Abstract
Muscular adaptation to physical exercise has previously been described as a repair process following tissue damage. Recently, evidence has been published to question this hypothesis. The purpose of this study was to investigate inflammatory processes in human skeletal muscle and epimysium after acute physical exercise with large eccentric components. Three groups of subjects (n= 19) performed 45 min treadmill running at either 4 deg (n= 5) or 8 deg (n= 9) downhill or 4 deg uphill (n= 5) and one group served as control (n= 9). One biopsy was taken from each subject 48 h post exercise. Blood samples were taken up to 7 days post exercise. Compared to the control group, none of the markers of inflammation in muscle and epimysium samples was different in any exercised group. Only subjects in the Downhill groups experienced delayed onset of muscle soreness (DOMS) and increased serum creatine kinase activity (CK). The detected levels of immunohistochemical markers for T cells (CD3), granulocytes (CD11b), leukaemia inhibitory factor (LIF) and hypoxia-inducible factor 1β (HIF-1β) were greater in epimysium from exercised subjects with DOMS ratings >3 (0–10 scale) compared to exercised subjects without DOMS but not higher than controls. Eccentric physical exercise (downhill running) did not result in skeletal muscle inflammation 48 h post exercise, despite DOMS and increased CK. It is suggested that exercise can induce DOMS by activating inflammatory factors present in the epimysium before exercise. Repeated physical training may alter the content of inflammatory factors in the epimysium and thus reduce DOMS.
Muscular adaptation to physical exercise has hitherto been explained by the classical damage–inflammation–repair pathway (MacIntyre et al. 1995; Tidball, 1995; Chambers & McDermott, 1996; Clarkson & Sayers, 1999). By definition, this process involves (a) exercise-induced muscle damage, (b) release of chemo-attractive factors, (c) vasodilatation, (d) leukocyte adhesion, (e) neutrophils and macrophages migration, and (f) activation of satellite cells (Tidball, 1995). Adaptation of adult skeletal muscle to physical exercise has also been compared with neonatal muscle development and regeneration following damage in the sense that satellite cell activation is believed to be inevitable (Chambers & McDermott, 1996). In a recent study in our laboratory several pieces of evidence indicated that muscle adaptation to physical exercise might not occur only via this classical pathway because similar inflammatory changes in skeletal muscle occurred in both the exercise and control groups (Malm et al. 2000). Gibala et al. (2000) and Stupka et al. (2000) have demonstrated a greater degree of ultra-structural damage in eccentrically versus concentrically exercised muscle, while Yu et al. could not detect any alteration in desmin structure after different modes of eccentric exercise (Yu et al., 2002, 2003). Stupka et al. (2000) also found an increased number of leukocyte common antigen (LCA/CD45)-positive cells in skeletal muscle 48 h after eccentric exercise. LCA (CD45) is expressed on all leukocytes but not all macrophages and the total number of inflammatory cells in each muscle section could not be evaluated in this study. Thus, it can be argued that exercise-induced disruption of the cytoskeleton is a sign of remodelling (Yu et al. 2003), and that the evidence for a consequent muscle inflammation should be viewed with caution.
Mechanical stretch of muscle tissue is most probably essential for normal muscle function and adaptation (Goldspink, 1999), and all non-circulating cells will in fact die without stretch or contact stimulus (Ruoslahti, 1997). The possibility of non-inflammatory adaptation of skeletal muscle tissue to physical exercise and local adaptation mechanisms within the muscle tissue should therefore be investigated more thoroughly. Several synergistic factors (growth factors, cytokines, hormones, oxygen radicals, receptor expression, etc.) are probably responsible for muscular adaptation. Insulin-like growth factor-1 (IGF-1) is known to have both systemic and local effects (Adams, 1998), and a splice variant found in human muscle has been termed mechano growth factor (Goldspink, 1999). IL-1β is found in human muscle tissue (Cannon et al. 1989; Malm et al. 2000) and can affect protein synthesis (Cooney et al. 1999). The oxygen-sensitive hypoxia-inducible factor-1α (HIF-1α) and HIF-1β are up-regulated in human skeletal muscle after exercise and can affect gene expression of vascular endothelial growth factor (VEGF) (Gustafsson et al. 1999). A local system comprising leukaemia inhibitory factor (LIF) and its receptor (LIF-R) probably also exists in muscle tissue, and can in part control muscle regeneration after injury (Barnard et al. 1994; Schoser et al. 1998; Cabanillas et al. 2000). Large amounts of hepatocyte growth factor/scatter factor (HGF) and its receptor (c-Met) are constitutively expressed in skeletal muscle, where it activates dormant satellite cells and is vital for normal neonatal skeletal muscle development (Birchmeier & Gherardi, 1998; Tatsumi et al. 1998). Other possible local adaptation systems have also been described (Husmann et al. 1996). Thus, the involvement of both local and systemic factors is likely to be necessary for skeletal muscle adaptation to physical exercise.
The primary purpose of this study was to investigate expression of inflammatory markers, growth factors and cytokines in human skeletal muscle and the peripheral blood after strenuous physical exercise, without the influence of previous muscle biopsies. In order to induce various proportions of concentric and eccentric muscle contractions, three different exercise protocols were used.
It was hypothesized that (1) muscle adaptation occurs without muscle inflammation, (2) expression of growth factors is affected by physical exercise, and (3) DOMS is an event in the epimysium.
Knowledge of the mechanisms governing skeletal muscle adaptation to altered physical demand is important for optimal skeletal muscle rehabilitation and exercise regimes for people suffering from muscular diseases and optimal training programmes for athletes.
Methods
Subjects
Twenty-five healthy male and three healthy female subjects (mean (range): age 27.8 (18–58) years; body mass 76.2 (62–94) kg) participated in the study. All subjects were physically active on a regular basis, with mean (range) maximal oxygen uptake during running, V̇O2max 52.8 (28.0–67.0) ml kg −1 min−1 and muscle fibre type compositions of 58 (30–84)% type 1 and 42 (16–70)% type 2. V̇O2max and fibre type composition values did not differ between the groups. After receiving oral and written information about the study, subjects signed an informed consent form and were randomly assigned to Downhill 4 deg (n= 5), Downhill 8 deg (n= 9), Uphill 4 deg (n= 5) or control (n= 9) groups. Due to the invasive nature of the study, the number of subjects allocated to each group was the smallest possible for statistical calculations. The study was approved by the Ethics Committee at the Karolinska Institutet (Dnr: 00-220) and all procedures used conformed to The Declaration of Helsinki.
Exercise protocol
Not later than 2 days before the 45 min running exercise each subject's V̇O2max was determined (AMIS 2001, Inovision A/S, Denmark). A standard incremental running test was performed on a treadmill (Rodby Electronics, Sweden). After a 10–15 min warm-up at a speed chosen by the subject and a brief resting period (approximately 5 min) the test started at an incline of 1 deg and an increase of 1 deg every minute until exhaustion. Running speed was constant and set at each subject's estimated 10 km racing pace. Subjects were asked not to perform any strenuous or unaccustomed exercise from 7 days before the 45 min running exercise until the muscle biopsy and last blood samples were taken (7 days post exercise).
Running speeds during the 45 min exercise were chosen based on V̇O2 measurements from each subject when running at two different speeds for 60–90 s at each speed at the assigned incline or decline. These measurements were performed as part of the warm-up for the V̇O2max test. The Downhill 4 deg group was intended to run at 50% of V̇O2max and the Uphill 4 deg group at 75% of V̇O2max. In the Downhill 8 deg group, concerns were raised regarding the risk of injury due to the steep decline. In order to maximize the eccentric component of the exercise while minimizing the risk of injury, subjects were asked to run at the maximum speed maintainable for 45 min. All subjects managed to run at the chosen speed for the 45 min.
Subjects reported to the laboratory between 08.00 and 10.00 h and were instructed to eat a light breakfast not later than 2 h before the exercise. Water was given ad libitum during exercise. The 45 min running exercise was preceded by a 10 min warm-up on the treadmill at 0 deg and an individually chosen speed. Between 20 and 23 min and 42 and 45 min of exercise V̇O2max and heart rate were recorded (Table 1).
Table 1.
Physiological variables before, during and after 45 min of downhill running at 4 deg or 8 deg or uphill running at 4 deg
| Variable | Down 4 deg (n= 5) | Down 8 deg (n= 9) | Up 4 deg (n= 5) |
|---|---|---|---|
| Running speed (km h−1) | 8.5 (6.49–10.5)* | 12.8 (11.1–14.6)*† | 9.1 (6.6–11.6)† |
| V̇O2 during exercise (ml min−1 kg−1) | 23.9 (19.3–28.5)* | 32.2 (26.9–37.4)† | 45.2 (37.2–53.2)*† |
| V̇O2 during exercise (l min−1) | 1.91 (1.49–2.33)* | 2.38 (2.04–2.73)† | 3.29 (2.19–4.39)*† |
| Percentage V̇O2max during exercise | 48 (42–55)* | 57 (51–62)† | 81 (77–85)*† |
| Heart rate (HR) during exercise (beats min−1) | 132 (108–155)* | 168 (150–185) | 184 (176–192)* |
| Percentage HRmax during exercise | 69 (59–79)*† | 99 (81–94)* | 92 (87–96)† |
| DOMSmax (0–10 scale)a | 2 (0–4) | 3 (1–9)* | 0 (0)* |
| Painmax (0–10 scale)a | 0 (0–3) | 4 (1–8)* | 0 (0–1)* |
| V̇O2 at 150 W cycling (l min−1) | 2.15 (2.05–2.26) | 1.89 (1.58–2.20) | 1.97 (1.70–2.24) |
| V̇O2 at 200 W cycling (l min−1) | 2.70 (2.66–2.75) | 2.48 (2.18–2.77) | 2.47 (2.07–2.87) |
| HR at 150 W cycling (beats min−1) | 126 (119–133) | 126 (108–143) | 138 (109–166) |
| HR at 200 W cycling (beats min−1) | 149 (132–166) | 144 (121–185) | 156 (126–185) |
| Isometric torque (N m) | |||
| Pre-exercise | 194 (139–250) | 210 (163–258) | 237 (156–318) |
| Post 24 h | 196 (137–256)* | 175* (143–206) | 234 (147–321) |
| Post 48 h | 201 (153–248) | 184 (146–221) | 236 (183–289) |
| Post 72 h | 203 (139–268) | 193 (153–232) | 245 (209–281) |
| Post 7 days | 198 (148–248) | 185 (152–217) | 259 (206–312) |
Mean (95% confidence interval). * and † indicate significant difference (ANOVA and Fisher's PLSD or unpaired t test) between groups (P < 0.05). a Mann–Whitney U test and median (minimum and maximum).
Muscle and epimysium biopsies
Muscle and epimysium biopsies were taken from the left vastus lateralis using the open forceps biopsy technique 48 h after exercise. Based on a previous study in our laboratory (Malm et al. 2000) the presence of neutrophils, macrophages and activated satellite cells peaked at this time point after eccentric exercise and multiple biopsies. After local epidermal anaesthesia (Carbocain 20 mg ml−1, ASTRA, Södertälje, Sweden) a 2.5 cm incision was made through the dermis, epimysium and perimysium. An approximately 3 mm × 5 mm piece of the epimysium was cut by a surgical scalpel after which a 50–100 mg muscle tissue sample was removed by the forceps biopsy technique. The muscle and epimysium samples were each placed in Tissue-Tek medium (Miles Laboratories, IN, USA), frozen in isopropanol in liquid nitrogen within 1 min and stored at −70°C before sectioning (6 μm). Sections were placed on Superfrost glass slides (Novakemi AB, Enskede, Sweden), air dried for 30 min and stored at −70°C until staining.
Muscle function
A computerized dynamometer (the SPARK System) (Seger et al. 1988) was used for a functional test of the knee extension muscles, including the vastus lateralis. Due to technical problems, only isometric measurements could be performed and a 45 deg knee angle was used with subjects seated in the SPARK unit. At each test session, three maximal voluntary isometric contractions with the right leg (not biopsied) were performed and the maximal force maintained for 0.5 s recorded. The test was performed twice during the week prior to the running exercise (the second test was recorded as the pre-exercise value) and 24 h, 48 h, 72 h and 7 days post exercise. All tests were performed immediately after collection of blood samples.
Muscle soreness
Before and 24 h, 48 h, 72 h and 7 days after exercise, muscle soreness at rest was estimated by the subject on a 0–10 subject rating scale (0 = no soreness and 10 = very, very sore). Delayed onset muscle soreness (DOMS) was estimated in the subjects' right thigh muscles when the subjects, in a prone, dorsal position, lifted the right foot 5 cm from the surface. Muscle pain was evaluated by placing a rubber cylinder (area 5 cm2) attached to a 5 kg weight (giving a pressure of 10 N cm−2) on the mid-section of the right vastus lateralis muscle. Pain was estimated by the subjects on the 0–10 subject rating scale.
Immunohistochemistry
The Histostain Plus kit (Zymed Laboratories Inc., South San Francisco, USA) and a modified staining protocol (Ulfgren et al. 1995) were used when investigating expression of antigens with monoclonal antibodies in muscle and fascia sections (Table 2 and Fig. 1). The main feature in the modified protocol is the use of saponin (Sigma-Aldrich, Stockholm, Sweden) and Hepes (Life Technologies Ltd, Paisley, Scotland) in the Earle's balanced salt solution (EBSS; Life Technologies Ltd). The optimal concentration of each antibody was determined before analysis. Briefly, the immunhistochemical staining procedure included the following main steps: (1) blocking of endogenous peroxidase with H2O2; (2) blocking of unspecific antigens with normal serum; (3) incubation with a primary antibody (mouse antihuman) for the antigen investigated; (4) incubation with biotinylated secondary antibody (goat antimouse); (5) incubation with an avidin–biotin–peroxidase complex; (6) developing with a peroxidase substrate (AEC); and (7) counterstaining with Mayer's Hematoxylin (Apoteksbolaget, Malmö, Sweden). Stained glasses were mounted with a cover slip over glycerol (BDH glycerol jelly, KEBO Laboratory AB, Spånga, Sweden) and stored in the dark until analysed. All antibodies were tested for functionality using the immunohistochemical method on 6 μm sections of human muscle, epimysium, umbilical cord and tonsil to determine staining pattern and background interference. All antibodies were monoclonal and thus specific for a selected epitope on each protein. The existence of this epitope on other proteins present in human skeletal muscle tissue cannot be ruled out. Positive control sections from tissues other than human skeletal muscle were not used because any cross-reactivity with muscle proteins would not be detected. Thus all antibodies were specific and functional, but cross-reactivity could not be completely ruled out. Human blood lymphocytes/monocytes stimulated for 3.5 h with Lipopolysaccharide(LPS). Distinct staining of a proportion of the cells was obtained. A negative control (rat IgG2a for interleukin-6 (IL-6) and mouse IgG1 for all others) was included in each experiment.
Table 2.
Staining panel for immunohistochemistry
| Antigen | Clone/Nr | Supplier | Dilution (μg ml−1) | Expression/function |
|---|---|---|---|---|
| CD3 | T3-4B5 | DP | 1: 400 | T cells, T cell receptor accessory molecule (see Table 2) |
| CD11b | 2LPM19c | DP | 1: 800 | Granulocytes, C3bi receptor (CR3) (Table 2) |
| CD56 | MOC-1 | DP | 1: 100 | NK cells, satellite cells, dendritic cells (N-CAM). Cell-cell adhesion |
| CD163 | Ber-MAC3 | DP | 1: 200 | Human macrophages, down-regulation of inflammation |
| IL-1β | Mix | CG | 1: 500 | Macrophages, fibroblasts, endothelial cell, smooth muscles. Pro-inflammatory |
| IL-6 | MQ2-6A3 | PH | 2 | Macrophages, T2 helper cells. Increases in response to inflammation |
| IGF-1 | 500-P11 | PT | 5 | Liver, smooth muscle. Cell differentiation and proliferation |
| LIF | 500-P39 | PT | 10 | Smooth muscle, Type 1 muscle fibres (?). Myoblast division, muscle regeneration |
| LIF-Rα | AF-249-NA | RD | 25 | LIF receptor. LIF mRNA expressed in muscle precursor cells. Signal transduction via gp130 |
| HIF-1β | NB 100–110 | NB | 1: 1000 | Many cell types. Oxygen sensing transcription factor with HIF-1α |
| Endothelium | EN4 | SB | 1: 100 | Human endothelium. Used as protocol control |
| Neg. control | IgG1 | DP | 1: 20 | Aspergillus niger glucose oxidase antibody |
CD: cluster of differentiation. DP: DAKOPATTS AB, Älsvjö, Sweden. CG: Immunocontact, Ciba-Geigen, Switzerland. Nr: number. PH: Pharmingen, USA. RD: R & D Systems, UK. PT: Peprotech EC Ltd, UK. NB: Novus Biologicals, USA. SB: SANBIO, Netherlands.
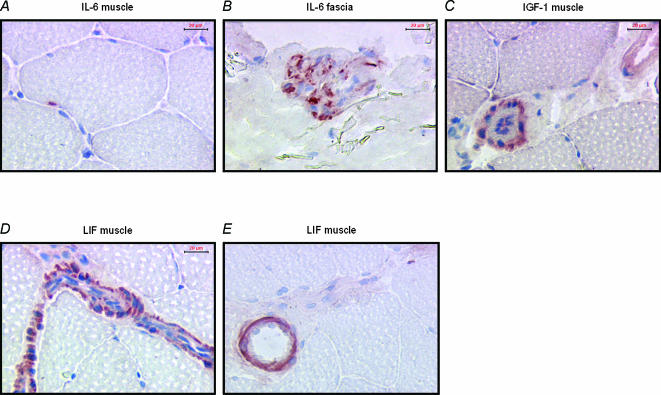
Figure 1. Antigen detection in muscle and epimysium.
A, IL-6 in muscle cell; B, IL-6 in epimysium; C, IGF-1 in smooth muscle cells in skeletal muscle tissue; D, LIF expression in smooth muscle cells in a blood vessel from skeletal muscle tissue; E, LIF expression in smooth muscle in a larger blood vessel.
Image analysis
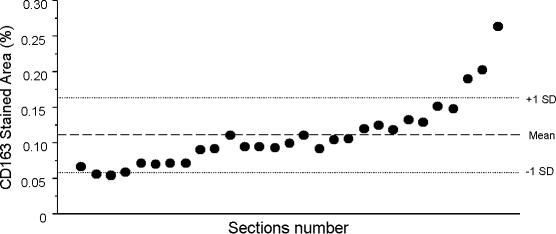
For quantification of positively stained tissue sections, a semiautomated image analysis system (Leica Microsystems, Sweden), previously described, was used (for details, see Malm et al. 2000). Analyses were performed on three separate areas of each muscle section giving a total area of approximately 1.5 mm2 per antigen and biopsy. The optimal threshold for each individual and antigen was determined. The coefficient of variance (standard deviation/mean) for repeated measurement of the same tissue section was 0.08% (n= 29). The coefficient of variance for analysis of 29 serial sections from the same muscle sample was 42%. These results indicate that measurement of the stained area by the image analysis system was accurate, but there was a large variation in antigen expression (in this case CD163) within a tissue sample (Fig. 2).
Figure 2. Detection of CD163 in 29 serial sections from the same muscle biopsy, demonstrating the variation of macrophage presence within one muscle tissue sample.
This variation stresses the importance of standardized sampling placement, checking for reproducibility within one sample, and performing subsequent power calculations before statistical analysis and interpretation is performed.
Blood samples
Venous blood samples were drawn from a forearm vein before, immediately after and 6 h, 24 h, 48 h, 4 days and 7 days post exercise. Five millilitres of blood were collected into Vacutainer tubes containing ethylendiaminetetraacetic acid (EDTA; Becton Dickinson, France) for leukocyte analysis by flow cytometry, 5 ml into untreated Vacutainer tubes for analysis of C-reactive protein (CRP), cortisol, albumin, sex hormone-binding globulin (SHBG), testosterone and CK.
Flow cytometry
Determination of different subsets of leukocytes (Table 3) was accomplished by flow cytometry with three-colour analysis. The method for three-colour flow cytometry analysis has been described in detail previously (Lenkei & Andersson, 1995) and is based on a high degree of standardization. Cell surface molecule density is expressed as molecules of equivalent soluble fluorochrome (MESF).
Table 3.
Antigens investigated on lymphocytes and monocytes in blood and serum cytokines
| Antigen | Function | Expression/production |
|---|---|---|
| Lymphocytes | ||
| CD3 | Associated with the T cell receptor. Involved in signal transduction | T cells |
| CD4 | Accessory molecule for TCR antigen recognition, MHC II interaction | T helper cells (TH), monocytes |
| CD8 | Accessory molecule for TCR antigen recognition, MHC I interaction | T cytotoxic/suppressor cells (Tc/s) |
| CD11b | Integrin, adhesion to vascular endothelium, receptor for C3bi | Granulocytes, monocytes, NK cells, T cells |
| CD45 | Cytoplasmic phosphatase activity, signal transduction, apoptosis | All leukocytes |
| CD62L | Selectin, homing receptor on leukocytes | Blood B, T and NK cells, monocytes, granulocytes |
| CD95 | (Fas), apoptotic signalling mediated by Fas ligand | Many different cell types |
| Ki-67 | Proliferation marker | All dividing cells |
| DR | MHC II subunit | Antigen-presenting cells |
| Monocytes | ||
| CD14 | LPS binding protein. Activation marker. Induces oxidative burst | Monocytes |
| Cytokines | ||
| IL-2 | Anti-inflammatory, T cell, B cell, NK cell, neutrophil and macrophage activation | TH1 cells, |
| IL-4 | Anti-inflammatory, macrophage suppression | TH2 cells, mast cells |
| IL-5 | Pro-inflammatory, B cell and eosinophil activation, | TC cells, mast cells, eosinophils |
| IL-6 | Anti-inflammatory (?), B cell differentiation, T cell activation, acute phase protein induction, energy utilization control (?) | Macrophages, TH2 cells, B cells, fibroblasts, vascular endothelial cells, muscle cells |
| IL-10 | Anti-inflammatory, matrix biosynthesis regulation, T cell, NK cell and macrophage suppression (anti-inflammatory activation), CD163 up-regulation, B and mast cell differentiation | Macrophages, TH2 cells |
| IFNγ | Pro-inflammatory, blocks viral RNA translation, CD163 down-regulation | TH1 cells, virus exposed cells |
| TNFα | Pro-inflammatory, anticancer effects | Monocytes/macrophages |
White blood cell count and differentials were estimated with a Coulter STKS haemocytometer (Coulter Electronics, USA). Because cell numbers were determined in whole blood, corrections for changes in plasma volume were not made.
Soluble CD8 (sCD8) was estimated by enzyme-linked immunosorbent assay (ELISA; Endogen, Inc. Woburn, MA, USA) according to the manufacturer's instructions.
Serum cytokines were measured by the cytometric bead array method for flow cytometry, with the Human Th1/Th2 Cytokine kit from Becton Dickinson (BD Pharmingen, San Diego, CA, USA) according to the manufacturer's instructions. The flow cytometric results were translated into cytokine concentrations using the software included in the kit.
The Ki-67 antigen (a marker for cell proliferation rate) was detected after lymphocyte permeabilization by using a whole blood method. The four-colour staining protocol included (a) in the first tube: isotype controls for FITC-, PE- and PerCP-conjugated mAbs, and CD3-APC which was used to gate the CD3+ T cells; (further details from Lenkei, et al. 1995) (b) in the second tube Ki-67-FITC, CD45RO-PE, CD8-PerCP and CD3-APC. Thus, Ki-67 was estimated in different lymphocyte subsets. Briefly, the cells were stained with the mAbs directed to antigens expressed on the lymphocyte surface, washed and treated with paraformaldehyde. This was followed by a permeabilization step with saponin buffer, and thereafter the cells were stained with Ki-67.
Serum hormones and proteins
CRP and albumin.
C-reactive protein (CRP) and albumin were analysed by means of particle-enhanced immunonephelometry (Dade Behring Marburg, Germany).
Cortisol.
Total serum cortisol was determined by a standard immunofluorescent method (Department of Clinical Chemistry, Karolinska Hospital, Solna, Sweden).
Testosterone, SHGB and albumin.
Serum testosterone and sex hormone binding globulin (SHGB) concentrations were determined by a standard time-resolved fluoroimmunoassay (Kit B050-101 and B070-101, respectively, AutoDelphiaTM, Wallac Oy, Finland). The biologically available (free) testosterone concentration (not bound to SHGB or albumin) was calculated as T(1 + 0.601C) where T is unbound testosterone and C is plasma albumin concentration (personal communication R. Solborg, Department of Clinical Chemistry, Karolinska Hospital).
Serum CK.
Serum CK activity was measured using a standard laboratory kit (CK MPR2, Boehringer-Mannheim, Germany) and a DU-70 spectrophotometer (Beckman Instruments AB, Bromma, Sweden).
Statistical analysis
The StatView software (Abacus Concepts, Inc., Berkeley, USA) was used for all statistical analysis. Due to the non-parametric distribution in resting numbers of most blood leukocyte phenotypes in a larger population (n= 57) (Lenkei & Andersson, 1995), as well as in blood and muscle samples in the present study (skewness > 2.0), all data were log transformed before applying ANOVA statistics with Fisher's PLSD or Student's unpaired t test for differences between groups. Student's unpaired t test was used for between-group comparisons at each time point when a difference between the groups over time (time × group effect) was detected by ANOVA. The non-parametric Mann–Whitney procedure tested for between-group differences in DOMS and muscle pain. Multiple regression was used to investigate relationships between variables, with R2 > 0.70 accepted in the model. Variables where one single outlier determined the correlation were excluded. Outliers were judged by a dependent versus fitted regression plot. Multiple predictor variables were used only if they were not correlated to each other (P > 0.1). A power calculation using data from a previous publication (Malm et al. 2000) was performed. In the previous study, the results from the immunohistochemical analysis (percentage stained area of total area analysed) were in the range 0.1–0.3 (mean) and 0.02–0.08 (standard deviation) for neutrophils and macrophages in human skeletal muscle at rest and 48 h after exercise and in the third biopsy from the same muscle. With α= 0.05 and β= 0.20, the number of subjects required to detect a change in the percentage stained area of 0.2–0.3 (a 2- to 3-fold change from the mean at rest) is three or four.
Results
Only subjects in the downhill running groups experienced DOMS (Table 1), and muscle function decreased (Fig. 3) and serum CK increased (Table 4) in the Downhill 8 deg group only. Compared to the control group, no increase in markers of inflammation was detected in skeletal muscle or epimysium 48 h after exercise (Table 5). However, when the exercised subjects were grouped according to the DOMS they experienced (>3 on a 0–10 scale) there was significantly higher expression of CD3, CD11b, HIF-1β and LIF observed in the epimysium from the DOMS group compared to the epimysium from the group without DOMS (Figs 4–7). No difference in muscle antigen expression in the skeletal muscle was detected between the ‘DOMS’ and the ‘no DOMS’ groups.
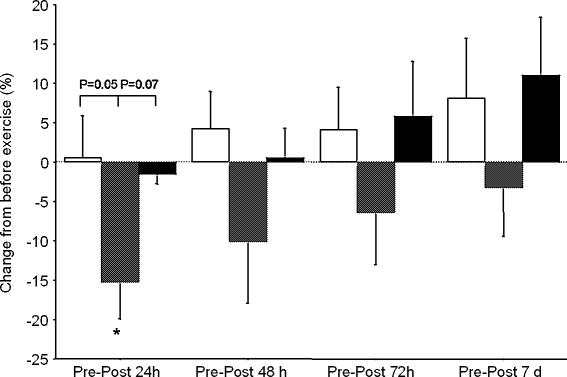
Figure 3. Change in maximal isometric torque from before to after running.
Open columns, downhill 4 deg; hatched columns, downhill 8 deg; filled columns, uphill 4 deg. * Significant difference from rest (paired t test, p= 0.02).
Table 4.
Hormones (nmol l−1) and CK (mKat) in serum before and after 45 min of downhill running at 4° or 8° or uphill running at 4 deg
| Variable | Group | ANOVA | Rest | Post | 6 h | 24 h | 48 h | 72 h | 7 days |
|---|---|---|---|---|---|---|---|---|---|
| Testosterone | All | Time | 17 | 20* | 15* | 16 | 18 | 17 | 17 |
| (12–21) | (15–25) | (11–19) | (12–21) | (14–22) | (12–21) | (12–21) | |||
| Free testosterone | All | Time | 9.9 | 11.9* | 8.5* | 9.4 | 10.3 | 9.4 | 9.7 |
| (7.0–12.8) | (8.5–15.3) | (6.3–10.8) | (7.0–11.8) | (7.5–13.0) | (7.0–11.9) | (7.0–12.3) | |||
| Cortisol | Down 4 | 508 | 371 | 342 | 493 | 394 | 448 | 489 | |
| (255–760) | (157–586) | (126–558) | (249–736) | (177–610) | (206–690) | (271–706) | |||
| Down 8 | Time × | 437 | 434¶ | 285 | 426 | 428 | 360 | 373 | |
| Group | (361–513) | (349–520) | (223–346) | (342–510) | (361–505) | (289–431) | (262–484) | ||
| Up 4 | 482 | 645¶ | 300 | 437 | 328 | 381 | 364 | ||
| (369–595) | (463–827) | (240–359) | (312–563) | (219–438) | (290–472) | (294–434) | |||
| CK | Down 4 | 1.3 | 1.6¶ | 2.1¶ | 2.2¶ | 1.6¶ | 1.6 | 1.2 | |
| (0.64–2.0) | (0.77–2.4) | (1.1–3.1) | (1.0–8.3) | (0.62–2.6) | (0.87–2.2) | (0.55–1.8) | |||
| Down 8 | Time × | 2.6 | 3.8¶ | 11.9¶ | 14.6¶ | 8.8¶ | 7.0 | 6.5 | |
| Group | (1.3–5.1) | (1.9–6.8) | (5.5–9.0) | (4.8–2.0) | (3.5–16.0) | (2.2–15.6) | (1.4–18.9) | ||
| Up 4 | 3.6 | 4.8 | 5.1† | 4.5† | 3.2† | 2.4 | 1.7 | ||
| (0.76–6.5) | (1.0–8.6) | (1.1–9.1) | (1.2–7.9) | (1.1–5.3) | (1.3–3.5) | (0.64–2.8) |
Mean (95% confidence interval). In the ANOVA column Time indicates change over time P < 0.01 and Group indicates a Time × Group effect. *Indicates significant difference compared to Rest (P < 0.05; Fsher PLSD). ¶ and † indicate P < 0.05 and P < 0.025 between groups (unpaired t test)
Table 5.
Immunohistochemical detection of antigens in muscle and epimysium after 45 min of downhill running at 4 deg or 8 deg or uphill running at 4 deg
| Antigen | Group | Muscle | Epimysium |
|---|---|---|---|
| CD3 (T-cells) | Down 4 deg | 0.05 (0.01–0.15) | 0.04 (0.02–0.06) |
| Down 8 deg | 0.02 (0–0.03) | 0.10 (0–0.54) | |
| Up 4 deg | 0.02 (0.01–0.04) | 0.04 (0.02–0.06) | |
| Control | 0.03 (0.01–0.05) | 0.15 (0.01–0.86) | |
| CD11b (granulocytes) | Down 4 deg | 0.03 (0.01–0.07) | 0.07 (0.01–0.15) |
| Down 8 deg | 0.02 (0.01–0.04) | 0.06 (0–0.23) | |
| Up 4 deg | 0.01 (0.01–0.02) | 0.05 (0.01–0.06) | |
| Control | 0.01 (0–0.03) | 0.15 (0.01–0.80) | |
| CD56a | Down 4 deg | 0.74 (0–2.1) | 0.03 (0–0.10) |
| Down 8 deg | 0.89 (0–3.8) | 0.19 (0–1.12) | |
| Up 4 deg | 0.38 (0–1.2) | 0.09 (0.03–0.18) | |
| Control | 1.7 (0–7.6) | 0.09 (0–0.38) | |
| CD163 (macrophages) | Down 4 deg | 0.08 (0.01–0.14) | 0.21 (0.06–0.37) |
| Down 8 deg | 0.05 (0.01–0.21) | 0.28 (0.06–0.85) | |
| Up 4 deg | 0.02 (0.01–0.03) | 0.24 (0.17–0.36) | |
| Control | 0.03 (0.01–0.08) | 0.20 (0.0–0.37) | |
| IGF-1 | Down 4 deg | 0.06 (0.01–0.14) | 0.27 (0.02–0.71) |
| Down 8 deg | 0.03 (0–0.1) | 0.77 (0.01–5.36) | |
| Up 4 deg | 0.04 (0.02–0.08) | 0.18 (0.01–0.35) | |
| Control | 0.03 (0.01–0.08) | 0.56 (0–2.20) | |
| LIF | Down 4 deg | 0.08 (0.01–0.14) | 0.04 (0–0.07) |
| Down 8 deg | 0.05 (0.01–0.21) | 0.13 (0–0.54) | |
| Up 4 deg | 0.02 (0.01–0.03) | 0.13 (0.03–0.36) | |
| Control | 0.03 (0.01–0.08) | 0.27 (0–1.63) | |
| LIF-R | Down 4 deg | 0.01 (0–0.01) | 0.06 (0–0.24) |
| Down 8 deg | 0.01 (0–0.04) | 0.11 (0–0.71) | |
| Up 4 deg | 0 (0–0.01) | 0.02 (0.01–0.05) | |
| Control | 0.01 (0–0.04) | 0.10 (0–0.27) | |
| HIF-1β | Down 4 deg | 0.10 (0–0.20) | 0.16 (0.02–0.36) |
| Down 8 deg | 0.05 (0–0.17) | 0.22 (0.01–0.79) | |
| Up 4 deg | 0.05 (0.01–0.09) | 0.11 (0–1.04) | |
| Control | 0.05 (0.01–0.14) | 0.24 (0–1.04) | |
| Down 4 deg | 0.75 (0.53–1.09) | 96 (31–162) | |
| IL-1β | Down 8 deg | 0.25 (0.01–1.13) | 125 (2–248) |
| Up 4 deg | 0.77 (0.58–1.01) | 65 (4–127) | |
| Control | 0.43 (0.04–1.32) | 128 (7–249) | |
| IL-6 | Down 4 deg | 0.01 (0–0.01) | 0.08 (0–0.47) |
| Down 8 deg | 0 (0–0.01) | 0.06 (0–0.37) | |
| Up 4 deg | 0.01 (0–0.02)* | 0.01 (0–0.02) | |
| Control | 0 (0)* | 0.09 (0.1–0.47) |
Mean (minimum and maximum). *P < 0.05 between groups (unpaired t test). Down 8 deg, n= 8; Down 4 deg, n= 5; Up 4 deg, n= 5; Control n= 8. a In muscle the number of stained non-leukocyte cells (satellite cells, myoblasts, myotubes, etc.) was counted and expressed as a percentage of muscle cell number.
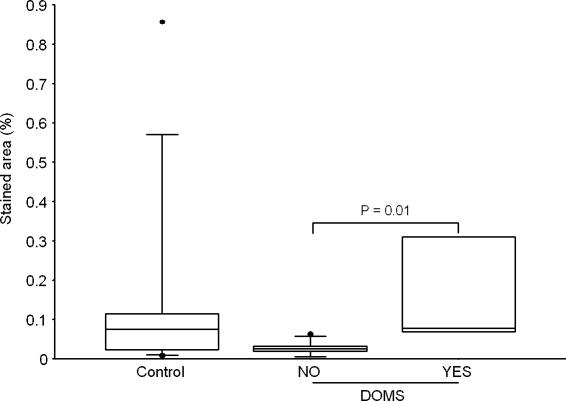
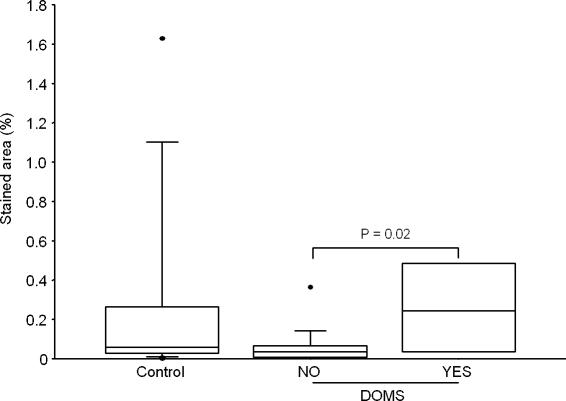
Figure 4. CD3 in epimysium.
Stained area of CD3 in the control group (Control, n= 9) and the exercise groups split into groups of subjects suffering from DOMS (YES, n= 4) or not (NO, n= 14), regardless of treadmill elevation.
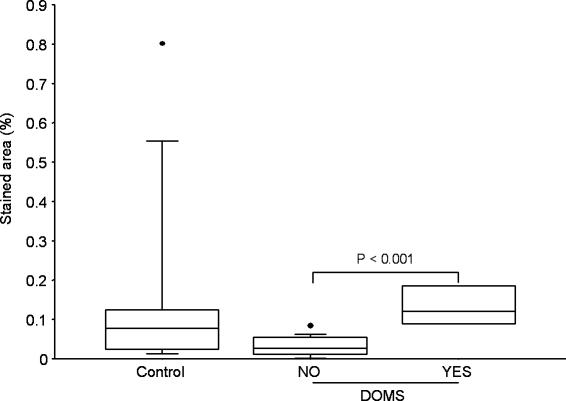
Figure 7. LIF in epimysium.
Stained area of LIF in the control group (Control, n= 9) and the exercise groups split into groups of subjects suffering from DOMS (YES, n= 4) or not (NO, n= 14), regardless of treadmill elevation.
Exercise intensity
Different slopes and speeds on the treadmill resulted in differences in relative exercise intensities and proportions of eccentric muscle contractions between individuals and groups (Table 1). The percentage of V̇O2max achieved during exercise was higher in the Uphill 4 deg group compared to the Downhill 4 deg and 8 deg groups. The percentage of maximal heart rate achieved during exercise was higher in the Uphill 4 deg and Downhill 8 deg groups compared to the Downhill 4 deg group. Based on the subject's rating of muscle soreness (DOMS) and pain, the Downhill 8 deg running put the most strain on the vastus lateralis muscle. These data indicate that Downhill 8 deg running was the most strenuous exercise protocol for the skeletal musculature while Uphill 4 deg running induced the highest total metabolic stress.
Muscle function
Maximal voluntary isometric contractions decreased in the Downhill 8 deg group compared to the Downhill 4 deg and Uphill 4 deg groups (Fig. 3).
Muscle soreness, muscle pain, serum CK, CRP and hormones
One subject in the Downhill 4 deg and four subjects in the Downhill 8 deg group experienced severe DOMS and muscle pain, which peaked at 24 h or 48 h after exercise (Table 1).
CK was significantly increased in the Downhill 8 deg group from 6 to 48 h after exercise (Table 4). C-reactive protein (CRP), a marker of systemic inflammation, did not change significantly in any group and was (mean and 95% confidence interval) 1.6 (0.2–2.9) μg ml−1 at rest.
Subjects were grouped in ‘DOMS’ (n= 5) and ‘no DOMS’ groups (n= 14) and ‘pain’ (n= 9) and ‘no pain’ (n= 10) groups (subjects with ratings higher than 3 on a 0–10 scale at any time after exercise were assigned to the ‘DOMS’ and ‘pain’ groups). Multiple regression analysis was used to investigate relationships between DOMS, pain and other variables investigated. A significant correlation between DOMS and granulocytes (CD11b) in the epimysium and maximal serum creatine kinase activity (CKmax) (R2= 0.78; P < 0.0001) was found. Pain was related to running speed, CD11b-positive cells in the epimysium and the proportion of cytotoxic T cells among all naïve lymphocytes at rest (R2= 0.97; P < 0.0001).
CKmax was related to running speed and the change in the total number of circulating leukocytes from rest to 6 h post exercise (R2= 0.65; P < 0.0001), but not treadmill slope. Serum CK did not correlate to exercise intensity (measured as percentage V̇O2max in l min−1 and l min−1 kg−1) or any indicator of inflammation in skeletal muscle or epimysium.
Hormones in serum.
Testosterone (total and free) in the serum increased immediately after exercise and decreased at 6 h (Table 4). Serum cortisol concentrations changed differently over time in the three groups (ANOVA) but did not differ between the groups at any given time point. The testosterone/cortisol ratio did not change significantly (data not shown).
Expression of inflammatory markers in muscle and epimysium (Table 5)
The levels of inflammatory markers detected in muscle and epimysium samples from the Downhill 8 deg group were not higher than in the control group, demonstrating a lack of skeletal muscle inflammation 48 h after strenuous dynamic, eccentric exercise. The amounts detected were comparable to data from muscle samples taken at rest in a previous study (Malm et al. 2000). One subject in the control group demonstrated a large infiltration of CD3-, CD11b- and CD163-positive cells, similar to biopsies from myositis patients. The subject was referred to the Department of Rheumatology at the Karolinska Hospital for a clinical investigation and was excluded from the study.
T cells (CD3).
Image analysis detected stained areas of 0−0.14% (minimum and maximum) in muscle tissue and 0–0.9% in epimysium.
Leukocytes/neutrophils (mainly) (CD11b).
CD11b, the complement 3bi receptor, was detected in 0–0.07% of the total muscle area and in 0.01–0.80% of the total epimysium section area (Table 5). The percentage of stained area in muscle was identical to previous findings in rested human skeletal muscle (Malm et al. 2000).
CD56-positive cells and structures.
Antibodies to CD56 stained between 0 and 0.1% (minimum and maximum) of the total muscle area and 0–1.1% of the total epimysium section area. Between 0 and 2% of the muscle fibres (0–4 fibres per section) were CD56 positive, and this number did not differ between the groups. CD56-positive muscle fibres are believed to be activated satellite cells, myoblasts or denervated muscle fibres (Illa et al. 1992) and therefore a sign of regeneration/degeneration. In muscle, very few non-muscle cells expressing CD56 are observed while CD56 expression in epimysium may be located in natural killer (NK) or neuroectodermal cells.
Macrophages (CD163).
Between 0 and 0.2% (minimum and maximum) of total muscle area and 0–0.8% of total epimysium section area expressed CD163. The percentage of stained area was similar in all four groups and identical to previous findings in rested human skeletal muscle (Malm et al. 2000).
IL-6.
In skeletal muscle expression of IL-6 was low, with a few muscle cell nuclei in some sections staining positive (Fig. 1A), while a few epimysium sections (possibly fibroblasts) expressed significant amounts of IL-6 (Fig. 1B). In positive control samples, IL-6 was readily detected in human lymphocytes 3.5 h post LPS stimulation (data not shown).
Many growth factors are constitutionally expressed in skeletal muscle, epimysium and endothelial cells. As with markers of inflammation, there was no difference between the groups in the detected amounts of any analysed growth factor.
IGF-1.
In muscle tissue, IGF-1 was detected mostly in non-muscle cells (Fig. 1C).
LIF and LIF receptor.
In muscle, LIF was exclusively detected in endothelial cells (Fig. 1D and E). The same levels of LIF were detected in both muscle and epimysium. In muscle, the LIF receptor was only found in some muscle samples and in very small amounts compared to other antigens. Detection of LIF receptors in epimysium was higher than in muscle (mean percentage in epimysium; 0.05%versus 0.007% in muscle P= 0.02).
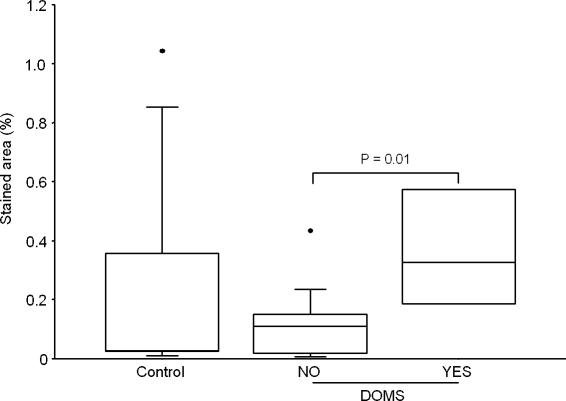
The areas staining positive for CD3, CD11b, LIF and HIF-1β were not different between the groups, but a higher percentage of area staining for these antigens was detected in the epimysium of exercised subjects suffering from DOMS compared to exercised subjects without DOMS (Figs 4–7).
Leukocytes and cytokines in blood (Tables 6 and 7)
Table 6.
Significantly changes in leukocyte phenotypes in blood before and after 45 min of downhill running at 4 deg or 8 deg or uphill running at 4 deg
| Antigen | Group | ANOVA | Rest | Post | 6 h | 24 h | 48 h |
|---|---|---|---|---|---|---|---|
| Leukocytes (cells × 103 ml−1) | All | Time | 6.2 | 8.5¶ | 9.1¶ | 5.9 | 6.6 |
| (5.7–6.9) | (7.5–9.6) | (7.7–10.5) | (5.4–6.4) | (6.0–7.2) | |||
| Lymphocytes (cells × 103μl−1) | Down 4 | Time × group | 2.1 | 2.2 | 2.2 | 2.1 | 2.1 |
| (1.2–3.0) | (1.3–3.1) | (1.7–2.7) | (1.3–2.8) | (1.8–2.4) | |||
| Down 8 | 2.2 | 3.1¶ | 2.4 | 1.8 | 2.3 | ||
| (1.8–2.6) | (2.6–3.8) | (1.7–3.1) | (1.7–2.0) | (1.8–2.7) | |||
| Up 4 | 1.9 | 3.6¶ | 1.7 | 1.8 | 1.8 | ||
| (1.5–2.4) | (2.6–4.8) | (1.4–2.0) | (1.4–2.2) | (1.3–2.2) | |||
| Granulocytes (cells × 103μl−1) | All | Time | 3.7 | 5.0¶ | 6.3¶ | 3.7 | 4.1 |
| (3.2–4.3) | (4.1–5.8) | (5.2–7.6) | (3.2–4.2) | (3.5–4.7) | |||
| MESF CD45 all cells (×103) | Down 4 | Time × group | 23 | 24* | 26*† | 26* | 25 |
| (22–25) | (21–26) | (22–29) | (24–28) | (23–27) | |||
| Down 8 | 21* | 21*† | 21* | 22* | 22 | ||
| (20–23) | (19–23) | (19–23) | (20–24) | (20–23) | |||
| Up 4 | 23* | 25† | 23† | 23 | 23 | ||
| (23–24) | (23–27) | (19–26) | (22–24) | (21–25) | |||
| Ratio granulo/lympho | Down 4 | Time × group | 1.6 | 1.8 | 2.3 | 1.6 | 1.8 |
| (0.8–2.5) | (1.2–2.4) | (1.6–3.0) | (0.8–2.4) | (1.5–2.0) | |||
| Down 8 | 1.9 | 1.9 | 2.9 | 2.3 | 1.9 | ||
| (1.4–2.4) | (1.2–2.6) | (1.5–4.4) | (1.7–2.9) | (1.4–2.4) | |||
| Up 4 | 2.0 | 1.5 | 4.2¶ | 2.0 | 2.5 | ||
| (1.4–2.6) | (1.0–1.9) | (3.3–5.1) | (1.4–2.7) | (0.9–4.1) | |||
| Lymphocytes | |||||||
| CD3+ (cells × 103μl−1) | Down 4 | Time × group | 1.5 | 1.4 | 1.4 | 1.4 | 1.3 |
| (0.8–2.1) | (0.9–1.9) | (1.0–1.9) | (0.9–2.0) | (1.0–1.6) | |||
| Down 8 | 1.5 | 2.0¶ | 1.5 | 1.2 | 1.4 | ||
| (1.2–1.8) | (1.5–2.5) | (1.2–1.9) | (1.1–1.4) | (1.1–1.6) | |||
| Up 4 | 1.3 | 1.9 | 1.0) | 1.1 | 1.0 | ||
| (0.6–1.9) | (1.0–2.9) | (0.3–1.6 | (0.4–1.8) | (0.6–1.4) | |||
| CD3+CD4+ (cells μl−1) | Down 4 | Time × group | 770 | 673* | 715 | 766 | 608 |
| (330–1211) | (309–1038) | (351–1079) | (365–1166) | (310–907) | |||
| Down 8 | 991 | 1305* | 999* | 875 | 971* | ||
| (725–1229) | (911–1700) | (780–1219) | (645–1106) | (686–1257) | |||
| Up 4 | 700 | 899 | 486* | 559 | 510* | ||
| (321–1080) | (485–1314) | (193–779) | (191–927) | (377–643) | |||
| CD3+CD8+ (cells μl−1) | Down 4 | Time × group | 611 | 631 | 640 | 573 | 618 |
| (252–969) | (278–983) | (305–975) | (320–826) | (384–851) | |||
| Down 8 | 554¶ | 867¶ | 610 | 465 | 585 | ||
| (449–660) | (586–1147) | (394–827) | (365–544) | (416–753) | |||
| Up 4 | 486¶ | 884¶ | 425 | 463 | 452 | ||
| (160–811) | (281–1487) | (91–759) | (155–771) | (178–725) | |||
| HLA-DR+ (%) | All | Time | 14 | 13¶ | 17¶ | 16 | 16 |
| (12–17) | (11–15) | (15–20) | (14–18) | (13–18) | |||
| Lymphocyte MESF (× 103) | |||||||
| CD62L on CD3+ | All | Time | 54 | 41¶ | 41¶ | 52 | 51 |
| (45–61) | (35–46) | (34–48) | (48–57) | (43–58) | |||
| CD62L on CD4+ | All | Time | 53 | 41¶ | 42¶ | 51 | 56 |
| (46–61) | (36–47) | (35–48) | (43–58) | (47–67) | |||
| CD62L on CD8+ | All | Time | 56 | 40¶ | 40¶ | 55 | 50 |
| (47–67) | (36–49) | (32–48) | (49–60) | (41–59) | |||
| CD95 (ABC) on Lymphocytes (× 103) | |||||||
| CD4+ CD45RA + | Down 4 | Group | 3.9* | 4.1* | 4.6* | 3.9* | 4.9 |
| (2.7–5.1) | (3.2–5.1) | (3.3–5.9) | (2.8–4.9) | (4.2–5.6) | |||
| Down 8 | 3.4† | 3.3† | 3.5† | 3.5 | 3.8 | ||
| (2.1–4.7) | (2.1–4.5) | (2.1–4.8) | (2.2–4.9) | (2.3–5.3) | |||
| Up 4 | 7.6*† | 7.4*† | 7.8*† | 5.8* | 6.8 | ||
| (6.0–9.1) | (4.7–10.1) | (5.3–10.4) | (4.9–6.8) | (4.6–9.1) | |||
| CD8+ CD45RO + | All | Time | 15 | 14 | 16¶ | 16 | 16¶ |
| (13–17) | (12–16) | (14–18) | (13–18) | (14–18) | |||
| Monoctyes | |||||||
| HLA-DR + (%) | All | Time | 88 | 87 | 91 | 80¶ | 87 |
| (85–91) | (85–90) | (88–94) | (75–85) | (85–89) | |||
| CD45 MESF | All | Time | 111 | 82¶ | 77¶ | 111 | 103 |
| (94–129) | (69–95) | (62–92) | (101–122) | (87–120) | |||
| Proportions (%) | |||||||
| CD4+ among CD3+ | All | Time | 56 | 52¶ | 54) | 54 | 53¶ |
| (52–61) | (46–57) | (50–59 | (50–59) | (48–58) | |||
| CD8+ among CD3+ | All | Time | 37 | 41¶ | 39¶ | 38 | 40¶ |
| (32–41) | (35–46) | (34–44) | (33–42) | (35–45) | |||
| CD62L among CD3+ | All | Time | 76 | 71¶ | 66¶ | 76 | 71 |
| (70–81) | (64–78) | (60–73) | (71–81) | (63–78) | |||
| CD45RA+CD45RO+ among CD4+ | All | Time | 24 | 23 | 28¶ | 24 | 23 |
| (18–30) | (17–28) | (21–34) | (18–29) | (18–28) | |||
| CD62L+ among CD4+ | All | Time | 83 | 80 | 74¶ | 83 | 80 |
| (80–87) | (76–84) | (69–79) | (79–87) | (75–84) | |||
| CD62L+ among CD8+ | All | Time | 64 | 59 | 56¶ | 66 | 61 |
| (57–70) | (52–66) | (50–63) | (60–72) | (52–70) | |||
| Down 4 | 94 | 94 | 95 | 96* | 93 | ||
| (88–100) | (91–98) | (93–97) | (94–97) | (89–96) | |||
| CD95+ among CD8+CD45RO+ | Down 8 | Group | 90 | 90 | 92 | 89 | 89 |
| (84–95) | (85–96) | (88–96) | (83–96) | (85–94) | |||
| Up 4 | 83 | 87 | 88 | 90* | 93 | ||
| (70–95) | (75–99) | (76–101) | (87–94) | (91–95) | |||
| Ratio CD4/CD8 | All | Time | 1.7 | 1.4¶ | 1.5 | 1.6 | 1.5¶ |
| (1.4–1.9) | (1–1.7) | (1.2–1.8) | (1.3–1.9) | (1.2–1.7) | |||
Mean (95% confidence interval). n= 19 (Down 4, n= 5; Down 8, n= 9; Up 4, n= 5). In the ANOVA column change over time (Time) and between groups over time (Time × group) are indicated (P < 0.01). ¶ indicates significant difference compared to rest (P < 0.05; Fisher PLSD). * and † indicate significant difference between groups (P < 0.025; unpaired t test). ABC, antibody binding capacity. MESF, molecules of equivalent soluble fluorochrome
Table 7.
Antigens not changed in response to exercise
| Antigen | Resting value | |
|---|---|---|
| Lymphocytes | CD3+ (%) | 65 (59–72) |
| CD45+ (%) | 99.6 (99.4–99.9) | |
| CD11b + (%) | 63 (52–74) | |
| Lymphocyte MESF or ABC (×103) | MESF CD3 | 174 (151–198) |
| MESF CD45 | 229 (221–236) | |
| MESF CD4+CD45RO + | 23 (20–27) | |
| MESF CD8+CD45RA + | 7.0 (6.1–7.9) | |
| ABC CD95 on CD4+CD45RA + | 42 (31–52) | |
| Monocytes | Monocytes (cells μl−1) | 409 (352–465) |
| CD11b + (%) | 95 (94–97) | |
| CD14+ (%) | 97 (96–98) | |
| CD4+ (%) | 99 (98–99) | |
| CD95+ (%) | 99 (99–100) | |
| Monocytes MESF (×103) | CD4 | 19 (18–25) |
| CD11b | 23 (18–27) | |
| CD14 | 91 (82–101) | |
| Monocyte proportions | CD4+ CD8+ among CD3+ | 1.8 (1.3–2.3) |
| CD45RO+ among CD4+ | 42 (35–49) | |
| CD11b+ among CD8+CD3+ | 9 (5–12) | |
| CD11b+ among CD8+CD3- | 80 (72–87) | |
| CD45RA+CD45RO+ among CD8+ | 25 (20–29) | |
| CD45RA+ among CD4+ | 34 (29–39) | |
| CD45RA+ among CD8+ | 58 (52–63) | |
| CD45RO+ among CD8+ | 17 (12–22) | |
| Proportion CD95+ among | CD4+CD45RA+ | 13 (9–17) |
| CD8+CD45R+ | 31 (23–40) | |
| CD4+CD45RO+ | 95 (92–97) | |
| Percent cells expressing Ki-67 | CD4+ | 1.5 (1.0–1.9) |
| CD4+RO+ | 2.5 (1.8–3.3) | |
| CD4+RO- | 0.6 (0.3–0.8) | |
| CD8+ | 1.2 (0.8–1.6) | |
| CD8+RA+ | 0.6 (0.4–0.8) | |
| CD8+RO+ | 2.1 (1.3–2.8) | |
| Cytokines (pg ml−1) | IFN-γ* | 100 (–67 to 268) |
| TNF-α | 6.9 (0–14) | |
| IL-2 | 2.5 (1.3–3.7) | |
| IL-4 | 7.1 (4.1–10) | |
| IL-5 | 4.3 (0.7–8.0) | |
| IL-6 | 2.4 (0.8–4.0) | |
| IL-10 | 5.6 (4.1–7.2) |
Blood values at rest. n= 19 (Down 4 deg, n= 5; Down 8 deg, n= 9; Up 4 deg, n= 5). Mean (95% confidence interval). ABC, antibody binding capacity. MESF, molecules of equivalent soluble fluorochrome. *One subject with 1531 pg ml −1. Omitting this person gives 21 (5–36) pg ml−1.
Compared to rest, the total number of circulating leukocytes increased significantly in all groups, with a peak at 6 h and a return to resting mean at 24 h post exercise (Table 6). The concentration of circulating leukocytes did not differ significantly between the groups, and the peak in leukocyte number at 6 h was correlated to the increase in granulocytes at 6 h (R2= 0.91; P < 0.0001).
Lymphocytes
Lymphocyte numbers changed differently between the groups (a time × group effect detected by ANOVA, P < 0.01; Table 6). There appeared to be a re-distribution of lymphocytes and a change in activation/adhesion status (CD62L cell surface density) over time regardless of exercise mode (Table 6).
Granulocytes
There was a significant granulocytosis after exercise, but no difference between the groups (Table 6). The ratio between lymphocytes and granulocytes was significantly higher in the Uphill 4 deg compared to the Downhill 4 deg group 6 h after exercise (P= 0.02).
Monocytes
Circulating numbers of monocytes did not differ between the groups, but the change from rest was significantly larger in the Downhill 8 deg group compared to the Downhill 4 deg group immediately after exercise (P < 0.01). The percentage distribution of the circulating monocyte population did not change (Table 7). Monocyte function may have changed because CD45 expression (signal transduction) was decreased up to 6 h after exercise (Table 6).
NK cells
Due to technical problems, CD16, CD56 and CD57 antigens were not analysed.
CD95 expression
The expression (antigen binding capacity, ABC) of the apoptotic signal transducer molecule CD95 (Fas/APO-1) on lymphocytes and monocytes only changed on CD8+CD45RO+ lymphocytes over time but there was no difference between the groups (Table 6). CD95 expression on CD4+CD45RA+ cells was initially higher in the Uphill 4 deg group compared to the other two groups. The percentage of monocytes expressing CD95 did not change over time (Table 7) and CD95 expression on lymphocytes and monocytes at rest did not correlate with resting serum concentrations of cytokines or hormones or with any physiological variable investigated.
Ki-67 on lymphocytes
As indicated by the percentage of cells expressing Ki-67, turnover rate among CD4+ and CD8+ lymphocytes did not change in response to the 45 min running exercise (Table 7). The percentage of Ki-67+ cells at rest did not correlate with resting serum concentrations of cytokines or hormones, or any physiological variable investigated.
Cytokines in serum
None of the cytokines investigated changed significantly in response to either running protocol (Table 7). When performing regression analysis, there was no strong correlation (R2 < 0.7) between Ki-67 or CD95 and cytokines in any blood sample. One subject displayed an extremely high concentration (6- to 150-fold greater than the mean value) of all cytokines in the resting sample and may have had a subclinical infection upon entering the study. All cytokine samples from this subject were excluded from the regression analysis.
Discussion
The exercise protocol used in this study resulted in decreased muscle function and inflicted severe muscle discomfort that hindered normal every day activities (Table 1 and Fig. 3). DOMS and muscle pain were related to markers of inflammation in the epimysium but not in skeletal muscle (Table 8). No signs of muscle inflammation 48 h after downhill or uphill running could be detected, despite significant increases in muscle soreness, blood granulocyte number and serum CK activity. A previous investigation in our laboratory demonstrated that multiple biopsies in the same muscle induced an increase in the detection of CD11b (neutrophils), CD163 (macrophages), IL-1β, IL-1α and CD56 antigens in muscle tissue (Malm et al. 2000). The correlations between immunological variables in human blood and skeletal muscle found in the previous study could not be reproduced in this study, indicating that they were the result of damage inflicted by prior muscle biopsies. In the present study, multiple regression analysis revealed correlations between cells and molecules detected in muscle, epimysium and blood (Table 8).
Table 8.
Multiple regression models
| Dependent | Predictors | N | P | t value | R2 |
|---|---|---|---|---|---|
| CD11b epimysium | CRP at 72 h | 15 | <0.0001 | 8.1 | 0.82 |
| Lymphocytes at 24 h | 0.0004 | −3.5 | |||
| HIF-1β epimysium | IL-6 at 6 h | 15 | 0.0018 | 4.0 | 0.86 |
| CRP at 72 h | 0.0001 | 5.7 | |||
| Pain | Running speed | 18 | <0.0001 | 9.0 | 0.87 |
| Epimysium CD11b | <0.0001 | 7.9 | |||
| Proportion CD+ among CD45RA+ at rest | 0.0008 | −4.2 | |||
| DOMS | Epimysium CD11b | 15 | <0.0001 | 6.8 | 0.78 |
| CK Rest-Max | <0.0001 | 3.8 |
Positive (+) or negative (−) correlation indicated by the t value. Rest-Max individual change in CK from rest to maximum value between 6 h and 48 h post exercise. The t value tests whether the true variable is zero; R2, multiple correlation coefficient adjusted for multiple predictor variables; P, individual probability for each predictor variable; Predictor variables not correlated (P >0.1).
We interpret these findings as indicating that: (1) muscular adaptation to physical exercise is fundamentally different from the muscle cell repair mechanisms activated by experimentally inflicted damage, (2) the sensation of DOMS and pain after unaccustomed physical exercise is confined to immunological reactions in muscle epimysium, and (3) individual differences between subjects (genetic, exercise training, infections, nutrition, etc.) result in larger deviations when investigating muscle adaptation to physical exercise than when tissue repair after trauma is studied.
Expression of inflammatory markers in muscle and epimysium (Table 5 and Figs 4–7)
The presence of leukocytes in muscle tissue is necessary both for normal muscle function and tissue repair following injury (Hohlfeld & Engel, 1994; Mitchell et al. 1995; Tidball, 1995; Chambers & McDermott, 1996; Lescaudron et al. 1999). Several studies have demonstrated an increased presence of leukocytes in muscle tissue after exercise in humans (Fielding et al. 1993; MacIntyre et al. 1996; Stupka et al. 2000) and animals (Armstrong et al. 1983). Data from the present study bring into question the interpretation of these findings. Even though neutrophils and macrophages were detected in muscle sections 48 h after exercise, the differences between muscles from exercised and control subjects were small and the results from the present study do not support the notion that exercise (especially eccentric exercise) causes leukocyte infiltration and inflammation in human skeletal muscle. When comparing human and rat muscle sections 48 h after downhill running in the present and a previous study (Armstrong et al. 1983), there appears to be a difference between the two species' responses to similar exercise protocols. No muscle section from any individual in the present study displayed infiltration of the magnitude demonstrated in the study by Armstrong et al. (1983). It cannot be ruled out that sedentary rats may be more prone to exercise-induced muscle inflammation than humans. Neutrophil infiltration in human skeletal muscle has been correlated to Z-band damage and IL-1β detection (Fielding et al. 1993). The findings by Fielding et al. (1993) could have been a result of the biopsies being taken in the same muscle before and after exercise, and the lack of an increase in CD11b detection 48 h after 45 min of downhill running at 8 deg in the present study indicates that any cytoskeletal disruptions/alternations did not result in infiltration of inflammatory cells. Subsequently, the biopsies from this study have been further analysed for cytoskeletal disruptions and the results were recently published by Yu et al. (2002). In this study it was concluded that the previously reported Z-band disruption is in fact a re-synthesis of desmin.
Significantly more T cells (CD3) and neutrophils (CD11b) were detected in epimysium from exercised subjects suffering from DOMS compared to exercised subjects not experiencing DOMS (Figs 4 and 5). However, neither group differed from the control subjects, and the highest percentage of both T cells and neutrophils was observed in a control subjects not suffering from DOMS. This suggests that an increase in T cells and neutrophils (i.e. infiltration/inflammation) is not the cause of DOMS. An alternative possibility is that activation of T cells and/or neutrophils already present in the epimysium before exercise may be necessary for development of DOMS. The low concentration of T cells and neutrophils in the epimysium of the exercised subjects who did not develop DOMS could be the result of previously performed eccentric exercise. Neither of these possibilities was tested in this study.
Figure 5. CD11b in epimysium.
Stained area of CD11b in the control group (Control, n= 9) and the exercise groups split into groups of subjects suffering from DOMS (YES, n= 4) or not (NO, n= 14), regardless of treadmill elevation.
Macrophages (CD163-positive cells) can have pro- and anti-inflammatory functions, and in mice alternatively activated macrophages (Stumpo et al. 1999) have been shown to increase antigen-presenting functions and support angiogenesis and healing, thus potentially serving to protect muscle tissue from inflammation. The exercise-induced muscle inflammation described in several publications (reviewed by MacIntyre et al. 1995; Clarkson & Sayers, 1999) involves intracellular neutrophils and macrophages within the muscle cells' basement membrane. Many of these studies were performed on mice, rats or rabbits and this phenomenon was never observed in the present or our previous study on humans (Malm et al. 2000), where all detected leukocytes were restricted to the intercellular space between muscle fibres.
No increase in CD56-positive cells and structures was observed in the present study. Typically, one to four muscle fibres (cells morphologically the shape and size of muscle fibres) in each section (200–400 muscle fibres) were CD56 positive. In our previous investigation (Malm et al. 2000) the number of CD56-positive muscle fibres increased similarly in exercised and control subjects (second and third biopsy in the same muscle, 24 h and 48 h after exercise, respectively). If the CD56-positive muscle cells are indicative of regeneration/degeneration of the muscle tissue, one conclusion is that this process occurs within 24 h after the trauma inflicted by a needle biopsy but not after physical exercise. Taken together, these two studies support the argument that acute physical exercise, even when it involves eccentric contractions, does not result in muscle regeneration involving satellite cell activation.
Insulin-like growth factor 1 (IGF-1) and leukaemia inhibitory factor (LIF) in human skeletal muscle were visualized in this study (Fig. 1C–E). Several studies have demonstrated that IGF-1 can induce skeletal muscle hypertrophy via a systemic, growth hormone-dependent pathway and via local autocrine/paracrine action (Adams, 1998). IGF-1 can also protect cardiomyocytes and fibroblasts from osmotic stress-induced apoptosis (Mockridge et al. 2000). In contrast to the hypothesis discussed by Adams (Adams, 1998) but in agreement with findings by Hellsten et al. (1996), IGF-1 was not detected within myofibres but was present in the smooth muscle surrounding small blood vessels (Fig. 1C). This local muscle IGF-1 may be a splice variant of the liver IGF-1 named mechano growth factor (MGF) and could be a link between muscle contraction and gene expression (McKoy et al. 1999).
Another potential modulator of skeletal muscle adaptation is leukaemia inhibitory factor (LIF), which has been detected in human skeletal muscle (Schoser et al. 1998) and in some cases (crush injury in mouse) (Barnard et al. 1994) but not all (bupivacaine injection in rat) (Gregorevic et al. 2000) has been shown to stimulate muscle regeneration after injury. In contrast to findings by Schoser et al. (1998), our analysis limits LIF expression in human skeletal muscle to smooth muscle cells (Fig. 1D and E). The use of different antibodies can be one explanation for the divergent findings, as can the use of saponin in our method. Some epimysium sections stained strongly for LIF (10 times the percentage area detected in muscle) and epimysium could be a potential source of LIF production, even though the levels of LIF detected in epimysium were not different between the four groups. The LIF-R was not detected in most muscle sections while epimysium sections displayed some staining. Neither LIF nor LIF-R was affected by exercise (Table 5).
Interleukin 1β (IL-1β) and hypoxia-inducible factor 1β (HIF-1β) were detected in both muscle and epimysium sections in equal amounts but did not differ between the groups (Table 5). One previous study on humans has reported increased staining for IL-1β after exercise (Fielding et al. 1993), but these findings were most likely due to a previous muscle biopsy, as indicated earlier (Malm et al. 2000) and confirmed in this study by the lack of an increase in IL-1β 48 h after exercise. As in our previous study (Malm et al. 2000), IL-1β was localized mostly to non-muscle cells. This is in agreement with an earlier human study (Authier et al. 1997) where IL-1β was associated with muscle regeneration and not inflammation. It has been demonstrated that HIF-1β mRNA can increase in human skeletal muscle 30 min after 45 min knee-extension exercise (Gustafsson et al. 1999). The lack of any increase in HIF-1β in the present study may be due to the different sampling times (30 min versus 48 h), mRNA versus protein detection or/and different methods (PCR/ELISA versus immunohistochemistry).
Leukocytes, cytokines and hormones in blood
Changes in leukocyte distribution, function and antigen expression in response to physical exercise are well described (Gabriel & Kindermann, 1997; Pedersen, 1997; Mackinnon, 1999; Malm et al. 1999) and the intention of this study was to investigate possible interactions between circulating leukocytes and immunological events in skeletal muscle. Leukocytosis after exercise is a common finding in most studies, including the present one (Table 6), and is usually attributed to an increase in the number of neutrophils and is explained as the release of these cells from the marginated pool. While previous studies have interpreted leukocytosis as an indication/result of muscle inflammation, data from the present study suggest that muscle inflammation is not the cause of the exercise-induced increase in circulating leukocyte numbers.
The total number of lymphocytes in the circulation increased during exercise and returned to normal 6 h after Downhill 8 deg and Uphill 4 deg running (Table 6), in accordance with many previous human studies (Pedersen, 1997). The increase was positively correlated to the change in cortisol concentration (R2= 0.55; P < 0.001).
The lymphocyte adhesion capacity (CD62L expression on CD4+ and CD8+ T cells, as well as the proportion of T cells expressing CD62L) decreased. As CD62L is shed from the cell surface upon activation, these findings indicate activation of CD4+ and CD8+ T cells. The proportion of CD8+ T cells increased while the proportion CD4+ T cells decreased, resulting in a decreased CD4/CD8 ratio. Thus, mobilization of activated cycotoxic T cells (TC) cells to the circulation appears to be one outcome of Downhill 8 deg and Uphill 4 deg running.
Of some interest are the minor changes in CD95/Fas expression despite the presumably large production of reactive oxygen species (ROS) and other stress-induced substances during strenuous exercise (Tables 6 and 7). ROS are known to affect apoptosis and cell cycling but no significant changes in CD95 or Ki-67 were detected, suggesting the presence of stress protection systems sufficient to inhibit changes in lymphocyte and monocyte apoptosis receptors and lymphocyte turnover rate. Mechanisms for regulating leukocyte apoptosis other than changed cell surface receptor expression have been described (Um et al. 1996). The changes in CD4+ and CD8+ phenotypic and functional properties were relatively small (<50% in most cases) and may not be of clinical relevance. According to the review by Gabriel & Kindermann (1997), T cells appear affected mostly by high-intensity (>100% of the anaerobic threshold) or long-duration endurance (100 km run) exercise. Our finding that the percentage of lymphocytes and monocytes expressing HLA-DR decreased could be interpreted as a decrease in the antigen-presenting capacity. Alternatively, the decrease in the percentage of CD62L and its expression on T cells, (activation) combined with the decrease in percentage of HLA-DR-positive lymphocytes (inactivation), could be caused by adhesion of CD62L+HLA-DR+ lymphocytes to endothelium and their disappearance from the blood. Ki-67, a common marker for cell proliferation rate (Boulton & Hodgson, 1995), was used to investigate the proportion of proliferating lymphocytes in response to physical exercise. From Table 7 it can be determined that 45 min of downhill or uphill running was not enough to influence the lymphocyte proliferation rate as measured in this study.
Monocyte numbers did not change in any of the exercised groups (Table 7) but CD45 expression on monocytes decreased for up to 6 h after exercise. The decrease in the expression of CD45 on monocytes, together with the lack of change in monocyte numbers, adhesion capacity and CD95/Fas expression, indicates a specific modulation (decrease) of signal transduction capacity among circulation monocytes.
Conclusion
In order to achieve a full appreciation of the divergent functions of the immune system, investigations in this field require analysis of multiple variables. The disadvantage of this approach is the accumulation of large sets of data and it is beyond the scope of this article to explore and explain all possible interpretations of all the results presented. An attempt has been made to focus on some important findings that may add to our understanding of skeletal muscle function and adaptations to changes in functional demand. Future investigations may also benefit from the data collected by focusing on some of the main findings summarized below. Because there is great variability in adaptation between individuals to similar physical exercise, it is suggested that the large individual differences in the immune system's response to a similar relative exercise load may be of greater clinical importance than significant changes on a group basis.
Based on findings in the present study and a previous study by our group (Malm et al. 2000), it must be concluded that physical exercise, even it involves substantial eccentric components, does not result in skeletal muscle inflammation, and, as indicated by several previous investigations (Warren et al. 1999), serum CK is not related to muscle inflammation.
Correlations between blood leukocytes, cytokines, growth factors and hormones were demonstrated and some of these may be of importance for skeletal muscle adaptation to physical exercise.
Two paracrine/autocrine growth factor systems were visualized in skeletal muscle and epimysium. IGF-1 and the IGF-1 receptor were detected in smooth muscle cells and muscle fibres in a juxtacrine fashion. IGF-1 and the IGF-1 receptors were also detected in epimysium. LIF was detected in smooth muscle cells in muscle and in epimysium. The LIF receptor was not consistantly detected in muscle sections and was more frequently detected in epimysium.
Based on findings in the present study, the hypothesis that skeletal muscle development, regeneration and adaptation are three distinct events with some similar and some different immunological mechanisms appears plausible.
One clinical application of these findings could be a re-evaluation of the possible beneficial effect of physical exercise on subjects suffering from inflammatory muscle diseases in the light of our demonstration that physical exercise does not induce inflammation in healthy muscle.
Figure 6. HIF-1β in epimysium.
Stained area of HIF-1β in the control group (Control, n= 9) and the exercise groups split into groups of subjects suffering from DOMS (YES, n= 4) or not (NO, n= 14), regardless of treadmill elevation.
Acknowledgments
This study is dedicated to our mentor Dr Bertil Sjödin, who passed away on September 9, 2003. We are grateful for the assistance from Elisabeth Ishizaki (Nova Medical Research, St. Görans Hospital, Stockholm, Sweden) for flow cytometry analyses of leukocytes and cytokines, Tönu Saartuk (Section of Sports Medicine, Karolinska Institutet, Stockholm, Sweden) for taking some of the muscle biopsies, Alexander Ovendal for helping with the SPARK measurements, and participating subjects for their donation of time, blood and muscle tissue. The study was supported in part by grants from Centrum för Idrottsforskning, Stockholm, Sweden and the Karolinska Institutet Research Foundation.
References
- Adams GR. Role of insulin-like growth factor-I in the regulation of skeletal muscle adaptation to increased loading. Exerc Sport Sci Rev. 1998;26:31–60. [PubMed] [Google Scholar]
- Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- Authier FJ, Mhiri C, Chazaud B, Christov C, Cherin P, Barlovatz-Meimon G, Gherardi RK. Interleukin-1 expression in inflammatory myopathies: evidence of marked immunoreactivity in sarcoid granulomas and muscle fibres showing ischaemic and regenerative changes. Neuropathol Appl Neurobiol. 1997;23:132–140. [PubMed] [Google Scholar]
- Barnard W, Bower J, Brown MA, Murphy M, Austin L. Leukemia inhibitory factor (LIF) infusion stimulates skeletal muscle regeneration after injury: injured muscle expresses lif mRNA. J Neurol Sci. 1994;123:108–113. doi: 10.1016/0022-510x(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8:404–410. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- Boulton RA, Hodgson HJ. Assessing cell proliferation: a methodological review. Clin Sci (Colch) 1995;88:119–130. doi: 10.1042/cs0880119. [DOI] [PubMed] [Google Scholar]
- Cabanillas F, Horning S, Kaminski M, Champlin R. Managing Indolent Lymphomas in Relapse: Working Our Way Through a Plethora of Options. Hematology (Am Soc Hematol Educ Program) 2000:166–179. doi: 10.1182/asheducation-2000.1.166. [DOI] [PubMed] [Google Scholar]
- Cannon JG, Fielding RA, Fiatarone MA, Orencole SF, Dinarello CA, Evans WJ. Increased interleukin 1 beta in human skeletal muscle after exercise. Am J Physiol. 1989;257:R451–R455. doi: 10.1152/ajpregu.1989.257.2.R451. [DOI] [PubMed] [Google Scholar]
- Chambers RL, McDermott JC. Molecular basis of skeletal muscle regeneration. Can J Appl Physiol. 1996;21:155–184. doi: 10.1139/h96-014. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Sayers SP. Etiology of exercise-induced muscle damage. Can J Appl Physiol. 1999;24:234–248. doi: 10.1139/h99-020. [DOI] [PubMed] [Google Scholar]
- Cooney RN, Maish GO, 3rd, Gilpin T, Shumate ML, Lang CH, Vary TC. Mechanism of IL-1 induced inhibition of protein synthesis in skeletal muscle. Shock. 1999;11:235–241. doi: 10.1097/00024382-199904000-00002. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Manfredi TJ, Ding W, Fiatarone MA, Evans WJ, Cannon JG. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am J Physiol. 1993;265:R166–R172. doi: 10.1152/ajpregu.1993.265.1.R166. [DOI] [PubMed] [Google Scholar]
- Gabriel H, Kindermann W. The acute immune response to exercise: what does it mean? Int J Sports Med. 1997;18(suppl. 1):S28–S45. doi: 10.1055/s-2007-972698. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, Interisano SA, Tarnopolsky MA, Roy BD, Macdonald JR, Yarasheski KE, Macdougall JD. Myofibrillar disruption following acute concentric and eccentric resistance exercise in strength-trained men. Can J Physiol Pharmacol. 2000;78:656–661. [PubMed] [Google Scholar]
- Goldspink G. Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat. 1999;194:323–334. doi: 10.1046/j.1469-7580.1999.19430323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorevic P, Hayes A, Lynch GS, Williams DA. Functional properties of regenerating skeletal muscle following LIF administration. Muscle Nerve. 2000;23:1586–1588. doi: 10.1002/1097-4598(200010)23:10<1586::aid-mus17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Gustafsson T, Puntschart A, Kaijser L, Jansson E, Sundberg CJ. Exercise-induced expression of angiogenesis-related transcription and growth factors in human skeletal muscle. Am J Physiol. 1999;276:H679–H685. doi: 10.1152/ajpheart.1999.276.2.H679. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Hansson HA, Johnson L, Frandsen U, Sjodin B. Increased expression of xanthine oxidase and insulin-like growth factor I (IGF-I) immunoreactivity in skeletal muscle after strenuous exercise in humans. Acta Physiol Scand. 1996;157:191–197. doi: 10.1046/j.1365-201X.1996.492235000.x. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R, Engel AG. The immunobiology of muscle. Immunol Today. 1994;15:269–274. doi: 10.1016/0167-5699(94)90006-X. [DOI] [PubMed] [Google Scholar]
- Husmann I, Soulet L, Gautron J, Martelly I, Barritault D. Growth factors in skeletal muscle regeneration. Cytokine Growth Factor Rev. 1996;7:249–258. doi: 10.1016/s1359-6101(96)00029-9. [DOI] [PubMed] [Google Scholar]
- Illa I, Leon-Monzon M, Dalakas MC. Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Ann Neurol. 1992;31:46–52. doi: 10.1002/ana.410310109. [DOI] [PubMed] [Google Scholar]
- Lenkei R, Andersson B. Determination of the antibody binding capacity of lymphocyte membrane antigens by flow cytometry in 58 blood donors. J Immunol Meth. 1995;183:267–277. doi: 10.1016/0022-1759(95)00064-h. [DOI] [PubMed] [Google Scholar]
- Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, Parrish E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord. 1999;9:72–80. doi: 10.1016/s0960-8966(98)00111-4. [DOI] [PubMed] [Google Scholar]
- MacIntyre DL, Reid WD, Lyster DM, Szasz IJ, McKenzie DC. Presence of WBC, decreased strength, and delayed soreness in muscle after eccentric exercise. J Appl Physiol. 1996;80:1006–1013. doi: 10.1152/jappl.1996.80.3.1006. [DOI] [PubMed] [Google Scholar]
- MacIntyre DL, Reid WD, McKenzie DC. Delayed muscle soreness. The inflammatory response to muscle injury and its clinical implications. Sports Med. 1995;20:24–40. doi: 10.2165/00007256-199520010-00003. [DOI] [PubMed] [Google Scholar]
- MacKinnon LT. Advances in Exercise Immunology. Champaign: Human Kinetics; 1999. [Google Scholar]
- McKoy G, Ashley W, Mander J, Yang SY, Williams N, Russell B, Goldspink G. Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J Physiol. 1999;516:583–592. doi: 10.1111/j.1469-7793.1999.0583v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm C, Lenkei R, Sjodin B. Effects of eccentric exercise on the immune system in men. J Appl Physiol. 1999;86:461–468. doi: 10.1152/jappl.1999.86.2.461. [DOI] [PubMed] [Google Scholar]
- Malm C, Nyberg P, Engstrom M, Sjodin B, Lenkei R, Ekblom B, Lundberg I. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol. 2000;529:243–262. doi: 10.1111/j.1469-7793.2000.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CA, Grounds MD, Papadimitriou JM. The genotype of bone marrow-derived inflammatory cells does not account for differences in skeletal muscle regeneration between SJL/J and BALB/c mice. Cell Tissue Res. 1995;280:407–413. doi: 10.1007/BF00307814. [DOI] [PubMed] [Google Scholar]
- Mockridge JW, Benton EC, Andreeva LV, Latchman DS, Marber MS, Heads RJ. IGF-1 regulates cardiac fibroblast apoptosis induced by osmotic stress. Biochem Biophys Res Commun. 2000;273:322–327. doi: 10.1006/bbrc.2000.2934. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. Exercise Immunology. Georgetown: R.G. Landes Company; 1997. [Google Scholar]
- Ruoslahti E. Stretching is good for a cell. Science. 1997;276:1345–1346. doi: 10.1126/science.276.5317.1345. [DOI] [PubMed] [Google Scholar]
- Schoser BG, Storjohann S, Kunze K. Immunolocalization of leukemia inhibitory factor in normal and denervated human muscle. Neuroreport. 1998;9:2843–2846. doi: 10.1097/00001756-199808240-00029. [DOI] [PubMed] [Google Scholar]
- Seger JY, Westing SH, Hanson M, Karlson E, Ekblom B. A new dynamometer measuring concentric and eccentric muscle strength in accelerated, decelerated, or isokinetic movements. Validity and reproducibility. Eur J Appl Physiol Occup Physiol. 1988;57:526–530. doi: 10.1007/BF00418457. [DOI] [PubMed] [Google Scholar]
- Stumpo R, Kauer M, Martin S, Kolb H. Alternative activation of macrophage by IL-10. Pathobiology. 1999;67:245–248. doi: 10.1159/000028102. [DOI] [PubMed] [Google Scholar]
- Stupka N, Lowther S, Chorneyko K, Bourgeois JM, Hogben C, Tarnopolsky MA. Gender differences in muscle inflammation after eccentric exercise. J Appl Physiol. 2000;89:2325–2332. doi: 10.1152/jappl.2000.89.6.2325. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc. 1995;27:1022–1032. doi: 10.1249/00005768-199507000-00011. [DOI] [PubMed] [Google Scholar]
- Ulfgren AK, Lindblad S, Klareskog L, Andersson J, Andersson U. Detection of cytokine producing cells in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:654–661. doi: 10.1136/ard.54.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um HD, Orenstein JM, Wahl SM. Fas mediates apoptosis in human monocytes by a reactive oxygen intermediate dependent pathway. J Immunol. 1996;156:3469–3477. [PubMed] [Google Scholar]
- Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999;27:43–59. doi: 10.2165/00007256-199927010-00004. [DOI] [PubMed] [Google Scholar]
- Yu JG, Furst DO, Thornell LE. The mode of myofibril remodelling in human skeletal muscle affected by DOMS induced by eccentric contractions. Histochem Cell Biol. 2003;119:383–393. doi: 10.1007/s00418-003-0522-7. [DOI] [PubMed] [Google Scholar]
- Yu JG, Malm C, Thornell LE. Eccentric contractions leading to DOMS do not cause loss of desmin nor fibre necrosis in human muscle. Histochem Cell Biol. 2002;118:29–34. doi: 10.1007/s00418-002-0423-1. [DOI] [PubMed] [Google Scholar]