Abstract
The intracellular signalling pathways and molecular mechanisms responsible for P2-purinoceptor-mediated chloride (Cl−) currents (ICl,ATP) were studied in mouse ventricular myocytes. In standard NaCl-containing extracellular solutions, extracellular ATP (100 μm) activated two different currents, ICl,ATP with a linear I–V relationship in symmetrical Cl− solutions, and an inwardly rectifying cation conductance (cationic IATP). Cationic IATP was selectively inhibited by Gd3+ and Zn2+, or by replacement of extracellular NaCl by NMDG; ICl,ATP was Cl− selective, and inhibited by replacement of extracellular Cl− by Asp−; both currents were prevented by suramin or DIDS pretreatment. In GTPγS-loaded cells, ICl,ATP was irreversibly activated by ATP, but cationic IATP was still regulated reversibly. GDPβS prevented activation of the ICl,ATP, even though pertussis toxin pretreatment did not modulate ICl,ATP. These results suggest that activation of ICl,ATP occurs via a G-protein coupled P2Y purinergic receptor. The ICl,ATP persistently activated by GTPγS, was inhibited by glibenclamide but not by DIDS, thus exhibiting known pharmacological properties of cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channels. In ventricular cells of cftr−/− mice, extracellular ATP activated cationic IATP, but failed to activate any detectable ICl,ATP. These results provide compelling evidence that activation of CFTR Cl− channels in mouse heart are coupled to G-protein coupled P2Y purinergic receptors.
Adenine nucleotides are continually present in variable amounts in the extracellular space of the heart, being normally released as a cotransmitter from sympathetic perivascular nerves (for review see Vassort, 2001). Extracellular ATP mainly acts on cell surface receptors of the P2X and P2Y subtypes, which are much more sensitive to ATP and ADP than to AMP and adenosine, and genes encoding seven ionotropic P2X nucleotide receptor subtypes and eight G-protein coupled P2Y nucleotide receptor subtypes have been identified in human and other mammalian tissues (Fredholm et al. 1994, 1997; Inscho, 2001; Vassort, 2001; North, 2002; Dubyak, 2003; Lee & O'Grady, 2003). In contrast to P2Y receptors, P2X receptors are ligand-gated ion channels permeable to small monovalent cations (Inscho, 2001; North, 2002). Based on their functional coupling to particular G-proteins and effector proteins, P2Y receptors can be broadly subdivided into five Gq-coupled subtypes (P2Y1–4, P2Y6, P2Y11) which can stimulate a variety of pathways including protein kinase C (PKC), and three Gi-coupled subtypes (P2Y12–14). Expression of several Gq-coupled subtypes have been reported in several mammalian species of cardiac myocytes (Vassort, 2001). It has been observed in neonatal and adult cardiac cells that extracellular ATP increases PKC activity through both ɛ- and δ-PKC, two Ca2+-insensitive PKC isoforms (Puceat et al. 1994; Vassort, 2001). In addition, the activation of P2-purinergic receptors in the heart has been shown to elevate cyclic AMP (cAMP) due to activation of a specific isoform (V) of adenylyl cyclase that may be different from the isoform activated by β-adrenergic receptor stimulation (Puceat et al. 1998).
In heart, stimulation of purinergic receptors is known to activate non-selective cation currents (IATP) as well as chloride (Cl−)-dependent currents (ICl,ATP) (Vassort, 2001). Extracellular nucleotides, including ATP, ADP, and ATPγS, but not adenosine nor AMP in mouse ventricular myocytes (Levesque & Hume, 1995), activated ICl,ATP similar to the ICl,ATP previously described in guinea-pig atrial (Matsuura & Ehara, 1992) and rat myocytes (Kaneda et al. 1994). In both rat and mouse ventricular myocytes, the activation of ICl,ATP appeared to be coupled to P2-purinoceptor stimulation. In a subsequent report (Duan et al. 1999) it was suggested that ICl,ATP in mouse heart may be due to activation of cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channels through a novel intracellular signalling pathway involving purinergic activation of PKC and protein kinase A (PKA), since the CFTR gene encodes a Cl− channel activated by both PKA and PKC (for reviews see Gadsby & Nairn, 1999; Jentsch et al. 2002). Although several cAMP activators (isoproterenol, forskolin and isobutyl methylxanthine) alone were unable to activate any detectable ICl.PKA in mouse ventricular myocytes, RT-PCR clearly confirmed expression of the mouse homologue of CFTR in heart, and P2-purinergic activation of the current was prevented by inhibition of either endogenous PKC or PKA activity (Duan et al. 1999). The possibility that ICl,ATP in mouse heart might be due to activation of CFTR Cl− channels was also supported by strong similarities in the electrophysiological properties of ICl,ATP and CFTR Cl− currents (ICl,CFTR), and the finding that constitutive PKC phosphorylation may be essential for acute activation of CFTR by PKA (Jia et al. 1997). More recently, in Xenopus oocytes coexpressing the P2Y6 receptor together with CFTR, it has been reported that extracellular nucleotides activated both Ca2+-activated Cl− currents and CFTR Cl− currents (Kottgen et al. 2003). It seems possible therefore that ICl,ATP and ICl,CFTR in heart might be generated by the same protein or by proteins molecularly related to CFTR.
In the present study, we used electrophysiological, pharmacological and transgenic approaches to further characterize the properties of cationic IATP and ICl,ATP in mouse heart. We first demonstrate that extracellular ATP activation of cationic IATP involves G-protein uncoupled purinergic receptors, whereas ATP activation of ICl,ATP involves a G-protein signalling pathway mediated by the P2Y purinergic receptor subtype. We then confirm that the pharmacological properties of ICl,ATP are similar to the known properties of cardiac ICl,CFTR, including sensitivity to block by glibenclamide and insensitivity to DIDS. Finally, we directly test the hypothesis that ICl,ATP may be mediated by cardiac CFTR Cl− channels by examining membrane currents activated by extracellular ATP in ventricular cells isolated from cftr−/− mouse.
Methods
Cell preparation
The Institutional Animal Use and Care Committee at the University of Nevada approved the use and treatment of all animals used in the experiments described here. Single ventricular myocytes from mouse hearts were isolated using an enzymatic dispersion technique as originally described (Levesque & Hume, 1995; Duan et al. 1999). Briefly, cftr+/+ (wild-type; C-57BL/6J/black inbred, 10–20 weeks, 34 animals; Jackson Laboratory, Bar Harbour, MA, USA) and cftr−/− mice (knockout; B6.129P2-cftrtmI Unc, 6.2 ± 0.4 weeks, 15.9 ± 1.6 g, 4 animals; Jackson Laboratory) were anaesthetized with sodium pentobarbitone (0.5 mg 10 g i.p.). The chest was opened, and the heart was rapidly removed and perfused by using a modified Langendorff technique, with a physiological saline solution (PSS; see Solutions and drugs) warmed to 37°C until free of blood and then with a nominally Ca2+-free PSS until the heart ceased to beat, and finally with the Ca2+-free solution containing 0.07% collagenase (CLSII, Sigma) and 1.0% bovine serum albumin (BSA) for 20–30 min. The ventricles were removed and cell dissociation was achieved by gentle mechanical agitation. After the enzyme treatment, the cells were dissociated in high-K+, low-Cl− storage (modified KB) solution (see Solutions and drugs) and stored in a refrigerator (4°C) for later use (within 8 h). Only rod-shaped myocytes with clear cross-striations and no blebs under isotonic conditions were used for electrophysiology studies.
Electrophysiological techniques
The tight-seal whole-cell patch-clamp technique was used to record whole-cell currents in isolated mouse ventricular myocytes. Patch pipettes (1.5 mm o.d. borosilicate glass electrodes) had a tip resistance of 1–3 MΩ when filled with pipette solution. Voltage-clamp recordings were performed using an Axopatch-200A patch-clamp amplifier (Axon Instruments, Union City, CA, USA) and membrane currents were filtered at a frequency of 1 kHz. Data acquisition and command potentials were controlled by pCLAMP 8.1 software (Axon Instruments). A 3 m KCl–agar bridge between the bath and the Ag–AgCl reference electrode was used to minimize changes in liquid junctional potential. When necessary, the current density was calculated by membrane capacitance, which was obtained using pCLAMP 8.1 software. Usually, 5 min was allowed for adequate cell dialysis after membrane rupture before beginning the voltage clamp protocol. All experiments were performed at room temperature.
Solutions and drugs
The PSS for cell preparation contained (mm): 126 NaCl, 10 glucose, 4.4 KCl, 5.0 MgCl2, 1.5 CaCl2, 20 taurine, 5.0 creatine, 5.0 sodium pyruvate, 1.0 Na2HPO4, 10 Hepes; pH 7.4 adjust with NaOH; 300 mosmol l−1 with mannitol. Ca2+-free PSS was prepared by simply omitting CaCl2 from the PSS. The modified KB (Kraft-Brühe) solution for cell storage contained (mm): 70 potassium glutamate, 20 KCl, 1.0 MgCl2, 10 KH2PO4, 10 Taurine, 10 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 10 glucose, 0.1% albumin, 10 β-hydroxybutyric acid and 10 Hepes; pH 7.2 with KOH (room temperature); 300 mosmol l−1 with mannitol. For ICl,ATP recording, the extracellular and intracellular solutions were chosen to maximize recording of Cl− currents and reduce possible contamination by cation currents and Ca2+-dependent currents. The standard extracellular solutions contained (mm): 77 NaCl, 0.8 MgCl2, 1.0 CaCl2, 5.0 CsCl, 2.0 BaCl2, 0.2 CdCl2, 5.5 glucose, 10 Hepes, 0.01 nicardipine; pH 7.4 adjusted with NaOH; total [Cl−]o = 90 mm; 320 mosmol l−1 with mannitol. In some experiments, extracellular NaCl and CsCl were replaced by an equimolar concentration (82 mmol l−1) of sodium aspartate or N′-methyl-d-glucamine (NMDG) chloride. The standard intracellular pipette solution contained (mm): 140 NMDG, 90 HCl, 5 MgATP, 0.1 Na2GTP, 20 EGTA, 10 Hepes; pH 7.3 adjusted with NMDG; total [Cl−]i = 90 mmol l−1; 280 mosmol l−1 using mannitol. Mannitol was used to adjust osmolarity in these solutions so that the activation of ICl,vol by hypotonic solutions could be easily tested in control experiments in cells from cftr−/− mice. In some experiments studying intracellular guanosine 5′-[γ-thio]triphosphate (GTPγS; Sigma, St Louis, MO, USA) or guanosine 5′-[β-thio]diphosphate (GDPβS; Sigma), 0.1 mm Na2GTP was replaced by 0.1 mm GTPγS or 1.0 mm GDPβS. The cells were preincubated for 2 h at room temperature in the modified KB solution (pH 7.4 at room temperature) containing 5.0 μg ml−1 pertussis toxin (PTX; Calbiochem, San Diego, CA, USA) in some experiments. The osmolarity of solutions was measured using a freezing point depression osmometer (model 3300; Advanced Instruments Inc., Norwood, MA, USA). Chemicals, including glibenclamide (Sigma), 4,4′-diisothiocyanostilbene-2,2′-disulphonate (DIDS; Sigma), phorbol 12,13-dibutyrate (PDBu; Sigma) and suramin (Sigma) were prepared as stock solutions in dimethyl sulphoxide (DMSO) and added to a known volume of superfusion solutions to produce the desired concentrations. Final concentration of DMSO was between 0.001 and 0.1% in solutions, which by itself had no effect on measured currents.
Data analysis
The straight line in the reversal potential−log [Cl−]o relationships (Fig. 2D), was fitted to the data according to a regression analysis with the least-squares method. Concentration–response curves (Fig. 2E) to extracellular ATP were analysed by using a Hill equation:
where [A] is the agonist concentration, EC50 is the agonist concentration to achieve 50% of the maximum response and nH is the Hill coefficient. Currents were normalized to the maximum difference current in each tested cell. The blocking effects of some inhibitors were calculated from the equation:
where Imax is the fully activated amplitudes of ATP-induced current, Idrug is the minimal amplitudes of ATP-induced current after application of the inhibitors and Icontrol is the basal current amplitudes in control solutions before exposure to ATP.
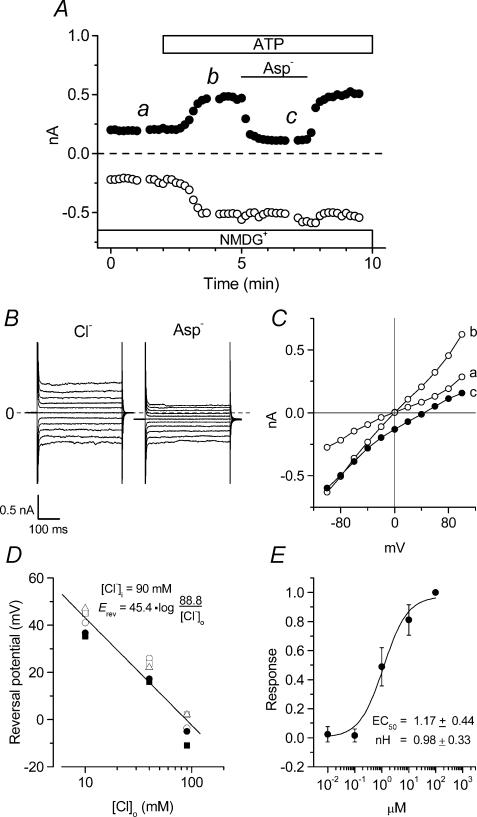
Figure 2. Anion sensitivity of extracellular ATP-induced membrane currents in mouse ventricular myocytes.
A, time course of extracellular ATP-induced whole-cell currents in NMDG-Cl solutions during application of 100 μm ATP to the bath. [Cl−]o was replaced to equimolar [Asp−]o during the period indicated by the bar. B, whole-cell current recordings at the time points b (Cl−) and c (Asp−) in A. C, I–V relationships of currents recorded at the time points indicated in A. D, reversal potential–log [Cl−]o relationships of whole-cell currents in the presence of extracellular ATP in 4 different cells in which [Cl−]o was changed from 90 to 10 mm in [NMDG+]-rich conditions. D, dose–response relationships of anion-sensitive [NMDG+ solutions] extracellular ATP-induced (difference) currents in 4 different cells. Responses were normalized to the maximum current density obtained at 100 μm ATP. The EC50 and nH coefficient correspond to the fitted curve.
Data are expressed as means ±s.e.m.; n indicates the number of cells. Statistical analysis was performed using paired or unpaired Student's t test where appropriate. Differences were considered to be significant with a two-tailed probability (P) of < 0.05.
Results
Extracellular ATP-induced currents in mouse ventricular myocytes
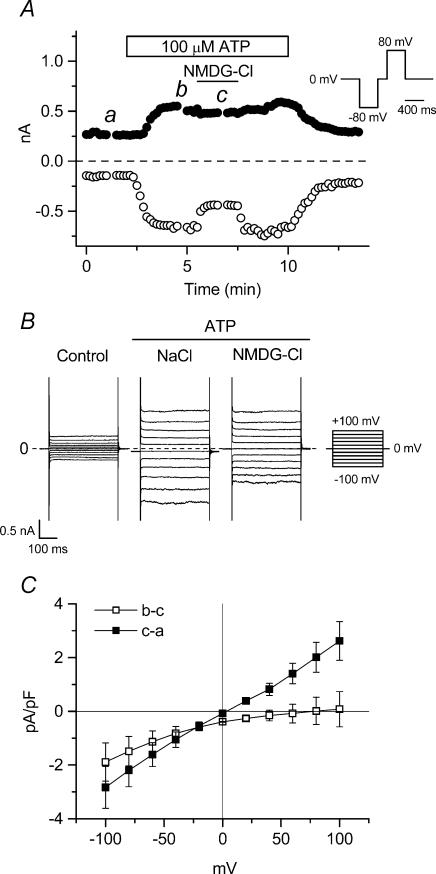
Under our experimental conditions with selected extracellular and intracellular solutions (see Methods), most cationic and exchange currents were inhibited and the standard intracellular solution contained 20 mm EGTA to minimize contamination by Ca2+-activated currents. Figure 1A shows the time course of extracellular ATP-induced whole-cell currents at +80 mV (filled circles) and −80 mV (open circles) in single ventricular cells isolated from mice. Only small whole-cell leak currents were observed in ventricular cells in the presence of standard extracellular solutions. Current amplitudes began to rapidly increase following a delay of ∼1 min after extracellular application of 100 μm ATP, and reached a steady state level within ∼2 min. Replacement of extracellular NaCl by NMDG-Cl was used to eliminate any ATP-induced non-selective cation current (IATP). After currents reached steady-state, subsequent replacement of extracellular NaCl by NMDG-Cl solution reversibly reduced the magnitude of inward currents (63.8 ± 3.3% of control at −100 mV, n = 6) while having little effect on the magnitude of outward currents (94.5 ± 4.2% of control at +100 mV, n = 6). Figure 1B shows examples of raw currents recorded in control (left), ATP with NaCl (middle), and ATP with NMDG-Cl (right) solutions. These currents were obtained at the time points a, b and c in Fig. 1A. Difference current–voltage (I–V) relationships are shown in Fig. 1C (n = 6). Extracellular Na+-dependent ATP-induced currents (b–c) exhibited inward rectification (0.02 ± 0.46 and −1.49 ± 0.65 pA pF−1 at +80 mV and −80 mV, respectively), and a mean reversal potential (Erev) of 63.4 ± 16.5 mV, consistent with activation of a non-selective cation current, IATP. Furthermore, the extracellular Na+-dependent ATP-induced inward currents were inhibited by 100 μm Gd3+ and 2 mm Zn2+ (inhibition = 22.8 ± 5.9 (n = 5) and 53.9 ± 2.0% (n = 3), respectively, at −100 mV), known blockers of non-selective cation channels (Nilius & Droogmans, 2001).
Figure 1. Cation sensitivity of extracellular ATP-induced membrane currents in mouse ventricular myocytes.
A, time course of extracellular ATP-induced whole-cell currents at +80 mV (•) and −80 mV (○) in single mouse ventricular cells exposed to standard extracellular solution during application of 100 μm ATP to the bath. [Na+]o and [Cs+]o were replaced with equimolar [NMDG+]o during the period indicated by the bar. Here and in subsequent similar figures, the pulse protocol is the same as shown in the inset of A. B, whole-cell current recordings induced by applying 400 ms voltage-clamp steps to membrane potentials between −100 mV and +100 mV in +20 mV steps from a holding potential of 0 mV every 2 s. In subsequent similar figures, the step-pulse protocol is the same as shown in the inset of B. Currents were recorded at the time points indicated in A. C, mean I–V relationships of [NMDG+]o-sensitive (b–c) and -insensitive (c–a) currents in 6 different cells.
In contrast to IATP, the Na+-independent ATP-induced currents (c–a) were almost linear (2.02 ± 0.47 and −2.25 ± 0.44 pA pF−1, at +80 mV and −80 mV, respectively), with a mean reversal potential (Erev: 3.0 ± 2.1 mV) close to the estimated Cl− equilibrium potential (ECl = 0 mV with symmetrical Cl− solutions). These properties of the Na+-independent currents are characteristic of ATP-activated Cl− currents, ICl,ATP, previously characterized in mouse cardiac myocytes (Levesque & Hume, 1995; Duan et al. 1999). Data presented in Fig. 2 further confirm the anionic nature of these currents.
Figure 2A shows the time course of the Na+-independent ICl,ATP at +80 mV (filled circles) and −80 mV (open circles) before (NMDG-Cl) and after replacement of extracellular Cl− (NMDG-aspartate). Figure 2B shows examples of raw currents recorded at the time points b and c in Fig. 2A, and Fig. 2C shows I–V relationships of currents obtained at the time points a, b and c in Fig. 2A. Currents activated by extracellular ATP had almost linear I–V relationships under NMDG-Cl conditions, as shown in Fig. 2C. Reduction of extracellular [Cl−] from 90 to 10 mm during ATP application, by replacing 80 mm NMDG-Cl with equimolar NMDG-aspartate, led to a positive shift of Erev, accompanied by a marked decrease in outward current as shown in Fig. 2A, B and C. Figure 2D summarizes the changes of Erev in the whole-cell currents at three different [Cl−]o levels, performed using the same ventricular myocytes (n = 4). The reversal potential−log [Cl−]o relation had a slope of 45.4 mV per 10-fold change in [Cl−]o, indicating that Cl− ions are the main charge carrier of the ATP-induced current component under NMDG-Cl conditions, as has been observed previously in guinea-pig atrial (Matsuura & Ehara, 1992) and rat ventricular (Kaneda et al. 1994) cells.
Next, to define further the ATP dependence of ICl,ATP, we determined the agonist concentration to achieve a 50% maximal response (EC50) and calculated the Hill coefficient (nH) for extracellular ATP (see Methods). Figure 2E shows the response–concentration relationship obtained at +100 mV with NMDG-Cl solutions (n = 4). This relationship was well fitted by a Hill equation, and extracellular ATP increased the Cl− current with an estimated EC50 value of 1.17 ± 0.44 μm and nH of 0.98 ± 0.33, suggesting that the binding of 1 ATP molecule to P2-receptors activates ICl,ATP in mouse ventricular cells. This EC50 value is similar to that observed previously for P2-purinergic receptor stimulation of ICl,ATP in rat ventricular cells (Kaneda et al. 1994), and IK in guinea-pig atrial (Matsuura et al. 1996) and ventricular (Matsubayashi et al. 1999) cells.
Involvement of PTX-insensitive G protein in activation of ICl,ATP
P2 purinergic receptors are divided into two groups: ligand-activated ion channels (P2X receptors) and GTP-binding protein (G protein) coupled receptors (P2Y receptors) (for reviews see Fredholm et al. 1994, 1997; North, 2002; Dubyak, 2003; Lee & O'Grady, 2003). To classify the P2-purinergic receptor involved in IATP, we examined the effects of intracellular dialysis of 100 μm GTPγS, a non-hydrolysable GTP analogue. Several previous reports have showed that intracellular GTPγS itself does not induce cardiac whole-cell currents under conditions similar to those used here (Hwang et al. 1992; Iyadomi et al. 1995; Hool et al. 1997). In contrast, it has been reported that intracellular dialysis of GTPγS in human endothelial cells transiently activates a Cl− current (Nilius et al. 1994). Therefore, we first confirmed that intracellular GTPγS dialysis failed to activate Cl− currents under basal conditions in mouse ventricular myocytes (n = 3, data not shown). We then tested whether intracellular GTPγS dialysis would alter the purinergic activation of IATP or ICl,ATP.
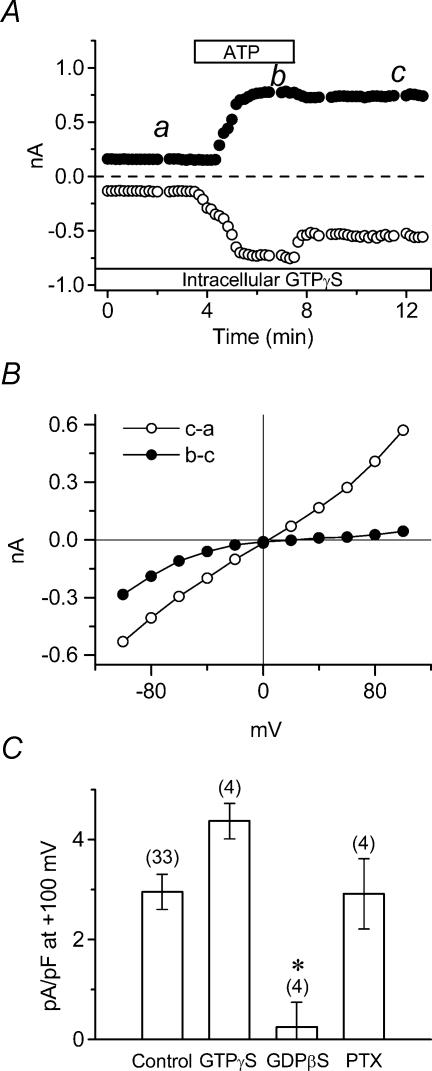
Figure 3A shows that, in GTPγS-loaded cells with standard extracellular (NaCl) solutions, the outward currents were irreversibly activated by brief exposure to 100 μm ATP, but the inward currents were partially decreased following brief exposure to ATP, as previously found in control cells (Fig. 1A). The different I–V relationships derived by using currents obtained at the time points a, b and c in Fig. 3A are shown in Fig. 3B. Intracellular GTPγS-dependent ICl,ATP (c–a) was almost linear with a reversal potential (3.6 mV) close to the estimated Cl− equilibrium potential (ECl = 0 mV with symmetrical Cl− solutions). In contrast, the previously identified Na+-dependent IATP (b–c), which exhibited inward rectification and a positive reversal potential (22.6 mV), appeared to be insensitive to intracellular GTPγS, as it reversed following washout of ATP.
Figure 3. Role of G-protein coupled purinergic receptors in activation of ICl,ATP.
A, time course of extracellular ATP-induced whole-cell currents in standard extracellular solution during application of 100 μm ATP to the bath. The tested cell was dialysed with 0.1 mm GTPγS, and was exposed to ATP as indicated by the bar. B, I–V relationships of ATP-induced currents (b–a) and the persistently activated ICl,ATP (c–a). Whole-cell currents were activated by voltage-clamp pulses as in Fig. 1B, at time points a, b and c in A. C, mean current densities at +100 mV of ICl,ATP in GTPγS-dialysed (n = 4), GDPβS-dialysed (n = 4) or PTX-pretreated (n = 4) cells. * signifies significantly smaller than control with P < 0.05.
To further clarify the GTP dependence of ICl,ATP, we measured current densities of ICl,ATP (at +100 mV) in control, GTPγS-dialysed, GDPβS (non-hydrolysable GDP analogue)-dialysed, and pertussis toxin (PTX; Gi/o protein inhibitor)-pretreated cells. Figure 3C compares ICl,ATP densities obtained under these four experimental conditions in a number of myocytes. The mean outward current density of ICl,ATP in control was 2.95 ± 0.35 pA pF−1 (n = 33), whereas mean outward current density of ICl,ATP in GTPγS-loaded cells and PTX-pretreated cells was 4.37 ± 0.36 (n = 4) and 2.91 ± 0.71 pA pF−1 (n = 4), respectively. In contrast, extracellular ATP induced very little activation of ICl,ATP in GDPβ-loaded cells (0.25 ± 0.50 pA pF−1 (n = 4), P < 0.05 versus control). The cationic IATP had no sensitivity to these GTP analogues (data not shown). These results suggest that cationic IATP is regulated by a G protein independent signalling pathway, whereas a G protein-coupled receptor (P2Y purinergic receptor coupled to a PTX-insensitive G protein pathway) plays a crucial role in the activation of ICl,ATP.
Absence of ICl,ATP in myocytes from cftr−/− mice
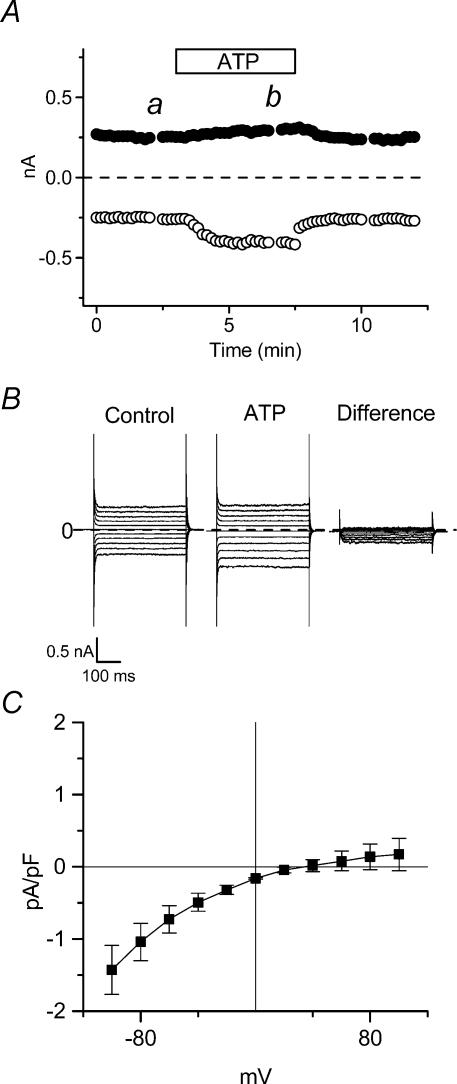
In a previous report (Duan et al. 1999) it was suggested that CFTR may be a molecular candidate responsible for ICl,ATP in mouse cardiac myocytes, based on: (1) the observation that ICl,ATP was regulated by a dual intracellular signal phosphorylation pathway involving both protein kinase A (PKA) and protein kinase C (PKC), (2) the observation that ICl,ATP was inhibited by 50 μm glibenclamide, a concentration known to inhibit CFTR Cl− currents, but not volume-regulated Cl− currents (ICl,vol) or Ca2+-activated Cl− currents (ICl,Ca) (Yamazaki & Hume, 1997), and (3) single channel properties (conductance and rectification) that resembled those of CFTR Cl− channels in cardiac myocytes (Ehara & Ishihara, 1990). In addition, RT-PCR and Northern blot analysis clearly showed CFTR mRNA expression in mouse atrium and ventricle. To further test this hypothesis, we examined the effects of extracellular ATP in single ventricular cells isolated from cftr−/− (CFTR knockout) mice. As shown in Fig. 4A, extracellular ATP activated cationic IATP in NaCl-containing solutions in cells from cftr−/− mice; however, activation of ICl,ATP, which was prominent in cells from cftr+/+ mice (cf. Figure 1), was not detectable. PDBu (100 nm) and isoprenaline (1.0 μm), which previously have been shown to significantly augment the amplitude of ICl,ATP in mouse cardiac myocytes (Duan et al. 1999), alone or in combination with ATP, also failed to activate ICl,ATP in cells from cftr−/− mice (data not shown). Despite the absence of ICl,ATP in cells from cftr−/− mice, exposure of these cells to hypotonic solutions elicited typical changes in ICl,vol with current densities, kinetics and rectification properties similar to ICl,vol in cells from normal cftr+/+ mice (data not shown). In Fig. 4B, representative raw currents activated by voltage clamp pulses obtained at the time points a and b in Fig. 4A, and the difference current, are shown. The mean I–V relationships (Fig. 4C) of the extracellular ATP-sensitive current (n = 10 from 4 cftr−/− mice) was inwardly rectifying (0.14 ± 0.18 and −1.04 ± 0.26 pA pF−1 at +80 and −80 mV, respectively) and the mean Erev was 36.3 ± 7.3 mV. These results indicate that typical cationic IATP can be recorded in myocytes from cftr−/− mice, and the absence of ICl,ATP in ventricular cells can be attributed to targeted inactivation of cftr.
Figure 4. Extracellular ATP-induced membrane currents in ventricular cells from cftr−/− mice.
A, time course of extracellular ATP-induced whole-cell currents in standard extracellular solution in ventricular cells from cftr−/− mice. B, whole-cell current recorded using same protocol as in Fig. 1B at time points a (control) and b (ATP) in panel A. Difference currents obtained by subtracting currents obtained at time b from currents obtained at time a. C, mean I–V relationships of ATP-induced difference currents in 10 cells from 4 cftr−/− mice.
Mechanism of block of ICl,ATP by DIDS
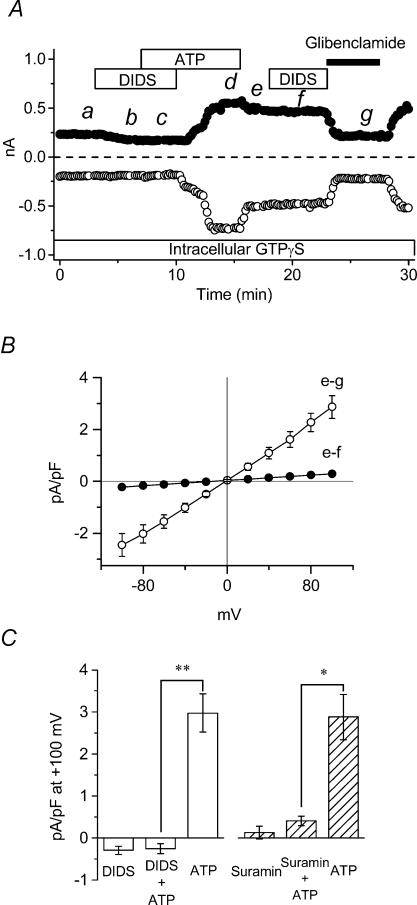
It was previously demonstrated (Kaneda et al. 1994) that ICl,ATP is potently inhibited by DIDS, which is a known inhibitor of several types of Cl− channels, excluding CFTR Cl− channels (Vandenberg et al. 1994). It is possible that these apparently contradictory effects on ICl,ATP might be due to the ability of DIDS to directly antagonize P2 purinergic receptors (Hume et al. 2000; Vassort, 2001; North, 2002). To directly distinguish between possible inhibitory effects of DIDS on ICl,ATP from DIDS antagonism of P2 purinergic receptors, we examined the effects of DIDS on ICl,ATP persistently activated in GTPγS-dialysed cells. As shown in Fig. 5A, 100 μm DIDS pretreatment completely prevented the activation of both cationic IATP and ICl,ATP in GTPγS-dialysed cells; both currents were activated in the continued presence of ATP following washout of DIDS. As previously shown in Fig. 3, ICl,ATP remained persistently activated following washout of ATP, and re-exposure to 100 μm DIDS failed to significantly alter the amplitude of the persistently activated ICl,ATP. This result rules out a direct effect of DIDS on ICl,ATP.Figure 5A also demonstrates that the DIDS-insensitive persistently activated ICl,ATP was nearly completely inhibited by 100 μm glibenclamide. Figure 5B illustrates I–V relationships of the DIDS-sensitive (e–f) and glibenclamide-sensitive (e–g) persistently activated ICl,ATP in GTPγS-dialysed cells. Overall, these results suggest that DIDS inhibition of ICl,ATP is due to P2 purinergic receptor antagonism, and that the channels mediating ICl,ATP are DIDS insensitive and glibenclamide sensitive, thus resembling the known properties of CFTR Cl− channels in cardiac cells (Vandenberg et al. 1994; Tominaga et al. 1995).
Figure 5. Effects of DIDS and suramin on ICl,ATP in ventricular cells of wild-type mice.
A, time course of extracellular ATP-induced whole-cell currents in standard extracellular solutions in intracellular GTPγS dialysed cell of wild-type mouse. ATP (100 μm), DIDS (100 μm) and glibenclamide (100 μm) were applied during the times indicated by each bar. B, mean I–V relationships of DIDS- (e–f) and glibenclamide-sensitive currents (e–g), which were obtained from whole-cell recordings induced using the same voltage clamp pulse protocol shown in Fig. 1B, at time points e, f and g in A. C, the left side shows the mean (n = 4) difference current density at +100 mV in DIDS, DIDS and ATP, and ATP following the removal of DIDS in control (absence of GTPγS) cells. The right side shows the mean (n = 4) difference current densities at +100 mV in suramin, suramin and ATP, and ATP following removal of suramin. * and ** signify significantly smaller than ATP alone with P < 0.05 and 0.01, respectively.
Figure 5C summarizes the effects of pretreatment of DIDS (100 μm, n = 4) and suramin (100 μm, n = 4), a P2 purinergic receptor antagonist, on ICl,ATP activation by extracellular ATP at +100 mV in control (absence of GTPγS) myocytes. These results show that pretreatment of DIDS or suramin strongly inhibited the activation of ICl,ATP by extracellular ATP. In addition, the activation of cationic IATP was also inhibited by DIDS (Fig. 5A) or suramin pretreatment (data not shown).
Discussion
The major findings of the present investigation include the following: (1) extracellular ATP activates both a cationic IATP and ICl,ATP in mouse ventricular myocytes; (2) the activation of cationic IATP involves a G protein-independent signalling pathway, whereas activation of ICl,ATP involves a PTX-insensitive G protein signalling pathway; (3) the absence of ICl,ATP in myocytes from cftr−/− mice provides direct evidence that CFTR Cl− channels are indeed responsible for ICl,ATP in mouse heart; and (4) the apparent inhibitory effects of the Cl− channel blocker DIDS on ICl,ATP can be attributed to antagonism of P2 purinergic receptors, and are not due to direct inhibition of ICl,ATP. Thus the demonstrated DIDS insensitivity and glibenclamide sensitivity of ICl,ATP are consistent with the known pharmacological properties of CFTR Cl− channels.
Role of P2Y purinergic receptors in the activation of ICl,ATP
Activation of ICl,ATP was clearly prevented by suramin, an antagonist of P2 purinergic receptors, showing that binding of extracellular ATP to the P2 purinergic receptor is essential for the activation of ICl,ATP in mouse ventricular myocytes. ICl,ATP was also found to be persistently activated by ATP in the GTPγS-dialysed cells, and its activation was abolished in the cells dialysed with GDPβS, suggesting an essential role of G-proteins in the activation of ICl,ATP. In previous reports (Matsuura & Ehara, 1992; Kaneda et al. 1994; Levesque & Hume, 1995; Duan et al. 1999), the activation of ICl,ATP appeared to be coupled to P2-purinoceptor stimulation, since ICl,ATP was activated by ATP, ADP and ATPγS, but not by adenosine or AMP in cardiac cells. However, the type of P2 receptor involved was not characterized in these previous reports. This agonist profile, together with the present demonstration of involvement of a G protein signalling pathway, and inhibition by suramin, provides evidence for an essential role of P2Y receptors for the activation of ICl,ATP in mouse ventricular myocytes.
Several types of cardiac ion channels are known to be influenced by P2Y purinergic receptor stimulation. Among these, the modulation of L-type Ca2+ current (for review see Vassort, 2001) and delayed rectifier K+ current (Matsuura et al. 1996, Matsubayashi et al. 1999) has been clearly shown to depend on P2Y purinergic receptors. In contrast, we demonstrated that extracellular ATP-activated cation currents (cationic IATP) were not influenced by GTPγS and GDPβS although the activation was prevented by pretreatment of suramin and DIDS. In addition, the cationic IATP was partially inhibited by 100 μm Gd3+ and 2 mm Zn2+, inhibitors of non-selective cation channels. These results suggest that the cationic IATP is most likely regulated by ionotropic P2X receptors, although the remote possibility that a yet to be identified G-protein-independent signalling pathway involving P2Y receptors may play a role cannot be eliminated. In general, it is reported that extracellular ATP elicits a rapid activating, fast desensitizing inward cation current via P2X receptors in several cardiac cells (Matsuura & Ehara, 1992; Vassort, 2001; North, 2002). However, the cationic IATP observed in the present study did not appear to exhibit rapid desensitization within a few of minutes of ATP application. A similar long-lasting ATP-induced inward cation current has been shown in rabbit sinoatrial cells (Shoda et al. 1997) and rat cardiomyocytes (Ugur & Vassort, 2001). However, the experimental conditions we used were different since bath and pipette solutions were selected to optimize recording of Cl− currents. In addition, in examining current responses to extracellular ATP, we did not employ a rapid extracellular solution exchange system. These factors therefore prevent us from reaching a definitive conclusion regarding desensitization of the cationic IATP.
Pharmacological properties of ICl,ATP
In a previous study (Duan et al. 1999) it was shown that ICl,ATP was inhibited by glibenclamide, Rp-cAMP (a specific PKA inhibitor) and bisindolylmaleimide (BIM; a specific PKC inhibitor) in mouse ventricular myocytes. These are all properties characteristic of CFTR Cl− channels (Gadsby & Nairn, 1999); however, the observed inhibition by DIDS is inconsistent with a role of CFTR channels being responsible for ICl,ATP (Schultz et al. 1999). DIDS also was found to block ICl,ATP in rat ventricular cells (Kaneda et al. 1994). However, since it is known that DIDS and other stilbene derivatives are potent antagonists of P2 purinergic receptors (Vassort, 2001; North, 2002), it is not clear whether the observed inhibition of ICl,ATP by DIDS is due to a direct inhibitory effect on the channels mediating ICl,ATP or is due to antagonism of P2 purinergic receptors. In order to distinguish between these possibilities, we examined the effect of DIDS on persistently activated ICl,ATP in cells dialysed with intracellular GTPγS, since these currents are maintained presumably in the absence of sustained P2 receptor stimulation. Under these conditions we found that DIDS had negligible effects on ICl,ATP, strongly suggesting that the inhibitory effects of DIDS on acutely activated ICl,ATP are due to P2 purinergic receptor antagonism.
Though it has been reported that stimulation of P2Y purinergic receptors or extracellular ATP itself can lead to an increase in intracellular Ca2+ in some cells (Vassort, 2001), thus possibly activating Ca2+-dependent Cl− channels, the pipette solution used for ICl,ATP recording in our experiments contained a high concentration (20 mm) of EGTA to chelate intracellular Ca2+. Other types of Cl− channels which have been shown to be activated by extracellular ATP include volume-regulated Cl− channels (ICl,vol) (Perez-Samartin et al. 2000) and a ‘novel’ ATP-gated Cl− channel (Arreola & Melvin, 2003). These are unlikely to be involved in the ICl,ATP measured in our experiments. With symmetrical Cl− solutions, it is well known that ICl,vol has an outwardly rectifying I–V relationship (Hume et al. 2000; Jentsch et al. 2002), whereas ICl,ATP in mouse ventricular myocytes and ICl,CFTR exhibit linear I–V relationships under these conditions. Moreover, DIDS, is a potent inhibitor of both ICl,Ca and ICl,vol. The recently described ATP-gated Cl− channel (Arreola & Melvin, 2003) is insensitive to glibenclamide, and its EC50 for ATP is 158 μm. In contrast, as reported here, the EC50 of ICl,ATP for ATP is 1.2 μm, and glibenclamide inhibited the current. These considerations make it highly unlikely that ICl,ATP in mouse ventricular myocytes is generated by activation of ICl,Ca, ICl,vol or ‘novel’ ATP-gated channels.
Absence of ICl,ATP in cardiac myocytes from cftr−/− mice
CFTR is expressed in various mammalian cell types including heart (Hume et al. 2000). Analysis of CFTR knockout mouse models has been useful to identify the functional role and potential interactions of CFTR. Several CFTR knockout mouse models have been generated in which the CFTR gene has been disrupted by insertion, duplication, or an in-frame stop codon, and all of these CFTR knockout mice have a defective cAMP-mediated Cl− conductance in the tissues which have been examined (for review see Devuyst & Guggino, 2002). In previous studies (Levesque & Hume, 1995; Duan et al. 1999), the possibility that ICl,ATP in mouse heart might be due to activation of CFTR Cl− channels was supported by strong similarities in electrophysiological, biophysical and pharmacological properties of ICl,ATP and ICl,CFTR. Furthermore, unitary channels associated with ICl,ATP resembled those CFTR unitary channels in terms of anion selectivity, rectification and conductance, and Northern blot analysis confirmed CFTR mRNA expression in mouse heart. The absence of detectable ICl,ATP in cardiac myocytes from cftr−/− mice demonstrated in the present study provides compelling and conclusive evidence that ICl,ATP in heart is mediated by CFTR.
While it is possible that extracellular ATP might be converted to adenosine by ectoenzymes, and then activate CFTR channels by increasing cAMP generation, this seems very unlikely in the present experiments because we have previously demonstrated (Levesque & Hume, 1995; Duan et al. 1999): (1) that ICl,ATP can be activated by extracellular application of ATPγS, an ATP analogue which is resistant to hydrolysis by ectoATPases, and (2) that extracellular application of adenosine alone fails to activate ICl,ATP. Future studies should reveal whether CFTR may be coupled to P2Y purinergic receptor stimulation in other tissues as well.
Acknowledgments
The authors would like to thank Susan Tamowski, Paul Scowen, Linda Ye, and Phillip Keller for excellent technical assistance. This study was supported by NIH grants HL49254 and NCRR P20RR15581.
References
- Arreola J, Melvin JE. A novel chloride conductance activated by extracellular ATP in mouse parotid acinar cells. J Physiol. 2003;547:197–208. doi: 10.1113/jphysiol.2002.028373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devuyst O, Guggino WB. Chloride channels in the kidney: lessons learned from knockout animals. Am J Physiol Renal Physiol. 2002;283:F1176–F1191. doi: 10.1152/ajprenal.00184.2002. [DOI] [PubMed] [Google Scholar]
- Duan D, Ye L, Britton F, Miller LJ, Yamazaki J, Horowitz B, Hume JR. Purinoceptor-coupled Cl− channels in mouse heart: a novel, alternative pathway for CFTR regulation. J Physiol. 1999;521:43–56. doi: 10.1111/j.1469-7793.1999.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak GR. Knock-out mice reveal tissue-specific roles of P2Y receptor subtypes in different epithelia. Mol Pharmacol. 2003;63:773–776. doi: 10.1124/mol.63.4.773. [DOI] [PubMed] [Google Scholar]
- Ehara T, Ishihara K. Anion channels activated by adrenaline in cardiac myocytes. Nature. 1990;347:284–286. doi: 10.1038/347284a0. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, Schwabe U, Williams M. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol Sci. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby DC, Nairn AC. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol Rev. 1999;79:S77–S107. doi: 10.1152/physrev.1999.79.1.S77. [DOI] [PubMed] [Google Scholar]
- Hool LC, Oleksa LM, Harvey RD. Role of G proteins in α1-adrenergic inhibition of the β-adrenergically activated chloride current in cardiac myocytes. Mol Pharmacol. 1997;51:853–860. doi: 10.1124/mol.51.5.853. [DOI] [PubMed] [Google Scholar]
- Hume JR, Duan D, Collier ML, Yamazaki J, Horowitz B. Anion transport in heart. Physiol Rev. 2000;80:31–81. doi: 10.1152/physrev.2000.80.1.31. [DOI] [PubMed] [Google Scholar]
- Hwang TC, Horie M, Nairn AC, Gadsby DC. Role of GTP-binding proteins in the regulation of mammalian cardiac chloride conductance. J General Physiol. 1992;99:465–489. doi: 10.1085/jgp.99.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inscho EW. P2 receptors in regulation of renal microvascular function. Am J Physiol Renal Physiol. 2001;280:F927–F944. doi: 10.1152/ajprenal.2001.280.6.F927. [DOI] [PubMed] [Google Scholar]
- Iyadomi I, Hirahara K, Ehara T. α-Adrenergic inhibition of the β-adrenoceptor-dependent chloride current in guinea-pig ventricular myocytes. J Physiol. 1995;489:95–104. doi: 10.1113/jphysiol.1995.sp021033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Jia Y, Mathews CJ, Hanrahan JW. Phosphorylation by protein kinase C is required for acute activation of cystic fibrosis transmembrane conductance regulator by protein kinase A. J Biol Chem. 1997;272:4978–4984. doi: 10.1074/jbc.272.8.4978. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Fukui K, Doi K. Activation of chloride current by P2-purinoceptors in rat ventricular myocytes. Br J Pharmacol. 1994;111:1355–1360. doi: 10.1111/j.1476-5381.1994.tb14894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottgen M, Loffler T, Jacobi C, Nitschke R, Pavenstadt H, Schreiber R, Frische S, Nielsen S, Leipziger J. P2Y6 receptor mediates colonic NaCl secretion via differential activation of cAMP-mediated transport. J Clin Invest. 2003;111:371–379. doi: 10.1172/JCI16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, O'Grady SM. Modulation of ion channel function by P2Y receptors. Cell Biochem Biophys. 2003;39:75–88. doi: 10.1385/CBB:39:1:75. [DOI] [PubMed] [Google Scholar]
- Levesque PC, Hume JR. ATPo but not cAMPi activates a chloride conductance in mouse ventricular myocytes. Cardiovasc Res. 1995;29:336–343. [PubMed] [Google Scholar]
- Matsubayashi T, Matsuura H, Ehara T. On the mechanism of the enhancement of delayed rectifier K+ current by extracellular ATP in guinea-pig ventricular myocytes. Pflugers Arch. 1999;437:635–642. doi: 10.1007/PL00008090. [DOI] [PubMed] [Google Scholar]
- Matsuura H, Ehara T. Activation of chloride current by purinergic stimulation in guinea pig heart cells. Circ Res. 1992;70:851–855. doi: 10.1161/01.res.70.4.851. [DOI] [PubMed] [Google Scholar]
- Matsuura H, Tsuruhara Y, Sakaguchi M, Ehara T. Enhancement of delayed rectifier K+ current by P2-purinoceptor stimulation in guinea-pig atrial cells. J Physiol. 1996;490:647–658. doi: 10.1113/jphysiol.1996.sp021174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- Nilius B, Oike M, Zahradnik I, Droogmans G. Activation of a Cl− current by hypotonic volume increase in human endothelial cells. J General Physiol. 1994;103:787–805. doi: 10.1085/jgp.103.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Perez-Samartin AL, Miledi R, Arellano RO. Activation of volume-regulated Cl− channels by ACh and ATP in Xenopus follicles. J Physiol. 2000;525:721–734. doi: 10.1111/j.1469-7793.2000.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puceat M, Bony C, Jaconi M, Vassort G. Specific activation of adenylyl cyclase V by a purinergic agonist. FEBS Lett. 1998;431:189–194. doi: 10.1016/s0014-5793(98)00747-9. [DOI] [PubMed] [Google Scholar]
- Puceat M, Hilal-Dandan R, Strulovici B, Brunton LL, Brown JH. Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J Biol Chem. 1994;269:16938–16944. [PubMed] [Google Scholar]
- Schultz BD, Singh AK, Devor DC, Bridges RJ. Pharmacology of CFTR chloride channel activity. Physiol Rev. 1999;79:S109–S144. doi: 10.1152/physrev.1999.79.1.S109. [DOI] [PubMed] [Google Scholar]
- Shoda M, Hagiwara N, Kasanuki H, Hosoda S. ATP-activated cationic current in rabbit sino-atrial node cells. J Mol Cell Cardiol. 1997;29:689–695. doi: 10.1006/jmcc.1996.0311. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Horie M, Sasayama S, Okada Y. Glibenclamide, an ATP-sensitive K+ channel blocker, inhibits cardiac cAMP-activated Cl− conductance. Circ Res. 1995;77:417–423. doi: 10.1161/01.res.77.2.417. [DOI] [PubMed] [Google Scholar]
- Ugur M, Vassort G. A novel nonspecific current activated by extracellular ATP in rat cardiomyocytes. Biophys J. 2001;80:586a. abstract. [Google Scholar]
- Vandenberg JI, Yoshida A, Kirk K, Powell T. Swelling-activated and isoprenaline-activated chloride currents in guinea pig cardiac myocytes have distinct electrophysiology and pharmacology. J General Physiol. 1994;104:997–1017. doi: 10.1085/jgp.104.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassort G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- Yamazaki J, Hume JR. Inhibitory effects of glibenclamide on cystic fibrosis transmembrane regulator, swelling-activated, and Ca2+-activated Cl− channels in mammalian cardiac myocytes. Circ Res. 1997;81:101–109. doi: 10.1161/01.res.81.1.101. [DOI] [PubMed] [Google Scholar]