA, Friedman A, Gutnick MJ. Slow inactivation of Na+ current and slow cumulative spike adaptation in mouse and guinea-pig neocortical neurones in slices. J Physiol. 1996;493:83–97. doi: 10.1113/jphysiol.1996.sp021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- Franceschetti S, Lavazza T, Curia G, Aracri P, Panzica F, Sancini G, et al. Na+-activated K+ current contributes to postexcitatory hyperpolarization in neocortical intrinsically bursting neurons. J Neurophysiol. 2003;89:2101–2111. doi: 10.1152/jn.00695.2002. [DOI] [PubMed] [Google Scholar]
- Fricker D, Verheugen JAH, Miles R. Cell-attached measurements of the firing threshold of rat hippocampal neurones. J Physiol. 1999;517:791–804. doi: 10.1111/j.1469-7793.1999.0791s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesemer D, Zawar C, Neumcke B. Cell-type specific depression of neuronal excitability in rat hippocampus by activation of ATP-sensitive potassium channels. Eur Biophys J. 2002;31:467–477. doi: 10.1007/s00249-002-0241-3. [DOI] [PubMed] [Google Scholar]
- Hanse E, Gustafsson B. Factors explaining heterogeneity in short-term synaptic dynamics of hippocampal glutamatergic synapses in the neonatal rat. J Physiol. 2001a;537:141–149. doi: 10.1111/j.1469-7793.2001.0141k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanse E, Gustafsson B. Vesicle release probability and pre-primed pool at glutamatergic synapses in area CA1 of the rat neonatal hippocampus. J Physiol. 2001b;531:481–493. doi: 10.1111/j.1469-7793.2001.0481i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harata N, Pyle JL, Aravanis AM, Mozhayeva M, Kavalali ET, Tsien RW. Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci. 2001;24:637–643. doi: 10.1016/s0166-2236(00)02030-0. [DOI] [PubMed] [Google Scholar]
- Hatt H, Smith DO. Synaptic depression related to presynaptic axon conduction block. J Physiol. 1976;259:367–393. doi: 10.1113/jphysiol.1976.sp011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zorumski CF, Mennerick S. Contribution of presynaptic Na(+) channel inactivation to paired-pulse synaptic depression in cultured hippocampal neurons. J Neurophysiol. 2002;87:925–936. doi: 10.1152/jn.00225.2001. [DOI] [PubMed] [Google Scholar]
- Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, Buzsaki G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J Neurophysiol. 2000;84:390–400. doi: 10.1152/jn.2000.84.1.390. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3. Sunderland, MA, USA: Sinauer Associates, Inc.; 2001. [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952a;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952b;116:497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol. 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard JI, Llinas R, Quastel DMJ. Electrophysiological analysis of synaptic transmission. In: Dawson H, Greenfield ADM, Whittam R, Brindley GS, editors. Monographs of the Physiological Society. London: Edward Arnold Publishers; 1969. pp. 265–293. [Google Scholar]
- Johnston D, Hoffman DA, Colbert CM, Magee JC. Regulation of back-propagating action potentials in hippocampal neurons. Curr Opin Neurobiol. 1999;9:288–292. doi: 10.1016/s0959-4388(99)80042-7. [DOI] [PubMed] [Google Scholar]
- Jung HY, Mickus T, Spruston N. Prolonged sodium channel inactivation contributes to dendritic action potential attenuation in hippocampal pyramidal neurons. J Neurosci. 1997;17:6639–6646. doi: 10.1523/JNEUROSCI.17-17-06639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi J, Ohta M, Terada Y. Evidence for the involvement of Na+-K+ pump and K+ conductance in the post-tetanic hyperpolarization of the tetrodotoxin-resistant C-fibers in the isolated bullfrog sciatic nerve. Neurosci Lett. 1997;236:171–174. doi: 10.1016/s0304-3940(97)00790-8. [DOI] [PubMed] [Google Scholar]
- Kopysova IL, Debanne D. Critical role of axonal A-type K+ channels and axonal geometry in the gating of action potential propagation along CA3 pyramidal cell axons: a simulation study. J Neurosci. 1998;18:7436–7451. doi: 10.1523/JNEUROSCI.18-18-07436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liley AW, North KAK. An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J Neurophysiol. 1953;16:509–527. doi: 10.1152/jn.1953.16.5.509. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Streit J, Lipp P, Lüscher H-R. Action potential propagation through embryonic dorsal root ganglion cells in culture. II. Decrease of conduction reliability during repetitive stimulation. J Neurophysiol. 1994;72:634–643. doi: 10.1152/jn.1994.72.2.634. [DOI] [PubMed] [Google Scholar]
- Madeja M. Do neurons have a reserve of sodium channels for the generation of action potentials? A study on acutely isolated CA1 neurons from the guinea-pig hippocampus. Eur J Neurosci. 2000;12:1–7. doi: 10.1046/j.1460-9568.2000.00871.x. [DOI] [PubMed] [Google Scholar]
- Meeks JP, Mennerick S. Selective effects of potassium elevations on glutamate signaling and action potential conduction in hippocampus. J Neurosci. 2004;24:197–206. doi: 10.1523/JNEUROSCI.4845-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Buchanan J, Holz RW, Smith SJ. Retrograde regulation of synaptic vesicle endocytosis and recycling. Nat Neurosci. 2003;6:925–932. doi: 10.1038/nn1114. [DOI] [PubMed] [Google Scholar]
- Mickus T, Jung H, Spruston N. Properties of slow, cumulative sodium channel inactivation in rat hippocampal CA1 pyramidal neurons. Biophys J. 1999;76:846–860. doi: 10.1016/S0006-3495(99)77248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterdorfer J, Bean BP. Potassium currents during the action potential of hippocampal CA3 neurons. J Neurosci. 2002;22:10106–10115. doi: 10.1523/JNEUROSCI.22-23-10106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, David G, Barrett JN, Barrett EF. Posttetanic hyperpolarization produced by electrogenic Na+-K+ pump in lizard axons impaled near their motor terminals. J Neurophysiol. 1993;70:1874–1884. doi: 10.1152/jn.1993.70.5.1874. [DOI] [PubMed] [Google Scholar]
- Nadim F, Manor Y. The role of short-term synaptic dynamics in motor control. Curr Opin Neurobiol. 2000;10:683–690. doi: 10.1016/s0959-4388(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA. Depression at thalamocortical synapses: the key for cortical neuronal adaptation? Neuron. 2002;34:331–332. doi: 10.1016/s0896-6273(02)00691-8. [DOI] [PubMed] [Google Scholar]
- Patil PG, Brody DL, Yue DT. Preferential closed-state inactivation of neuronal calcium channels. Neuron. 1998;20:1027–1038. doi: 10.1016/s0896-6273(00)80483-3. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Mennerick S. Selective depression of low-release probability excitatory synapses by sodium channel blockers. Neuron. 2000;26:671–682. doi: 10.1016/s0896-6273(00)81203-9. [DOI] [PubMed] [Google Scholar]
- Raastad M, Shepherd GM. Single-axon action potentials in the rat hippocampal cortex. J Physiol. 2003;548:745–752. doi: 10.1113/jphysiol.2002.032706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang HP, Ritchie JM. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968;196:183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV, Vogel W. Properties and functions of Na+-activated K+ channels in the soma of rat motoneurones. J Physiol. 1996;497:727–734. doi: 10.1113/jphysiol.1996.sp021803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3 –– > CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleng AF, Chiu K, Raastad M. Unmyelinated axons in the rat hippocampus hyperpolarize and activate an H current when spike frequency exceeds 1 Hz. J Physiol. 2003a;552:459–470. doi: 10.1113/jphysiol.2003.048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleng AF, Raastad M, Andersen P. Conduction latency along CA3 hippocampal axons from rat. Hippocampus. 2003b;13:953–961. doi: 10.1002/hipo.10141. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, Hausser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 1997;20:125–131. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle revisited. Neuron. 2000;28:317–320. doi: 10.1016/s0896-6273(00)00109-4. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Molecular frequency filters at central synapses. Prog Neurobiol. 2000;62:159–196. doi: 10.1016/s0301-0082(00)00008-3. [DOI] [PubMed] [Google Scholar]
- Vara H, Martinez B, Santos A, Colino A. Thyroid hormone regulates neurotransmitter release in neonatal rat hippocampus. Neuroscience. 2002;110:19–28. doi: 10.1016/s0306-4522(01)00541-3. [DOI] [PubMed] [Google Scholar]
- Vara H, Munoz-Cuevas J, Colino A. Age-dependent alterations of long-term synaptic plasticity in thyroid-deficient rats. Hippocampus. 2003;13:816–825. doi: 10.1002/hipo.10132. [DOI] [PubMed] [Google Scholar]
- Von Gersdorff H, Schneggenburger R, Weis S, Neher E. Presynaptic depression at a calyx synapse: the small contribution of metabotropic glutamate receptors. J Neurosci. 1997;17:8137–8146. doi: 10.1523/JNEUROSCI.17-21-08137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Changes in action potential duration alter reliance of excitatory synaptic transmission on multiple types of Ca2+ channels in rat hippocampus. J Neurosci. 1996;16:2226–2237. doi: 10.1523/JNEUROSCI.16-07-02226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AYC, Graham BP, Billups B, Forsythe ID. Distinguishing between presynaptic and postsynaptic mechanisms of short-term depression during action potential trains. J Neurosci. 2003;23:4868–4877. doi: 10.1523/JNEUROSCI.23-12-04868.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]