Abstract
In mammals, expiration is lengthened by mid-expiratory lung inflation (Breuer-Hering Expiratory reflex; BHE). The central pathway mediating the BHE is paucisynaptic, converging on neurones in the rostral ventrolateral medulla. An in vitro neonatal rat brainstem–lung preparation in which mid-expiratory inflation lengthens expiration was used to study afferent modulation of respiratory neurone activity. Recordings were made from respiratory neurones in or near the pre-Bötzinger Complex (preBötC). Respiratory neurone membrane properties and BHE-induced changes in activity were characterized. Our findings suggest the following mechanisms for the BHE: (i) lung afferent signals strongly excite biphasic neurones that convey these signals to respiratory neurones in ventrolateral medulla; (ii) expiratory lengthening is mediated by inhibition of rhythmogenic and (pre)motoneuronal networks; and (iii) pre-inspiratory (Pre-I) neurones, some of which project to abdominal expiratory motoneurones, are excited during the BHE. These findings are qualitatively similar to studies of the BHE in vivo. Where there are differences, they can largely be accounted for by developmental changes and experimental conditions.
In behaving mammals, respiration is continuously modulated as a function of metabolic demand, state of arousal, posture, temperature, etc. Signals related to these variables, conveyed by descending and sensory afferents, converge on respiratory rhythm-generating networks in the ventrolateral medulla. Respiratory afferent modulation has been studied extensively in humans (q.v., Widdicombe & Lee, 2001) and, more invasively, in a variety of juvenile or adult mammalian preparations (von Euler, 1983; Lindsey et al. 1987, 2000; Schwarzacher et al. 1995; Gray et al. 2001, Mitchell & Johnson, 2003; Dutschmann & Paton, 2003). A limitation of these in vivo studies is that the respiratory networks are relatively inaccessible, and basic rhythmogenic mechanisms remain poorly understood. To overcome these limitations, neonatal rodent in vitro preparations that produce respiratory-related rhythm unmodulated by sensory feedback have been developed (Smith & Feldman, 1987; Onimaru & Homma, 1987; Smith et al. 1991) and extensively used to investigate essential rhythmogenic mechanisms (Smith et al. 1993; Rekling & Feldman, 1998).
A basic problem impeding our understanding of respiratory rhythm generation and modulation is that while mappings between in vitro and in vivo data have been proposed (Feldman et al. 1990; Richter & Spyer, 2001), they are difficult to test experimentally: differences due to experimental conditions and development interact and it is difficult to control for them. Here we reincorporate lung afferent feedback in vitro so as to be able to compare cellular and systems level responses in vitro to similar studies carried out in vivo (Hayashi et al. 1996), and to assess whether afferent perturbations to respiratory rhythm can be used to differentiate between rhythmogenic and sensory relay networks.
We use an in vitro neonate rat brainstem–spinal cord preparation in which the lungs and their vagal innervation are retained (Murakoshi & Otsuka, 1985; Mellen & Feldman, 1997). In this preparation, lung inflation at pressures in the physiological range (2–5 mmH2O; Widdicombe, 1961) modulates respiratory rhythm. Transient lung inflation during inspiration shortens inspiration (Mellen & Feldman, 2000, 2001) and sustained mid-expiratory lung inflation lengthens expiration (Mellen & Feldman, 1997). These responses match those seen in mammals (Breuer, 1868) and are referred to as the Breuer-Hering inspiratory reflex (BHI) and Breuer-Hering expiratory reflex (BHE), respectively (von Euler, 1983, Feldman, 1986).
The Breuer-Hering reflexes are elicited by activation of slowly adapting pulmonary receptors (SARs; Adrian, 1933, Schelegle & Green, 2001). These provide glutamatergic input (Bonham et al. 1993) to second-order neurones in nucleus tractus solitarii (NTS). These second order neurones project to the ventrolateral medulla, as far rostral as the caudal margin of the facial nucleus (Ezure & Tanaka, 1996; Ezure et al. 2002) and also inhibit rapidly adapting relay neurones in the caudal NTS (Ezure & Tanaka, 2000).
In adult cats, electrical stimulation of the vagus nerve produces short latency EPSPs in late inspiratory (Late-I; Feldman & Cohen, 1978; Cohen et al. 1993) and decrementing expiratory (E-Dec; Feldman & Cohen 1978) neurones, demonstrating vagal-mediated excitatory drive to expiratory neurones in the ventrolateral medulla. E-Dec neurones, in turn, inhibit a broad range of inspiratory neurones (Lindsey et al. 1987; Segers et al. 1987). Similar observations were made in rats (Ezure & Manabe, 1988; Manabe & Ezure, 1988; Parkes et al. 1994). Thus, electrical stimulation of the vagus nerve reliably elicits IPSPs in inspiratory neurones and lung inflation reduces respiratory-related phasic depolarization in all inspiratory neurones (Hayashi et al. 1996). These results, taken together, suggest that the BHE in vivo is mediated by a widespread inhibition of inspiratory neurones by E-Dec neurones (Hayashi et al. 1996).
Respiratory-modulated rhythmogenic networks functional in vitro are postulated to constitute the kernel of the larger network active in vivo (Feldman et al. 1990), localized in the preBötzinger Complex (preBötC) just caudal and ventral to the compact division of the rostral nucleus ambiguus (Smith et al. 1991). Inspiratory neurones predominate in this region, and can be classified based on: (i) the presence of delayed excitation (Type 1 neurones) or sag-rebound (Type 2 neurones) properties in the transverse slice (Rekling et al. 1996); and (ii) peri-inspiratory activity, such as Type III inspiratory (hyperpolarized before and after, but active during inspiration) and preinspiratory (active before and after, but hyperpolarized during inspiration; Pre-I) neurones in the en bloc preparation (Onimaru & Homma, 1992).
Type 1 and Pre-I neurones are proposed to play a causal role in respiratory rhythmogenesis (Rekling et al. 1996; Rekling & Feldman, 1998; Onimaru et al. 1988, 1997). We recorded from them, as well as other respiratory neurone types. If the BHE occurred exclusively via inhibition of rhythmogenic neurones, we predicted that only a subset of respiratory neurones would be hyperpolarized during the BHE, and that BHE-lengthened cycles would not be integer multiples of the control period. Instead, if the BHE occurred exclusively by disruption of respiratory drive but not of rhythmogenic mechanisms, we predicted that periods of cycles in which the BHE-lengthened cycles would be integer multiples of the control period, as seen when respiratory drive, but not rhythmogenic networks, are disrupted (Mellen et al. 2003). Preliminary results have appeared in abstract form (Mellen & Feldman, 1999).
Methods
Dissection
Sprague–Dawley rats (0–3 days old; n= 58) were used. In accordance with methods approved by the Institutional Animal Care and Use Committee, UCLA, rat pups were cooled to 5°C, decerebrated immediately rostral to the superior colliculus and transferred to a bath continuously perfused with artificial cerebrospinal fluid (ACSF) containing (mm): 128.0 NaCl, 3.0 KCl, 1.5 CaCl2, 1.0 MgSO4, 21.0 NaHCO3, 0.5 NaH2PO4 and 30.0 glucose, equilibrated with 95% O2–5% CO2, at 5°C.
The neuraxis with the heart, oesophagus, carotid artery, trachea, right vagus nerve, and lungs were retained as follows: after exposing the dorsal surface of the neuraxis, the animal was pinned out ventral surface upwards; the sternohyoid and sternomastoid muscles were cut to expose the trachea, vagi and carotid arteries. Musculature underneath and lateral to the right vagus nerve was removed (cleidomastoid, clavotrapezius and ornohyoid muscles). The trachea was cut at the larynx and separated from the oesophagus beneath it. Without damaging the carotid artery or the vagus nerve, the digastric and masseter muscles were removed to expose the ventral surface of the skull, and arteries rostral to the tympanic bulla were cut. The skull was transected rostral to the tympanic bulla, and the left vagus and carotid artery were cut. The occipital bone was removed to expose the ventral surface of the brainstem, and what remained of the skull was removed from the left side. The thorax was then split, and the lungs, heart, oesophagus and trachea were freed. The spinal column was then removed and the spinal cord was transected at the third thoracic segment.
The brainstem was pinned out ventral surface upwards on a Sylgard™ platform, and the lungs stabilized by pinning down the oesophagus at the rostral and caudal ends. After removing the dura from the ventral surface, the medulla was transected just rostral to the vagus nerve, which corresponded to a transection through the facial nucleus. A saline-filled cannula (22 gauge) connected to a computer-controlled precision syringe pump (Carnegie Medecin M100), with a branch to a manometer, was inserted into the trachea approximately 2 mm caudal to the larynx, and held in place with a suture (Fig. 1). The bath was warmed to 26–28°C before recording activity. The dissection, from start to finish, was routinely completed in under an hour.
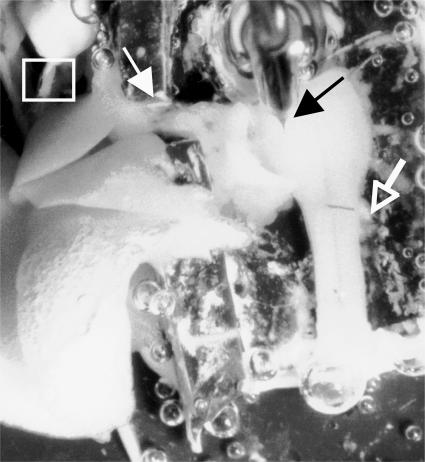
Figure 1. View of the preparation in the recording chamber.
The brainstem–spinal cord preparation is pinned out on a Sylgard™ (Dow Corning) platform. An intracellular recording electrode (black arrow) is positioned for recording in the rostral ventrolateral medulla. Population activity is recorded off ventral roots C1–C3 (white hollow arrow). The lungs and the right vagus nerve (thin white arrow) are visible on the right. Pressure changes to the lungs are applied via a cannula inserted in the trachea (white box, upper left).
Because respiratory efforts persisted following decerebration, the lungs were filled with ACSF so that they did not collapse following opening of the thorax. As a result, we were able to obtain the BHE from the outset with pressure changes in the physiological range (2–3 cmH2O). In cases in which expiratory lengthening did not occur, we gradually increased lung volume by withdrawing slightly less ACSF from the lungs than was injected. Once expiratory lengthening was observed, lung inflation and deflation were made symmetric.
Recording methods
Ventral root recordings.
Inspiratory activity was recorded from ventral roots C1–C3 using a saline-filled low resistance glass suction electrode (100 kΩ). Signals were amplified 15 000–30 000 times, and bandpass filtered (0.3–3 kHz) using Grass P5 differential amplifiers (Grass Instruments, Quincy, MA, USA).
Intracellular recordings.
Intracellular current-clamp recordings were carried out using the blind whole-cell patch clamp technique (Blanton et al. 1989) from inspiratory and expiratory neurones in a region extending rostrocaudally from the rostral ventral respiratory group to the Bötzinger complex (Smith et al. 1991), to depths of 200–600 μm from the ventral surface, near the base of the hypoglossal rootlets. Electrodes were pulled from filamented glass capillary tubing (1.5 mm o.d., 0.86 mm i.d.; A-M Systems). Lucifer Yellow CH (0.2%, Molecular Probes) and biocytin (0.2%, Molecular Probes) were added to the intracellular solution for subsequent identification of recording locations. In order to mitigate sampling bias towards larger cells, electrodes with resistances ranging from 4 to 12 MΩ were used.
Recordings were carried out in current-clamp bridge mode using the Axoclamp-2A amplifier (Axon Instruments). Because of the high input resistance, a 0.01X gain headstage was used. Capacitance was neutralized. An increase in the voltage deflection associated with a high-frequency (100 Hz, 0.2 duty cycle) 120 pA square wave passed through the electrode indicated contact with the ventral surface of the brainstem. The electrode was then quickly advanced 200–500 μm while applying strong positive pressure. Thereafter, the electrode was advanced in 3–5 μm steps, while applying moderate positive pressure and passing 120 pA pulses through the tip. A sudden increase in voltage deflection indicated the close proximity of a cell. Pulse amplitude was then decreased to 20 pA. To facilitate membrane rupture, a hyperpolarizing bias current was passed to hold the electrode at –90 mV. When less than 10 pA of current was required to maintain −90 mV, the membrane was ruptured by applying negative pressure to the electrode tip. Access resistance was 10–20 MΩ. Only neurones with Vm <−40 mV and action potential overshoot were studied. Series resistance was balanced before applying bias currents. Input resistance (Rin) was estimated by measuring the voltage deflection associated with a 2 s 20 pA hyperpolarizing pulse, applied in mid-expiration. Liquid junction potentials were not compensated. Step currents were applied using a pulse generator (AMPI Master 8), triggered by the instrumentation control computer (see below).
Bath application of drugs
In some experiments, pharmacological manipulations were carried out by adding a concentrated agonist–antagonist solution to the ACSF to obtain the appropriate bath concentration. The following pharmacological agents were used (μm): 10 glycine receptor antagonist strychnine (STR), 10 GABAA antagonist bicuculline (BIC), 20 non-NMDA ionotropic glutamate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), and 10 NMDA receptor antagonist (±)-2-amino-5-phosphonopentanoic acid (APV). Bath application of each drug was followed by an equilibration period of at least 5 min.
Data acquisition, signal processing and instrumentation control
Ventral root recordings, intracellular voltage recordings, applied bias currents and square pulses used to control the syringe pump and pulse generator were digitized at 20 kHz using an A/D–D/A board (AT-MIO-64-E3; National Instruments, Austin, TX, USA) running on a Pentium-based computer under a Windows operating system. These data were displayed using virtual instrumentation developed in LabView™ (National Instruments) and written to hard disk. In addition, time stamps associated with inspiratory onsets and lung inflation and deflation were written to hard disk.
A second computer, equipped with an A/D–D/A board (AT-MIO-16E-10; National Instruments) was used for instrumentation control. Lung inflation and current pulse application were triggered off inspiration with a 3–5 s delay, so that all stimuli were applied before mid-expiration.
Experimental protocol
Lung inflation protocols.
Lungs were inflated to volumes of 0.2–0.4 ml at ∼0.1 ml s−1, which resulted in steady-state changes of 2–5 mmH2O within the physiological range (4–7 mmH2O; Widdicombe, 1961) as measured using a manometer connected to the cannula. Lungs were held inflated until the subsequent inspiratory burst. Inflation protocols were carried out in bouts. Within each bout, lung inflation was repeated three to five times, and cycles with inflation were separated by at least five respiratory cycles in which no inflation was applied. In order to calculate the reversal potential of inflation-induced hyperpolarization in individual neurones, depolarizing and hyperpolarizing bias currents were applied before lung inflation.
Measurement of membrane properties.
Delayed excitation, consistent with the presence of transient outward currents (IA; Connor & Stevens, 1971), and sag rebound response consistent with an h-type current (Ih; Pape et al. 1989), were assessed. These step de- and hyperpolarizations were applied 2–4 s after inspiratory onset, i.e. in mid-expiration, so that membrane responses would not be obscured by de- or hyperpolarizing inspiratory drive currents. For expiratory neurones a small hyperpolarizing bias was applied (<30 pA), just sufficient to block impulse activity.
To test for IA, a hyperpolarizing bias current sufficient to bring the membrane potential below −80 mV was applied, the bridge was balanced, and a depolarizing step current was applied sufficient to elicit tonic impulse activity. To test for Ih, a series of step hyperpolarizations were applied from the resting membrane potential. In addition, this protocol tested for the presence of low-voltage-activated Ca2+ conductances.
Data analysis
Motor output analysis.
For both control and test cycles within a bout, the cycle period was defined as the interval from one inspiratory burst onset to the next. Within each bout, mean control (n > 20) and test (n > 7) cycle periods were calculated. Inflation-induced expiratory lengthening was tested using Student's paired t test on experiment means within Origin (Microcal™). The modulo function within Excel (Microsoft) was used to test whether test cycle periods were integer multiples of the mean control period within each bout.
Single neurone analysis.
Neurones were classified based on their conductances (see Measurement of membrane properties, above) and their membrane trajectories in relation to the respiratory motor pattern (see Introduction). This was done by burst-triggered averaging, i.e. averaging single neurone activity in a 1.5 s peri-inspiratory window, centred on ventral root inspiratory onset. In order to identify how intrinsic properties and synaptic inputs shaped the baseline activity pattern of respiratory neurones, three criteria were used: (i) membrane properties using methods described above; (i) voltage trajectory in control respiratory cycles; and (iii) voltage trajectory in test cycles.
Histology
At the end of each experiment, the brainstem was fixed in 10% formalin for at least 24 h, then sectioned (200 μm thick) with a Vibratome. Sections were first viewed under fluorescence to locate the Lucifer Yellow-labelled somata. The section containing the soma, as well as three or four neighbouring sections (for a total of 800–1000 μm), were then processed using an avidin–biotin immunoperoxidase kit (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA, USA), mounted on slides, counterstained with Neutral Red and coverslipped in mounting medium (Cytoseal 60, Stephens Scientific). Soma locations were then compiled using a reference set of drawings made from scanned serial sections of a brainstem and a standard neonate rat brain atlas (Altman & Bayer, 1995). Soma locations in relation to the mediolateral and dorsoventral axes were identified based on the relative distance of the soma with respect to the compact formation of the nucleus ambiguus, the inferior olive and the ventral surface; the rostrocaudal locations were estimated based on the number of sections away from the rostral margin of the inferior olive, which in the angle of sectioning used here, was caudal to the facial nucleus. We display our data with reference to the obex, which we define as the point where the central canal opens. Because of tissue damage during sectioning, not all neurones recorded from were included for analysis.
Results
Respiratory motor output response to lung inflation
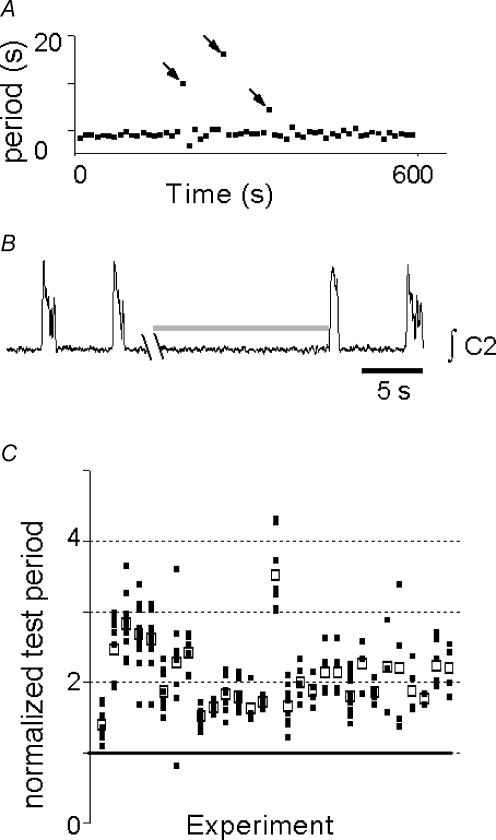
The mean control respiratory cycle period across experiments (n= 58) was 9.5 ± 0.5 s and was stable within each experiment (Fig. 2A). Mid-expiratory lung inflation consistently lengthened the test cycle period (Fig. 2B and C 20.0 ± 1.3 s, P < 0.01). While the mean of test cycle period means was a near-integer multiple of the control period, the individual test cycles were widely dispersed (Fig. 2C).
Figure 2. Mid-expiratory inflation lengthened expiration.
A, raster plot of periods collected over 10 min. Cycles with inflation (i.e. test cycles) are indicated by arrows. B, rectified integrated C2 ventral root activity (∫C2) showing typical response to inflation (shaded bar). Syringe pump noise during inflation has been removed for clarity. C, test periods, normalized so that control period = 1 (thick continuous line); □, individual normalized test cycle periods; ▪, mean test cycle period for each experiment. If expiratory lengthening were due only to suppression of inspiratory drive to motoneurones, then normalized periods would cluster at integer values. While normalized test period means tended to cluster at 2, the individual normalized test period means were dispersed, suggesting that inflation-induced expiratory lengthening is at least in part due to resetting of rhythmogenic networks.
Anatomical location of recorded neurones
Neurones (n= 67) for which anatomical location could be determined were recorded in and around the preBötC (Fig. 3, right). Neurones were classified as biphasic (n= 10), expiratory (n= 9), inspiratory (n= 36) and preinspiratory (n= 11). Inspiratory neurones were further classified into subgroups (see Methods). While most inspiratory neurones were clustered at the obex or 240 μm rostral to it, other neurone types were dispersed along the ventral respiratory column (Fig. 3, left).
Figure 3. Location of recorded cell somata.
Sections are 60 μm apart, with the most caudal segment (top) at the level of the obex. Diamonds, hyperpolarized inspiratory neurones; crosses, excited inspiratory neurones; circles, Pre-I neurones; asterisks, biphasic neurones, hypothesized to correspond to decrementing expiratory neurones in vivo; triangles, hyperpolarized expiratory neurones. Abbreviations: CN, cuneate nucleus; N12, hypoglossal nucleus; IO, inferior olive; VN, vestibular nucleus; N5, trigeminal nucleus; N10, dorsal motor nucleus of vagus; NA, nucleus ambiguus. Graph on the left shows normalized cell counts at each section level.
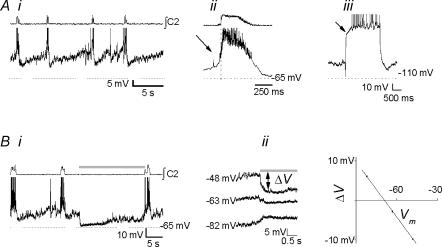
Biphasic neurones.
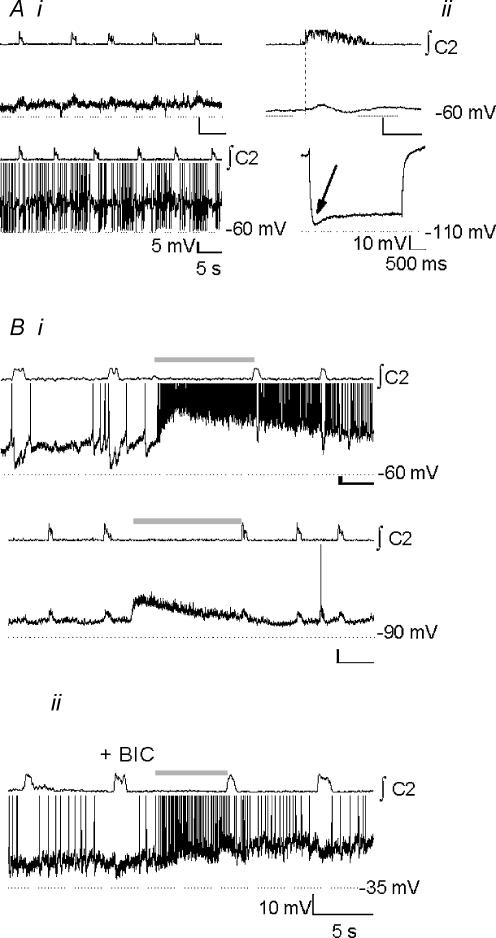
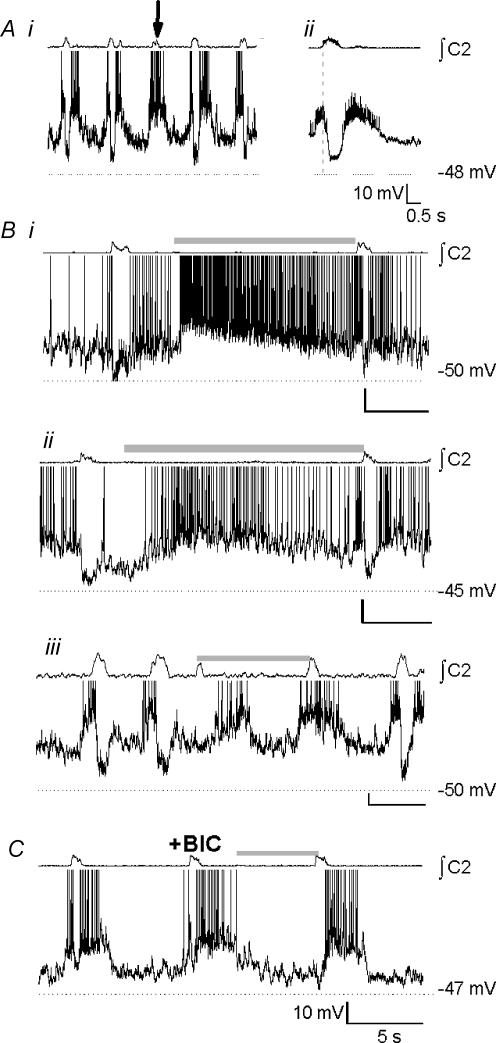
Biphasic neurones (n= 10) were silent in control cycles (Fig. 4Ai, top), and fired during expiration when depolarized (Fig. 4Ai, bottom). The burst-triggered average revealed a mixture of excitatory and inhibitory drive during inspiration (Fig. 4Aii, top). These neurones showed a sag-rebound response to step hyperpolarization (5/9; Fig. 4Aii, bottom). The average membrane potential during the expiratory period of control cycles was −53.7 ± 1.9 mV, with a mean input resistance of 762 ± 153 MΩ. All these neurones were excited during the BHE, and fired briskly during and after inflation (Fig. 4Bi, top); the maximal firing rate was 11.8 ± 3.7 Hz. When hyperpolarized below spike threshold, strong inflation-induced depolarizing currents were apparent (Fig. 4Bi, bottom). After BIC was applied to block the BHE, these neurones continued to be strongly depolarized during inflation (Fig. 4Bii).
Figure 4. Biphasic neurones.
Ai, baseline activity at resting membrane potential (top) and with depolarizing bias to −47 mV (bottom) reveals inhibition during inspiration. Aii, top, burst-triggered average activity reveals biphasic activation (n= 8 control cycles); bottom, sag-rebound response consistent with an Ih-like current (arrow) was elicited by step hyperpolarization to −100 mV. Bi, top, neurones fired briskly with little adaptation during inflation (shaded bar); bottom, inflation-induced depolarization persisted when the neurone was hyperpolarized below spiking threshold. Bii, in the presence of BIC (10 μm), the BHE was blocked, but excitatory drive to biphasic neurones was not.
Expiratory neurones
Expiratory neurones (n= 9) were hyperpolarized during inspiratory bursts, and otherwise fired late in (Fig. 5A), or throughout (Fig. 5B), expiration at 3.9 ± 2.0 Hz. Membrane potential during expiration was −45.0 ± 5.7 mV, with a mean input resistance of 896 ± 345 MΩ. The BHE-induced hyperpolarization (n= 3; Fig. 5Ai, bottom), cessation of spiking (n= 4; Fig. 5B, bottom), or no change from baseline (n= 2; not shown). In neurones hyperpolarized during inflation, the reversal potential of the inflation-induced hyperpolarization was −65.0 ± 4.1 mV, more negative than the reversal potential of inspiratory inhibition (− 59.0 ± 4.5 mV, P < 0.05; Fig. 5Aii).
Figure 5. Expiratory neurones.
Ai, control activity (top), and inflation-induced inhibition (bottom, shaded bar). Aii, left, de- and hyperpolarizing bias currents during inspiratory inhibition (shaded traces) and inflation-induced inhibition (black traces) were applied. Based on the resulting change in membrane potential accompanying inhibitory drive (ΔV) the reversal potential (Vrev) for both can be calculated; right: Vrev of BHE inhibition was more negative than Vrev of inspiratory inhibition. B, other expiratory neurones with uniform firing frequency under baseline conditions (top) showed reduced spike frequency during BHE, but little hyperpolarization (bottom).
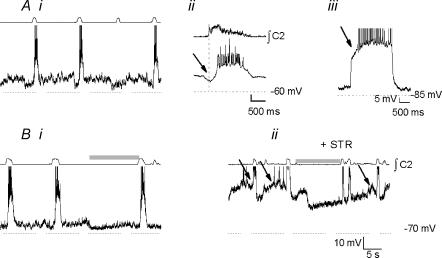
Inspiratory neurones
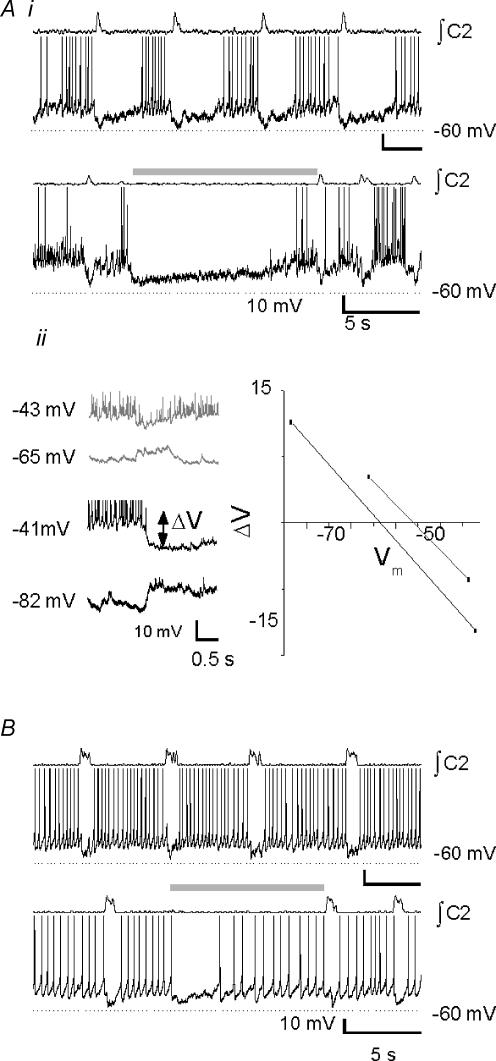
Type-1 inspiratory neurones (n= 12; Rekling et al. 1996) are characterized by ramp-like depolarization during expiration (Fig. 6Ai), early onset of inspiratory depolarization (280 ± 44 ms before inspiratory burst onset; Fig. 6Aii), and delayed excitation consistent with an IA-like current (Fig. 6Aiii). They were strongly hyperpolarized during the BHE (Fig. 6Bi). Applied bias currents (Fig. 6Bii, left) amplified or reversed BHE-induced hyperpolarization (ΔV; Fig. 6Bii, left), allowing estimation of its reversal potential (Vrev; −66.0 ± 3.5 mV; n= 9; Fig. 6Bii, right). The average membrane potential of these neurones was −53.0 ± 1.0 mV, with a mean input resistance of 616 ± 100 MΩ. Disruption of fast synaptic transmission by bath application of BIC, STR, CNQX and APV revealed endogenous bursting properties in 3/12 neurones (not shown).
Figure 6. Type-1 inspiratory neurones (Rekling et al. 1996).
Ai, control activity. Aii, burst-triggered average activity (n= 8 control cycles); note preinspiratory depolarization (arrow). Aiii, delayed excitation consistent with an IA-like current (arrow) was elicited by step depolarization to −30 mV from −85 mV. Bi, these neurones were strongly hyperpolarized during mid-expiratory inflation (shaded bar). Bii, by applying bias currents, inflation-induced hyperpolarization (shaded bar) could be reversed. ΔV, difference between holding membrane potential and membrane potential during inflation (double-headed arrow). The reversal potential (i.e. ΔV= 0) was calculated to be −66 mV.
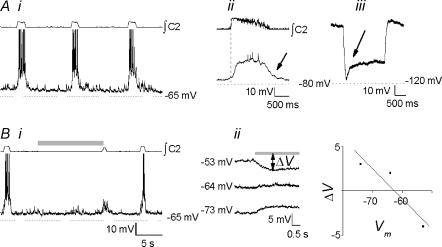
Type III inspiratory neurones (Onimaru et al. 1997; n= 8) are characterized by pre- and postinspiratory hyperpolarization (Fig. 7Ai and Aii); under our experimental conditions, postsynaptic hyperpolarization was typically only apparent when depolarizing bias currents were applied (not shown). They displayed delayed excitation consistent with an IA-like current (Fig. 7Aiii), and were hyperpolarized by inflation (Fig. 7Bi). STR did not block hyperpolarization (10 μm, n= 2), but did abolish peri-inspiratory hyperpolarization (Fig. 7Bii). These neurones had an average membrane potential of −56.0 ± 2.0 mV and a mean input resistance of 553 ± 105 MΩ.
Figure 7. Type-III inspiratory neurones (Onimaru et al. 1997).
Ai, baseline activity. Note signature pre- and postinspiratory hyperpolarization, particularly apparent in the cycle in which the neurone failed to fire. Aii, burst-triggered average activity (n= 8 control cycles). Note preinspiratory inhibition (arrow). Aiii, delayed excitation consistent with an IA-like current (arrow) was elicited by step depolarization to −30 mV from −79 mV. Bi, neurones were hyperpolarized by mid-expiratory inflation (shaded bar). Bii, following bath application of STR (10 μm), preinspiratory inhibition was blocked (arrows), unmasking the expiratory ramp, the BHE was unaffected, and inflation-induced hyperpolarization persisted.
Type 2 neurones (n= 14) are characterized by flat membrane trajectory during expiration (Fig. 8Ai), depolarization onset coincident with inspiratory onset (Fig. 8Aii), and sag-rebound responses to hyperpolarizing current pulses consistent with an Ih current (Fig. 8Aiii; Rekling et al. 1996). These neurones were weakly hyperpolarized during the BHE (Fig. 8Bi). The poor linear fit to ΔV values obtained by applying bias currents during the BHE (Fig. 8Bii) may be due to activation of Ih currents at Vm <−70 mV, which would shunt the reversed inflation-induced current. Thus, the estimated Vrev of the inflation-induced hyperpolarization (−62.5 ± 0.7 mV; n= 4; Fig. 8Bii, left) is probably too negative. These neurones had an average membrane potential of −56.0 ± 3.0 mV and a mean input resistance of 574 ± 89 MΩ.
Figure 8. Type 2 neurones (Rekling et al. 1996).
Ai, baseline activity. Note flat membrane trajectory during expiration. Aii, burst-triggered average activity (n= 8 control cycles). Note postinspiratory depolarization (arrow). Aiii, sag-rebound response consistent with an Ih-like current was obtained by application of step hyperpolarization to −115 mV from resting mid-expiratory membrane potential (arrow). Bi, inflation (shaded bar) produced weak inhibition, but subthreshold inspiratory drive during the first inspiratory burst following inflation offset (arrow). Bii, by applying de- and hyperpolarizing bias currents during BHE (shaded bar), inflation-induced inhibition was reversed. ΔV, difference between holding membrane potential and membrane potential during inflation (double-headed arrow). The reversal potential was calculated to be −62.5 mV. This value was probably skewed towards more hyperpolarized values by the shunt associated with the Ih-like current.
Two inspiratory neurones with control activity similar to Type III inspiratory neurones (Fig. 9Ai) showed preinspiratory hyperpolarization (Fig. 9Aii), and displayed both IA-like (Fig. 9Bi), and Ih-like (Fig. 9Bii) properties. They were strongly excited during the BHE (Fig. 9C), attaining maximal firing rates of 13.5 ± 3.2 Hz; because of this BHE response they need to be classified separately from hyperpolarized Type III neurones. These neurones had an average membrane potential of −58.0 ± 2.8 mV and a mean input resistance of 750 ± 70 MΩ.
Figure 9. Inspiratory neurones excited by inflation.
Ai, baseline activity. Aii, burst-triggered baseline average activity (n= 8 control cycles); reveals preinspiratory inhibition (arrow) similar to type III neurones. Bi, delayed excitation consistent with an IA-like current (arrow) was elicited by step depolarization to −30 mV from −80 mV. Bii, sag-rebound response consistent with an Ih-like current was obtained by application of step hyperpolarization to −110 mV from resting mid-expiratory membrane potential (arrow). C, neurones fired briskly during inflation (shaded bar).
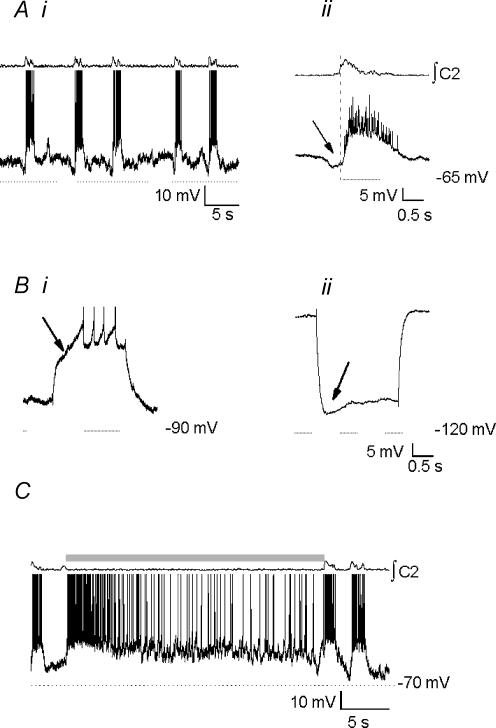
Pre-I neurones
Pre-I neurones (n= 11) fire before and after, and are inhibited during inspiration (Onimaru et al. 1988; Fig. 10A. Pre-inspiratory firing lasted 690 ± 10 ms and postinspiratory firing lasted 2480 ± 230 ms; inspiratory hyperpolarization began 237 ± 60 ms after inspiratory onset and ended 232 ± 70 ms after inspiratory offset. In cycles in which the inspiratory burst was not accompanied by inhibition of Pre-I neurones, peri-inspiratory excitatory drive was apparent (Fig. 10A, arrow). Because strong synaptic drives were present throughout the respiratory cycle, it was impossible to test for Ih or IA. The response to the BHE was variable. Of 11 neurones recorded, one was immediately excited (Fig. 10Bi), four were depolarized within 1 s (680 ± 10 ms; Fig. 10Bii) and six were depolarized more than 1 s after inflation (2450 ± 930 ms; Fig. 10Biii). Inflation-induced depolarization was blocked following addition of BIC (10 μm; n= 3, Fig. 10C). These neurones had an average membrane potential of −48.0 ± 1.0 mV and a mean input resistance of 460 ± 100 MΩ. Following disruption of fast synaptic transmission using STR (10 μm), BIC (10 μm), CNQX (20 μm) and APV (20 μm), 2/11 Pre-I neurones exhibited endogenous bursting properties.
Figure 10. Pre-inspiratory neurones.
Ai, baseline activity. Note peri-inspiratory firing in cycle lacking inspiratory inhibition (arrow). Aii, burst-triggered average activity. Neurones were depolarized before and after, and hyperpolarized during inspiration. Inspiratory inhibition began 240 ms after inspiratory onset (average of 8 cycles). Both fast (Bi) and slow (Bii and iii) excitation was observed during inflation (shaded bar). C, inflation-induced depolarization of Pre-I neurone was blocked by addition of BIC to the perfusate, for a final concentration of 10 μm.
Discussion
Significance of systems level responses
In an en bloc in vitro preparation with the lungs attached and innervated, we were able to reproduce the BHE. Appropriate expiratory lengthening followed lung inflations at pressures in the physiological range, in contrast to the minutes-long apnoeas obtained in response to lung hyperinflation (Murakoshi & Otsuka, 1985). Neurones were excited or hyperpolarized by inflation, similar to changes accompanying lung inflation but not electrical stimulation of the vagus nerve in vivo (Hayashi et al. 1996). This suggests that SAR afferents, which underlie the BHE in vivo (Adrian, 1933), were selectively activated. The effect of inflation on respiratory frequency and on respiratory-modulated neurones was enhanced compared to responses in the anaesthetized adult rat (Hayashi et al. 1996) or cat (Feldman & Cohen, 1978) in vivo. This difference may be due to stronger mechanoreceptor activation in fluid-filled (and hence more compliant) lungs, greater sensitivity to pulmonary mechanoreceptor feedback in neonates (Fedorko et al. 1988), or anaesthesia-induced neuronal depression in adult in vivo preparations (Stucke et al. 2002). Furthermore, the consistent and robust expiratory lengthening obtained here validates the in vitro preparation as a model for eupnoeic breathing, as SAR afferent modulation of respiratory rhythm, proposed as the defining criterion of eupnoea (Pluta & Romaniuk, 1990), is not observed during hypoxia-induced gasping in vivo (Remmers, 1999; Richter, 2003).
Since our en bloc preparation does not include the pons, our results show that medullary networks alone are sufficient to obtain the BHE. Similarly, the BHI is not under control of the pons (Karius et al. 1991; Bianchi et al. 1995; Mellen & Feldman, 2001), and BH reflexes can be induced following depression of the pons (Feldman et al. 1992). Thus the basic circuitry for the BH reflexes is contained in the medulla, with the pons playing a modulatory role.
We considered the possibility that the BHE bypasses rhythmogenic networks to act exclusively via inhibition of motor output. If this were the case, then test cycle periods would cluster at integer multiples of the control period, as seen when respiratory drive but not rhythm generation is disrupted (Mellen et al. 2003). This was not the case, since individual test cycle periods were widely dispersed (Fig. 2C). This suggests that the BHE (also) affects rhythmogenic networks.
Anatomical distribution of recorded neurones
Our recording locations spanned the ventral respiratory column (Alheid et al. 2002), including regions caudal and rostral to the preBötC that contain premotor networks projecting to cranial and spinal motoneurones. We recorded from Pre-I neurones both rostral to and at the caudal margin of the preBötC. Pre-I neurones at or caudal to the preBötC are bulbospinal abdominal premotoneurones that are driven by more rostral Pre-I neurones (Janczewski et al. 2002). Thus, because of the distribution of recording locations, we surmise that our sample includes rhythmogenic and (pre)motor neurones.
One of the goals of this study was to establish whether the BHE could be used to distinguish rhythmogenic from other networks. Because of the heterogeneity of neurones sampled, we are able to evaluate whether this distinction is possible. In the sections that follow, we interpret the responses of expiratory, inspiratory and Pre-I neurones in the context of findings in vivo and in vitro.
Biphasic neurones
Biphasic neurones showed weak respiratory modulation (Fig. 4A), but were strongly excited by lung inflation. The strong depolarizing drive revealed when these neurones were hyperpolarized by current injection (Fig. 4Bi, bottom) indicates that lung inflation-induced firing was due to increased synaptic drive. These attributes closely match those of SAR relay neurones in adult rats in vivo, which are found in the dorsal medulla (Bonham & McCrimmon, 1990; Ezure et al. 2002). Because of their location in the ventral respiratory column, biphasic neurones are likely to be third-order neurones in the pathway mediating the BHE. Based on the time course of inflation-induced hyperpolarization of expiratory (Fig. 5) and inspiratory neurones (Figs 6–8), we hypothesize that biphasic neurones inhibit respiratory neurones involved in generating and relaying respiratory drive.
In adult mammals in vivo, two classes of neurones are hypothesized to have the same inhibitory function as we ascribe to biphasic neurones in vitro: E-Dec neurones (Hayashi et al. 1996), identified as glycinergic (Ezure et al. 2003), and Late-I neurones (Cohen et al. 1993; Haji et al. 2002), inferred to be GABAergic (Haji et al. 1999,2002). The finding that the BHE in vitro was unaffected by the glycine receptor antagonist strychnine (Fig. 7) would suggest that biphasic neurones are GABAergic, and thus correspond to Late-I neurones. One caveat is that the transmitter phenotype of biphasic neurones could undergo a developmentally regulated transition from GABA to glycine, as observed elsewhere in the rat CNS (Bruning et al. 1990; Colin et al. 1998; Turecek & Trussell, 2002; Nabekura et al. 2004). Relating biphasic neurones in neonates in vitro to adult in vivo neurone types is further complicated by the fact that biphasic neurones show weaker respiratory modulation and stronger afferent feedback modulation than either E-Dec or I-Late neurones in adults in vivo. The weak respiratory modulation of biphasic neurones could result from the absence in our preparation of the pons, which contains neurones that fire in the postinspiratory phase (Dick et al. 1994). The stronger afferent modulation of biphasic neurones may also be due to the absence of anaesthesia-induced increase in GABAergic inhibition (Olsen, 1998; Stucke et al. 2002), or their higher input resistance, due to fewer dendritic branches and synaptic inputs in neonatal rat medullary neurones (Hilaire & Duron, 1999).
Expiratory neurones
Most expiratory neurones were hyperpolarized during the BHE. This is consistent with the hypothesis that propriobulbar expiratory neurones provide excitatory drive to rhythmogenic preBötC networks (Smith et al. 1993). Alternatively, some of these neurones may have been cranial motoneurones, which are active during expiration and are inhibited by electrical stimulation of the vagus (Hayashi & McCrimmon, 1996). We found that the reversal potential of the BHE-induced hyperpolarization was more negative than that accompanying inspiratory bursts (Fig. 5Aii). Inhibition of expiratory neurones during inspiration is glycinergic in neonatal rats (Shao & Feldman, 1997). We hypothesize that hypepolarization necessary for the BHE is GABAergic. This is supported by the observation that the BHE persisted in the presence of strychnine (Fig. 7Bii), but was blocked by bicuculline (Figs 4Bii and 10C). The more negative reversal potential of the BHE-induced inhibition may be due to coactivation of K+-permeable GABAB receptor-linked channels in preBötC neurones in the neonatal mouse (Zhang et al. 1999).
Inspiratory neurones
Inspiratory neurones were hyperpolarized during the BHE. Type 1 and Type III neurones, which display delayed excitation consistent with IA but not sag-rebound properties consistent with Ih, were strongly hyperpolarized by lung inflation (Fig. 6Bi). For these neurones, their ΔV/V plots were well fitted by straight lines (Fig. 6Bii). Inflation-induced hyperpolarization would partially deinactivate IA, and so could amplify inflation-induced inhibition by delaying the postinflation return to resting Vm.
By contrast, inflation-induced hyperpolarization was markedly smaller in Type 2 neurones. The reversal potential for BHE-induced hyperpolarization in Type 2 neurones is likely to be less negative than the estimates we obtained; by biasing Vm below −70 mV, inflation-induced hyperpolarization was reversed, but because of partial activation of Ih (Thoby-Brisson et al. 2000), it would also be shunted. This may account for the poor linear fit to points in ΔV/Vm plots that included values at holding potentials at which Ih was weakly, then more strongly activated (Fig. 8Bii). While the reversal potential for Cl−-mediated hyperpolarization (∼−75 mV in neonatal rats; Shao & Feldman, 1997) is well below the Ih activation threshold (−55 mV; Thoby-Brisson et al. 2000), the sag-rebound characteristic of Ih activation (Fig. 8Aiii) is not apparent during inflation-induced hyperpolarization (Fig. 8Bi and Bii), suggesting that weak hyperpolarizations during the BHE in Type 2 neurones is not due to immediate Ih-induced shunting of inhibitory drive.
A subset of I neurones, qualitatively similar to Type III neurones, were strongly excited during the BHE (Fig. 9). Thus, despite their similar baseline activity pattern, it is unlikely that they are functionally equivalent to Type III neurones hyperpolarized during the BHE. Their strong activation during the BHE suggests that they mediate restoration of expiratory flow.
Type 1 neurones are proposed to play a causal role in respiratory rhythmogenesis (Rekling et al. 1996). Respiratory period is hypothesized to be strongly modulated by changes in membrane potential of rhythmogenic neurones (Smith et al. 1993). A generic prediction of models of bursting mechanisms is that cycle period varies with rhythmogenic neurone membrane potential; above some threshold, cycle period lengthens as membrane potential hyperpolarizes (ibid.). Thus, the strong hyperpolarization of Type 1 neurones during the BHE is consistent with their hypothesized role as constituents of the respiratory rhythm generator (Rekling et al. 1996; Rekling & Feldman, 1998). By the same logic, the weaker hyperpolarization seen in Type 2 neurones suggests that they are not constituents of a voltage-dependent rhythmogenic network, consistent with the conjecture that they are premotoneurones (Rekling et al. 1996).
Although the strong hyperpolarization of Type 1 neurones provides a mechanism for the BHE's action on rhythmogenic networks, this evidence is weak. If Type 1 neurones were the only class of inspiratory neurones hyperpolarized by the BHE, then the inference that Type 1 neurones were necessary for respiratory rhythmogenesis would be relatively straightforward. The observation that the majority of inspiratory neurones were hyperpolarized suggests that both rhythmogenic and relay neurones are modulated during the BHE, and strength of hyperpolarization must be interpreted carefully. Thus functional inferences based solely on responses during the BHE are by necessity incomplete. When combined with other criteria, including immunohistochemistry (Gray et al. 1999, Stornetta et al. 2003) and morphology, responses during the BHE may support stronger functional inferences.
Relating in vitro inspiratory neurone classes to those used in vivo on the basis of their responses during the BHE is difficult because, while differences in BHE-induced hyperpolarization were apparent in distinct inspiratory neurone classes in vitro (Figs 6–8), such differences are not observed across inspiratory neurone classes in vivo. This may be due to the developmental changes (Hilaire & Duron, 1999) or experimental differences (Stucke et al. 2002) indicated above.
Pre-I neurones
Pre-I neurones are proposed as essential constituents of respiratory rhythm-generating networks (Onimaru & Homma, 1987). Pharmacological manipulations (Mellen et al. 2003) and optical recordings (Onimaru & Homma, 2003) suggest that respiratory rhythm is generated by the interaction between preBötC and rostral Pre-I networks, each of which, under appropriate conditions, can determine inspiratory burst timing. The causal role of Pre-I neurones is supported by the consistent observation of Pre-I firing immediately preceding inspiratory burst onset during control cycles. During the BHE, however, that coupling was lost; lung inflation induced either an immediate (Fig. 10Bi) or delayed (Fig. 10Bii and Biii) Pre-I neurone firing, followed not immediately but rather seconds later by the subsequent inspiratory burst onset. Mid-expiratory Pre-I firing during the BHE suggests that in test cycles, Pre-I activity does not determine inspiratory burst onset. This mid-expiratory activity is probably due to disinhibition rather than excitatory drive from second- or third-order relay neurones, since it is blocked by BIC (Fig. 10C). Because caudal Pre-I neurones project bulbospinally to motoneurones innervating expiratory abdominal muscles (Janczewski et al. 2002), Pre-I neurone activity during the BHE may underlie an airway-clearing manoeuvre via abdominal muscle contraction. Such a response is seen in dogs, in response to moderate airway pressure changes (Koepchen et al. 1973; Bajic et al. 1992; reviewed by Iscoe, 1998). Pre-I and biphasic neurones are both hyperpolarized during inspiration and excited during the BHE; thus, these neurones could represent extremes of a continuum. The following observations suggest that this is not the case. In the presence of inflation during inspiration, biphasic neurones fired during inspiration, whereas Pre-I neurones were hyperpolarized during inspiration (Mellen & Feldman, 2001). In addition, bicuculline had no effect on the biphasic neurone response to lung inflation (Fig. 4) but blocked the Pre-I neurone response (Fig. 10). Thus, these neurone classes appear to be distinct. Based on their responses to phasic inflation, Pre-I neurones may correspond to augmenting expiratory neurones in vivo (Parkes et al. 1994; Hayashi et al. 1996).
Mappings between in vitro and in vivo preparations
An early discussion of the relationship between in vitro and in vivo preparations (Feldman et al. 1990) was framed in terms of two alternative hypotheses. In the reductionist hypothesis, the basic mechanisms for respiratory rhythm generation were stipulated to be the same in vivo at all ages and in vitro, so that differences between them were due to removal of nonessential complexity in vitro; in the transformational hypothesis, mechanisms for respiratory rhythm generation in neonatal rodents in vitro and in adult rodents in vivo were stipulated to be qualitatively different because of differences in milieu, afferent modulation and development. More recent findings do not support the strong form of either hypothesis. On the one hand, important morphological and electrophysiological changes occur over the course of development (Richter & Spyer, 2001). On the other hand, several key observations driving hypotheses about rhythmogenic mechanisms in neonate in vitro rodent preparations are congruent with those in adult rats in vivo (Gray et al. 2001; Mellen et al. 2003), suggesting that despite developmental transformations, neonatal in vitro preparations are useful model systems for studying eupnoea in adult mammals in vivo.
Despite the differences in adult in vivo and neonate in vitro respiratory neurone activity during the BHE, changes in activity during the BHE are qualitatively the same: neurones hyperpolarized during inspiration are depolarized during lung inflation, and inspiratory neurones are inhibited. More importantly, the observed differences could be accounted for by developmental or methodological differences proposed by others as factors that need to be taken into account when comparing in vitro and in vivo data (Richter & Spyer, 2001), suggesting that the transformation of cellular and network properties from in vitro to in vivo conditions obeys identified mechanisms.
Acknowledgments
This research was supported by the following grants: The American Lung Association, RG 105-N; and National Institutes of Health, HL40959 and HL37941.
References
- Adrian ED. Afferent impulses in the vagus and their effect on respiration. J Physiol. 1933;79:332–358. doi: 10.1113/jphysiol.1933.sp003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol. 2002;31:693–717. doi: 10.1023/a:1025799830302. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Atlas of Prenatal Rat Brain Development. Boca Raton, FL, USA: CRC Press; 1995. [Google Scholar]
- Bajic J, Zuperku EJ, Tonkovic-Capin M, Hopp FA. Expiratory bulbospinal neurons of dogs. I. Control of discharge patterns by pulmonary stretch receptors. Am J Physiol. 1992;262:R1075–R1086. doi: 10.1152/ajpregu.1992.262.6.R1075. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Meth. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Bonham AC, Coles SK, McCrimmon DR. Pulmonary stretch receptor afferents activate excitatory amino acid receptors in the nucleus tractus solitarii in rats. J Physiol. 1993;464:725–745. doi: 10.1113/jphysiol.1993.sp019660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham AC, McCrimmon DR. Neurons in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J Physiol (Lond) 1990;427:261–280. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer J. Self-steering of respiration through the nervus vagus [English transl.] In: Porter R, editor. Breathing: Hering-Breuer Centenary Symposium. Churchill, London: 1868. pp. 365–394. 1970. [Google Scholar]
- Bruning G, Bauer R, Baumgarten HG. Postnatal development of [3H]flunitrazepam and [3H]strychnine binding sites in rat spinal cord localized by quantitative autoradiography. Neurosci Lett. 1990;110(1–2):6–10. doi: 10.1016/0304-3940(90)90778-8. [DOI] [PubMed] [Google Scholar]
- Cohen MI, Huang WX, Barnhardt R, See WR. Timing of medullary late-inspiratory neuron discharges: vagal afferent effects indicate possible off-switch function. J Neurophysiol. 1993;69(5):1784–1787. doi: 10.1152/jn.1993.69.5.1784. [DOI] [PubMed] [Google Scholar]
- Colin I, Rostaing P, Augustin A, Triller A. Localization of components of glycinergic synapses during rat spinal cord development. J Comp Neurol. 1998;398(3):359–372. [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Bellingham MC, Richter DW. Pontine respiratory neurons in anesthetized cats. Brain Res. 1994;636(2):259–269. doi: 10.1016/0006-8993(94)91025-1. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Paton JF. Whole cell recordings from respiratory neurones in an arterially perfused in situ neonatal rat preparation. Exp Physiol. 2003;88(6):725–732. doi: 10.1113/eph8802639. [DOI] [PubMed] [Google Scholar]
- von Euler C. On the origin and pattern control of breathing rhythmicity in mammals. Symp Soc Exp Biol. 1983;37:469–485. [PubMed] [Google Scholar]
- Ezure K, Manabe M. Decrementing expiratory neurons of the Bötzinger complex. II. Direct inhibitory synaptic linkage with ventral respiratory group neurons. Exp Brain Res. 1988;72(1):159–166. doi: 10.1007/BF00248511. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Pump neurons of the nucleus of the solitary tract project widely to the medulla. Neurosci Lett. 1996;215(2):123–126. [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Lung inflation inhibits rapidly adapting receptor relay neurons in the rat. Neuroreport. 2000;11(8):1709–1712. doi: 10.1097/00001756-200006050-00023. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Kondo M. Glycine is used as a transmitter by decrementing expiratory neurons of the ventrolateral medulla in the rat. J Neurosci. 2003;23(26):8941–8948. doi: 10.1523/JNEUROSCI.23-26-08941.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Saito Y, Otake K. Axonal projections of pulmonary slowly adapting receptor relay neurons in the rat. J Comp Neurol. 2002;446(1):81–94. doi: 10.1002/cne.10185. [DOI] [PubMed] [Google Scholar]
- Fedorko L, Kelly EN, England SJ. Importance of vagal afferents in determining ventilation in newborn rats. J App Physiol. 1988;65:1033–1039. doi: 10.1152/jappl.1988.65.3.1033. [DOI] [PubMed] [Google Scholar]
- Feldman J. Neurophysiology of breathing in mammals. In: Bloom F, editor. Handbook of Physiology; Section I: The Neurons System. IV. Bethesda MD: American Physiological Society; 1986. pp. 463–524. [Google Scholar]
- Feldman JL, Cohen MI. Relation between expiratory duration and rostral medullary expiratory neuronal discharge. Brain Res. 1978;141(1):172–178. doi: 10.1016/0006-8993(78)90627-3. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Smith JC, Ellenberger HH, Connelly CA, Liu GS, Greer JJ, Lindsay AD, Otto MR. Neurogenesis of respiratory rhythm and pattern: emerging concepts. Am J Physiol. 1990;259:R879–R886. doi: 10.1152/ajpregu.1990.259.5.R879. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Windhorst U, Anders K, Richter DW. Synaptic interaction between medullary respiratory neurones during apneusis induced by NMDA-receptor blockade in cat. J Physiol. 1992;450:303–323. doi: 10.1113/jphysiol.1992.sp019128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P, Rekling J, Bocchiaro C, Feldman J. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4(9):927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji A, Okazaki M, Takeda R. GABA (A) receptor-mediated inspiratory termination evoked by vagal stimulation in decerebrate cats. Neuropharmacology. 1999;38(9):1261–1272. doi: 10.1016/s0028-3908(99)00057-x. [DOI] [PubMed] [Google Scholar]
- Haji A, Okazaki M, Yamazaki H, Takeda R. Physiological properties of late inspiratory neurons and their possible involvement in inspiratory off-switching in cats. J Neurophysiol. 2002;87(2):1057–1067. doi: 10.1152/jn.00470.2001. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, McCrimmon DR. Respiratory neurons mediating the Breuer-Hering reflex prolongation of expiration in rat. J Neurosci. 1996;16:6526–6536. doi: 10.1523/JNEUROSCI.16-20-06526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, McCrimmon DR. Respiratory motor responses to cranial nerve afferent stimulation in rats. Am J Physiol. 1996;271:R1054–R1062. doi: 10.1152/ajpregu.1996.271.4.R1054. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Duron B. Maturation of the mammalian respiratory system. Physiol Rev. 1999;79(2):325–360. doi: 10.1152/physrev.1999.79.2.325. [DOI] [PubMed] [Google Scholar]
- Iscoe S. Control of abdominal muscles. Prog Neurobiol. 1998;56(4):433–450. doi: 10.1016/s0301-0082(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol. 2002;545(3):1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karius DR, Ling LM, Speck DF. Lesions of the rostral dorsolateral pons have no effect on afferent-evoked inhibition of inspiration. Brain Res. 1991;559(1):22–28. doi: 10.1016/0006-8993(91)90282-z. [DOI] [PubMed] [Google Scholar]
- Koepchen HP, Klüssendorf D, Philipp U. Mechanisms of central transmission of respiratory reflexes. Acta Neurobiol Exp. 1973;33:287–299. [PubMed] [Google Scholar]
- Lindsey BG, Morris KF, Segers LS, Shannon R. Respiratory neuronal assemblies. Respir Physiol. 2000;122:183–196. doi: 10.1016/s0034-5687(00)00158-4. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Segers LS, Shannon R. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. II. Evidence for inhibitory actions of expiratory neurons. J Neurophysiol. 1987;57(4):1101–1117. doi: 10.1152/jn.1987.57.4.1101. [DOI] [PubMed] [Google Scholar]
- Manabe M, Ezure K. Decrementing expiratory neurons of the Bötzinger complex. I. Response to lung inflation and axonal projection. Exp Brain Res. 1988;72(1):150–158. doi: 10.1007/BF00248510. [DOI] [PubMed] [Google Scholar]
- Mellen NM, Feldman JL. Vagal stimulation induces expiratory lengthening in the in vitro neonate rat. J App Physiol. 1997;83:1607–1611. doi: 10.1152/jappl.1997.83.5.1607. [DOI] [PubMed] [Google Scholar]
- Mellen NM, Feldman JL. Functional analysis of respiratory neurons using the Breuer-Hering reflex in vitro. Soc Neurosci Abstract. 1999;24(1):532. [Google Scholar]
- Mellen NM, Feldman JL. Phasic lung inflation shortens inspiration and respiratory period in the lung-attached neonate rat brain stem spinal cord. J Neurophysiol. 2000;83:3165–3168. doi: 10.1152/jn.2000.83.5.3165. [DOI] [PubMed] [Google Scholar]
- Mellen NM, Feldman JL. Phasic vagal sensory feedback transforms respiratory neuron activity in vitro. J Neurosci. 2001;21(18):7363–7371. doi: 10.1523/JNEUROSCI.21-18-07363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37(5):821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94(1):358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Murakoshi T, Otsuka M. Respiratory reflexes in an isolated brainstem–lung preparation of the newborn rat: possible involvement of gamma-aminobutyric acid and glycine. Neurosci Lett. 1985;62:63–68. doi: 10.1016/0304-3940(85)90285-x. [DOI] [PubMed] [Google Scholar]
- Nabekura J, Katsurabayashi S, Kakazu Y, Shibata S, Matsubara A, Jinno S, Mizoguchi Y, Sasaki A, Ishibashi H. Developmental switch from GABA to glycine release in single central synaptic terminals. Nature Nsci. 2004;7(1):17–23. doi: 10.1038/nn1170. [DOI] [PubMed] [Google Scholar]
- Olsen RW. The molecular mechanism of action of general anesthetics: structural aspects of interactions with GABA (A) receptors. Toxicol Lett. 1998;100–101:193–201. doi: 10.1016/s0378-4274(98)00185-4. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Primary respiratory rhythm generator in the medulla of brainstem–spinal cord preparation from newborn rat. Brain Res. 1988;445(2):314–324. doi: 10.1016/0006-8993(88)91194-8. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Neuronal mechanisms of respiratory rhythm generation: an approach using in vitro preparation. Jap J Physiol. 1997;47:385–403. doi: 10.2170/jjphysiol.47.385. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Respiratory rhythm generator neurons in medulla of brainstem–spinal cord preparation from newborn rat. Brain Res. 1987;403:380–384. doi: 10.1016/0006-8993(87)90080-1. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Whole cell recordings from respiratory neurons in the medulla of brainstem–spinal cord preparations isolated from newborn rats. Pflügers Arch. 1992;420(3–4):399–406. doi: 10.1007/BF00374476. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23(4):1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Parkes MJ, Lara-Munoz JP, Izzo PN, Spyer KM. Responses of ventral respiratory neurones in the rat to vagus stimulation and the functional division of expiration. J Physiol. 1994;476(1):131–139. [PMC free article] [PubMed] [Google Scholar]
- Pluta R, Romaniuk JR. Recovery of breathing pattern after 15 min of cerebral ischemia in rabbits. J App Physiol. 1990;69:1676–1681. doi: 10.1152/jappl.1990.69.5.1676. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Champagnat J, Denavit-Saubié M. Electroresponsive properties and membrane potential trajectories of three types of inspiratory neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1996;75:795–810. doi: 10.1152/jn.1996.75.2.795. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Remmers JE. Central neural control of breathing. In: Altose M, Kawami Y, editors. Lung Biology in Health and Disease: Control of Breathing in Health and Disease. New York, NY: M. Dekker; 1999. pp. 1–41. [Google Scholar]
- Richter DW. Commentary on eupneic breathing patterns and gasping. Respir Physiol Neurobiol. 2003;139(1):121–130. doi: 10.1016/s1569-9048(03)00196-4. [DOI] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 2001;24(8):464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- Schelegle ES, Green JF. An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir Physiol. 2001;125:17–31. doi: 10.1016/s0034-5687(00)00202-4. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. J Neurophysiol. 1995;73(4):1452–1461. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- Segers LS, Shannon R, Saporta S, Lindsey BG. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. I. Evidence for excitatory and inhibitory actions of inspiratory neurons. J Neurophysiol. 1987;57(4):1078–1100. doi: 10.1152/jn.1987.57.4.1078. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Bötzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophys. 1997;77(4):1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem–spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Meth. 1987;21:321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Smith JC, Funk G, Johnson S, Feldman JL. Cellular and synaptic mechanisms generating respiratory rhythm: insights from in vitro and computational studies. In: Speck DF, Dekin MS, Revelette WR, Frazier DT, editors. Respiratory Control: Central and Peripheral Mechanisms. Lexington KY, USA: The University Press of Kentucky; 1993. pp. 39–42. [Google Scholar]
- Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Bötzinger complex. J Comp Neurol. 2003;455(4):499–512. doi: 10.1002/cne.10504. [DOI] [PubMed] [Google Scholar]
- Stucke AG, Stuth EA, Tonkovic-Capin V, Tonkovic-Capin M, Hopp FA, Kampine JP, Zuperku EJ. Effects of halothane and sevoflurane on inhibitory neurotransmission to medullary expiratory neurons in a decerebrate dog model. Anesthesiology. 2002;96(4):955–962. doi: 10.1097/00000542-200204000-00025. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Telgkamp P, Ramirez J-M. The role of the hyperpolarization-activated current in modulating rhythmic activity in the isolated respiratory network of mice. J Neurosci. 2000;20(8):2994–3005. doi: 10.1523/JNEUROSCI.20-08-02994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Reciprocal developmental regulation of presynaptic ionotropic receptors. Proc Natl Acad Sci U S A. 2002;99(21):13884–13889. doi: 10.1073/pnas.212419699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JG. Respiratory reflexes in man and other mammalian species. Clin Sci. 1961;21:163–170. [PubMed] [Google Scholar]
- Widdicombe J, Lee LY. Airway reflexes, autonomic function, and cardiovascular responses. Environ Health Perspect. 2001;109(Suppl. 4):579–584. doi: 10.1289/ehp.01109s4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Elsen F, Barnbrock A, Richter DW. Postnatal development of GABAB receptor-mediated modulation of voltage-activated Ca2+ currents in mouse brain-stem neurons. Eur J Neurosci. 1999;11(7):2332–2342. doi: 10.1046/j.1460-9568.1999.00655.x. [DOI] [PubMed] [Google Scholar]