Abstract
Mitochondria sequester and release calcium (Ca2+) and regulate intracellular Ca2+ concentration ([Ca2+]i) in eukaryotic cells. However, the regulation of different Ca2+ signalling modalities by mitochondria in smooth muscle cells is poorly understood. Here, we investigated the regulation of Ca2+ sparks, Ca2+ waves and global [Ca2+]i by mitochondria in cerebral artery smooth muscle cells. CCCP (a protonophore; 1 μm) and rotenone (an electron transport chain complex I inhibitor; 10 μm) depolarized mitochondria, reduced Ca2+ spark and wave frequency, and elevated global [Ca2+]i in smooth muscle cells of intact arteries. In voltage-clamped (−40 mV) cells, mitochondrial depolarization elevated global [Ca2+]i, reduced Ca2+ spark amplitude, spatial spread and the effective coupling of sparks to large-conductance Ca2+-activated potassium (KCa) channels, and decreased transient KCa current frequency and amplitude. Inhibition of Ca2+ sparks and transient KCa currents by mitochondrial depolarization could not be explained by a decrease in intracellular ATP or a reduction in sarcoplasmic reticulum Ca2+ load, and occurred in the presence of diltiazem, a voltage-dependent Ca2+ channel blocker. Ru360 (10 μm), a mitochondrial Ca2+ uptake blocker, and lonidamine (100 μm), a permeability transition pore (PTP) opener, inhibited transient KCa currents similarly to mitochondrial depolarization. In contrast, CGP37157 (10 μm), a mitochondrial Na+–Ca2+ exchange blocker, activated these events. The PTP blockers bongkrekic acid and cyclosporin A both reduced inhibition of transient KCa currents by mitochondrial depolarization. These results indicate that mitochondrial depolarization leads to a voltage-independent elevation in global [Ca2+]i and Ca2+ spark and transient KCa current inhibition. Data also suggest that mitochondrial depolarization inhibits Ca2+ sparks and transient KCa currents via PTP opening and a decrease in intramitochondrial [Ca2+].
Intracellular calcium (Ca2+) signals regulate a diverse array of physiological functions including secretion, contraction, transcription, and apoptosis (Berridge et al. 2000; Duchen, 2000). To control Ca2+-dependent physiological processes, cells have developed a variety of mechanisms that regulate the intracellular free Ca2+ concentration ([Ca2+]i) (Berridge et al. 2000). Although it has been known for many years that mitochondria can transport Ca2+, recent evidence has suggested that these organelles are important physiological modulators of intracellular Ca2+ signalling (Berridge et al. 2000; Duchen, 2000).
Mitochondria sequester Ca2+ from the cytosol via a low-affinity, high-capacity inner membrane uniporter (Bernardi, 1999; Gunter et al. 2000). The driving force for Ca2+ uptake is created by the mitochondrial membrane potential (ΔΨm), which is generated by the extrusion of protons (H+) by the electron transport chain and is ∼150–200 mV more negative than the cytosol (Bernardi, 1999; Gunter et al. 2000). Conversely, mitochondrial Ca2+ release can occur via a number of mechanisms, including the inner membrane Na+–Ca2+ exchanger and the permeability transition pore (PTP) (Bernardi, 1999). The spatial location of mitochondria nearby sites of intracellular Ca2+ release exposes these organelles to elevated microdomains of [Ca2+]i and establishes communication with the sarcoplasmic/endoplasmic reticulum (SR/ER) (Rizzuto et al. 1999).
In arterial smooth muscle cells, several intracellular Ca2+ signalling modalities occur that differ in respect to spatial localization, temporal kinetics, and physiological function (Jaggar et al. 2000). Ca2+ sparks are spatially restricted [Ca2+]i transients that occur due to the opening of multiple SR ryanodine-sensitive Ca2+ release (RyR) channels (Jaggar et al. 2000). In smooth muscle cells, Ca2+ sparks induce a local micromolar [Ca2+]i elevation and activate several large-conductance Ca2+-sensitive K+ (KCa) channels to elicit a transient outward K+ current (Jaggar et al. 2000; Perez et al. 2001; ZhuGe et al. 2002), which has also been termed a ‘spontaneous transient outward current’ or ‘STOC’ (Benham & Bolton, 1986). In arteries at physiological levels of pressure, inhibition of Ca2+ sparks or KCa channels leads to membrane depolarization, activation of voltage-dependent Ca2+ channels, an elevation in arterial wall [Ca2+]i and constriction (Nelson et al. 1995; Jaggar, 2001). Ca2+ waves are propagating, asynchronous intracellular [Ca2+]i elevations that contribute Ca2+ for contraction (Kasai et al. 1997; Sward et al. 2002), although Ca2+ waves that do not induce vasoconstriction have also been observed (Miriel et al. 1999). In arterial smooth muscle cells, Ca2+ wave frequency is increased by RyR channel activation and vasoconstrictors that elevate inositol trisphosphate (Iino et al. 1994; Miriel et al. 1999; Jaggar & Nelson, 2000; Heppner et al. 2002). Global [Ca2+]i is the cytosolic [Ca2+] of arterial smooth muscle cells that results from Ca2+ entry through voltage-dependent Ca2+ channels and Ca2+ release from intracellular stores (for review see Jaggar et al. 2000). An elevation in global [Ca2+]i leads to vasoconstriction (Jaggar, 2001).
Global [Ca2+]i transients elevate intramitochondrial [Ca2+] in arterial smooth muscle cells, suggesting that mitochondria function to reduce [Ca2+]i in this tissue (Drummond & Tuft, 1999; Monteith & Blaustein, 1999; Gurney et al. 2000; Szado et al. 2003). Similarly, agents that depolarize mitochondria prolong the decay of [Ca2+]i transients and elevate spatially averaged [Ca2+]i (Kamishima et al. 2000; Kamishima & Quayle, 2002; Wang et al. 2003). Recent studies also demonstrate that electron transport chain blockers activate Ca2+ sparks in pulmonary artery smooth muscle cells (Wang et al. 2003) and modify agonist-induced Ca2+ waves in tail artery smooth muscle cells (Sward et al. 2002). Collectively, these studies suggest that mitochondria regulate local and global Ca2+ signalling events in smooth muscle cells.
In the present study, we investigated the regulation of local and global [Ca2+]i signalling modalities by mitochondria in cerebral artery smooth muscle cells. Mitochondrial depolarization with rotenone or CCCP blocked Ca2+ sparks and Ca2+ waves and elevated global [Ca2+]i in smooth muscle cells of intact arteries. In voltage-clamped smooth muscle cells, mitochondrial depolarization reduced transient KCa currents by decreasing Ca2+ spark frequency, amplitude, and spatial spread, and by reducing the effective coupling of Ca2+ sparks to KCa channels. Data also suggest that mitochondrial depolarization inhibits Ca2+ sparks and transient KCa currents by inducing PTP opening and a decrease in intramitochondrial [Ca2+].
Methods
Tissue preparation
Procedures involving animals were approved by the Animal Care and Use Committee policies at the University of Tennessee. Sprague-Dawley rats (200–250 g) of either sex were killed by peritoneal injection of a sodium pentobarbital overdose (150 mg kg−1). The brain was removed and placed into ice-cold (4°C), oxygenated (21% O2–5% CO2), physiological saline solution (PSS) containing (mm): 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 1.6 CaCl2, 1.2 MgSO4, 0.023 EDTA and 11 glucose. Posterior cerebral and cerebellar arteries (50–200 μm in diameter) were removed, cleaned of connective tissue and maintained in ice-cold PSS. Individual smooth muscle cells were dissociated from cerebral arteries using an enzyme procedure similar to that previously described (Jaggar, 2001).
Confocal Ca2+ imaging
Arterial segments (1–2 mm in length) or isolated smooth muscle cells were placed into Hepes-buffered PSS containing (mm): 134 NaCl, 6 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes and 10 glucose (pH 7.4, NaOH) containing 10 μm fluo-4 AM and 0.05% pluronic F-127 for 1 h or 15 min, respectively, at 22°C. Arterial segments or cells were then placed into Hepes-buffered PSS for 30 min to allow indicator de-esterification. Smooth muscle cells were imaged using a Noran Oz laser scanning confocal microscope (Noran Instruments, Middleton, WI, USA) and a × 60 water immersion objective (NA = 1.2) attached to a Nikon TE300 microscope by illuminating with a krypton-argon laser at 488 nm and capturing emitted light >500 nm. Confocal imaging of isolated voltage-clamped (−40 mV) smooth muscle cells was done using a Hepes-buffered PSS (described above) bath solution. Smooth muscle cells in intact arterial segments were imaged in a 30 mm K+ bath solution containing (mm): NaCl 110; KCl 30; Hepes 10; CaCl2 2; MgCl2 1; and glucose 10 (pH 7.4, NaOH) (see Jaggar et al. 1998; Cheranov & Jaggar, 2002 for similar procedure). 30 mm K+ depolarizes arterial smooth muscle cells to ∼−40 mV (Harder, 1980), which is similar to the voltage used in patch-clamp experiments in this study. Images (56.3 μm × 52.8 μm) were recorded every 8.3 ms (120 images s−1) in isolated cells or every 16.7 ms (60 images s−1) in intact arteries. For imaging smooth muscle cells in arteries, at least two different representative areas of the same segment were scanned for at least 10 s under each condition. The same part of the artery was scanned only once to avoid any laser-induced changes in Ca2+ signalling, and the effects of drugs were measured in paired experiments. In experiments where confocal microscopy was used in combination with patch clamp, electrophysiological and fluorescence measurements were synchronized using a light emitting diode placed above the recording chamber that was triggered during acquisition. Each isolated smooth muscle cell was imaged for at least 10 s under each condition. Ca2+ sparks were detected in smooth muscle cells using custom analysis software and manual analysis (see Cheranov & Jaggar, 2002 for detailed description). Detection of Ca2+ sparks was performed by dividing an area 1.54 μm (7 pixels) × 1.54 μm (7 pixels) (i.e. 2.37 μm2) in each image (F) by a baseline (F0) which was determined by averaging 10 images without Ca2+ spark activity. The entire area of each image was analysed to detect Ca2+ sparks. A Ca2+ spark was identified as a local increase in F/F0 that was greater than 1.2. The calculated root mean square of baseline fluorescence noise is 0.06. Ca2+ spark spatial spread was calculated as the full width at half-maximal amplitude (FWHM). Ca2+ waves were analysed by placing 2.2 × 2.2 μm boxes in individual smooth muscle cells and refer to a change in F/F0 of > 1.2 that propagated for at least 20 μm. Global Ca2+ fluorescence was calculated from the same images used for Ca2+ spark analysis and was the mean pixel value of 100 different images acquired over 10 s. Changes in local or global [Ca2+]i were calculated using the pseudo ratio method (Cheng et al. 1993):
where K is the apparent affinity of fluo 4 for Ca2+ (770 nm; Woodruff et al. 2002), R is the fractional fluorescence increase (F/F0), and [Ca2+]rest is the cytosolic [Ca2+]i at F0. In isolated cells, Ca2+ spark amplitude in control and CCCP was calculated as the local [Ca2+]i increase (i.e. Δ[Ca2+]spark=[Ca2+]spark−[Ca2+]global) within a 1.54 × 1.54 μm region. Arterial wall [Ca2+]rest used for Ca2+ calibrations was measured using a photomultiplier tube (Ionoptix, Milton, MA, USA) and the ratiometric Ca2+ indicator, fura-2 (see below for detailed methodology). Elevation of extracellular K+ from 6 to 30 mm increased arterial wall [Ca2+]i from 92 ± 6 nm to 193 ± 8 nm (n= 7).
Fura-2 imaging
Isolated cerebral artery smooth muscle cells were incubated with the ratiometric fluorescent Ca2+ indicator fura-2 AM (2 μm) and 0.02% pluronic F-127 for 20 min, followed by a 15 min wash. All experiments in isolated cells were performed using a 6 mm K+ Hepes-buffered bath solution (composition described above). Smooth muscle cells were alternately exited at 340 or 380 nm using a PC-driven hyperswitch (Ionoptix). Background corrected ratios were collected every 1 s at 510 nm using a Dage MTI integrating CCD camera (Ionoptix). [Ca2+]SR was estimated by rapidly applying a high concentration of caffeine (10 mm), a RyR channel activator, and measuring the amplitude of the [Ca2+]i transient (i.e. Δ[Ca2+]i). [Ca2+]i concentrations were calculated using the following equation (Grynkiewicz et al. 1985):
where R is the 340/380 nm ratio, Rmin and Rmax are the minimum and maximum ratios determined in Ca2+-free and saturating Ca2+ solutions, respectively, Sf2/Sb2 is the Ca2+-free/Ca2+-replete ratio of emissions at 380 nm excitation, and Kd is the dissociation constant for fura-2 (224 nm, Grynkiewicz et al. 1985). Rmin, Rmax, Sf2 and Sb2 were determined at the end of experiments and in separate experiments by increasing the Ca2+ permeability of smooth muscle cells with ionomycin (10 μm), and perfusing cells with a high Ca2+ (50 mm) or Ca2+-free (no added Ca2+, 10 mm EGTA) solution.
TMRM imaging
Isolated cerebral artery smooth muscle cells were incubated with TMRM (1 μm) for 15 min followed by a 15 min wash. TMRM is a cationic fluorescent potentiometric indicator that accumulates in mitochondria due to their negative ΔΨm. All experiments in isolated cells were performed using a 6 mm K+ Hepes-buffered bath solution (composition described above). Smooth muscle cells were excited with 535 nm light and background corrected fluorescence intensity was collected every 2 s at 610 nm using a Dage MTI integrating CCD camera (Ionoptix).
Patch-clamp electrophysiology
Isolated cells were allowed to adhere to a glass coverslip in the bottom of a chamber for 10 min prior to experimentation. Potassium currents were measured using either the conventional whole-cell or perforated-patch configuration of the patch-clamp technique using an Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA). Bath solution was 6 mm K+ Hepes-buffered PSS (composition described above). For perforated-patch experiments, the pipette solution contained (mm): 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 Hepes, 0.05 EGTA (pH 7.2 with KOH). For conventional whole-cell experiments, the pipette (i.e. intracellular) solution contained (mm): 140 KCl, 1.9 MgCl2, 0.037 CaCl2, 0.1 EGTA, 10 Hepes, 2 Na2ATP (pH 7.2 with KOH); the calculated free Ca2+ and free Mg2+ concentrations of this solution are 100 nm and 1 mm, respectively (WEBMAXC, Stanford University, CA, USA). The perforated-patch configuration was used, unless stated otherwise. All experiments were performed with a holding potential of −40 mV, unless stated otherwise. Membrane currents were recorded with a sample rate of 2.5 kHz and filtered at 1 kHz. In experiments where the activity of single KCa channel currents was measured, Ca2+ sparks, and hence transient KCa currents, were abolished with thapsigargin, a SR Ca2-ATPase inhibitor (Nelson et al. 1995). In each patch under each condition, single KCa channel activity (NPo) was calculated from 5 min of continuous gap-free data using Fetchan 6 (Axon Instruments). NPo was calculated from the following equation: NPo= (tii)/T, where ti is the time at each channel level i and T is the total time of analysis. Transient KCa current analysis was performed off-line using methodology described elsewhere (Cheranov & Jaggar, 2002). A transient KCa current was defined as the simultaneous opening of three or more KCa channels.
Statistical analysis
Values are expressed as mean ± standard error of the mean. Student's t tests were used for comparing paired or unpaired data, and Student-Newman-Keuls test was used for comparing multiple data sets. First-order polynomial linear fits were used to calculate statistical correlation between the amplitude of Ca2+ sparks and evoked transient KCa currents (Origin, OriginLab Corp., Northampton, MA, USA). ANCOVA of first-order polynomial best fits was used to compare amplitude correlation data sets (Graphpad Prizm, San Diego, CA, USA). P < 0.05 was considered significant.
Chemicals
Unless otherwise stated, all chemicals used in this study were obtained from Sigma Chemical Company (St Louis, MO, USA). Papain was purchased from Worthington Biochemical Co. (Lakewood, NJ, USA), fura-2 AM, fluo-4 AM and pluronic F-127 from Molecular Probes (Eugene, OR, USA), bongkrekic acid and Ru360 from Calbiochem (La Jolla, CA, USA) and CGP37157 from Tocris Cookson Inc. (Ellisville, MO, USA).
Results
Rotenone and CCCP depolarize mitochondria in cerebral artery smooth muscle cells
Mitochondrial Ca2+ uptake is driven by the negative mitochondrial potential (Gunter et al. 2000). Rotenone, an electron transport chain complex I inhibitor, and CCCP, a protonophore, depolarize mitochondria by inducing H+ retention and by permeabilizing the inner membrane to H+, respectively. To evaluate the time course of mitochondrial depolarization caused by rotenone and CCCP in cerebral artery smooth muscle cells, mitochondrial potential was measured using the fluorescent indicator TMRM.
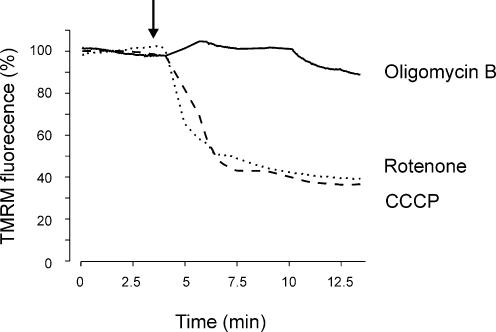
Rotenone and CCCP gradually decreased TMRM fluorescence, which reached a plateau ∼0.4 of control after approximately 5 min (Fig. 1). In contrast, oligomycin B (1 μm), an F1F0-ATP synthase blocker, did not alter TMRM fluorescence over the same time course (Fig. 1). These data indicate that rotenone and CCCP depolarize mitochondria in cerebral artery smooth muscle cells via a mechanism that is independent of a decrease in mitochondrial ATP synthesis.
Figure 1. Rotenone and CCCP depolarize mitochondria in cerebral artery smooth muscle cells.
Original recordings illustrating the mean percentage change in TMRM fluorescence in isolated arterial smooth muscle cells following application (indicated by arrow) of rotenone (10 μm), CCCP (1 μm) or oligomycin B (1 μm). Each trace represents the mean fluorescence change of 7, 10, and 11 cells for rotenone, CCCP and oligomycin B, respectively.
Mitochondrial depolarization inhibits Ca2+ sparks and waves and elevates global [Ca2+]i in smooth muscle cells of intact cerebral arteries
To investigate the regulation of intracellular Ca2+ signalling modalities by mitochondria, smooth muscle cells within the wall of cerebral arteries were imaged in control and 5 min after sustained application of rotenone (10 μm), CCCP (1 μm) or oligomycin B (1 μm). To compare confocal imaging data with electrophysiological recordings presented in this study, Ca2+ sparks in smooth muscle cells of arterial segments were measured in an extracellular solution containing 30 mm K+ (see Jaggar et al. 1998; Cheranov & Jaggar, 2002 for similar procedure). Thirty millimolar K+ depolarizes arterial smooth muscle cells to ∼−40 mV (Harder, 1980).
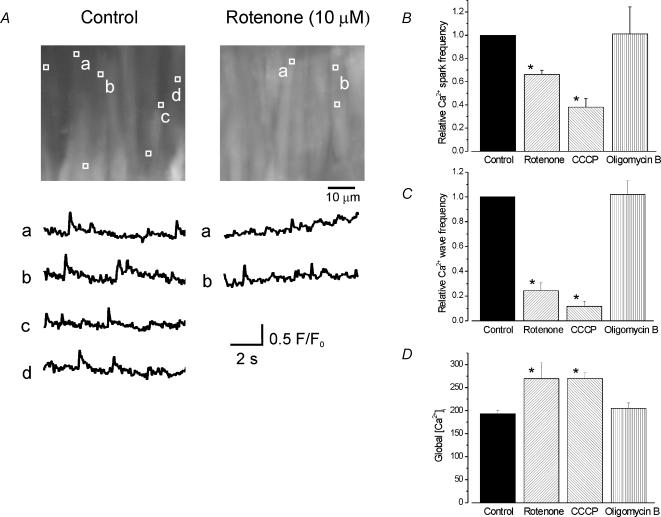
Rotenone decreased mean Ca2+ spark frequency and amplitude to ∼0.66 and ∼0.75 of control, respectively, and reduced mean Ca2+ wave frequency to ∼0.24 of control (Fig. 2A–C). In contrast, rotenone increased mean global [Ca2+]i in the same cells from ∼193 nm (determined with fura-2 in separate experiments; see Methods) to 255 nm (Fig. 2A). In a separate series of experiments, CCCP similarly inhibited Ca2+ sparks and Ca2+ waves, and elevated global [Ca2+]i (Fig. 2B and C). In contrast, oligomycin B did not alter Ca2+ sparks, Ca2+ waves, or global [Ca2+]i (Fig. 2B and C). These data suggest that mitochondrial depolarization inhibits Ca2+ sparks and Ca2+ waves and elevates global [Ca2+]i in cerebral artery smooth muscle cells via a mechanism that does not involve a decrease in ATP.
Figure 2. Regulation of Ca2+ sparks, Ca2+ waves and global [Ca2+]i in smooth muscle cells of intact cerebral arteries by rotenone, CCCP and oligomycin B.
A, average fluorescence (100 of 600 images) over 10 s of two different 56.3 μm × 52.8 μm areas of the same cerebral artery in control and 5 min after application of rotenone (10 μm). The locations of Ca2+ sparks that occurred during 10 s are indicated by white boxes (1.54 μm × 1.54 μm). Representative localized F/F0 changes over time are illustrated below respective images and labelled accordingly. Average relative effects on Ca2+ spark (B) and wave frequency (C) and global [Ca2+]i (D) of a 5 min application of rotenone, CCCP or oligomycin B. Rotenone decreased mean Ca2+ spark frequency from 1.18 ± 0.23 to 0.75 ± 0.12 Hz, Ca2+ spark amplitude (F/F0) from 1.31 ± 0.01 to 1.24 ± 0.01, and wave frequency from 0.39 ± 0.07 to 0.09 ± 0.06 Hz (n= 6 arteries). CCCP decreased mean Ca2+ spark frequency from 1.05 ± 0.25 to 0.33 ± 0.01 Hz, spark amplitude (F/F0) from 1.40 ± 0.01 to 1.25 ± 0.01, and wave frequency from 0.31 ± 0.04 to 0.04 ± 0.02 Hz (n= 6 arteries). Oligomycin B did not alter mean Ca2+ spark frequency (control, 1.03 ± 0.12; oligomycin B, 1.00 ± 0.38 Hz), spark amplitude (control, 1.34 ± 0.02; oligomycin B, 1.37 ± 0.02), or wave frequency (control, 0.31 ± 0.05; oligomycin B, 0.30 ± 0.04 Hz (n= 4 arteries). *P < 0.05 using Students t test.
Rotenone and CCCP block transient KCa currents in isolated arterial smooth muscle cells
In arterial smooth muscle cells, a Ca2+ spark activates several KCa channels, resulting in a transient KCa current (Jaggar et al. 2000). If mitochondrial depolarization inhibits Ca2+ sparks, then this should also decrease transient KCa currents.
At −40 mV, rotenone (10 μm) and CCCP (1 μm) similarly reduced transient KCa current frequency and amplitude when using either the perforated-patch configuration, or the conventional whole-cell configuration with 2 mm ATP included in the pipette solution (Fig. 3A and C). For example, when using the perforated-patch configuration, rotenone decreased mean transient KCa current frequency from ∼0.74 to 0.15 Hz in 5 min, or to ∼0.28 of control (Fig. 3A and C and 10B). Rotenone also reduced mean transient KCa current amplitude from ∼15.1 to 10.2 pA, or to ∼0.69 of control over the same time course. Ten minutes after rotenone or CCCP application, transient KCa currents were almost completely abolished (Fig. 3A, also see Fig. 10C). In contrast, oligomycin B (1 μm) did not inhibit transient KCa currents over the same time course. Five minutes after application of oligomycin B, transient KCa currents were unaltered, although prolonged application (∼15 min) ultimately decreased the frequency and amplitude of these events (Figs 3B and C). Simultaneous application of CCCP and oligomycin B, which prevents mitochondrial consumption of ATP during depolarization (Duchen, 2000), blocked transient KCa currents similarly to when CCCP was applied alone (Fig. 3C). These results suggest mitochondrial depolarization inhibits transient KCa currents in arterial smooth muscle cells via a mechanism that does not involve a decrease in cytosolic ATP.
Figure 3. Regulation of transient KCa currents in isolated voltage-clamped arterial smooth muscle cells by rotenone, CCCP and oligomycin B.
A and B, original recordings of transient KCa currents in cerebral artery smooth muscle cells voltage-clamped at −40 mV using the perforated-patch configuration. Rotenone (10 μm, A) reduced transient KCa current frequency and amplitude, whereas oligomycin B (1 μm, B) did not alter transient KCa currents. C, average relative changes in transient KCa current frequency (left panel) and amplitude (right panel) when compared with control of a 5 min application of: rotenone (10 μm, p-p, n= 7), rotenone (10 μm, w-c, n= 10), CCCP (1 μm, p-p, n= 6), CCCP (1 μm, w-c, n= 7), CCCP (1 μm) + oligomycin B (1 μm, p-p, n= 7), and oligomycin B (1 μm) at 5 and 15 min (p-p, n= 6 for each). p-p, perforated-patch; w-c, conventional whole-cell configuration. *P < 0.05 using Students t test.
Figure 10. Mitochondrial PTP blockers reduce rotenone inhibition of transient KCa currents.
A, original recording of transient KCa currents in a cerebral artery smooth muscle cell voltage-clamped at −40 mV. Bongkrekic acid (10 μm) reduced inhibition of transient KCa currents by rotenone (10 μm). B, frequency histogram illustrating the time course of transient KCa current inhibition by rotenone applied alone (from Fig. 3A, black bars) or in the presence of bongkrekic acid (from Fig. 10A, hatched bars). C, average relative effects on transient KCa current frequency (left panel) and amplitude (right panel) of bongkrekic acid (BA, 10 μm, n= 3), cyclosporin A (CsA, 1 μm, n= 4), rotenone (10 μm, n= 5) alone, or rotenone applied in the presence of bongkrekic acid (10 μm, n= 6) or cyclosporin A (1 μm, n= 7). *P < 0.05 using Student-Newman-Keuls test.
CCCP alters Ca2+ spark spatial and temporal properties and attenuates the effective coupling of Ca2+ sparks to KCa channels
To examine mechanisms by which mitochondrial depolarization inhibits Ca2+ sparks and transient KCa currents, Ca2+ spark spatial-temporal properties and the signalling relationship between sparks and transient KCa currents were measured in the same voltage-clamped (−40 mV) cells in control and 5 min following application of CCCP.
CCCP decreased Ca2+ spark amplitude (ΔCa2+]i) from 671 ± 67 nm in control to 433 ± 41 nm, reduced spatial spread (FWHM) from 3.8 ± 0.2 μm to 3.1 ± 0.1 μm, and accelerated the rate of decay (t1/2) from 72 ± 4 ms to 55 ± 4 ms (P < 0.05 for each). In addition, CCCP reduced the slope of the amplitude correlation between Ca2+ sparks and evoked transient KCa currents from 0.018 to 0.011 or to 0.61 of control (P < 0.05, Fig. 4A and B). In contrast, CCCP increased mean global [Ca2+]i in smooth muscle cells from ∼193 to 253 ± 32 nm. These data suggest that in voltage-clamped arterial smooth muscle cells, mitochondrial depolarization alters the spatial and temporal properties of Ca2+ sparks, reduces the effective coupling of Ca2+ sparks to KCa channels, and elevates global [Ca2+]i.
Figure 4. CCCP attenuates the coupling relationship between Ca2+ sparks and transient KCa currents.
A, original simultaneous recordings of Ca2+ sparks and transient KCa currents in the same voltage-clamped (−40 mV) cerebral artery smooth muscle cell. The black trace illustrates whole cell K+ current. Red and green traces illustrate fluorescence changes (F/F0) measured in two different areas of the cell where Ca2+ sparks occurred. Traces show activity in control and 5 min after CCCP (1 μm) and oligomycin B (1 μm). B, scatter plot of Ca2+ spark and evoked transient KCa current amplitude at −40 mV obtained in the same cells in control (black), and after CCCP (red, n= 5 cells). Ca2+ spark amplitude is calculated as the local elevation in [Ca2+]i (i.e. Δ[Ca2+]i) reported by fluo-4. First-order polynomial linear fits for control and CCCP data are illustrated with confidence bands. CCCP reduced the slope of the amplitude correlation between a spark and the evoked transient KCa current from 0.018 ± 0.001 to 0.011 ± 0.001 (P < 0.05). Ca2+ spark and associated transient KCa current amplitudes were significantly correlated for control and CCCP (P < 0.05 for each), but CCCP reduced the correlation coefficient (r) from 0.61 to 0.32.
In the absence of Ca2+ sparks, rotenone and CCCP activate KCa channels
KCa channels are exposed to micromolar [Ca2+]i during a spark (Perez et al. 2001; ZhuGe et al. 2002). A CCCP-induced decrease in the effective coupling of Ca2+ sparks to KCa channels suggests that mitochondrial depolarization inhibits KCa channels. To investigate this hypothesis, the regulation of KCa channel activity by mitochondrial depolarization was measured using the perforated-patch clamp configuration. To block Ca2+ sparks and transient KCa currents, experiments were performed in cells pretreated with thapsigargin, a SR Ca2+ ATPase inhibitor (Jaggar et al. 2000).
At 0 mV, rotenone increased KCa channel activity (NPo) ∼2.6-fold (Fig. 5A and B). In a separate series of experiments, CCCP increased KCa channel activity ∼2-fold (Fig. 5B). Thus, in the absence of Ca2+ sparks, mitochondrial depolarization elevates KCa channel activity, presumably by elevating global [Ca2+]i (see Figs 2 and 6).
Figure 5. Rotenone and CCCP activate KCa channels.
A, original recordings of single KCa channels measured at 0 mV using the perforated-patch configuration. Ca2+ sparks, and thus transient KCa currents, were abolished with thapsigargin (100 nm). Rotenone increased KCa channel activity. ‘C’ indicates closed level. B, average relative effect of rotenone (10 μm) or CCCP (1 μm) on KCa channel activity (NPo). Rotenone increased mean NPo from 0.017 ± 0.003 to 0.044 ± 0.024 (n= 7 cells). CCCP increased mean NPo from 0.021 ± 0.005 to 0.042 ± 0.016 (n= 5 cells). *P < 0.05 using Students t test.
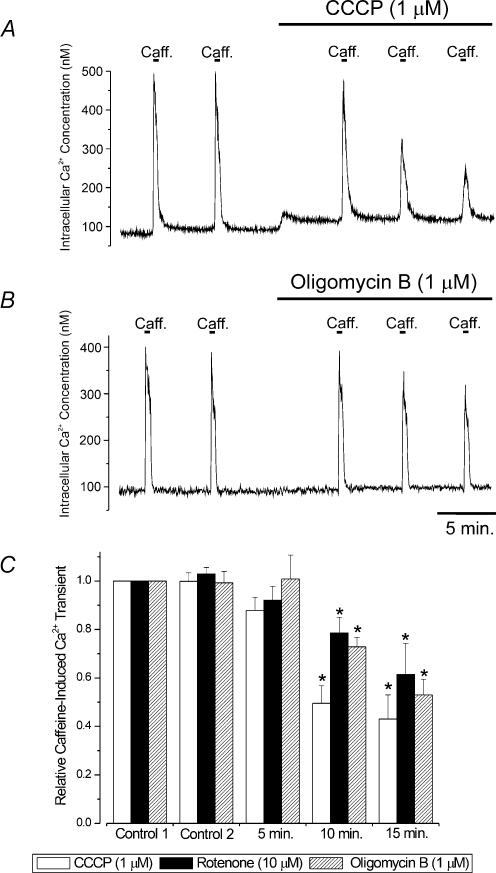
Figure 6. Regulation of SR Ca2+ load by CCCP, rotenone and oligomycin B.
A and B, original traces illustrating the regulation of intracellular Ca2+ concentration and caffeine (10 mm)-induced [Ca2+]i transients in single cerebral artery smooth muscle cells by CCCP (1 μm, A) or oligomycin B (1 μm, B). C, average relative caffeine-induced [Ca2+]i transients in control (two applications 5 min apart), and five, 10 and 15 min after CCCP (1 μm, n= 9 cells), rotenone (10 μm, n= 5 cells) or oligomycin B (1 μm, n= 11 cells). *P < 0.05, when compared with control using Student-Newman-Keuls test.
Regulation of sarcoplasmic reticulum Ca2+ load by rotenone, CCCP and oligomycin B
In HeLa and HEK293 cells, mitochondria supply the ER with a local source of Ca2+ for sequestration (Arnaudeau et al. 2001). If a similar process exists in smooth muscle cells, mitochondrial depolarization may disrupt this supply mechanism, which would decrease SR Ca2+ load and inhibit Ca2+ sparks and transient KCa currents (Cheranov & Jaggar, 2002). To investigate this hypothesis, the regulation of global [Ca2+]i and SR Ca2+ load by CCCP (1 μm), rotenone (10 μm), and oligomycin B (1 μm) was measured in isolated cells.
Rotenone and CCCP elevated [Ca2+]i, whereas oligomycin B did not change [Ca2+]i (Fig. 6). Five minutes after application of either CCCP, rotenone, or oligomycin B, the mean amplitude of caffeine-induced [Ca2+]i transients were unaltered (Fig. 6). However, 10 and 15 min after application of CCCP, rotenone, or oligomycin B, caffeine-induced [Ca2+]i transients were significantly smaller when compared with control (Fig. 6). Because transient KCa currents (Figs 3 and 4) were reduced to ∼0.25 of control after a 5 min application of rotenone or CCCP, but SR Ca2+ load was unchanged, these data suggest that mitochondrial depolarization does not inhibit Ca2+ sparks and transient KCa currents due to a decrease in SR Ca2+ load.
Mitochondrial depolarization inhibits transient KCa currents independently of voltage-dependent Ca2+ channels
Voltage-dependent Ca2+ channel blockers inhibit Ca2+ sparks and transient KCa currents in arterial smooth muscle cells (Jaggar et al. 1998; Cheranov & Jaggar, 2002). Conceivably, mitochondrial depolarization may inhibit Ca2+ sparks and transient KCa currents by blocking voltage-dependent Ca2+ channels. To investigate this hypothesis, rotenone was applied in the presence of diltiazem (50 μm), a voltage-dependent Ca2+ channel blocker. Diltiazem reduced mean transient KCa current frequency to ∼0.46 of control, but did not change mean transient KCa current amplitude (Fig. 7A and B). In the same cells in the presence of diltiazem, rotenone reduced mean transient KCa current frequency and amplitude to ∼0.25 and ∼0.5 of control, respectively (Fig. 7A and B). These results suggest mitochondrial depolarization inhibits transient KCa currents via a mechanism that is independent of Ca2+ entry via voltage-dependent Ca2+ channels.
Figure 7. Rotenone blocks transient KCa currents when applied in the continued presence of diltiazem, and H2O2 activates transient KCa currents.
Average relative effects of diltiazem (50 μm, n= 6), rotenone (10 μm) applied in the continued presence of diltiazem (50 μm, n= 6), or H2O2 (100 μm, n= 7) on transient KCa current frequency (A) and amplitude (B). *P < 0.05 using using Student-Newman-Keuls test.
H2O2 activates transient KCa currents
In arterial smooth muscle cells, mitochondrial depolarization generates reactive oxygen species (ROS) (Michelakis et al. 2002). To investigate if mitochondrial depolarization inhibits Ca2+ sparks and transient KCa currents via an increase in ROS, the regulation of transient KCa currents by H2O2 was examined. At −40 mV, H2O2 (100 μm) increased mean transient KCa current frequency ∼1.6-fold, but did not alter mean transient KCa current amplitude (Fig. 7A and B). These data suggest mitochondrial depolarization does not inhibit transient KCa currents due to an increase in H2O2.
Differential regulation of transient KCa currents by inhibitors of mitochondrial Ca2+ uptake and release
Mitochondrial depolarization reduces the driving force for Ca2+ uptake, leading to a decrease in intramitochondrial [Ca2+] (for reviews see Gunter et al. 2000; Duchen, 2000). Conceivably, rotenone and CCCP could inhibit transient KCa currents by decreasing intramitochondrial [Ca2+]. To investigate this hypothesis, the regulation of transient KCa currents by pharmacological blockers of mitochondrial Ca2+ uptake and release was measured. Ru360 (10 μm), a selective mitochondrial Ca2+ uniporter blocker that reduces [Ca2+]mito (Matlib et al. 1998), decreased mean transient KCa current frequency and amplitude to ∼0.42 and ∼0.64 of control, respectively (Fig. 8B). In contrast, CGP37157 (10 μm), a selective mitochondrial Na+/Ca2+ exchange blocker that elevates [Ca2+]mito (Cox & Matlib, 1993), increased mean transient KCa current frequency and amplitude ∼1.82- and ∼1.31-fold, respectively (Fig. 8A and B). In addition, activation of transient KCa currents by CGP37157 was reversed by Ru360 (10 μm) (Fig. 8A and B). These results show that inhibitors of mitochondrial Ca2+ uptake and release differentially regulate transient KCa currents in arterial smooth muscle cells, and suggest that mitochondrial potential regulates Ca2+ sparks and transient KCa currents by modulating intramitochondrial [Ca2+].
Figure 8. Differential regulation of transient KCa currents by Ru360 and CGP37157.
A, original recording obtained in a voltage-clamped (−40 mV) cerebral artery smooth muscle cell illustrating activation of transient KCa currents by CGP37157 (10 μm) and inhibition by Ru360 (10 μm). B, average relative effects on transient KCa current frequency (left panel) and amplitude (right panel) of Ru360 (n= 6), CGP37157 (n= 9), and Ru360 applied in the continued presence of CGP37157 (n= 5). *P < 0.05 using Students t test.
Lonidamine, a PTP opener, blocks transient KCa currents
In addition to reducing the driving force for mitochondrial Ca2+ uptake, mitochondrial depolarization leads to PTP opening (Bernardi, 1999). Conceivably, mitochondrial depolarization may inhibit Ca2+ sparks and transient KCa current due to PTP opening. To investigate this hypothesis, the regulation of transient KCa currents by lonidamine, a PTP opener (Ravagnan et al. 1999), was studied. At −40 mV, lonidamine (100 μm) reduced transient KCa current frequency and amplitude to ∼0.28 and ∼0.64 of control, respectively (Fig. 9A and B). These results suggest that PTP opening inhibits transient KCa currents in arterial smooth muscle cells.
Figure 9. Lonidamine inhibits transient KCa currents.
A, original recording illustrating inhibition of transient KCa currents by lonidamine (100 μm) in a cerebral artery smooth muscle cell voltage-clamped at −40 mV. B, average effects of lonidamine on transient KCa current frequency and amplitude (n= 10). *P < 0.05 using Students t test.
PTP blockers reduce rotenone inhibition of transient KCa currents
We sought to determine if mitochondrial depolarization inhibits transient KCa currents due to PTP opening. To investigate this hypothesis, the regulation of transient KCa currents by rotenone was determined in the absence and presence of the PTP blockers bongkrekic acid or cyclosporin A. PTP blockers were applied 5 min prior to rotenone application.
In the absence of a PTP blocker, a 10 min application of rotenone essentially abolished transient KCa currents and reduced frequency and amplitude to ∼0.04 and ∼0.42 of control, respectively (see Fig. 10B and C). Transient application (<15 min) of bongkrekic acid (10 μm) or cyclosporin A (1 μm) did not alter transient KCa current frequency and amplitude (Fig. 10A and B). However, 15 min after application of cyclosporin A or bongkrekic acid, transient KCa current frequency and amplitude were slightly reduced (Fig. 10C). In contrast to the marked inhibitory effects of rotenone when applied alone, when applied in the presence of either bongkrekic acid or cyclosporin A, rotenone (10 μm) was a less effective transient KCa current inhibitor (Fig. 10A–C). In the presence of bongkrekic acid, rotenone reduced transient KCa current frequency and amplitude to ∼0.42 and ∼0.71 of control, respectively, after 10 min (Fig. 10C). Similarly, in the presence of cyclosporin A, rotenone reduced transient KCa current frequency and amplitude to ∼0.43 and ∼0.62 of control, respectively (Fig. 10C). Thus, both PTP blockers reduced rotenone inhibition of transient KCa currents. These data suggest that mitochondrial depolarization-induced PTP opening contributes to transient KCa current inhibition.
Discussion
In this study, the regulation of local and global [Ca2+]i signalling modalities by mitochondria was investigated in cerebral artery smooth muscle cells. The following novel findings are presented: (1) mitochondrial depolarization inhibits Ca2+ sparks and Ca2+ waves and elevates global [Ca2+]i in smooth muscle cells of intact arteries; (2) in voltage-clamped smooth muscle cells, mitochondrial depolarization reduces transient KCa current frequency and amplitude by decreasing Ca2+ spark frequency, amplitude, spatial spread, and the effective coupling of Ca2+ sparks to KCa channels; (3) inhibition of transient KCa currents by mitochondrial depolarization does not occur via a decrease in intracellular ATP or SR Ca2+ load and occurs in the presence of a voltage-dependent Ca2+ channel blocker; (4) blockers of the mitochondrial Ca2+ uniporter or mitochondrial Na+–Ca2+ exchanger inhibit or activate transient KCa currents, respectively; (5) lonidamine, a PTP opener, blocks transient KCa currents; and (6) inhibition of transient KCa currents by mitochondrial depolarization is attenuated by PTP blockers. Collectively, these data suggest mitochondria regulate local and global Ca2+ signals in arterial smooth muscle cells and mitochondrial depolarization leads to PTP opening and a decrease in intramitochondrial [Ca2+] that inhibits Ca2+ sparks and transient KCa currents.
Mitochondrial regulation of Ca2+ sparks and transient KCa currents
Data in this study are consistent with the prior observation that CCCP rapidly blocked spontaneous transient inward currents (STICs) in rabbit portal vein myocytes (Greenwood et al. 1997). A STIC occurs due to the activation of a number of Ca2+-activated Cl− channels by a Ca2+ spark (Jaggar et al. 2000). In contrast, cyanide, an electron transport chain complex IV blocker, activated Ca2+ sparks and STICs in pulmonary artery smooth muscle cells (Wang et al. 2003). The contrasting results obtained in systemic and pulmonary artery smooth muscle cells may occur due to different mitochondrial physiology in these preparations (e.g. see Michelakis et al. 2002), or the effects of inhibiting different electron transport chain complexes on Ca2+ signalling.
In the present study, mitochondrial depolarization reduced Ca2+ spark frequency, amplitude, and spatial spread, and accelerated spark decay. Collectively, these findings suggest that mitochondrial depolarization reduces RyR channel activity. These results also suggest mitochondrial Ca2+ uptake does not directly contribute to spark decay, consistent with previous evidence that sparks decay primarily due to diffusion (Jaggar et al. 2000; Cheranov & Jaggar, 2002). Mitochondrial depolarization decreased transient KCa current amplitude via two mechanisms: (1) a reduction in Ca2+ spark amplitude and spatial spread, and (2) a decrease in the effective coupling of Ca2+ sparks to KCa channels. Ca2+ sparks of smaller amplitude and spatial spread will have a lower impact on KCa channel activity and activate fewer channels, and therefore induce smaller amplitude transient KCa currents. Since KCa channels are typically exposed to micromolar [Ca2+]i during a Ca2+ spark (Perez et al. 2001; ZhuGe et al. 2002), mitochondrial depolarization presumably reduces effective coupling to Ca2+ sparks by decreasing the micromolar Ca2+-sensitivity of KCa channels. In the absence of Ca2+ sparks, rotenone and CCCP activated KCa channels, presumably by inducing a voltage-independent nanomolar elevation in global [Ca2+]i.
Mitochondrial depolarization leads to opening of the PTP, a non-selective, high conductance complex comprised of several components including the voltage-dependent anion channel, the adenine nucleotide translocase (ANT) and cyclophilin D (Duchen, 2000). PTP opening leads to the release of mitochondrial factors less than 1.5 kDa, including Ca2+ and cytochrome c (Bernardi, 1999). Bongkrekic acid blocks the ANT and stabilizes the closed state of the PTP. Cyclosporin A inhibits the PTP by binding to cyclophilin D (Bernardi, 1999), but also inhibits calcineurin (protein phosphatase 2B) (Bernardi, 1999; Duchen, 2000). Acute application (<15 min) of bongkrekic acid and cyclosporin A did not inhibit transient KCa currents, but prolonged application (>15 min) decreased the frequency and amplitude of these events. One explanation for this inhibitory effect is that PTP blockers ultimately decrease cytosolic ATP, particularly since the time course of block was similar to that with oligomycin B.
In arterial smooth muscle cells, mitochondrial depolarization elevates ROS (Michelakis et al. 2002). Rather than inhibiting transient KCa currents, exogenous application of H2O2 activated these events, similarly to effects in cat tracheal smooth muscle cells (Bauer et al. 1997). H2O2-activation of transient KCa currents was presumably due to RyR channel and/or KCa channel activation (Boraso & Williams, 1994; Barlow & White, 1998). However, oxidative stress also leads to PTP opening (e.g. see Teshima et al. 2003), raising the question of why H2O2 did not block transient KCa currents? One explanation is that in cardiac myocytes, significant mitochondrial depolarization does not occur until tens of minutes after H2O2 exposure (Teshima et al. 2003). Conceivably, prolonged exposure to H2O2 that leads to PTP opening may inhibit Ca2+ sparks and transient KCa currents in arterial smooth muscle cells. Regardless, 5 min after rotenone or CCCP application transient KCa currents were blocked, whereas 5 min after H2O2 application transient KCa currents were activated, arguing against the possibility that mitochondrial depolarization blocked transient KCa currents due to an increase in ROS.
Mitochondrial regulation of Ca2+ waves and global Ca2+
Mitochondrial depolarization blocked spontaneous Ca2+ waves, which occur due to RyR channel activation under the conditions used in our experiments (Jaggar et al. 1998). In colonic smooth muscle cells, CCCP also blocked IP3-evoked [Ca2+]i transients (McCarron & Muir, 1999). However, in tail artery smooth muscle cells electron transport chain blockers increased the frequency, but reduced the amplitude of agonist-induced Ca2+ waves (Sward et al. 2002). Collectively, these studies indicate that mitochondrial depolarization modifies Ca2+ waves in smooth muscle, but the spatial and temporal changes that occur may depend on the relative contribution of RyR channels and IP3-gated Ca2+ release channels to the events.
Mitochondrial depolarization elevated global [Ca2+]i in agreement with previous studies performed in vascular and non-vascular smooth muscle cells (Taggart et al. 1997; Kamishima et al. 2000; Kamishima & Quayle, 2002; Wang et al. 2003). Data in the present study suggest that mitochondrial depolarization elevates global [Ca2+]i via two mechanisms: (1) a voltage-independent pathway, and (2) via inhibition of Ca2+ sparks and transient KCa currents, which would lead to membrane depolarization, activation of voltage-dependent channels, and Ca2+ influx (Jaggar et al. 2000). Mechanisms by which mitochondrial depolarization leads to a voltage-independent elevation in global [Ca2+]i are unclear, but may involve the elimination of a mitochondrial Ca2+ sink. In addition, mitochondrial depolarization may release or generate mitochondrial mediators that stimulate Ca2+ influx and/or release and/or inhibit Ca2+ removal. In pulmonary and mesenteric artery smooth muscle cells, metabolic inhibition with cyanide elevates global [Ca2+]i by stimulating Ca2+ release from the SR and mitochondria (Wang et al. 2003). In cerebral artery smooth muscle cells, mitochondrial depolarization inhibited Ca2+ sparks and Ca2+ waves, suggesting that global [Ca2+]i did not increase due to RyR channel activation.
Transient and sustained mitochondrial depolarizations may regulate Ca2+ signalling in arterial smooth muscle
In cardiac muscle, local SR Ca2+ release leads to a transient elevation in intramitochondrial Ca2+ concentration termed a ‘Ca2+ mark’ (Pacher et al. 2002) and a transient mitochondrial depolarization termed a ‘flicker’ (Duchen et al. 1998). Flickers have been attributed to a number of mechanisms, including PTP opening (Huser & Blatter, 1999), Ca2+ influx via the Ca2+ uniporter (Duchen et al. 1998), and H+ entry via the F1F0-ATPase (Buckman & Reynolds, 2001). Recently, flickers have been described in Bufo marinus stomach smooth muscle cells, where mitochondria flicker independently of one another (O'Reilly et al. 2003). In smooth muscle cells, individual flickers last for up to 30 s and range from less than 10 mV to greater than 100 mV (O'Reilly et al. 2003). Conceivably, the regulation of RyR channel activity by mitochondrial flickers may be an ongoing feedback process that continually regulates Ca2+ spark generation via mechanisms described here, i.e. via intramitochondrial [Ca2+]. In a recent study that measured the spatial arrangement of the SR and mitochondria within venous smooth muscle cells, TMRE fluorescence could not be observed within the vicinity of a Ca2+ spark release site (Singh et al. 2003). The authors suggested that local Ca2+ released from the SR may be sequestered into mitochondria, leading to depolarization and a loss of TMRE fluorescence at these sites (Singh et al. 2003). Conceivably, in arterial smooth muscle cells mitochondrial Ca2+ uptake during a spark could lead to depolarization and the initiation of a localized negative-feedback mechanism that reduces Ca2+ spark occurrence.
Several stimuli may regulate local and global Ca2+ signalling by inducing sustained mitochondrial depolarization. For example, mitochondria may act as an oxygen sensor (Waypa et al. 2001; Michelakis et al. 2002). In systemic artery smooth muscle cells, hypoxia depolarizes mitochondria (Michelakis et al. 2002). Similarly, vasoconstrictors may regulate smooth muscle Ca2+ signalling, in part, by modulating mitochondrial potential. Vasoconstrictor-stimulated Ca2+ release elevates mitochondrial [Ca2+] in smooth muscle cells (Monteith & Blaustein, 1999; Szado et al. 2003), which would lead to mitochondrial depolarization (Bernardi, 1999; Duchen, 2000). Vasoconstrictors also inhibit Ca2+ sparks (Jaggar & Nelson, 2000; Mauban et al. 2001). Conceivably, a vasoconstrictor-induced mitochondrial depolarization may contribute to Ca2+ spark inhibition by the mechanisms we describe in this study. Thus, mitochondria may regulate Ca2+ signalling and arterial diameter under resting conditions and in response to diverse stimuli.
Mechanisms by which mitochondrial depolarization may inhibit Ca2+ sparks and transient KCa currents
Mitochondria regulate the activity of both IP3-gated Ca2+ release channels and RyR channels, but regulation appears to be cell specific. For example, mitochondrial depolarization accelerates IP3-mediated Ca2+ waves in cortical astrocytes (Duchen, 2000), but blocks IP3-evoked Ca2+ release in colonic smooth muscle cells (McCarron & Muir, 1999). In cardiac and skeletal muscle, inhibition of mitochondrial Ca2+ uptake stimulates Ca2+ sparks, suggesting that mitochondria normally suppress RyR channel activity in these tissues (Pacher et al. 2002; Isaeva & Shirokova, 2003). Mechanisms by which mitochondrial depolarization inhibits RyR and KCa channels in arterial smooth muscle cells remain to be established, but both local and global regulatory mechanisms must be considered. Mitochondria are spatially located within 20 nm of the ER/SR in many cell types, including arterial smooth muscle cells (Rizzuto et al. 1999; Duchen, 2000; Szado et al. 2003). Although slight differences occur between subtypes, RyR channels typically exhibit a bell-shaped Ca2+ dependence and are activated by 1–10 μm Ca2+ and inhibited by 1–10 mm Ca2+ (Fill & Copello, 2002). Due to the bell-shaped Ca2+ dependence of RyR channels, an increase or a decrease in [Ca2+]i can activate or inhibit Ca2+ sparks depending on the concentration that is reached. Conceivably, in smooth muscle cells mitochondria may normally buffer, and thus lower, cytosolic Ca2+ in the immediate vicinity of RyR channels. Mitochondrial depolarization may remove this localized buffering, leading to high local [Ca2+]i, RyR channel inhibition and a decrease in Ca2+ spark occurrence. Alternatively, mitochondria may normally stimulate Ca2+ sparks by releasing Ca2+ within the vicinity of RyR channels. Mitochondrial depolarization would remove this stimulatory mechanism, which would also decrease RyR channel activity and inhibit Ca2+ sparks. Whether mitochondrial depolarization elevates or reduces the local cytosolic [Ca2+]i in the vicinity of RyR channels in smooth muscle cells remains to be determined. If mitochondria stimulate Ca2+ sparks by releasing Ca2+ within the vicinity of RyR channels, data suggest that this does not occur due to Ca2+ released via the PTP or the Na+–Ca2+ exchanger. Acute application of PTP blockers did not alter transient KCa currents and CGP37157 activated transient KCa currents. However, data with CGP37157 support a localized regulatory mechanism. CGP37157 elevates intramitochondrial [Ca2+] (Cox & Matlib, 1993), which would reduce the buffering capacity of mitochondria and increase the driving force for mitochondrial Ca2+ efflux. Both of these effects may elevate local cytosolic Ca2+ in the vicinity of RyR channels.
Mitochondrial depolarization not only reduces the driving force for Ca2+ uptake, but leads to PTP opening and mitochondrial Ca2+ efflux (Gunter & Pfeiffer, 1990; Bernardi, 1999; Duchen, 2000). PTP blockers may reduce depolarization-induced inhibition of transient KCa currents by blocking this mitochondrial Ca2+ efflux pathway and attenuating the loss of intramitochondrial Ca2+. However, PTP opening may also release intramitochondrial components that inhibit RyR and/or KCa channels. One potential RyR/KCa channel inhibitor released upon PTP opening is cytochrome c (Bernardi, 1999). Cytochrome c blocks Ca2+-dependent inhibition of IP3-gated Ca2+ release channels (Boehning et al. 2003) and activates voltage-dependent K+ channels in pulmonary artery smooth muscle cells (Platoshyn et al. 2002). Thus mitochondrial depolarization may inhibit RyR and KCa channels via local and global mechanisms.
Physiological relevance of mitochondrial regulation of local and global Ca2+ signalling in arterial smooth muscle cells
Mitochondria generate metabolic energy, directly and indirectly regulate Ca2+ signalling (Rizzuto et al. 1999), play a central role in the survival and programmed death of eukaryotic cells (Gunter et al. 2000; Duchen, 2000), and function as an oxygen sensor (Michelakis et al. 2002; Waypa et al. 2002). Therefore, changes in mitochondrial potential that regulate local and global Ca2+ signalling in arterial smooth muscle cells may be important under both physiological and pathophysiological conditions and directly and indirectly regulate diverse cellular functions, including contractility and apoptosis.
In summary, our data indicate that mitochondria regulate local and global Ca2+ signals in cerebral artery smooth muscle cells. The data suggest that mitochondrial depolarization inhibits Ca2+ sparks and transient KCa currents via PTP opening and a reduction in intramitochondrial [Ca2+]. These findings suggest that changes in mitochondrial potential may regulate a range of Ca2+-dependent cellular functions by modulating local and global Ca2+ signalling modalities.
Acknowledgments
We thank Dr C. W. Leffler, Dr Q. Xi, and Miss X. Cheng for helpful comments on the manuscript. This study was supported by grants (to J.H.J) from the National Institutes of Health (HL67061) and American Heart Association National Center (0130190 N).
References
- Arnaudeau S, Kelley WL, Walsh JV, Jr, Demaurex N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem. 2001;276:29430–29439. doi: 10.1074/jbc.M103274200. [DOI] [PubMed] [Google Scholar]
- Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol. 1998;275:H1283–H1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- Bauer V, Oike M, Tanaka H, Inoue R, Ito Y. Hydrogen peroxide induced responses of cat tracheal smooth muscle cells. Br J Pharmacol. 1997;121:867–874. doi: 10.1038/sj.bjp.0701202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham CD, Bolton TB. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5(12):1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- Boraso A, Williams AJ. Modification of the gating of the cardiac sarcoplasmic reticulum Ca2+-release channel by H2O2 and dithiothreitol. Am J Physiol. 1994;267:H1010–H1016. doi: 10.1152/ajpheart.1994.267.3.H1010. [DOI] [PubMed] [Google Scholar]
- Buckman JF, Reynolds IJ. Spontaneous changes in mitochondrial membrane potential in cultured neurons. J Neurosci. 2001;21:5054–5065. doi: 10.1523/JNEUROSCI.21-14-05054.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cheranov SY, Jaggar JH. Sarcoplasmic reticulum calcium load regulates rat arterial smooth muscle calcium sparks and transient KCa currents. J Physiol. 2002;544:71–84. doi: 10.1113/jphysiol.2002.025197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DA, Matlib MA. Modulation of intramitochondrial free Ca2+ concentration by antagonists of Na+-Ca2+ exchange. Trends Pharmacol Sci. 1993;14:408–413. doi: 10.1016/0165-6147(93)90063-P. [DOI] [PubMed] [Google Scholar]
- Drummond RM, Tuft RA. Release of Ca2+ from the sarcoplasmic reticulum increases mitochondrial [Ca2+] in rat pulmonary artery smooth muscle cells. J Physiol. 1999;516:139–147. doi: 10.1111/j.1469-7793.1999.139aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, Leyssens A, Crompton M. Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J Cell Biol. 1998;142:975–988. doi: 10.1083/jcb.142.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Greenwood IA, Helliwell RM, Large WA. Modulation of Ca2+-activated Cl− currents in rabbit portal vein smooth muscle by an inhibitor of mitochondrial Ca2+ uptake. J Physiol. 1997;505:53–64. doi: 10.1111/j.1469-7793.1997.053bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000;28:285–296. doi: 10.1054/ceca.2000.0168. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Gurney AM, Drummond RM, Fay FS. Calcium signalling in sarcoplasmic reticulum, cytoplasm and mitochondria during activation of rabbit aorta myocytes. Cell Calcium. 2000;27:339–351. doi: 10.1054/ceca.2000.0124. [DOI] [PubMed] [Google Scholar]
- Harder DR. Comparison of electrical properties of middle cerebral and mesenteric artery in cat. Am J Physiol. 1980;239:C23–C26. doi: 10.1152/ajpcell.1980.239.1.C23. [DOI] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Santana LF, Nelson MT. Alkaline pH shifts Ca2+ sparks to Ca2+ waves in smooth muscle cells of pressurized cerebral arteries. Am J Physiol. 2002;283:H2169–H2176. doi: 10.1152/ajpheart.00603.2002. [DOI] [PubMed] [Google Scholar]
- Huser J, Blatter LA. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J. 1999;343:311–317. [PMC free article] [PubMed] [Google Scholar]
- Iino M, Kasai H, Yamazawa T. Visualization of neural control of intracellular Ca2+ concentration in single vascular smooth muscle cells in situ. EMBO J. 1994;13:5026–5031. doi: 10.1002/j.1460-2075.1994.tb06831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaeva EV, Shirokova N. Metabolic regulation of Ca2+ release in permeabilized mammalian skeletal muscle fibres. J Physiol. 2003;547:453–462. doi: 10.1113/jphysiol.2002.036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH. Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol. 2001;281:C439–C448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Nelson MT. Differential regulation of Ca2+ sparks and Ca2+ waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol. 2000;279:C1528–C1539. doi: 10.1152/ajpcell.2000.279.5.C1528. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Stevenson AS, Nelson MT. Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am J Physiol. 1998;274:C1755–C1761. doi: 10.1152/ajpcell.1998.274.6.C1755. [DOI] [PubMed] [Google Scholar]
- Kamishima T, Davies NW, Standen NB. Mechanisms that regulate [Ca2+]i following depolarization in rat systemic arterial smooth muscle cells. J Physiol (Lond) 2000;522:285–295. doi: 10.1111/j.1469-7793.2000.t01-2-00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamishima T, Quayle JM. Mitochondrial Ca2+ uptake is important over low [Ca2+]i range in arterial smooth muscle. Am J Physiol. 2002;283:H2431–H2439. doi: 10.1152/ajpheart.00865.2001. [DOI] [PubMed] [Google Scholar]
- Kasai Y, Yamazawa T, Sakurai T, Taketani Y, Iino M. Endothelium-dependent frequency modulation of Ca2+ signalling in individual vascular smooth muscle cells of the rat. J Physiol. 1997;504:349–357. doi: 10.1111/j.1469-7793.1997.349be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron JG, Muir TC. Mitochondrial regulation of the cytosolic Ca2+ concentration and the InsP3-sensitive Ca2+ store in guinea-pig colonic smooth muscle. J Physiol. 1999;516:149–161. doi: 10.1111/j.1469-7793.1999.149aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, Phillips R, Altschuld R, Katsube Y, Sperelakis N, Bers DM. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- Mauban JR, Lamont C, Balke CW, Wier WG. Adrenergic stimulation of rat resistance arteries affects Ca2+ sparks, Ca2+ waves, and Ca2+ oscillations. Am J Physiol. 2001;280:H2399–H2405. doi: 10.1152/ajpheart.2001.280.5.H2399. [DOI] [PubMed] [Google Scholar]
- Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res. 2002;90:1307–1315. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- Miriel VA, Mauban JR, Blaustein MP, Wier WG. Local and cellular Ca2+ transients in smooth muscle of pressurized rat resistance arteries during myogenic and agonist stimulation. J Physiol (Lond) 1999;518:815–824. doi: 10.1111/j.1469-7793.1999.0815p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith GR, Blaustein MP. Heterogeneity of mitochondrial matrix free Ca2+: resolution of Ca2+ dynamics in individual mitochondria in situ. Am J Physiol. 1999;276:C1193–C1204. doi: 10.1152/ajpcell.1999.276.5.C1193. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- O'Reilly CM, Fogarty KE, Drummond RM, Tuft RA, Walsh JV., Jr Quantitative analysis of spontaneous mitochondrial depolarizations. Biophys J. 2003;85:3350–3357. doi: 10.1016/S0006-3495(03)74754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Thomas AP, Hajnoczky G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci U S A. 2002;99:2380–2385. doi: 10.1073/pnas.032423699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez GJ, Bonev AD, Nelson MT. Micromolar Ca2+ from sparks activates Ca2+-sensitive K+ channels in rat cerebral artery smooth muscle. Am J Physiol. 2001;281:C1769–C1775. doi: 10.1152/ajpcell.2001.281.6.C1769. [DOI] [PubMed] [Google Scholar]
- Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Cytochrome c activates K+ channels before inducing apoptosis. Am J Physiol Cell Physiol. 2002;283:C1298–C1305. doi: 10.1152/ajpcell.00592.2001. [DOI] [PubMed] [Google Scholar]
- Ravagnan L, Marzo I, Costantini P, Susin SA, Zamzami N, Petit PX, Hirsch F, Goulbern M, Poupon MF, Miccoli L, Xie Z, Reed JC, Kroemer G. Lonidamine triggers apoptosis via a direct, Bcl-2-inhibited effect on the mitochondrial permeability transition pore. Oncogene. 1999;18:2537–2546. doi: 10.1038/sj.onc.1202625. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Brini M, Chiesa A, Filippin L, Pozzan T. Mitochondria as biosensors of calcium microdomains. Cell Calcium. 1999;26:193–199. doi: 10.1054/ceca.1999.0076. [DOI] [PubMed] [Google Scholar]
- Singh H, Hudman D, Lawrence CL, Rainbow RD, Lodwick D, Norman RI. Distribution of Kir6.0 and SUR2 ATP-sensitive potassium channel subunits in isolated ventricular myocytes. J Mol Cell Cardiol. 2003;35:445–459. doi: 10.1016/s0022-2828(03)00041-5. [DOI] [PubMed] [Google Scholar]
- Sward K, Dreja K, Lindqvist A, Persson E, Hellstrand P. Influence of mitochondrial inhibition on global and local [Ca2+]i in rat tail artery. Circ Res. 2002;90:792–799. doi: 10.1161/01.res.0000015214.40360.84. [DOI] [PubMed] [Google Scholar]
- Szado T, Kuo KH, Bernard-Helary K, Poburko D, Lee CH, Seow C, Ruegg UT, van Breemen C. Agonist-induced mitochondrial Ca2+ transients in smooth muscle. FASEB J. 2003;17:28–37. doi: 10.1096/fj.02-0334com. [DOI] [PubMed] [Google Scholar]
- Taggart MJ, Menice CB, Morgan KG, Wray S. Effect of metabolic inhibition on intracellular Ca2+, phosphorylation of myosin regulatory light chain and force in rat smooth muscle. J Physiol. 1997;499:485–496. doi: 10.1113/jphysiol.1997.sp021943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima Y, Akao M, Jones SP, Marban E. Cariporide (HOE642), a selective Na+-H+ exchange inhibitor, inhibits the mitochondrial death pathway. Circulation. 2003;108:2275–2281. doi: 10.1161/01.CIR.0000093277.20968.C7. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zheng YM, Abdullaev I, Kotlikoff MI. Metabolic inhibition with cyanide induces calcium release in pulmonary artery myocytes and Xenopus oocytes. Am J Physiol. 2003;284:C378–C388. doi: 10.1152/ajpcell.00260.2002. [DOI] [PubMed] [Google Scholar]
- Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91:719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- Woodruff ML, Sampath AP, Matthews HR, Krasnoperova NV, Lem J, Fain GL. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J Physiol. 2002;542:843–854. doi: 10.1113/jphysiol.2001.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZhuGe R, Fogarty KE, Tuft RA, Walsh JV., Jr Spontaneous transient outward currents arise from microdomains where BK channels are exposed to a mean Ca2+ concentration on the order of 10 microM during a Ca2+ spark. J General Physiol. 2002;120:15–27. doi: 10.1085/jgp.20028571. [DOI] [PMC free article] [PubMed] [Google Scholar]