Abstract
Cytoskeleton proteins play important roles in regulating vascular smooth muscle (VSM) contraction and relaxation. We tested the hypotheses that the expression levels of several of these proteins change significantly during the course of development, and that these changes contribute to age-related changes in contractile responses. In cerebral arteries from 95-day (d) gestation and 140-d fetus, newborn lambs, and adult sheep, by Western immunoblot (n = 5 for each age) we quantified the relative expression of α-actin, α-tubulin, cyclophilin A, and proliferating cell nuclear antigen (PCNA). In addition, we examined middle cerebral artery tension responses to phenylephrine (PHE) stimulation in the absence or presence of cytochalasin D (3 × 10−7 m) and nocodazole (3 × 10−6 m), inhibitors of α-actin and α-tubulin polymerization, respectively. The expression levels of α-actin and cyclophilin A varied little during the course of development. In contrast, α-tubulin expression was ∼2.5-fold greater in both fetal age groups as compared to adult. Also, as compared to adult and as expected, expression of PCNA was several-fold greater in cerebral arteries of the 95-d fetus (×8), 140-d fetus (×5), and newborn (×3). In both adult and fetal middle cerebral artery, cytochalasin d-induced inhibition of actin polymerization decreased PHE-induced contraction, to ∼60 and ∼40% of control, respectively (despite no significant change in expression level). In contrast, α-tubulin inhibition by nocodazole showed little effect on PHE-induced tension (in spite of the age-related decrease in expression). In conclusion, expression levels of α-actin, a thin filament protein involved in contraction, remained relatively constant during the course of development, as did the effects of inhibition of its polymerization on contractility. In contrast, α-tubulin, important in intracellular protein trafficking, showed a significant age-related decrease in expression and played a relatively minor role in contractility. The present studies suggest that other cytoskeletal structural proteins and/or elements of pharmaco-mechanical coupling are important to developmental differences in cerebrovascular contractility. In addition, the relatively constant expression levels of α-actin and cyclophilin A with development, suggest that these are useful internal standards for studies of cytosolic protein expression.
A major challenge in cell biology is biocomplexity, i.e. an understanding of the manner in which cell and tissue behaviours emerge from the interactions within complex molecular networks. Linear models of signal transduction pathways have provided valuable information on the role of various receptors, second messengers, enzymes, and other elements of the signalling cascade in terms of cell function and dynamics. For instance, for vascular smooth muscle (VSM) such analysis has provided many useful insights (see Horowitz et al. 1996; Somlyo & Somlyo, 2003). Nonetheless, increasingly it is becoming evident that a more complex model, which incorporates interactions among multiple molecular components including those commonly viewed as ‘just structural’, will be required to understand emergent properties of diverse cell activity, and the underlying basis of biocomplexity (Ingber, 2003a,b; Pollard, 2003).
One aspect of understanding the relation of function to structure in VSM requires knowledge of the relative roles of thin filaments (actin) and thick filaments (myosin) in contraction/relaxation responses (Pollard, 2003; Somlyo & Somlyo, 2003). As a corollary, such understanding requires knowledge of the relative abundance of these and other cytoskeletal components. Considerable evidence suggests that the amount and function of many of these cytoskeletal proteins differ in a tissue-specific and age-dependent manner (Ingber, 1997; Janmey, 1998; Geiger et al. 2001). During the past decade, our group has reported on a number of factors of importance in the functional aspects of cerebral artery contraction/relaxation mechanisms, and the manner in which these differ significantly as a function of developmental age (Longo et al. 1996, 2000; Zhou et al. 1997; Long et al. 1999, 2000, 2002; Lin et al. 2003; Zhao et al. 2003; Geary et al. 2004).
From these studies, an important question arises as to the extent to which the expression levels of several of the key cytoskeletal proteins change with developmental age, and the manner in which disruption of their structure affects vascular contractility. Thus, for α-actin, a key structural protein often used to normalize expression, we tested the hypothesis that cytochalasin D-induced inhibition of polymerization, as measured by the phenylephrine (PHE)-induced contraction pattern, would help to elucidate its role in vascular function. In a similar manner, for α-tubulin, a protein involved in intracellular trafficking, we examined the role of nocodazole-induced inhibition of polymerization on PHE-induced contractile response. In addition, we tested the hypothesis that expression levels of several key elements of the cytoskeleton change dramatically with developmental maturation from pre-term, to term fetus, to newborn, and to adult. To test this latter thesis, we measured the expression of several cytosolic proteins: α-actin, α-tubulin, and the ‘housekeeping’ protein cyclophilin A. Although these proteins are used widely for normalization, their stability with development is unknown. Finally, we quantified levels of proliferative cell nuclear antigen (PCNA), which would be expected to be elevated in proliferating cells, as a ‘positive control’ for age-related changes. These measurements are important, we believe, in terms of establishing appropriate internal standards when measuring various proteins, the levels of which might vary with developmental age. Thus, these studies serve as a prelude to studies on the role of other related cytoskeletal proteins in agonist-induced cerebrovascular contractile responses, and their role in developmental changes. Importantly, we believe, the study helps to lay the groundwork for studies of the functional role of thin filament regulation in VSM, particularly as regards the developing organism.
Methods
Tissue preparation
We obtained cerebral arterial samples from pre-term (∼95 days gestation) and near-term (∼140 days) fetal sheep, newborn lambs (7–10 days), and young female non-pregnant ewes (< 2 year) (n = 5 sets of samples for each age group). Sheep were obtained (Nebeker Ranch, Lancaster, CA, USA) and were killed using 100 mg kg−1 intravenous pentobarbital sodium. Immediately after kill the main branch anterior, middle and posterior cerebral arteries, as well as common carotid arteries, were dissected out, cleaned in Krebs buffer, and wrapped in aluminium foil, snap-frozen in liquid nitrogen, and stored at −80°C until use. In addition, for studies of cerebral artery contractility we obtained branches of the middle cerebral artery (∼200 μm in diameter, 4 mm in length) for measurement of tension in response to agonist ± inhibitor. We have shown that this method of dissection has no significant effect on vascular contractility (Pearce et al. 1991; Longo et al. 1996). All surgical and experimental procedures were performed within the regulations of the Animal Welfare Act, strictly adhered to the principles outlined in The National Institutes of Health's Guide for the Care and Use of Laboratory Animals and The Guiding Principals in the Care and Use of Animals, approved by the Council of the American Physiological Society, and were governed by the Animal Care and Use Committee of Loma Linda University.
Western immunoblot assay
For each protein studied, we obtained tissue from at least 10 separate fetuses of each age group, and five newborns and adults. For each immunoblot assay, cerebral arteries from two fetal sheep, one adult, or one newborn were pooled separately to obtain ∼0.5 g of tissue. Frozen tissue samples were homogenized in the lysing buffer (20 mm Tris-HCl, 1 mm EDTA, 1.5 mm MgCl2, 10 mm KCl, 1 mm dithiothreitol, 1 μg ml−1 pepstaten, 1 μg ml−1 leupeptin, 1 μg ml−1 aprotinin, pH 7.4) with a glass tissue grinder. Homogenized samples then were centrifuged at 1000 ×g for 15 min. Nuclei and debris were discarded, and the supernatant stored at −20°C.
A 10% polyacrylamide gel was loaded with 15 μg of protein mixed with an equal volume of 2 ×electro-phoresis sample buffer per lane. We demonstrated this concentration to be on the linear part of the protein concentration–density curve (data not shown), and determined the protein concentration by a modification of the Bradford method (Bradford, 1976). Before loading, the samples were boiled for 5 min, and then electrophoresed at 90 V for 2.5 h. We used a Mini Trans-Blot Electrophoretic Transfer Cell system (Bio-Rad Laboratories, Hercules, CA, USA) to transfer proteins from the gel to a nitrocellulose membrane at 100 V for 1 h. As an aside, we used the same membrane to quantify expression of each of the four proteins.
We performed blocking for non-specific binding by incubating the membrane overnight in blotting solution (5% non-fat milk in 1 × tris-buffered saline (TBS) with 0.1% Tween-20 (TTBS)) at 4°C. Then we performed a 3 h incubation of primary antibodies in blotting solution at room temperature (22°C). To establish the levels of VSM α-actin, we used a 1: 16 000 dilution of mouse monoclonal anti-smooth muscle specific α-actin (Sigma Chemical Co., St Louis, MO, USA). To establish the expression level of microtubules in cerebral arteries, we quantified α-tubulin by Western immunoblots using a 1: 200 dilution of mouse monoclonal anti-α-tubulin antibody (C-20; SC 7394; Santa Cruz Biotechnology, Santa Cruz, CA, USA). For cyclophilin A, we used a 1: 2000 dilution of rabbit polyclonal anti-cyclophilin A antibody (United States Biological, Swampscott, MA, USA). For PCNA, we used a 1: 200 dilution of rabbit polyclonal anti-PCNA antibody (FL-261; SC-7907; Santa Cruz Biotechnology). We then washed the membrane three times with TTBS, and incubated it with horseradish perioxidase (HRP)-conjugated secondary antibody for 1.5 h at a 1: 1000 dilution at room temperature. Following the secondary antibody incubation, the membrane was washed 3 times in TTBS, for 5 min each time. The membrane was then incubated with a chemiluminescent reagent (Santa Cruz Biotechnology) for 1 min, and the protein band was detected and the density determined by use of the ChemiImager (Alpha Innotech, San Leandro, CA, USA). To minimize variation due to protein loading, we used the same membrane for measurement of the four proteins studied. The adult expression level was given the value of unity, and the expression levels of the other age groups expressed as a fraction of the adult value. Values for the adult expression levels varied by ±10% (s.e.m.). Unless otherwise noted, all chemical compounds were purchased from Sigma Chemical Co.
Measurement of isometric tension
As noted above, we isolated and removed without stretching middle cerebral arteries from near-term fetal and non-pregnant adult sheep. From each animal we obtain four artery segments and removed the endothelium as we have previously described (Longo et al. 1996, 2000). Four-millimetre segments of each vessel were cannulated with tungsten mounting wires and suspended between a force transducer (159901-A, Radnoti, Monrovia, CA, USA) and a micrometer-driven post used to control resting tension. The vessels were suspended in an oxygenated standard Krebs solution containing (mm): 122 NaCl, 25.6 NaHCO3, 5.56 dextrose, 5.17 KCl, 2.49 MgSO4 1.60 CaCl2, 0.114 ascorbic acid, and disodium 0.027 EDTA. The bath chambers were bubbled with 95% O2–5% CO2 at 37°C. We allowed 30 min for equilibration at optimum resting tension. Based on our previous studies, the optimum resting tension was 0.6 g for fetal and 0.7 g for adult middle cerebral arteries (Pearce et al. 1991; Long et al. 1999, 2000).
Relative roles of α-actin and α-tubulin
To determine the potential role of α-actin and α-tubulin in modulating PHE-induced changes in middle cerebral artery vascular tension of the two age groups, we quantified this variable in the absence or presence of selective inhibitors of polymerization. For all studies, after initial K+ (120 mm) depolarization to determine maximum tension achieved at 120 mm K+ (Kmax), we plotted PHE dose–response curves to establish the maximum contraction, percentage Kmax, and pD2 (n = 5 for each). Then, to examine the effect of α-actin in tension development, and on the basis of a preliminary study to determine optimal dose, we gave 3 × 10−7 m cytochalasin D and 15 min later performed a PHE dose–response study (10−9–10−3 m). To examine the effect of α-tubulin on contraction, we quantified PHE-induced changes in tension after administration of the inhibitor nocodazole (3 × 10−6 m). From these data we plotted the shift in the PHE dose–response curves.
Statistical analysis
All individually shown experiments are representative of a least five separate experiments in as many animals, performed in duplicate and subjected to analysis of variance and Dunnett's multiple comparison. Results were considered statistically significant with P < 0.05.
Results
α-actin
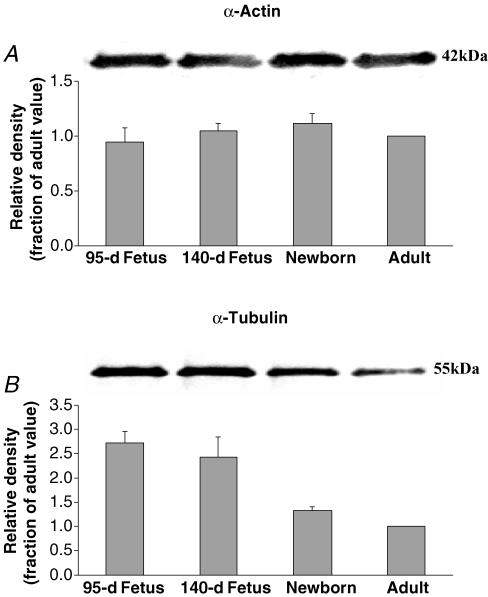
As seen in (Fig. 1A) the density of the Western immunoblots for α-actin were remarkably stable with age from 95-d fetus, to term fetus, to newborn, and to adult. The lower panel shows the mean values of densitometric analysis normalized to the value for the adult (n = 5 each). See Table 1 for values of total cytosolic protein relative to that of the adult.
Figure 1. α-actin and α-tubulin expression in ovine cerebral arteries.
A, Western immunoblot (upper panel) and densitometric analysis (lower panel) for the α-actin in fractions of homogenized 95-d and 140-d fetus, newborn, and adult cerebral arteries. Densitometry measurements were normalized to the density of the α-actin band for the adult (n = 5, see Methods for details). B, Western immunoblot (upper panel) and densitometric analysis (lower panel) for the α-tubulin in fractions of homogenized 95-d and 140-d fetus, newborn, and adult cerebral arteries. α-Tubulin densitometry measurements were normalized to the density of the adult value (n = 5, see Methods for details). Symbols same as in A.
Table 1.
Cerebral artery expression of cytoskeletal proteins α-actin, α-tubulin, cyclophilin A, and proliferative cell nuclear antigen (PCNA) relative to that of the adult
| 95 days | 140 days | Newborn | Adult | |
|---|---|---|---|---|
| α-Actin | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.0 |
| α-Tubulin | 2.7 ± 0.3† | 2.4 ± 0.4† | 1.3 ± 0.1 | 1.0 |
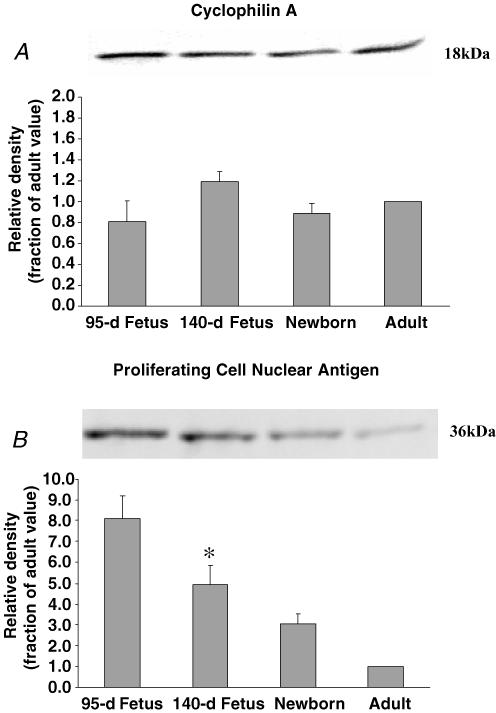
| Cyclophillin A | 0.8 ± 0.2 | 1.2 ± 0.2 | 0.9 ± 0.1 | 1.0 |
| PCNA | 8.1 ± 1.1† | 4.9 ± 1.0* | 3.0 ± 1.1 | 1.0 |
Values given as fraction of that of adult ± s.e.m.; n = 5 for each measurement from Western immunoblot.
P < 0.01
P < 0.05, as compared to adult. See Methods for details.
α-Tubulin
Figure 1B shows the immunoblots for α-tubulin in cerebral arteries of the four age groups. The lower panel shows the densitometric analysis normalized to the value for the adult (n = 5 each). Clearly maturation was associated with significant decreases in α-tubulin expression (Table 1).
α-actin and PHE-induced contraction
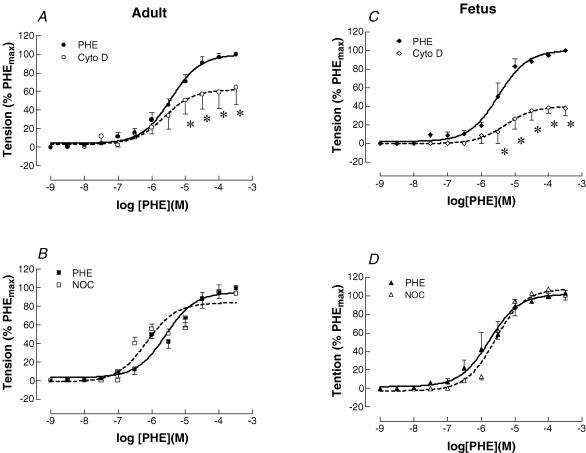
Figure 2 shows control phenylephrine dose–response curves in both adult and term fetal middle cerebral arteries. As also seen in Fig. 2, inhibition of actin polymerization with cytochalasin D (3 × 10−7 m) decreased PHE-induced contraction from control values in both adult (Fig. 2A) and fetal (Fig. 2C) cerebral arteries. In the adult artery, cytochalasin D decreased the PHE-induced maximum (PHEmax) contraction to 62%, while in fetus PHEmax dropped to 40% of control. This difference in response was not significantly greater in the fetus than in the adult (P < 0.07). The pD2 values for adult control and cytochalasin D dose–response curves were 5.4 ± 0.1 and 5.6 ± 0.1, respectively. For the fetus these values were 5.5 ± 0.1 and 5.3 ± 0.1, respectively (n = 7 each for adult and fetus).
Figure 2. Phenylephrine (PHE) dose-response relationships for adult and fetal cerebral arteries.
PHE-induced contraction in adult (A) and term-fetal (C) middle cerebral arteries with or without inhibition of actin polymerization. The continuous line represents dose-dependent, PHE-induced contraction (10−9–10−3 m); the dashed line represents PHE-induced, dose-dependent contraction with inhibition of α-actin polymerization by cytochalasin D (3 × 10−7 m). Data were normalized as a percentage of maximal contraction in response to PHE (n = 5 each, *P < 0.05). PHE-induced contraction in adult (B) and term-fetal (D) middle cerebral arteries with or without α-tubulin inhibition. The continuous line represents dose-dependent, PHE-induced contraction, the dashed line represents PHE-induced, dose-dependent contraction with inhibition of α-tubulin polymerization by nocodazole (3 × 10−6 m). Data were normalized as a percentage of maximal contraction in response to PHE (n = 5; *P < 0.05).
α-tubulin and PHE-induced contraction
As also seen in (Fig. 2) in contrast to the effect of α-actin inhibition, inhibition of tubulin with nocodazole (3 × 10−6 m) did not change PHE-induced maximal contraction in either adult (Fig. 2B) or fetal (Fig. 2D) cerebral arteries. The pD2 values also were not changed significantly (n = 5 each for adult and fetus).
Cyclophilin A
Figure 3A shows the immunoblots for cyclophilin A in ovine cerebral arteries of the four age groups. The lower panel shows the densitometric analysis for each of five sets of blots, normalized to the adult value. Table 1 gives the mean ± s.e.m. for each age group relative to that of the adult.
Figure 3. Cyclophillin A and proliferating cell nuclear antigen expression in ovine cerebral arteries.
A, Western immunoblot (upper panel) and densitometric (lower panel) analysis for cyclophilin A antigen protein in fractions of homogenized 95-d and 140-d fetus, newborn, and adult cerebral arteries. Cyclophilin A densitometry measurements were normalized to the density of the adult value (n = 5, see Methods for details). B, Western immunoblot (upper panel) and densitometric analysis (lower panel) for the proliferative cell nuclear antigen protein in fractions of homogenized 95-dat and 140-d fetus, newborn, and adult cerebral arteries. PCNA densitometry measurements were normalized to the density of the adult value (n = 5, see Methods for details). †P < 0.01; *P < 0.05; significantly greater than adult.
Proliferating cell nuclear antigen
Figure 3B shows the Western immunoblots for PCNA in cerebral arteries of 95-d and 140-d fetus, newborn, and adult sheep. The lower panel shows the densitometric analysis of blots normalized to the value for the adult (n = 5 each). As is evident, the density of this protein decreased significantly with developmental age from ∼8-fold greater than adult at 95-d to 3-fold greater in the newborn.
Discussion
Vascular smooth muscle
Vascular (and other) smooth muscle cells possess a contractile apparatus of actin (thin) and myosin (thick) filaments, which, following phosphorylation of myosin light chain, and in association with a number of effector proteins, produce contraction (Small, 1995; Horowitz et al. 1996; Small & Gimona, 1998; Somlyo & Somlyo, 2003). Vascular smooth muscle internal scaffolding cytoskeleton consists of three classes of filamentous assemblies: actin microfilaments (∼70Å diameter), intermediate filaments (70–110Å diameter), and microtubules (∼300Å diameter). In addition, there exists a well-developed membrane skeleton, which provides the interface between the extracellular matrix, plasma membrane, and the contractile structure within the cells (Small & Gimona, 1998; Carpenter, 2000; Pollard, 2003). Because of the apparent lack of high structural order of smooth muscle cells (as compared with skeletal or cardiac muscle), the architecture of the contractile units, the nature of coupling of the contractile apparatus to the cytoskeleton, the exact role of the cytoskeleton in the mechanical properties of the cell and its acute regulation, and the organization of the contractile apparatus per se, are poorly understood. In addition, the changes in the cytoskeleton and associated proteins during the course of development have not been described.
In the present studies, we show for the first time the relative constant abundance of the key structural protein α-actin, during the course of development from pre-term fetus to adult. Importantly, when actin polymerization was blocked, PHE-induced contraction was significantly inhibited in the middle cerebral artery of both term fetus and adult. Although this effect was greater in the fetus, the age-related difference was not significant statistically. We also found that the expression of cyclophilin A, a ‘housekeeping’ protein widely used for normalization, was relatively constant with developmental maturation. In contrast, expression of α-tubulin, involved in intracellular protein trafficking, showed a significant decrease in abundance with age. Nonetheless, following blockage of microtubule polymerization, this change was not reflected in a significant decrease in the PHE-induced contractile response. These findings are of importance because of their structure–function implications in terms of the biocomplexity of the contractile apparatus, and because, in addition to the cytoskeletal proteins per se, other elements of pharmaco-mechanical coupling play major roles in developmental changes in cerebrovascular contractility.
The studies also are of importance, because analysis of protein abundance requires an internal control or norminative standard. When considering various tissues and/or cell types, it is essential to minimize errors due to variations in loading efficiency. Even for the same tissue, this is the case when considering responses to different treatment regimens. In addition, this is of particular relevance in physiological/biochemical studies in the developing organism, in which cell size, cell number, and other variables in a given tissue may change during the course of maturation.
Actin and actin-associated proteins
In VSM the ‘thin’ filament α-actin, a 42 kDa myofibrillar protein, interacts with the ‘thick’ filament myosin to produce contraction. In solutions of low ionic strength, the actin monomer assumes a globular shape, G-actin. As ionic strength (K+, Cl−, and so forth), increases to physiological levels, actin polymerizes into a fibrous F-form, which is a helix of actin monomers, the thin filaments. Smooth muscle contraction is regulated by the degree to which the myosin light chains are phosphorylated (Carpenter, 2000; Somlyo & Somlyo, 2003), and the extent to which a number of proteins, such as caldesmon, calponin, gelsolin, et cetera, inhibit the binding properties of actin to myosin (Morgan & Gangopadhyay, 2001; Dos Remedios et al. 2003; Somlyo & Somlyo, 2003). Actin filaments thus can form stable and labile structures and, with myosin, are a crucial component of the VSM contractile apparatus. In the present studies, we show for the first time that following cytochalasin D-induced inhibition of actin polymerization, PHE-induced contraction in both adult and fetal cerebral arteries decreased significantly. This suggests that for both age groups actin polymerization plays an important role in regulating agonist-induced contraction. This is of importance because of the structure–function implications of both change and relative constancy in structural protein expression. In addition, the stable level of α-actin expression during the course of development from 95-d fetus to adult, makes it a reasonable choice as an internal control for studies of protein expression.
Microtubules
These hollow, cylindrical structures are formed by two similar, but alternating, 55 kDa subunits, α- and β-tubulin, arranged in a helical array. Importantly, microtubules form a network that participates in the movement of vesicles and organelles within the cell (Yildiz et al. 2004). In this study, we showed that inhibition of α-tubulin polymerization had no significant effect on PHE-induced contraction. Although this suggests that α-tubulin appears not to play a major role in regulating agonist-induced contraction in cerebral arteries, several studies have reported on this role for tubulin in other vessels (Sheridan et al. 1996; Leite & Webb, 1998; Platts et al. 1999; Paul et al. 2000). The pronounced decrease in protein expression level with developmental age raises the question of functional significance in terms of cytoplasmic transport and cell signalling mechanisms, and the effect of this change in vascular contraction–relaxation mechanisms. Answers to these questions await future studies. Additionally, this marked decrease in α-tubulin expression during the course of development from fetus to adult makes it an inappropriate choice as an internal control for protein expression.
Functional correlates of actin–tubulin interactions
In vascular smooth muscle, actin thin filaments (6–8 nm in diameter), in concert with intermediary filaments and myosin thick filaments (15–18 nm) play a key role in cross-bridge regulation of contraction (Morgan & Gangopadhyay, 2001). Additionally, a number of actin binding proteins such as caldesmon and calponin interact to modulate this process (for review see Gunst & Tang, 2000). In essence the contraction–relaxation cycle involves polymerization and depolymerization of actin, as well as microtubules (∼24 nm) (Somlyo, 1980). Several studies have examined the effect of disruption of actin filaments and microtubules on VSM cell function. For instance in cultured A7γ5 cells derived from rat aorta, whole cell voltage-clamp analysis demonstrated ∼36% inhibition of L-type Ca2+ current by cytochalasin D (10−4 m; Nakamura et al. 2000). In contrast, disruption of microtubules by nocodazole (1. 3 × 10−6 m) showed no such inhibition (Nakamura et al. 2000). Also in VSM cells from Sprague-Dawley rats, cytochalasin D (4 × 10−7 m for 60 min) inhibited stretch-induced activation of extracellular signal-regulated kinases 1/2 (Numaguchi et al. 1999). Several studies have demonstrated the effect of disruption of actin polymerization in other cell types (for instance see Adler et al. 1983; Stevenson & Begg, 1994; Tseng et al. 1997; Battistella-Patterson et al. 1997; Filipe & Nunes, 2002; Hinz et al. 2003).
Cytoplasmic microtubules, in contrast, are believed to serve as a network by which intracellular vesicles, organelles and/or kinesin can travel (Yildiz et al. 2004). In addition, according to the ‘tensegrity’ model of cytoskeletal structure, microtubules are believed to provide structural stability, in transferring extracellular mechanical or other stimuli within the cell (see Ingber, 1991, 3a,b; Kolodney & Elson, 1995; Paul et al. 2000). Several reports suggest that disruption of microtubular polymerization increases force in intact arteries (Sheridan et al. 1996; Leite & Webb, 1998; Platts et al. 1999), although one study reported a decrease in force (Battistella-Patterson et al. 1997). In porcine coronary artery, microtubules appear to contribute minimally to VSM mechanical characteristics, but play a key role in modulating intracellular Ca2+ signal transduction (Paul et al. 2000). The present study suggests that, at least in adult and fetal middle cerebral artery, microtubular disruption by nocodazole had little effect on PHE-induced contraction.
Cyclophilin A
Cyclophilin A is a 18 kDa cyclosporin A binding protein that catalyses the isomerization of proline imidic peptide bonds. As a cytoplasmic enzyme, it accelerates protein folding (Stamnes et al. 1992). The fact that expression of cyclophilin A, a classical ‘housekeeping’ protein, changes so little with developmental age also makes it a reasonable choice as an internal marker for protein expression.
Proliferating cell nuclear antigen
PCNA, also known as cyclin (Mathews et al. 1984; Waseem & Lane, 1990), is a 36 kDa polymerase δ-associated protein (Bravo et al. 1987) synthesized during early G1 and S phases of the cell cycle (Bravo & Macdonald-Bravo, 1985; Woods et al. 1991). The protein is strongly associated with the cell nucleus during periods of DNA transcription (Mathews et al. 1984; Bravo & Macdonald-Bravo, 1985; Bravo et al. 1987). In fact, at least a dozen antigenically distinct PCNA epitopes exist, and these are localized to different compartments of the nucleus (Waseem & Lane, 1990). As expected, the present study demonstrated much greater PCNA levels in VSM of the two fetal age groups, as compared to the adult. This supports the idea of using PCNA as an index of proliferating VSM cell activity during the course of development.
Perspective
In previous studies, using several approaches, we have demonstrated the many respects in which contractility of developing cerebral arteries differs from that of the adult. These differences involve both Ca2+-dependent and Ca2+-independent pathways, and are a consequence, in part, of significant differences in both thin filament and thick filament regulation. (For instance see Pearce et al. 1991; Longo et al. 1996, 2000; Zhou et al. 1997; Long et al. 1999, 2000; Lin et al. 2003; Zhao et al. 2003; Geary et al. 2004.)
Our demonstration that the relative expression of some cellular proteins changes dramatically with developmental age, while that of others shows only minimal change, should come as no surprise. Growth and development is a dynamic process characterized by changes in expression of a number of cell cycle-associated proteins (Nurse, 2002) and cytoskeletal proteins (Geiger et al. 2001). Nonetheless, whether considering various tissues or cell types, a given tissue in response to various physiological or pharmacological interventions, or as a function of developmental age, for comparison of results of a given protein, mRNA, and so forth, one requires an internal control. The present study demonstrates that some components of VSM cytoskeleton such as α-actin and the cytosolic protein cyclophilin A, may serve as proper internal controls for equal protein loading in studies of expression patterns during the course of development. In contrast, use of α-tubulin would not be appropriate in this regard. These findings may be of value in studies of ontogeny of structure–function relations in vascular smooth muscle.
In addition to their structural contribution per se, α-actin and α-tubulin play important roles in cell plasticity, tensegrity, and function (Ingber, 2003a,b; Pollard, 2003; Somlyo & Somlyo, 2003). The present study supports the hypothesis that the thin filament protein α-actin plays a key role in cerebral artery contraction, a quality of VSM which persists from fetus to adult. However, the study rejects the hypothesis that α-actin constitutes a significant factor in the age-related contractility changes. Additionally, the studies suggest that expression of α-tubulin, a reflector of cell maturation and protein trafficking, is not closely related to contractility per se. Thus, we believe that findings of the present study have important implications in regard to age-related changes in vascular function, and emphasize the importance of many components of the signal transduction cascade to vascular smooth muscle function–structure relations. Quite obviously, the regulation of expression of these proteins, and their roles in cellular metabolic and signal transduction, in thin filament versus thick filament regulation, and in myofilament Ca2+ sensitivity, are issues that require further study.
Acknowledgments
This study was supported, in part, by USPHS grant HD-03807. We thank Brenda Kreutzer for assistance with preparation of the manuscript.
References
- Adler KB, Krill J, Alberghini TV, Evans JN. Effect of cytochalasin D on smooth muscle contraction. Cell Motil. 1983;3:545–551. doi: 10.1002/cm.970030521. [DOI] [PubMed] [Google Scholar]
- Battistella-Patterson AS, Wang S, Wright GL. Effect of disruption of the cytoskeleton on smooth muscle contraction. Can J Physiol Pharmacol. 1997;75:1287–1299. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-δ. Nature. 1987;326:515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Bravo R, Macdonald-Bravo H. Changes in the nuclear distribution of cyclin (PCNA) but not its synthesis depend on DNA replication. EMBO J. 1985;4:665–661. doi: 10.1002/j.1460-2075.1985.tb03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CL. Actin cytoskeleton and cell signaling. Crit Care Med. 2000;28:N94–N99. doi: 10.1097/00003246-200004001-00011. [DOI] [PubMed] [Google Scholar]
- Dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin binding proteins: Regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- Filipe JAC, Nunes JPL. Functional importance of the actin cytoskeleton in contraction of bovine iris sphincter muscle. Autonomic Autocoid Pharmacol. 2002;22:155–159. doi: 10.1046/j.1474-8673.2002.00255.x. [DOI] [PubMed] [Google Scholar]
- Geary GG, Osol GJ, Longo LD. Development affects in vitro vascular tone and calcium sensitivity in ovine cerebral arteries. J Physiol. 2004 doi: 10.1113/jphysiol.2003.056945. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane extracellular matrix-cytoskeleton crosstalk. Nature Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Gunst SJ, Tang DD. The contractile apparatus and mechanical properties of airway smooth muscle. Eur Respir J. 2000;15:600–616. doi: 10.1034/j.1399-3003.2000.15.29.x. [DOI] [PubMed] [Google Scholar]
- Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. α-Smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell. 2003;14:2508–2519. doi: 10.1091/mbc.E02-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev. 1996;76:967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991;3:841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003a;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003b;116:1397–1408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- Janmey PA. The cytoskeleton and cell signaling. Component localization and mechanical coupling. Physiol Rev. 1998;78:763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- Kolodney MS, Elson EL. Contraction due to microtubule disruption is associated with increased phosphorylation of myosin regulatory light chain. Proc Natl Acad Sci U S A. 1995;92:10252–10256. doi: 10.1073/pnas.92.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite R, Webb RC. Microtubule disruption potentiates phenylephrine-induced vasoconstriction in rat mesenteric arterial bed. Eur J Pharmacol. 1998;351:R1–R3. doi: 10.1016/s0014-2999(98)00358-6. [DOI] [PubMed] [Google Scholar]
- Lin M, Hessinger DA, Pearce WJ, Longo LD. Developmental differences in Ca2+-activated K+ channel activity in ovine basilar artery. Am J Physiol Heart Circ Physiol. 2003;285:H701–H709. doi: 10.1152/ajpheart.00138.2003. [DOI] [PubMed] [Google Scholar]
- Long W, Zhang L, Longo LD. Fetal and adult cerebral artery KATP- and KCa-channel responses to long-term hypoxia. J Appl Physiol. 2002;92:1692–1701. doi: 10.1152/japplphysiol.01110.2001. [DOI] [PubMed] [Google Scholar]
- Long W, Zhao Y, Zhang L, Longo LD. Role of Ca2+ channels in NE-induced increase in [Ca2+]i and tension in fetal and adult cerebral arteries. Am J Physiol. 1999;277:R286–R294. doi: 10.1152/ajpregu.1999.277.1.R286. [DOI] [PubMed] [Google Scholar]
- Long W, Zhao Y, Zhang L, Longo LD. Cerebral artery sarcoplasmic reticulum Ca2+ stores and contractility: changes with development. Am J Physiol. 2000;279:R860–R873. doi: 10.1152/ajpregu.2000.279.3.R860. [DOI] [PubMed] [Google Scholar]
- Longo LD, Ueno N, Zhao Y, Pearce WJ, Zhang L. Developmental changes in α1-adrenergic receptors, IP3 responses, and NE-induced contraction in cerebral arteries. Am J Physiol. 1996;71:H2313–H2319. doi: 10.1152/ajpheart.1996.271.6.H2313. [DOI] [PubMed] [Google Scholar]
- Longo LD, Zhao Y, Long W, Miguel C, Windemuth RS, Cantwell AM, Nanyonga AT, Saito T, Zhang L. Dual role of PKC in modulating pharmacomechanical coupling in fetal and adult cerebral arteries. Am J Physiol. 2000;279:R1419–R1429. doi: 10.1152/ajpregu.2000.279.4.R1419. [DOI] [PubMed] [Google Scholar]
- Mathews MB, Bernstein RM, Franza BR, Jr, Garrels JI. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984;309:374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- Morgan KG, Gangopadhyay SS. Signal transduction in smooth muscle. Invited Review: Cross-bridge regulation by thin filament-associated proteins. J Appl Physiol. 2001;91:953–962. doi: 10.1152/jappl.2001.91.2.953. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Sunagawa M, Kusugi T, Sperelakis N. Actin filament disruption inhibits L-type Ca2+ channel current in cultured vascular smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C480–C487. doi: 10.1152/ajpcell.2000.279.2.C480. [DOI] [PubMed] [Google Scholar]
- Numaguchi K, Eguchi S, Yamakawa T, Motley ED, Inagami T. Mechanotransduction of rat aortic vascular smooth muscle cells requires RhoA and intact actin filaments. Circ Res. 1999;85:5–11. doi: 10.1161/01.res.85.1.5. [DOI] [PubMed] [Google Scholar]
- Nurse P. Cyclin dependent kinases and cell cycle control (Nobel Lecture) Chembiochem. 2002;3:596–603. doi: 10.1002/1439-7633(20020703)3:7<596::AID-CBIC596>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Paul RJ, Bowman PS, Kolodney MS. Effects of microtubule disruption on force, velocity, stiffness and [Ca2+]i in porcine coronary arteries. Am J Physiol Heart Circ Physiol. 2000;179:H2493–H2501. doi: 10.1152/ajpheart.2000.279.5.H2493. [DOI] [PubMed] [Google Scholar]
- Pearce WJ, Hull AD, Long DM, Longo LD. Developmental changes in ovine cerebral vessels composition and reactivity. Am J Physiol. 1991;261:R458–R465. doi: 10.1152/ajpregu.1991.261.2.R458. [DOI] [PubMed] [Google Scholar]
- Platts SH, Falcone JC, Holton WT, Hill MA, Meininger GA. Alteration of microtubule polymerization modulates arteriolar vasomotor tone. Am J Physiol Circ Physiol. 1999;277:H100–H106. doi: 10.1152/ajpheart.1999.277.1.H100. [DOI] [PubMed] [Google Scholar]
- Pollard TD. The cytoskeleton cellular motility, and the reductionist agenda. Nature. 2003;422:741–745. doi: 10.1038/nature01598. [DOI] [PubMed] [Google Scholar]
- Sheridan BC, McIntyre RC, Jr, Meldrum DR, Cleveland JC, Jr, Agrafojo J, Banerjee A, Harken AH, Fullerton DA. Microtubules regulate pulmonary vascular smooth muscle contraction. J Surg Res. 1996;62:284–287. doi: 10.1006/jsre.1996.0209. [DOI] [PubMed] [Google Scholar]
- Small JV. Structure-function relationships in smooth muscle: the missing links. Bioessays. 1995;17:785–792. doi: 10.1002/bies.950170908. [DOI] [PubMed] [Google Scholar]
- Small JV, Gimona M. The cytoskeleton of the vertebrate smooth muscle cell. Acta Physiol Scand. 1998;164:341–348. doi: 10.1046/j.1365-201X.1998.00441.x. [DOI] [PubMed] [Google Scholar]
- Somlyo AV. Handbook of Physiology, section 2, The Cardiovascular System, Vascular Smooth Muscle, chap. 2. II. MD, USA: American Physiological SocietyBethesda; 1980. Ultrastructure of vascular smooth muscle; pp. 33–67. [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Stamnes MA, Rutherford SL, Zuker CS. Cyclophilins: a new family of proteins involved in intracellular folding. Trends Cell Biol. 1992;2:272–276. doi: 10.1016/0962-8924(92)90200-7. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Begg DA. Concentration-dependent effects of cytochalasin D on tight junctions and actin filaments in MDCK epithelial cells. J Cell Sci. 1994;107:367–375. doi: 10.1242/jcs.107.3.367. [DOI] [PubMed] [Google Scholar]
- Tseng S, Kim R, Kim T, Morgan KG, Hai CM. F-actin disruption attenuates agonist-induced [Ca2+], myosin phosphorylation, and force in smooth muscle. Am J Physiol. 1997;272:C1960–C1967. doi: 10.1152/ajpcell.1997.272.6.C1960. [DOI] [PubMed] [Google Scholar]
- Waseem NH, Lane DP. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990;96:121–129. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- Woods AL, Hall PA, Shepherd NA, Hanby AM, Waseem NH, Lane DP, Levison DA. The assessment of proliferating cell nuclear antigen (PCNA) immunostaining in primary gastrointestinal lymphomas and its relationship to histological grade, S+G2+M phase fraction (flow cytometric analysis) and prognosis. Histopathol. 1991;19:21–27. doi: 10.1111/j.1365-2559.1991.tb00890.x. [DOI] [PubMed] [Google Scholar]
- Yildiz A, Tomishige M, Vale RD, Selvin PR. Kinesin walks hand-over-hand. Science. 2004;303:676–678. doi: 10.1126/science.1093753. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Long W, Zhang L, Longo LD. Extracellular signal-regulated kinases and contractile responses in ovine in adult and fetal cerebral arteries. J Physiol (Lond) 551. 2003;2:691–703. doi: 10.1113/jphysiol.2003.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhao Y, Nijland R, Zhang L, Longo LD. Ins (1,4,5) P3 receptors in cerebral arteries: changes with development and high altitude hypoxia. Am J Physiol. 1997;272:R1954–R1959. doi: 10.1152/ajpregu.1997.272.6.R1954. [DOI] [PubMed] [Google Scholar]