Abstract
Forearm vascular responses to intra-arterial infusions of endothelium-dependent and -independent vasodilators have been thoroughly characterized in humans. While the forearm is a well-established experimental model for studying human vascular function, it is of limited consequence to systemic cardiovascular control owing to its small muscle mass and blood flow requirements. In the present study we determined whether these responses could be generalized to the leg. Based upon blood pressure differences between the leg and arm during upright posture, we hypothesized that the responsiveness to endothelium-dependent vasodilators would be greater in the forearm than the leg. Brachial and femoral artery blood flow (Q, ultrasound Doppler) at rest and during intra-arterial infusions of endothelium-dependent (acetylcholine and substance P) and -independent (sodium nitroprusside) vasodilators were measured in eight healthy men (22–27 years old). Resting blood flows in the forearm before infusion of acetylcholine, substance P or sodium nitroprusside were 25 ± 4, 30 ± 7 and 29 ± 5 ml min−1, respectively, and in the leg were 370 ± 32, 409 ± 62 and 330 ± 30 ml min−1, respectively. At the highest infusion rate of acetylcholine (16 μg (100 ml tissue)−1 min−1) there was a greater (P < 0.05) increase in Q to the forearm (1864 ± 476%) than to the leg (569 ± 86%). Similarly, at the highest infusion rate of substance P (125 pg (100 ml tissue)−1 min−1) there was a greater (P < 0.05) increase in Q to the forearm (911 ± 286%) than to the leg (243 ± 58%). The responses to sodium nitroprusside (1 μg (100 ml tissue)−1 min−1) were also greater (P < 0.05) in the forearm (925 ± 164%) than in the leg (326 ± 65%). These data indicate that vascular responses to both endothelium-dependent and -independent vasodilator agents are blunted in the leg compared to the forearm.
The endothelium plays a pivotal role in the regulation of vascular tone, angiogenesis and thrombus formation (Cooke, 1992). The responses of the forearm vasculature to intra-arterial infusions of endothelium-dependent vasodilators, such as acetylcholine, substance P and bradykinin, have been thoroughly characterized in healthy humans (Taddei et al. 1997a,b; DeSouza et al. 2000,2002). Unfortunately, it is not known whether these findings can be generalized to the vasculature of the legs. While the forearm is a well-established experimental model for studying human vascular function, it is of limited consequence to systemic cardiovascular control due to its small muscle mass and blood flow requirements.
Upright posture and bipedal locomotion create a haemodynamic challenge for humans. The hydrostatic pressure gradient created by the earth's gravitational force exposes the vasculature of the lower extremities to pressures approximately 65 mmHg greater than those experienced by the upper extremities (Rowell, 1993; Malhotra et al. 2002). The impact of this elevated blood pressure on endothelial vasodilator function in the legs is still unknown. However, based on research using coarctation of the aorta to induce hypertension in animals it seems plausible that endothelial vasodilator function in the leg is decreased relative to the forearm. This model of hypertension has parallels to the upright human condition because it exposes the vasculature proximal to the coarctation to elevated blood pressures while the blood pressure distal to the coarctation remains intact. Research using this model of hypertension suggests that elevated blood pressure induces vessel remodelling by altering both endothelial (Hollander et al. 1976; Owens & Reidy, 1985; Ueno et al. 2000) and vascular smooth muscle cells (Berry & Greenwald, 1976; Bevan, 1976; Bevan et al. 1976; Hollander et al. 1976; Owens & Reidy, 1985; Ueno et al. 2000). Specifically, arterial segments harvested proximal to the coarctation exhibit increased wall thickness (Berry & Greenwald, 1976; Hollander et al. 1976; Ueno et al. 2000) due to proliferation of smooth muscle (Bevan, 1976; Bevan et al. 1976; Owens & Reidy, 1985; Ueno et al. 2000). This proliferation of vascular smooth muscle is a consequence of reductions in nitric oxide synthase (Ueno et al. 2000) and presumably the bioavailability of nitric oxide. These findings are supported by experimental studies which reported impaired endothelium-dependent relaxation in the vasculature proximal to the obstruction in animals subjected to aortic coarctation (Lockette et al. 1986; Miller et al. 1987; Lai et al. 1989; Bell & Bohr, 1991; Bell, 1993). Similar findings have also been reported in the forearm vasculature of humans following the successful repair of aortic coarctation (Gardiner et al. 1994).
Evidence for decreased endothelial vasodilator function in the leg is also indirectly supported by literature describing the genesis of peripheral arterial disease. It is well established that peripheral arterial disease develops first in the vasculature of the lower extremities before progressing to the vasculature of the upper extremities (Moore, 2002). These observations may be indicative of greater endothelial dysfunction and accelerated atherogenesis in the legs.
The purpose of the present investigation was to determine whether endothelial vasodilator function is different in the upper and lower extremities of humans. Based upon limb differences in blood pressure during upright posture, we hypothesized that the endothelium of the lower extremities is less responsive to intra-arterial infusions of endothelial agonists, acetylcholine and substance P, than the upper extremities.
Methods
Subjects
Studies were performed on eight healthy young men (age 22–27 years). All subjects were free of hypertension (blood pressure < 140/90 mmHg), hypercholesterolaemia (fasting cholesterol < 200 mg dl−1), diabetes (fasting glucose <120 mg dl−1) and cardiovascular disease (12 lead electrocardiogram at rest and during exercise). All subjects had a negative smoking history and were currently not taking medication. Subjects were excluded from the study if they had a body mass index (BMI) ≥ 30 kg m−2 and/or a V̇CO2 > 70th percentile (46.8 ml kg−1 min−1) of age group norms (American College of Sports Medicine, 2002). Before participation, each subject was verbally informed of the potential risks and discomforts associated with the study and signed a written informed consent form approved by the Institutional Review Board of the Milton S. Hershey Medical Center.
Experimental design
This study was separated into three visits (screening, study 1 and study 2). The purpose of the screening visit was to ensure that subjects were healthy but sedentary. During this visit subjects gave a blood sample for analysis (complete blood count with differential, lipid profile, electrolytes, uric acid, glucose, creatinine and blood urea nitrogen) and performed a graded exercise test on a cycle ergometer (Monark 829E; Monark Exercise AB, Varberg, Sweden). Metabolic measurements (Sensormedics Vmax 229; Sensormedics, Yorba Linda, CA, USA), in addition to heart rate, rhythm (Sensormedics) and blood pressure were obtained during rest and graded exercise.
The purpose of study 1 was to measure limb volume and blood flow in the non-dominant forearm and leg. Limb volume was measured in the forearm and leg using water displacement. Measurements of limb blood flow were made using Doppler ultrasound (ATL 5000. 12–15 MHz non-invasive transducer) at rest and after 10 min of ischaemia (i.e. peak reactive hyperaemia) in the forearm and leg.
The purpose of study 2 was to measure the vascular responses of the forearm and leg to endothelium-dependent and -independent vasodilators. Vascular responsiveness was determined by measuring blood flow in the forearm and leg during rest, vehicle infusion (saline), and during increasing doses of acetylcholine, substance P or sodium nitroprusside.
Measurements
Limb volume
Forearm volume was measured from the head of the ulna to the olecranon of the non-dominant arm using water displacement. Leg volume was measured from the medial malleolus to approximately 6–8 cm below the femoral bifurcation. This location was determined by taking two-thirds the distance from the proximal patella to the anterior superior iliac spine.
Blood flow and vascular conductance
Brachial and femoral arterial blood flows were measured in the experimental arm and leg with high resolution Doppler ultrasound (ATL 5000; Philips Medical Systems, Bothwell, WA, USA). A 12–5 MHz transducer was positioned approximately 1–2 cm proximal to the tip of the intra-arterial infusion catheter. During study 1 the transducer position over the brachial artery was moved proximally by approximately 10 cm to accommodate the position of the occlusion cuff. Mean blood velocity was measured at an insonation angle of ≤60°. The sample volume was maximized in an attempt to minimize overestimation of mean blood velocity (Radegran, 1997; Lott et al. 2001,2002). Arterial diameter was measured using a longitudinal view of the artery. Measurement of arterial diameter was performed at the end of diastole (determined by ECG) by measuring the distance between near and far wall intima–media interface. Blood flow was calculated as:
where Q is blood flow (ml min−1), r is radius (cm) and MBV is mean blood velocity (cm s−1). Arterial diameter and MBV were collected and analysed over a 30 s period immediately following cuff deflation for measurements of peak reactive hyperaemia and during the last minute of drug infusion.
Brachial and femoral vascular conductance were calculated during rest, saline infusion and drug infusions as:
where Q is blood flow (ml min−1) and MAP is mean arterial pressure (mmHg). Mean arterial pressure was continuously measured intra-arterially between minutes three and five of saline or drug infusion.
The percentage increase in blood flow during both peak reactive hyperaemia and intra-arterial infusion interventions was calculated as:
where Qintervention is either the limb blood flow response to 10 min of ischaemia or drug infusion and Qbaseline is the blood flow before intervention. The same calculation was used to assess percentage increase in conductance.
Venous occlusion plethysmography
A calibrated mercury-in-Silastic™ strain gauge was used to express blood flow measurements as millilitres per 100 millilitres of tissue per minute (DE Hokanson, Inc., Bellevue, WA, USA) (Proctor et al. 1996; Groothuis et al. 2003). Briefly, the subject's lower leg and forearm were positioned above heart level and a mercury-in-Silastic™ strain gauge was placed around the widest portion of the limb segment. To exclude blood flow to the hand and foot a Hokanson TMC-7 cuff was placed around the wrist and ankle and inflated to 200 mmHg. During blood flow measurements a Hokanson SC-10 cuff positioned above the elbow or knee was inflated to 50 mmHg. Four blood flow measurements separated by 20 s were taken during minutes three and five of all drug infusions.
Interventions
Peak reactive hyperaemia (study 1)
Blood flow responses to 10 min of ischaemia were measured in the brachial and femoral arteries. Briefly, cuffs were positioned around the wrist and above the elbow of the non-dominant arm and inflated to 200 mmHg. After 10 min, the upper cuff was rapidly deflated and brachial artery blood flow was measured for 30 s using Doppler ultrasound. Peak limb blood flow elicited by 10 min of arterial occlusion has been reported to occur within 10–15 s (Wascher et al. 1998; Pawelczyk & Levine, 2002). Similar methodology was used to obtain peak blood flows in the non-dominant leg. Cuffs were placed around the ankle and upper thigh. The position of the quadriceps cuff was immediately distal to the femoral bifurcation. Both cuffs were inflated for 10 min to 200 mmHg. Peak blood flow was measured in the femoral artery upon deflation of the quadriceps cuff. The arm and leg were positioned approximately 20 cm above heart level during the trials.
Intra-arterial infusions (study 2)
Each subject reported at 07.30 to the Milton S. Hershey Medical Center's General Clinical Research Center in a postabsorptive state and abstained from caffeine. Upon arrival subjects were directed to a temperature-controlled room and placed in a supine position. Under aseptic conditions, two polyethylene catheters (22 and 20 gauge, Arrow International, Inc., Reading, PA, USA) were inserted proximally into the brachial (2 cm proximal to antecubital crease) and common femoral (1 cm proximal to bifurcation) artery of the non-dominant limb under local anaesthesia (1% lignocaine). Catheters were used for both intra-arterial drug infusions and measurements of brachial artery blood pressure. To ensure accurate arterial blood pressure measurements the pressure transducer (Abbott) was positioned at heart level and calibrated (OHMEDA, XCaliber) to 250 mmHg. Blood flow in the control limbs was measured via strain-gauge venous occlusion plethysmography and arterial pressure was also monitored for systemic drug effects.
Separate intra-arterial infusions of acetylcholine and substance P were used to assess endothelium-dependent vasodilatation. Acetylcholine was infused at 1, 4 and 16 μg (100 ml limb tissue)−1 min−1 and substance P at 8, 31 and 125 pg (100 ml limb tissue)−1 min−1. Sodium nitroprusside was infused intra-arterially to assess endothelium-independent vasodilatation. Sodium nitroprusside was infused at rates of 0.063, 0.25 and 1 μg (100 ml limb tissue)−1 min−1. These drug infusion rates were chosen based on pilot studies that revealed marked increases in limb blood flow without significant systemic effects.
Each dose of endothelium-dependent and -independent vasodilators was infused for 5 min. A 2 min washout period was allowed between drug infusions. The sequence of drugs was randomized to avoid any ordering effect.
Drug infusion rates were manipulated by adjusting the speed of infusion of a Harvard Apparatus syringe pump to the predetermined infusion rates. A vehicle (saline) was infused at the highest infusion rate of the dose–response curve to determine what effect infusion rate had on blood flow measurements.
Acetylcholine (Novartis Ophthalamics, Duluth, GA, USA) substance P (Clinalfla AG, Laufelfingen, Switzerland) and sodium nitroprusside (Abbott Laboratories, Chicago, IL, USA) were diluted in saline to the desired concentration before drug infusion. Both substance P and sodium nitroprusside were protected from light by wrapping aluminium and wire insulation around the syringe and connective tubing.
Statistical analysis
Repeated-measures two-way ANOVA models were applied to compare limb differences in response to intra-arterial infusions of acetycholine, substance P and sodium nitroprusside. For multiple comparisons of simple effects at the different drug infusion rates a Bonferroni correction was made. Repeated-measures one-way ANOVA and Dunnett's test were applied to compare variables to baseline. Statistical significance was set at P < 0.05. All data are presented as means ±s.e.m.
Results
Subject characteristics
The subject characteristics are (age, height, weight, BMI, blood pressure, cholestrol, VO2peak, limb volume) reported in Table 1.
Table 1.
Subject characteristics
| Variable | Mean ±s.e.m. |
|---|---|
| Age (years) | 24 ± 1 |
| Height (cm) | 181 ± 2 |
| Weight (kg) | 82 ± 2 |
| BMI (kg m−2) | 25 ± 1 |
| Systolic BP (mmHg) | 113 ± 4 |
| Diastolic BP (mmHg) | 74 ± 4 |
| Total cholesterol (mg dl−1) | 146 ± 8 |
| V̇O2peak (ml kg−1 min−1) | 34 ± 2 |
| Forearm volume (ml) | 1232 ± 25 |
| Leg volume (ml) | 8903 ± 288 |
Blood flow at rest
During study 1, baseline blood flow was significantly greater in the leg than in the arm (393 ± 78 versus 47 ± 11 ml min−1, P < 0.05). Baseline blood flows during study 2 are reported in Tables 2 and 3.
Table 2.
Haemodynamic responses in forearm to vasodilators
| Drug | Dose | BAD (cm) | FBF (infusion) (ml min−1) | MAP (mmHg) | FVC (ml min−1 mmHg−1) | HR (b.p.m.) | FBF (control) (ml (100 ml)−1 min−1) |
|---|---|---|---|---|---|---|---|
| Acetylcholine | Base | 0.45 ± 0.01 | 25 ± 4 | 84 ± 4 | 0.30 ± 0.05 | 61 ± 3 | 1.7 ± 0.2 |
| Veh | 0.45 ± 0.01 | 30 ± 4 | 87 ± 4* | 0.36 ± 0.06 | 61 ± 2 | 1.7 ± 0.2 | |
| 1 | 0.44 ± 0.01 | 78 ± 16 | 84 ± 4 | 0.90 ± 0.16 | 60 ± 3 | 1.5 ± 0.2 | |
| 4 | 0.46 ± 0.01 | 221 ± 42* | 85 ± 5 | 2.54 ± 0.42* | 61 ± 3 | 1.3 ± 0.1* | |
| 16 | 0.48 ± 0.01* | 394 ± 47* | 88 ± 5* | 4.48 ± 0.52* | 61 ± 3 | 1.3 ± 0.1* | |
| Substance P | Base | 0.45 ± 0.02 | 30 ± 7 | 81 ± 3 | 0.36 ± 0.07 | 57 ± 3 | 1.0 ± 0.1 |
| Veh | 0.44 ± 0.02 | 31 ± 5 | 84 ± 4 | 0.37 ± 0.05 | 58 ± 3 | 1.0 ± 0.2 | |
| 8 | 0.44 ± 0.02 | 75 ± 17 | 81 ± 3 | 0.95 ± 0.22 | 58 ± 3 | 1.1 ± 0.1 | |
| 31 | 0.46 ± 0.01 | 172 ± 29* | 80 ± 4 | 2.16 ± 0.33* | 59 ± 3 | 1.2 ± 0.1 | |
| 125 | 0.46 ± 0.01 | 225 ± 29* | 84 ± 3 | 2.76 ± 0.45* | 62 ± 3* | 1.5 ± 0.1* | |
| Nitroprusside | Base | 0.44 ± 0.01 | 29 ± 5 | 87 ± 4 | 0.33 ± 0.06 | 60 ± 3 | 1.3 ± 0.1 |
| Veh | 0.44 ± 0.01 | 30 ± 5 | 88 ± 4 | 0.35 ± 0.06 | 60 ± 3 | 1.2 ± 0.1 | |
| 0.063 | 0.45 ± 0.02 | 48 ± 5 | 85 ± 4 | 0.57 ± 0.08 | 60 ± 3 | 1.1 ± 0.1 | |
| 0.25 | 0.45 ± 0.01 | 132 ± 9* | 86 ± 4 | 1.54 ± 0.08* | 61 ± 3 | 1.1 ± 0.1 | |
| 1 | 0.48 ± 0.01* | 243 ± 15* | 85 ± 4 | 2.89 ± 0.20* | 64 ± 3 | 1.4 ± 0.2 |
BAD, brachial artery diameter; FBF (infusion), forearm blood flow in infused limb; MAP, mean arterial pressure; FVC, forearm vascular conductance; HR, heart rate; FBF (control), forearm blood flow in control limb. Values are means ±s.e.m.
Significantly different from baseline (P < 0.05).
Table 3.
Haemodynamic responses in leg to vasodilators
| Drug | Dose | FAD (cm) | LBF (infusion) (ml min−1) | MAP (mmHg) | LVC (ml min−1 mmHg−1) | HR (b.p.m.) | LBF (control) (ml (100 ml)−1 min−1) |
|---|---|---|---|---|---|---|---|
| Acetylcholine | Base | 0.96 ± 0.03 | 370 ± 32 | 88 ± 5 | 4.47 ± 0.54 | 56 ± 3 | 1.3 ± 0.1 |
| Veh | 0.96 ± 0.04 | 409 ± 41 | 88 ± 4 | 4.89 ± 0.75 | 57 ± 3 | 1.4 ± 0.1 | |
| 1 | 0.98 ± 0.04 | 940 ± 114* | 86 ± 5 | 10.75 ± 1.29* | 62 ± 3* | 1.4 ± 0.2 | |
| 4 | 0.99 ± 0.04 | 1836 ± 239* | 87 ± 5 | 20.88 ± 2.49* | 63 ± 3* | 1.3 ± 0.1 | |
| 16 | 0.99 ± 0.04 | 2355 ± 249* | 83 ± 7 | 27.94 ± 2.59* | 69 ± 4* | 1.4 ± 0.1 | |
| Substance P | Base | 0.96 ± 0.04 | 409 ± 62 | 84 ± 4 | 4.86 ± 0.65 | 56 ± 3 | 1.5 ± 0.2 |
| Veh | 0.95 ± 0.04 | 399 ± 74 | 84 ± 4 | 4.84 ± 0.94 | 57 ± 3 | 1.7 ± 0.2 | |
| 8 | 0.95 ± 0.04 | 761 ± 84* | 83 ± 4 | 9.28 ± 1.13* | 57 ± 3 | 1.3 ± 0.2 | |
| 31 | 0.96 ± 0.04 | 1052 ± 112* | 80 ± 4 | 13.43 ± 1.85* | 61 ± 3 | 1.4 ± 0.2 | |
| 125 | 0.94 ± 0.03 | 1230 ± 51* | 74 ± 4* | 17.09 ± 1.59* | 74 ± 4* | 1.9 ± 0.4* | |
| Nitroprusside | Base | 0.96 ± 0.04 | 330 ± 30 | 88 ± 4 | 3.79 ± 0.32 | 58 ± 3 | 1.3 ± 0.1 |
| Veh | 0.97 ± 0.04 | 318 ± 44 | 88 ± 4 | 3.41 ± 0.46 | 58 ± 3 | 1.4 ± 0.2 | |
| 0.063 | 0.98 ± 0.04 | 641 ± 88* | 88 ± 4 | 7.24 ± 1.09 | 58 ± 3 | 1.2 ± 0.2 | |
| 0.25 | 0.98 ± 0.04 | 1025 ± 116* | 84 ± 5 | 12.34 ± 2.00* | 61 ± 3 | 1.1 ± 0.2* | |
| 1 | 1.00 ± 0.03 | 1301 ± 140* | 76 ± 5* | 16.61 ± 2.06* | 74 ± 3* | 1.1 ± 0.1 |
FAD, femoral artery diameter; LBF (infusion), leg blood flow in infused limb; MAP, mean arterial pressure; LVC, leg vascular conductance; HR, heart rate; LBF (control), leg blood flow in control limb. Values are meas ±s.e.m.
Significantly different from baseline (P < 0.05).
Peak reactive hyperaemia
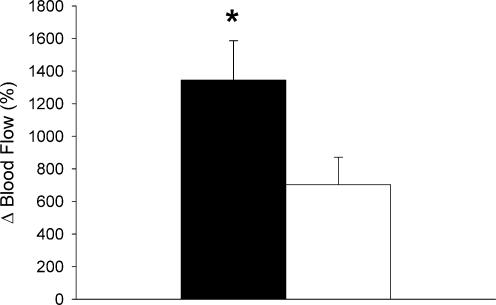
Peak blood flow in response to 10 min of arterial occlusion was significantly greater in the leg than in the forearm (2575 ± 192 versus 546 ± 49 ml min−1, P < 0.05). In contrast, 10 min of arterial occlusion caused a significantly greater relative increase in blood flow above baseline in the forearm compared to the leg (1345 ± 241 versus 720 ± 169%, P < 0.05; Fig. 1)
Figure 1. Peak reactive hyperaemia blood flow in forearm (▪) and leg (□).
Values are means ±s.e.m. and are expressed as a percentage above baseline. * Significant difference between forearm and leg (P < 0.05).
Acetylcholine infusions
Responses to acetylcholine infusions in the forearm and leg are reported in Tables 2 and 3, respectively. During forearm infusions brachial artery diameter was significantly greater (P < 0.05) than baseline at the infusion rate of 16 μg (100 ml tissue)−1 min−1. Forearm blood flow and vascular conductance were significantly (P < 0.05) higher than baseline during infusions in the forearm at 4 and 16 μg (100 ml tissue)−1 min−1. Mean arterial pressure was significantly (P < 0.05) elevated above baseline during infusions in the forearm of saline and at 16 μg (100 ml tissue)−1 min−1 acetylcholine. Blood flow to the control forearm was significantly decreased below baseline at acetylcholine infusion rates of 4 and 16 μg (100 ml tissue)−1 min−1. Heart rate was not significantly changed during infusions of acetylcholine in the forearm.
Leg blood flow, vascular conductance and heart rate were significantly (P < 0.05) increased above baseline during all three infusion rates of acetylcholine in the leg. However, femoral artery diameter, mean arterial pressure and control leg blood flow were not significantly (P < 0.05) altered during infusions of acetylcholine in the leg.
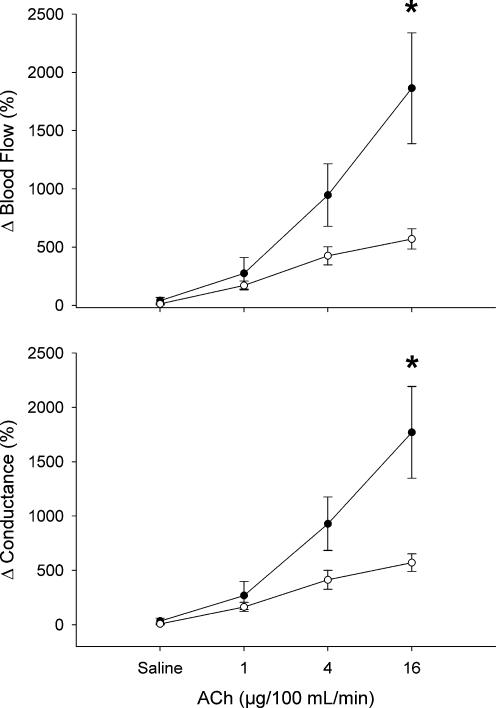
Acetylcholine at 16 μg (100 ml tissue)−1 min−1 caused a significantly greater relative increase in blood flow to the forearm than to the leg (1864 ± 476 versus 569 ± 86%, P < 0.05; Fig. 2). Forearm vascular conductance was increased greater than in the leg at 16 μg (100 ml tissue)−1 min−1 (1760 ± 421 versus 571 ± 81%, P < 0.05; Fig. 2).
Figure 2. Blood flow and conductance responses to acetycholine in forearm (‥) and leg (○).
Values are means ±s.e.m. and are expressed as a percentage above baseline. * Significant difference between forearm and leg (P < 0.05).
Substance P infusions
Responses to substance P infusions in the forearm and leg are reported in Tables 2 and 3, respectively. At infusion rates of 31 and 125 pg (100 ml tissue)−1 min−1, substance P significantly (P < 0.05) increased forearm blood flow and vascular conductance above baseline. Heart rate and blood flow to the control forearm were significantly (P < 0.05) increased above baseline at the highest infusion rates of substance P. Infusions of substance P in the forearm caused no significant (P < 0.05) change in either brachial artery diameter or mean arterial pressure.
Leg blood flow and vascular conductance were significantly (P < 0.05) increased above baseline during all three infusion rates of substance P. Heart rate and blood flow to the control leg were significantly (P < 0.05) increased above baseline at the highest infusion rate of substance P. However, mean arterial pressure was significantly (P < 0.05) decreased below baseline at the highest infusion rate of substance P. Infusions of substance P in the leg caused no significant (P < 0.05) change in femoral artery diameter.
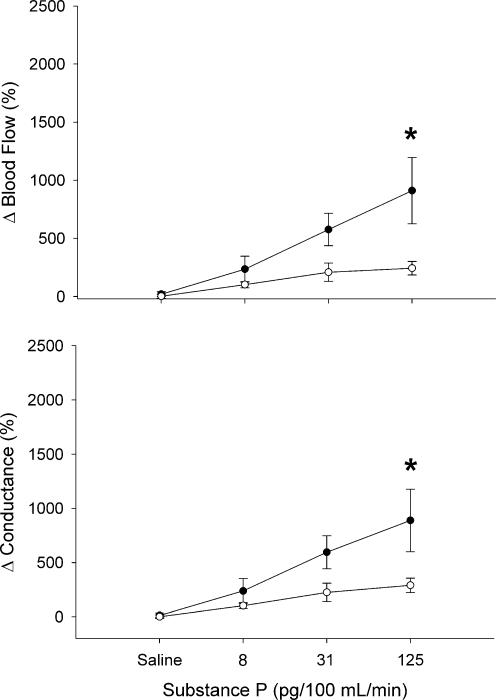
Substance P at 125 pg (100 ml tissue)−1 min−1 caused a significantly greater relative increase in blood flow to the forearm than to the leg (911 ± 286 versus 243 ± 58%, P < 0.05; Fig. 3). Forearm vascular conductance was increased greater than in the leg at 125 pg (100 ml tissue)−1 min−1 (890 ± 289 versus 291 ± 66%, P < 0.05; Fig. 3).
Figure 3. Blood flow and conductance responses to substance P in forearm (‥) and leg (○).
Values are means ±s.e.m. and are expressed as a percentage above baseline. * Significant difference between forearm and leg (P < 0.05).
Sodium nitroprusside infusions
Responses to sodium nitroprusside infusions in the forearm and leg are reported in Tables 2 and 3, respectively. At infusion rates of 0.25 and 1 μg (100 ml tissue)−1 min−1, sodium nitroprusside (P < 0.05) increased forearm blood flow and vascular conductance above baseline. Brachial artery diameter was significantly (P < 0.05) increased above baseline at the highest infusion rate of sodium nitroprusside. However, infusions of sodium nitroprusside in the forearm caused no significant (P < 0.05) change in mean arterial pressure, heart rate or blood flow in the control forearm.
Leg blood flow was significantly (P < 0.05) increased above baseline during all three infusion rates of sodium nitroprusside. However, leg vascular conductance was significantly (P < 0.05) increased above baseline at 0.25 and 1 μg (100 ml tissue)−1 min−1. Heart rate was significantly (P < 0.05) increased above baseline at 1 μg (100 ml tissue)−1 min−1. In addition, blood flow to the control leg and mean arterial pressure were significantly (P < 0.05) decreased below baseline at 0.25 and 1 μg (100 ml tissue)−1 min−1, respectively. Infusions of sodium nitroprusside in the leg caused no significant (P < 0.05) change in femoral artery diameter.
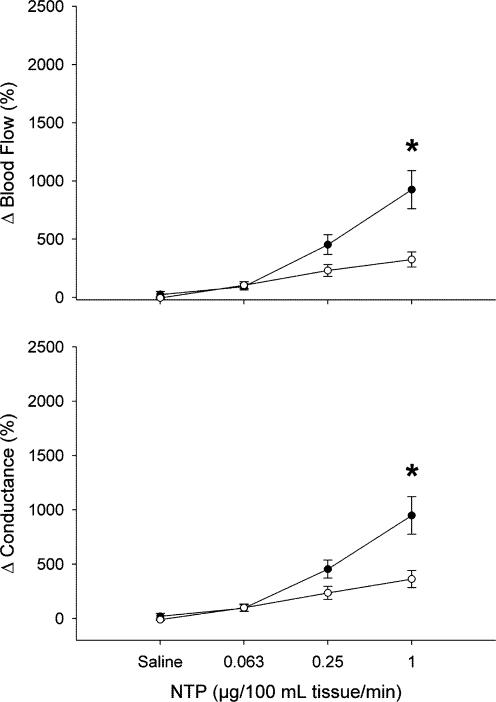
Sodium nitroprusside at 1 μg (100 ml tissue)−1 min−1 caused a significantly greater relative increase in blood flow to the forearm than to the leg (925 ± 164 versus 326 ± 65%, P < 0.05; Fig. 4). Forearm vascular conductance was increased greater than in the leg at 1 μg (100 ml tissue)−1 min−1 (948 ± 173 versus 363 ± 79%, P < 0.05; Fig. 4).
Figure 4. Blood flow and conductance responses to sodium nitroprusside in forearm (‥) and leg (○).
Values are means ±s.e.m. and are expressed as a percentage above baseline. * Significant difference between forearm and leg (P < 0.05).
Discussion
Previous studies have characterized endothelium-dependent and -independent vasodilatation in either the forearm or the leg. Unique to this investigation is the fact that we were able to characterize endothelium-dependent and -independent vasodilatation in both the forearm and the leg of our subjects on the same day. Our findings demonstrate that the leg exhibits smaller relative increases in blood flow and vascular conductance than the forearm to both pharmacological and physiological vasodilator stimuli. Specifically, the leg exhibits smaller relative increases in blood flow and vascular conductance than the forearm to intra-arterial infusions of the endothelium-dependent vasodilators acetylcholine and substance P, and the endothelium-independent vasodilator sodium nitroprusside. In addition, the vasculature of the leg exhibits smaller relative increases in blood flow and vascular conductance than the forearm in response to 10 min of ischaemia. To our knowledge, this is the first study to characterize limb-specific vascular responses to endothelium-dependent or -independent vasodilators in humans.
Endothelium-dependent and independent vasodilatation in previous studies
The human forearm has become the preferred model for investigation of vascular function because intra-arterial infusions of vasoactive compounds can be limited to the forearm and systemic effects can be avoided. This model is often used to assess endothelial and vascular smooth muscle function in the forearm of healthy subjects and patients with disease. It is important to note that our brachial artery blood flow responses to intra-arterial infusions of acetylcholine, substance P and sodium nitroprusside are similar to those previously reported in the forearm of healthy subjects (Taddei et al. 1997a,b; DeSouza et al. 2000,2002). However, direct comparisons of these data are limited owing to methodological variations in blood flow measurement techniques and drug doses between studies.
While the forearm is a well-established experimental model for studying human vascular function, it may be of limited consequence to systemic cardiovascular control because of its small muscle mass and blood flow requirements. However, the leg requires a greater percentage of cardiac output than the forearm owing to the fact that it is approximately 10 times the size of the forearm. Relatively few studies have used the leg as a model for investigating vascular function because of the complexity of femoral artery catheterization and the likelihood of systemic drug effects. However, the vasculature of the leg is the preferred model when examining skeletal muscle blood flow during exercise. Fortunately, a few studies investigating exercise hyperaemia have examined the effects of infused endothelium-dependent and -independent vasodilators into the femoral artery during rest. Our absolute femoral artery blood flow responses to intra-arterial infusions of acetylcholine and sodium nitroprusside are similar to those reported in this literature (Radegran & Saltin, 1999; Kingwell et al. 2003). Once again, direct comparisons of these data are limited owing to methodological variations in blood flow measurement techniques and drug doses between studies.
The similarities between our blood flow responses to both endothelium-dependent and -independent vasodilators and those reported elsewhere support our findings that the leg exhibits smaller relative increases in blood flow and vascular conductance than the forearm.
Mechanisms
A number of studies in the literature suggest that the human forearm and leg differ in their blood flow response to a variety of stimuli (Imadojemu et al. 2001; Pawelczyk & Levine, 2002). In addition, studies performed in miniature swine have demonstrated heterogeneous vascular responses to endothelium-dependent vasodilators in the brachial and femoral arteries (Laughlin et al. 1998). These findings of heterogeneous vascular responses in both humans and animals suggest phenotypic differences in vascular endothelium and smooth muscle throughout the vascular tree. Moreover, it is widely accepted that both endothelial and vascular smooth muscle cell function are not ubiquitous throughout the vasculature (Hill et al. 2001). These differences in function have been attributed to variations in a number of factors, such as receptor expression and activation, structural proteins and proteins associated with signal transduction processes (Hill et al. 2001). However, it is unclear whether these regional differences in endothelial and vascular smooth muscle cell function are predetermined during embryonic development or governed by the microenvironment in which they reside (Aird, 2003).
In support of the latter of these two hypotheses, research using coarctation of the aorta to induce forelimb hypertension in animals has demonstrated attenuated vascular responses to endothelium-dependent vasodilators in vascular rings proximal to the obstruction (Lockette et al. 1986; Miller et al. 1987; Lai et al. 1989; Bell & Bohr, 1991; Bell, 1993). This model of hypertension has parallels to the upright human condition because it creates blood pressure differences in the forelimb and hindlimb of approximately 55–65 mmHg (Bell & Bohr, 1991; Bell, 1993). These blood pressure differences are similar to those reported between the arms and legs of upright humans (Rowell, 1993; Malhotra et al. 2002). It is not surprising then that our findings of smaller relative increases in blood flow and vascular conductance in the leg in response to endothelium-dependent vasodilators are in agreement with data collected in the aortic coarctation model. Taken together, these findings suggest that increased blood pressure in the leg during upright posture may be one potential mechanism underlying reductions in endothelial vasodilator function. However, it is important to acknowledge that other factors, such as fluid shear stress, may also play a pivotal role in endothelial cell phenotype (Topper & Gimbrone, 1999).
Our blood flow and vascular conductance responses to sodium nitroprusside also may suggest phenotypic differences in vascular smooth muscle function. As previously mentioned, it is well established that the vasculature is made up of a mosaic of phenotypically different vascular smooth muscle cells (Archer, 1996; Hill et al. 2001). It is thought that the interaction of the vascular smooth muscle cells with their environment may contribute to their heterogeneity (Daemen & De Mey, 1995; Hill et al. 2001). In support of this, functional changes of vascular smooth muscle have been reported in animals (Lockette et al. 1986; Otsuka et al. 1988; Lai et al. 1989) and humans (Gardiner et al. 1994) exposed to elevated blood pressures through aortic coarctation. Specifically, vessels exposed to elevated blood pressures have been reported to have a decreased sensitivity (Lockette et al. 1986; Otsuka et al. 1988; Lai et al. 1989) and maximal responsiveness (Gardiner et al. 1994) to a variety of nitrovasodilators. Our responses to sodium nitroprusside in the leg are in agreement with these reported changes. These findings indicate that differences in blood pressure during upright posture may potentially bring about phenotypic changes to the vascular smooth muscle, which in turn may alter their response to endothelium-derived relaxing factors. However, it is important to note that phenotypic differences in vascular smooth muscle function and morphology have been reported within branches of the same vascular bed, which are exposed to equal blood pressures (Daemen & De Mey, 1995; Hill et al. 2001). This suggests that blood pressure is just one of many factors which may contribute to vascular smooth muscle heterogeneity.
Part of the differences we observed in forearm and leg responses to vasodilators may be attributed to a 'ceiling effect' if resting blood flow represents a higher percentage of maximal blood flow in the leg than the arm. Our data suggest that resting blood flow, when expressed as a percentage of peak reactive hyperaemia, was approximately 8% greater in leg than forearm. However, this calculation relies on the assumption that reactive hyperaemia elicited by 10 min of ischaemia in the arm or leg produces a maximal blood flow response. Although no previous studies have addressed this assumption, many of our subjects' blood flow responses to intra-arterial infusions of acetylcholine exceeded their responses to 10 min of ischaemia. Therefore, although a ceiling effect is possible, defining the maximal blood flow is problematic.
Limitations
All infusion rates were normalized to limb volume to ensure equal concentrations in the vasculature of the forearm and leg. However, an important assumption of the present study is that the percentages of skin, fat and muscle tissue in the forearm and leg are relatively equal. Based upon surface-to-volume ratio calculations the forearm is comprised of a larger percentage of skin tissue than the leg. These differences in tissue composition may have played a potential role in our observed forearm and leg responses to endothelium-dependent and -independent vasodilators. However, based upon research describing relatively equal distribution of lean and fat tissue in the arms and legs of young men when measured by dual-energy X-ray absorptiometry, we believe this is unlikely (Nindl et al. 2002). Another important assumption when comparing the responsiveness of two distinct vascular beds to intra-arterial infusions of endothelium-dependent and -independent vasodilators is that both the microvascular and receptor (muscarinic and neurokinin) density are similar. Unfortunately, there is no literature that we know of that compares these variables in the forearm and leg.
We chose to express our data as a percentage increase in blood flow above baseline based upon literature suggesting that it is the preferred method of expression when interventions cause vasodilatation or vasoconstriction and baseline blood flow values are statistically different (Thomas et al. 1994; Buckwalter & Clifford, 2001; Tschakovsky et al. 2002; Rosenmeier et al. 2003). A potential problem of expressing our data in this manner is that any errors in our baseline measurements will directly affect the magnitude of responses observed. However, we are confident that this did not occur based upon our ability to consistently reproduce our baseline blood flows between interventions (Tables 2 and 3). In addition, our baseline blood flows are consistent with those previously reported in the brachial and femoral arteries (Radegran, 1997; Dinenno et al. 1999; Tschakovsky et al. 2002).
When intra-arterial infusions are used to elicit regional vascular responses of the forearm and leg, the baroreflex-mediated increases in muscle sympathetic nerve activity are minimized (Pawelczyk & Levine, 2002). However, we still found evidence of small systemic effects, as mean arterial pressure was reduced at the highest infusion rates of substance P and sodium nitroprusside in the leg (Table 3). These data suggest that a baroreflex-mediated increase in muscle sympathetic nerve activity may have opposed vasodilatation in the leg during the highest infusion rates of substance P and sodium nitroprusside. However, we believe that it is highly unlikely that baroreflex-mediated increases in muscle sympathetic nerve activity can account for the marked limb differences we observed in blood flow and vascular conductance. This is based on the findings that blood flow in the leg opposite the drug infusion was not reduced during infusions of acetylcholine and substance P.
Possible clinical significance
Recently, Malhotra et al. (2002) suggested that the higher susceptibility of the lower extremities to plaque formation when compared to the upper extremities was a direct function of blood pressure differences between these limbs during upright posture (Malhotra et al. 2002). This hypothesis is based upon the findings that the extent and severity of atherosclerosis is directly associated with the degree of hypertension (Glagov et al. 1961). The effects of hypertension on endothelial cell integrity and function have long been hypothesized to play a pivotal role in atherogenesis (Moore, 2002). Specifically, decreased nitric oxide availability due to attenuated endothelial cell function has been linked to smooth muscle cell proliferation, which is a crucial component in the formation of atherosclerosis. Our data are consistent with the possibility that stimulated nitric oxide release through intra-arterial infusions of acetylcholine and substance P is reduced in the vasculature of the leg compared to the forearm. These findings support the hypothesis of Malhotra et al. (2002) by suggesting that differences in limb blood pressure during upright posture may negatively impact the endothelium of the lower extremities, rendering them less effective at protecting the vasculature from plaque formation. However, it is important to note that the carotid arteries of upright humans are exposed to lower blood pressures than the arms and yet appear to be particularly susceptible to peripheral arterial disease (Moore, 2002). Based on these findings it is clear that other factors, such as turbulent blood flow and wall shear stress, may also play a significant role in the genesis of peripheral arterial disease between limbs.
Conclusion
In conclusion, our findings suggest that the leg exhibits smaller relative increases in blood flow and vascular conductance than the forearm in response to intra-arterial infusions of endothelium-dependent and -independent vasodilators. We hypothesize that these findings could be a consequence of elevated blood pressure in the leg during upright posture, which leads to phenotypic alterations of both the endothelial and vascular smooth muscle cells. Our results also suggest that future studies investigating peripheral vascular function in humans should take functional vascular heterogeneity into account.
Acknowledgments
The authors would like to thank Kristen S. Gray, Michael D. Herr and Shelly A. Silber for excellent technical support. The authors would also like to thank Chester A. Ray and Kevin D. Monahan for their critical review of this manuscript. This study was supported by RO1 AG 18246 (D. N. Proctor), RO1 HL 68699 (U. A. Leuenberger), T32 AG 00048 (S. C. Newcomer) and M01 RR 10732 (General Clinical Research Center).
References
- Aird WC. Endothelial cell heterogeneity. Crit Care Med. 2003;31:S221–S230. doi: 10.1097/01.CCM.0000057847.32590.C1. [DOI] [PubMed] [Google Scholar]
- Franklin BA, editor. American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. Philadelphia, PA, USA: Lippincott; 2002. [Google Scholar]
- Archer SL. Diversity of phenotype and function of vascular smooth muscle cells. J Laboratory Clin Med. 1996;127:524–529. doi: 10.1016/s0022-2143(96)90142-0. [DOI] [PubMed] [Google Scholar]
- Bell DR. Vascular smooth muscle responses to endothelial autacoids in rats with chronic coarctation hypertension. J Hypertens. 1993;11:65–74. doi: 10.1097/00004872-199301000-00010. [DOI] [PubMed] [Google Scholar]
- Bell DR, Bohr DF. Endothelium in functional aortic changes of coarctation hypertension. Am J Physiol. 1991;260:H1187–H1193. doi: 10.1152/ajpheart.1991.260.4.H1187. [DOI] [PubMed] [Google Scholar]
- Berry CL, Greenwald SE. Effects of hypertension on the static mechanical properties and chemical composition of the rat aorta. Cardiovasc Res. 1976;10:437–451. doi: 10.1093/cvr/10.4.437. [DOI] [PubMed] [Google Scholar]
- Bevan RD. An autoradiographic and pathological study of cellular proliferation in rabbit arteries correlated with an increase in arterial pressure. Blood Vessels. 1976;13:100–128. doi: 10.1159/000158083. [DOI] [PubMed] [Google Scholar]
- Bevan RD, van Marthens E, Bevan JA. Hyperplasia of vascular smooth muscle in experimental hypertension in the rabbit. Circ Res. 1976;38:58–62. doi: 10.1161/01.res.38.6.58. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev. 2001;29:159–163. doi: 10.1097/00003677-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Cooke JP. Endothelium-derived factors and peripheral vascular disease. Cardiovasc Clin. 1992;22:3–17. [PubMed] [Google Scholar]
- Daemen MJ, De Mey JG. Regional heterogeneity of arterial structural changes. Hypertension. 1995;25:464–473. doi: 10.1161/01.hyp.25.4.464. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol. 2002;542:255–262. doi: 10.1113/jphysiol.2002.019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Gardiner HM, Celermajer DS, Sorensen KE, Georgakopoulos D, Robinson J, Thomas O, Deanfield JE. Arterial reactivity is significantly impaired in normotensive young adults after successful repair of aortic coarctation in childhood. Circulation. 1994;89:1745–1750. doi: 10.1161/01.cir.89.4.1745. [DOI] [PubMed] [Google Scholar]
- Glagov S, Rowley DA, Kohut RI. Atherosclerosis of human aorta and its coronary and renal arteries. A consideration of some hemodynamic factors which may be related to the marked differences in atherosclerotic involvement of the coronary and renal arteries. Arch Pathol. 1961;72:558–571. [PubMed] [Google Scholar]
- Groothuis JT, van Vliet L, Kooijman M, Hopman MT. Venous cuff pressures from 30 mmHg to diastolic pressure are recommended to measure arterial inflow by plethysmography. J Appl Physiol. 2003;95:342–347. doi: 10.1152/japplphysiol.00022.2003. [DOI] [PubMed] [Google Scholar]
- Hill CE, Phillips JK, Sandow SL. Heterogeneous control of blood flow amongst different vascular beds. Med Res Rev. 2001;21:1–60. doi: 10.1002/1098-1128(200101)21:1<1::aid-med1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hollander W, Madoff I, Paddock J, Kirkpatrick B. Aggravation of atherosclerosis by hypertension in a subhuman primate model with coarctation of the aorta. Circ Res. 1976;38:63–72. doi: 10.1161/01.res.38.6.63. [DOI] [PubMed] [Google Scholar]
- Imadojemu VA, Lott ME, Gleeson K, Hogeman CS, Ray CA, Sinoway LI. Contribution of perfusion pressure to vascular resistance response during head-up tilt. Am J Physiol Heart Circ Physiol. 2001;281:H371–H375. doi: 10.1152/ajpheart.2001.281.1.H371. [DOI] [PubMed] [Google Scholar]
- Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care. 2003;26:899–904. doi: 10.2337/diacare.26.3.899. [DOI] [PubMed] [Google Scholar]
- Lai FM, Cobuzzi A, Shepherd C, Tanikella T, Hoffman A, Cervoni P. Endothelium-dependent basilar and aortic vascular responses in normotensive and coarctation hypertensive rats. Life Sci. 1989;45:607–614. doi: 10.1016/0024-3205(89)90046-5. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, McAllister RM, Jasperse JL, Hitchcock LS, Bonagura JD. Acetylcholine is a vasodilator of porcine skeletal muscle arteries. Comp Biochem Physiol A Mol Integr Physiol. 1998;120:345–354. doi: 10.1016/s1095-6433(98)10035-1. [DOI] [PubMed] [Google Scholar]
- Lockette W, Otsuka Y, Carretero O. The loss of endothelium-dependent vascular relaxation in hypertension. Hypertension. 1986;8(II):61–66. doi: 10.1161/01.hyp.8.6_pt_2.ii61. [DOI] [PubMed] [Google Scholar]
- Lott ME, Herr MD, Sinoway LI. Effects of transmural pressure on brachial artery mean blood velocity dynamics in humans. J Appl Physiol. 2002;93:2137–2146. doi: 10.1152/japplphysiol.00443.2002. [DOI] [PubMed] [Google Scholar]
- Lott ME, Hogeman CS, Vickery L, Kunselman AR, Sinoway LI, MacLean DA. Effects of dynamic exercise on mean blood velocity and muscle interstitial metabolite responses in humans. Am J Physiol. 2001;281:H1734–H1741. doi: 10.1152/ajpheart.2001.281.4.H1734. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Cohen D, Syms C, Townsend RR. Blood pressure changes in the leg on standing. J Clin Hypertens (Greenwich) 2002;4:350–354. doi: 10.1111/j.1524-6175.2002.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Pinto A, Mullane KM. Impaired endothelium-dependent relaxations in rabbits subjected to aortic coarctation hypertension. Hypertension. 1987;10:164–170. doi: 10.1161/01.hyp.10.2.164. [DOI] [PubMed] [Google Scholar]
- Moore WS. Vascular Surgery: a Comprehensive Review. Philadelphia: W.B. Saunders Company; 2002. [Google Scholar]
- Nindl BC, Scoville CR, Sheehan KM, Leone CD, Mello RP. Gender differences in regional body composition and somatotrophic influences of IGF-I and leptin. J Appl Physiol. 2002;92:1611–1618. doi: 10.1152/japplphysiol.00892.2001. [DOI] [PubMed] [Google Scholar]
- Otsuka Y, DiPiero A, Hirt E, Brennaman B, Lockette W. Vascular relaxation and cGMP in hypertension. Am J Physiol. 1988;254:H163–H169. doi: 10.1152/ajpheart.1988.254.1.H163. [DOI] [PubMed] [Google Scholar]
- Owens GK, Reidy MA. Hyperplastic growth response of vascular smooth muscle cells following induction of acute hypertension in rats by aortic coarctation. Circ Res. 1985;57:695–705. doi: 10.1161/01.res.57.5.695. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect. J Appl Physiol. 2002;92:2105–2113. doi: 10.1152/japplphysiol.00979.2001. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Halliwill JR, Shen PH, Vlahakis NE, Joyner MJ. Peak calf blood flow estimates are higher with Dohn than with Whitney plethysmograph. J Appl Physiol. 1996;81:1418–1422. doi: 10.1152/jappl.1996.81.3.1418. [DOI] [PubMed] [Google Scholar]
- Radegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol. 1999;276:H1951–H1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. α1- and α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol. 2003;547:971–976. doi: 10.1113/jphysiol.2002.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Cyclooxygenase inhibition restores nitric oxide activity in essential hypertension. Hypertension. 1997a;29:274–279. doi: 10.1161/01.hyp.29.1.274. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997b;29:736–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. Inhibition of α2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol. 1994;266:H920–H929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- Topper JN, Gimbrone MA., Jr Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today. 1999;5:40–46. doi: 10.1016/s1357-4310(98)01372-0. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle. J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H, Kanellakis P, Agrotis A, Bobik A. Blood flow regulates the development of vascular hypertrophy, smooth muscle cell proliferation, and endothelial cell nitric oxide synthase in hypertension. Hypertension. 2000;36:89–96. doi: 10.1161/01.hyp.36.1.89. [DOI] [PubMed] [Google Scholar]
- Wascher TC, Bammer R, Stollberger R, Bahadori B, Wallner S, Toplak H. Forearm composition contributes to differences in reactive hyperaemia between healthy men and women. Eur J Clin Invest. 1998;28:243–248. doi: 10.1046/j.1365-2362.1998.00270.x. [DOI] [PubMed] [Google Scholar]