Abstract
Termination of a muscle contraction is as important a part of movement as muscle activation yet the mechanisms responsible are less well understood. In the present experiments we examined the possible role of intracortical inhibitory circuits in terminating a 20% maximum isometric contraction of the first dorsal interosseous muscle (FDI) in eight healthy subjects. Subjects performed the task simultaneously with both hands and received single or pairs (at an interstimulus interval of 3 ms to evaluate short interval intracortical inhibition, SICI) of transcranial magnetic stimuli (TMS) via a focal coil over the motor hand area of the left hemisphere at different times before and after the onset of relaxation. The amplitude of the motor-evoked potential (MEP) following a single or a pair of TMS pulses was measured in the right FDI and plotted relative to the onset of relaxation as estimated from the surface electromyogram (EMG) of the left FDI. MEPs were larger during contraction than after relaxation whereas SICI was absent during contraction and reappeared after relaxation. We found that in all subjects, the time course of MEP changes during relaxation was closely fitted by a Boltzmann sigmoidal curve which allowed us to estimate the mean MEP amplitudes as well as the ratio of the amplitudes after single or pairs of TMS pulses (i.e.%SICI) at any time in the task. The data showed that the amplitude of MEPs to single pulse TMS had started to decline at about the same time as the onset of EMG silence. Furthermore, the size of the MEPs evoked by paired pulses decreased up to 30 ms beforehand. The latter suggests that an increase in SICI occurs prior to the onset of MEP changes, and hence that increased cortical inhibition may play a role in suppressing corticospinal excitability during relaxation. A subsidiary experiment showed that the time relations of changes in SICI and MEP were unchanged by a period of 10 min training on the task.
Termination of an ongoing muscle contraction (‘muscle relaxation’) is an important part of movement control, particularly during rapid sequences of movements when activation must switch between different sets of contracting muscles. Indeed slowness in relaxing is characteristic of neurological conditions such as stroke, Parkinson's disease and dystonia, and may well contribute to the problems that these patients experience (Corcos et al. 1996). Although we have a good deal of information about spinal mechanisms of movement inhibition, such as are provided, for example, by Ia reciprocal inhibition or presynaptic inhibition (Schieppati & Crenna, 1985), much less is known about possible cortical contributions to muscle relaxation. Both EEG and PET studies have shown that cortical activations accompany (and precede) voluntary relaxation of muscle, but whether this activity simply reflects withdrawal of an excitatory motor command or a process of active inhibition is unknown (Terada et al. 1995; Rothwell et al. 1998; Toma et al. 1999, 2000).
Transcranial magnetic stimulation (TMS) has been used extensively to study the organization and physiology of the human motor cortex and can assess not only the excitability of the corticospinal pathway, but also the excitability of a variety of intracortical circuits. One such circuit is that activated by the paired pulse method of Kujirai et al. (1993) which gives information about the excitability of local GABAergic connections in motor cortex (SICI). Reynolds & Ashby (1999) showed that the excitability of this circuit decreases just prior to the onset of a contraction, at a time when it could contribute to increased cortical excitability in the target muscle. Two recent studies have also shown that the same circuit may be involved in preventing unwanted muscle contraction. Thus, the excitability of this pathway is increased after a no-go signal in go/no-go reaction task, consistent with a role in actively suppressing execution of prepared movements (Sohn et al. 2002). Similarly in some subjects, there is an increase in SICI to relaxed muscles that surround a focal contraction that may help prevent unwanted spread of muscle activation (Stinear & Byblow, 2003).
In the present experiments we test whether this pathway may play a role in the active termination of an ongoing muscle contraction. In addition, since it is possible that the mechanisms of muscle relaxation may depend on the instruction or practice given to subjects, we also tested whether a period of practice would change the mechanisms involved, perhaps by increasing the relative contribution of active inhibition to removal of ongoing excitation in order to speed up the process of relaxation.
Methods
We studied eight healthy subjects (3 males and 5 females; aged 26–33 years, mean ± s.d. 28.75 ± 3.73) without known neurological or psychiatric disease and taking no psychoactive drug. All subjects gave their informed consent and the experiment was approved by the local ethics committee and performed according to the Declaration of Helsinki.
Recordings and stimulation
EMG activity was recorded bilaterally from the first dorsal interosseus muscle (FDI) using pairs of surface electrodes (Ag–AgCl cups, diameter 9 mm) in the usual belly tendon montage. EMG signals were amplified (× 1000) and filtered with a band-pass filter of 20 Hz to 2 kHz. The data were collected through a CED 1401 laboratory interface (Cambridge Electronic Design (CED), Cambridge, UK), digitized with a sampling rate of 5 kHz and stored on a computer using a data collection program (Signal; CED). For further analysis off-line we used in-house software.
Paired-pulse TMS was performed using three Magstim 200 connected by a TriStim unit (Magstim Co, Whitland, Dyfed, UK), through a figure-of-eight shaped coil (external wing diameter 9 cm). The coil was placed over the hand area of the left motor cortex with the handle pointing backwards and laterally approximately 45 deg to the interhemispheric line and the stimuli were applied over the lowest threshold point (the ‘hot spot’) for evoking EMG responses in right FDI muscle at rest.
According to Rossini et al. (1994), motor thresholds (MTs) were defined as the lowest stimulus intensity that evoked an MEP with an amplitude > 50–100 μV in at least 5 of 10 successive stimuli at rest (relaxed motor threshold, RMT) and during contraction (active motor threshold, AMT). To evoke the maximal M-wave in the right FDI the ulnar nerve was stimulated at the wrist with stimuli of increasing intensity and constant duration of 0.5 ms.
Intracortical excitability was investigated according to the paired-pulse protocol described by Kujirai et al. (1993) with a subthreshold conditioning magnetic stimulus preceding a suprathreshold test stimulus. A single interstimulus interval of 3 ms was used to test the activity in intracortical inhibitory circuits (short interval intracortical inhibition; SICI). Since there is possible contamination of SICI at this interval with short latency I-wave facilitation (Ziemann & Rothwell, 2000) we used two intensities of conditioning stimulus (80% or 100% AMT) in the main series of experiments. We reasoned that since the threshold for I-wave facilitation is higher than that of SICI any I-wave effects would show up as a difference in behaviour at the two conditioning intensities. The stimulus intensity of the test pulse was selected so as to produce an MEP approximately 1 mV peak-to-peak amplitude.
An audio tone burst (50 ms duration) was used as the imperative signal during the task. A frequency of 1500 Hz signalled that subjects should contract the FDI muscle, whereas a frequency of 750 Hz signalled relaxation.
Experimental procedure
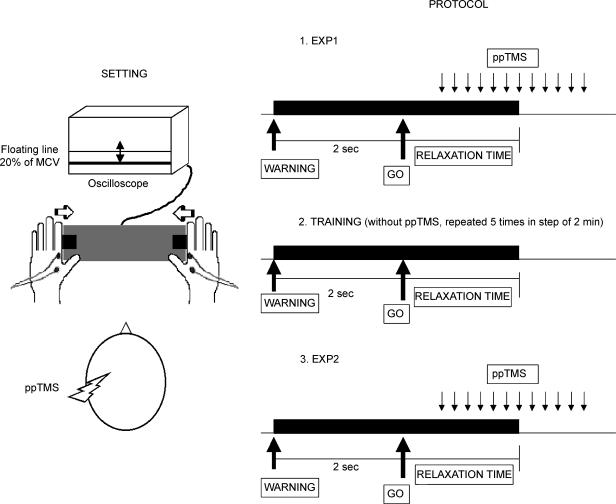
The subject was seated at a table with both forearms resting on the surface. The elbow was flexed to an angle of 90 deg and wrist pronated. The medial side of each index finger was positioned against a block so that subjects could make isometric abduction movements against the block. On the right side, the block contained a force transducer, the output of which was displayed on an oscilloscope in front of the subject. The voltage corresponding to a muscle maximum contraction was established as the best of three trials at the start of the experiment and a DC level representing 20% of this value was displayed on the oscilloscope so that the subject could sustain a constant level of contraction when required (Fig. 1, left).
Figure 1. The experimental setting (left) and the experimental protocol (right).
Baseline values of RMT, AMT and SICI were determined with the subject relaxed in this position. SICI at rest was studied with four intensities of conditioning (60, 80, 100, 120% of AMT) so that it was possible to define an intensity curve for intracortical inhibiton.
Experimental protocol
The experiment was divided into three parts (Fig. 1, right). In the first (‘experiment 1’) and in the third part (‘experiment 2’) at the acoustic go signal (1500 Hz) the subject had to make a bilateral isometric contraction to the pre-specified level with the FDI. This was sustained for 2 s, and followed by a second tone (750 Hz) that signalled that the subject should relax as quickly as possible and await the next trial. To avoid fatigue the intertrial interval was always 6 s.
During the process of relaxation, immediately after the second tone, SICI was evaluated at eight randomized different intervals (between 40 and 220 ms with respect to the second tone). For each interval 8–10 stimuli were applied through the ‘Tristim’ in each condition of the SICI protocol: test stimulus alone (T), test stimulus preceded by a conditioning stimulus at 80% AMT (c80), test stimulus preceded by a conditioning stimulus at 100% AMT (c100). Randomly, there were also 30 trials without any acoustic signals in which we checked SICI at rest (rest trial). The percentage SICI was calculated as the ratio between the mean amplitudes of the conditioned and test responses.
The second part of the experiment was a training session in which the subject had to practice to relax as fast as possible after the imperative signal without any TMS in five blocks of 2 min each.
Measurement and statistics
The peak-to-peak amplitude of the motor-evoked potential (MEP) in each trial was automatically measured by computer program and expressed as a ratio to the maximal compound muscle action potential (CMAP) evoked by supramaximal ulnar nerve stimulation (MEP/CMAP).
In experiments 1 and 2 the latency of the relaxation time (RT) was measured bilaterally in each trial. The RT of right and left FDI muscles was approximately equal in control trials without any MEPs. Thus we could use the left RT and assume it would be equal to that in the right hand in trials in which TMS was given. The time of the TMS stimulus was then expressed relative to the onset of relaxation in the left hand, identified in the surface EMG using an interactive cursor on the computer visual display. This method avoids problems in estimating the onset of relaxation in the presence of a large MEP in the right hand. In experiments 1 and 2, the timing of the responses was divided into 25 ms bins relative to the onset of relaxation and averaged. We then applied a curve fitting process to identify the best equation describing the modification of MEP amplitudes of both test and conditioned responses during execution of the task.
Different models (linear, asymmetric sigmoid, symmetric sigmoid and polynomial of second, third and fourth order) were fitted to these data and goodness of fit estimated. Then acceptable models were compared using an F test. The best fitting curve was the symmetric sigmoid model represented for its high goodness of fit (r2) from the Boltzmann equation:
in which the parameters a and b represent the minimum and maximum MEP amplitudes during the task, and t the time relative to onset of EMG relaxation. The parameter c represents the time at which the MEP amplitude is equal to half of the difference between a and b. Finally the parameter d represents the slope of the curve reflecting the rate of change of the MEP amplitude.
In each subject in both experiments (experiment 1 and experiment 2) and at each intensity of conditioning shock this equation was fitted to all the data points for (a) MEPs to the test stimulus alone (T), (b) MEPs conditioned by a preceding stimulus at 80% AMT (C80), and (c) MEPs conditioned by a preceding stimulus at 100% AMT (C100). The curve was accepted as a reasonable description of the data if its goodness of fit (r2) was greater than 0.9. Values of MEP amplitude that were estimated from this curve (rather than the actual data points themselves) we termed estimated MEPs (eMEPs). These allowed us to extrapolate data points for all times during the relaxation task. In addition, by calculating the ratio between the estimated MEP amplitudes of the test and conditioned responses, we could obtain an estimate of the time course of SICI (eSICI).
For each parameter obtained from this equation, analysis of variance (two-factor ANOVA with ‘MEP TYPE’ and ‘EXPERIMENT’ as factor between subjects) was used to evaluate the effect of conditioning stimulus and the effect of the training on the three sets of mesaurements (T, C80, C100).
In each millisecond of the considered range (100 ms before and after the onset of relaxation), mean eSICI and mean SICI measured at rest before the task and similarly mean control eMEP and mean MEP at rest were compared by using a paired t test to define the time of no statistical difference (TNSD) between estimated and real values at rest.
The significance level was set at P < 0.05 for all experiments.
Results
TMS parameters and SICI at rest
The mean (± s.d.) RMT and AMT were 48 ± 8 and 39 ± 6% of the maximum stimulator output, respectively. The mean amplitude of test stimulus was 0.90 ± 0.35 mV and the mean value of intensity needed to obtain it was 59 ± 16% maximum output.
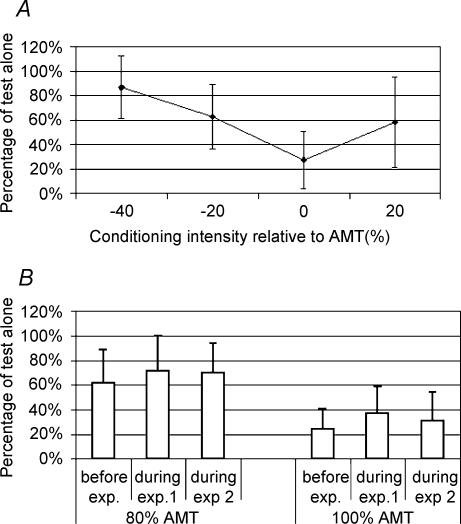
Figure 2A shows the effect of varying the conditioning shock intensity on SICI at 3 ms ISI in the relaxed FDI. The data are similar to those described by other authors (e.g. Kujirai et al. 1993; Ridding et al. 1995a; Butefisch et al. 2003). A one-factor ANOVA showed a significant effect of intensity (F = 9.14, DF = 3, 8; P < 0.002) with maximal inhibition (24% of test alone) if the conditioning intensity was 100% AMT.
Figure 2. SICI at 3 ms in the relaxed FDI.
A shows the effect of varying the intensity of the conditioning shock on the percentage SICI. Intensities are expressed relative to active motor threshold (AMT). Each point represents the mean value (± s.d.) of inhibition for eight subjects. B shows that the baseline amount of SICI in subjects at rest before and in control trials during each experiment was the same. The percentage SICI is shown for conditioning stimulus intensities of 80% and 100% AMT. Each bar represents the mean value (± s.d.) of eight subjects.
We also checked that SICI remained constant during the rest trials that were interspersed during the relaxation task in experiments 1 and 2. Figure 2B shows that SICI was the same during task performance when subjects were at rest as in the control period before the relaxation task (one-way ANOVA, P > 0.05) although there was a tendency for inhibition to be greatest in the control period when no movement was required of the subjects. SICI at 100% AMT was more variable (coefficient of variation, CV = 70%) compared with that at 80% AMT (CV = 43%), but this may be due to the smaller value of the mean percentage SICI with a conditioning stimulus of 100% AMT (see, e.g. Orth et al. 2003).
Relaxation reaction times
The relaxation task required subjects to stop contracting the FDI muscle in both hands simultaneously. In experiments 1 and 2 we used the relaxation time as measured in the left hand to estimate the time of relaxation in the right hand since the data on the right was often obscured by the EMG responses to TMS. To confirm that the reaction times in both hands are the same in this task, we measured the mean RTs for each subject in each of the five phases of the intermediate training session (average ± s.d. of mean RTs of the five phases of all subjects was 133 ± 36 ms for right side and 134 ± 37 for the left side) and performed a two-way ANOVA (with SIDE as a between subjects factor and TIME as a within subjects factor) with the mean values of RT of each subject. There was no significant main effect of SIDE or TIME and no interaction between them. Thus we could rely on using the RT in the left FDI to estimate the RT on the right.
The onset of relaxation potentially can be defined as the time at which the EMG during contraction begins to decline or as the time when the EMG activity has disappeared. Because estimation of the former on individual trials was difficult we used the latter as a default solution. However, in order to give some indication of how this estimate related to the onset of the decline in EMG activity we constructed a rectified average EMG of all the trials in each subject aligned on the identified point of relaxation (i.e. the absence of EMG). We could then measure the difference in latency between the time at which EMG began to decline below its average value, and our identified time of relaxation. Across subjects, this gave values of −7 ± 7 ms (s.d.) and −16 ± 19 ms in experiment 1 and 2, respectively.
MEPs obtained during the relaxation task
The mean amplitude (± s.d.) of the test MEPs during experiments varied from a maximum of 12.1 ± 2.2 mV during contraction to a minimum of 0.4 ± 0.3 mV after complete relaxation. The mean amplitude of the maximal M-wave was 21.8 ± 4.9 mV.
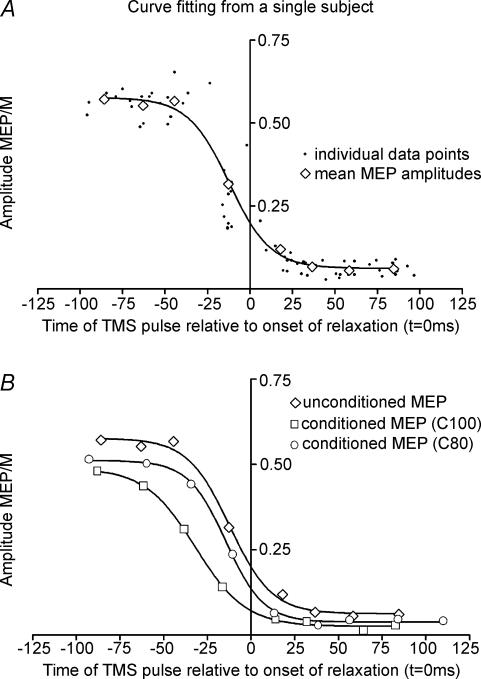
Figure 3A shows data from one subject in whom the amplitude of the test MEP has been plotted at different times relative to the onset of relaxation. The data were then grouped into 25 ms time bins and the mean value of the MEP and the mean time interval of the data in that bin have been plotted as the larger superimposed diamonds. Before the onset of relaxation, the MEP was large, but declined quickly around the time of relaxation.
Figure 3. Example from one subject.
A, change in amplitude of the test MEP as a function of the time of the TMS pulse relative to the onset of muscle relaxation (0 ms). The amplitude of the MEP is expressed as a fraction of the amplitude of the maximal peripheral M-wave. Small dots are the data from each single trial; the diamonds represent the mean value of the amplitude and the timing of the MEPs in bins of 25 ms. B, the mean data from the top graph have been plotted with two other lines describing the behaviour of MEPs conditioned by a preceding pulse at 3 ms. Conditioning intensities of 80% (C80) and 100% AMT (C100) were used. Mean data points from each 25 ms time bin are represented by diamonds fitted with a Boltzmann curve. Note the earlier decline in MEPs conditioned by pulses at 100% AMT.
The next step involved fitting a curve to the data so that we could extrapolate the MEP amplitude at all times during the task. As described in the Methods we tried a variety of curve types, and we compared estimates of goodness of fit (r2) estimated from the pooled data of all subjects with an F test. The Boltzmann sigmoid fitted the data much better than the other equations, and therefore we used this in all the analysis below. Table 1 gives values of r2 for some equations tested and values of F when these equations were compared to the Boltzmann equation. The curve drawn through the single subject data in Fig. 3A is the Boltzmann curve.
Table 1.
Example of Goodness of fit (r2) for exp 1
| Comparison of r2 between each model and Boltzmann model | |||
|---|---|---|---|
| Model | Goodness of fit (r2) | F ratio | P-value |
| Boltzmann | 0.98 | — | — |
| Fourth order polynomial | 0.94 | Simpler model fits better | not necessary |
| Third-order polynomial | 0.90 | Simpler model fits better | not necessary |
| Second-order polynomial | 0.89 | 55.84 (1,13) | P < 0.0001 |
| Sigmoidal dose–response | 0.77 | 133.7 (1,12) | P < 0.0001 |
| Linear | 0.72 | 86.07 (2,13) | P < 0.0001 |
Goodness of fit (r2) for the models used to fit the mean data in experiment 1 are shown in the second column and sorted by decreasing value. The third and fourth columns show the results of comparing the r2 between each model and the Boltzmann model using an F test. This takes into account not only the differences in sum-of-squares of each model but also differences in the number of parameters in each model. Note that for third and fourth order polynomial models the F test is not necessary because the Boltzmann model has the higher r2 and it is also the simpler model.
Figure 3B from the same subject illustrates the behaviour of the conditioned MEPs with conditioning stimuli of 80% and 100% AMT. Mean data points from the 25 ms time bins are shown together with the Boltzmann curves. It is clear that not only are the conditioned MEPs smaller than the test MEP, but also that they begin to decline in amplitude earlier relative to the onset of relaxation than the test MEP alone. This can be compared most directly by using the parameter c of the Boltzmann equation, which is the time at which the MEP has declined by 50% (i.e. the midpoint of the sigmoidal slope).
The data from all subjects are summarized in Table 2 which gives the mean (± s.d.) of all four parameters of the Boltzmann curve in all subjects for experiment 1 and experiment 2. Differences between test and conditioned values for each parameter were first analysed by a two-factor ANOVA with MEP TYPE (i.e. T, C80, or C100) and EXPERIMENT (i.e. experiment 1 or 2) as main factors. There were no main or interaction effects on d, the slope of the curve, nor on b, the maximum point of the curve. The former indicates that the rate of change of conditioned and test eMEPs during relaxation was the same in both experiments whilst the latter indicates that there was no significant SICI while the muscle was contracted, before relaxation began (i.e. when the MEPs were largest). The two-way analysis revealed a main effect of MEP TYPE on parameter a, but no other changes. We therefore combined the data from experiment 1 and 2 and performed a follow-up one-way ANOVA on mean values of a calculated for test and conditioned MEPs at 80% and 100% AMT. This showed a significant difference between values (F = 12.4, DF 2, 14, P < 0.001). Post hoc paired t tests (with Bonferroni's correction) showed that this part of the curve was lower than the test eMEP for both the 80% AMT (P < 0.008) and the 100% AMT (P < 0.007) conditioning stimulus. This indicates that SICI again became significant after the end of contraction (i.e. when the MEPs were smallest).
Table 2.
Parameters of the Boltzmann equation
| Experiment 1 | Experiment 2 | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | c100 | T | c80 | c100 | T | c80 | |
| a | Mean | 0.044 | 0.025 | 0.021 | 0.017 | 0.069 | 0.045 |
| s.d. | 0.02 | 0.01 | 0.06 | 0.03 | 0.376 | 0.02 | |
| b | Mean | 0.447 | 0.02 | 0.436 | 0.362 | 0.480 | 0.421 |
| s.d. | 0.17 | 0.20 | 0.15 | 0.16 | 0.18 | 0.14 | |
| c | Mean | −3.210 | −9.345 | −28.408 | −10.514 | −14.528 | −38.211 |
| s.d. | 11.60 | 18.37 | 12.98 | 14.75 | 21.63 | 16.31 | |
| d | Mean | 13.569 | 15.149 | 12.478 | 14.245 | 13.802 | 13.829 |
| s.d. | 10.56 | 7.92 | 7.25 | 3.87 | 4.10 | 5.12 | |
Data from all subjects. All four parameters of the Boltzmann curve, fitted for test and conditioned MEPs, are summarized (mean and s.d.) for experiments 1 and 2.
Finally, in both experiments parameter c was significantly influenced by the presence of a conditioning stimulus. The two-factor ANOVA revealed a significant main effect of MEP TYPE (F = 37.1, DF = 6, 7, P < 0.0004) but not of EXPERIMENT. Post hoc paired comparisons of combined data from the two experiments showed that there was a significant difference in the value of c for all MEP TYPES. Thus c was more negative for MEPs conditioned with either 80% or 100% AMT than for the test MEP alone (P < 0.05). In addition c was more negative with a conditioning intensity of 100% AMT than at 80% AMT (P < 0.005). The implication is that SICI starts to decline before the MEP, particularly when tested with conditioning stimuli of 100% AMT.
Time course of estimated intracortical inhibition
By using the values of estimated MEPs amplitudes from the curves above, it is possible to calculate the ratio between conditioned values (conditioned by stimuli at 80% and at 100% AMT) and control values in order to estimate the percentage SICI (eSICI) during the task. Note that this estimate is not corrected for the change in amplitude of the test response, and the calculation here is given mainly to visualize the time course of the effect in Fig. 4. However, since the percentage SICI usually increases with larger test responses (Chen et al. 1998), our calculation of the increase in SICI as the test response becomes smaller is likely to have underestimated the true magnitude of the effect in these experiments.
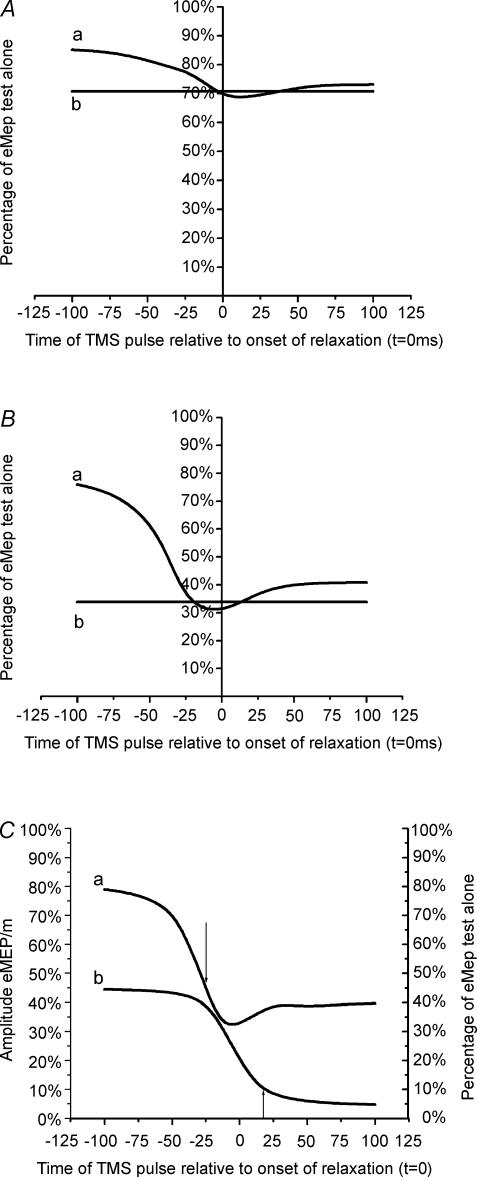
Figure 4. Mean time courses of the estimated SICI (eSICI; curve a) and the mean value of SICI at rest during the experiment (curve b) in eight subjects.
A shows data with conditioning intensities of 80% AMT. B shows data with conditioning intensities of 100% AMT. C, compares the time course of eSICI at 100% AMT (right Y-axis, curve a) with the time course of the change of the unconditioned eMEP (left Y-axis, curve b). The arrows indicate the TNSD in the eSICI and the eMEP.
Figure 4 shows the mean time course of eSICI in eight subjects: Fig. 4A illustrates the data with 80% AMT conditioning intensity and Fig. 4B the data with 100% AMT. Both estimates show that there was less SICI during the contraction than at rest (horizontal lines), but that the amount of inhibition increased around the time of relaxation and approached the resting levels at the end of relaxation. Figure 4C compares the time course of eSICI at 100% AMT with the time course of the change in the test eMEP.
These curves were analysed by using t tests to calculate the time point when the eSICI or eMEP was no longer any different from the size measured in the control period at rest (‘the time of no statistical difference’; TNSD). The values of TNSD are given in Table 3. They show that the amount of SICI returned to resting values before the MEP, and that this was particularly true for conditioning with 100% AMT.
Table 3.
Comparison between estimated values during the experiments and real values at rest
| Time of no statistical difference (TNSD) | TNSD differences | ||||
|---|---|---|---|---|---|
| eSICI with 80%aMT | eSICI with 100% aMT | eMep | TNSDSICI 80 -Mep | TNSDSICI 100 -Mep | |
| Before training | −44 | −29 | +18 | 62 | 47 |
| After training | −88 | −34 | +15 | 103 | 49 |
TNSD is the time at which eSICI or eMEP were not statistically different from the size measured in the control period at rest. Signs minus and plus indicate, respectively, ‘before’ and ‘after’ the end of contraction. The units are milliseconds.
Effect of training
Training had no significant effect on any of the parameters of the task. The reaction times to onset of relaxation were 159 ± 52 and 143 ± 25 in experiments 1 and 2, respectively. As noted above, the estimates of c were not different before and after training and the estimated TNSDs were very similar.
Discussion
The excitability of SICI is lower during voluntary contraction of a target muscle than it is at rest (Ridding et al. 1995a). Reynolds & Ashby (1999) showed that just before the onset of a voluntary contraction, SICI decreases, and hence by removing ongoing inhibition from corticospinal projections could contribute to the increased excitability of the cortical motor pool at that time. The results of the present study suggest that the reverse happens just prior to termination of a contraction. SICI increases and hence may assist in reducing cortical excitability and promote relaxation of the target muscle. The data complement two sets of previous studies that have shown that SICI increases in two situations that require prevention of unwanted muscle contraction: (a) in the no-go phase of a go/no-go reaction task (Sohn et al. 2002) and (b) in relaxed muscles surrounding the onset of a focal muscle contraction (Stinear et al. 2003).
Curve-fitting methodology
Given the variability of subjects' reaction times to the relaxation signal, we decided that rather than try to attempt to measure SICI at particular time points during/before relaxation, we would maximize the use of all data points by fitting a curve to the time course of MEP changes relative to the onset of relaxation on each trial. The approach was based on that used by Capaday (1997) and Devanne et al. (1997) to describe the relationship of MEP to stimulus intensity. This has been shown to be reliable with test–retest methods (Carroll et al. 2001) and has been used successfully to describe changes in corticospinal excitability in leg muscles during walking (Capaday et al. 1999).
In the present experiments, we found the time course of both test and conditioned MEPs (using either the 80% or 100% AMT conditioning pulse) was well fitted by a Boltzmann sigmoidal curve. These curves are described by four different parameters, a, b, c and d. Parameter d is related to the maximum slope of the curve, which was the same for both test and conditioned MEPs. This indicates that test or conditioned eMEPs take an equal overall time to change from their state during contraction to the state at rest. Parameter b represents the maximum value of the eMEPs. Again, this was equal for both the test and conditioned MEPs, indicating that there is no SICI during contraction (Ridding et al. 1995a). Parameter a describes the minimum eMEP size, which was smaller for conditioned (80% or 100% AMT) than test MEPs indicating that SICI had reappeared by the end of relaxation. Finally, parameter c is the time when the curves have declined through 50% of their range, and represents the position of the curves along the time axis relative to the onset of EMG suppression. This value was negative for both test and conditioned MEPs. According to the values in Table 2, this means that if the test TMS pulse was given 3–10 ms before the onset of EMG relaxation, then the MEPs recorded some 20–25 ms later were much smaller than those evoked during tonic contraction. Effectively this means that MEPs decline at approximately the same time as the ongoing level of EMG activity. The result complements the well-known increase in MEP amplitudes that occurs slightly in advance of EMG during muscle contraction (e.g. MacKinnon & Rothwell, 2000).
The difference in the value of c for test and conditioned eMEPs indicates that conditioned eMEPs began to decline up to 25 ms (when evaluated with a conditioning pulse of 100% AMT) before test eMEPs. This is consistent with the idea that SICI increases in advance of the reduction in test MEP. We also estimated the lead of SICI over MEPs by dividing the curves describing test and conditioned MEP amplitudes to give a value for the estimated percentage SICI (eSICI) at all timings. We then tested when the value of eSICI was no longer significantly different from the value of SICI at rest. These calculations yielded slightly longer lead times of SICI over MEP of up to 103 ms. The latter values are similar to those calculated by Reynolds & Ashby (1999) as the lead of SICI changes before onset of a contraction.
Whichever estimate is correct, it is clear that increases in SICI precede termination of an active contraction, and hence could contribute to reduced corticospinal drive even though the causality between these phenomena is only suggestive and it is likely that this is just one of many mechanisms that contribute to relaxation. Direct withdrawal of excitatory input to cortex is a likely candidate as well as withdrawal of spinal mechanisms of facilitation. For example, it has been suggested (Schieppati & Crenna, 1984, 1985; Schieppati et al. 1986) that relaxation of postural muscles may be accompanied by an increase in the level of pre-synaptic inhibition of excitatory Ia inputs.
It should be noted that we did not attempt to adjust the intensity of the test shock when evaluating the time course of changes in SICI. In many experiments, SICI is measured using a constant amplitude of test MEP in order to avoid the possibility that any changes in SICI are secondary to changes in test amplitude (Chen et al. 1998). However, in the present experiments, the fact that conditioned MEPs decrease in size in advance of test MEPs means that the change cannot be explained by this mechanism. Finally, there was no difference between the rate of decline (i.e. parameter a in the Boltzmann curve fit) between SICI using conditioning stimuli of either 80% or 100% AMT. This is consistent with the idea that the main effect of relaxation was on short interval inhibitory processes rather than on possible I-wave interactions. If the latter had been important then the rate of decay may have been different for conditioning stimuli at 100% AMT (which are around the threshold for producing short latency I-wave interaction effects; Ziemann & Rothwell, 2000) and 80% AMT. The difference in the parameter c with the two intensities of conditioning stimuli is probably due to the fact that the effect size (i.e. the percentage SICI) is larger with conditioning of 100% AMT, and hence allows clearer identification of the onset of changes in SICI.
Relation to SICI changes in other types of voluntary movement
As noted above, SICI has been shown to decline prior to the onset of a voluntary contraction (Reynolds & Ashby, 1999) and could therefore contribute both to the facilitation of corticospinal drive as well as to its suppression. SICI may also be involved in preventing contraction of passive muscle. Thus SICI increases in the no-go phase of a go/no-go task (Sohn et al. 2002), and has also been shown recently to increase in relaxed muscles surrounding a focal contraction (Stinear et al. 2003; Zoghi et al. 2003). In both these cases, the implication is that relaxation can be an active phenomenon that requires central inhibition to prevent ‘overflow’ of excitatory inputs to other muscles. Indeed, pathologically reduced SICI in patients with dystonia has been proposed to be one element that contributes to the excessive co-contraction and overflow of activity to unintended muscles that is characteristic of this condition (Ridding et al. 1995b).
Cortical involvement in termination of muscle activity
SICI is generally believed to test excitability of a GABAA-ergic system in the motor cortex, so that the changes we have described are likely to involve cortical rather than subcortical mechanisms. Several other approaches have also investigated cortical involvement in muscle relaxation. Luders et al. (1995) reported that direct electrical stimulation of inhibitory cortical areas termed ‘primary negative motor areas’ anterior to primary motor cortex can suppress ongoing movement. Studies of movement-related cortical potentials (MRCP) and functional neuroimaging have shown that voluntary muscle relaxation is preceded and accompanied by activation of primary and supplementary motor areas (Terada et al. 1995; Dimitrov et al. 1996; Rothwell et al. 1998; Yazawa et al. 1998; Toma et al. 1999). Furthermore, Toma et al. (2000) using magnetoencephalography (MEG) showed a modulation of 20-Hz central cortical rhythm temporally related to muscle relaxation suggesting a specific involvement in this task of mechanisms in primary motor cortex.
However, in these studies it was not clear whether the activation of motor areas was related to an increase of activity in corticospinal neurones followed by activation of spinal inhibitory interneurones or by the withdrawal of ongoing input from pre-motor areas to pyramidal neurones (Rothwell et al. 1998) or by a reduction of motor output mediated by the intracortical inhibitory neurones acting in the primary motor cortex (Toma et al. 1999). It is interesting to note that Rothwell et al. (1998) demonstrated in an isometric task that MRCPs were smaller preceding relaxation than before contraction. As observed by Toma et al. (1999) this finding suggests that there is an increase of the activity of intracortical inhibitory interneurones that do not generate potentials because their dendrites spread in many different directions, cancelling out the potentials generated by each individual neurone (Niedermeyer & Lopes da Silva, 1993).
Effect of training
Unfortunately it was not possible to demonstrate an effect of training on either task performance or the MEP parameters. Reaction times did tend to decrease slightly in experiment 2 compared with experiment 1, but this was not significant. This was probably because the number of trials in each experiment had to be quite large in order for us to obtain sufficient data to explore the time course of relaxation. It may well have been that in experiment 1 subjects received sufficient practice to reach a plateau of performance, limiting the effects of any subsequent training. Nevertheless, it is interesting to note that the lack of behavioural change was also associated with a lack of physiological effects.
Some neurological conditions such as stroke or Parkinson's disease and dystonia are characterized by difficulty in terminating or suppressing unwanted muscle activity or by slowness in relaxing. The present findings on mechanisms underlying voluntary muscle relaxation in healthy subjects may therefore be relevant to a better understanding of not only this essential feature of the normal motor control but also the abnormalities that occur in patients with movement disorders.
Acknowledgments
We would like to thank Professor E. Favale for his helpful suggestions and Mr P. Asselman for his invaluable assistance with the equipment used in these experiments. The work was supported by the grant ‘Anna e Francesco Nuti’ from the University of Genoa and by the Medical Research Council, UK.
References
- Butefisch CM, Netz J, Wessling M, Seitz RJ, Homberg V. Remote changes in cortical excitability after stroke. Brain. 2003;126:470–481. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- Capaday C. Neurophysiological methods for studies of the motor system in freely moving human subjects. J Neurosci Meth. 1997;74:201–218. doi: 10.1016/s0165-0270(97)02250-4. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J Neurophysiol. 1999;81:129–139. doi: 10.1152/jn.1999.81.1.129. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Riek S, Carson RG. Reliability of the input-output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. J Neurosci Meth. 2001;112:193–202. doi: 10.1016/s0165-0270(01)00468-x. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Chen CM, Quinn NP, McAuley J, Rothwell JC. Strength in Parkinson's disease: relationship to rate of force generation and clinical status. Ann Neurol. 1996;39:79–88. doi: 10.1002/ana.410390112. [DOI] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- Dimitrov B, Gantchev GN, Popivanov D. Brain macropotentials associated with distinct phases of voluntary sustained isometric contraction in man. Int J Psychophysiol. 1996;22:35–44. doi: 10.1016/0167-8760(96)00012-8. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders HO, Dinner DS, Morris HH, Wyllie E, Comair YG. Cortical electrical stimulation in humans. The negative motor areas. Adv Neurol. 1995;67:115–129. [PubMed] [Google Scholar]
- MacKinnon CD, Rothwell JC. Time-varying changes in corticospinal excitability accompanying the triphasic EMG pattern in humans. J Physiol. 2000;528:633–645. doi: 10.1111/j.1469-7793.2000.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E, Lopes da Silva F. Electroencephalography. Basic Principles, Clinical Applications, and Related Fields. 3. Baltimore: Williams & Wilkins; 1993. [Google Scholar]
- Orth M, Snijders AH, Rothwell JC. The variability of intracortical inhibition and facilitation. Clin Neurophysiol. 2003;114:2362–2369. doi: 10.1016/s1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology. 1999;53:730–735. doi: 10.1212/wnl.53.4.730. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J Neurol Neurosurg Psychiatry. 1995b;59:493–498. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995a;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencheph Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Higuchi K, Obeso JA. The offset cortical potential: an electrical correlate of movement inhibition in man. Mov Disord. 1998;13:330–335. doi: 10.1002/mds.870130221. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Crenna P. From activity to rest: gating of excitatory autogenetic afferences from the relaxing muscle in man. Exp Brain Res. 1984;56:448–457. doi: 10.1007/BF00237985. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Crenna P. Excitability of reciprocal and recurrent inhibitory pathways after voluntary muscle relaxation in man. Exp Brain Res. 1985;59:249–256. doi: 10.1007/BF00230904. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Nardone A, Musazzi M. Modulation of the Hoffmann reflex by rapid muscle contraction or release. Hum Neurobiol. 1986;5:59–66. [PubMed] [Google Scholar]
- Sohn YH, Wiltz K, Hallett M. Effect of volitional inhibition on cortical inhibitory mechanisms. J Neurophysiol. 2002;88:333–338. doi: 10.1152/jn.2002.88.1.333. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Role of intracortical inhibition in selective hand muscle activation. J Neurophysiol. 2003;89:2014–2020. doi: 10.1152/jn.00925.2002. [DOI] [PubMed] [Google Scholar]
- Terada K, Ikeda A, Nagamine T, Shibasaki H. Movement-related cortical potentials associated with voluntary muscle relaxation. Electroencephalogr Clin Neurophysiol. 1995;95:335–345. doi: 10.1016/0013-4694(95)00098-j. [DOI] [PubMed] [Google Scholar]
- Toma K, Honda M, Hanakawa T, Okada T, Fukuyama H, Ikeda A, et al. Activities of the primary and supplementary motor areas increase in press and execution of voluntary muscle relaxation: an event-related fMRI study. J Neurosci. 1999;19:3527–3534. doi: 10.1523/JNEUROSCI.19-09-03527.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma K, Nagamine T, Yazawa S, Terada K, Ikeda A, Honda M, et al. Desynchronization and synchronization of central 20-Hz rhythms associated with voluntary muscle relaxation: a magnetoencephalographic study. Exp Brain Res. 2000;134:417–425. doi: 10.1007/s002210000483. [DOI] [PubMed] [Google Scholar]
- Yazawa S, Ikeda A, Kunieda T, Mima T, Nagamine T, Ohara S, et al. Human supplementary motor area is active in press for both voluntary muscle relaxation and contraction: subdural recording of Bereitschaftspotential. Neurosci Lett. 1998;244:145–148. doi: 10.1016/s0304-3940(98)00149-9. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. I-waves in motor cortex. J Clin Neurophysiol. 2000;17:397–405. doi: 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Zoghi M, Pearce SL, Nordstrom MA. Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle. J Physiol. 2003;550:933–946. doi: 10.1113/jphysiol.2003.042606. [DOI] [PMC free article] [PubMed] [Google Scholar]