Abstract
Gibberellins (GAs) in developing seeds of morning glory (Pharbitis nil) were quantified and localized by immunostaining. The starch grains began to be digested after the GA contents had increased and reached a plateau. Immunohistochemical staining with the antigibberellin A1-methyl ester-antiserum, which has high affinity to biologically active GAs, showed that GA1 and/or GA3 were localized around starch grains in the integument of developing young seeds, suggesting the participation of GA-inducible α-amylase in this digestion. We isolated an α-amylase cDNA (PnAmy1) that was expressed in the immature seeds, and using an antibody raised against recombinant protein, it was shown that PnAmy1 was expressed in the immature seeds. GA responsiveness of PnAmy1 was shown by treating the young fruits 9 d after anthesis with GA3. RNA-blot and immunoblot analyses showed that PnAmy1 emerged soon after the rapid increase of GA1/3. An immunohistochemical analysis of PnAmy1 showed that it, like the seed GA1/3, was also localized around starch grains in the integument of developing young seeds. The localization of GA1/3 in the integument coincident with the expression of PnAmy1 suggests that both function as part of a process to release sugars for translocation or for the further development of the seeds.
GAs play important roles in many aspects of plant growth, e.g. stem elongation, seed germination, and flower development. In the germination of cereal seeds, GAs are synthesized in the embryos of imbibed seeds, diffuse to the aleurone layers, and induce such hydrolytic enzymes as α-amylase. The mechanism for the induction of α-amylase by GA in aleurone cells has been extensively studied, and information on the signal transduction pathway has accumulated. GA has been shown to up-regulate the transcription of GAMyb, a protein that binds to the promoter region of the high pI α-amylase gene and activates transcription (Gubler et al., 1995). GA also induced the elevation of cytoplasmic Ca2+, which is involved in the secretion of this enzyme (Bush and Jones, 1987, 1988). G-Protein (Jones et al., 1998; Ashikari et al., 1999; Fujisawa et al., 1999) and cGMP (Penson et al., 1996) signaling have also been suggested to be involved in GA responses in the aleurone.
In contrast to the accumulated information on the roles of GA in the germination of cereal seeds, little is known about the physiological roles of GAs in the seeds of dicotyledonous plants. Developing seeds of dicotyledonous plants such as the Convolvulaceae and Leguminosae contain large amounts of GAs. It has been shown that the na gene mutation of pea (Pisum sativum) blocked the synthesis of GAs and decreased the GA contents in the shoots and pods but not in the developing seeds and that there was little possibility for GA to leak from the developing seeds to other tissues (Potts, 1986).
The high levels of GAs in immature seeds generally decreased during seed maturation (McComb, 1961; Murakami, 1961; Skene and Carr, 1961; Corcoran and Phinney, 1962; Ogawa, 1963; Hashimoto and Rappaport, 1966; Rudrapal et al., 1992), which suggests that GAs in immature seeds play a primary role early in the process of seed development. The content of endogenous GAs dropped in shoots of lh-2 mutant of pea, in which GA biosynthesis is blocked, resulting in a dwarf phenotype compared with wild-type plants (Swain et al., 1993). The endogenous GAs also dropped in the embryo and endosperm of the lh-2 mutant a few days after anthesis, resulting in reduced seed weight and survival at harvest; whereas the ls and le-3 mutants neither reduced the GA levels at the early stage nor affected seed development. These observations suggest that GAs are involved in the early stage of seed development in pea (Swain et al., 1995). On the other hand, Groot et al. (1987) have reported that exogenous GA increased the seed weight and delayed seed dehydration of the GA-deficient ga-1 mutant of tomato (Lycopersicon esculentum), which suggests that GAs played a role even in the later stages of fruit and seed development in the tomato.
Scialabba et al. (1999) have observed starch grains in the integument of developing seeds of Brassica macrocarpa and their gradual degradation and disappearance during the process of seed maturation. A similar phenomenon has been reported for Arabidopsis (Windsor et al., 2000). The existence of starch grains in the integument of developing seeds has also been reported for Petunia inflata (Pai et al., 1997) and ginkgo (Ginkgo biloba; Nakao et al., 1999). However, little is known about the elements involved in starch degradation in the integument of these dicotyledonous plants.
The expression of the GA 20-oxidase gene, cv20Ox, has been reported in integument tissues of the watermelon (Citrullus lanatus), but not in the embryos, endosperms, or cotyledons (Kang et al., 1999). This observation suggests that GAs occur in the integument and are there to participate in the induction of hydrolytic enzymes required for the degradation of starch grains.
We report here on the variation in the content of biologically active GAs and show their localization during seed development in morning glory (Pharbitis nil Choisy cv Violet) using immunohistochemical methods. We also describe the cloning of cDNA of the GA-inducible α-amylase, PnAmy1, periodic changes in the expression of PnAmy1, and the immunohistochemical localization of this GA-inducible enzyme.
RESULTS
Variation of Active GAs in the Developing Seeds of Morning Glory
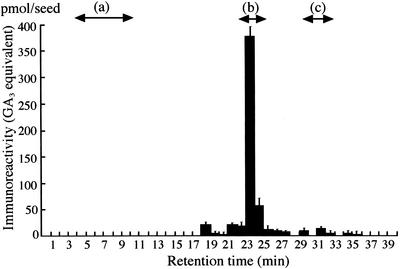
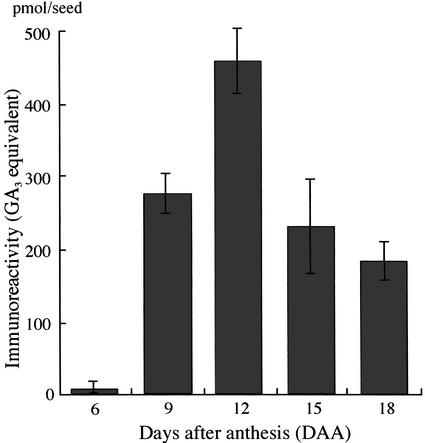
In advance of the immunoassay to quantify endogenous GAs in the immature seeds, we examined the immunoreactants in seeds 6 to 18 d after anthesis (DAA) and detected no contaminants disturbing the specific quantification of GAs. The methanol extracts of the seeds were subjected to octadecylsilanized silica gel (ODS)-HPLC without any pretreatment to avoid technical loss. We analyzed the amounts of GAs in the fractions from HPLC by ELISA, using an antiserum that has high affinity to the methyl esters of GA1, GA3, GA4, and GA7. Clear immunoreactivity was detected in the fraction of Rt 23 to 24 min (Fig. 1), where GA1 and GA3 were definitely identified by gas chromatography/mass spectrometry (GC/MS). The variation in the total immunoreactivity is shown in GA3 equivalent in Figure 2, because GA3 was predominant in the seeds 12 DAA and its methyl ester was used as a standard for calibration in ELISA. GA1/3 were hardly detectable in the seeds of 6 DAA, gradually increased until 12 DAA, and then decreased.
Figure 1.
Evaluation of biologically active GAs in developing seeds (12 DAA) of morning glory. GAs were extracted from developing seeds and fractionated by ODS-HPLC. Active GAs that had been methylated with diazomethane in each fraction were subjected to ELISA with the anti-GA1-Me antibody. Arrows a through c show the retention times of GA-glucosides, GA1/3, and GA4/7, respectively.
Figure 2.
Evaluation of endogenous GAs in the seed from 6 to 18 DAA by using the anti-GA1-Me antibody. Extracts from immature seeds in an 80% (v/v) MeOH solution were applied to a C18-cartridge, and GAs in the extracts were measured by ELISA.
Immunohistochemical Localization of Active GAs
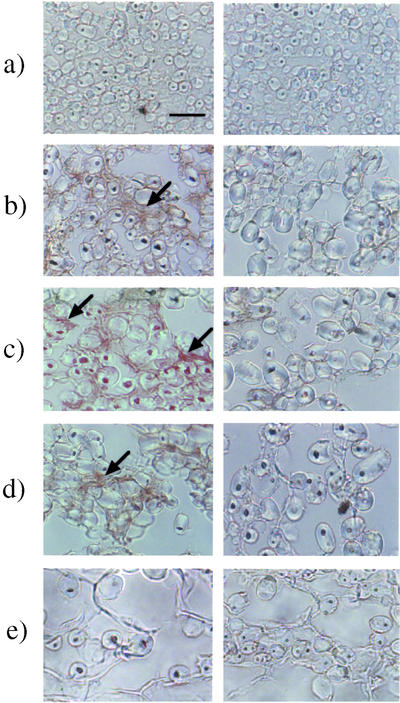
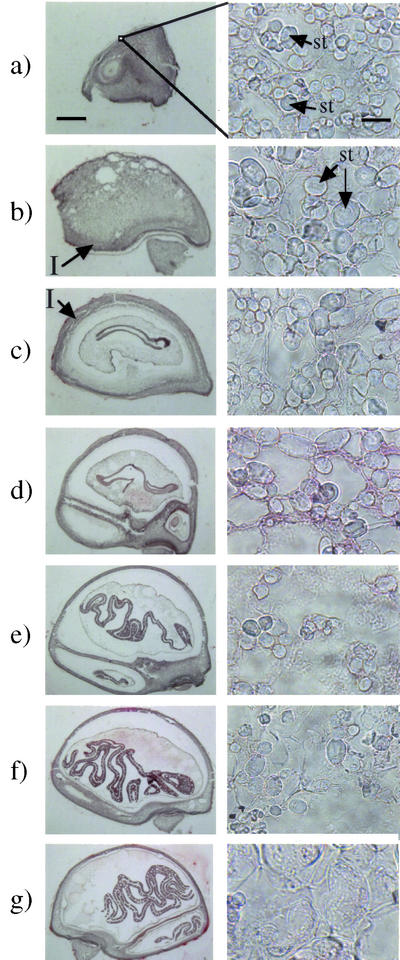
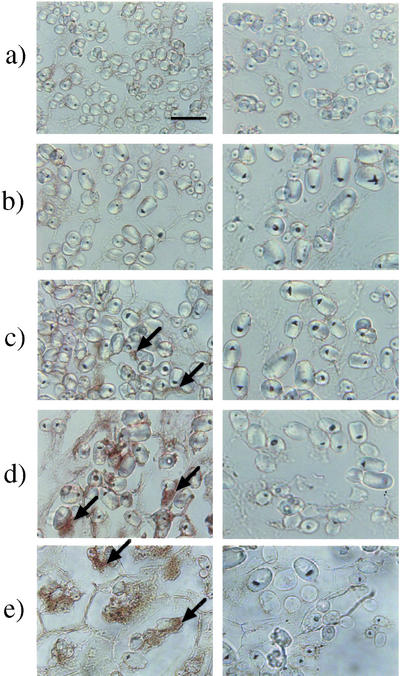
The localization of biologically active GAs, which were expected to be GA1 and/or GA3, in immature seeds of morning glory was visualized by immunohistochemical staining with an anti-GA1-Me-antiserum, which followed the method reported by Hasegawa et al. (1995) as described in “Materials and Methods.” As shown in Figure 3, specific staining due to GA1/3 was observed around the starch grains in the integument of the seeds. No staining was apparent in the control experiments with the same antiserum that had been presaturated with GA4-Me that shows as high as approximately 50% cross-reactivity to the antiserum, suggesting that the positive staining was specific for immobilized GA1 and/or GA3. The localization was chronologically analyzed by staining the sections of the seeds at different degrees of maturity. The GA-specific staining began to be recognizable under an optical microscope around the starch grains in the integument of the seeds 9 to 15 DAA. The integument occupied the major area in the immature seeds of 6 DAA and gradually became smaller relative to the other seed organs. Starch grains could already be seen in the seeds of 6 DAA, and the population of the grains had decreased in the seeds of 18 DAA (Fig. 4).
Figure 3.
Immunohistochemical analysis of active GAs in developing seeds of morning glory. Developing seeds were lyophilized, fixed with diisopropylcarbodiimide (DIPC), and embedded in paraffin. Sections (10 μm) were made for an immunohistochemical analysis of active GAs with the anti-GA1-Me antibody (left column). The antibody whose GA recognition sites were saturated with GA4-Me, which shows high cross-reactivity (48%) to the antibody, was used for control staining (right column). a, 6 DAA; b, 9 DAA; c, 12 DAA; d, 15 DAA; e, 18 DAA. The stained images have been magnified 1,600 times with an optical microscope (the scale bar is 10 μm). Arrows show the staining specific for active GAs.
Figure 4.
Sections from 6 to 24 DAA morning glory seeds stained with acetocarmine. Developing seeds were fixed in a glutaraldehyde-paraformaldehyde solution and embedded in paraffin. Sections were stained with acetocarmine for 5 min. The left column shows whole seeds magnified 10 times (the scale bar is 1 mm), whereas the right column shows the integument magnified 1,600 times (the scale bar is 10 μm). a, 6 DAA; b, 9 DAA; c, 12 DAA; d, 15 DAA; e, 18 DAA; f, 21 DAA; g, 24 DAA. I, Integument tissue; st, starch grain.
Cloning of cDNA of PnAmy1, a Homolog of α-Amylase
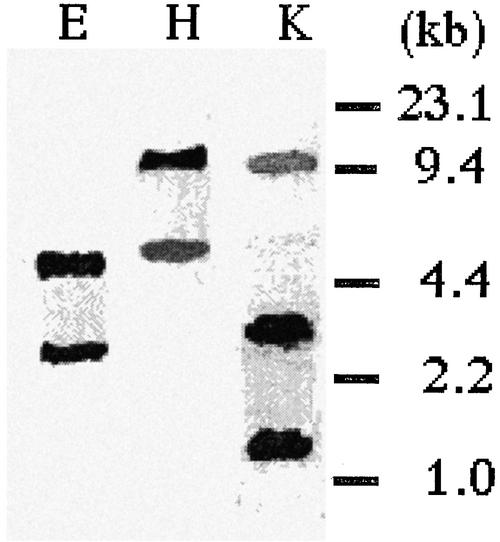
Degenerate primers for PCR were constructed according to the well-conserved sequences of known α-amylase genes from various plant species. We prepared the cDNA library from developing seeds of morning glory and amplified the PCR products with the constructed primers. A cDNA fragment of about 0.6 kb was obtained as the main product, this being used for 5′-RACE and 3′-RACE to generate a full-length cDNA of 1.6 kb (accession no. AB077387). A homology search by the BLAST service revealed that the deduced amino acid sequence of the PnAmy1 gene had 60% to 80% homology to that of other well-known α-amylases (shown in Table I). We performed a genomic DNA-blot analysis to identify the copy number of the PnAmy1 gene within the genome (Fig. 5). Morning glory genomic DNA was prepared from young leaves and digested with EcoRI, HindIII, and KpnI, respectively. The result showed that one or two major bands and some weakly hybridizing bands were present, indicating that more than two genes highly homologous to PnAmy1 existed in the genome and that PnAmy1 homologs possibly existed in the seeds.
Table I.
Identity of the deduced amino acid sequence for PnAmy1 cDNA to well-known α-amylase derived from plant species
| Homology | Gene | Botanical Name |
|---|---|---|
| % | ||
| 85 | CUS AMY2 | Cuscuta reflexa (precursor) |
| 73 | amyVm1 | Vigna mungo |
| 72 | Phaseolus vulgaris | |
| 67 | Zea mays | |
| 66 | RAmy3B | Oryza sativa |
Figure 5.
Genomic DNA-blot analysis of PnAmy1. Ten micrograms of genomic DNA extracted from leaves of morning glory was digested with EcoRI (E), HindIII (H), and KpnI (K) and then electrophoresed with 0.8% (w/v) agarose gel. Size markers are shown on the right-hand side in kilobases.
RNA-Blot Analyses of PnAmy1
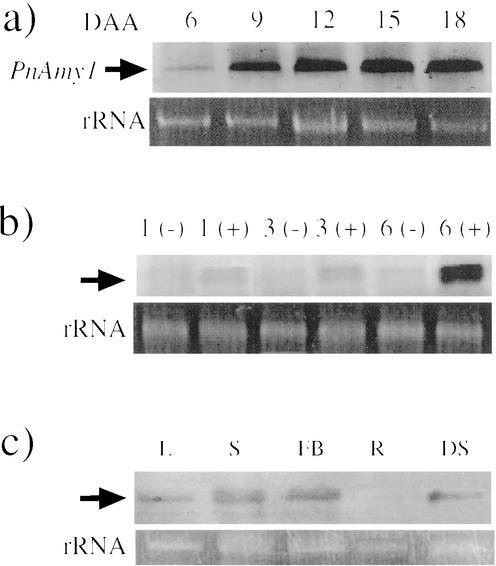
An RNA probe was prepared for RNA-blot analyses by referring to the sequence unique to PnAmy1. The expression pattern of PnAmy1 during seed maturation is shown in Figure 6a. A slight but clear signal of PnAmy1 mRNA appeared at 6 DAA. This mRNA level had significantly increased by 9 DAA and maintained a gradual increase until 18 DAA. The GA responsiveness of PnAmy1 was examined by using fruits of 9 DAA in which the expression of the gene was clear but not saturated (Fig. 6b). The transcriptional level of the gene was slightly up-regulated 1 to 3 h after administering GA3 to the fruit and significantly so 6 h after the administration. We examined the organ specificity of the expression of PnAmy1 (Fig. 6c). PnAmy1 was expressed in the leaf, stem, and flower buds, as well as in the developing seed.
Figure 6.
RNA-blot analyses of PnAmy1. a, Temporal expression of PnAmy1 (top, arrows). Total RNA (2 μg) was extracted from seeds at 6 to 18 DAA, transferred to a nylon membrane, and hybridized with the same probe as that used in the DNA-blot analysis. Bottom, Total RNA in developing seeds. b, Responsiveness of PnAmy1 to applied GAs. Total RNA (2 μg) was extracted from developing seeds that had been incubated with exogenously applied 0.1 μm GA3 dissolved in 1% (v/v) MeOH for 1, 3, and 6 h. Immature seeds treated with a 1% (v/v) MeOH solution for the same periods of time were used as controls. c, Spatial expression of PnAmy1. Total RNA was extracted from the leaf (L), stem (S), flower bud (FB), root (R), and developing seed (DS). Two micrograms of RNA was blotted onto the membrane and hybridized as already described.
Detection of Enzymatic Activity of PnAmy1 and Immunoblot Analysis
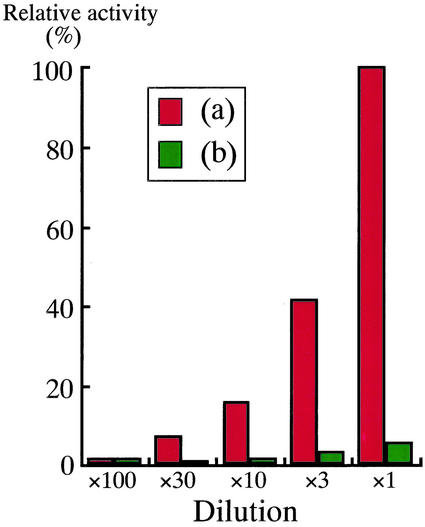
The PnAmy1-glutathione S-transferase (GST) fusion protein and GST protein alone were prepared as respective soluble proteins by using an Escherichia coli expression system. Both crude extracts were subjected to an α-amylase assay by the iodine-starch reaction. PnAmy1-GST showed amylase activity, whereas protein from E. coli containing only the GST construct was without amylase activity (Fig. 7). This result confirms that the PnAmy1 encodes an α-amylase expressed in morning glory seeds early in development.
Figure 7.
Assay of the α-amylase activity of PnAmy1 with a soluble fusion protein. a, GST-PnAmy1; b, GST alone as a control. One hundred micrograms per milliliter of each of these protein solutions (×1) was prepared and properly diluted. Each was subjected to the iodine-starch reaction, and A590 was measured. The enzyme activity has been converted to the relative activity compared with that of 100 μg mL−1 of GST-PnAmy1.
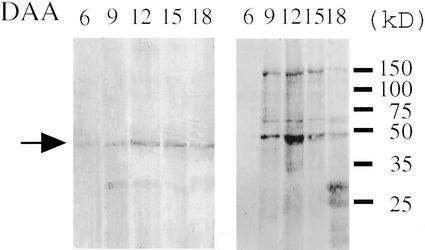
An immunoblot analysis of PnAmy1 in the developing seeds of morning glory was performed with a polyclonal antibody that had been raised against the recombinant PnAmy1-GST protein. The crude soluble proteins extracted from the seeds were subjected to SDS-PAGE, blotted on a nylon membrane, and immunostained with the antibody. A single, clear band attributable to PnAmy1 was observed at 45 kD, as well as weak staining at 30 kD (Fig. 8).
Figure 8.
Immunoblot analysis of PnAmy1 in the soluble protein fraction of developing seeds of morning glory. Soluble fractions from developing seeds of 6 to 18 DAA were extracted with phosphate-buffered saline (PBS), precipitated by an equivalent volume of a saturated ammonium sulfate solution, transferred to a nitrocellulose membrane, and detected with the anti-PnAmy1 antiserum. Left, The arrow at around 45 kD shows the staining due to PnAmy1. Right, Staining with Coomassie Brilliant Blue after SDS-PAGE of the soluble fraction, and size markers are shown on the right side in kD.
Immunohistochemistry of PnAmy1
The antibody against recombinant PnAmy1-GST was purified in advance by passing through a column packed with the PBS-insoluble residue of seeds to remove the proteins that had bound to the nonextractable seed sediment. This pretreatment effectively reduced non-specific staining on the paraffin sections of the seeds (data not shown).
The seeds were fixed in an aldehyde solution and submitted to an immunohistochemical analysis of PnAmy1. The specificity of this staining was confirmed by a control experiment showing no signals when stained with the preimmune antibody instead of the specific one. No specific staining for PnAmy1 was apparent in the seeds at 9 DAA (Fig. 9), whereas specific staining for GA1/3 was clear in the seeds at this developmental stage (Fig. 3). The specific localization signal around the starch grains began to appear in the seeds at 12 DAA.
Figure 9.
Temporal analysis of PnAmy1 in the integument by an immunohistochemical technique. Sections fixed in an aldehyde solution were used for the analysis. The left column shows staining with the anti-PnAmy1 antiserum, and the right column shows that with the preimmune serum. a, 6 DAA; b, 9 DAA; c, 12 DAA; d, 15 DAA; and e, 18 DAA. The scale bar is 10 μm. Arrows show specific staining due to PnAmy1.
DISCUSSION
Before the immunohistochemical analysis of GAs in immature seeds of morning glory, we examined the immunoreactive components in the seeds. This was necessary because we previously found that an abundance of 17-O-glucosylated derivatives of GA4 and GA7 found in rice anthers interfered with the specific staining of the free GAs (Hasegawa et al., 1994). Our previous analysis of immature seeds of morning glory showed that they contained a large amount of 2- or 3-O-glucosylated GAs (Yokota et al., 1969, 1970). Thus, we used HPLC analysis of methanol extracts without solvent fractionation and showed that immunoreactive materials other than the targeted GAs were hardly detectable in the extracts and that the quantification by immunoassay was not disturbed. This was reasonable because the antibody is very selective in its recognition of the structure of the A-ring in the GA molecule, and 2- or 3-O-glucosyl GAs showed little cross-reactivity to the antibody (Yamaguchi et al., 1990). The GAs detected by ELISA were identified as GA1 and GA3 by GC/MS.
Free forms of GAs are generally lipophylic but still water-soluble. We have, therefore, paid attention to avoiding any drift of GAs from their original localization during the fixation process for immunostaining and have succeeded in fixing GAs in their original tissues (Hasegawa et al., 1995). We adopted the same procedure, in which the seeds were initially lyophilized and then exposed to gaseous DIPC under reduced pressure for fixation. We did not examine the fixation efficiency with this procedure; if some GAs remained unfixed, they would neither have been stained nor disturbed the results, because the cross-reactivity of free GAs to the antibody was negligible.
Starch grains were already observable in the integument of the seeds at 6 DAA before GAs could be detected by immunohistochemistry. The starch grains began to decay, and their edges became obscure after the appearance of GA-positive staining (Fig. 3). This series of events observed in the integument was reminiscent of the process occurring in germinating cereal grains, in which GAs synthesized in the embryo induced α-amylase in aleurone, which diffused to the endosperm and degraded the starch.
The α-amylase activity in the whole seeds became higher with the increase in GA1/3 (data not shown). We cloned a cDNA of an α-amylase homolog (PnAmy1) by PCR and examined the GA responsiveness of PnAmy1, which was predominant in the seeds (Fig. 6b). A GA responsive increase of PnAmy1 mRNA was clearly observed in the seeds 6 h after the GA3 treatment, whereas no signal was apparent in the control seeds treated with 1% (v/v) methanol.
Genomic DNA blotting showed that at least two homologous copies existed in the genome and also that the polyclonal antibody raised against PnAmy1 possibly cross-reacted with the homologs. In the immunoblot analysis shown in Figure 8, the minor band was detected at 30 kD. This band could be a degradation product, because the relative intensity of the band to that at 45 kD varied in every experiment.
We examined whether the combination of lyophilization and gaseous fixation, which was effective for immunostaining GAs, would be useful or not for immunostaining PnAmy1, and found that PnAmy1 was denatured and became insensitive to the antibody. We then adopted another fixation protocol using a formaldehyde solution and succeeded in specific staining (data not shown). This fixation procedure generally used for proteins conserved the cell structure well, and we could observe the starch grains attached to the inner surface of the plasma membrane (Fig. 4). This conservation of the cell structure was not achievable by the procedure of lyophilization and DIPC gas fixation, and we could not identify the cytochemical localization of GAs, but only their histochemical localization. We observed that GA1/3 and PnAmy1 were localized in the integuments where many starch grains existed.
On the basis of following results, (a) the spatial similarity between the sites of GA1/3 and PnAmy1 localization, (b) the time lag between their appearances in the tissues of GA1/3 preceding to PnAmy1 and the subsequent degradation of the starch grains, and (c) that PnAmy1 was responsive to GA, we hypothesize that a process similar to that observed with the digestion of starch in the endosperm of cereal grain proceeded in the immature seeds of morning glory; namely that GA induced α-amylase in the integument or neighboring tissues and that the enzyme secreted into the integument digested the starch grains. The digested starch could then be used as a source for the development of cotyledons. The sites for GA biosynthesis and expression of the PnAmy1 gene need to be determined, and an in situ hybridization analysis is planned for this purpose.
MATERIALS AND METHODS
Plant Material
Morning glory (Pharbitis nil Choisy cv Violet) plants were grown in a cultivation chamber at 25°C with alternating continuous fluorescent light (13 h) and continuous dark (11 h). The flower induction conditions under which the plant had been cultivated for 4 d after the cotyledons had opened were opposite to the usual photoperiodic conditions. The developed seeds were harvested, frozen in liquid nitrogen, and stored at −85°C.
Application of GA
The top (approximately one-tenth) of the fruits (9 DAA) on the runner of morning glory was cut off with a razor to facilitate the liquid injection by avoiding the increase of inner pressure, and 10 μL of 0.1 μm GA3 solution in 1% (v/v) MeOH was applied to each fruit of 9 DAA using a 100-μL microsyringe (Hamilton, Reno, NV). On application, the needle of a syringe was carefully addressed to the basal part of a fruit through lower part of husk, and GA solution was applied. Seeds to which 1% (v/v) MeOH was applied were used as controls. The seeds were harvested 1, 3, and 6 h after the application of the GA solution.
Immunoassay of GAs in the Seeds of Morning Glory
Immature seeds of morning glory (20 seeds) were homogenized in 5 mL of 80% (v/v) MeOH. The extract was applied to a Sep-Pak C18 cartridge (Waters, Milford, MA), which was immediately eluted with 6 mL of 80% (v/v) MeOH. The resulting eluate was dried in vacuo, a portion of the extract was dissolved in 1 mL of 10% (v/v) acetonitrile containing 0.5% (v/v) acetic acid (solvent A), and 100 μL of the resulting solution was subjected to reverse-phase HPLC with a Pegasil ODS C18 column (6 mm i.d., 150 mm long; Senshu Co., Tokyo). Elution was performed according to the previously reported method (Hasegawa et al., 1994), except that the flow rate was 1.5 mL min−1. Each fraction was methylated with diazomethane and subjected to ELISA with an Immuron 4 microplate (Dynatech Laboratories, Chantilly, VA). The procedure for ELISA was as follows: (a) incubating with 100 μL of 50 μg mL−1 goat antibody to rabbit γ-globulin (Calbiochem-Novabiochem) in a 50 mm sodium hydrogencarbonate buffer (pH 9.6) per well at 4°C overnight; (b) washing the wells with distilled water three times; (c) incubating with 100 μL of the 1/20,000-diluted anti-GA1-Me antiserum (Yamaguchi et al., 1990) in PBS at 37°C for 2 h; (d) washing the wells with distilled water three times; (e) incubating with 50 μL of a sample solution and 50 μL of a tracer (1/60-diluted alkaline-phosphatase-conjugated GA3 prepared according to the method described previously [Yamaguchi et al., 1990] in Tris-buffered saline) at room temperature for 2 h; (f) washing the wells with distilled water five times; (g) incubating with 100 μL of 1 mg mL−1 p-nitrophenylphosphate in a 50 mm sodium hydrogencarbonate buffer (pH 9.6) containing 1 mm MgCl2 at 37°C for 1 h; and (h) detecting A410 with a microplate reader (MR5000; Dynatech).
Identification of GA1 and GA3
The immunoreactive fraction (Rt 23–24 min) from ODS-HPLC of the extract of seeds 9 DAA was concentrated and dissolved in methanol. An aliquot of the solution was treated with ethereal diazomethane and concentrated. To the concentrate, 20 μL of N-methyl-N-trimethylsilyl trifluoroacetamide was added and heated with a hair dryer for a few minutes for trimethylsilylation. The methylated and trimethylsilylated sample was subjected to GC/MS analysis (instrument, M-4100 mass spectrometer connected with a HP-5890 gas chromatograph [Hitachi, Tokyo]; column: DB-5 [0.25 μm thick, 0.25 mm × 15 m, J&W Scientific, Folsom, CA]; temperature, 60°C, 2 min; 60°C–200°C, 30°C min−1; 200°C–300°C, 5°C min−1; He, 1 mL min−1).
The Rt of GA1-methyl ester-trimethylsilyl ether was 12.9 min (Kovat's retention index, 2,747) and that of GA3-methyl ester-trimethylsilyl ether 13.2 min (Kovat's retention index, 2,753). Their mass spectra were superimposable to those of authentic samples.
RNA Isolation and Construction of the cDNA Library
Total RNA was extracted from the developing seeds of morning glory with a buffer of 1× MES (2-[N-morpholino]ethanesulfonic acid containing 8 m guanidine hydrochloride and 50 mm 2-mercaptoethanol). The RNA fraction was subjected to further extraction three times with a mixture of phenol:chloroform:isoamyl alcohol (25:24:1, v/v). The resulting fraction was precipitated by a one-fifth volume of 1 m acetic acid and four-fifths volume of ethanol. This precipitate was dissolved in 0.05% (v/v) diethylpyrocar-bonate-treated sterilized water. Poly(A+) RNA was purified with a Dynabeads mRNA purification kit (Dynal, Oslo), and cDNA was synthesized with a Marathon cDNA amplification kit (CLONTECH Laboratories, Palo Alto, CA) according to the manufacturers' protocols.
Cloning of PnAmy1 Full-Length cDNA
A pair of degenerate primers was designed on the basis of the well-conserved amino acid sequence among α-amylase genes derived from dicotyledonous plants. The following primers were synthesized: forward primer, 5′-GCNGAYRTNGTNATHAAYCAYMG-3′; and reverse primer, 5′-CCNGGRTGNGTNARDATRTANGC-3′. The cDNA library from immature seeds of morning glory was used as template. The PCR reaction conducted with PCR Thermal Cycler (TaKaRa, Kyoto) was initiated by heating to 94°C for 3 min and then subjected to 30 cycles of 94°C for 1 min, 55°C for 1 min 45 s, and 72°C for 2 min. The reaction was completed by a 10-min incubation at 72°C. The products were purified by 1% (w/v) agarose gel electrophoresis and cloned into the pGEM-T Easy vector (Promega, Madison, WI). A Thermo Sequenase cycle DNA sequencing kit (Shimadzu, Columbia, MD) and a DNA sequencer (DSQ-1000L, Shimadzu) were used to determine the nucleotide sequence, and PnAmy1 full-length cDNA was obtained by 5′- and 3′-RACE. A homology search was performed with the BLAST program of the DNA Databank of Japan.
Genomic DNA and RNA-Blot Analyses
Total genomic DNA was extracted from young leaves (10 g) of morning glory with a buffer containing 0.1 m Tris, 0.05 m EDTA (pH 8.0), 0.5 m sodium chloride, and 0.2% (w/v) SDS. Ten micrograms of DNA was treated overnight at 37°C with restriction enzymes (EcoRI, HindIII, and KpnI), separated with 0.8% (w/v) agarose gel, and transferred to a nylon membrane (Hybond-N+, Amersham-Pharmacia Biotech, Uppsala). A probe (RNA) solution was prepared with a DIG RNA labeling kit (Boehringer Mannheim/Roche, Basel) and 1/2,000-diluted in the hybridization buffer according to the manufacturer's procedure. After the membrane had been prehybridized at 50°C for 3 h, hybridization was performed overnight at 50°C before detection. The isolated total RNA was purified with 2 m lithium chloride. Two micrograms of total RNA was electrophoresed with 1% (w/v) agarose gel containing 5% (v/v) formaldehyde and transferred to a nylon membrane. The protocols for hybridization and detection were the same as those used for the genomic DNA-blot analysis, except that the temperature for hybridization was 68.3°C.
Preparation of Recombinant PnAmy1 and Its Specific Antiserum
The PnAmy1 cDNA clone was inserted into the SmaI site of the pGEX-4T-2 vector (Pharmacia Biotech, Piscataway, NJ), generating GST-PnAmy1 fusion (PnAmy1-GST). The fusion protein and GST alone were obtained from Escherichia coli JM109, and it was confirmed by SDS-PAGE that the proteins were included in the soluble fraction. The amylase activity was then assayed. Thirty microliters each of a 1% (w/v) starch solution was transferred to microtubes, and 180 μL of the sample solution was added to each. After holding the microtubes at room temperature for 1 h, 300 μL of 50 μg mL−1 iodine in a 0.05% (w/v) potassium iodide solution was added, and A590 was detected. PnAmy1-GST was also used as an antigen for preparing the anti-PnAmy1 antiserum. This fusion protein was immunized into New Zealand white rabbits, and the polyclonal antibody was raised against it.
Immunoblot Analysis
Soluble proteins were extracted from developing seeds of morning glory with PBS and purified with 50% (v/v) saturated ammonium sulfate. This fraction was desalted in a gel filtration column (Nap-5) purchased from Pharmacia Biotech, size-fractionated with SDS-PAGE, and transferred to a nitrocellulose membrane. The protocol used for immunostaining was as follows: (a) incubating the membrane with a blocking buffer (1% [w/v] bovine serum albumin [BSA] in PBS) at room temperature overnight; (b) incubating with the 1/500-diluted anti-PnAmy1 antiserum in PBS at room temperature for 2 h; (c) rinsing twice for 15 min each in a washing buffer of 25 mm Tris-HCl (pH 7.4) containing 0.2% (w/v) BSA, 1% (w/v) NaCl, 0.02% (w/v) KCl, 0.2% (w/v) polyvinylpyrrolidone (K = 90), and 0.05% (v/v) Tween 20; (d) incubating with the 1/1,000-diluted alkaline phosphatase-conjugated goat antibody to rabbit IgG (ICN/Cappel) in the washing buffer at room temperature for 1 h; (e) rinsing three times in Tris-buffered saline containing 0.1% (v/v) Tween 20 for 10 min; and (f) colorizing with a substrate solution of 1 mg mL−1 naphthol AS-MX phosphate (Sigma, St. Louis) and 5 mg mL−1 Fast Red TR (Sigma) dissolved in 0.2 m Tris-HCl (pH 8.2).
Purification of the Antibody to Immunohistochemical Grade
The anti-GA1-Me antiserum had been previously prepared (Yamaguchi et al., 1990) and purified in HiTrap hydroxysuccinimide-activated affinity columns (Pharmacia, Uppsala). Five milligrams of BSA or the soluble protein derived from morning glory was used to couple to 1 mL of gel. About 1 mL of the 1/100-diluted anti-GA1-Me antiserum in PBS was applied to the column at 4°C. The antiserum was eluted with PBS and further purified in a column loaded with the MeOH-insoluble fraction from developing seeds of morning glory. Six milliliters of the eluate was obtained, and an equivalent volume of glycerol was added to it. The anti-PnAmy1 antiserum was purified in a column loaded with the water-insoluble fraction from immature seeds of morning glory. One milliliter of the one-tenth-diluted anti-PnAmy1 antiserum in PBS was applied to this column. After leaving at room temperature for 1 h, the eluate with 2 mL of PBS was collected.
Preparation of Tissue Sections
The immature seeds of morning glory were harvested and immediately frozen in liquid nitrogen. The samples to be used for the immunohistochemical analysis of active GAs were lyophilized in vacuo with a VFD-300 device (Vacuum Device Inc., Ibaraki, Japan). The temperature conditions during lyophilization were as follows: (a) maintaining at −120°C (1 h); (b) elevating from −120°C to −90°C (24 h, linear gradient); (c) maintaining at −90°C (24 h); (d) elevating from −90°C to −60°C (6 h, linear gradient); (e) maintaining at −60°C (12 h); and (f) elevating from −60°C to 25°C (6 h, linear gradient). Samples were fixed with DIPC and embedded in paraffin by the method described previously (Hasegawa et al., 1995), before sections (10 μm) were prepared and dewaxed. The samples for PnAmy1 and tissue staining were fixed in a glutaraldehyde-paraformaldehyde solution by evacuating with an aspirator for 2 h and then rinsed in a 50 mm cacodylate buffer. The method for preparing the sections was similar to that for the immunohistochemical analysis of GAs.
Tissue Staining
Sections fixed with the aldehyde solution were dewaxed as already described. About 100 μL of acetocarmine was dropped onto each section, and the sections were incubated at room temperature for 5 min. After acetocarmine had been removed, the sections were mounted in glycerol.
Immunohistochemical Analysis
All operations for staining were carried out in a moist chamber at room temperature. A drop of each reagent (100 μL) was put on one section, preventing its spread with an Immunopen (Wako Pure Chemical Industries, Osaka). The staining procedure was as follows: (a) incubating with the blocking buffer used in the immunoblot analysis for 1 h; (b) incubating with the one-twentieth-diluted purified primary antibody (the anti-GA1-Me antiserum for GA staining and anti-PnAmy1 antiserum for PnAmy1 staining) in a dilution buffer (PBS containing 0.1% [v/v] Tween 20 [PBS-T]: blocking buffer [1:1, v/v]) for 2 h; (c) rinsing three times in PBS-T (10 min each); (d) incubating with the 1/300-diluted biotin-conjugated donkey anti-rabbit IgG antibody in the dilution buffer for 1 h; (e) rinsing three times in PBS-T (10 min each); (f) incubating with a Vectastain Elite ABC peroxidase complex solution (Vector) for 50 min; (g) rinsing three times in PBS-T (10 min each) before rinsing once in PBS (10 min); (h) incubating with PBS containing 0.2 mg mL−1 of 3,3′-diaminobenzidine tetrahydrochloride and 0.005% (w/v) hydrogen peroxide for 15 min; and (i) dehydrating and mounting by the method previously described (Hasegawa et al., 1995).
Immunohistochemical Controls
The anti-GA1-Me antiserum containing 1 μm GA4-Me was used as a primary control antibody for staining of active GAs, whereas the nonimmunized rabbit serum was used for PnAmy1 staining.
ACKNOWLEDGMENTS
We thank Prof. Jerry D. Cohen for critical reading of the manuscript and helpful suggestions, Prof. Naoko Nishizawa for valuable immunohistochemical advice and interpretations of the pictures, and Prof. Junko Yamagishi for growing morning glory.
Footnotes
This work was supported by a grant from the Bio-oriented Technology Research Advancement Institution.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010921.
LITERATURE CITED
- Ashikari M, Wu JZ, Yano M, Sasaki T, Yoshimura A. Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the α-subunit of GTP-binding protein. Proc Natl Acad Sci USA. 1999;96:10284–10289. doi: 10.1073/pnas.96.18.10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DS, Jones RL. Measurement of cytoplasmic calcium in aleurone protoplasts using indo-1 and fura-2. Cell Calcium. 1987;8:455–472. doi: 10.1016/0143-4160(87)90029-7. [DOI] [PubMed] [Google Scholar]
- Bush DS, Jones RL. Cytoplasmic calcium and α-amylase secretion from barley aleurone protoplasts. Eur J Cell Biol. 1988;46:466–469. [Google Scholar]
- Corcoran MR, Phinney BO. Changes in amount of gibberellin-like substances in developing seed of Echinocystis, Lupinus and Phaseolus. Physiol Plant. 1962;15:252–262. [Google Scholar]
- Fujisawa Y, Kato T, Ohki S, Ishikawa A, Kitano H, Sasaki T, Asahi T, Iwasaki Y. Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc Natl Acad Sci USA. 1999;96:7575–7580. doi: 10.1073/pnas.96.13.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot SPC, Bruinsma J, Karssen CM. The role of endogenous gibberellin in seed and fruit development of tomato: studies with a gibberellin-deficient mutant. Physiol Plant. 1987;71:184–190. [Google Scholar]
- Gubler F, Kalla R, Roberts JK, Jacobsen JV. Gibberellin-regulated expression of a Myb gene in barley aleurone cells-evidence for Myb transactivation of a high-pI alpha-amylase gene promoter. Plant Cell. 1995;7:1879–1891. doi: 10.1105/tpc.7.11.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Hashimoto N, Zhang J, Nakajima M, Takeda K, Yamaguchi I, Murofushi N. Immunohistochemistry of gibberellins in rice anthers. Biosci Biotech Biochem. 1995;59:1925–1929. [Google Scholar]
- Hasegawa M, Nakajima M, Takeda K, Yamaguchi I, Murofushi N. A novel gibberellin glucoside, 16α,17-dihydroxy-16,17-dihydrogibberellin A4-17-O-β-D-glucopyranoside, from rice anthers. Phytochemistry. 1994;37:629–634. [Google Scholar]
- Hashimoto T, Rappaport L. Variations in endogenous gibberellins in developing bean seeds: I. Occurrence of neutral and acidic substances. Plant Physiol. 1966;41:623–628. doi: 10.1104/pp.41.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HD, Smith SJ, Desikan R, Plakidou-Dymock S, Lovegrove A, Hooley R. Heterotrimeric G proteins are implicated in gibberellin induction of α-amylase gene expression in wild oat aleurone. Plant Cell. 1998;10:245–253. doi: 10.1105/tpc.10.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HG, Jun SH, Kim J, Kawaide H, Kamiya Y, An G. Cloning and molecular analysis of a gibberellin 20-oxidase gene expressed specifically in developing seeds of watermelon. Plant Physiol. 1999;121:373–382. doi: 10.1104/pp.121.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb AJ. Bound gibberellin in mature runner bean seeds. Nature. 1961;192:575–576. [Google Scholar]
- Murakami Y. Paper chromatographic studies on charge in gibberellins during seed development and germination in Pharbitis nil. Bot Mag. 1961;74:241–247. [Google Scholar]
- Nakao Y, Shiozaki S, Ogata T, Kawase K, Horiuchi S. Changes in carbohydrate and water content with ovule growth of Ginkgo biloba L. J Hortic Sci Biotechnol. 1999;74:60–63. [Google Scholar]
- Ogawa Y. Gibberellin-like substances occurring in the seed of Pharbitis nil Chois. and their change in contents during the seed development. Plant Cell Physiol. 1963;4:217–225. [Google Scholar]
- Pai H, Mariani C, Kao TH. Cytological study of pollen tube growth and early seed development in Petunia inflata. J Plant Biol. 1997;40:212–219. [Google Scholar]
- Penson SP, Schuurink RC, Fath A, Gubler F, Jacobsen J, Jones RL. cGMP is required for gibberellic acid-induced gene expression in barley aleurone. Plant Cell. 1996;8:2325–2333. doi: 10.1105/tpc.8.12.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts WC. Gibberellin in light-grown shoots of Pisum sativum and the influence of reproductive development. Plant Cell Physiol. 1986;27:997–1004. [Google Scholar]
- Rudrapal D, Okubo H, Uemoto S, Fujieda K. Comparison of the anatomy and physiology of seeds of two varieties of winged bean. Sci Hortic. 1992;51:13–24. [Google Scholar]
- Scialabba A, Di Liberto C, Bellani LM, Muccifora S. Structural and histochemical changes during seed development of Brassica macrocarpa Guss. Eur J Histochem. 1999;43:147–159. [PubMed] [Google Scholar]
- Skene KGM, Carr DJ. A quantitative study of the gibberellin content of seeds of Phaseolus vulgaris at different stages in their development. Aust J Biol Sci. 1961;14:13–25. [Google Scholar]
- Swain SM, Reid JB, Ross JJ. Seed development in Pisum: the lhi allele reduces gibberellin levels in developing seeds, and increases seed abortion. Planta. 1993;191:482–488. [Google Scholar]
- Swain SM, Ross JJ, Reid JB, Kamiya Y. Gibberellins and pea seed development. Planta. 1995;195:426–433. [Google Scholar]
- Windsor JB, Symonds VV, Mendenhall J, Lloyd AM. Arabidopsis seed coat development: morphological differentiation of the outer integument. Plant J. 2000;22:483–493. doi: 10.1046/j.1365-313x.2000.00756.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi I, Nakazawa H, Nakagawa R, Suzuki Y, Kurogochi S, Murofushi N, Takahashi N, Weiler EW. Identification and semi-quantification of gibberellins from the pollen and anthers of Zea mays by immunoassay and GC/MS. Plant Cell Physiol. 1990;31:1063–1069. [Google Scholar]
- Yokota T, Murofushi N, Takahashi N. Structure of new gibberellin glucosides in immature seeds of Pharbitis nil. Tetrahedron Lett. 1970;18:1489–1491. doi: 10.1016/s0040-4039(01)98003-7. [DOI] [PubMed] [Google Scholar]
- Yokota T, Takahashi N, Murofushi N, Tamura S. Structure of new gibberellin glucosides in immature seeds of Pharbitis nil. Tetrahedron Lett. 1969;25:2081–2084. doi: 10.1016/s0040-4039(01)98003-7. [DOI] [PubMed] [Google Scholar]