Abstract
PKA-dependent phosphorylation of cardiac troponin I (cTnI) contributes significantly to β-adrenergic agonist-induced acceleration of myocardial relaxation (lusitropy). However, the role of PKA-dependent cTnI phosphorylation in the positive inotropic response to β-adrenergic stimulation is unclear. We studied the contractile response to isoprenaline (10 nm) in isolated hearts and isolated cardiomyocytes from transgenic mice with cardiac-specific expression of slow skeletal TnI (ssTnI, which lacks the N-terminal protein extension containing PKA-sensitive phosphorylation sites in cTnI) and matched wild-type littermate controls. As expected, the lusitropic effect of isoprenaline was significantly blunted in ssTnI hearts. However, the positive inotropic response to isoprenaline was also blunted in ssTnI hearts. This effect was especially prominent for ejection-phase indices in isolated auxotonically loaded ssTnI hearts whereas the positive inotropic response of isovolumic hearts or unloaded isolated myocytes was much less affected. Isoprenaline decreased left ventricular end-systolic volume in wild-type hearts (10.6 ± 1.6 to 6.2 ± 0.4 μl at a preload of 20 cmH2O; P < 0.05) but not transgenic hearts (11.4 ± 1.3 to 10.9 ± 1.3 μl; P = n.s.). Likewise, isoprenaline increased stroke work in control hearts (14.5 ± 1.0 to 22.5 ± 1.8 mmHg μl mg−1; P < 0.05) but not transgenic hearts (15.4 ± 1.3 to 18.3 ± 1.2 mmHg μl mg−1; P = n.s.). The end-systolic pressure–volume relation was increased by isoprenaline to a greater extent in control than transgenic hearts. However, isoprenaline induced a similar rise in intracellular Ca2+ transients in transgenic and non-transgenic cardiomyocytes. These results indicate that cTnI has a pivotal role in the positive inotropic response of the murine heart to β-adrenergic stimulation, an effect that is highly dependent on loading conditions and is most evident in the auxotonically loaded ejecting heart.
The thin filament protein troponin I (TnI) has a key regulatory role in cardiac muscle contraction through its inhibition of actomyosin crossbridge formation. Upon elevation of cytosolic Ca2+ levels and the binding of Ca2+ to troponin C (TnC), the affinity of TnI for TnC increases while its affinity for actin decreases thereby relieving this inhibition (reviewed by Solaro, 2001). Mice with gene-targeted ablation of cardiac TnI (cTnI) demonstrate a reduced myofilament Ca2+ responsiveness and diastolic dysfunction, and develop heart failure and death at a young age (Huang et al. 1999). The pathophysiological importance of cTnI is underlined by observations that cTnI proteolysis contributes to contractile depression during myocardial stunning (Gao et al. 1997) and that mutations in cTnI are linked to contractile dysfunction in familial hypertrophic cardiomyopathy (Westfall et al. 2002).
The phosphorylation of cTnI constitutes a major physiological mechanism through which myofilament properties are modulated. In particular, β-adrenergic agonists promote cAMP-dependent protein kinase (PKA)-mediated phosphorylation of cTnI, which causes a reduction in myofilament Ca2+ sensitivity and contributes to an acceleration of relaxation, i.e. a lusitropic effect (reviewed by Solaro, 2001). The PKA phosphorylation sites responsible for this effect, namely serines 23 and 24, are found in the 27–33 amino acid N-terminal extension found in cTnI. The slow skeletal isoform of TnI (ssTnI) lacks this 27–33 amino acid N-terminal extension and thus cannot be phosphorylated by PKA. Indeed, studies in gene-modified murine models in which cTnI is replaced by a non-phosphorylatable isoform have confirmed that cTnI phosphorylation contributes significantly to β-adrenergic agonist-induced lusitropy (Fentzke et al. 1999; Kentish et al. 2001; Wolska et al. 2002; Pi et al. 2002). Alterations in the phosphorylation status of cTnI have been linked to contractile dysfunction in systemic sepsis (Tavernier et al. 2000) and heart failure (Bodor et al. 1997).
β-Adrenergic stimulation induces PKA-dependent phosphorylation of several other proteins, such as myosin-binding protein C, L-type Ca2+ channels and phospholamban (reviewed by Bers, 2001). The positive inotropic response to β-adrenergic agonists is thought to mainly involve the phosphorylation of L-type Ca2+ channels (which increases sarcolemmal Ca2+ entry) and of phospholamban, which leads to increased sarcoplasmic reticulum (SR) Ca2+ uptake and Ca2+ loading. In contrast, cTnI phosphorylation has not generally been considered important in the positive inotropic response to β-adrenergic stimulation. However, it is well established that PKA-dependent cTnI phosphorylation increases crossbridge cycling rate and maximum unloaded shortening velocity (Vmax), which contributes to the lusitropic effects of β-adrenergic stimulation (e.g. Hoh et al. 1988; Strang et al. 1994; Kentish et al. 2001). In theory, an increased shortening velocity could also contribute to positive inotropy, since the power output of muscle is determined by the product of force and velocity. Indeed, PKA treatment of skinned cardiac myocytes performing loaded shortening has been found to increase absolute peak power, in part by speeding loaded crossbridge cycling rates (Herron et al. 2001).
The inotropic effects of an increased crossbridge cycling rate are likely to be significantly influenced by the mode of contraction studied. Whereas experimental studies are often performed, for good technical and other reasons, using externally unloaded cardiomyocyte shortening or isometric/isovolumic contraction, the heart in vivo undergoes ‘auxotonic’ contraction consisting of isovolumic contraction, ejection, isovolumic relaxation and refilling phases. In addition, technical limitations often necessitate isolated myocyte and muscle experiments to be undertaken at non-physiological temperatures and stimulation frequencies, which may also influence crossbridge kinetics (Janssen et al. 2002). Indeed, the contribution of myofilament properties to the relaxant effect of β-stimulation has been suggested to be load dependent (Li et al. 2000; Wolska et al. 2002; Layland & Kentish, 2002), consistent with the long-established notion of the load dependence of relaxation (Brutsaert & Sys, 1989). Likewise, any involvement of cTnI in the positive inotropic response to β-stimulation may be more apparent under specific conditions of loading and at physiological temperature.
In the present study, we therefore systematically investigated the contractile response to β-adrenergic stimulation using ex vivo perfused ejecting (i.e. auxotonically loaded) mouse hearts at physiological temperature and frequency, as well as isolated unloaded cardiomyocytes and isolated isovolumic hearts. An ex vivo approach was chosen in preference to in vivo assessment in order to avoid the potential confounding effects of neurohumoral influences, anaesthesia and variable loading. In order to specifically address the contribution of the cTnI isoform, we compared the contractile response to β-stimulation in hearts from transgenic mice with cardiac-specific expression of ssTnI and hearts from matched littermate controls containing the normal cTnI isoform. In the ssTnI transgenic mouse, cTnI is fully and stoichiometrically replaced by the slow skeletal isoform, which lacks the 27–33 amino acid N-terminal extension that contains the PKA phosphorylation sites (Fentzke et al. 1999). We report the novel finding that cTnI plays a pivotal role in the positive inotropic response to β-adrenergic stimulation during auxotonic contraction in the ejecting heart but much less so in the isovolumic heart or the unloaded cardiomyocyte.
Methods
Animals
All experiments were performed in accordance with UK Home Office regulations.
Adult male transgenic mice (TG) expressing the slow skeletal isoform of TnI were obtained from the laboratory of R. J. Solaro where they were generated as previously described (Fentzke et al. 1999). These founder TG mice were bred with female adult CD-1 mice (containing normal cTnI) to establish a colony of heterozygous TG mice, giving either non-transgenic (NTG, cTnI) or transgenic (TG, ssTnI) offspring in the same litter. Littermates were genotyped using specific PCR primers directed against the inserted transgene as previously described (Fentzke et al. 1999). Hearts from TG mice showed full stoichiometric replacement of cTnI with ssTnI (Fentzke et al. 1999). These TG mice are known to be fertile and viable, and show no signs of increased mortality or cardiovascular pathology up to 18 months of age (Fentzke et al. 1999). In the present study, male TG or NTG littermates, age 6–10 weeks, were killed by an overdose of sodium pentobarbitone (120 mg kg−1i.p.).
Langendorff-perfused hearts
Hearts were retrogradely perfused with Krebs-Henseleit buffer (KHB) containing (mm): NaCl 118, KCl 3.8, KH2PO4 1.18, NaHCO3 25, MgSO4 1.19, CaCl2 1.25, glucose 10, sodium pyruvate 5.0, ascorbic acid 0.03, and EGTA 0.01; and bubbled with 95% O2–5% CO2 at 37°C. A constant coronary flow rate was used, adjusted to achieve a perfusion pressure of 75 mmHg. Hearts were paced at 588 beats min−1 via the atria. Isovolumic left ventricular pressure (LVP) was measured using a water-filled polythene balloon inserted into the LV. The balloon was inflated in 5 μl increments and LVP measured at each volume. Data were sampled at 1 kHz via a PowerLab module (AD Instruments, Hastings, UK) running Chart software (version 4.1.2). Measurements of maximum left ventricular pressure (max LVP), minimum LVP (min LVP), LV end-diastolic pressure (LVEDP) and LV developed pressure (LVDP = max LVP – LVEDP) were derived from the resulting LVP trace. The Chart software also displayed the derivative of LVP on-line, from which the maximum rates of LVP rise (LV dP/dtmax) and decline (LV dP/dtmin) could be measured. Individual hearts were exposed to either isoprenaline (Iso, 10 nm) or saline.
Isolated ejecting hearts
Hearts were initially perfused in Langendorff mode with KHB containing 1.5 mm CaCl2 at 37°C and a perfusion pressure of 50 mmHg, and paced at 500 beats min−1 via the right atrium. A custom-made 1.4 F microconductance catheter-manometer (SPR-853, Millar Instruments, Houston, TX, USA) was positioned in the LV via the apex to record pressure and volume. As for Langendorff experiments, pressure and volume data were sampled at 1 kHz via a PowerLab module (AD Instruments) running Chart software (version 4.1.2). The catheter was calibrated for each heart using a series of fluid-filled reservoirs of known volume. Parallel conductance was measured and corrected for, following the method described by Baan et al. (1981). The left atrium was cannulated via the largest pulmonary vein and the heart was switched to ejecting mode with afterload set at 80 cmH2O using a hydrostatic column. Contractile parameters were assessed after 20 min stabilization. Left atrial pressure (preload) was varied between 10 and 25 cmH2O to generate Starling curves. Conductance data were analysed using Millar Aria software (Millar Instruments). For individual hearts, LV pressures and volumes at each preload were measured under baseline conditions and then following exposure to Iso (10 nm). Measurements of LVDP, LVEDP, min LVP, LV dP/dtmax and LV dP/dtmin were made as described for Langendorff-perfused hearts. τ, the time constant of isovolumic left ventricular pressure decline, was calculated by fitting an exponential to the decline of pressure during isovolumic relaxation using Millar Aria software. LV end-systolic volume and end-diastolic volume were measured using the Millar Aria software and used to calculate stroke volume (end-diastolic volume – end-systolic volume) and ejection fraction (i.e. stroke volume as a percentage of end-diastolic volume). LV stroke work was calculated by the Millar Aria software as the area enclosed within the pressure–volume loop and expressed per unit LV wet weight (mg).
Isolated cardiomyocytes
Murine ventricular myocytes were isolated essentially as described by Terracciano et al. 1998). Briefly, hearts were perfused with a Tyrode solution containing 1.25 mm CaCl2 at 50–60 mmHg perfusion pressure for 3 min; then with a ‘low calcium’ solution containing 20 mm taurine and 10 mm 2,3-butanedione monoxime for 5 min; followed by an ‘enzyme solution’ containing 0.15 mg ml−1 protease Type XXIV (Sigma) for 1 min and finally a second ‘enzyme solution’ containing 0.05 mg ml−1 collagenase (Type II, Worthington, 268 U mg−1) and 0.125 mg ml−1 hyaluronidase (Sigma) for 8 min. The ventricles were then removed and cut into small pieces. The tissue was digested for a further 3–6 min in the collagenase and hyaluronidase solution, followed by gentle trituration and filtering through a nylon mesh. Collagenase and hyaluronidase were removed from the cell suspensions by gentle centrifugation (30 g, 1 min) and removal of supernatant. The final myocyte suspension was stored at room temperature in a Hepes-buffered solution containing 0.2 mm CaCl2 (no 2,3-butanedione monoxime) and used within 5–6 h. Some cells were loaded with the acetoxymethyl ester form of the fluorescent Ca2+ indicator indo-1 (indo-1 AM; 2 μm) as previously described (Layland et al. 2002). Indo-1-loaded myocytes were protected from light until use. This collagenase digestion protocol typically yielded 60–70% rod-shaped, viable, Ca2+-tolerant myocytes.
Single myocyte contractility and indo-1 fluorescence were studied on the stage of an inverted fluorescence microscope (Nikon Diaphot) coupled to a dual emission spectrophotometer (Cairn Research, Faversham, Kent) as previously described (Layland et al. 2002). Myocytes were superfused at 1–2 ml min−1 with Hepes buffer containing (mm) NaCl 117, KCl 5.7, NaH2PO4 1.2, MgSO4 0.66, glucose 10, sodium pyruvate 5, creatine 10, Hepes 20, ascorbic acid 0.03, EGTA 0.01, CaCl2 1.25, pH 7.4. All experiments were performed at 32 ± 0.5°C. Myocytes were field stimulated at 1 Hz (isolated stimulator unit model S48, Grass Instrument Co., Quincy, MA, USA) via two platinum electrodes positioned on either side of the chamber. Myocytes were observed using a × 40 oil immersion Fluor objective (Nikon, NA 1.3) and the image relayed via a video camera and frame grabber to a PC running IonWizard software (IonOptix Corp. Milton, MA, USA). Cell contractility was assessed by measuring the changes in sarcomere spacing using an IonOptix algorithm. Myocytes were selected for study according to previously established criteria (Capogrossi et al. 1986). Baseline contractility or Ca2+ transients were recorded following a 10 min period of stabilization with continuous stimulation at 1 Hz. The effects of 10 nm Iso on contractility or Ca2+ transients were assessed after a 10–15 min period of exposure to the drug. Differences in the intracellular Ca2+ transients were assessed as relative changes in the indo-1 410 nm/480 nm ratio. Typical maximal indo-1 fluorescence ratios in isolated mouse cardiomyocytes during tetanic contractions (10 Hz stimulation, sarcoplasmic reticulum inhibited with 1 μm thapsigargin, extracellular Ca2+ raised to 10 mm) were 2.2 ± 0.06 (n = 3), well above the indo-1 ratios recorded after exposure to isoprenaline.
Steady-state twitches and Ca2+ transients were averaged over 30 s periods. Cell twitch amplitude was expressed as percentage of diastolic sarcomere length. Twitch kinetics were quantified by measuring the time to peak shortening and the time from peak shortening to 50% relaxation (RT50). Comparable measurements were derived to quantify Ca2+ transient kinetics.
Chemicals and solutions
All chemicals used were of analytical grade and were obtained from BDH (Poole, Dorset, UK) or Sigma (Poole, Dorset, UK). Collagenase type II was purchased from Worthington Biochemical Corporation (Twyford, Reading, UK). Indo-1 AM was obtained from Calbiochem (Nottingham, UK) or Molecular Probes (Eugene, OR, USA).
Statistics
All data are presented as mean ±s.e.m. Student's paired or unpaired t tests were used for comparison of averaged myocyte twitch and transient data. Curves were compared using repeated measures (RM) ANOVA. Statistical analyses were performed using StatView (version 5) and P < 0.05 was considered statistically significant with P = n.s. indicating no significant difference.
Results
TG (n = 25) and NTG (n = 26) animals had similar body weights (31.8 ± 5.0 g and 33.7 ± 1.2 g, respectively; P = n.s.) and heart/body weight ratios (4.69 ± 0.05 and 4.72 ± 0.07, respectively; P = n.s.).
Isovolumic Langendorff hearts
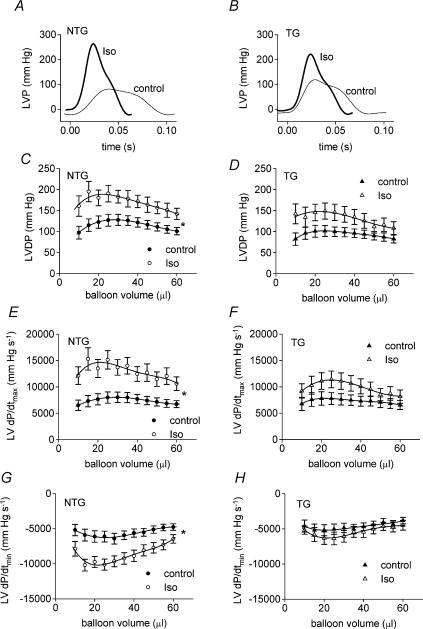
Examples of left ventricular pressure traces (25 μl balloon volume) for baseline contractility and during stimulation with Iso are illustrated in Fig. 1A (NTG) and B (TG). Coronary flows were similar in TG and NTG hearts (data not shown). There were no statistically significant differences between NTG and TG hearts in baseline LV developed pressure (LVDP), LV dP/dtmax, LV dP/dtmin, minimum LVP or LV end-diastolic pressure (LVEDP) across a range of LV end-diastolic volumes (Figs 1 and 2). TG hearts tended to have a lower LV dP/dtmin than NTG hearts at all ventricular volumes under baseline conditions but this was not statistically significant (RM ANOVA, P = 0.211).
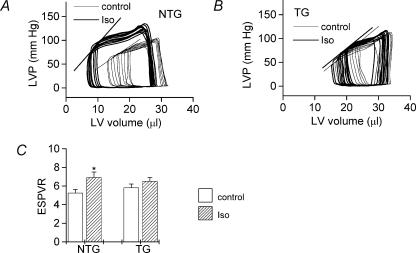
Figure 1.
Examples of isovolumic pressure traces measured during Langendorff experiments (25 μl balloon volume) in NTG (A) and TG (B) hearts. Thin lines indicate the pressure signal in baseline conditions and thick lines indicate the signal during β-adrenergic stimulation with isoprenaline (10 nm). Effects of isoprenaline (Iso, 10 nm) on LV developed pressure (LVDP, C and D), LV dP/dtmax (E and F) and LV dP/dtmin (G and H) in isolated Langendorff-perfused isovolumic hearts from non-transgenic (NTG) mice (C, E and G) and ssTnI-expressing transgenic (TG) mice (D, F and H). *P < 0.05 for difference between curves.
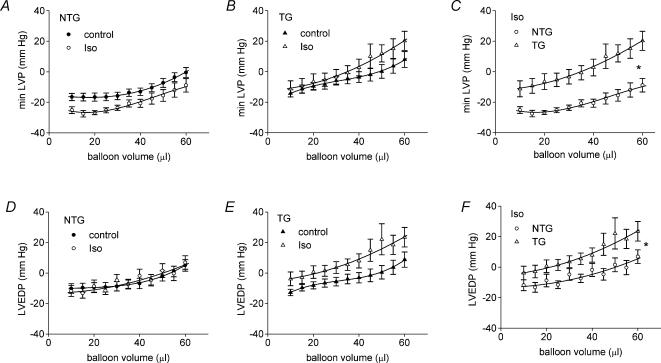
Figure 2.
Effects of isoprenaline (Iso, 10 nm) on diastolic properties in isolated Langendorff-perfused hearts. NTG, non-transgenic; TG, ssTnI-expressing transgenic. *P < 0.05 for difference between curves.
Stimulation of NTG hearts with the β-adrenoceptor agonist Iso (10 nm) substantially increased LVDP, LV dP/dtmax and LV dP/dtmin (Fig. 1C, E and G). As expected, the lusitropic effect of Iso (manifest as a greater LV dP/dtmin,Fig. 1G) was markedly inhibited in TG hearts (Fig. 1H). Accordingly dP/dtmin was significantly lower in TG hearts compared to NTG hearts during stimulation with Iso (P < 0.01, RM ANOVA). Surprisingly, the increases in LVDP and LV dP/dtmax with Iso were not statistically significant in the TG hearts (Fig. 1D and F), suggesting a blunting of the positive inotropic effect of Iso in hearts expressing ssTnI. In the presence of Iso, dP/dtmax tended to be higher in NTG compared to TG hearts (compare Fig. 1E and F), although the differences between the curves were not statistically significant (P = 0.074, RM ANOVA).
Iso tended to reduce minimum LVP in NTG hearts (Fig. 2A) whereas in TG hearts both minimum LVP and LVEDP tended to be increased by Iso (Fig. 2B and E). Consequently, in the presence of Iso, minimum LVP and LVEDP were both significantly higher in TG hearts compared with NTG hearts (Fig. 2C and F). These data suggest that cTnI in the NTG hearts allows for more complete relaxation between beats during β-adrenergic stimulation. The increased EDP in the TG hearts most probably reflects an increase in myofilament Ca2+ response to the elevated intracellular [Ca2+] during β-stimulation, since the ssTnI TG is known to be more Ca2+ sensitive than NTG (Fentzke et al. 1999).
Isolated auxotonically loaded ejecting hearts
Contractile parameters under baseline conditions (1.5 mm CaCl2) in TG and NTG ejecting hearts are shown in Table 1. There were no significant differences between groups apart from the time constant of isovolumic relaxation, τ, which was prolonged in TG hearts. As in the Langendorff-perfused hearts, LV dP/dtmin tended to be reduced in TG compared to NTG hearts but this was not found to be statistically significant (RM ANOVA, P = 0.14). The increases in LV end-diastolic volume produced by raising preload were not significantly different between NTG and TG hearts.
Table 1.
Baseline parameters in isolated ejecting hearts of non-transgenic (NTG) and ssTnI-expressing transgenic animals (TG) in either 1.5 mm or 3 mm bathing Ca2+ (in the absence of isoprenaline)
| Baseline 1.5 mm Ca2+ | Baseline 3.0 mm Ca2+ | |||
|---|---|---|---|---|
| NTG (n = 6) | TG (n = 7) | NTG (n = 6) | TG (n = 6) | |
| Peak LVP (mmHg) | 90.0 ± 5.0 | 89.8 ± 2.0 | 95.5 ± 1.4 | 94.0 ± 1.5 |
| LV dP/dtmax (mmHg s−1) | 7319 ± 949 | 7366 ± 525 | 7908 ± 336 | 8196 ± 214 |
| LV dP/dtmin (mmHg s−1) | −3943 ± 242 | −3255 ± 292 | −3394 ± 189 | −3131 ± 151 |
| LVEDP (mmHg) | 3.2 ± 0.6 | 3.3 ± 0.2 | 4.0 ± 0.8 | 4.8 ± 0.9 |
| τ (ms) | 6.9 ± 0.5 | 11.0 ± 1.1* | 9.0 ± 0.9 | 11.7 ± 2.1 |
| Coronary blood flow (ml min−1 g−1) | 35.2 ± 4.2 | 38.3 ± 3.9 | 36.7 ± 3.8 | 40.7 ± 2.3 |
| LV end-systolic volume (μl) | 10.6 ± 1.6 | 11.4 ± 1.3 | 6.2 ± 0.7 | 9.7 ± 0.8* |
| LV ejection fraction (%) | 64.3 ± 4.3 | 63.9 ± 3.6 | 81.5 ± 1.9 | 70.5 ± 2.2* |
| LV stroke work (mmHg μl mg−1) | 14.5 ± 1.0 | 15.3 ± 1.3 | 19.3 ± 1.1 | 17.4 ± 0.7 |
| ESPVR | 5.3 ± 0.3 | 5.8 ± 0.4 | 6.6 ± 0.4 | 6.7 ± 0.4 |
Data shown were measured at a preload of 20 cmH2O.
P < 0.05 for TG versus NTG at equivalent bathing [Ca2+]. LVP, left ventricular pressure; LV dP/dtmax and LV dP/dtmin, maximum rates of LVP rise and decline, respectively; LVEDP, LV end-diastolic pressure; ESPVR, end-systolic pressure–volume relationship.
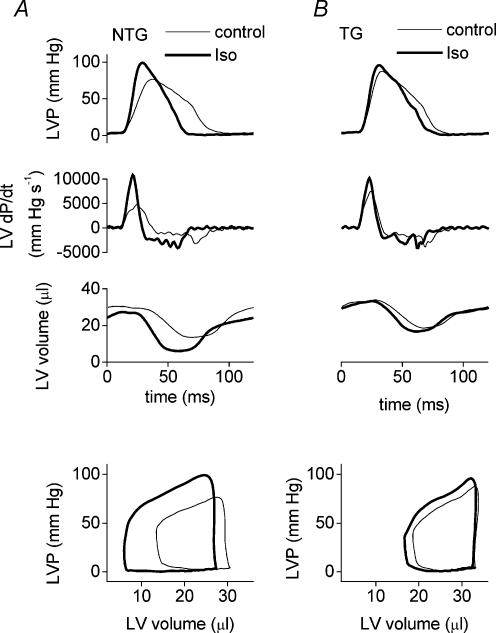
Figure 3 shows typical traces for left ventricular pressure (top pair of panels), the derivative of left ventricular pressure (second set of panels), and left ventricular volume (third set of panels) under baseline conditions and after Iso for a typical NTG heart (Fig. 3A) and TG heart (Fig. 3B) recorded by the Millar conductance catheter during ejecting heart experiments (20 cmH2O preload). The bottom pair of panels of Fig. 3A and B show the corresponding steady-state pressure–volume loops derived from the pressure and volume records illustrated above.
Figure 3.
Examples of LV pressure traces (top set of panels), their derivatives (second set of panels), and the corresponding LV volume (third set of panels) recorded by a Millar microconductance catheter-manometer during ejecting heart experiments at 20 cmH2O preload. The bottom set of panels illustrate the steady-state pressure–volume loops derived from the instantaneous LV pressure and volume traces above (first and third panels). Thin lines indicate baseline conditions and thick lines indicate the signal during stimulation with isoprenaline (10 nm).
Iso significantly increased LV dP/dtmax and LV dP/dtmin at all ventricular volumes in both NTG and TG hearts. As expected, in the presence of Iso LV dP/dtmin was significantly lower at all preloads in TG hearts compared to NTG (RM ANOVA, P < 0.05, Fig. 4B), consistent with a critical role for cTnI phosphorylation in the lusitropic effect of Iso in the ejecting mouse heart. The increase in LV dP/dtmax with Iso also appeared to be greater in the NTG group than the TG (Fig. 4A), similar to our findings in the Langendorff experiments. However, comparison of the LV dP/dtmax curves of NTG hearts and TG hearts in the presence of Iso did not reveal a statistically significant difference (P = 0.15, RM ANOVA).
Figure 4.
Effect of isoprenaline (Iso, 10 nm) on LV dP/dtmax (A) and LV dP/dtmin (B) in isolated ejecting hearts from non-transgenic (NTG) mice (left panel) and ssTnI-expressing trangenic mice (TG, right panel). *P < 0.05 for difference between curves.
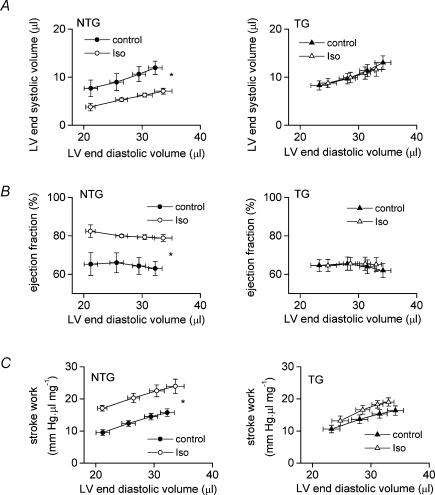
Figure 3 illustrates that LV dP/dtmax is achieved during the isovolumic phase of the heart beat in the ejecting heart. Although the effects of Iso on LV dP/dtmax were not significantly different between NTG and TG hearts, striking differences in the response to Iso were revealed when we examined indices relating to the ejection phase (i.e. end-systolic volume, ejection fraction and stroke work, Figs 5 and 3). Iso significantly reduced LV end-systolic volume and increased LV ejection fraction in NTG hearts but both these effects were completely abolished in TG hearts (Fig. 5A and B; and Fig. 3, third set of panels). Consequently, in the presence of Iso, LV end-systolic volume was significantly lower (RM ANOVA, P < 0.01) and ejection fraction significantly higher (RM ANOVA, P < 0.01) in NTG than TG hearts at all preloads. Hence, NTG hearts shortened more during the ejection phase in response to β-adrenoceptor stimulation than did TG hearts.
Figure 5.
Effect of isoprenaline (Iso, 10 nm) on LV end-systolic volume (A), ejection fraction (B) and stroke work (C) in isolated ejecting hearts from non-transgenic (NTG, left panel) mice and ssTnI-expressing transgenic mice (TG, right panel). *P < 0.05 for difference between curves.
As illustrated in the steady-state LV pressure–volume (P–V) loops (Fig. 3, bottom panels), Iso increased both systolic pressure and extent of ejection in the NTG heart, resulting in a much larger P–V loop. In contrast, the P–V loop was little altered in the TG heart. Figure 5C shows data for LV stroke work calculated from the area enclosed within steady-state P–V loops. Iso significantly increased stroke work at each preload in NTG hearts whereas stroke work was not significantly altered in TG hearts. In the presence of Iso, stroke work tended to be higher in NTG than TG hearts but this was not quite significant (P = 0.059, RM ANOVA).
The effect of Iso on the LV end-systolic pressure–volume relationship (ESPVR) was also determined (Fig. 6) from P–V loops recorded during ∼5 s of aortic occlusion to produce beat-to-beat changes in load (Georgakopoulos et al. 1998). The ESPVR was generated by fitting a line through the end-systolic points of sequential pressure–volume loops recorded during the occlusion period (e.g. see Fig. 6A and B) using custom-designed software (Aria, Millar Instruments), the slope of the line representing a load-independent index of contractility. Iso significantly increased the slope of the ESPVR in NTG hearts but not in TG hearts (Fig. 6C). The slope of the ESPVR under baseline conditions appeared to be greater for TG than for NTG hearts (Fig. 6C) but this difference was not statistically significant (P > 0.05).
Figure 6.
Examples illustrating the effects of isoprenaline (Iso, 10 nm) on LV pressure–volume relations in isolated ejecting hearts from non-transgenic mice (NTG, A) and ssTnI-expressing transgenic mice (TG, B). Thin lines indicate baseline conditions and thick lines indicate the signal during stimulation with isoprenaline (10 nm). Averaged data for the slope of the end-systolic pressure volume relation (ESPVR) is given in C. Open columns, baseline; hatched columns, isoprenaline. *P < 0.05 for difference between groups.
Isolated cardiomyocytes
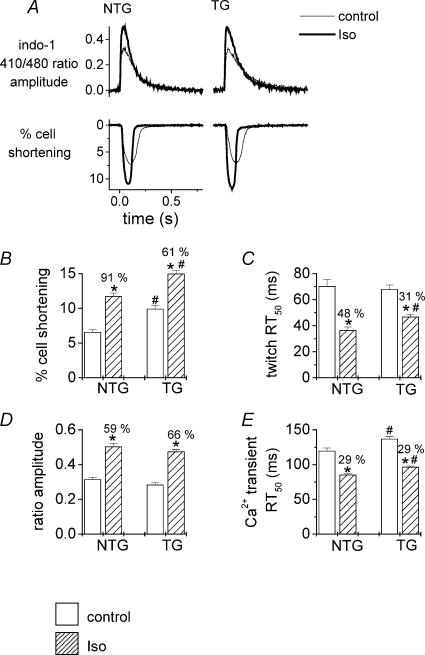
In order to exclude the possibility that the differing response to Iso between NTG and TG hearts may reflect differences in the effects of Iso on intracellular Ca2+ transients, we measured cell sarcomere shortening and indo-1 fluorescence transients in single cardiomyocytes. Examples of typical twitches and Ca2+ transients for baseline and Iso are illustrated in Fig. 7A (left panel, NTG, and right panel, TG). Baseline unloaded sarcomere shortening was significantly greater in TG compared with NTG myocytes (Fig. 7B), as previously described in this model (Fentzke et al. 1999). Iso increased sarcomere shortening by 91% in NTG myocytes and 61% in TG myocytes (Fig. 7B), although the final amplitude of sarcomere shortening with Iso was still significantly higher in TG than NTG myocytes.
Figure 7.
A, examples of Ca2+ transients (indo-1 fluorescence ratio, top panel) and cell shortening traces (bottom panel) from typical myocytes isolated from non-transgenic hearts (NTG, left panel) or ssTnI-expressing transgenic hearts (TG, right panel). Thin lines indicate baseline conditions and thick lines indicate the signal during stimulation with isoprenaline (10 nm). Average data illustrating the effects of isoprenaline (10 nm) on isolated cardiac myocyte shortening (B and C) and indo-1 fluorescence transients (D and E). NTG, non-transgenic mice; TG, ssTnI-expressing trangenic mice. RT50, time from peak to 50% relaxation. Open columns, baseline; hatched columns, isoprenaline. *P < 0.05 for difference between groups.
Iso increased the peak indo-1 410 nm/480 nm ratio in both NTG and TG myocytes, i.e. from 1.19 ± 0.02 to 1.45 ± 0.03 for NTG (P < 0.01) and from 1.19 ± 0.03 to 1.50 ± 0.04 for TG (P < 0.01). Iso also produced a small increase in diastolic indo-1 410 nm/480 nm ratio in both NTG (from 0.87 ± 0.01 to 0.94 ± 0.01, P < 0.01) and TG myocytes (from 0.91 ± 0.02 to 1.03 ± 0.03, P < 0.01). Hence, Iso increased the amplitude (peak ratio – diastolic ratio) of the indo-1 fluorescence transient to a similar level in TG and NTG myocytes (Figs 7D, P = n.s.). Furthermore, the baseline transient in the absence of Iso was also similar between groups (P = n.s.). Hence, the observed differences in contractility between unloaded NTG and TG myocytes most likely reflect an increased myofilament Ca2+ responsiveness in the TG cells expressing ssTnI, as previously reported (e.g. Fentzke et al. 1999).
In baseline conditions, there was no significant difference in twitch relaxation times (assessed as the time from peak twitch to 50% relaxation, twitch RT50) between NTG and TG cells (Fig. 7C). Iso had greater lusitropic effects in NTG cells compared with TG cells, reducing twitch RT50 by 48% in NTG myocytes compared with 31% in TG myocytes (Fig. 7C). The average reduction in twitch RT50 by Iso was 33 ± 4 ms (n = 35 cells) in NTG and 21 ± 2 ms (n = 33 cells) in TG (P < 0.01). In contrast, Iso accelerated Ca2+ transient decline (reduced transient RT50) to a similar extent in both NTG and TG myocytes (Fig. 7D), indicating that the alterations in twitch relaxation were not attributable to differing effects on Ca2+ transient kinetics but most likely reflect differing myofilament properties between NTG and TG. Interestingly, the Ca2+ transient RT50 was slightly prolonged in TG myocytes compared to NTG myocytes (baseline and Iso) as previously reported (Fentzke et al. 1999).
Response to elevated bathing [Ca2+]per se in isolated ejecting hearts
It is well documented that the expression of ssTnI in cardiac myocytes increases myofilament Ca2+ sensitivity (e.g. Fentzke et al. 1999; Kentish et al. 2001) and, as described above, this would account for the increased contractility of TG compared to NTG myocytes under externally unloaded conditions, despite similar Ca2+ transient amplitudes (Fig. 7). Paradoxically, it could also be argued that an increase in baseline myofilament Ca2+ sensitivity in TG ejecting hearts may limit their ability to respond to an increased Ca2+ transient upon stimulation with Iso since they may be operating near the peak of the force–Ca2+ relationship under baseline conditions and hence have little contractile reserve (opposite to what was found in the isolated cardiomyocyte setting). Contrary to this hypothesis, there were no baseline differences in indices of systolic function in TG compared to NTG hearts (Table 1). Furthermore, Iso produced a significant increase in LV dP/dtmax in both NTG and TG ejecting hearts (Fig. 4A), the major differences in inotropic responsiveness only becoming apparent when ejection phase indices were examined (Fig. 5A, B and C). Nevertheless, in order to assess the capacity of the TG ejecting heart to respond to increased [Ca2+]per se, contractile function was compared between NTG and TG hearts when perfusate Ca2+ concentration was increased to 3 mm (Table 1). An increase in bathing [Ca2+] significantly increased peak LVP, LV dP/dtmax, LV ejection fraction, LV stroke work and ESPVR and significantly decreased LV end-systolic volume in TG as well as NTG hearts (all P < 0.05). There were no significant differences between NTG and TG hearts in peak LVP, LV dP/dtmax, LV stroke work and ESPVR in 3 mm bathing Ca2+. However, the ejection fraction was significantly lower in TG than NTG hearts at 3 mm Ca2+, despite the increase in the former group. Taken together, these data indicate that TG ejecting hearts are not operating at maximal inotropic state and are indeed capable of exhibiting a positive inotropic response to increased [Ca2+]per se, although their capacity to shorten against a load during the ejection phase is somewhat reduced compared to NTG hearts.
Discussion
It is well established that cTnI is phosphorylated by PKA in response to β-adrenergic stimulation, and that this is associated with a reduction in myofilament Ca2+ sensitivity and an acceleration of myocardial relaxation (reviewed by Solaro, 2001). The phosphorylation of cTnI by PKA also increases crossbridge cycling rate and maximum shortening velocity (e.g. Hoh et al. 1988; Strang et al. 1994; Kentish et al. 2001). However, the functional significance of these latter changes, especially for the contractile performance of the whole heart, remain unclear. In the present study we report the novel finding that, under conditions resembling in vivo auxotonic contraction, cTnI plays a major role not only in the lusitropic effects of β-adrenoceptor stimulation but also in the positive inotropic response of the whole heart to a β-adrenoceptor agonist. Furthermore, the contribution of cTnI to the β-adrenoceptor-induced positive inotropic effect is highly dependent on loading conditions, being most evident for ejection-phase indices in the auxotonically contracting ejecting heart and much less apparent in the isovolumic heart or in externally unloaded isolated cardiomyocyte.
Novel role for cTnI in positive inotropic responsiveness in the ejecting heart
In isolated myocytes performing externally unloaded shortening, stimulation of the β-adrenergic receptors produced significant positive inotropic effects in both NTG and TG groups (Fig. 7). For a similar increase in Ca2+ transient amplitude, Iso increased twitch contraction (% cell shortening) to a slightly greater extent in TG than NTG myocytes (Fig. 7), reflecting an increased myofilament Ca2+ responsiveness when ssTnI is expressed (Fentzke et al. 1999; Kentish et al. 2001; Wolska et al. 2002; Konhilas et al. 2003). These results are fully in accordance with previous studies (e.g. Fentzke et al. 1999; Pi et al. 2002) in that cTnI phosphorylation is clearly not a critical requirement for the increase in unloaded cell shortening following β-adrenergic stimulation.
However, no previous studies have examined the role of cTnI in the positive inotropic response to β-adrenergic stimulation under conditions of auxotonic loading, i.e. conditions analogous to those that occur in vivo. In the current study, dramatic differences between the positive inotropic responsiveness of NTG and TG ejecting hearts were found for ejection phase indices. Hence, the effects of Iso to reduce LV end-systolic volume and to increase ejection fraction and stroke work in NTG hearts were severely blunted in TG hearts (Fig. 5). Furthermore, the Iso-induced increase in ESPVR observed in NTG hearts was also significantly blunted in TG hearts. ESPVR represents probably the best index of systolic function in the ejecting heart (Georgakopoulos et al. 1998), taking into account both developed pressure and extent of shortening at the end of ejection. In contrast, the effect of Iso on isovolumic indices of systolic function (such as LV dP/dtmax) was much more similar between NTG and TG hearts, both in the Langendorff-perfused heart and in the ejecting heart. Collectively, these observations lead us to propose a novel role for cTnI in determining positive inotropic responsiveness to Iso during loaded shortening, as occurs in the ejection phase.
Mechanisms underlying reduced positive inotropic responsiveness to Iso in TG hearts
Given the increased basal myofilament Ca2+ sensitivity of TG mice, it may be thought paradoxical that ejecting TG hearts should exhibit blunted positive inotropic responses to Iso. An increased myofilament Ca2+ sensitivity might predict an enhanced positive inotropic response to the increased Ca2+ transient following β-stimulation, especially since PKA-mediated reduction in myofilament Ca2+ sensitivity is lost in the ssTnI-overexpressing mice (Fentzke et al. 1999; Konhilas et al. 2003). In the following discussion we consider possible reasons why expression of ssTnI in place of cTnI could reduce loaded shortening during β-stimulation.
The blunted positive inotropic effect of Iso in the ejecting TG hearts cannot be explained by a reduction in β-adrenoceptor density, since this is known to be unaltered in the ssTnI-expressing mice (Wolska et al. 2002). It is also unlikely to reflect a reduced effect of β-adrenoceptor stimulation on intracellular Ca2+ transients, since cardiac myocytes isolated from NTG and TG hearts had similar Ca2+ transient amplitudes in the presence of Iso (Fig. 7A and D). However, it should be noted that Ca2+ transients were assessed during unloaded cell shortening at 1 Hz and 32°C, conditions found to be optimal for the stability of isolated mouse myocytes, but differing significantly from those used for ejecting heart experiments (8.33 Hz, 37°C, physiological loading). It is therefore possible, although unlikely, that Iso-induced rises in intracellular [Ca2+] in the ejecting heart may have been different in TG compared to NTG.
Another possibility to consider is that contractile reserve could be compromised by the increased Ca2+ responsiveness of TG hearts since they could be operating at (or close to) the maximum of their force–Ca2+ relationship under baseline conditions. However, the isolated myocyte data clearly demonstrate that TG cells do have significant contractile reserve (Fig. 7). Furthermore, there were no baseline differences in systolic function between NTG and TG hearts in the absence of Iso (Table 1). TG ejecting hearts also demonstrated significant contractile reserve since Iso significantly increased LV dP/dtmax, an isovolumic index of contractility (Fig. 4A). Finally, increasing bathing [Ca2+]per se (i.e. independent of PKA-induced phosphorylation) also produced significant increases in LV dP/dtmax, ejection fraction and ESPVR in TG hearts (Table 1), indicating that elevated myofilament Ca2+ sensitivity is not a limiting factor.
Considering the above, perhaps the most plausible explanation for the differing effects of β-stimulation during ejection in NTG and TG hearts is a lack of TnI phosphorylation in TG hearts. Although cTnI phosphorylation was not directly assessed in the present study, it has previously been shown that there is no detectable Iso-induced TnI phosphorylation in the ssTnI transgenic mouse heart, which lacks the PKA-sensitive phosphorylation sites present in the N-terminal of cTnI (Fentzke et al. 1999; Kentish et al. 2001). Evidence suggests that phosphorylation of cTnI by PKA increases crossbridge cycling rate and maximum shortening velocity (e.g. Hoh et al. 1988; Strang et al. 1994) and that these effects are abolished in the ssTnI TG mice (Fentzke et al. 1999; Kentish et al. 2001). An increased shortening velocity following β-stimulation in the NTG hearts would allow a greater extent of shortening during ejection than in TG hearts (velocity unchanged), as observed in the present study (Fig. 5A). This would explain the lack of effect of β-stimulation on LV end-systolic volume and ejection fraction in TG hearts.
The expression of ssTnI per se (independent of differences in PKA-dependent phosphorylation) is unlikely to account for the difference in shortening velocity since previous studies found no differences in the maximum rate of unloaded shortening (Fentzke et al. 1999) or intrinsic rate of crossbridge cycling (Kentish et al. 2001) between NTG and TG hearts in the absence of β-adrenergic stimulation. Likewise, in the present study, there were no significant differences in LV end-systolic volumes (reflecting the extent of shortening) or ejection fraction between NTG and TG hearts in the absence of Iso (Fig. 5A and B, Table 1). We did observe, however, that although ssTnI TG hearts demonstrated positive inotropic responses to an increase in bathing [Ca2+], the absolute values of ejection fraction were significantly lower in TG compared to NTG hearts. This suggests either (a) that the presence of ssTnI per se may to some extent contribute to a reduced inotropic responsiveness during loaded shortening, independent of differences in PKA-mediated phosphorylation, or (b) that a low level of cTnI phosphorylation in the absence of added Iso contributes to positive inotropic effects during loaded shortening in the normal setting. In order to distinguish between these possibilities, it would be necessary to undertake studies in transgenic mice expressing mutant TnI lacking PKA-sensitive phosphorylation sites but with no other differences compared to native cTnI. The current results leave open the possibility that part of the difference between the positive inotropic response to Iso in TG and NTG hearts may reflect a more general difference between cTnI and ssTnI. However, regardless of precise underlying mechanisms, it is clear from the data presented that the cTnI isoform is a critical requirement in the positive inotropic response to β-adrenergic stimulation in the auxotonically loaded ejecting heart.
Contractile reserve in the murine heart
Several recent studies have suggested that the increase in contractility in response to β-adrenergic stimulation in murine hearts is relatively small compared to other species (Michele et al. 2002; Stull et al. 2002). In contrast, the present study clearly demonstrated a substantial increase in systolic function following β-adrenergic stimulation of isolated myocytes, Langendorff-perfused hearts and isolated ejecting hearts alike. Although the reasons for these differences are not entirely clear, at least part of the difference may reflect high pre-existing sympathetic activity during in situ assessment (e.g. by catheterization in the study by Michele et al. 2002) whereas the use of isolated preparations in the present study minimizes these influences.
Role of cTnI phosphorylation in the lusitropic response to β-stimulation
The effect of β-adrenergic stimulation to accelerate relaxation is believed to involve a combination of an increased rate of sarcoplasmic reticulum Ca2+ uptake due to phospholamban phosphorylation, and a reduction in myofilament Ca2+ sensitivity and an increase in crossbridge cycling rate due to cTnI phosphorylation (Koss & Kranias, 1996; Solaro, 2001). Previous studies using gene-modified models in which the effects of phospholamban phosphorylation or cTnI phosphorylation can be studied independently have confirmed the contribution of both these mechanisms (e.g. Fentzke et al. 1999; Li et al. 2000; Kentish et al. 2001; Pi et al. 2002; Wolska et al. 2002). The present study also confirmed the pivotal role of cTnI in the lusitropic effects of β-stimulation, not only in isolated myocytes but also in the more physiological ejecting heart preparation. In addition, we found that LVEDP and minimum LVP were higher in TG compared with NTG hearts after Iso, suggesting that cTnI phosphorylation contributes to a reduction in diastolic force during β-adrenergic stimulation, possibly by allowing more complete relaxation between beats.
Conclusion
In summary, this study reports the novel finding that the cTnI isoform is critical in the positive inotropic response to β-adrenergic stimulation in isolated hearts performing auxotonic contractions at physiological temperature and frequency. Our data indicate that the contribution of cTnI phosphorylation to the positive inotropic effect of Iso is highly dependent on loading conditions, such that it is most apparent when there is both pressure development and ejection (i.e. loaded shortening in ejecting hearts) than in isovolumic hearts or externally unloaded myocytes. These data underscore the importance of an integrative approach to the examination of myocardial contractile performance in a range of preparations. This is the first study to indicate that cTnI phosphorylation may influence systolic contractile performance in the intact ejecting heart in addition to its well-recognized lusitropic effects. The physiological and pathophysiological significance of this novel role of cTnI warrants further investigation.
Acknowledgments
We are grateful to Professor Sian Harding and Dr Cesare Terracciano for invaluable advice and assistance in setting up the protocol for mouse ventricular myocytes isolation. This work was supported by British Heart Foundation (BHF) Programme Grant RG/98008 (A.M.S.) and NIH R37 HL 22231 (R.J.S.). A.M.S. holds the BHF Chair of Cardiology in King's College London.
References
- Baan J, Aouw A, Jong TT, Kerkhof PLM, Moene RJ, Van Dijk AD, van der Velde ET, Koops J. Continuous stroke volume and cardiac output from intra-ventricular dimensions obtained with conductance catheter. Cardiovasc Res. 1981;15:328–334. doi: 10.1093/cvr/15.6.328. [DOI] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2nd edn. Dordrecht, The Netherlands: Kluwer, Academic Publishers; 2001. [Google Scholar]
- Bodor GS, Oakeley AE, Allen PD, Ladenson DL, Anderson PA. Troponin phosphorylation in the normal and failing adult human heart. Circulation. 1997;96:1495–1500. doi: 10.1161/01.cir.96.5.1495. [DOI] [PubMed] [Google Scholar]
- Brutsaert DL, Sys SU. Relaxation and diastole of the heart. Physiol Rev. 1989;69:1228–1315. doi: 10.1152/physrev.1989.69.4.1228. [DOI] [PubMed] [Google Scholar]
- Capogrossi MC, Kort AA, Spurgeon HA, Lakatta EG. Single adult rabbit and rat cardiac myocytes retain the Ca2+ and species dependent systolic and diastolic contractile properties of intact muscle. J General Physiol. 1986;88:589–613. doi: 10.1085/jgp.88.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, Martin AF, Solaro RJ, Moss RL, Leiden JM. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol. 1999;517:143–157. doi: 10.1111/j.1469-7793.1999.0143z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WD, Atar D, Liu YG, Perez NG, Murphy AM, Marban E. Role of troponin I proteolysis in the pathogenesis of stunned myocardium. Circ Res. 1997;80:393–399. [PubMed] [Google Scholar]
- Georgakopoulos D, Mitzner WA, Chen CH, Byrne BJ, Millar HD, Hare JM, Kass DA. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol. 1998;274:H1416–H1422. doi: 10.1152/ajpheart.1998.274.4.H1416. [DOI] [PubMed] [Google Scholar]
- Herron TJ, Korte FS, McDonald KS. Power output is increased after phosphorylation of myofibrillar proteins in rat skinned cardiac myocytes. Circ Res. 2001;89:1184–1190. doi: 10.1161/hh2401.101908. [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Rossmanith GH, Kwan LJ, Hamilton AM. Adrenaline increases the rate of cycling of crossbridges in rat cardiac muscle as measured by pseudo-random binary noise-modulated perturbation analysis. Circ Res. 1988;62:452–461. doi: 10.1161/01.res.62.3.452. [DOI] [PubMed] [Google Scholar]
- Huang XP, Pi YQ, Lee KJ, Henkel AS, Gregg RG, Powers PA, Walker JW. Cardiac troponin I gene knockout. A mouse model of myocardial troponin I deficiency. Circ Res. 1999;84:1–8. doi: 10.1161/01.res.84.1.1. [DOI] [PubMed] [Google Scholar]
- Janssen PML, Stull LB, Marban E. Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat. Am J Physiol Heart Circ Physiol. 2002;282:H499–H507. doi: 10.1152/ajpheart.00595.2001. [DOI] [PubMed] [Google Scholar]
- Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Phosphorylation of troponin-I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res. 2001;88:1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, de Tombe PP. Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol. 2003;547:951–961. doi: 10.1113/jphysiol.2002.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss KL, Kranias EG. Phospholamban: a prominent regulator of myocardial contractility. Circ Res. 1996;79:1059–1063. doi: 10.1161/01.res.79.6.1059. [DOI] [PubMed] [Google Scholar]
- Layland J, Kentish JC. Myofilament-based relaxant effect of isoprenaline revealed during work-loop contractions in rat cardiac trabeculae. J Physiol. 2002;544:171–182. doi: 10.1113/jphysiol.2002.022855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland J, Li J-M, Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in isolated cardiac myocytes. J Physiol. 2002;540:457–467. doi: 10.1113/jphysiol.2001.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, DeSantiago J, Chu GX, Kranias EG, Bers DM. Phosphorylation of phospholamban and troponin I in β-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol. 2000;278:H769–H779. doi: 10.1152/ajpheart.2000.278.3.H769. [DOI] [PubMed] [Google Scholar]
- Michele DE, Gomez CA, Hong KE, Westfall MV, Metzger JM. Cardiac dysfunction in hypertrophic cardiomyopathy mutant tropomyosin mice is transgene-dependent, hypertrophy-independent and improved by β-blockade. Circ Res. 2002;91:255–262. doi: 10.1161/01.res.0000027530.58419.82. [DOI] [PubMed] [Google Scholar]
- Pi Y, Kemnitz KR, Zhang D, Kranias EG, Walker JW. Phosphorylation of troponin I controls cardiac twitch dynamics. Evidence from phosphorylation site mutants expressed on a troponin-I null background. Circ Res. 2002;90:649–656. doi: 10.1161/01.res.0000014080.82861.5f. [DOI] [PubMed] [Google Scholar]
- Solaro RJ. Modulation of cardiac myofilament activity by protein phosphorylation. In: Page E, Fozzard H, Solaro RJ, editors. Handbook of Physiology, section 2, The Cardiovascular System. Vol. 1. New York: Oxford University Press; 2001. pp. 264–300. The Heart. [Google Scholar]
- Strang KT, Sweitzer NK, Greaser ML, Moss RL. β-Adrenergic receptor stimulation increases unloaded shortening velocity of skinned single ventricular myocytes from rats. Circ Res. 1994;74:542–549. doi: 10.1161/01.res.74.3.542. [DOI] [PubMed] [Google Scholar]
- Stull LB, Leppo MK, Marban E, Janssen PML. Physiological determinants of contractile force generation and calcium handling in mouse myocardium. J Mol Cell Cardiol. 2002;34:1367–1376. doi: 10.1006/jmcc.2002.2065. [DOI] [PubMed] [Google Scholar]
- Tavernier B, Li J-M, El-Omar MM, Lanone S, Yang Z-K, Trayer IP, Mebazaa A, Shah AM. Cardiac contractile impairment associated with increased phosphorylation of troponin I in endotoxaemic rats. FASEB J. 2000 doi: 10.1096/fj.00-0433fje. DOI: 10.1096/fj.00-0433fje. [DOI] [PubMed] [Google Scholar]
- Terracciano CMN, Desouza AI, Philipson KD, Macleod KT. Na+−Ca2+ exchange and sarcoplasmic reticular Ca2+ regulation in ventricular myocytes from transgenic mice overexpressing the Na+−Ca2+ exchanger. J Physiol. 1998;512:651–667. doi: 10.1111/j.1469-7793.1998.651bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall MV, Borton AR, Albayya FP, Metzger JM. Myofilament calcium sensitivity and cardiac disease. Insights from troponin I isoforms and mutants. Circ Res. 2002;91:525–531. doi: 10.1161/01.res.0000034710.46739.c0. [DOI] [PubMed] [Google Scholar]
- Wolska BM, Arteaga GM, Pena JR, Nowak G, Phillips RM, Sahai S, de Tombe PP, Martin AF, Kranias EG, Solaro RJ. Expression of slow skeletal troponin I in hearts of phospholamban knockout mice alters the relaxant effect of β-adrenergic stimulation. Circ Res. 2002;90:882–888. doi: 10.1161/01.res.0000016962.36404.04. [DOI] [PubMed] [Google Scholar]